Abstract
Background:
Intraarticular corticosteroid injection is an adjunct to core treatments for relief of moderate-to-severe pain in osteoarthritis (OA) patients. This randomized controlled trial was conducted to determine the effect of dexamethasone phonophoresis (DxPh) on knee OA.
Patients and Methods:
Forty six female patients with knee OA were randomized into two equal groups. The study group received DxPh over the medial side of the knee, transcutaneous electrical nerve stimulation (TENS), and quadriceps strengthening exercises. Control group received ultrasound therapy and the same TENS and exercise program. Pain was assessed using the visual analog scale (VAS) and the pain subscale of Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pre- and posttreatment. Functional mobility was assessed by the Timed Up and Go (TUG) test, total WOMAC, and the joint stiffness and physical function subscales of WOMAC. The minimal clinically identifiable difference was used to calculate treatment effect sizes of both modalities, which was compared to intraarticular steroid injections.
Results:
The VAS, TUG, and WOMAC scores improved with both modalities. Pain intensity improved by 50.6%–58.0% in the study group (VAS and pain subscale of WOMAC, respectively) compared to 17.8%–28.6% for the control group. Functional mobility showed a higher rate of improvement in the DxPh group compared to control (37.7 vs. 17.5% for TUG and 53.2 vs. 23.0 and 56.1 vs. 26.4% for the joint stiffness and physical function subscales of WOMAC, respectively). Posttreatment results revealed statistically and clinically significant improvement in pain intensity and functional mobility in the DxPh group.
Conclusion:
DxPh resulted in a greater improvement in pain and function in patients with knee OA than therapeutic ultrasound combined with exercise and TENS. The effect size of phonophoresis was clinically significant and higher than that reported for intraarticular steroid injection from pooled data in the literature.
Keywords: Knee osteoarthritis, pain and function, phonophoresis, physical therapy, patient-reported outcome measures, Western Ontario and McMaster Universities Osteoarthritis Index
Introduction
Osteoarthritis (OA) is characterized by the breakdown of articular cartilage over time. Although cartilage change is the major disease characteristic, OA affects all joint tissues, including the synovial membrane, which is usually associated with increased pain and joint dysfunction.1,2 Evidence-based guidelines on the conservative treatment of knee OA are riddled with inconclusive and consensus recommendations due to the inadequacy of clinical trials addressing certain aspects of the treatment modalities.3,4 A very recent study found that only 25% of nonsurgical trials registered on ClinicalTrials.gov were actually relevant to recommendations made within the AAOS conservative management guidelines, with the greatest number of new or ongoing trials addressing a recommendation which is already supported by strong evidence.4 The authors of this article called for continued attention to research gaps in the current guidelines (AAOS) and praised investigators of ongoing trials designed to explore the 8 (44%) inconclusive or consensus recommendations.4 Two of those inconclusive recommendations (nonsurgical 3B and 8) relate to the use of physical agents (including electrotherapeutic modalities) and intraarticular corticosteroids in patients with symptomatic knee OA.
Phonophoresis is widely used as a physical enhancer of absorption of drugs by ultrasound waves. It enhances the absorption of topically applied drugs by increasing skin permeability to topical medications. Phonophoresis is applied in the same manner as ultrasound, except that a medication is used in the coupling agent.5,6
Intraarticular corticosteroid injection is considered an adjunct treatment to core treatments for the relief of moderate-to-severe pain in OA patients.7 Corticosteroids produce antiinflammatory and immunosuppressive effects by reducing vascular permeability, inhibiting the accumulation of inflammatory cells and preventing the synthesis and secretion of several inflammatory mediators.8,9 A recent network meta-analysis of high- and moderate-quality studies ranked intraarticular corticosteroids as the most likely conservative treatment to reduce pain based on cumulative probability, with nonsteroidal anti-inflammatory drugs (NSAIDs) having a higher probability of improving function.10 Drug delivery through the skin avoids adverse effects that may occur with other routes such as oral or intraarticular injection. Dexamethasone phonophoresis (DxPh) was shown to improve the pain and function of patients with several musculoskeletal conditions,11 including knee OA.12,13 However, previous studies concentrated only on gait parameters,12 rather than reporting functional improvement, which might be more relevant from the patient's perspective.
The purpose of this study was to investigate the additive effect that dexamethasone has on ultrasound therapy for improving the pain and function of patients with knee OA using patient-reported outcome measures (PROMS) and objective functional tests. We also aimed to indirectly compare the effect size of each treatment to that of intraarticular corticosteroids generated from network meta-analysis reports.
Patients and Methods
Study subjects
This study was conducted as a double-blind, randomized, controlled trial (full details including ethical approval are available under the Pan African Clinical Trial Registration number PACTR201711002410392). It included patients with bilateral medial tibiofemoral OA, who were referred by the orthopedic department. All the patients were diagnosed and referred by a physician according to the following criteria: bilateral mild-to-moderate medial tibiofemoral OA based on the American College of Rheumatology criteria14,15 and a radiological image (Kellgren–Lawrence Grade II–III).16,17 All the patients were informed about the study and consented to participate in it.
Patients were excluded from the study if they had rheumatologic conditions such as rheumatoid arthritis, severe knee OA, thrombosis of the lower limbs, physiotherapy treatment of the knee in the previous 6 months, a history of injections in the knee joint during the last 6 months, balance disorders, neuropathy or sensory disorders, skin damage around the knee, previous surgery on the knee joint, or a previous fracture of the lower extremity with knee joint involvement. Patients were also excluded if they had any contraindications or precautions for the use of corticosteroids (e.g., high blood pressure, osteoporosis, or diabetes) or ultrasound (e.g., infection, heart problems, pacemaker, metal implants, open epiphysis, pregnancy, thrombophlebitis, or impaired sensation).
Significant age-related differences in performance were found in tests of coordinated stability, near tandem balance, six-meter walk, alternate step, five repetitions sit to stand, and stair negotiation, with older women performing worse than older men in all tests.18
Forty six female patients with bilateral knee OA whose ages ranged from 40 to 65 years and body mass indexes ranged from 25 to 35 kg/m2 met the eligibility criteria and were randomized into two treatment groups using computer-generated numbers in sealed envelopes. The patients were blinded to the group allocation. Group A received DxPh and conventional physical therapy treatment12,19 in the form of transcutaneous electrical nerve stimulation (TENS) current and exercise (study group). Group B received ultrasound therapy and the same conventional physical therapy treatment (control group).
Evaluation procedure
The primary outcome measure was knee pain measured by the visual analog scale (VAS) and Western Ontario and McMaster Universities OA Index (WOMAC) pain subscale. The secondary outcome was functional mobility measured using the stiffness and physical function subscales of the WOMAC, total WOMAC, and TUG. Each patient was assessed 1 day before starting the first session and after the last session according to the same criteria by an assessor who was blinded to the patient's allocation. Initially, all patients were questioned about their personal data, including age, weight, height, onset and duration of knee pain, and any history of chronic diseases. The measured outcomes included the VAS within the last 24 h, WOMAC, and Timed Up and Go (TUG) test scores. The VAS (0–100 mm) ranges from “no pain” to “extreme pain.“20 The WOMAC questionnaire consists of three subscales that are scored as follows: pain = 0–20, stiffness = 0–8, and physical function = 0–68. The total WOMAC score consists of the sum of the items for all three subscales. Higher WOMAC scores indicate worse pain, stiffness, and functional limitations.21 The TUG test measures the time, in seconds, required for an individual to stand up from a standard arm chair, walk a distance of 3 meters, turn, walk back to the chair, and sit down again. The patient walks at a safe pace with proper footwear and uses an assistive aid if needed.22
Treatment procedure
Treatment was conducted from August 2017 to November 2017 in the physical therapy outpatient clinic. The total duration of treatment was 4 weeks, and the treatments were conducted on alternate days with a frequency of 3 sessions/week. The treatment targeted the most affected and painful knee. For patients with equal pain in both knees, the dominant knee was treated.23
Both groups received the same exercise program during every session for strengthening the quadriceps muscle. The dose was three sets of ten repetitions, with the starting weight being matched to the ten-repetition maximum weight of each participant. The following quadriceps exercises were used: (1) quads over a roller (inner range knee extension) using ankle weights for resistance; (2) knee extension in sitting (sitting with the knee at 90° flexion and performing full extension using ankle weights for resistance); (3) straight leg raise (starting in the supine position and raising the leg to 30° hip flexion using ankle weights for resistance); and (4) outer range knee extension (sitting with the knee at 90° flexion and extending to 60° against the resistance of an elastic band).24,25 Patients were not instructed to use any special footwear or insole during treatment to reduce variables and interactions as much as possible.
TENS was applied to both groups at a frequency of 80 Hz for 20 min (using the Endomed 182 ENRAF NONIUS, Netherlands) through four adhesive electrodes placed over the medial side of the knee region with pulse width constant among all patients and an intensity in the tactile sensation threshold.26 Ultrasound was applied to the medial tibiofemoral joint in a continuous mode at a frequency of 1 MHz and an intensity of 1 W/cm2 for a treatment duration of 10 min (Pulson 100 Gymna Uniphy, Belgium) using ultrasound gel for the control group and 0.4% dexamethasone gel (locally prepared at the Faculty of Pharmacy, Cairo University) for the phonophoresis.
Dexamethasone gel (0.4%) was locally prepared using a colloid mill by incorporating the active dexamethasone (purchased from “Zhejiang Xianju Xianle Pharmaceutical Co., China“) into the gel matrix. The prepared gel was tested for its rheological properties and drug content to ensure proper consistency and active drug concentration. The active gel was then used as a coupling medium for phonophoresis study group.23,27,28,29,30 The participants were positioned in a supine position with a roller under the affected knee to maintain flexion at 90°, and the ultrasound device was held over the tibiofemoral joint medial to the patellar tendon to enhance energy penetration into the joint space. The optimum position for the transducer was near the joint line.31,32
Statistical analysis
Sample size estimation was performed a priori for the pain scores (primary outcome measure) using G power 3.1 software (Department of Psychology, Dusseldorf University, Germany). A change of 18-mm in VAS score was considered as the minimum clinically important difference in pain, based on previously published literature.33 When setting the alpha level to 0.05 and the test power to 90%, a minimum of 42 participants was required for the whole study. Forty six participants were recruited to allow for loss to followup.
The statistical analysis was conducted using SPSS for Windows, version 22 (SPSS, Inc., Chicago, IL, USA). Before the final analysis, the data were screened for normality assumptions, homogeneity of variance, and presence of extreme scores. This exploration was performed as a prerequisite for parametric calculations of the analysis of variance. Box and whisker plots of each of the tested variables were constructed to detect any outliers. A descriptive analysis using histograms with a normal distribution curve showed that the data were normally distributed and did not violate the parametric assumption for each of the measured dependent variables.
All of the above-mentioned tests confirmed normality and homogeneity, which allowed the researchers to conduct a parametric analysis. Accordingly, a 2 × 2 mixed-design multivariate analysis of variance (MANOVA) was used to compare the tested variables of interest for the two groups and at various measurement times with an initial alpha level set at 0.05. The current study involved two independent variables. The first was the treatment (between-subject factor), which had two levels (Group A, which received DxPh, and Group B, which received ultrasound therapy). The second variable was the measurement time (within-subject factor), which had two levels (pre- and posttreatment). In addition, the study involved five tested dependent variables (VAS, TUG test, WOMAC pain, stiffness, and physical function subscale scores). A paired t-test was used to compare the total WOMAC score before and after treatment for each group. Unpaired t-tests were conducted to compare results between the two groups using an alpha level of 0.05.
In order to allow indirect comparison with other nonsurgical treatment modalities, the WOMAC function score was converted to a 0–100 scale and the effect size of each treatment was calculated by dividing the uniform pain (0–100 VAS) and function (converted WOMAC function scale) scores by minimal clinically important difference (MCID) units. The latter was quoted in a recent network meta-analysis of nonsurgical treatment of knee OA to be a reduction of 19.1 units on a 100-mm VAS scale and a reduction of 8.0 units on a 100-mm WOMAC function scale. An effect size >0.5 indicates a statistically significant difference between treatments with potential clinical significance for an appreciable number of patients and an effect size >1 indicates a clinically significant difference between treatments.10
Results
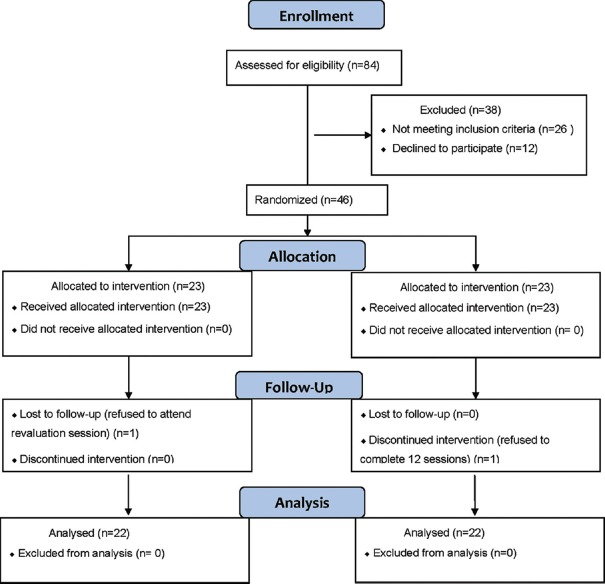
The flow diagram of the participants throughout the trial is shown in Figure 1. A total of 84 patients were assessed. Twenty six patients who did not meet the inclusion criteria and 12 who declined to participate were excluded. One patient in each group was lost to followup [reasons indicated in Figure 1]. The study population consisted of 44 female patients suffering from knee OA who were assigned randomly to two equal groups. As indicated by the independent t-test, no significant differences (P > 0.05) were observed in the mean values for age, body mass, or height between the two tested groups [Table 1].
Figure 1.
Flow diagram of the present study
Table 1.
Summary of the physical characteristics of both patient groups
| Items | Group A | Group B | Comparison | Significance | |
|---|---|---|---|---|---|
| Mean±SD | Mean±SD | t | P | ||
| Age (years) | 53.09±5.46 | 50.59±6.77 | 1.347 | 0.185 | NS |
| Body mass (kg) | 76.90±8.30 | 76.54±10.27 | 0.129 | 0.898 | NS |
| Height (cm) | 156.77±7.29 | 160.63±10.18 | -1.446 | 0.156 | NS |
| BMI (kg/m2) | 31.25±2.22 | 29.65±2.8 | 2.096 | 0.05 | NS |
*SD: standard deviation; P: probability; S: significant; NS: non-significant
The statistical analysis using the 2 × 2 mixed-design MANOVA indicated significant effects of the treatment (the first independent variable) on all tested dependent variables (the VAS, TUG, and WOMAC subscales) for the two groups. Similarly, significant effects were observed for the measurement times (the second independent variable) on the tested dependent variables. In addition, the interaction between the two independent variables was significant [Table 2], which indicated that the effect of the treatment (first independent variable) on the dependent variables was influenced by the measurement time (second independent variable).
Table 2.
The 2×2 mixed-design multivariate analysis of variance (MANOVA) for all dependent variables between the two groups at different measurement times
| Source of variation | F | P |
|---|---|---|
| Groups | 3.286 | 0.015* |
| Measurement times | 105.908 | 0.0001* |
| Interaction | 18.031 | 0.0001* |
*Significant at an alpha level of <0.05
The within-groups comparison revealed a significant posttreatment reduction in the VAS and pain subscale of the WOMAC compared to the pretreatment scores for both groups [Table 3]. The study group had a 50.56%–58.05% improvement in pain intensity (VAS and pain subscale of WOMAC, respectively) compared to 17.8%–28.64% for the control group (mean difference [MD]: −19.82; 95% confidence interval [CI]: −30.43–−9.21 for VAS and MD: −2.68; CI: −4.64–−0.72 for pain subscale).
Table 3.
Summary of the pre-treatment and post-treatment mean±SD and P values of the outcome measures for both groups
| Outcome measures | Group A | Group B | P (between groups) | |
|---|---|---|---|---|
| Mean±SD | Mean±SD | |||
| VAS | Pre-treatment | 80.81±13.92 39.95±16.1** |
72.72±17.53 59.77±18.68** |
0.098 0.001* |
| Post-treatment | ||||
| TUG | Pre-treatment | 12.07±1.85 7.51±1.27** |
10.81±2.38 8.91±2.78** |
0.058 0.038* |
| Post-treatment | ||||
| WOMAC pain | Pre-treatment | 14.4±3.17 6.04±2.14** |
12.22±4.2 8.72±4.01** |
0.059 0.008* |
| Post-treatment | ||||
| WOMAC stiffness | Pre-treatment | 6.31±1.28 2.95±1.5** |
5.31±1.93 4.09±1.84** |
0.05 0.032* |
| Post-treatment | ||||
| WOMAC function | Pre-treatment | 45.45±9.43 19.95±7.22** |
44.54±9.21 32.77±10.91** |
0.748 0.0001* |
| Post-treatment | ||||
| WOMAC total | Pre-treatment | 66.45±12.03 28.95±8.69** |
61.63±13.36 46.04±15.35** |
0.216 0.0001* |
| Post-treatment | ||||
*Significance level is set at an alpha level of <0.05. SD: standard deviation. P: Probability value. **Statistically significant difference from the pre-treatment value for the same group
The TUG test and stiffness and physical function subscales of the WOMAC were significantly improved posttreatment compared to the pretreatment values for both groups (multiple pairwise post hoc comparison tests used for all). Functional mobility likewise showed a higher rate of improvement in the phonophoresis group compared to control (37.69% vs. 17.48% for TUG [MD: −1.40; CI: −2.72–−0.08] and 53.24 vs. 22.97 [MD: −1.136; CI: −2.17–−0.11] and 56.1 vs. 26.42% [MD: −12.82; CI: −18.45–−7.18] for the joint stiffness and physical function subscales of the WOMAC, respectively). Significant differences were observed for the posttreatment mean values for all outcome measures between the two groups, favoring group A. Comparison of the mean pretreatment values showed no significant differences between the two groups.
A significant reduction in the total WOMAC score was observed after treatment compared to the pretreatment value for both groups (paired t-test). An unpaired t-test revealed that the mean values for each parameter were not significantly different between the two groups before treatment. However, significant differences were found in the mean values after treatment between the two groups, which favored group A. The rate of improvement of the total WOMAC after treatment was 56.43% for group A compared to 25.29% for Group B (MD: −17.09; CI: −24.68–−9.5).
Both treatment groups demonstrated a clinically significant improvement in function [Table 4]. Whereas the phonophoresis group reached a clinically significant effect size (−2.14), the ultrasound group demonstrated only a potential to be clinically significant in reducing pain (effect size −0.68). Direct comparison of both the groups showed a clinically significant pain reduction and improvement in function in favor of steroid phonophoresis (effect size −1.02 and −2.52, respectively).
Table 4.
Summary of pain reduction and function improvement for each group and between groups and their clinical significance
| Comparison | Pain reduction | Effect size | Function improvement | Effect size |
|---|---|---|---|---|
| Mean (95% CI) | MCID units | Mean (95% CI) | MCID units | |
| Group A (post vs. pre-treatment) | -40.86 (-46.49 to -35.24) | -2.14** | -37.5 (-42.88 to -32.12) | -4.69** |
| Group B (post vs. pre-treatment) | -12.96 (-18.58 to -7.33) | -0.68* | -17.31 (-23.27 to -11.35) | -2.16** |
| Group A vs. Group B | -19.82 (-30.43 to -9.21) | -1.02** | -20.19 (-28.45 to -11.93) | -2.52** |
*≥0.5 indicates a statistically significant difference between treatments with potential clinical significance for an appreciable number of patients. **≥1 indicates a clinically significant difference between treatments
Discussion
This study was conducted to report the effect of dexamethasone combined with therapeutic ultrasound using PROMs and objective functional tests. Our results showed that DxPh had a positive influence on pain and function in patients with mild-to-moderate knee OA. Both the groups improved in terms of pain (VAS and the pain subscale of the WOMAC) and function (TUG test, stiffness and physical function subscales of the WOMAC, and the total WOMAC) following the treatment. However, the improvement was significantly greater in the phonophoresis group. The study was limited to female patients to avoid gender-related differences in the performance of functional assessment.18
The results of the present study are consistent with those of Elshazly et al.,12 who reported a significant improvement with DxPh in patients with knee OA compared to therapeutic ultrasound alone. However, these authors studied only the pain and gait parameters and did not study the patients’ perceptions of their own conditions as measured by PROMs. In contrast to their study, we recruited female patients with knee OA, given the higher prevalence of knee OA in women than men.34 However, Akinbo et al.13 did not demonstrate an increased therapeutic efficacy of DxPh compared to iontophoresis using the same drug in the treatment of knee OA. In that study, ultrasound phonophoresis was applied to the targeted knee for a shorter duration (5 min) compared to that in our study (10 min). The discrepancy in findings is not surprising, given the positive interaction between the treatment period and outcome measures observed in the present study. We were able to demonstrate significant efficacy of DxPh using a longer treatment duration (10 min), which is consistent with the results reported by Elshazly et al.12
Several studies showed a significant additive effect of corticosteroids and ultrasound therapy for the treatment of different musculoskeletal disorders, such as carpal tunnel syndrome11 and chronic hemophilic synovitis of the knee.35 However, other studies failed to show any significant effect of corticosteroid phonophoresis compared to ultrasound27 or exercise and cryotherapy36 for the treatment of soft tissue disorders (epicondylitis, tendinitis, and tenosynovitis).
The drug selection for phonophoresis seems to be as important as the ultrasound parameters for the success of the treatment [Table 5]. A greater accumulation of dexamethasone in the serum was demonstrated with the use of ultrasound facilitation compared to sham ultrasound applied over an occlusive dressing.37 However, hydrocortisone acetate absorption did not seem to be affected by ultrasound waves.38 This might explain the faster effect of clobetasol than hydrocortisone on OA knee joint pain that was reported by Sedghimehr and Bahrpeima.39 Similarly, the lack of efficacy in the study by Klaiman et al.27 might, at least in part, be attributed to drug selection (fluocinonide).
Table 5.
Summary of the selected studies
| Study | Drug | Treated condition | Intervention vs. comparator | Ultrasound parameters | Outcome measures | Conclusion |
|---|---|---|---|---|---|---|
| Elshazly et al. 10 | Dexamethasone sodium phosphate | Knee osteoarthritis | Phonophoresis vs. ultrasound | Phonophoresis: continuous mode; duration, 5 min; frequency, 1 MHz; intensity, 1 W/cm² Ultrasound: continuous mode; duration, 5 min; frequency, 1 MHz; intensity 0.8, W/cm² |
VAS Gait parameters |
Both effective. Phonophoresis significantly better |
| Akinbo et al. 11 | Dexamethasone sodium phosphate | Knee osteoarthritis | Phonophoresis vs. iontophoresis | Phonophoresis: duration, 5 minutes; frequency, 1 MHz | WOMAC, 20 m ambulatory time, and knee ROM | Both effective. No significant difference |
| Bakhtiary et al. 9 | Dexamethasone sodium phosphate | Carpal tunnel syndrome | Phonophoresis vs. iontophoresis | Phonophoresis: pulse mode, 1:4; duration, 5 minutes; frequency, 1 MHz; intensity, 1 W/cm² | VAS, pinch and grip strength and electro-neurographic measurement. | Phonophoresis more effective |
| Saliba et al. 34 | Dexamethasone | Drug absorption | Phonophoresis vs. occlusive dressing and sham phonophoresis | Phonophoresis: pulse mode, 1:1; duration, 5 minutes; frequency, 3 MHz; intensity, 1W/cm² | Dexamethasone concentration in serum | Phonophoresis: higher dexamethasone serum levels. Negligible with sham phonophoresis |
| Sedghimehr and Bahrpeima 36 | Hydrocortisone and clobetasol | Knee osteoarthritis | Phonophoresis vs. ultrasound, sham ultrasound and sham phonophoresis | None available (paper in Persian; abstract only in English) | VAS, Knee ROM, oedema, 20 m walking test | Phonophoresis more effective. Clobetasol had a faster effect |
| Klaiman et al. 24 | Fluocinonide | Soft tissue disorders (epicondylitis, tendinitis, and tenosynovitis) | Phonophoresis vs. ultrasound | Phonophoresis: continuous mode; duration, 8 min; intensity, 1.5 W/cm² | VAS and pressure algometry | Both modalities effective. No significant difference |
| Saraf and Singh 32 | Betamethasone | Chronic haemophilic knee synovitis | Phonophoresis | Phonophoresis: pulse mode, 1:1; duration, 5-6 min; frequency, 1 MHz | Degree of swelling, ROM, and frequencies of joint bleeding and joint tenderness | Phonophoresis effective |
| Gurney et al. 35 | Hydrocortisone acetate | Drug absorption | Phonophoresis vs. sham phonophoresis | Phonophoresis: continuous mode; duration, 6 min; frequency, 1 MHz; intensity, 1 W/cm² | Cortisone level in connective tissue | No significant difference |
| Penderghest et al. 33 | Dexamethasone- lidocaine | Tendinitis | Phonophoresis vs. sham phonophoresis | Pulsed mode | Pain (visual perceived pain scale, VPPS) and punctate tenderness gauge (PTG) | No significant difference. Effectiveness attributed to stretching, strengthening, and cryotherapy. |
A recent network meta-analysis of high- and moderate-quality studies ranked intraarticular corticosteroids as the most likely conservative treatment to reduce pain based on cumulative probability, with NSAIDs having a higher probability of improving function. When divided by the MCID, the effect size of intraarticular steroid demonstrated the “potential to be clinically significant” at decreasing pain (effect size ≥0.5). The only clinically significant treatment (effect size ≥1) for increasing function in this meta-analysis, however, was naproxen, with other NSAIDs demonstrating only a potential to be clinically significant.10 Using similar calculations, the effect size of phonophoresis compared to ultrasound in the present study was clinically significant and higher than either intraarticular corticosteroids in reducing pain (−1.02 vs. −0.55) or naproxen (−2.52 vs. −1.18) for improving function. The above authors depended on the function subscale of the WOMAC to measure improvement in function and did not include any physical or electrotherapeutic modalities. The present study, however, used an extended battery of tools to quantify functional improvement (TUG, stiffness, and function subscales of the WOMAC). Although we did not directly compare steroid phonophoresis to intraarticular corticosteroids, it would still be fair to say that phonophoresis might be even more effective based on an indirect comparison of effect sizes and would, therefore, be a valid (and more convenient) alternative to intraarticular steroid injections.
This study was limited to comparing the short term effects of DxPh with ultrasound therapy. However, the followup period of the study is consistent with the inclusion criteria of a recent network meta-analysis of best available evidence for nonsurgical treatment of knee OA.10 It might be recommended for future studies to investigate the long term effects of such treatment as well. Nevertheless, it provides a valuable building block in the body of evidence meant to fill research gaps in the recommendations of conservative management guidelines currently supported by inconclusive evidence.3,4
Conclusion
Our results showed that both DxPh and ultrasound therapy combined with conventional physical therapy in the form of TENS and therapeutic exercises had a beneficial effect on pain and functional mobility in patients with knee OA; however, significantly greater improvement was observed with DxPh.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors wish to thank Nature Research Editing Service from Springer Nature for their expert language editing. This service was provided free of charge through the Egyptian Knowledge Bank, as part of their initiative to assist Egyptian researcher gain international recognition.
References
- 1.Scanzello CR, Goldring SR. The role of synovitis in osteoarthritis pathogenesis. Bone. 2012;51:249–57. doi: 10.1016/j.bone.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 3.Jevsevar DS, Brown GA, Jones DL, Matzkin EG, Manner PA, Mooar P, et al. The American Academy of Orthopaedic Surgeons evidence-based guideline on: Treatment of osteoarthritis of the knee, 2nd edition. J Bone Joint Surg Am. 2013;95:1885–6. doi: 10.2106/00004623-201310160-00010. [DOI] [PubMed] [Google Scholar]
- 4.Scott J, Checketts JX, Horn JG, Cooper C, Vassar M. “Knee osteoarthritis and current research for evidence-are we on the right way?”. Int Orthop. 2018;42:2105–12. doi: 10.1007/s00264-018-3932-9. [DOI] [PubMed] [Google Scholar]
- 5.Gutenbrunner C, Bender T, Cantista P, Karagülle Z. A proposal for a worldwide definition of health resort medicine, balneology, medical hydrology and climatology. Int J Biometeorol. 2010;54:495–507. doi: 10.1007/s00484-010-0321-5. [DOI] [PubMed] [Google Scholar]
- 6.Polat BE, Hart D, Langer R, Blankschtein D. Ultrasound-mediated transdermal drug delivery: Mechanisms, scope, and emerging trends. J Control Release. 2011;152:330–48. doi: 10.1016/j.jconrel.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conaghan PG, Dickson J, Grant RL Guideline Development Group. Care and management of osteoarthritis in adults: Summary of NICE guidance. BMJ. 2008;336:502–3. doi: 10.1136/bmj.39490.608009.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ostergaard M, Halberg P. Intraarticular corticosteroids in arthritic disease: A guide to treatment. BioDrugs. 1998;9:95–103. doi: 10.2165/00063030-199809020-00002. [DOI] [PubMed] [Google Scholar]
- 9.Creamer P. Intraarticular corticosteroid treatment in osteoarthritis. Curr Opin Rheumatol. 1999;11:417–21. doi: 10.1097/00002281-199909000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Jevsevar DS, Shores PB, Mullen K, Schulte DM, Brown GA, Cummins DS. Mixed treatment comparisons for nonsurgical treatment of knee osteoarthritis: A Network meta-analysis. J Am Acad Orthop Surg. 2018;26:325–36. doi: 10.5435/JAAOS-D-17-00318. [DOI] [PubMed] [Google Scholar]
- 11.Bakhtiary AH, Fatemi E, Emami M, Malek M. Phonophoresis of dexamethasone sodium phosphate may manage pain and symptoms of patients with carpal tunnel syndrome. Clin J Pain. 2013;29:348–53. doi: 10.1097/AJP.0b013e318255c090. [DOI] [PubMed] [Google Scholar]
- 12.Elshazly FA, Shimaa A, Azab R, Lotfy Radwan N, Salah W, Mahmoud ED. Effect of phonophoresis on selected gait parameters in patients with knee osteoarthritis. J Am Sci. 2013;99:679–90. [Google Scholar]
- 13.Akinbo SR, Aiyejusunle CB, Akinyemi OA, Adesegun SA, Danesi MA. Comparison of the therapeutic efficacy of phonophoresis and iontophoresis using dexamethasone sodium phosphate in the management of patients with knee osteoarthritis. Niger Postgrad Med J. 2007;14:190–4. [PubMed] [Google Scholar]
- 14.Altman RD. Classification of disease: Osteoarthritis. Semin Arthritis Rheum. 1991;20:40–7. doi: 10.1016/0049-0172(91)90026-v. [DOI] [PubMed] [Google Scholar]
- 15.Altman RD. Criteria for the classification of osteoarthritis of the knee and hip. Scand J Rheumatol Suppl. 1987;65:31–9. doi: 10.3109/03009748709102175. [DOI] [PubMed] [Google Scholar]
- 16.Kohn MD, Sassoon AA, Fernando ND. Classifications in brief: Kellgren-Lawrence classification of osteoarthritis. Clin Orthop Relat Res. 2016;474:1886–93. doi: 10.1007/s11999-016-4732-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butler AA, Menant JC, Tiedemann AC, Lord SR. Age and gender differences in seven tests of functional mobility. J Neuroeng Rehabil. 2009;6:31. doi: 10.1186/1743-0003-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pietrosimone BG, Saliba SA, Hart JM, Hertel J, Kerrigan DC, Ingersoll CD. Effects of transcutaneous electrical nerve stimulation and therapeutic exercise on quadriceps activation in people with tibiofemoral osteoarthritis. J Orthop Sports Phys Ther. 2011;41:4–12. doi: 10.2519/jospt.2011.3447. [DOI] [PubMed] [Google Scholar]
- 20.Huskisson EC. Measurement of pain. Lancet. 1974;2:1127–31. doi: 10.1016/s0140-6736(74)90884-8. [DOI] [PubMed] [Google Scholar]
- 21.Bellamy N. WOMAC Osteoarthritis Index User Guide. Version VII. Brisbane, Australia; 2005; quoted in: Bellamy, N. The WOMAC Knee and Hip Osteoarthritis Indices: development, validation, globalization and influence on the development of the AUSCAN Hand Osteoarthritis Indices. Clin Exp Rheumatol. 2005;23:S148–53. [PubMed] [Google Scholar]
- 22.Podsiadlo D, Richardson S. The timed “Up and go”: A test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–8. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 23.Luksurapan W, Boonhong J. Effects of phonophoresis of piroxicam and ultrasound on symptomatic knee osteoarthritis. Arch Phys Med Rehabil. 2013;94:250–5. doi: 10.1016/j.apmr.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 24.Lim BW, Hinman RS, Wrigley TV, Sharma L, Bennell KL. Does knee malalignment mediate the effects of quadriceps strengthening on knee adduction moment, pain, and function in medial knee osteoarthritis? A randomized controlled trial. Arthritis Rheum. 2008;59:943–51. doi: 10.1002/art.23823. [DOI] [PubMed] [Google Scholar]
- 25.Day ML, McGuigan MR, Brice G, Foster C. Monitoring exercise intensity during resistance training using the session RPE scale. J Strength Cond Res. 2004;18:353–8. doi: 10.1519/R-13113.1. [DOI] [PubMed] [Google Scholar]
- 26.Atamaz FC, Durmaz B, Baydar M, Demircioglu OY, Iyiyapici A, Kuran B, et al. Comparison of the efficacy of transcutaneous electrical nerve stimulation, interferential currents, and shortwave diathermy in knee osteoarthritis: A double-blind, randomized, controlled, multicenter study. Arch Phys Med Rehabil. 2012;93:748–56. doi: 10.1016/j.apmr.2011.11.037. [DOI] [PubMed] [Google Scholar]
- 27.Klaiman MD, Shrader JA, Danoff JV, Hicks JE, Pesce WJ, Ferland J. Phonophoresis versus ultrasound in the treatment of common musculoskeletal conditions. Med Sci Sports Exerc. 1998;30:1349–55. doi: 10.1097/00005768-199809000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Darrow H, Schulthies S, Draper D, Ricard M, Measom GJ. Serum dexamethasone levels after decadron phonophoresis. J Athl Train. 1999;34:338–41. [PMC free article] [PubMed] [Google Scholar]
- 29.Boucaud A, Montharu J, Machet L, Arbeille B, Machet MC, Patat F, et al. Clinical, histologic, and electron microscopy study of skin exposed to low-frequency ultrasound. Anat Rec. 2001;264:114–9. doi: 10.1002/ar.1122. [DOI] [PubMed] [Google Scholar]
- 30.Benson HA. Transdermal drug delivery: Penetration enhancement techniques. Curr Drug Deliv. 2005;2:23–33. doi: 10.2174/1567201052772915. [DOI] [PubMed] [Google Scholar]
- 31.Yeǧin T, Altan L, Kasapoǧlu Aksoy M. The effect of therapeutic ultrasound on pain and physical function in patients with knee osteoarthritis. Ultrasound Med Biol. 2017;43:187–94. doi: 10.1016/j.ultrasmedbio.2016.08.035. [DOI] [PubMed] [Google Scholar]
- 32.White D, Evans JA, Truscott JG, Chivers RA. Can ultrasound propagate in the joint space of a human knee? Ultrasound Med Biol. 2007;33:1104–11. doi: 10.1016/j.ultrasmedbio.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Todd KH, Funk JP. The minimum clinically important difference in physician-assigned visual analog pain scores. Acad Emerg Med. 1996;3:142–6. doi: 10.1111/j.1553-2712.1996.tb03402.x. [DOI] [PubMed] [Google Scholar]
- 34.Roos EM, Arden NK. Strategies for the prevention of knee osteoarthritis. Nat Rev Rheumatol. 2016;12:92–101. doi: 10.1038/nrrheum.2015.135. [DOI] [PubMed] [Google Scholar]
- 35.Saraf SK, Singh OP. Management of chronic hemophilic synovitis in children by phonophoresis. Indian J Orthop. 2005;39:47. [Google Scholar]
- 36.Penderghest CE, Kimura IF, Gulick DT. Double-blind clinical efficacy study of pulsed phonophoresis on perceived pain associated with symptomatic tendinitis. J Sport Rehabil. 1998;7:9–19. [Google Scholar]
- 37.Saliba S, Mistry DJ, Perrin DH, Gieck J, Weltman A. Phonophoresis and the absorption of dexamethasone in the presence of an occlusive dressing. J Athl Train. 2007;42:349–54. [PMC free article] [PubMed] [Google Scholar]
- 38.Gurney AB, Wascher D, Schenck R, Tennison A, Jaramillo B. Absorption of hydrocortisone acetate in human connective tissue using phonophoresis. Sports Health. 2011;3:346–51. doi: 10.1177/1941738111405970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sedghimehr T, Bahrpeima F. Comparison of the effects of topical hydrocortison and clobetasole phonophoresis on reduction of pain in osteoarthritic knee joint. Physiol Pharmacol. 2006;10:247–58. [Google Scholar]