Abstract
Background
Plasmodium vivax malaria requires a 2-week course of primaquine (PQ) for radical cure. Evidence suggests that the hepatic isoenzyme cytochrome P450 2D6 (CYP2D6) is the key enzyme required to convert PQ into its active metabolite.
Methods
CYP2D6 genotypes and phenotypes of 550 service personnel were determined, and the pharmacokinetics (PK) of a 30-mg oral dose of PQ was measured in 45 volunteers. Blood and urine samples were collected, with PQ and metabolites were measured using ultraperformance liquid chromatography with mass spectrometry.
Results
Seventy-six CYP2D6 genotypes were characterized for 530 service personnel. Of the 515 personnel for whom a single phenotype was predicted, 58% had a normal metabolizer (NM) phenotype, 35% had an intermediate metabolizer (IM) phenotype, 5% had a poor metabolizer (PM) phenotype, and 2% had an ultrametabolizer phenotype. The median PQ area under the concentration time curve from 0 to ∞ was lower for the NM phenotype as compared to the IM or PM phenotypes. The novel 5,6-ortho-quinone was detected in urine but not plasma from all personnel with the NM phenotype.
Conclusion
The plasma PK profile suggests PQ metabolism is decreased in personnel with the IM or PM phenotypes as compared to those with the NM phenotype. The finding of 5,6-ortho-quinone, the stable surrogate for the unstable 5-hydroxyprimaquine metabolite, almost exclusively in personnel with the NM phenotype, compared with sporadic or no production in those with the IM or PM phenotypes, provides further evidence for the role of CYP2D6 in radical cure.
Clinical Trials Registration
Keywords: Primaquine; pharmacokinetics; CYP2D6; genotype; phenotype; metabolism; military; 5,6-ortho-quinone
The World Health Organization (WHO) estimates there were 8 550 000 cases of Plasmodium vivax infection in 2016, with regional prevalence rates of 23%–64% [1]. P. vivax infection is characterized by the presence of latent hypnozoites in the liver, which can cause malaria relapses unless a 2-week course (or, in individuals with glucose 6-phosphate dehydrogenase [G6PD] deficiency, a once-weekly course for 8 weeks) of the 8-aminoquinoline primaquine (PQ) is given for radical cure [2]. Tafenoquine, an 8-aminoquinoline recently approved by the Food and Drug Administration, is not yet in widespread use.
Radical cure treatment failure is well described [3], and potential contributing mechanisms were revealed when 2 malaria-naive volunteers experienced relapse following P. vivax controlled human malaria infection despite receiving PQ at 30 mg per day for 14 days under directly observed therapy. Investigations demonstrated that these 2 volunteers had genetic polymorphisms in the hepatic isoenzyme cytochrome P450 2D6 (CYP2D6), which likely led to impaired metabolism of PQ [4]. If a search of the The Pharmacogene Variation Consortium database (available at: https:www.pharmvar.org [formerly, the Human CYP Allele Nomenclature Database]) lists known CYP2D6 genotypes, and an activity score (AS-A) ranging between 0 and 1 can be assigned to each allele based on expected enzyme activity. [5]. The additive diplotype score can then be used to predict one of 4 different metabolism phenotypes: no enzyme activity (the poor metabolizer [PM] phenotype), reduced/variable metabolism (the intermediate metabolizer [IM] phenotype), normal metabolism (the normal metabolizer [NM] phenotype), and accelerated metabolism (the ultrarapid metabolizer [UM] phenotype) [5–7].
By using recombinant CYP2D6, in vitro experiments have been able to detect phenolic metabolites of PQ [8], and in vivo studies in mice have confirmed CYP2D metabolism in the PQ pathway by measuring metabolites such as 2-, 3-, and 4-hydroxyprimaquine and 5,6-ortho-quinone in the blood [9–11]. Recently, a human PQ pharmacokinetic (PK) study detected 5,6-ortho-quinone in the urine, although the CYP2D6 genotype of these 7 healthy adult volunteers was not determined [12]. Although the 5,6-ortho-quinone is not active itself, it is a stable surrogate marker for the presence of 5-hydroxyprimaquine, the unstable PQ metabolite generated through 2D6, which is believed to be necessary for radical cure through the generation of oxidative stress from redox cycling [13]. Work over the past 60 years suggests this redox activity may also be the mechanism behind the hemotoxicity of PQ, particularly in G6PD-deficient individuals, who are less able to manage intraerythrocytic oxidative stress [14–18].
The association of CYP2D6 and relapse has proved harder to confirm in areas of endemicity [19–24]. Two clinical trials found an increased risk of relapse with an AS-A of ≤1.0 [20, 21], yet in Australian soldiers contracting P. vivax while deployed, relapse was not associated with activity score [22]. This latter study, as well as 2 studies in Thailand [23, 24], found the NM phenotype in the majority of patients with relapse. In March 2019, the pharmacogenomics guidelines for CYP2D6 interpretation were adjusted [25], and as such, defining parameters for the CYP2D6 phenotype, PQ PK, and risk of relapse is very much a work in progress. The aim of this study was to determine the CYP2D6 genotype and predicted phenotype in a cohort of US military personnel, a group at imminent risk of contracting P. vivax or requiring presumptive antirelapse therapy. This was followed by a smaller nested PQ PK study, representing the first assessment of the impact of CYP2D6 status on production of the suspected active phenolic metabolites of PQ.
METHODS
Design
Volunteers were recruited from active duty populations stationed in the Washington, D. C., area through 2 clinical trial centers (the Clinical Trial Center at Walter Reed Army Institute of Research [WRAIR; Silver Spring, MD] and the Uniformed Services University of the Health Sciences [USUHS; Bethesda, MD]). Signed informed consent was obtained from all volunteers, and ethical approval for the study was obtained from the WRAIR and USUHS institutional review boards. Part 1 of the study (performed from February 2016 to November 2016) involved collection of 1 blood specimen for CYP2D6 genotyping from individuals who met the inclusion criteria of being an active duty service member aged 18–60 years. In part 2, volunteers were enrolled on a rolling basis between March 2016 and June 2017 as genotyping results became available. See the Supplementary Materials for inclusion/exclusion criteria and screening procedures.
CYP2D6 Characterization
Genotyping was performed with the xTAG CYP2D6 kit, version 3 (Luminex, Austin, TX), according to the manufacturer’s instructions, at each variant position (*1, *2, *3, *4, *5, *6, *7, *8, *9, *10, *11, *15, *17, *29, *35, and *41), and a diplotype was assigned. If duplication of an allele was found, “(DUP)” was added to the diplotype. If a full diplotype was not able to be determined, the results were classified as “no calls.” Subjects were then categorized into one of four predicted phenotypes using the AS-A Model and Clinical Pharmacogenomics Implementation Consortium (CPIC) guidelines [5, 26, 27]. At time of study execution, an AS-A of 0 was considered indicative of a PM phenotype; 0.5, an IM phenotype; and 1.0, 1.5, and 2, an NM phenotype. Goal enrollments for part 2 were 10 individuals with the NM phenotype, 22 with the IM phenotype, and 22 with the PM phenotype (Supplementary Materials). After the study was completed, new CPIC guidelines were released, designating an AS-A of 1.0 as indicative of an IM phenotype and the *10 allele as having a score of 0.25 [25], and the completed data set was reanalyzed.
PK Analysis
The morning of the PK study, breakfast was provided, baseline (ie, time 0) plasma and urine samples were collected, and the volunteer was then administered 30 mg of PQ orally under direct observation. Peripheral blood specimens (volume, 3 mL) were collected in lithium heparin tubes at 1, 2, 4, 6, 8, 10, and 24 hours after dosing, and spot urine specimens were collected 4, 10, and 24 hours after dosing. Plasma and urine levels of PQ, carboxyprimaquine (cPQ), 2-hydroxyprimaquine, 3-hydroxyprimaquine, 4-hydroxyprimaquine, and 5,6-ortho-quinone were measured using high-performance liquid chromatography with mass spectrometry. All samples were analyzed using a triple-quadrupole mass spectrometer (Waters, Milford, MA). Chromatographic separations were achieved using a Waters XTerra mass spectrometry C-18 analytical column (length, 50 mm; internal diameter, 2.1 mm; particle size, 3 µm), a Waters I class liquid chromatography system with a flow rate of 0.40 mL/minute, and a 6-minute linear gradient of 5%–98% acetonitrile (0.1% formic acid). Mass spectrometry conditions were optimized for each analyte. All samples were prepared for analysis by extraction with 2 volumes of acetonitrile containing internal standard (mefloquine) for each volume of sample. Mean plasma drug concentration versus time curves were prepared. For determination of PK parameters for PQ, cPQ, and the different phenolic metabolites in plasma, a compartmental analysis was performed using WinNonlin/Phoenix, version 6.4 (Pharsight, Mountain View, CA; Supplementary Materials).
Statistical Analysis
Prism, version 8.0, was used for statistical analyses. Means, standard deviations, medians, and ranges of PK parameters were calculated for each of the 3 predicted phenotype groups and compared using 1-way analysis of variance or the Kruskal-Wallis test, with the Tukey post hoc test.
RESULTS
Demographic Characteristics
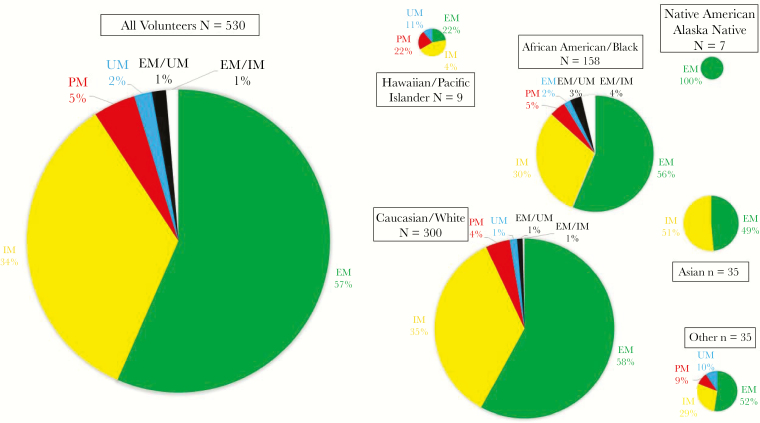
For part 1, of the 550 active duty volunteers enrolled, the mean age was 30.2 years (median, 28 years; range, 18–59 years). The racial/ethnic characteristics of the cohort are shown in Supplementary Figure 1. Overall demographic patterns were similar to percentages reported in 2014 by the US Department of Defense (DoD) for the Armed Forces [28] and in 2016 by the US Census Bureau [29] (Supplementary Table 1), although female sex was reported for 32% of participants (n=176) in the WRAIR study, compared with 15% in the DoD report.
CYP2D6 Genotype
Results for CYP2D6 genotyping were successful in 530 of 550 volunteers (96%), with 20 “no calls.” Table 1 shows the allelic haplotypes per race/ethnicity. There were 76 different CYP2D6 genotypes among the 530 volunteers, with the following 4 genotypes having a prevalence of ≥5%: *1/*2 (13.2% of volunteers [70]), *1/*1 (in 12.8% [68]), *1/*4 (in 8.5% [45]), and *1/*41 (in 5% [27]). The top 3 most frequent genotypes differed slightly among the racial groupings (Supplementary Table 2; see Supplementary Table 3 for all genotypes). The *10 allele, having been reattributed a score of 0.25, was found in 10% of our cohort (53 volunteers); this was paired with most often with a normal activity allele (in 28 volunteers [53%]), followed by a reduced activity allele (in 15 [28%]) and a null allele (in 10 [19%]). A gene duplication DUP was present in 40 volunteers (7.5%), among whom 19 different CYP2D6 genotypes were observed (Supplementary Table 4). The ratio of male to female participants shifted to 50:50, and most reported African American/black race/ethnicity (65%) [26].
Table 1.
CYP2D6 Allelic Frequencies Among Volunteers, Overall and by Race/Ethnicity
| Allele | Overall, No. break/ (%) (n = 530) | Caucasian/ break/ White (n = 300) | African American/ break/ Black (n = 158) | Asian break/ (n = 35) | Native American/ break/ Alaska Native (n = 7) | Hawaiian/Pacific break/ Islander (n = 9) | Other Race/Ethnicity break/ (n = 21) | Hispanic/ break/ LatinXa (n = 94) |
|---|---|---|---|---|---|---|---|---|
| *1 | 396 (37.4) | 241 (40.2%) | 99 (31.3) | 21 (30.0) | 10 (71.4) | 6 (33.3) | 19 (45.2) | 78 (41.5) |
| *2 | 180 (17.0) | 102 (17.0) | 62 (19.6) | 6 (8.6) | 2 (14.3) | 0 | 8 (19.0) | 38 (20.2) |
| *3 | 13 (1.2) | 11 (1.8) | 1 (0.3) | 0 | 0 | 0 | 1 (2.4) | 2 (1.1) |
| *4 | 133 (12.5) | 86 (14.3) | 34 (10.8) | 4 (5.7) | 0 | 2 (11.1) | 7 (16.7) | 17 (9.0) |
| *5 | 45 (4.2) | 20 (3.3) | 16 (5.1) | 3 (4.3) | 0 | 4 (22.2) | 2 (4.8) | 9 (4.8) |
| *6 | 8 (0.8) | 6 (1.0) | 2 (0.6) | 0 | 0 | 0 | 0 | 0 |
| *7 | 1 (0.1) | 1 (0.2) | 0 | 0 | 0 | 0 | 0 | 0 |
| *9 | 19 (1.8) | 18 (3.0) | 1 (0.3) | 0 | 0 | 0 | 0 | 3 (1.6) |
| *10 | 64 (6.0) | 10 (1.7) | 15 (4.7) | 32 (47.1) | 0 | 6 (33.3) | 1 (2.4) | 10 (5.3) |
| *14 | 1 (0.1) | 0 | 0 | 1 (1.4) | 0 | 0 | 0 | 0 |
| *17 | 55 (5.2) | 4 (0.7) | 49 (15.5) | 0 | 0 | 0 | 2 (4.8) | 5 (2.7) |
| *29 | 26 (2.5) | 1 (0.2) | 24 (7.6) | 0 | 0 | 0 | 1 (2.4) | 3 (1.6) |
| *35 | 41 (3.9) | 37 (6.2) | 3 (0.9) | 0 | 1 (7.1) | 0 | 0 | 6 (3.2) |
| *41 | 78 (7.4) | 63 (10.5) | 10 (3.2) | 3 (4.3) | 1 (7.1) | 0 | 1 (2.4) | 17 (9.0) |
Data are no. (%) of volunteers. The 20 volunteers with a “no call” result of the 2D6 genotyping assay are excluded. In the 40 volunteers with CYP2D6 gene duplication, only the 2 alleles identified by the xTAG CYP2D6 kit, version 3, are used in the calculation.
aSome volunteers reported Hispanic/LatinX along with another race/ethnicity is included. This column shows the frequencies only for those who reported Hispanic/LatinX ethnicity.
CYP2D6 Phenotype
Of the 530 genotypes successfully identified, 515 had a single predicted phenotypic designation, while 15 had gene duplications creating 2 possible AS-As, each predicting a different phenotype: the phenotype for 8 subjects could be NM or UM and for 7 was NM or IM. Of the 515 single phenotypes, 300 (58%) were NMs, 181 (35%) were IMs, 24 (5%) were PMs, and 10 (2%) were UMs. Results of comparisons of the number of subjects in each AS-A group and phenotype, using the former and most recent CPIC guidelines, are shown in the Supplementary Materials, and the percentage of phenotypes for each race/ethnicity is given in Figure 1.
Figure 1.
Predicted CYP2D6 metabolizer phenotypes according to race/ethnicity. Percentages of each phenotype are listed next to pie sections. The PM phenotype was not detected in Asian or Native American/Alaska Native volunteers.
PK Profiles and Parameters
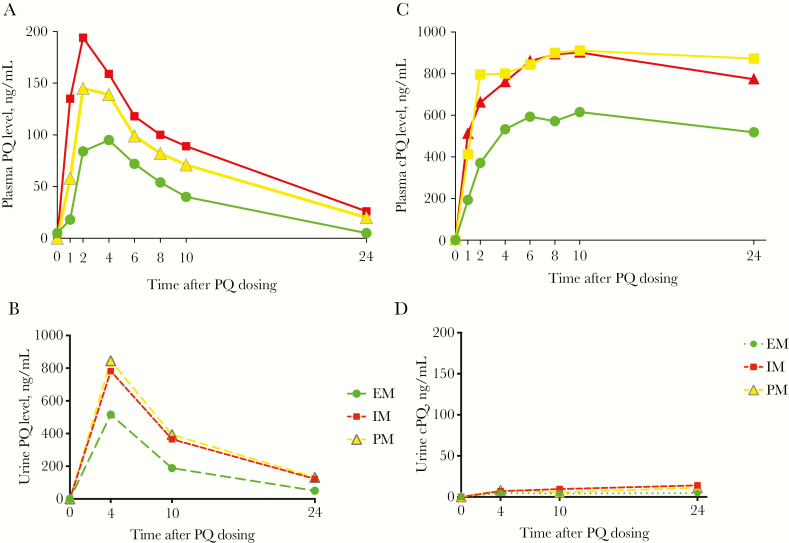
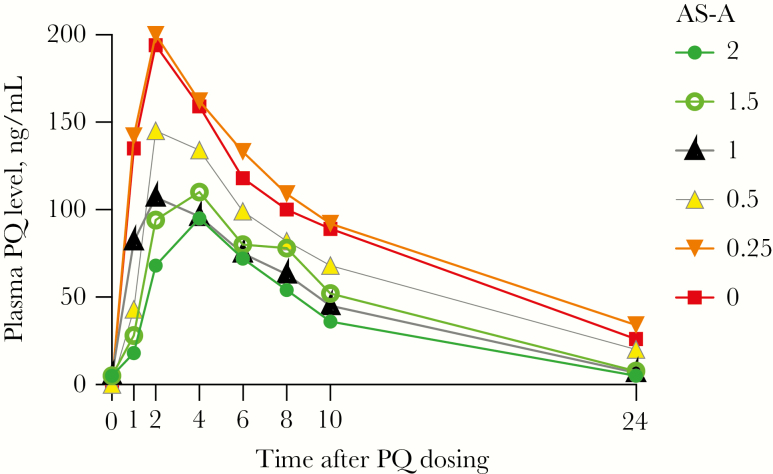
Forty-five individuals were enrolled for the PK portion of the study: 8 had the NM phenotype, 21 had the IM phenotype, and 16 had the PM phenotype (Supplementary Figure 2). One volunteer was later excluded from PK analysis since potential inhibition of CYP2D6 by a concomitant medication could not be ruled out (Supplementary Table 5). Plasma concentrations of PQ and carboxyprimaquine per phenotype are shown in Figure 2A and 2B, with plasma PK parameters presented in Table 2A and 2B. All plasma PK parameters for the NM group were statistically significantly less than those for the PM group, except for the time to achievement of the maximum concentration (Cmax). Parameters for carboxyprimaquine were more similar among the 3 phenotypes, although with a lower Cmax for the NM phenotype. Urinary PK profiles of PQ and carboxyprimaquine are shown in Figure 2C and 2D. The urine PQ concentration followed the same pattern as plasma PK, while urinary carboxyprimaquine concentrations were low to undetectable for the entire 24-hour period, supporting further biotransformation of this compound. We also plotted the plasma PQ PK profiles according to AS-A (Figure 3); those with AS-As of 2, 1.5, and 1 had similar profiles, while the 5 volunteers in the IM group with an AS-A of 0.25 (*10/null allele) had a PK profile that was almost identical to that for the PM group (Supplementary Table 5).
Figure 2.
Pharmacokinetic profiles in plasma (A and C) and urine (B and D, dotted lines) specimens from volunteers with the normal metabolizer (NM) phenotype (circles; n = 8), the intermediate metabolizer (IM) phenotype (triangles; n = 20), and the poor metabolizer (PM) phenotype (squares; n = 16) for primaquine (PQ; A and B) and carboxyprimaquine (cPQ; C and D) at scheduled time points after administration of 30 mg of PQ orally. Data are median values.
Table 2.
Plasma Pharmacokinetic Parameters for Primaquine (PQ) and Carboxyprimaquine (cPQ), by Metabolizer Phenotype
| Compound, Parameter | Normal Metabolizer (n = 8) | Intermediate Metabolizer (n = 20) | Poor Metabolizer (n = 16) | P a | ||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Median (Range) | Mean ± SD | Median (Range) | Mean ± SD | Median (Range) | ANOVA | K-W | |
| PQ | ||||||||
| Half-life, h | 5.1 ± 1.7b | 4.9 (2.3–7.9)b | 8.5 ± 2.6 | 8.5 (4.8–14) | 9.1 ± 2.3 | 9.0 (5.1–13) | .001 | .0013 |
| Tmax, h | 2.5 ± 1.3 | 2.0 (1.0–4.0) | 2.4 ± 0.88 | 2.0 (1.0–4.0) | 2.4 ± 1.0 | 2.0 (1.0–4.0) | .938 | .996 |
| Cmax, ng/mL | 124 ± 65 | 105 (63–266)c | 161 ± 47 | 150 (85–274) | 219 ± 92d | 194 (116–410) | .006 | .0099 |
| AUC∞, ng*h/mL | 1166 ± 571 | 950 (656–2385)b | 1998 ± 550 | 1809 (1000–2802) | 2947 ± 1585d | 2378 (1255–6612) | .001 | .0008 |
| Cl/F, mL/h/kg | 30 112 ± 11 039b | 31 811 (12 580–45 746)b | 16 256 ± 4991 | 16 584 (10 708–30 010) | 12 732 ± 5617 | 12 620 (4537–23 901) | <.0001 | .0008 |
| cPQ | ||||||||
| Half-life, h | 44 ± 27 | 48 (10–98) | 59 ± 46 | 37 (11–178) | 48 ± 32 | 44 (8–133) | .546 | .824 |
| Tmax, h | 7.8 ± 2.7 | 9 (4–10) | 8.6 ± 7.2 | 6 (1–24) | 7.9 ± 2.7 | 8 (2–10) | .102 | .537 |
| Cmax, ng/mL | 713 ± 191b | 623 (535–1030)e | 1014 ± 156 | 1023 (704–1429) | 938 ± 159 | 830 (633–1160) | .0004 | .004 |
| AUC∞, ng*h/mLf | 49 492 ± 29 192 | 42 625 (10 222–95 329) | 93 696 ± 62 913 | 68 112 (22 990–242 110) | 70 763 ± 33 119 | 68 896 (25 350–135 397) | .09 | .183 |
| Cl/F, mL/h/kgf | 975 ± 881e | 705 (315–2935) | 481 ± 309 | 440 (124–1305) | 538 ± 287 | 439 (222–1183) | .04 | .183 |
Abbreviations: AUC∞, area under the concentration time curve from 0 to ∞; Cl, clearance; Cmax, maximum concentration; F, bioavailability; Tmax, time to achievement of the maximum concentration.
aBy 1-way analysis of variance of means (ANOVA) or the Kruskal-Wallis test of medians (K-W), followed by the Tukey post hoc test.
b P < .05, for pairwise comparisons to volunteers with the intermediate or poor metabolizer phenotype.
c P < .05, for pairwise comparison to volunteers with the poor metabolizer phenotype.
d P < .05, for pairwise comparisons to volunteers with the normal or intermediate metabolizer phenotypes.
e P < .05, for pairwise comparison to volunteers with the intermediate metabolizer phenotype.
fData for the intermediate metabolizer group are for 19 volunteers because they could not be calculated for 1 volunteer.
Figure 3.
Pharmacokinetic profile of primaquine (PQ) in plasma specimens according to activity score (AS-A). Data are median plasma PQ concentrations after administration of 30 mg of PQ orally. Measurements were made at 0, 1, 2, 4, 6 8, 10 and 24 hours. Data are for 6 volunteers with an AS-A of 2 (solid circles), 2 with an AS-A of 1.5 (open circles), 2 with an AS-A of 1.0 (solid black triangles), 12 with an AS-A of 0.5 (triangles with a gray border), 5 with an AS-A of 0.25 (upside-down triangles), and 16 with an AS-A of 0 (squares). Two volunteers with duplications were not included.
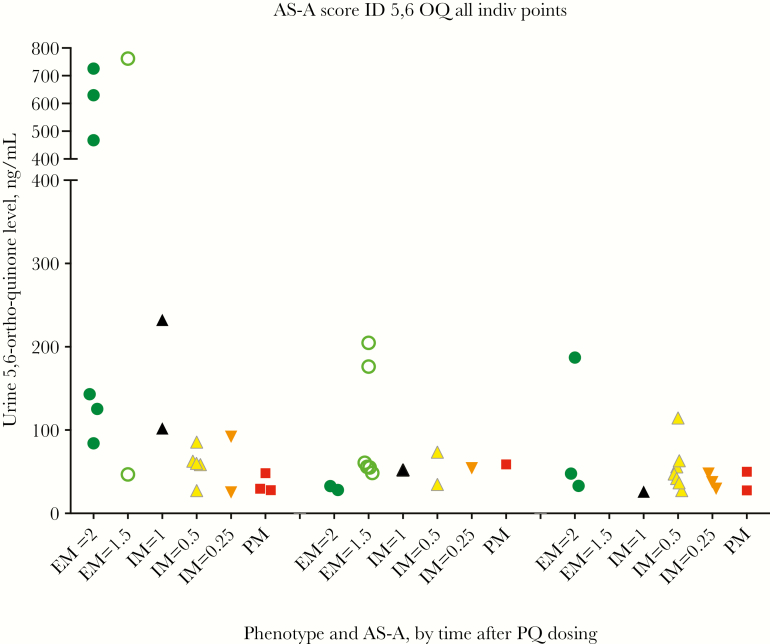
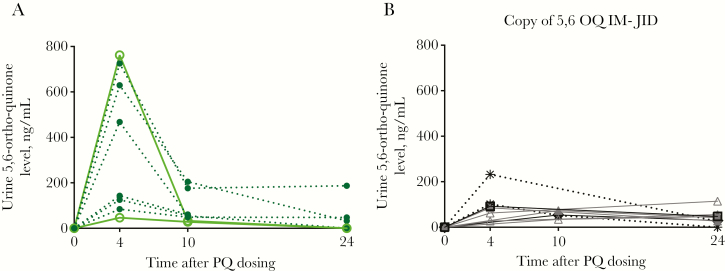
Other known PQ metabolites arising from the CYP2D6 pathway were also assessed in both plasma and urine specimens. The major phenolic metabolite of interest, 5,6-ortho-quinone, was not found in plasma samples from any volunteers; however, it was detected in urine specimens from all volunteers with the NM phenotype, among whom the time to Cmax was 4 hours but the range of Cmax values was wide (47–762 ng/mL; Figures 4 and 5A and 5B). Fifteen of 20 volunteers with the IM phenotype produced 5,6-ortho-quinone; the Cmax range was much lower than that for the NM group (25.2–232.2 ng/mL), and times to Cmax varied from 4 to 24 hours. 5,6-ortho-quinone was detected in only 3 of 16 volunteers with the PM phenotype, with 2 testing positive at the 4-hour time point (29.6 and 48.4 ng/mL) and 1 testing positive at 3 time points (Cmax, 58.9 ng/mL). The other metabolites, 2-, 3-, and 4-hydroxyprimaquine, were also only detected in urine specimens (Supplementary Figure 3). Most volunteers produced 3-hydroxyprimaquine, only those with the IM or PM phenotype produced 2-hydroxyprimaquine, and 4-hydroxyprimaquine was only seen in a few NMs. We also did exploratory post hoc comparisons of plasma PK parameters with respect to urine Cmax and comparisons of plasma and urine PK parameters with respect to weight-based PQ dose, which ranged from 0.26–0.57 mg/kg (Supplementary Figures 4 and 5). There was not a strong association for any of the parameters with 5,6-ortho-quinone. The CYP2D6 status, demographic group, PQ dose (in mg/kg), and PK parameters for all 45 volunteers are shown in Supplementary Table 6.
Figure 4.
Concentrations of 5,6-ortho-quinone in urine specimens collected 4, 10, and 24 hours after primaquine (PQ) dosing from volunteers with a normal metabolizer (NM) phenotype and an activity score (AS-A) of 2 (dark circles) or 1.5 (open circles); from those with an intermediate metabolizer (IM) phenotype and an AS-A score of 1.0 (black triangles), 0.5 (light triangle with gray outline), or 0.25 (upside down triangles); and from those with a poor metabolizer (PM) phenotype (squares).
Figure 5.
Pharmacokinetic profile of urinary 5,6-ortho-quinone 0, 4, 10, and 24 hours after primaquine (PQ) dosing, by CYP2D6 metabolizer phenotype. A, 5,6-ortho-quinone levels in all volunteers with a normal metabolizer (NM) phenotype (n = 8). Those with an activity score (AS-A) of 2 are indicated by dotted lines and filled circles, and those with an AS-A of 1.5 are indicated by solid lines and open circles. B, 5,6-ortho-quinone levels in volunteers from the intermediate metabolizer (IM) group in whom 5,6-ortho-quinone was detected at ≥2 time points (n = 9). Two volunteers from the IM group with an AS-A of 1.0 are indicated by dotted black lines and stars. The 5 volunteers with an AS-A of 0.5 are indicated by gray triangles (for those with a maximum concentration [Cmax] at 4 hours) or open triangles (for those with a Cmax at 24 hours). Two volunteers from the IM group had an AS-A of 0.25, of whom one had a Cmax at 4 hours (open square) and the other had a Cmax at 10 hours (open diamond). Not shown are data for 1 volunteer with an IM phenotype who had 5,6-ortho-quinone detected only at 4 hours, data for 2 who had 5,6-ortho-quinone detected only at 10 hours, and data for 2 who had 5,6-ortho-quinone detected only at 24 hours. 5,6-ortho-quinone was not detected in 5 volunteers from the IM group.
DISCUSSION
This study was an opportunity to assess whether 2D6 polymorphisms influence the production of oxidative metabolites posited to be necessary for radical cure of P. vivax infection and, thus, to better understand their potential pharmacogenomic liability. The major CYP2D6 allelic frequencies were similar to what has been reported with larger data sets [30–32] (Supplementary Table 7). Finding gene duplications in 7.5% of our volunteers was somewhat unexpected, although others have reported comparable percentages [32–34]. In our initial analysis of predicted phenotype, we used CPIC criteria to classify an AS-A of 1.0 as an NM phenotype [5, 27, 28, 35]. Additionally, in a study by Bennett et al, one of 3 volunteers with a score of 0.5 experienced relapse, while none of the 21 with scores of ≥1.0 did so [4]. There are alternative pharmacogenomic interpretations of 2D6 activity; the Dutch Pharmacogenetics Working Group classifies an AS-A of 1.0 as an IM phenotype [35], and some have advocated for nomenclature revisions, such as NM-slow (indicated by an AS-A of 1.0) and NM-fast (indicated by an AS-A of 1.5–2) [36]. This year, a CPIC consensus guideline was released, reclassifying an AS-A of 1.0 as an IM phenotype, although by a narrow margin, 41% to 38% [25]. Upon our reanalysis, the percentage of predicted NM and IM phenotypes shifted dramatically, from 87% to 58% and from 7% to 35%, respectively. A recent meta-analysis of genotype-to-phenotype interpretation reported almost the same shift when grouping AS-As of 1.0 with the IM phenotype [30].
Our range of PK parameters for a 30-mg oral dose of PQ in healthy adults were similar to what has been published in the past [37, 38], although these studies did not stratify PK parameters by CYP2D6 status. Although the number of subjects in our study was small, we saw a decrease in half-lives, Cmax values, and areas under the concentration time curve from 0 to ∞ (AUC∞) across the phenotype groups, with the highest values in the NM group and the lowest in the PM group, similar to what was modeled by Gonçalves et al [39] and what was seen by Bennett et al [4] (Supplementary Table 8). There was little change in PK parameters or profiles among the NM, IM, and PM groups regardless of whether prior or new CPIC guidelines for an AS-A of 1.0 were applied (data not shown); however, only 2 volunteers (both with an AS-S score of 1.0) were assigned a new phenotype on the basis of the new guidelines. Although there were only 2 such volunteers, neither had a PK profile that clearly fell into the IM or EM category. The median AUC∞ for an AS-A of 1 (950 hours*ng/mL) was not significantly different than that for AS-As of 1.5 and 2 combined (1163 hours*ng/mL; P = .99, by the t test). In contrast, the median AUC∞ was 1794 hours*ng/mL among those with an AS-A of 0.5 and 2726 hr*ng/mL among volunteers with an AS-A of 0.25 (Supplementary Table 5). The 2 volunteers with an AS-A of 1.0 also had higher concentrations of the urinary 5,6-ortho-quinone metabolite at 4 hours, compared with all others with an IM phenotype (AS-A range, 0.25–0.5). There can be large variation within phenotypes [34, 40, 41], and this may be especially pertinent for those associated with an AS-A of 1.0 [5]. Future PK studies enrolling larger numbers of volunteers in each AS-A group, rather than by phenotypic group, may help better define the interplay of CYP2D6 and PQ metabolism.
5,6-ortho-quinone is a stable surrogate compound that can be measured in lieu of the unstable PQ metabolite 5-hydroxyprimaquine, which is thought be involved in redox cycling and could therefore be responsible for the hepatic activity of PQ, as well as the hemolytic toxicity [9–18]. The distinctive finding of 5,6-ortho-quinone in all volunteers with the NM phenotype and the notable reduced level or absence of 5,6-ortho-quinone in the IM and PM groups provides additional support that radical cure is accomplished through the 2D6 pathway. The large variability in the 5,6-ortho-quinone Cmax in the NM group may be due to a small sample size (ie, data from too few time points) or to host differences in urinary excretion; we did not detect any associations with sex, race/ethnicity, AS-A, PQ dose (in mg/kg), or PQ PK parameters. The highest 5,6-ortho-quinone levels in urine specimens for all volunteers with the NM phenotype were detected at 4 hours, whereas the Cmax varied among volunteers in the IM group with detectable 5,6-ortho-quinone. This may suggest that the primary pharmacodynamic effects from PQ metabolites are driven by an early burst (Cmax) of redox active species, rather than by overall exposure. Studies in areas of endemicity may be able to identify whether there is a therapeutic level of 5,6-ortho-quinone excretion that reflects hypnozoite clearance in the liver or whether detection of the compound is sufficient evidence.
Carboxyprimaquine, which is produced through the monoamine oxidase pathway [13] and not thought to have antimalarial activity, had a lower Cmax and AUC∞ in the NM group, perhaps reflecting its increased metabolism through the CYP2D6 pathway. Levels of the 2-, 3- and 4-hydroxyprimaquine metabolites and 5,6-ortho-quinone were not appreciable in plasma specimens, unlike past murine studies at WRAIR [10], although levels were similar to those from a recent PK study in 7 healthy volunteers in the United States [12]. The inherent instability of the compounds, loss during processing and freezing, or location in other compartments (eg, erythrocytes and tissues) represent possible explanations.
In the study by Bennett et al, the total and daily doses of PQ seemed to influence radical cure outcome: of two volunteers with same genotype (*4/*41, an IM phenotype), only 1 experienced relapse [4]. Our study had 4 *4/*41 volunteers, of whom 1 had an AUC∞ between that of the IM and PM groups and received a lower PQ dose (0.29 mg/kg) and, thus, could be at possible risk for relapse. This volunteer had urinary 5,6-ortho-quinone detected at 10 and 24 hours, but we do not know whether this would translate into efficacy. It was intriguing to see the PQ dose–dependent increase in the 5,6-ortho-quinone Cmax among volunteers with the IM phenotype (Supplementary Figure 5F), supporting the use of higher PQ doses in such volunteers. However, at PQ doses greater than approximately 0.5 mg/kg, the association was lost, and this observation does not reflect data from the 5 volunteers from the IM group who had no detectable 5,6-ortho-quinone. Supplementary Figure 5F also shows that 4 of 5 subjects with the *10/null diplotype (AS-A, 0.25) produced 5,6-ortho-quinone, despite this group having a plasma PQ PK profile similar to that of the PM group. While this is encouraging for P. vivax–endemic areas such as Southeast Asia, where the frequency of the *10 allele commonly approaches 50% [42, 43], prospective studies need to be conducted to delineate metabolic parameters in those who carry the *10 allele and the relationship of these parameters to radical cure. These field studies may also be able to assess any associations of 5,6-ortho-quinone production with a risk of acute hemolytic anemia in G6PD-deficient patients and/or P. falciparum gametocyte clearance.
Not determining a urinary metabolic ratio with a 2D6 probe drug limited our ability to definitively assign a metabolizer phenotype to our volunteers, and a recent study in Indonesia found that 17 of 18 P. vivax relapses had a log metabolic ratio of dextromethorphan of −1.0 or greater [21]. The xTAG assay we used detects single-nucleotide polymorphisms or deletions for only 16 alleles; thus, in our study, the proportion with the default genotype *1 allele may have been overestimated, with rare genotypes missed. We observed 1 volunteer in the NM group who had a *1/*1 genotype but an AUC∞ and Cmax values more similar to those in the PM group. Paradoxically, the amount of 5,6-ortho-quinone produced by this volunteer was the third highest of all volunteers in the NM group. The explanation may lie within the large variation that may be seen within phenotypes, even if a 2D6 probe drug is used to determine phenotype [34, 41]. Contemporary investigations are unveiling the complex influences of single-nucleotide polymorphism enhancers, transcription factors, and microRNA on the 2D6 metabolism phenotype [41, 44]. Whole-genome sequencing (WGS) could be of benefit; however, sequencing of the 2D6 gene is complicated by copy number, high G-C content, and pseudogenes (CYP2D7 and CYP2D8) [45]. This level of complexity will preclude WGS in many laboratories, particularly those in malaria-endemic areas.
The importance of CYP2D6 status is important not only for PQ, but also for other 2D6-metabolized medications, such as codeine, selective serotonin reuptake inhibitors (SSRIs), bupropion for smoking cessation, and many antihypertensive agents. The impact of polypharmacy was recently demonstrated in a study by Avula et al, in which mice that were coadministered SSRIs and PQ died of malaria, owing to inhibition of 2D6 by the SSRI [12]. Baird et al estimated the percentage of populations in malaria-endemic countries refractory to PQ treatment due to CYP2D6, G6PD deficiency, age <6 months, pregnancy, and lactation to be 38.8% [46]. All US active duty personnel are tested for G6PD deficiency at induction, with an estimated prevalence rate of 4.1%–4.3% [47, 48]. This prevalence, combined with the 5% prevalence of the PM phenotype detected in our study, suggests that PQ could not be effective in almost 10% of service personnel. Moreover, the limitations of PQ therapy due to CYP2D6 polymorphisms will likely exceed 5%, since some commonly used medications can render the NM phenotype as a functional PM phenotype, and additionally, some individuals with the IM phenotype will not respond to PQ therapy [4, 19]. Knowing a soldier’s predicted phenotype could aid in more-tailored prescription of PQ, reduce questions of compliance, and better prepare combatant command leaders and medical providers for potential episodes of PQ treatment failure due to host pharmacogenomic characteristics.
We now have an initial PQ PK study demonstrating differential production of 5,6-ortho-quinone, a surrogate for the presumed active metabolite 5-hydroxyprimaquine, in individuals with the NM phenotype. Tafenoquine, which also originated through DoD-sponsored studies, has recently been approved for use by the Food and Drug Administration but, as an 8-aminquinoline, may require the same 2D6 metabolism pathway [49]. WRAIR has a dedicated program to develop new drugs for radical cure of P. vivax, including other 8-aminoquinolines not subject to 2D6 metabolism. The results from these collective studies will inform not only the DoD, but also clinicians and public health policymakers worldwide, hopefully resulting in improved tools to ensure eventual elimination of P. vivax.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Presented in part: Annual Meeting of the American Society for Tropical Medicine and Hygiene, Atlanta, Georgia, 13–17 November 2016; Annual Military Health System Research Symposium, Kissimmee, Florida, 27–30 August 2017.
Acknowledgments.We thank Andrea Robichaud and Naveeta George, for their tireless work in volunteer recruitment; Ms Dutchabong Shaw, COL Paul Keiser, MAJ Erin Milner, LTC Melinda Hamer, LTC Adrian Kress, Dr. Sam Dickson, COL (retired) Robert Paris, and COL (retired) Mark Hickman, and, from the Defense Health Program, Dr Joan Cmarik, for continued support and help with this project; the volunteers who participated in the study; and all staff of the WRAIR and USUHS clinical trial centers.
Disclaimer. Material has been reviewed by the Walter Reed Army Institute of Research. There is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the author, and are not to be construed as official, or as reflecting true views of the Department of the Army, the Department of Defense, the Food and Drug Administration (Dr. Marcsisin works at FDA) or the US government. The investigators have adhered to the policies fro protection of human subjects as prescribed in AR 70-25.
The funding source had no role in the analysis or interpretation of data, preparation of the manuscript or the decision to publish.
Financial support. This work was supported by the Defense Health Program, US Army Medical Research and Materiel Command (grant D6.7_14_I_14_J9_837).
Potential conflicts of interest. P. T. is an employee of and owns stock in Roche Holding. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization (WHO), World malaria report 2017. Geneva: World Health Organization, 2017. [Google Scholar]
- 2. Hill DR, Baird JK, Parise ME, Lewis LS, Ryan ET, Magill AJ. Primaquine: report from CDC expert meeting on malaria chemoprophylaxis I. Am J Trop Med Hyg 2006; 75:402–15. [PubMed] [Google Scholar]
- 3. Baird JK, Hoffman SL. Primaquine therapy for malaria. Clin Infect Dis 2004; 39:1336–45. [DOI] [PubMed] [Google Scholar]
- 4. Bennett JW, Pybus BS, Yadava A, et al. . Primaquine failure and cytochrome P-450 2D6 in Plasmodium vivax malaria. N Engl J Med 2013; 369:1381–2. [DOI] [PubMed] [Google Scholar]
- 5. Gaedigk A, Simon SD, Pearce RE, Bradford LD, Kennedy MJ, Leeder JS. The CYP2D6 activity score: translating genotype information into a qualitative measure of phenotype. Clin Pharmacol Ther 2008; 83:234–42. [DOI] [PubMed] [Google Scholar]
- 6. Zhou SF. Polymorphism of human cytochrome P450 2D6 and its clinical significance: part I. Clin Pharmacokinet 2009; 48:689–723. [DOI] [PubMed] [Google Scholar]
- 7. Frank D, Jaehde U, Fuhr U. Evaluation of probe drugs and pharmacokinetic metrics for CYP2D6 phenotyping. Eur J Clin Pharmacol 2007; 63:321–33. [DOI] [PubMed] [Google Scholar]
- 8. Pybus BS, Sousa JC, Jin X, et al. . CYP450 phenotyping and accurate mass identification of metabolites of the 8-aminoquinoline, anti-malarial drug primaquine. Malar J 2012; 11:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pybus BS, Marcsisin SR, Jin X, et al. . The metabolism of primaquine to its active metabolite is dependent on CYP 2D6. Malar J 2013; 12:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Potter BM, Xie LH, Vuong C, et al. . Differential CYP 2D6 metabolism alters primaquine pharmacokinetics. Antimicrob Agents Chemother 2015; 59:2380–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jin X, Potter B, Luong TL, et al. . Pre-clinical evaluation of CYP 2D6 dependent drug-drug interactions between primaquine and SSRI/SNRI antidepressants. Malar J 2016; 15:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Avula B, Tekwani BL, Chaurasiya ND, et al. . Metabolism of primaquine in normal human volunteers: investigation of phase I and phase II metabolites from plasma and urine using ultra-high performance liquid chromatography-quadrupole time-of-flight mass spectrometry. Malar J 2018; 17:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marcsisin SR, Reichard G, Pybus BS. Primaquine pharmacology in the context of CYP 2D6 pharmacogenomics: current state of the art. Pharmacol Ther 2016; 161:1–10. [DOI] [PubMed] [Google Scholar]
- 14. Tarlov AR, Brewer GJ, Carson PE, Alving AS. Primaquine sensitivity. Glucose-6-phosphate dehydrogenase deficiency: an inborn error of metabolism of medical and biological significance. Arch Intern Med 1962; 109:209–34. [DOI] [PubMed] [Google Scholar]
- 15. Bowman ZS, Oatis JE Jr, Whelan JL, Jollow DJ, McMillan DC. Primaquine-induced hemolytic anemia: susceptibility of normal versus glutathione-depleted rat erythrocytes to 5-hydroxyprimaquine. J Pharmacol Exp Ther 2004; 309:79–85. [DOI] [PubMed] [Google Scholar]
- 16. Vasquez-Vivar J, Augusto O. Hydroxylated metabolites of the antimalarial drug primaquine. J Biol Chem 1992; 267:6848–54. [PubMed] [Google Scholar]
- 17. Vásquez-Vivar J, Augusto O. Oxidative activity of primaquine metabolites on rat erythrocytes in vitro and in vivo. Biochem Pharmacol 1994; 47:309–16. [DOI] [PubMed] [Google Scholar]
- 18. Ganesan S, Tekwani BL, Sahu R, Tripathi LM, Walker LA. Cytochrome P(450)-dependent toxic effects of primaquine on human erythrocytes. Toxicol Appl Pharmacol 2009; 241:14–22. [DOI] [PubMed] [Google Scholar]
- 19. Ingram RJ, Crenna-Darusallam C, Soebianto S, Noviyanti R, Baird JK. The clinical and public health problem of relapse despite primaquine therapy: case review of repeated relapses of Plasmodium vivax acquired in Papua New Guinea. Malar J 2014; 13:488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brasil LW, Rodrigues-Soares F, Santoro AB, et al. . CYP2D6 activity and the risk of recurrence of Plasmodium vivax malaria in the Brazilian Amazon: a prospective cohort study. Malar J 2018; 17:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baird JK, Louisa M, Noviyanti R, et al. . Association of impaired cytochrome P450 2D6 activity genotype and phenotype with therapeutic efficacy of primaquine treatment for latent Plasmodium vivax malaria. JAMA Netw Open 2018; 1:e181449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen N, Dowd S, Gatton ML, Auliff A, Edstein MD, Cheng Q. Cytochrome P450 2D6 profiles and their relationship with outcomes of primaquine anti-relapse therapy in Australian Defence Force personnel deployed to Papua New Guinea and East Timor. Malar J 2019; 18:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maneerattanasak S, Gosi P, Krudsood S, et al. . Genetic diversity among Plasmodium vivax isolates along the Thai-Myanmar border of Thailand. Malar J 2016; 15:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Longley RJ, Sripoorote P, Chobson P, et al. . High efficacy of primaquine treatment for Plasmodium vivax in Western Thailand. Am J Trop Med Hyg 2016; 95:1086–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. https://cpicpgx.org/wp-content/uploads/2019/03/Final-Consensus-CYP2D6-genotype-to-phenotype-table_-final_Mar2019.pdf. Accessed 31 May 2019.
- 26. Hicks JK, Bishop JR, Sangkuhl K, et al. ; Clinical Pharmacogenetics Implementation Consortium Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2D6 and CYP2C19 genotypes and dosing of selective serotonin reuptake inhibitors. Clin Pharmacol Ther 2015; 98:127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Crews KR, Gaedigk A, Dunnenberger HM, et al. ; Clinical Pharmacogenetics Implementation Consortium Clinical Pharmacogenetics Implementation Consortium guidelines for cytochrome P450 2D6 genotype and codeine therapy: 2014 update. Clin Pharmacol Ther 2014; 95:376–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Department of Defense. Demographics: profile of the military community. Washington, DC: Office of the Deputy Assistant Secretary of Defense (Military Community and Family Policy), 2016. http://download.militaryonesource.mil/12038/MOS/Reports/2014-Demographics-Report.pdf. Accessed 14 March 2019. [Google Scholar]
- 29. https://www.census.gov/quickfacts/fact/table/US/PST045216. Accessed 29 January 2018.
- 30. Gaedigk A, Sangkuhl K, Whirl-Carrillo M, Klein T, Leeder JS. Prediction of CYP2D6 phenotype from genotype across world populations. Genet Med 2017; 19:69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhou Y, Ingelman-Sundberg M, Lauschke VM. Worldwide distribution of Cytochrome P450 alleles: a meta-analysis of population-scale sequencing projects. Clin Pharmacol Ther 2017; 102:688–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Del Tredici AL, Malhotra A, Dedek M, et al. . Frequency of CYP2D6 alleles including structural variants in the United States. Front Pharmacol 2018; 9:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Beoris M, Amos Wilson J, Garces JA, Lukowiak AA. CYP2D6 copy number distribution in the US population. Pharmacogenet Genomics 2016; 26:96–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gaedigk A. Complexities of CYP2D6 gene analysis and interpretation. Int Rev Psychiatry 2013; 25:534–53. [DOI] [PubMed] [Google Scholar]
- 35. Bank PCD, Caudle KE, Swen JJ, et al. . Comparison of the guidelines of the clinical pharmacogenetics implementation consortium and the Dutch Pharmacogenetics Working Group. Clin Pharmacol Ther 2018; 103:599–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hicks JK, Swen JJ, Gaedigk A. Challenges in CYP2D6 phenotype assignment from genotype data: a critical assessment and call for standardization. Curr Drug Metab 2014; 15:218–32. [DOI] [PubMed] [Google Scholar]
- 37. Mihaly GW, Ward SA, Edwards G, Nicholl DD, Orme ML, Breckenridge AM. Pharmacokinetics of primaquine in man. I. Studies of the absolute bioavailability and effects of dose size. Br J Clin Pharmacol 1985; 19:745–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cuong BT, Binh VQ, Dai B, et al. . Does gender, food or grapefruit juice alter the pharmacokinetics of primaquine in healthy subjects? Br J Clin Pharmacol 2006; 61:682–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gonçalves BP, Pett H, Tiono AB, et al. . Age, weight, and CYP2D6 genotype are major determinants of primaquine pharmacokinetics in African children. Antimicrob Agents Chemother 2017; 61:e02590–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Montané Jaime LK, Lalla A, Steimer W, Gaedigk A. Characterization of the CYP2D6 gene locus and metabolic activity in Indo- and Afro-Trinidadians: discovery of novel allelic variants. Pharmacogenomics 2013; 14:261–76. [DOI] [PubMed] [Google Scholar]
- 41. Gaedigk A, Dinh JC, Jeong H, Prasad B, Leeder JS. Ten years’ experience with the CYP2D6 activity score: a perspective on future investigations to improve clinical predictions for precision therapeutics. J Pers Med 2018; 8:E15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sistonen J, Sajantila A, Lao O, Corander J, Barbujani G, Fuselli S. CYP2D6 worldwide genetic variation shows high frequency of altered activity variants and no continental structure. Pharmacogenet Genomics 2007; 17:93–101. [DOI] [PubMed] [Google Scholar]
- 43. McGraw J, Waller D. Cytochrome P450 variations in different ethnic populations. Expert Opin Drug Metab Toxicol 2012; 8:371–82. [DOI] [PubMed] [Google Scholar]
- 44. Yang Y, Botton MR, Scott ER. Sequencing the CYP2D6 gene: from variant allele discovery to clinical pharmacogenetic testing. Pharmacogenomics 2017; 18:673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Twist GP, Gaedigk A, Miller NA, et al. . Constellation: a tool for rapid, automated phenotype assignment of a highly polymorphic pharmacogene, CYP2D6, from whole-genome sequences. NPJ Genom Med 2016; 1:15007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Baird JK, Battle KE, Howes RE. Primaquine ineligibility in anti-relapse therapy of Plasmodium vivax malaria: the problem of G6PD deficiency and cytochrome P-450 2D6 polymorphisms. Malar J 2018; 17:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chinevere TD, Murray CK, Grant E Jr, Johnson GA, Duelm F, Hospenthal DR. Prevalence of glucose-6-phosphate dehydrogenase deficiency in U.S. Army personnel. Mil Med 2006; 171:905–7. [DOI] [PubMed] [Google Scholar]
- 48. Thomas JE, Kang S, Wyatt CJ, et al. . Glucose-6-phosphate dehydrogenase deficiency is associated with cardiovascular disease in U.S. Military Centers. Tex Heart Inst J 2018; 45:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vuong C, Xie LH, Potter BM, et al. . Differential cytochrome P450 2D metabolism alters tafenoquine pharmacokinetics. Antimicrob Agents Chemother 2015; 59:3864–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.