Abstract
Bisphenol A (BPA) is a widely used chemical that has been detected in follicular fluid and associated with adverse reproductive effects. Granulosa cells have an important role in follicular growth and oocyte maturation, however, little is known about the biological mechanisms of BPA toxicity on human granulosa cells. In this study, we exposed primary granulosa cells to different concentrations of BPA (0, 20, 200, 2000, and 20 000 ng/ml) and used quantitative polymerase chain reaction to measure the expression levels of miRNAs enriched in extracellular vesicles (EV-enriched miRNAs), and cellular levels of selected target genes of differentially expressed EV-enriched miRNAs. We found that exposure to 20 000 ng/ml BPA was associated with decreased levels of EV-miR-27b-3p (FC = 0.58, p = .04) and increased levels of its biologically relevant target genes FADD (FC = 1.22, p = .01), IGF1 (FC = 1.59, p = .06), and PPARG (FC = 1.73, p = .001) as compared with the control. In addition, we observed that under the same exposure conditions, the expression levels of miR-27b-3p in granulosa cells were also downregulated (FC = 0.65, p = .03) as compared with the control. Our findings suggest that both cellular and extracellular changes in gene expression may mediate BPA toxicity in granulosa cells.
Keywords: granulosa cells, bisphenol A, extracellular vesicles, miRNA, ovarian follicle
Bisphenol A (BPA) is one of the industrial synthetic chemicals produced at the highest volume worldwide. Potential sources of BPA exposure include ingestion of foods and beverages previously in contact with the lining of cans used for food and beverages, polycarbonates bottles, thermal receipts, dust, water, etc. (Vandenberg et al. 2007). Human exposure to BPA is ubiquitous and the chemical has been detected in various biofluids including urine, serum, amniotic fluid, and follicular fluid (Calafat et al. 2008; Ikezuki et al. 2002). Animal and human data have shown that BPA has toxic effects on oocyte maturation and spindle formation (Can et al. 2005; Eichenlaub-Ritter et al. 2008; Hunt et al. 2003, 2009; Lenie et al. 2008; Machtinger et al. 2013). We have recently shown in vitro that BPA alters granulosa cell steroidogenesis and global gene expression in a supra-physiological dose; however, the underlying mechanism remains unclear (Mansur et al. 2016, 2017).
Extracellular vesicles (EVs) are nanosized (<1000 nm) membrane vesicles released by various cells in both physiological and pathological conditions (Raposo and Stoorvogel 2013), and are involved in cellular communication by transferring biological molecules such as cytokines, growth factors, lipids, proteins, and nucleic acids (eg, RNAs) (Raposo and Stoorvogel 2013; Zhang et al. 2009). Recent studies have shown that EVs and their cargo respond to environmental toxins, however, these studies mainly focused on air pollutants, tobacco smoking, and alcohol (Harischandra et al. 2017; Neven et al. 2017). Extensive gap junctions has been known as the main mode of communication within and between the cellular compartments in the ovarian follicle (cumulus, granulosa cells, and the oocyte) (Granot and Deket, 2002; Sela-Avramovich et al., 2006). Recently, EVs were identified in follicular fluid as a novel mode of communication in the ovarian follicle by regulating genes involved in follicular development, meiotic resumption, and ovulation (da Silveira et al. 2015; Machtinger et al. 2016, 2017; Santonocito et al. 2014; Sohel Md. Mahmodul et al. 2013). EVs are enriched in noncoding RNAs (ncRNAs), especially micro-RNAs (miRNAs) that can directly regulate gene expression by targeting the messenger RNA and inhibit its translation to protein (Bartel 2004). Not only can EV-enriched miRNAs be transferred from cell to cell, but they are also found to be active regulators of gene expression in the recipient cells (Shivdasani 2006). Few studies have shown that BPA can deregulate miRNA levels in tissues (eg, placenta and endometrium) and that these changes can be detected extracellularly (Ehrlich et al. 2016; Reed et al. 2018); however, no studies have investigated whether BPA can alter the expression of cellular and extracellular miRNAs in granulosa cells. Based on our previous results showing that BPA can alter oogenesis also in doses relevant to human exposure (Machtinger et al. 2013), we aimed at determining whether BPA exposure alters the profile of EV-enriched miRNAs secreted by the granulosa cells, thus impair pathways that are associated with oogenesis.
MATERIALS AND METHODS
Sample collection
Granulosa cells, routinely aspirated during oocyte retrieval and then discarded, were collected from women undergoing in vitro fertilization. Inclusion criteria were patients ≤38 years. old, BMI ≤ 30 kg/m2, who were undergoing fertility treatments for male infertility factor or unexplained infertility, as well as fertile couples undergoing IVF for pre-implantation genetic diagnosis (PGD) of autosomal recessive diseases. We excluded patients with PCOS, diminished ovarian reserve, or endometriosis, which might impair granulosa cell gene expression. This study was approved by our local IRB (8707-11) and all patients signed a written informed consent.
Cell culture
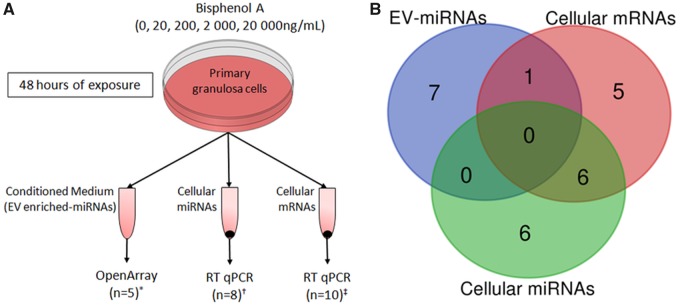
Cells were pooled before culture, purified, and counted using a hemocytometer and cell viability was determined using Trypan Blue according to the manufacturer’s protocol before seeding as described previously (Mansur et al. 2016). In brief, cells were cultured in 12-well plates for 48 h at 37°C in DMEM/F12 (Cat. Number 21041-025, GIBCO, USA) with 1% glutamax, 10% fetal bovine serum (Cat. Number 12657-029, GIBCO, Brazil), 1% penicillin/streptomycin (Cat. Number 15140-122, GIBCO, USA), and 1 µM 4-Androsten-3,17-dione (Cat. Number 46033, Sigma-Aldrich, USA) in a humidified atmosphere of 5% CO2 and 20% O2 for 48 h as described previously (Mansur et al. 2016). The medium was then replaced by the same medium formulation containing follicle stimulating hormone (FSH) (Merck-Serono, Switzerland) (Kwintkiewicz et al. 2010; Peretz and Flaws 2013) at a final concentration of 73.3 ng/ml and BPA at concentrations ranging from 0, 20, 200, 2000, or 20 000 ng/ml) for 48 h. The lowest BPA concentration was equivalent to that previously detected in human follicular fluid (3); supra-physiological concentrations matched those tested in previous in vitro studies (Grasselli et al. 2010; Machtinger et al. 2013; Peretz et al. 2011). DMSO 99% (Cat Number 2417B030, AMRESCO, Canada) served as a vehicle with a final DMSO concentration of 0.05%. The control contained the same concentration of 0.05% DMSO, but no BPA (0 ng/ml). After 48 h of culture, the conditioned media was collected and immediately frozen at −80°C until subsequent miRNA analysis was performed. BPA-exposed cells were also collected and gene expression microarray analyses were performed as described previously (Mansur et al. 2017). A summary of the experimental design as well as a Venn diagram showing the overlap between patients contributing granulosa cells to the biological replicates are shown in Figure 1.
Figure 1.
A, Schematic diagram summarizing the experimental design of our study. Granulosa cells were isolated from patients (n = 25) and cultured prior to their exposure to a range of Bisphenol A concentrations (ie, 0, 20, 200, 2000, and 20 000 ng/ml) for 48 h. A different set of patients contributed cells for each of the cellular and extracellular RNA analyses. For the EV-enriched miRNA analysis (marked with *), granulosa cells from eight patients were cultured and then pooled into five biological replicates (1–3 women per replicate) prior to exposure. Similarly, for the cellular miRNA (marked with †), granulosa cells from 12 patients were cultured and pooled into 8 biological replicates (1–2 women per replicate), and for the mRNA analyses (marked with ‡), granulosa cells from 12 patients were cultured and pooled into 10 biological replicates (1–2 women per replicate). B, Venn diagram showing the overlap between patients contributing granulosa cells to the biological replicates used in EV-enriched miRNA and cellular mRNA and miRNA analyses.
Measurement of EV-miRNA expression by real-time PCR
EVs were isolated according to a previously described protocol, which was further validated in the context of this study by Nanoparticle Tracking Analysis and flow cytometry (Supplementary Figure 1) (Pergoli et al. 2017). In brief, granulosa cell media (n = 5) from each exposure group (0, 20, 200, 2000, and 20 000 ng/ml BPA) were collected and centrifuged three times at 1000, 2000, and 3000 × g for 15 min at 4°C to remove any remaining cellular debris. To prepare EV-enriched pellets for miRNA extraction, we transferred the conditioned media to a 10.4-ml polycarbonate ultracentrifuge tube (Beckman Coulter), containing PBS and ultracentrifuged (BeckmanCoulter Optima-MAX-XP) the samples at 110 000 × g for 75 min at 4°C. Next, we carefully discarded the supernatant and extracted EV-enriched miRNAs from the pellet using the miRNeasy and RNeasy CleanUp commercial kits (Qiagen) per manufacturer’s instructions. Because our primary focus was on EV-enriched miRNAs, only extracellular RNA (conditioned media) and not cellular RNA was screened for the full miRNA panel.
Expression levels of EV-enriched miRNAs were measured using the TaqMan OpenArray Human miRNA Panel on the QuantStudio 12K Flex OpenArray Platform as described previously (Pergoli et al. 2017). The panel screens for 754 EV-enriched miRNAs and 4 internal controls (ath-miR159a, RNU48, RNU44, and U6). For the expression analysis, we included qPCR expression data that passed the following criteria: (1) Crt value <28, (2) amplification score ≥1.24, and (3) detected in ≥2 samples (out of 5) per exposure group. Data that did not meet these criteria were considered as missing values, and therefore not included in the analysis. To normalize our data, we used the global mean method (ΔCrt_miRNAi = (Crt_miRNAi − Crt_miRNAi_global_mean)), as described by Mestdagh et al. (2009).
Measurement of cellular mRNA and miRNA expression by real-time PCR
Total RNA was extracted from granulosa cells from each exposure group using a Quick-RNA Microprep Kit (Zymo Research, Irvine, California) according to the manufacturer’s instructions. RNA concentrations were assessed using a NanoDrop 2000C spectrophotometer (Thermo Scientific, Waltham, Massachusetts). For mRNA expression analysis, 25 ng of RNA was added to the reverse-transcription reaction mix to produce cDNA by high-capacity cDNA RT kit (Applied Biosystems, Foster City, California) according to the manufacturer’s instructions. Fluorescent SYBR Green PCR mix (Applied Biosystems) was used to quantify PCR products. For the PCR reaction, we used 0.4 µM of the primers for each gene in a final volume of 10 µl. Each assay was run in triplicates and β-actin was selected as the reference gene. We used the Ingenuity Pathway Analysis software (Qiagen Inc., Valencia, California) to identify genes that are experimentally validated targets of the differentially expressed EV-enriched miRNAs, and that they are biologically relevant as well. Primers were designed using Nucleotide (https://www.ncbi.nlm.nih.gov/nucleotide/) (Supplementary Table 1). Amplification and detection of each mRNA transcript were performed using the StepOnePlus real-time PCR system (Applied Biosystems) with the following profile: 1 cycle at 95°C for 20 s, 40 cycles each at 95°C for 3 s, and 60°C for 30 s. One microliter of cDNA was used per reaction in a 10-µl total reaction volume.
For miRNA expression analysis, the qScript microRNA cDNA synthesis kit (Quantabio, Beverly, Massachusetts) was used to convert miRNAs into cDNA per manufacturer’s protocol. In brief, 2 μl of RNA (25 ng/μl) were added to the tailing reaction mix together with 1 μl of qScript Reverse Transcriptase. The mix was vortexed gently and centrifuged briefly prior to incubation for 20 min at 42°C followed by 5 min at 85°C. The real-time PCR was performed by using two primers: the specific miRNA primer (Quantabio) (Supplementary Table 2) and the universal PCR primer. For each reaction, 2 μl of miRNA cDNA were added to reaction mix which contains 10 μl SYBR green SuperMix X 2, 0.4 μl of specific mRNA primer (10 μM), 0.4 μl of universal primer (10 μM), and 7.2 μl of nuclease-free water in 96-well’s plate. The plate was centrifuged and activated for 2 min at 95°C followed 40 cycles of 95°C for 5 s, and 60°C at 30 s. To normalize our miRNA data across samples, we used the U6. U6 was chosen for normalization as it is one of the most widely used normalizers for cellular miRNA studies and have been shown to be relatively stable across different conditions and experimental design.
Statistical analysis
Relative expression levels for all assays were calculated using the fold change (FC) method (2−ΔΔCt) (Livak and Schmittgen 2001). Results are expressed as FC ± standard errors (SE) with respect to the experimental control. To test our data for normality, we used the Shapiro-Wilk test. For all FC comparisons between the exposure groups, we used the Mann-Whitney U test and significance was defined as p ≤ .05. All statistical analyses were performed in SAS 9.4 (SAS Institute Inc., Cary, North Carolina).
RESULTS
Membrana granulosa cells were collected from follicular aspirates from 25 patients, all of whom contributed cells to our previous studies (Mansur et al. 2016, 2017). Patients’ mean age was 31.1 (±4.1 standard deviation). Of them, 15 underwent IVF for pregestational diagnosis of recessive disease, 7 were diagnosed as cases of male factor infertility, and 3 were diagnosed as cases of unexplained infertility. Out of the 754 miRNAs screened in EVs from the conditioned media of BPA-exposed granulosa cells, 105 miRNAs passed our detection criteria and were therefore considered in the data analysis (Supplementary Table 3). When we compared the expression levels of EV-enriched miRNAs in BPA-exposed groups to the control, we identified several miRNAs that were differentially expressed across exposure groups (Figure 2). Although no dose-response pattern was observed, we found increased levels of let-7g-5p in the conditioned media of granulosa cells across all BPA exposure groups, which were significantly increased in the 20 ng/ml (FC = 1.51, p = .04) and 20 000 ng/ml (FC = 2.42, p = .005) BPA groups. We also found increased levels of miR-191-5p (FC = 1.39, p = .04) in the 200 ng/ml, and miR-532 (FC = 2.96, p = .04) in the 2000 ng/ml BPA groups as compared with the control group. On the contrary, compared with the control, we observed decreased levels of: (1) miR-125b (FC = 0.65, p = .02) and miR-212-3p (FC = 0.66, p = .05) in the 20 ng/ml BPA group; (2) miR-324-5p (FC = 0.36, p = .02) in the 200 ng/ml BPA group; and (3) miR-27b-3p (FC = 0.58, p = .04), miR-335 (FC = 0.60, p = .05), and miR-572 (FC = 0.41, p = .05) in the 20 000 ng/ml group.
Figure 2.
Differentially expressed EV-enriched miRNAs in conditioned media collected from BPA-exposed granulosa cells. All comparisons were performed considering granulosa cells exposed to 0 ng/ml of BPA as our control. MiRNAs with p value <.05 are labeled and shown in black dots.
In silico pathway analysis identified interaction between three EV-enriched miRNAs (let-7g-5p, miR-212-3p, and miR-27b-3p) and their mRNA targets (AKAP8, ATP6V1F, FADD, IGF1, MECP2, and PPARG). These genes were found to be enriched for pathways regulating cell cycle progression, proliferation of gonadal cell lines, and in vitro fertilization of oocytes (Figure 3). We then measured these genes in RNA extracted from primary cultures granulosa cells and observed overexpression of AKAP8 (FC = 1.14, p = .01) and IGF1 (FC = 1.38, p = .001) in the 20 ng/ml BPA group; AKAP8 (FC = 1.13, p = .05) in the 2000 ng/ml BPA group; and ATP6V1F (FC = 1.22, p = .02), FADD (FC = 1.22, p = .01), IGF1 (FC = 1.59, p = .06), and PPARG (FC = 1.73, p = .001) in the 20 000 ng/ml BPA versus control groups (Figure 4). In addition, we leveraged global gene expression data from our previous experiments in BPA-exposed granulosa cells under the same conditions (Mansur et al. 2017) and found very similar relative expression levels (Supplementary Figure 2).
Figure 3.
In silico pathway analysis (Ingenuity Pathway Analysis software, Qiagen Inc. Valencia, California) showing interactions between EV-enriched miRNAs that are associated with BPA exposures, their experimentally validated mRNA targets, and biologically relevant pathways that are enriched for.
Figure 4.
Fold change (±SE) of the expression levels of selected genes in granulosa cells exposed to 20, 200, 2000, and 20 000 ng/ml of BPA as compared with the control (0 ng/ml). An asterisk (*) denotes a p value <.05.
Last, to explore any associations between the expression levels of cellular and extracellular miRNAs, we additionally measured let-7g-5p, miR-212-3p, and miR-27b-3p in BPA-exposed granulosa cells, miRNAs that were predicted to target our genes of interest (Figure 3). We found that cellular miR-27b-3p was significantly downregulated (FC = 0.65, p = .03) in granulosa cells exposed to 20 000 ng/ml BPA compared with the control group (Figure 5).
Figure 5.
Fold change (±SE) of the expression levels of let-7g-5p, miR-212-3p, and miR-27b-3p in granulosa cells exposed to 20, 200, 2000, and 20 000 ng/ml of BPA as compared with the control (0 ng/ml). An asterisk (*) denotes a p value <.05.
DISCUSSION
To our knowledge, this study has investigated for the first time the association between BPA exposure (20–20 000 ng/ml) and EV-enriched miRNAs from human primary granulosa cells. Our findings suggest that exposure of granulosa cells to supraphysiological concentrations of BPA (20, 000 ng/ml) alters the expression levels of specific EV-enriched miRNAs. We found negative associations between the expression levels of granulosa-derived EV-enriched miRNAs and their cellular gene targets, which could potentially regulate key biological pathways in follicular development, oocyte maturation, and human reproduction.
BPA is an endocrine disruptor and potent stimulator of PPARG that further regulates IGF1 in granulosa cells (Kwintkiewicz et al. 2010). Various studies have shown that PPARG is directly regulated by miR-27b-3p, but Song et al. found that this interaction plays a critical role in oocyte maturation in pigs (Song et al. 2016). In this study, we found that cellular levels of PPARG and IGF1 were upregulated and that both cellular and EV-enriched miR-27b-3p levels were downregulated when granulosa cells were exposed to the highest BPA concentration (20 000 ng/ml). These findings are also in line with the in silico pathway analysis that identified both PPARG and IGF1 as targets of miR-27b-3p (Figure 3). In the same setting, we recently reported that IGFBP1, a protein that binds to IGF1 and activates IGFR1 pathway, was also upregulated in granulosa cells following BPA exposure (20 000 ng/ml) (Mansur et al. 2017). IGFBP1 was found to be involved in biological processes important in female reproduction such as follicular growth, steroidogenesis, ovulation, and implantation (Fowler et al. 2000).
In addition, we found that FADD, which is also a target of miR-27b-3p, was upregulated in granulosa cells exposed to 20 000 ng/ml BPA that is consistent with the concurrent downregulation of miR-27b-3p (Figure 3). FADD is an adaptor molecule that plays a key role in Fas-mediated apoptosis modulation and signaling by recruiting and activating procaspase-8 or procaspase-10 (Curtin and Cotter 2003). Inoue et al. showed that overexpression of FADD and procaspase-8 induces apoptosis in porcine granulosa cells, a key biological process in the development of follicular atresia (Inoue et al. 2007). Previous studies have shown that FADD is upregulated in response to various cellular proapoptotic stimuli and promotes apoptosis (Kim et al. 2002). Recently, Zhang et al. showed that exposure to environmental particulate matter leads to the activation of Fas-mediated apoptosis by upregulation of FADD and caspase-8, which resulted in decreased sperm quantity and quality in rats (Zhang et al. 2018). Here, we show for the first time that FADD in primary human granulosa cells is sensitive to BPA exposure, suggesting that it may be implicated in female reproduction in response to environmental toxins.
We also found that the most robustly deregulated EV-miRNA across all concentrations of BPA exposure in granulosa cell media was let-7g-5p. Previous studies found that BPA disrupts critical granulosa cell functions such as cell cycle progression, proliferation, apoptosis, and estradiol secretion (Kwintkiewicz et al. 2010; Lenie et al. 2008; Xu et al. 2002), which are independently regulated by miRNAs, including let-7g. Zhou et al. showed that overexpression of let-7g induces apoptosis and autophagy by inhibiting the expression of IGF1R in vitro (Zhou et al. 2016). In another study, Cao et al. also showed in vitro that overexpression of let-7g induces apoptosis in granulosa cells by inhibiting MAP3K1 (Cao et al. 2015). The fact that cellular let-7g-5p is sensitive to BPA exposures and regulates important biological pathways in granulosa cells (Avissar-Whiting et al. 2010; Tilghman et al. 2012), together with our findings that is upregulated in EVs from the conditioned media in which we cultured granulosa cells, may indicate its importance in BPA-induced cellular toxicity. However, despite the increase in EV-enriched let-7g-5p levels in our study, we neither observed a significant change in its cellular levels, nor a decrease in the mRNA levels of AKAP8 and ATP6V1F, both of which known to be targets of let-7g-5p (Figure 3).
In addition, we found that EV-enriched miR-212-3p was downregulated in granulosa cell culture media after exposure of cells to 20 ng/ml of BPA. A previous study found that miR-212-3p was present in granulosa and cumulus cells, as well as in follicular fluid EVs in mares (da Silveira et al. 2012). Two studies showed that miR-212-3o is regulated by hCG/LH in granulosa cells in mouse and mares, and that it has a role in cell signaling and proliferation (Fiedler et al. 2008; Schauer et al. 2013). According to our in silico analysis, miR-212-3p targets MECP2 (Figure 3) that is involved in cell cycle progression, and it was also reported to regulate cell signaling, differentiation, and apoptosis (da Silveira et al. 2018). However, when we measured the expression levels of both MECP2 and miR-212-3p in all BPA-exposed granulosa cell groups, we did not find any significant changes. Other EV-enriched miRNAs that were deregulated by BPA in granulosa cells and had been found by others to be biologically relevant are miR-191-5p and miR-125b. Zhang et al. found that miR-191-5p was highly expressed in granulosa cells from dominant and subordinate follicles collected from human participants (Zhang et al. 2017). Together with miR-27b-3p, miR-191-5p can efficiently target BDNF, a member of the MAPK signaling pathway that is critical in the development of follicles. Last, miR-125b was detected in follicles (McBride et al. 2012), found to be enriched in follicular fluid EVs (da Silveira et al. 2012; Machtinger et al. 2017), and also found to control apoptosis in granulosa cells (Sen et al. 2014). Despite their differential expression in response to BPA exposures extracellularly, we did not see any changes in their cellular levels, neither for their targets.
Our study has certain limitations. First, due to the small sample size of our study, we could not correct for multiple comparisons in our EV-enriched miRNA microarray screening experiments, and therefore, we acknowledge that differentially expressed EV-enriched miRNAs in response to BPA exposures may have been false positive findings. In order to decrease as much as possible the chances for spurious results, we added further validations of miRNAs and mRNAs levels in granulosa cells exposed to the same concentrations of BPA. We found, however, a negative association between the expression levels of miR-27b-3p and its mRNA targets (PPARG, IGF, and FADD) that supports our findings for miR-27b-3p. We also acknowledge that these are associations at the miRNA/mRNA level and that additional experiments (ie, luciferase assays) to confirm regulation of these genes by the proposed miRNAs are warranted. Second, by using primary granulosa cells from a heterogeneous group of women has probably contributed to increased biological variability, which may explain the modest effect and statistical significance in our experiments. In order to control for these cofounders, we excluded women with polycystic ovaries and endometriosis as these conditions might affect miRNA expression. However, we are aware of the fact that ovarian stimulation itself, as well as granulosa cells deriving from oocytes with various maturation statuses might alter miRNA profiles. In addition, as we used human discarded material, we were limited by studying supernatant from granulosa cells only and not the whole follicle including the oocytes. Therefore, our recipient cells might be granulosa cells too. Last, we acknowledge that with our centrifugation-based isolation method we cannot preclude the likelihood of coprecipitation of other compartments (eg, Ago2 and LDL/HDL) that carry miRNAs in the extracellular space.
In summary, we showed that exposure to supra-physiological BPA levels changes the levels of specific EV-enriched miRNAs in the conditioned media of primary granulosa cells, which were also associated with changes in expression of their cellular targets genes. Our findings suggest that cellular and extracellular miRNAs may be a biological mechanism implicated in BPA toxicity in the ovarian follicle. Further studies validating our preliminary findings and elucidating the biological role of granulosa cell miRNAs in the association between BPA exposure and female infertility are warranted. Successful understanding of these mechanisms may lead to novel interventions to improve fertility outcomes.
CONFLICT OF INTEREST
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
National Institutes of Environmental Health Sciences (R21-ES024236, P30 ES009089) from the Center for Environmental Health in Northern Manhattan, HSPH-NIEHS Center for Environmental Health (P30 ES000002), Israeli Science Foundation (1936/12), and the Environmental Health Fund, Israel (1301).
Supplementary Material
REFERENCES
- Avissar-Whiting M., Veiga K. R., Uhl K. M., Maccani M. A., Gagne L. A., Moen E. L., Marsit C. J. (2010). Bisphenol A exposure leads to specific microRNA alterations in placental cells. Reprod. Toxicol. 29, 401–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel D. P. (2004). MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 116, 281–297. [DOI] [PubMed] [Google Scholar]
- Calafat A. M., Ye X., Wong L. Y., Reidy J. A., Needham L. L. (2008). Exposure of the U.S. Population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ. Health Perspect. 116, 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Can A., Semiz O., Cinar O. (2005). Bisphenol-A induces cell cycle delay and alters centrosome and spindle microtubular organization in oocytes during meiosis. Mol. Hum. Reprod. 11, 389–396. [DOI] [PubMed] [Google Scholar]
- Cao R., Wu W., Zhou X., Liu K., Li B., Huang X., Zhang Y., Liu H. (2015). Let-7g induces granulosa cell apoptosis by targeting map3k1 in the porcine ovary. Int. J. Biochem. Cell Biol. 68, 148–157. [DOI] [PubMed] [Google Scholar]
- Curtin J. F., Cotter T. G. (2003). Live and let die: Regulatory mechanisms in Fas-mediated apoptosis. Cell Signal. 15, 983–992. [DOI] [PubMed] [Google Scholar]
- da Silveira J. C., de Andrade G. M., Nogueira M. F., Meirelles F. V., Perecin F. (2015). Involvement of miRNAs and cell-secreted vesicles in mammalian ovarian antral follicle development. Reprod. Sci. 22, 1474–1483. [DOI] [PubMed] [Google Scholar]
- da Silveira J. C., de Avila A., Garrett H. L., Bruemmer J. E., Winger Q. A., Bouma G. J. (2018). Cell-secreted vesicles containing microRNAs as regulators of gamete maturation. J. Endocrinol. 236, R15–R27. [DOI] [PubMed] [Google Scholar]
- da Silveira JC, Veeramachaneni D. N., Winger Q. A., Carnevale E. M., Bouma G. J. (2012). Cell-secreted vesicles in equine ovarian follicular fluid contain miRNAs and proteins: A possible new form of cell communication within the ovarian follicle. Biol. Reprod. 86, 71, 1–10. [DOI] [PubMed] [Google Scholar]
- da Silveira J. C., Winger Q. A., Bouma G. J., Carnevale E. M. (2015). Effects of age on follicular fluid exosomal microRNAs and granulosa cell transforming growth factor-beta signalling during follicle development in the mare. Reprod. Fertil. Dev. 27, 897–905. [DOI] [PubMed] [Google Scholar]
- Ehrlich S., Lambers D., Baccarelli A., Khoury J., Macaluso M., Ho S. M. (2016). Endocrine disruptors: A potential risk factor for gestational diabetes mellitus. Am. J. Perinatol. 33, 1313–1318. [DOI] [PubMed] [Google Scholar]
- Eichenlaub-Ritter U., Vogt E., Cukurcam S., Sun F., Pacchierotti F., Parry J. (2008). Exposure of mouse oocytes to bisphenol A causes meiotic arrest but not aneuploidy. Mutat. Res. 651, 82–92. [DOI] [PubMed] [Google Scholar]
- Fiedler S. D., Carletti M. Z., Hong X., Christenson L. K. (2008). Hormonal regulation of microRNA expression in periovulatory mouse mural granulosa cells. Biol. Reprod. 79, 1030–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler D. J., Nicolaides K. H., Miell J. P. (2000). Insulin-like growth factor binding protein-1 (igfbp-1): A multifunctional role in the human female reproductive tract. Hum. Reprod. Update 6, 495–504. [DOI] [PubMed] [Google Scholar]
- Granot I., Dekel N. (2002). The ovarian gap junction protein connexin43: Regulation by gonadotropins. Trends Endocrinol. Metab. 13, 310–313. [DOI] [PubMed] [Google Scholar]
- Grasselli F., Baratta L., Baioni L., Bussolati S., Ramoni R., Grolli S., Basini G. (2010). Bisphenol A disrupts granulosa cell function. Domest. Anim. Endocrinol. 39, 34–39. [DOI] [PubMed] [Google Scholar]
- Harischandra D. S., Ghaisas S., Rokad D., Kanthasamy A. G. (2017). Exosomes in toxicology: Relevance to chemical exposure and pathogenesis of environmentally linked diseases. Toxicol. Sci. 158, 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt P. A., Koehler K. E., Susiarjo M., Hodges C. A., Ilagan A., Voigt R. C., Thomas S., Thomas B. F., Hassold T. J. (2003). Bisphenol A exposure causes meiotic aneuploidy in the female mouse. Curr. Biol. 13, 546–553. [DOI] [PubMed] [Google Scholar]
- Hunt P. A., Susiarjo M., Rubio C., Hassold T. J. (2009). The bisphenol A experience: A primer for the analysis of environmental effects on mammalian reproduction. Biol. Reprod. 81, 807–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikezuki Y., Tsutsumi O., Takai Y., Kamei Y., Taketani Y. (2002). Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum. Reprod. 17, 2839–2841. [DOI] [PubMed] [Google Scholar]
- Inoue N., Matsuda-Minehata F., Goto Y., Sakamaki K., Manabe N. (2007). Molecular characteristics of porcine Fas-associated death domain (FADD) and procaspase-8. J. Reprod. Dev. 53, 427–436. [DOI] [PubMed] [Google Scholar]
- Kim P. K. M., Wang Y., Gambotto A., Kim Y.-M., Weller R., Zuckerbraun B. S., Hua Y., Watkins S. C., Billiar T. R. (2002). Hepatocyte Fas-associating death domain protein/mediator of receptor-induced toxicity (FADD/MORT1) levels increase in response to pro-apoptotic stimuli. J. Biol. Chem. 277, 38855–38862. [DOI] [PubMed] [Google Scholar]
- Kwintkiewicz J., Nishi Y., Yanase T., Giudice L. C. (2010). Peroxisome proliferator-activated receptor-gamma mediates bisphenol A inhibition of FSH-stimulated IGF-1, aromatase, and estradiol in human granulosa cells. Environ. Health Perspect. 118, 400–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenie S., Cortvrindt R., Eichenlaub-Ritter U., Smitz J. (2008). Continuous exposure to bisphenol A during in vitro follicular development induces meiotic abnormalities. Mutat. Res. 651, 71–81. [DOI] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative pcr and the 2(-delta delta c(t)) method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Machtinger R., Combelles C. M. H., Missmer S. A., Correia K. F., Williams P., Hauser R., Racowsky C. (2013). Bisphenol-A and human oocyte maturation in vitro. Hum. Reprod. 28, 2735–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machtinger R., Laurent L. C., Baccarelli A. A. (2016). Extracellular vesicles: Roles in gamete maturation, fertilization and embryo implantation. Hum. Reprod. Update 22, 182–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machtinger R., Rodosthenous R. S., Adir M., Mansour A., Racowsky C., Baccarelli A. A., Hauser R. (2017). Extracellular microRNAs in follicular fluid and their potential association with oocyte fertilization and embryo quality: An exploratory study. J. Assist. Reprod. Genet. 34, 525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansur A., Adir M., Yerushalmi G., Hourvitz A., Gitman H., Yung Y., Orvieto R., Machtinger R. (2016). Does BPA alter steroid hormone synthesis in human granulosa cells in vitro? Hum. Reprod. 31, 1562–1569. [DOI] [PubMed] [Google Scholar]
- Mansur A., Israel A., Combelles C. M. H., Adir M., Racowsky C., Hauser R., Baccarelli A. A., Machtinger R. (2017). Bisphenol-A exposure and gene expression in human luteinized membrana granulosa cells in vitro. Hum. Reprod. 32, 409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride D., Carré W., Sontakke S. D., Hogg C. O., Law A., Donadeu F. X., Clinton M. (2012). Identification of miRNAs associated with the follicular-luteal transition in the ruminant ovary. Reproduction 144, 221–233. [DOI] [PubMed] [Google Scholar]
- Mestdagh P., Van Vlierberghe P., De Weer A., Muth D., Westermann F., Speleman F., Vandesompele J. (2009). A novel and universal method for microRNA RT-QPCR data normalization. Genome Biol. 10, R64.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neven K. Y., Nawrot T. S., Bollati V. (2017). Extracellular vesicles: How the external and internal environment can shape cell-to-cell communication. Curr. Environ. Health Rep. 4, 30–37. [DOI] [PubMed] [Google Scholar]
- Peretz J., Flaws J. A. (2013). Bisphenol A down-regulates rate-limiting cyp11a1 to acutely inhibit steroidogenesis in cultured mouse antral follicles. Toxicol. Appl. Pharmacol. 271, 249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz J., Gupta R. K., Singh J., Hernandez-Ochoa I., Flaws J. A. (2011). Bisphenol A impairs follicle growth, inhibits steroidogenesis, and downregulates rate-limiting enzymes in the estradiol biosynthesis pathway. Toxicol. Sci. 119, 209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergoli L., Cantone L., Favero C., Angelici L., Iodice S., Pinatel E., Hoxha M., Dioni L., Letizia M., Albetti B., et al. (2017). Extracellular vesicle-packaged miRNA release after short-term exposure to particulate matter is associated with increased coagulation. Part. Fibre Toxicol. 14, 32.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G., Stoorvogel W. (2013). Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 200, 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed B. G., Babayev S. N., Chen L. X., Carr B. R., Word R. A., Jimenez P. T. (2018). Estrogen-regulated miRNA-27b is altered by bisphenol A in endometrial stromal cells. Reproduction. 156, 559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santonocito M., Vento M., Guglielmino M. R., Battaglia R., Wahlgren J., Ragusa M., Barbagallo D., Borzì P., Rizzari S., Maugeri M., et al. (2014). Molecular characterization of exosomes and their microRNA cargo in human follicular fluid: Bioinformatic analysis reveals that exosomal microRNAs control pathways involved in follicular maturation. Fertil. Steril. 102, 1751–U1590. [DOI] [PubMed] [Google Scholar]
- Schauer S. N., Sontakke S. D., Watson E. D., Esteves C. L., Donadeu F. X. (2013). Involvement of miRNAs in equine follicle development. Reproduction 146, 273–282. [DOI] [PubMed] [Google Scholar]
- Sen A., Prizant H., Light A., Biswas A., Hayes E., Lee H.-J., Barad D., Gleicher N., Hammes S. R. (2014). Androgens regulate ovarian follicular development by increasing follicle stimulating hormone receptor and microRNA-125b expression. Proc. Natl. Acad. Sci. U.S.A. 111, 3008–3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sela-Abramovich S., Edry I., Galiani D., Nevo N., Dekel N. (2006). Disruption of gap junctional communication within the ovarian follicle induces oocyte maturation. Endocrinology 147, 2280–2286. [DOI] [PubMed] [Google Scholar]
- Shivdasani R. A. (2006). MicroRNAs: Regulators of gene expression and cell differentiation. Blood 108, 3646–3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohel Md. Mahmodul H., Hoelker M., Noferesti S. S., Salilew-Wondim D., Tholen E., Looft C., et al. (2013). Exosomal and non-exosomal transport of extra-cellular microRNAs in follicular fluid: Implications for bovine oocyte developmental competence. PLoS One 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C., Yao J., Cao C., Liang X., Huang J., Han Z., Zhang Y., Qin G., Tao C., Li C., et al. (2016). Ppargamma is regulated by mir-27b-3p negatively and plays an important role in porcine oocyte maturation. Biochem. Biophys. Res. Commun. 479, 224–230. [DOI] [PubMed] [Google Scholar]
- Tilghman S. L., Bratton M. R., Segar H. C., Martin E. C., Rhodes L. V., Li M., McLachlan J. A., Wiese T. E., Nephew K. P., Burow M. E., et al. (2012). Endocrine disruptor regulation of microRNA expression in breast carcinoma cells. PLoS One 7, e32754.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg L. N., Hauser R., Marcus M., Olea N., Welshons W. V. (2007). Human exposure to bisphenol A (BPA). Reprod. Toxicol. 24, 139–177. [DOI] [PubMed] [Google Scholar]
- Xu J., Osuga Y., Yano T., Morita Y., Tang X., Fujiwara T., Takai Y., Matsumi H., Koga K., Taketani Y., et al. (2002). Bisphenol A induces apoptosis and g2-to-m arrest of ovarian granulosa cells. Biochem. Biophys. Res. Commun. 292, 456–462. [DOI] [PubMed] [Google Scholar]
- Zhang B., Chen L., Feng G., Xiang W., Zhang K., Chu M., Wang P. (2017). MicroRNA mediating networks in granulosa cells associated with ovarian follicular development. Biomed. Res. Int. 2017, 4585213.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Liu J., Ren L., Wei J., Duan J., Zhang L., Zhou X., Sun Z. (2018). Pm2.5 induces male reproductive toxicity via mitochondrial dysfunction, DNA damage and ripk1 mediated apoptotic signaling pathway. Sci. Total Environ. 634, 1435–1444. [DOI] [PubMed] [Google Scholar]
- Zhang M., Ouyang H., Xia G. (2009). The signal pathway of gonadotrophins-induced mammalian oocyte meiotic resumption. Mol. Hum. Reprod. 15, 399–409. [DOI] [PubMed] [Google Scholar]
- Zhou J., Yao W., Liu K., Wen Q., Wu W., Liu H., Li Q. (2016). MicroRNA let-7g regulates mouse granulosa cell autophagy by targeting insulin-like growth factor 1 receptor. Int. J. Biochem. Cell Biol. 78, 130–140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.