Abstract
2,4,6-tribromophenol (TBP, CAS No. 118-79-6) is widely used as a brominated flame retardant and wood antifungal agent. TBP is frequently detected in environmental matrices, biota, and humans. In female SD rats, systemically available TBP (10 µmol/kg, IV) was rapidly excreted primarily via urine, with approximately 61% of the dose recovered after 4 h, and 89%–94% in 24 h; 5% was recovered in feces; and 1%–2% in blood/tissues. TBP administered to female SD rats (0.1–1000 µmol/kg) by gavage was well absorbed, with approximately 25% eliminated via urine after 4 h and approximately 88% after 24 h. Approximately 11% of a single oral dose was recovered in bile. Male SD rats and B6C3F1/J mice of both sexes had similar disposition profiles when administered a single oral dose of TBP (10 µmol/kg). Following administration, fecal recoveries varied only slightly by dose, sex, or species. TBP readily passed unchanged through both human (ex vivo only) and rat skin with between 55% and 85% of a 100 nmol/cm2 passing into or through skin. Concentrations of TBP in blood fit a two-compartment model after IV-dosing and a one-compartment model after oral dosing. Urine contained a mixture of TBP, TBP-glucuronide, and TBP-sulfate. Fecal extracts contained only parent TBP whereas bile contained only TBP-glucuronide. TBP did not appear to bioaccumulate or alter its own metabolism after repeated administration. TBP was readily absorbed at all doses and routes tested with an oral bioavailability of 23%–27%; 49% of TBP is expected to be dermally bioavailable in humans. From these data, we conclude that humans are likely to have significant systemic exposure when TBP is ingested or dermal exposure occurs.
Keywords: 2,4,6-tribromophenol; disposition; pharmacokinetics; bioavailability; brominated flame retardant; persistent organic pollutant; Chemical compounds studied in this article: 2,4,6-tribromophenol (PubChem CID: 1483; CAS No. 118-79-6, FW: 330.801 g/mol, LogP: 4.13) (Hansch et al., 1995)
2,4,6-Tribromophenol (TBP, CAS No. 118-79-6) is a widely used brominated flame retardant (BFR), precursor for other BFRs, and wood antifungal agent (Gutierrez et al., 2005; Thomsen et al., 2001, 2002). TBP is also naturally occurring in some marine fishes, such as Pacific salmon (Boyle et al., 1992). Due to this wide variety of natural and anthropogenic sources, TBP is a frequently detected (60%–100% detection frequency) contaminant in environmental matrices and biota, including human breast milk, placenta, and serum (Butt et al., 2016; Dufour et al., 2017; Fujii et al., 2014, 2018; Gutierrez et al., 2005; Koch and Sures, 2018; Leonetti et al., 2016a,b; Oliveira et al., 2009; Thomsen et al., 2002; Whitfield et al., 1999; Xiong et al., 2015, 2016). High levels of TBP have been reported in indoor air and in household dust, highlighting the potential for TBP exposure via inhalation and ingestion of dust (Sha et al., 2018; Takigami et al., 2009). In addition to potential oral or dermal exposure through dust, TBP is likely to be consumed in a diet rich in wild-caught fish (Fuller et al., 2008).
Brominated flame retardants are a prominent source of TBP. TBP has been shown to be formed as a degradation product of the polymeric flame retardant, PolyFR, a butadiene styrene brominated copolymer (Koch et al., 2016). Experimental systems designed to degrade the BFR Tetrabromobisphenol A (TBBPA) resulted in the formation of TBP (Qu et al., 2015). Furthermore, biotic degradation of TBBPA or other brominated phenolic chemicals results in the formation of TBP and other phenolic byproducts (Gu et al., 2018).
TBP is found as a naturally occurring bromophenol in saltwater sportfish (Whitfield et al., 1998) and wild-harvested seafood such as Pacific salmon, Dungeness crab, and clams (Boyle et al., 1992; Oliveira et al., 2009) and contributes to the “shrimp” flavor in processed foodstuffs (Fuller et al., 2008; Oliveira et al., 2009). In one study, coastal residents with high marine fish consumption had higher serum TBP concentrations than those seen in electronics recyclers, indicating dietary intake can be a major source of the chemical (Eguchi et al., 2012). TBP and other bromophenols are not biosynthesized in finfish and shellfish that are commercially harvested and consumed; biosynthesis of TBP occurs further down the food chain, most significantly in polychaetes (marine bristle worms) and bryozoans (filter feeding aquatic invertebrates).
TBP has been shown to be formed as a degradation product of the polymeric flame retardant, PolyFR, a butadiene styrene brominated copolymer (Koch et al., 2016). Experimental systems designed to degrade the brominated flame retardant Tetrabromobisphenol A (TBBPA) resulted in the formation of TBP (Qu et al., 2015). Furthermore, biotic degradation of TBBPA or other brominated phenolic chemicals results in the formation of TBP and other phenolic byproducts (Gu et al., 2018).
In exposed workers, circuit board producers and electronics disassemblers had blood concentrations that ranged from 14.2 to 244.9 pmol TBP/g lipid (Thomsen et al., 2001). Sawmill workers exposed to TBP were found to have urinary concentrations of 5.7–37.2 µmol TBP/g creatinine (Gutierrez et al., 2005). In nonoccupational exposures, serum levels of TBP correlated positively with those of polybrominated diphenyl ethers (PBDEs), suggesting similar sources of exposure, or that TBP may result from metabolism of PBDEs (Butt et al., 2016). Accumulation of TBP in human placenta is expected to contribute to prenatal exposures (Leonetti et al., 2016b).
In experimental animals, TBP exposures resulted in neurological, reproductive, and developmentally toxic outcomes and is a suspected endocrine disrupter (Deng et al., 2010; Halden et al., 2010). Pregnant rats exposed to aerosolized TBP gave birth to offspring that exhibited skeletal malformations (Lyubimov et al., 1998). Zebrafish exposed to TBP had negatively altered gonad morphology and reduced fertility (Deng et al., 2010; Halden et al., 2010). In vitro, TBP reduced the transcriptional activity of both estrogen and androgen receptor at low micromolar (IC50 = 4–14 µM) concentrations (Ezechias et al., 2012). TBP binds the thyroid hormone transport protein, transthyretin (prealbumin), potentially altering thyroid hormone signaling (Suzuki et al., 2008). TBP also inhibits estrogen sulfotransferase activity (Kester et al., 2000), possibly via the same mechanism as TBBPA (Gosavi et al., 2013).
However, no studies have comprehensively assessed TBP disposition or kinetics, an omission noted in the recent review of TBP by Koch and Sures (2018). Therefore, we assessed the disposition of TBP after oral, intravenous, and dermal administration. Single oral doses of [14C]-radiolabeled TBP were administered to female Hsd: Sprague Dawley SD (SD) rats by gavage over a 10 000-fold dose range (1000, 10, and 0.1 µmol/kg). The bioaccumulation and enzyme induction potential of TBP were investigated using 5-day repeated oral dosing (10 µmol/kg/d). To determine route-dependent disposition, in addition to oral dosing, TBP was administered intravenously (10 µmol/kg) or applied to rat skin (100 nmol/cm2). TBP blood-time concentration profiles were analyzed using pharmacokinetic modeling to determine oral bioavailability. In vivo dermal absorption study, data were compared with data from ex vivo human and rat skin studies to estimate human dermal bioavailability. Finally, to investigate basic sex- and species-dependent disposition, male SD rats and both sexes of B6C3F1/J mice were administered a single dose (10 µmol/kg) by gavage and disposition was evaluated over 24 h.
MATERIALS AND METHODS
Chemicals
[14C]-radiolabeled TBP (ring-labeled; Figure 1) was purchased as a crystalline solid from Chemdepo (Camarillo, California). [14C]-TBP had a radiochemical purity of >98% (confirmed by radiochemical HPLC) with a specific activity of 65 mCi/mmol; chemical purity was determined by HPLC to be >99% as compared with a TBP reference standard (Sigma-Aldrich, St. Louis, Missouri). [14C]-TBP was dissolved in acetone to a stock concentration of 0.75 mCi/ml. Scintillation cocktails were obtained from MP Biomedicals (Ecolume; Santa Ana, California) or Perkin-Elmer (Ultima Gold, Ultima-Flo M, Hionic Fluor, and PermaFluor E+; Waltham, Massachusetts). Food-grade corn oil was purchased from Sigma-Aldrich. Isoflurane was obtained from Piramal Healthcare (Mumbai, India). All other reagents used in these studies were high performance liquid chromatography (HPLC) or analytical grade.
Figure 1.
TBP structure and primary in vivo metabolites, TBP-glucuronide and TBP-sulfate.
Animal models and dosing
Hsd: Sprague Dawley SD rats (SD; 10 weeks, approximately 200 g; Harlan Laboratories, Raleigh, North Carolina) or B6C3F1/J mice (10 weeks; approximately 20 g; Jackson Labs, Bar Harbor, Maine) were used in this study. Conventional (nonsurgically altered) animals were maintained in an AAALAC-approved animal care facility and were acclimated to the facility for 1 week prior to dosing in polycarbonate shoebox cages (Techniplast, West Chester, Pennsylvania) with Sani-Chip bedding (PJ Murphy Forest Products, Montville, New Jersey) prior to being placed on study (humidity: approximately 49%, room temperature: approximately 72°F, 12 h light/dark cycle). Feed (NIH No. 31; Ziegler Brothers, 2010) and tap water (City of Durham, North Carolina) were provided for ad libitum consumption. The tap water was treated by reverse-osmosis in the animal facility before use. Animals with an indwelling jugular vein or common bile duct-cannula were surgically altered by the vendor and cannula patency was checked upon notification of arrival. Surgically altered animals were acclimated for 24 h in an AAALAC approved NIEHS facility prior to dosing. All procedures were approved by the NIEHS Institutional Care and Use committee.
To determine the fate of systemically available TBP in female SD rats, a single intravenous (IV) bolus was injected into a lateral tail vein. IV dosing solutions (10 µmol/kg, 1 ml/kg, N = 4 animals), were composed of [14C]-TBP in acetone added to 1 part of Cremophore EL (Sigma-Aldrich) followed by evaporation of acetone under a stream of nitrogen followed by the addition of 1 part of ethanol and 3 parts of water. Animals were preacclimated in plastic metabolism cages (Techniplast) for 24 h prior to dosing. Immediately following dosing, animals were returned to plastic metabolism cages for collection of urine and feces. Animals were not fasted prior to dosing in any studies.
To determine the fate of orally administered TBP, a single dose of TBP by gavage (PO) at dose levels of 0.1, 10, or 1000 µmol/kg (approximately 0.03–330 mg/kg; 4 ml/kg in corn oil; N = 4/dose group) was administered to groups of female SD rats. Distribution to tissues and cumulative excretion of [14C]-radioactivity in excreta were evaluated at 24-h postexposure for these doses. Dosing solutions (10 or 1000 µmol/kg, 4 ml/kg) were formulated to provide approximately 100 μCi/kg/rat of [14C]-TBP and contained nonradiolabeled TBP for delivery of doses up to 1000 µmol/kg. 0.1 µmol/kg of dosing solutions provided 6.5 μCi/kg/animal of [14C]-TBP (specific activity limited total radioactivity dosed per animal). Biliary excretion of TBP or its metabolites was studied using bile duct cannulated female SD rats; 4 rats were administered TBP (10 μmol/kg) by gavage or lateral tail vein and bile was collected at hourly intervals for 8 h then continuously for the 8–12 h and 12–24 h intervals and the rats were euthanized 24 h after dosing.
The bioaccumulation potential of TBP was investigated by administering 5 consecutive daily doses of [14C]-labeled TBP (10 µmol/kg/d; 100 µCi/kg/d) to female SD rats and collecting excreta at 24 h intervals; accumulation of TBP-dependent radioactivity in tissues was assessed 24 h after the final dose administration. To identify potential sex- or species-related differences in the in vivo fate of TBP, a group of male SD rats, male B6C3F1/J mice, and female B6C3F1/J mice (N = 4/group) received a single dose of 10 µmol/kg (4 ml/kg) by gavage and excreta were collected between 0 and 24 h. Urine and feces were collected for at least 8 h prior to dosing to determine background contamination in all studies. A summary of experiments is shown in Table 1.
Table 1.
Summary of Sex, Species, Doses, and Routes Investigated
| Female SD Rat | Male SD Rat | Female B6C3F1/J Mouse | Male B6C3F1/J Mouse |
|---|---|---|---|
| 0.1 µmol/kg PO | — | — | — |
| 0.1 µmol/cm2 Dermal | — | — | — |
| 10 µmol/kg IV | — | — | — |
| 10 µmol/kg PO | 10 µmol/kg PO | 10 µmol/kg PO | 10 µmol/kg PO |
| 1000 µmol/kg PO | — | — | — |
Pharmacokinetic modeling
An assessment of TBP pharmacokinetics in blood was performed using female SD rats containing an indwelling (Harlan Laboratories) jugular vein cannula (10 μmol/kg, 100 µCi/kg, N = 4). Blood (approximately 100 µl/time point) was obtained through the cannula using heparinized syringes. Sampled blood was replaced with equal amounts of saline. Pharmacokinetic modeling following a single oral or IV dose (10 μmol/kg) was based on 9 data points (7.5, 15, and 30 min, then at 1, 2, 4, 8, 12, and 24 h). Final pharmacokinetic values for free TBP in blood was determined by radiochemical HPLC analyses of blood collected from individual animals at each time point described earlier. These data were used to construct a time-concentration data table for each animal. These data were fit to established pharmacokinetic models using the Phoenix WinNonlin (Certara USA, Inc, St. Louis, Missouri) software package. Time-concentration data from animals administered TBP by gavage were fit to a one-compartment model with first order input and output whereas the time-concentration data from animals administered TBP intravenously was fit to a two-compartment model. Goodness of fit for the models and weighting schemes was assessed by comparing the sum of squared residuals.
Sample collections
Following administration of the compound by gavage or IV, excreta and cage rinses were collected at intervals of 0–4, 4–8, 8–12, and 12–24 h from the metabolism cages. Samples were collected at room temperature. Reverse osmosis water (Picopure, Hydro Service and Supplies, Inc, Durham, North Carolina) was used for cage washes. When bladder urine was recovered during necropsy, it was added to the final urine collection vial. Initial experiments (1000 µmol/kg) allowed for the collection of [14C]-radioactivity excreted in expired air using a method described previously (Sanders et al., 2000). However, [14C]-labeled CO2 and/or exhaled volatile organic compounds were not detected after administration of the highest dose; therefore, the collection of expired air was discontinued for all subsequent experiments. Euthanasia was achieved by CO2 asphyxiation and confirmed by unresponsiveness to forceps pinch applied to the hindpaw. Blood samples were collected into heparinized syringes via cardiac puncture immediately following euthanasia for determination of [14C]-radioactivity in whole blood. Plasma was isolated from the remaining heparinized blood by centrifugation (5 min at 2300×g). Tissues (adipose [pooled], adrenals, brain, heart, kidneys, large intestine, large intestine contents, liver, lung, muscle (quadriceps), pancreas, ovaries/testes, skin (ears), small intestine, small intestine contents, spleen, stomach, stomach contents, thymus, thyroid, urinary bladder, and uterus/vas deferens and epididymis) were dissected and placed in labeled preweighed vials and weights determined gravimetrically. Total mass of dispersed tissues were calculated as a fraction of body weight using previously published values (Birnbaum et al., 1980). Samples were maintained at −20°C until aliquoting and oxidation. After aliquoting, samples were stored at −80°C until further analyses.
Excreta and tissues containing [14C]-radioactivity were sampled and subjected to extractions using a previously described method (Knudsen et al., 2016). Briefly, aliquots (approximately 250 mg) of ground, dried feces were placed in labeled preweighed glass screw-top tubes and resuspended in 5 ml acetone and mixed by vortexing for 1 min. Samples were then centrifuged at 830×g for 10 min using a Sorvall RC6+ centrifuge (Thermo Scientific, Inc, Waltham, Massachusetts) with a SH-3000 swing bucket rotor to sediment solids; supernatants were transferred before repeating the process twice. When recoveries were <95% of expected values, the pellet was further extracted with ethyl acetate (3×5 ml) and 95% ethanol (3 × 5 ml). At each step, the samples were centrifuged, and the supernatants transferred and pooled. Extracts were concentrated to approximately 100 µl using a Savant SPD1010 SpeedVac (Thermo Scientific) without heating, and aliquots were analyzed by UV/radiometric HPLC. Remaining sample pellets were air dried, weighed, and [14C]-radioactivity remaining in the sample (unextracted fraction) was quantified by sample combustion followed by LSC counting. Fecal extraction efficiencies approached 100%.
Dermal dosing studies
Dermal TBP uptake and penetrance were investigated as previously described (Knudsen et al., 2015). Ex vivo studies were conducted using split-thickness skin (ie, epidermis and upper portion of the dermis) from human cadaver donors (dorsal scapular skin; National Disease Research Interchange, Philadelphia, Pennsylvania, N = 4, 3 males, 1 female, 57–69 years old) and female SD rats (Harlan Bioproducts for Science, Indianapolis, Indiana, hair clipped 24 h prior to excision, N = 4, 11 weeks old) to match the sex, species, and strain used in the in vivo studies; age and sex of human donors were determined by available tissues. Briefly, in vivo human bioavailability was estimated as a function of ex vivo human exposure multiplied by a normalization factor based on the same dose applied to rat skin in vivo and ex vivo. Flux (Jss) was calculated as described previously and derived from Fick’s first law of diffusion (Hughes and Edwards, 2010; Niedorf et al., 2008). Briefly, the maximal flux (pmol-eq per square centimeter per hour) was derived from the slopes of the penetrated mass across each barrier plotted versus sampling period. Error was calculated as described previously (Caldwell and Vahidsafa, 2015).
For in vivo dermal studies, the dorsal scapular region of isoflurane-anaesthetized female SD rats was clipped 24 h prior to dosing. On the day of dosing, rats (N = 4) were anaesthetized with isoflurane, and the dosing area was marked with a felt-tipped marker. A single dose of [14C]-TBP dissolved in acetone (100 nmol/cm2; 10 µl applied to approximately 1 cm2, 100 nmol/animal, 300 pg/animal) was applied to the dorsal scapular surface, dosing area covered using a perforated steel cap, and rats were returned to their metabolism cages. Urine, cage washes, and feces were collected at 0–6, 6–12, and 12–24 h intervals. After 24 h, animals were euthanized by CO2 inhalation and subjected to necropsy as described earlier. Dosed skin with the attached steel mesh cap was excised and set aside for washing and tape stripping. Animals were subjected to complete necropsies, with excreta and tissues processed and analyzed as described for all other studies.
Ex vivo absorption and penetration of [14C]-labeled TBP was determined using a ILC07 Automated flow-through system (PermeGear, Hellertown, Pennsylvania). A single dose of [14C]-TBP (100 nmol/cm2; 6.4 μl applied to 0.64 cm2) dissolved in acetone was applied to split-thickness dorsal scapular skin and perfusion media was collected for 24 h at 6 h intervals. For ex vivo studies, a single dose of [14C]-labeled TBP was applied to split-thickness rat or human skin with thicknesses of 356 ± 12 and 385 ± 57 μm for rat and human skin, respectively. Human skin samples were subjected to an additional barrier test using tritiated water (1 μCi, 100 μl, 5-min exposure; PerkinElmer) prior to application of [14C]-labeled TBP to determine water permeability of the samples. Penetrance of <0.05% of applied radioactivity over 1 h was indicative of an intact barrier.
After application of TBP, solvent evaporation was facilitated by gentle airflow over the dosing area and samples remained open to the air for the duration of the studies. After 24 h of perfusion media collection, the skin samples were washed in triplicate (500 μl, 1:1 Joy liquid soap: water), then 6 times with water (500 μl), and air-dried for 24 h. The flow-through system cells were rinsed in triplicate with 500 μl of ethanol. On the following day, skin samples were tape stripped 10 times to remove the stratum corneum and chemically solubilized (Soluene 350, PerkinElmer). [14C]-labeled radioactivity in aliquots of perfusion media, washes, dissolved skin and tapes strips were analyzed by liquid scintillation spectrometry.
Analytical methods
Final concentrations of [14C]-radioactivity in all dosing solutions were determined by assaying aliquots of dosing solution in triplicate by liquid scintillation spectrometry using a LS6500 Scintillation Counter (Beckman Coulter, Brea, California). Quantitative analyses of total [14C]-radioactivity content in plasma (100 μl), urine (10–100 μl), and cage rinses (1 ml) were determined by liquid scintillation counting (LSC). [14C]-radioactivity was quantified in whole blood (100 μl) by combustion of 50–100 μl aliquots in a Model 307 Biological Sample Oxidizer (Perkin-Elmer) followed by LSC counting. Feces were dried in a fume hood, weighed, and ground to a powder using a mortar and pestle. Aliquots of feces and tissues (approximately 25 mg) were weighed and [14C]-radioactivity was quantified by combustion. All samples were assayed in triplicate. Values reported for percent total dose excreted in urine include [14C]-radioactivity from the corresponding cage wash.
Feces (dried, ground, and weighed) and dosed skin (minced and weighed) were extracted to determine the chemical nature of recovered [14C]-radioactivity. Aliquots (approximately 250 mg) of air-dried samples were placed in labeled preweighed glass screw-top tubes and resuspended in 5 ml acetone and then centrifuged at 830×g for 10 min using a Sorvall RC6+ centrifuge (Thermo Fisher Scientific, Waltham, Massachusetts) with a SH-3000 swing bucket rotor. This process was repeated twice, with the supernatants transferred and pooled. Each pellet was further extracted with ethyl acetate (3×5 ml) and 1% HCl in 95% ethanol (3 × 5 ml). At each step, the samples were centrifuged, and the supernatants transferred and pooled. Extracts were concentrated using a Savant SPD1010 SpeedVac (Thermo Fisher Scientific) without heating, and aliquots were analyzed by UV/radiometric HPLC.
Prior to HPLC-radiometric or MS analyses, urine and plasma samples diluted 1:1 (v/v) with 0.2% formic acid in acetonitrile and filtered using PVDF 0.22 µm centrifugal filters (Ultrafree MC GV, Amicon Ultra; Tullagreen, IRL). Aliquots of samples were initially profiled by HPLC with radiometric detection. To determine the nature of phase II metabolites of TBP, undiluted urine samples were incubated at 37°C for up to 24 h with β-glucuronidase (5000 U/ml) or aryl-sulfatase (11 U/ml) as described previously (Andersen et al., 1999). Control urine was incubated with buffer (200 μM ammonium acetate in water, pH 5.0). Reactions were terminated by addition of equal volumes of acetonitrile followed by HPLC-radiometric analyses.
TBP was quantified by UV/Vis absorbance and radiochemical detection following HPLC separation. The HPLC system was composed of a Waters (Watertown, Massachusetts) Alliance 2695 separation module with a Waters 2487 dual wavelength detector, Phenomenex (Torrance, California) Luna 150×4.6 mm C18 column and an in-line Radiomatic 610TR Flow Scintillation Analyzer (PerkinElmer). HPLC control and analysis software was Laura4 (LabLogic, Brandon, Florida). Mobile phases consisted of (1) 0.2% formic acid in water and (2) 0.2% formic acid in acetonitrile. Sample separations were performed using a gradient from A to B; initial conditions (90% A) were reduced to 0% A over 5 min then held at 100% B for 5 min before returning the column to initial conditions and equilibrating the column for 2 min before reuse. HPLC flow rates were 1 ml/min and scintillation cocktail flow rates were 2 ml/min.
Qualitative analyses of extracted metabolites (described earlier) were carried out using ion-trap mass spectrometry with atmospheric pressure chemical ionization in negative ion mode. The mass spectrometer (MS) system was LTQXL ion trap MS coupled to an Ultimate 3000 binary UPLC (Thermo Fisher Scientific) with an Agilent (Santa Clara, California) Zorbax 150×4.6 mm C18 column. HPLC conditions were the same as above. MS conditions were: vaporizer temperature: 250°C; sheath gas flow rate: 20; auxiliary gas flow rate: 10; sweep gas flow rate: 2; discharge current: 5 µA; capillary temp: 275°C; capillary voltage: −15 V; tube lens voltage: −125 V.
Parallelogram calculations for human bioavailability
In vivo human dermal bioavailability was calculated using a parallelogram approach as described previously (Knudsen et al., 2017; van Ravenzwaay and Leibold, 2004). Briefly, in vivo human bioavailability was estimated as a function of ex vivo human exposure multiplied by a normalization factor based on the same dose applied to rat skin in vivo and ex vivo. Flux (Jss) and SD were calculated as described previously (Caldwell and Vahidsafa, 2015; Niedorf et al., 2008).
Statistical methods
The data were subjected to statistical analysis using paired t tests or two-way ANOVA followed by the Tukey-Kramer test for pairwise comparisons (Graphpad Prism 7, Graphpad Software, Inc, La Jolla CA). Values were significantly different at p < .05.
RESULTS
TBP Disposition After Single IV Dosing in Female SD Rats: Dose Recovery and Metabolite Analyses
Female SD rats were used to investigate TBP disposition after systemic (IV) administration (Figure 2). Systemically available TBP was rapidly excreted primarily via urine, with approximately 61% of the dose recovered in urine between 0 and 4 h. By 24 h, 89%–94% of the dose was recovered in urine with an additional 5% recovered in feces; the balance (1%–2%) was recovered in blood and tissues. A summary of dose recoveries after 24 h is shown in Table 2. A summary of tissue recoveries can be found in Supplementary Tables 1 and 2.
Figure 2.
Cumulative dose recoveries in urine and feces after a single IV administration of TBP (0.1 μmol/kg) to female SD rats (N = 4). Data represent mean±SD.
Table 2.
Dose (%) Recovered After 24 h Following a Single IV Administration of TBP to Female SD Rats
| Mean ±SD | |
|---|---|
| Urine | 91 ±2 |
| Feces | 4 ±3 |
| Blood | 0.7 ±0.3 |
| Non-GI tissues | 1 ±0.1 |
| GI tract and contents | 1.3 ±0.6 |
| Total recovery | 97 ±3 |
Data represent mean ±SD (N = 4).
TBP Disposition After Single Oral Dosing
Dose escalation in female SD rats dose recovery and metabolite analyses at 3 dose levels.
Conventional Studies
Female SD rats were used in studies investigating dose-dependent differences in TBP disposition. In these studies, TBP appeared to be well absorbed and excreted primarily in urine at all doses tested (Figure 3). Orally administered TBP was well absorbed from the gut, with approximately 25% eliminated via urine in the first 4 h after dosing at all dose levels. Urinary excretion was essentially unchanged when elimination curves for 0.1 and 10 µmol/kg doses were compared; 1000 µmol/kg urinary elimination appeared to be slightly delayed between 4 and 12 h, possibly indicating overwhelmed or impaired absorption or evidence of enterohepatic circulation of biliary metabolites but was statistically indistinguishable from that of the other doses by 12 h; the dose volume was the same for all doses so we deduced this was not due to delayed gastric emptying. Fecal recoveries varied only slightly by dose, but the differences were not significant. A summary of dose recoveries after 24 h is shown in Table 3. A summary of tissue recoveries can be found in Supplementary Tables 1 and 2.
Figure 3.
Cumulative dose recoveries in (■) urine and (●) feces after a single oral administration of TBP to female SD rats. A, 0.1 µmol/kg PO (N = 4); B, 10 µmol/kg PO (N = 4); C, 1000 µmol/kg PO (N = 4). Recovery in urine was significantly lower at 8-h postdose after oral administration of 1000 µmol/kg compared with 10 and 100 µmol/kg. Data represent mean±SD (N = 4). Cumulative recovery was significantly lower in the highest dose group than other doses at 8 h (p < .05).
Table 3.
Dose (%) Recovered after 24 h Following a Single Oral Administration of TBP to Female SD Rats
| Dose | 0.1 µmol/kg | 10 µmol/kg | 1000 µmol/kg |
|---|---|---|---|
| Mean ±SD | Mean ±SD | Mean ±SD | |
| Feces | 7 ±2 | 8 ±2 | 9 ±4 |
| Urine | 89 ±4 | 89 ±2 | 88 ±4 |
| Blood | 0.1 ±0.03 | 0.2 ±0.02 | 0.2 ±0.04 |
| Non-GI tissues | 0.3 ±0.5 | 1 ±0.5 | 1 ±0.4 |
| GI tract and content | 0.5 ±0.4 | 1 ±0.5 | 1 ±1 |
| Total recovery | 96 ±2 | 98 ±1 | 98 ±1 |
Data represent mean ±SD (N = 4).
HPLC-radiometric analyses of urine found 2 clear peaks: 1 peak that coeluted with the parent TBP standard and a second more hydrophilic peak (Figure 4). Mass spectral analyses of urine determined the major hydrophilic peak had a mass consistent with that of TBP-glucuronide (Figure 5). In addition, mass spectral analyses of urine found a third moiety that eluted immediately before the TBP standard that had a mass consistent with TBP-sulfate. Fecal extracts contained a single peak that coeluted with parent TBP (Figure 4).
Figure 4.
Representative HPLC-radiochromatograms of urine and fecal extracts following oral administration of TBP (1000 µmol/kg). A, [14C]-TBP standard. B, Urine. C, Fecal extract.
Figure 5.
Mass spectra of urine collected after oral administration of TBP (1000 µmol/kg). A, Extracted ion chromatogram for peak 1 (Retention time = 4.2–4.6 min). B, Extracted ion chromatogram for peak 2 (Retention time = 6–6.5 min).
Bile Duct Cannulated Studies
To determine whether the dose fraction recovered in feces represented an unabsorbed fraction, an absorbed then hepatically cleared fraction, or a combination of the 2, female SD rats with an indwelling common bile duct cannula were orally administered a single dose of TBP (10 µmol/kg, 100 µCi/kg, 4 ml/kg). After dosing, animals were placed in plastic metabolism cages for collection of excreta whereas bile was collected for quantitative and qualitative analyses. After 24 h, the rats were euthanized, blood removed by cardiac puncture, and the animals were necropsied. In this study, more than 80% of the dose was found to be absorbed and systemically available (eg, recovered in urine and tissues), consistent with previous studies using conventional animals. Bile fractions contained approximately 11% of the dose, indicative of an absorbed then hepatically cleared fraction. Feces and GI tract tissues and contents contained the remaining 4%–6% of the administered dose, indicative of an unabsorbed fraction (Figure 6). A small percentage of parent was also excreted in urine from conventional animals (Figure 7). HPLC-radiometric analyses of bile samples collected between 0- and 24-h postdosing showed only a single peak that was determined to be TBP-glucuronide.
Figure 6.
Cumulative dose recovered in urine, feces, and bile after oral administration of TBP (10 µmol/kg) to bile duct cannulated female SD rats. Data represent mean±SD (N = 4).
Figure 7.
Representative HPLC-radiochromatograms of urine and bile following administration of [14C]-TBP (10 µmol/kg, PO). A, 0–4 h urine. B, 0–1 h bile. Analyses of all other time points showed similar chromatography for urine and bile.
Dose Recovery in Male SD Rats, Male and Female B6C3F1/J Mice
Disposition in SD rats and B6C3F1/J mice of both sexes was compared at a single dose (10 µmol/kg) administered by intragastric (oral) gavage (Table 4). Animals were dosed, placed in plastic metabolism cages, and excreta collected for 24 h. Male SD rats and female B6C3F1/J mice urinary elimination overlaid that of female SD rats at all time points tested (Figure 8). Male B6C3F1/J mice displayed highly variable urinary output and corresponding overall elimination between 0 and 8 h, but by 12 h, urinary recoveries mirrored that of the other test groups. Mice eliminated more TBP in feces through 12 h but were not significantly different by 24 h. A summary of tissue recoveries can be found in Supplementary Tables 1–3.
Table 4.
Dose (%) Recovered After 24 h Following a Single Oral Administration of TBP to SD Rats or B6C3F1/J Mice (10 µmol/kg)
| Dose | Female SD Rats | Male SD Rats | Female B6C3F1/J Mice | Male B6C3F1/J Mice |
|---|---|---|---|---|
| Mean ±SD | Mean ±SD | Mean ±SD | Mean ±SD | |
| Feces | 8 ±2 | 9 ±2 | 10 ±7 | 14 ±6 |
| Urine | 89 ±2 | 87 ±3 | 86 ±7 | 82 ±8 |
| Blood | 0.2 ±0.02 | 0.1 ±0.02 | 0.1 ±0.02 | 0.1 ±0.002 |
| Non-GI tissues | 1 ±0.5 | 0.4 ±0.2 | 0.5 ±0.2 | 0.4 ±0.1 |
| GI tract and content | 1 ±0.5 | 1 ±0.2 | 0.5 ±0.2 | 0.2 ±0.1 |
| Total recovery | 98 ±1 | 97 ±3 | 98 ±2 | 96 ±3 |
Data represent mean ±SD (N = 4).
Figure 8.
Cumulative dose recoveries in rats (A) and mice (B) after a single oral administration of TBP (10 µmol/kg, 100 µCi/kg) to SD rats or B6C3F1/J mice. ■: female rat (N = 4); ●: male rat (N = 4); ▲: female mouse (N = 4); ▼: male mouse (N = 4). Closed symbols: urine; open symbols: feces. Male and female rat fecal recoveries were analogous and overlay almost exactly. Data represent mean±SD.
Pharmacokinetics After Single Oral or IV Dosing
TBP systemic pharmacokinetics were determined following a single oral or IV dose of TBP in female SD rats. Rats were administered 10 µmol/kg (100 µCi/kg, 4 or 1 ml/kg, respectively) as described earlier and blood sampled serially between 7.5 min and 24 h. TBP concentrations in blood were fit to one- (oral) or two-compartment (IV) models (Figure 9). After oral administration, there was an observed rapid rise to Cmax, followed by an up-down-up oscillating concentration plateau (15 min to 4 h) that may be due to enterohepatic cycling of TBP and its metabolites. After IV administration, biphasic clearance was observed, likely due to a rapid distribution to liver as indicated by the initial 18 min half-life and an elimination half-life of 9.6 h and a final clearance of 0.5 ml/min. After oral dosing, absorption was rapid, with an absorption half-life of 18 min, a Cmax of 2.4 nmol/ml occurring at 75-min postdose; the elimination half-life was 4.5 h with a final clearance of 2.1 ml/min. From the areas under the curves (AUC), TBP parental bioavailability (F) was calculated to be 23%–27%. Preliminary examinations of TBP present in blood, especially at late time points, indicate TBP associates with plasma protein(s) which retains a small but measurable fraction for an appreciable amount of time.
Figure 9.
TBP time-concentration curves following a single dose of TBP (10 µmol/kg) to female SD rats (■: Oral; ●: IV). Lines show the predicted regression curve based on best-fit. Data represent mean±SD (N = 4). Inset: detail of apparent oscillation in concentrations between 15 min and 4 h after oral dosing.
TBP Disposition After Repeated Oral Dosing
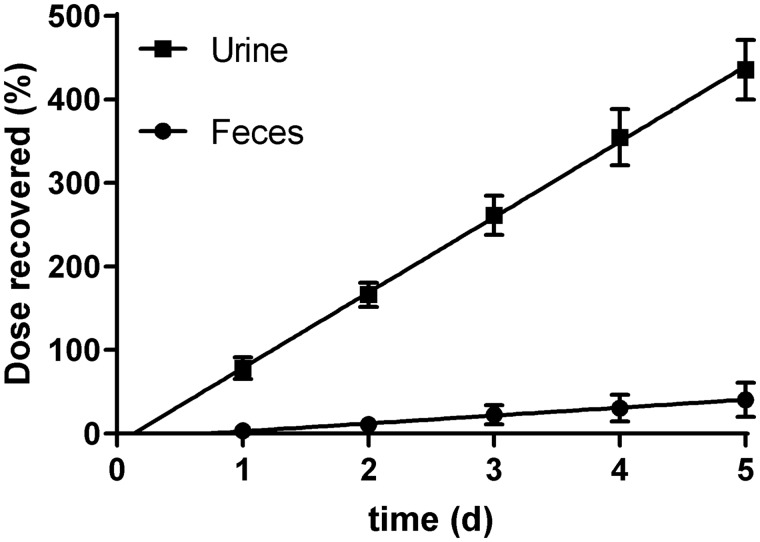
Female SD rats were administered TBP (10 µmol/kg/d, 100 µCi/kg, 4 ml/kg) for 5 days and excreta were collected at 24 h intervals to determine bioaccumulation and metabolic enzyme alteration potential for TBP. Interday recoveries were between 79% and 94% for urine and cumulative recoveries were linear with no apparent changes in excretion patterns to indicate bioaccumulation or enzyme alteration at the dose tested (Figure 10). Urine and feces contained the same proportions of parent and metabolites as that seen in single dose studies (Supplementary Table 4).
Figure 10.
Cumulative dose recovery (%) after daily repeated oral administration of TBP (10 µmol/kg/d) in female SD rats. Data represent mean±SD (N = 4).
TBP Disposition After Single Dermal Dosing
Dermal uptake of TBP was tested ex vivo using female SD rat and human skin and in vivo using female SD rats (Figure 11). Maximal TBP flux (slopes of the penetrated mass across each barrier plotted vs sampling period) through rat skin occurred between 0- and 6-h postapplication and was 5 ± 1 nmol/cm2/h ex vivo and was 3 ± 1 nmol/cm2/h after in vivo dosing. Maximal flux for ex vivo human skin was 2 ± 1 nmol/cm2/h and was reached between 6 and 12 h. Computed maximal flux for in vivo human skin was 4 ± 2 nmol/cm2/h.
Figure 11.
Cumulative penetration of [14C]-TBP through rat or human skin. A, Dose recovery in excreta after dermal application of TBP to female SD rats. B, Cumulative penetrance of TBP through in vivo and ex vivo skin samples. Estimated penetrance for in vivo human skin was calculated using the parallelogram method described earlier. Data represent mean±SD (N = 4).
Recoveries over time showed the dose penetrating the skin increased slowly throughout the experimental 24 h for all skin matrices. Over a 24-h period, 43 ± 12% and 63 ± 3% of the dose that was administered to ex vivo human and rat skin penetrated the skin (Table 5). The maximum absorbed fractions for these human and rat skin samples were 13 ± 2% and 18 ± 5%, respectively. In vivo dermal exposure to TBP found similar amounts were absorbed into the skin (17 ± 10%) and penetrated it (48 ± 4%). TBP that penetrated the skin in the in vivo study was largely eliminated in urine (40 ± 4%) or feces (3 ± 1%). Untreated skin (from the ears) contained 3 ± 2% of the dose (95 ± 57 pmol/g) whereas large intestine/cecum contents (0.6 ± 0.1%), spleen (0.5 ± 0.3%), and liver (0.1 ± 0.04%) were the only other tissues that contained >0.1% of the dose. In addition to undosed skin, kidney (49 ± 48 pmol/g, 0.07 ± 0.07%) and liver (12 ± 2 pmol/g) retained notable concentrations of TBP after 24 h of in vivo dermal exposure. Based on the parallelogram calculation, a risk-averse estimate indicates approximately 49 ± 13% of TBP may be absorbed into human skin in vivo with 39 ± 12% expected to reach systemic circulation after 24 h of continuous exposure. A summary of measured and calculated recoveries for individual fractions is shown in Supplementary Tables 1, 2, and 5.
Table 5.
Recoveries in Individual Fractions From Dermal Ex Vivo and In Vivo Studies
| Rat | Rat | Human | Human | ||||
|---|---|---|---|---|---|---|---|
| (ex vivo) | (in vivo) | (ex vivo) | (in vivo) | ||||
| Measured | Measured | Measured | Calculated | ||||
| Unabsorbed | Dosing cell | % | 0.9±0.3 | 15±8 | 2±1 | ||
| (nmol) | (0.6±0.2) | (16± 10) | (2±0.4) | ||||
| Wash | % | 15±1 | 18±3 | 37±10 | 44±15 | ||
| (nmol) | (10±1) | (19±3) | (23±8) | (44±16) | |||
| Total unabsorbed | % | 16±1 | 33±6 | 40±11 | 44±14 | ||
| Absorbed | Tape strips | % | 9±4 | 10±5 | 2±0.4 | 2±1 | |
| (nmol) | (6±3) | (10±5) | (1±0.3) | (2±1) | |||
| Skin | % | 9±6 | 7±5 | 11±2 | 9±9 | ||
| (nmol) | (6±4) | (7±5) | (7±5) | (8±8) | |||
| Total absorbed | % | 18±5 | 17±10 | 13±2 | 11±7 | ||
| Penetrated | Perfusate/excreta and tissues | 0–6 h | % | 31±7 | 19±6 | 9±5 | 5±4 |
| (nmol) | (20±5) | (19±5) | (5±3) | (5±3) | |||
| 6–12 h | % | 17±3 | 14±2 | 14±5 | 12±5 | ||
| (nmol) | (11±2) | (14±4) | (9±3) | (12±5) | |||
| 12–18 h | % | 10±2 | — | 11±2 | n.d. | ||
| (nmol) | (6±2) | — | (7±1) | (n.d.) | |||
| 18–24 h | % | 6±1 | 15±6 | 9±1 | 22±10 | ||
| (nmol) | (4±1) | (16±7) | (5±0.4) | (22±11) | |||
| % | 63±3 | 48±4 | 43±12 | 39±12 | |||
| % | 97±2 | 97±3 | 95±8 | 95±8 | |||
| (nmol) | (62±1) | (72±11) | (58±7) | (67±13) | |||
| (fdermal, %) | 81±3 | 64±7 | 55±13 | 49±13 | |||
Estimates for in vivo human skin were also calculated. Dermal bioavailability was calculated as the sum of absorbed and penetrated fractions. Data represent mean±SD (N = 4).
TBP was not metabolized in the skin in these studies. HPLC separation and radiochemical analysis of perfusate media and extracts from skin detected only a single peak that coeluted with the parent TBP (Figure 12). Analyses of excreta collected in these studies found that dermally applied TBP was partially conjugated to TBP-glucuronide but was primarily eliminated in the urine as parent TBP. Feces contained only parent TBP (data not shown). A summary of tissue recoveries can be found in Supplementary Table 1 and recoveries from individual fractions are shown in Supplementary Table 5.
Figure 12.
Representative HPLC-radiochromatograms of [14C]-radioactivity in studies of TBP applied to skin. A, [14C]-TBP standard. B, Rat urine collected after dermal application of TBP. C, Extracts from dosed in vivo rat skin. D, [14C]-TBP standard 2; diluted ex vivo dosing solution. E, Ex vivo rat skin perfusate. F, Ex vivo human skin perfusate. A single peak that coeluted with parent TBP was detected in skin extracts and perfusate whereas both parent TBP and metabolite TBP-glucuronide were detected in urine samples.
DISCUSSION AND CONCLUSIONS
This is the first comprehensive report of the disposition of TBP and these data support previously described high dose disposition studies of TBP (WHO, 2005). TBP is classified as a high-volume chemical by the US EPA; exact production volumes are not publicly available but were estimated to be 2500 metric tons per year in Japan and 9500 metric tons per year worldwide in 2001 (OECD-SIDS, 2004). TBP is a current-use flame retardant and a contaminant in both environmental and indoor exposures, especially to small children who are prone to ingesting dust through hand-mouth contact (Stapleton et al., 2014). One difficulty in comprehensively assessing TBP risk is that TBP dust concentrations are infrequently reported (Sha et al., 2018; Takigami et al., 2009). This is in stark contrast to other legacy BFRs (polybrominated diphenyl ethers and hexabromocyclododecane) and currently used or novel BFRs (TBBPA, 2-ethylhexyl tetrabromobenzoate and bis(2-ethylhexyl) tetrabromophthalate) even though TBP use appears to exceed that of some of the novel BFR (Hakk and Letcher, 2003).
TBP was extensively absorbed following oral and dermal administration, the 2 most likely routes of real-world exposure. TBP is readily metabolized and eliminated primarily via renal clearance into urine but also by hepatobiliary clearance into feces. TBP had a systemic oral bioavailability of almost 30% and based on dose recoveries in bile, has a hepatic exposure of >50%. In a prospective study where male and female SD rats were administered 0.5 µmol/kg of TBP and animals were euthanized at 4-h postdose, TBP blood concentrations were found to be highly predictable based on a linear relationship between dose and concentration. This was taken to support an assumption of dose-linear systemic exposure to TBP administration, but further studies are warranted at occupational and environmentally measured doses. There was no apparent sex- or species-dependent difference found in overall disposition. In addition, the relative distributions of peak areas did not markedly change over time, or with dose, repetition, sex, or route of administration, indicating consistent and well-conserved metabolic pathways. At least 2 metabolites were present in urine: TBP-glucuronide and TBP-sulfate. Urinary metabolite profiles differed little over a dose range from 0.1 to 1000 µmol/kg (approximately 0.03–330 mg/kg), indicating negligible saturation of one or more metabolic pathways. We deduced that there is a role for gut microflora in modulating enterohepatic circulation through deconjugation of biliary metabolites because although fecal extracts contained only parent TBP, bile contained only TBP-glucuronide. Enterohepatic circulation was further supported by the appearance of a plateau in the blood concentrations of TBP between 10 and 120 min after oral dosing. TBP was readily absorbed from the gut when orally administered to female SD rats and is likely to be absorbed when ingested by humans. Limited tissue retention of TBP or its metabolites was observed and would indicate a low likelihood of bioaccumulation after a single dose. However, these studies also show that parent TBP is present in circulation and low, persistent levels of TBP circulate up to 24 h after a single oral or dermal exposure. Although the potential for acute toxic effects is likely low due to TBP’s 30% oral bioavailability, long-term exposure may still be a concern. As has been shown in the case of TBBPA, another phenolic brominated chemical that has an even lower bioavailability (2%), long term exposure to TBBPA results in neoplasia and cancer in the NTP 2-year bioassay, and it has been judged to be a probable carcinogen to humans (IARC, 2018; NTP, 2014). Based on other studies in our laboratory that are described below, we expect TBP to possess a similar risk profile to TBBPA.
Current studies in our laboratory indicate TBP decreases efflux transporter function at the blood-brain barrier, with significant implications for adverse drug reactions and mechanisms for endocrine disruption by TBP (Trexler et al., 2018). Future studies will include investigation of the disposition of TBP in pregnant and nursing animals as well as its impacts on thyroid stimulating hormone and thyroid hormone levels, as thyroid signaling pathways have been implicated by in vitro assessments (Hamers et al., 2006, 2008). The related compound TBBPA has been shown to directly interact with the ERα receptor while also altering estrogen metabolism by competing for access to tissue-specific sulfotransferases, eg, SULT1E1 (Gosavi et al., 2013), which in turn can result in alteration in estrogen signaling pathways necessary for the uterine neoplasia demonstrated in the 2-year cancer bioassay (NTP, 2014). The structural similarity of TBP to TBBPA coupled with its 10-fold greater bioavailability make it reasonable to be concerned about human exposure to TBP. More work is needed to determine the nature of these signaling pathways following repeated exposures to TBP, especially in susceptible populations and across life stages.
Ultimately, humans are likely to have an appreciable systemic exposure to ingested or dermally encountered TBP given its high volume of use, presence in seafood, and ubiquity in the environment. Although we found that TBP is unlikely to bioaccumulate, because exposure to TBP is likely to be continuous, it is probable that these chronic TBP exposures could lead to similar long-term toxicities observed for other brominated phenolic chemicals, most notably cancer.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Dr Ronald Cannon, Ms Sherry Coulter, and Ms Pegah Khosravi-Kamrani for technical assistance. This research was supported in part by the Intramural Research Program of the National Institutes of Health and National Cancer Institute (Project ZIA BC 011476). This article has been reviewed in accordance with the policy of the National Health and Environmental Effects Research Laboratory, U.S. Environmental Protection Agency, and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use. The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
REFERENCES
- Andersen A., Holte H., Slordal L. (1999). Pharmacokinetics and metabolism of doxorubicin after short-term infusions in lymphoma patients. Cancer Chemother. Pharmacol. 44, 422–426. [DOI] [PubMed] [Google Scholar]
- Birnbaum L. S., Decad G. M., Matthews H. B. (1980). Disposition and excretion of 2, 3, 7, 8-tetrachlorodibenzofuran in the rat. Toxicol. Appl. Pharmacol. 55, 342–352. [DOI] [PubMed] [Google Scholar]
- Boyle J. L., Lindsay R. C., Stuiber D. A. (1992). Bromophenol distribution in salmon and selected seafoods of fresh- and saltwater origin. J. Food Sci. 57, 918–922. [Google Scholar]
- Butt C. M., Miranda M. L., Stapleton H. M. (2016). Development of an analytical method to quantify PBDEs, OH-BDEs, HBCDs, 2, 4, 6-TBP, EH-TBB, and BEH-TEBP in human serum. Anal. Bioanal. Chem. 408, 2449–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell J., Vahidsafa A. (2015). Propagation of error. Available at: http://chemwiki.ucdavis.edu/Analytical_Chemi stry /Quantifying_Nature/Significant_Digits/Propagation_of_Error#Arithmetic_Error_Propagation. Accessed October 8, 2015.
- Deng J., Liu C., Yu L., Zhou B. (2010). Chronic exposure to environmental levels of tribromophenol impairs zebrafish reproduction. Toxicol. Appl. Pharmacol. 243, 87–95. [DOI] [PubMed] [Google Scholar]
- Dufour P., Pirard C., Charlier C. (2017). Determination of phenolic organohalogens in human serum from a Belgian population and assessment of parameters affecting the human contamination. Sci. Total Environ. 599–600, 1856–1866. [DOI] [PubMed] [Google Scholar]
- Eguchi A., Nomiyama K., Devanathan G., Subramanian A., Bulbule K. A., Parthasarathy P., Takahashi S., Tanabe S. (2012). Different profiles of anthropogenic and naturally produced organohalogen compounds in serum from residents living near a coastal area and e-waste recycling workers in India. Environ. Int. 47, 8–16. [DOI] [PubMed] [Google Scholar]
- Ezechias M., Svobodova K., Cajthaml T. (2012). Hormonal activities of new brominated flame retardants. Chemosphere 87, 820–824. [DOI] [PubMed] [Google Scholar]
- Fujii Y., Kato Y., Masuda N., Harada K. H., Koizumi A., Haraguchi K. (2018). Contamination trends and factors affecting the transfer of hexabromocyclododecane diastereomers, tetrabromobisphenol A, and 2, 4, 6-tribromophenol to breast milk in japan. Environ. Pollut. 237, 936–943. [DOI] [PubMed] [Google Scholar]
- Fujii Y., Nishimura E., Kato Y., Harada K. H., Koizumi A., Haraguchi K. (2014). Dietary exposure to phenolic and methoxylated organohalogen contaminants in relation to their concentrations in breast milk and serum in Japan. Environ. Int. 63, 19–25. [DOI] [PubMed] [Google Scholar]
- Fuller S. C., Frank D. C., Fitzhenry M. J., Smyth H. E., Poole S. E. (2008). Improved approach for analyzing bromophenols in seafood using stable isotope dilution analysis in combination with SPME. J. Agric. Food Chem. 56, 8248–8254. [DOI] [PubMed] [Google Scholar]
- Gosavi R. A., Knudsen G. A., Birnbaum L. S., Pedersen L. C. (2013). Mimicking of estradiol binding by flame retardants and their metabolites: A crystallographic analysis. Environ. Health Perspect. 121, 1194–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C., Wang J., Guo M., Sui M., Lu H., Liu G. (2018). Extracellular degradation of tetrabromobisphenol A via biogenic reactive oxygen species by a marine Pseudoalteromonas sp. Water Res. 142, 354–362. [DOI] [PubMed] [Google Scholar]
- Gutierrez M., Becerra J., Godoy J., Barra R. (2005). Occupational and environmental exposure to tribromophenol used for wood surface protection in sawmills. Int. J. Environ. Health Res. 15, 171–179. [DOI] [PubMed] [Google Scholar]
- Hakk H., Letcher R. J. (2003). Metabolism in the toxicokinetics and fate of brominated flame retardants – A review. Environ. Int. 29, 801–828. [DOI] [PubMed] [Google Scholar]
- Halden A. N., Nyholm J. R., Andersson P. L., Holbech H., Norrgren L. (2010). Oral exposure of adult zebrafish (Danio rerio) to 2, 4, 6-tribromophenol affects reproduction. Aquat. Toxicol. 100, 30–37. [DOI] [PubMed] [Google Scholar]
- Hamers T., Kamstra J. H., Sonneveld E., Murk A. J., Kester M. H., Andersson P. L., Legler J., Brouwer A. (2006). In vitro profiling of the endocrine-disrupting potency of brominated flame retardants. Toxicol. Sci. 92, 157–173. [DOI] [PubMed] [Google Scholar]
- Hamers T., Kamstra J. H., Sonneveld E., Murk A. J., Visser T. J., Van Velzen M. J., Brouwer A., Bergman A. (2008). Biotransformation of brominated flame retardants into potentially endocrine-disrupting metabolites, with special attention to 2, 2′, 4, 4′-tetrabromodiphenyl ether (BDE-47). Mol. Nutr. Food Res. 52, 284–298. [DOI] [PubMed] [Google Scholar]
- Hansch C., Leo A., Hoekman D.. 1995. Exploring Qsar – Hydrophobic, Electronic, and Steric Constants. American Chemical Society, Washington, DC. [Google Scholar]
- Hughes M. F., Edwards B. C. (2010). In vitro dermal absorption of pyrethroid pesticides in human and rat skin. Toxicol. Appl. Pharmacol. 246, 29–37. [DOI] [PubMed] [Google Scholar]
- IARC. (2018). Iarc monographs – 115, tetrabromobisphenol A. Available at: https://monographs.iarc.fr/ENG/Monographs/vol115/mono115-07.pdf. Accessed December 20, 2018.
- Kester M. H., Bulduk S., Tibboel D., Meinl W., Glatt H., Falany C. N., Coughtrie M. W., Bergman A., Safe S. H., Kuiper G. G., et al. (2000). Potent inhibition of estrogen sulfotransferase by hydroxylated PCB metabolites: A novel pathway explaining the estrogenic activity of PCBs. Endocrinology 141, 1897–1900. [DOI] [PubMed] [Google Scholar]
- Knudsen G. A., Hughes M. F., McIntosh K. L., Sanders J. M., Birnbaum L. S. (2015). Estimation of tetrabromobisphenol A (TBBPA) percutaneous uptake in humans using the parallelogram method. Toxicol. Appl. Pharmacol. 289, 323–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen G. A., Sanders J. M., Birnbaum L. S. (2016). Disposition of the emerging brominated flame retardant, bis(2-ethylhexyl) tetrabromophthalate, in female Sprague Dawley rats: Effects of dose, route and repeated administration. Xenobiotica 311, 117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen G. A., Sanders J. M., Hughes M. F., Hull E. P., Birnbaum L. S. (2017). The biological fate of decabromodiphenyl ethane following oral, dermal or intravenous administration. Xenobiotica 47, 894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C., Dundua A., Aragon-Gomez J., Nachev M., Stephan S., Willach S., Ulbricht M., Schmitz O. J., Schmidt T. C., Sures B. (2016). Degradation of polymeric brominated flame retardants: Development of an analytical approach using PolyFR and UV irradiation. Environ. Sci. Technol. 50, 12912–12920. [DOI] [PubMed] [Google Scholar]
- Koch C., Sures B. (2018). Environmental concentrations and toxicology of 2, 4, 6-tribromophenol (TBP). Environ. Pollut. 233, 706–713. [DOI] [PubMed] [Google Scholar]
- Leonetti C., Butt C. M., Hoffman K., Hammel S. C., Miranda M. L., Stapleton H. M. (2016a). Brominated flame retardants in placental tissues: Associations with infant sex and thyroid hormone endpoints. Environ. Health 15, 113.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonetti C., Butt C. M., Hoffman K., Miranda M. L., Stapleton H. M. (2016b). Concentrations of polybrominated diphenyl ethers (pbdes) and 2, 4, 6-tribromophenol in human placental tissues. Environ. Int. 88, 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyubimov A. V., Babin V. V., Kartashov A. I. (1998). Developmental neurotoxicity and immunotoxicity of 2,4,6-tribromophenol in Wistar rats. Neurotoxicology 19, 303–312. [PubMed] [Google Scholar]
- Niedorf F., Schmidt E., Kietzmann M. (2008). The automated, accurate and reproducible determination of steady-state permeation parameters from percutaneous permeation data. Altern. Lab. Anim. 36, 201–213. [DOI] [PubMed] [Google Scholar]
- NTP. (2014). Toxicology studies of tetrabromobisphenol A (casrn 79-94-7) in f344/ntac rats and b6c3f1/n mice and toxicology and carcinogenesis studies of tetrabromobisphenol A in wistar han [crl: Wi(han)] rats and b6c3f1/n mice (gavage studies). Available at: https://ntp.Niehs.Nih.Gov/results/pubs/longterm/reports/longterm/tr500580/listedreports/tr587/index.Html. National Toxicology Program, Health and Human Services. Accessed January 18, 2017.
- OECD-SIDS. (2004). 2, 4, 6-tribromophenol Cas No.: 118-79-6. UNEP Publications, Arona, Italy. [Google Scholar]
- Oliveira A. S., Silva V. M., Veloso M. C., Santos G. V., Andrade J. B. (2009). Bromophenol concentrations in fish from Salvador, BA, Brazil. An. Acad. Bras. Cienc. 81, 165–172. [DOI] [PubMed] [Google Scholar]
- Qu R., Feng M., Wang X., Huang Q., Lu J., Wang L., Wang Z. (2015). Rapid removal of tetrabromobisphenol A by ozonation in water: Oxidation products, reaction pathways and toxicity assessment. PLoS One 10, e0139580.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders J. M., Chen L. J., Burka L. T., Matthews H. B. (2000). Metabolism and disposition of luminol in the rat. Xenobiotica 30, 263–272. [DOI] [PubMed] [Google Scholar]
- Sha B., Dahlberg A.-K., Wiberg K., Ahrens L. (2018). Fluorotelomer alcohols (FTOHS), brominated flame retardants (BFRS), organophosphorus flame retardants (OPFRS) and cyclic volatile methylsiloxanes (CVMSS) in indoor air from occupational and home environments. Environ. Pollut. 241, 319–330. [DOI] [PubMed] [Google Scholar]
- Stapleton H. M., Misenheimer J., Hoffman K., Webster T. F.. 2014. Flame retardant associations between children’s handwipes and house dust. Chemosphere 116, 54–60. [DOI] [PMC free article] [PubMed]
- Suzuki G., Takigami H., Watanabe M., Takahashi S., Nose K., Asari M., Sakai S. (2008). Identification of brominated and chlorinated phenols as potential thyroid-disrupting compounds in indoor dusts. Environ. Sci. Technol. 42, 1794–1800. [DOI] [PubMed] [Google Scholar]
- Takigami H., Suzuki G., Hirai Y., Sakai S-i. (2009). Brominated flame retardants and other polyhalogenated compounds in indoor air and dust from two houses in Japan. Chemosphere 76, 270–277. [DOI] [PubMed] [Google Scholar]
- Thomsen C., Lundanes E., Becher G. (2001). Brominated flame retardants in plasma samples from three different occupational groups in Norway. J. Environ. Monit. 3, 366–370. [DOI] [PubMed] [Google Scholar]
- Thomsen C., Lundanes E., Becher G. (2002). Brominated flame retardants in archived serum samples from Norway: A study on temporal trends and the role of age. Environ. Sci. Technol. 36, 1414–1418. [DOI] [PubMed] [Google Scholar]
- Trexler A. W., Cannon R. E., Knudsen G. A., Birnbaum L. S. (2018). 2, 4, 6-tribromophenol (TBP) alters efflux transporter activity in rat brain microvessels. Soc. Toxicol. 162, Abstract no. 3041. [Google Scholar]
- van Ravenzwaay B., Leibold E. (2004). A comparison between in vitro rat and human and in vivo rat skin absorption studies. Hum. Exp. Toxicol. 23, 421–430. [DOI] [PubMed] [Google Scholar]
- Whitfield F. B., Drew M., Helidoniotis F., Svoronos D. (1999). Distribution of bromophenols in species of marine polychaetes and bryozoans from eastern Australia and the role of such animals in the flavor of edible ocean fish and prawns (shrimp). J. Agric. Food Chem. 47, 4756–4762. [DOI] [PubMed] [Google Scholar]
- Whitfield F. B., Helidoniotis F., Shaw K. J., Svoronos D. (1998). Distribution of bromophenols in species of ocean fish from eastern Australia. J. Agric. Food Chem. 46, 3750–3757. [DOI] [PubMed] [Google Scholar]
- WHO. 2005. 2, 4, 6-Tribromophenol and Other Simple Brominated Phenols. Wissenchaftliche Verlagsgesellschaft mbH, Stuttgart, Germany. [Google Scholar]
- Xiong J., An T., Zhang C., Li G. (2015). Pollution profiles and risk assessment of pbdes and phenolic brominated flame retardants in water environments within a typical electronic waste dismantling region. Environ. Geochem. Health 37, 457–473. [DOI] [PubMed] [Google Scholar]
- Xiong J., Li G., An T., Zhang C., Wei C.. 2016. Emission patterns and risk assessment of polybrominated diphenyl ethers and bromophenols in water and sediments from the Beijiang river, South China. Environ. Pollut.219, 596–603. [DOI] [PubMed]
- Ziegler Brothers I. (2010). Rodent NIH-31 Open Formula Auto Available at: http://www.zeiglerfeed.com/product_literature/lab%20research%20literature_Rodent/Rodent%20NIH-31%20Open.pdf. Accessed March 28, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.