Abstract
Fine ambient particulate matter (PM2.5) is able to induce sympathetic activation and inflammation in the brain. However, direct evidence demonstrating an essential role of sympathetic activation in PM2.5-associated disease progression is lacking. We assess the contribution of α2B-adrenergic receptor (Adra2b) in air pollution-associated hypertension and behavioral changes in this study. Wild-type mice and Adra2b-transgenic mice overexpressing Adra2b in the brain (Adra2bTg) were exposed to concentrated PM2.5 or filtered air for 3 months via a versatile aerosol concentrator exposure system. Mice were fed with a high salt diet (4.0% NaCl) for 1 week at week 11 of exposure to induce blood pressure elevation. Intra-arterial blood pressure was monitored by radio-telemetry and behavior changes were assessed by open field, light-dark, and prepulse inhibition tests. PM2.5 exposure increased Adra2b in the brain of wild-type mice. Adra2b overexpression enhanced the anxiety-like behavior and high salt diet-induced blood pressure elevation in response to air pollution but not filtered air exposure. Adra2b overexpression induced upregulation of inflammatory genes such as TLR2, TLR4, and IL-6 in the brain exposed to PM2.5. In addition, there were increased frequencies of activated effector T cells and increased expression of oxidative stress-related genes, such as SOD1, NQO1, Nrf2, and Gclm in Adra2bTg mice compared with wild-type mice. Our results provide new evidence of distinct behavioral changes consistent with anxiety and blood pressure elevation in response to high salt intake and air pollution exposure, highlighting the importance of centrally expressed Adra2b in the vulnerability to air pollution exposure.
Keywords: adrenergic receptor, behavior, air pollution, particulate matter, blood pressure
Noncommunicable diseases account for over 70% of air pollution deaths. Air pollution is responsible for 19% of all cardiovascular deaths worldwide and indeed has been increasingly implicated as an important, and as yet poorly characterized risk factor for neurodevelopmental disorders in children and neurodegenerative disorders in elderly humans (Kioumourtzoglou et al., 2016; Wu et al., 2015). An intermediate factor that may potentially help explain the association between air pollution and susceptibility to cardiovascular events is increase in blood pressure. Although dietary and environmental factors are widely recognized as facilitators of hypertension, the precise mechanisms continue to be studied and debated. Studies by our group have indicated that exposure to PM2.5, a ubiquitous environmental risk, is akin to chronic stress and is associated with neuroinflammation and sympathetic activation (Liu et al., 2014). Chronic exposure to PM2.5 elevated blood pressure in wild-type (WT) mice and humans, which are paralleled by increases in surrogate markers of sympathetic activation (Liu et al., 2014; Munzel et al., 2017). Conversely, central nervous system (CNS) inhibition of inhibitor kappa-B kinase (IKK)/nuclear factor-kappaB (NF-κB) in air pollution-exposed mice, reversed CNS inflammation and restored peripheral energy homeostasis and insulin sensitivity (Liu et al., 2014). In both rodents and humans, stress is known to engage fear and threat circuitry in the brain resulting in a stereotypical biological and behavioral response, typified by include elevation of blood pressure, anxiety, and depression (Munzel et al., 2017). Studies examining the neurocircuitry of stress in rodents indicate that multiple stress responsive areas are activated, including the prefrontal cortex, hypothalamus, amygdala, and the hippocampus. Activation of this circuitry results in sympathetic nervous system (SNS) activation which in turn results in peripheral immune alterations.
To investigate the role of the sympathetic system in air pollution “stress”-induced adverse health effects, we examined the blood pressure and behavior changes in WT mice and brain α2B-adrenergic receptor (Adra2b)-overexpressing mice (Kintsurashvili et al., 2009). Activation of α2A and α2C in the CNS inhibits the SNS and lowers blood pressure, whereas α2B activation in the CNS activates SNS and increases blood pressure (Kanagy, 2005). We hypothesized that air pollution will potentiate hypertension, induce adverse, behavioral changes, and enhance inflammation in peripheral organs by activating sympathetic adra2b signaling. Air pollution exposure is a life-long unavoidable risk factor to most people living in polluted areas. As such, identifying the genes/conditions that predispose the subjects to air pollution may help to develop treatments for air pollution-associated adverse health effect. FVB/N mice have been shown to be less vulnerable to exposure of air pollutants such as ozone (Savov et al., 2004). In this study, we used brain-specific Adra2b-overexpressing FVB/N mice (Adra2bTg) to examine the role of sympathetic activation in regulating air pollution sensitivity. We found that adra2b overexpression in the brain enhanced air pollution-induced anxiety-like behavior and high salt diet-induced blood pressure elevation. Adra2b overexpression induced an upregulation of inflammatory genes such as TLR2, TLR4, and IL-6 in the brain exposed to PM2.5. In addition, there were increased frequencies of activated effector T cells and increased expression of oxidative stress-related genes, such as SOD1, Nrf2, and Gclm in Adra2bTg mice compared with WT mice.
METHODS
Animals and animal care
Adra2b transgenic (Adra2bTg) mice (Kintsurashvili et al., 2009) on FVB/N background were purchased from Jackson Laboratories (Bar Harbor, Maine) and bred in the animal facilities at the University of Maryland School of Medicine and Case Western Reserve University. Eight weeks old male Adra2b transgenic positive FVB/N mice and negative FVB/N (WT) were used in this study. Animals were maintained in the animal facility at 21°C on a 12-h light/12-h dark cycle with free access to water and food (normal chow diet). High salt intake is an important acute hypertensive stressor that induces blood pressure elevation in susceptible individuals (Tobian, 1991). Mice were randomly assigned to normal salt diet (0.75% NaCl) or high salt diet (4.0% NaCl, Teklad diet) treatment for 1 week after 11 weeks of exposure when the effects of air pollution have been established. Blood pressure was measured by radio-telemetry (Data Sciences International, St. Paul, Minnesota) as detailed below. The protocols and the use of animals were approved by and in accordance with the Institutional Animal Care and Use Committee at the University of Maryland School of Medicine and Case Western Reserve University.
Whole-body inhalational exposure to concentrated ambient fine particulate matter
Mice were exposed by inhalation to either filtered air (FA) or concentrated ambient PM2.5 at University of Maryland, Baltimore for 6 h/day, 5 days/week, for a total of 12 weeks, in a Versatile Aerosol Concentrator and Enrichment System (VACES) (Rao et al., 2014). This whole-body small animal air pollution exposure system allows 8- to 10-fold concentration of ambient PM2.5 particles. All mice, including both FA and PM2.5 exposure groups, were exposed at exactly the same time. FA-exposed mice received an identical protocol with the addition of a high-efficiency particulate air filter (Pall Life Sciences, East Hills, New York) positioned in the inlet valve to remove PM2.5 particles in the FA stream, as described previously (Liu et al., 2014). The monitoring of the exposure environment and ambient aerosol was described previously in our publications (Rao et al., 2014; Sun et al., 2009).
PM2.5 concentration and composition in the exposure chamber
Teflon filters (PTFE, 37 mm, 2 μm pore; PALL Life Sciences, Ann Arbor, Michigan) used in the VACES were collected and weighed before and after sampling. The filters were weighted after temperature and humidity acclimation on a Mettler Toledo Excellence Plus XP microbalance in a temperature- and humidity-controlled weighing room. The filter membranes were changed every week and concentration of each membrane was measured by gravimetric analysis. Weight gains were used to calculate exposure mass concentrations of PM2.5 in the exposure chambers. Elemental composition of the PM2.5 particles was measured by high-resolution inductively coupled plasma-mass spectrometry (ICSP-MS) (ELEMENT2, Thermo Finnigan, San Jose, California), as previously described by us (Xu et al., 2011).
Open-field test
For open-field test, Adra2bTg mice and WT mice were placed in 50 cm × 50 cm × 38 cm arenas under 30–35 lux illumination with an overhead video-tracking system (San Diego Instruments) after 12 weeks of exposure to FA or PM2.5. Locomotion of animals (distance traveled) was then continuously monitored by the experimenter throughout the 60-min habituation period. Travel distance and duration in the center were analyzed by TopScan v2.0 (CleverSys, Inc.).
Prepulse inhibition
The prepulse inhibition was performed, as previously described (Zanos et al., 2016). Following air pollution exposure for 12 weeks, the mice were placed in the startle chambers (SR-LAB, San Diego Instruments, San Diego, California) for a habituation period of 30 min. Each chamber consists of a Plexiglas cylinder (8.9 cm length × 2.8 cm diameter on a 20.4 × 12.7 cm plastic base) located within a ventilated enclosure (internal dimensions: 35 × 38.6 × 32 cm). After the habituation period, the mice were exposed to a 5-min adaptation period with a constant background noise (67 dB), followed by 5 initial startle stimuli (120 dB, 40 ms duration each). Subsequently, the mice were stimulated with 5 different trial types: pulse alone trials (120 dB, 40 ms duration), 3 prepulse trials of 76, 81, and 86 dB of white noise bursts (20 ms duration) preceding a 120 dB pulse by 100 ms, and background (67 dB) no-stimuli trials. Each of these trials was randomly presented 5 times. Startle response was measured by recording the rapid force change of body reaction in the startle chambers using the SR-LAB software. The percentage prepulse inhibition (% PPI) was calculated as follows: [(magnitude on pulse alone trial – magnitude on prepulse + pulse trial)/magnitude on pulse alone trial] × 100.
Light-dark box test
For the light-dark box (LDB) test, the mice were placed in an arena (35 × 35 × 35 cm) that is divided into two by a partition with a door. The larger chamber (23 × 35 × 35 cm) was illuminated brightly (400 lux) and the adjacent smaller chamber (12 × 35 × 35 cm) was darker (<5 lux). The mice were allowed to move freely between the 2 chambers through the door connecting the 2 chambers. The mice were monitored and videotaped for 10 min after placing them into the dark chamber. The transition numbers through the door, the time stayed in the light side, and the latency to enter the light chamber were recorded and analyzed using Anno Star software (CleverSys Inc., Reston, Virginia).
Radiotelemetry measurement of mouse blood pressure
Mouse radiotelemetry (Data Sciences International) was used for blood pressure and heart rate measurements, as we previously described (Liu et al., 2014). Briefly, the right common carotid artery was cannulated with the catheter of the transmitter under anesthesia. The transmitter body was placed in a subcutaneous pocket in the flank. The surgery was carried out in a dedicated surgery area which was previously disinfected with UV light and 70% alcohol. After surgery, the mice were housed individually with access to food/water ad libitum and allowed to recover for 1 week. After 1 week of recovery, the blood pressure, heart rate, and physical activities data were collected and stored. Dataquest A.R.T. software (Data Sciences) was used to analyze the data.
α2B-adrenergic antagonist imiloxan (1 mg/kg) was injected intraperitoneally into FA- or PM2.5-exposed WT and Adra2bTg mice to block α2B signaling and evaluate if PM-induced blood pressure elevation in Adra2bTg mice is dependent on α2B. Continuous beat-by-beat blood pressure values were recorded for 30 min before and 2 h after administration of imiloxan. The values from the 45th to the 60th minutes after drug injection were used to characterize the response and to avoid measuring stress-induced blood pressure changes.
Urine collection and norepinephrine analysis
Urine samples were collected during the week before implantation of telemetry transmitters. The mice were placed into the urine collection cages immediately after daily exposures and the urine was then collected the next morning. The urine was stored at –80°C until analysis. The concentrations of norepinephrine (NE) and vanillylmandelic acid (VMA) in the urine were analyzed using ELISA kit (Abnova, Taipei City, Taiwan), according to the manufacturer’s instructions.
Quantitative real time-PCR
Total RNA was extracted from whole-brain tissues using Trizol (Invitrogen, Carlsbad, California) and cDNA was reversely transcribed using a High Capacity cDNA Transcription kit (Applied Biosystems, Carlsbad, California), according to the manufacturers’ protocols. Quantitative real time polymerase chain reaction (qPCR) was performed in triplicate on a Lightcycler 480 system (Roche, Indianapolis, Indiana). The expression of target genes was calculated using the ΔΔCt method relative to housekeeping gene β-actin (Livak and Schmittgen, 2001).
Flow cytometry
The cells were stained with anti-CD3, anti-CD44, anti-CD62L, anti-Ly6C, and F4/80 at 4°C for 30 min, followed by 3 washes with 1× PBS and subsequently resuspended in flow buffer (1× PBS containing 2% FBS). The cells were then run on an Aminis Flowsight imaging flow cytometer (Millipore Sigma, Billerica, Massachusetts) and data were analyzed using Amnis IDEAS software (Millipore Sigma). All antibodies were purchased from Biolegend, eBioscience, or BD Bioscience.
Data analysis
Data are expressed as means ± standard error of the mean (SEM) unless otherwise specified. Unpaired 2-tailed t-tests were used for the comparison of adrenergic receptor expression or PM2.5 concentration between FA and PM2.5 using Graphpad Prism software (Version 7). Two-way analysis of variance (ANOVA) followed by Bonferroni’s post hoc test was used to compare the means among WT (FA), WT (PM), Adra2bTg (FA), and Adra2bTg (PM) in behavior study, detections of inflammatory marker and oxidative stress, using Graphpad Prism software (Version 7). Repeated measure 2-way ANOVA with Bonferroni’s post hoc test was used for the analysis of blood pressure. A p value of <.05 was considered statistically significant.
RESULTS
Characterization of Concentrated Air Pollution Exposures
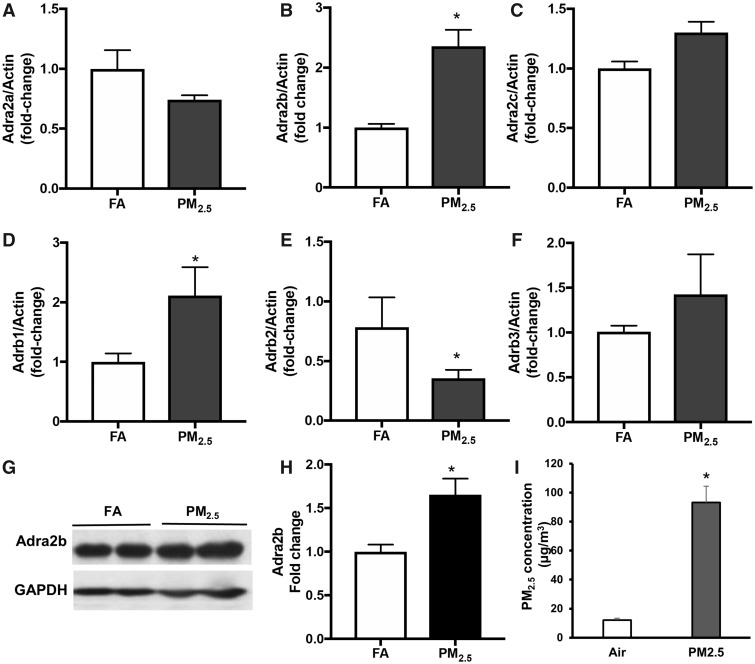
First, we examined the gene and protein expression of Adrenergic receptors in the brain of FA or PM2.5 exposed mice (Figure 1). PM2.5 exposure increased the gene expression of Adra2b more than 2-fold in the brain of WT mice by real-time PCR (Figure 1B). By contrast, there were no significant differences in the expressions of α2A and α2C adrenergic receptors between FA and PM group (Figs. 1A and 1C). We also examined the gene expression of β adrenergic receptors in the brain tissues. After PM2.5 exposure, gene expression of β1 adrenergic receptor increased (Figure 1D), whereas β2 adrenergic receptor decreased in the brain tissue of WT mice (Figure 1E). There was no change in β3 adrenergic receptor level after PM2.5 exposure (Figure 1F). These results suggest that a2B, β1, and β2 adrenergic receptors may participate in central sympathetic remodeling in response to PM2.5 (Figs. 1B, 1D, and 1E). Because α2B is the major adrenergic receptor in the CNS to activate SNS and increase blood pressure(Kanagy, 2005), we further examined the protein level of Adra2b in the brain after PM2.5 exposure. Adra2b protein expression in the brain increased ∼1.5-fold (representative bands in Figure 1G and bar graph in Figure 1H) in PM2.5 exposed mice compared with FA group.
Figure 1.
Effect of PM2.5 exposure on the gene expression of adrenergic receptors: Adra2a (A), Adra2b (B), Adra2c (C) mRNA levels in the brain from FA- or PM2.5-exposed WT mice. Adrb1 (D), Adrb2 (E), and Adrb3 (F) mRNA levels in the brain from FA- or PM2.5-exposed WT mice. Representative image (G) and statistic bar graph (H) showing Adra2b protein levels in the brain from FA- or PM2.5-exposed mice. I, PM2.5 concentration in ambient air and PM2.5 exposure chamber. Data are presented as means ± standard error (SE). N = 5–7 for each group. *p < .05.
To test the role of sympathetic activation in air pollution-associated adverse health effect, a mouse model with brain-selective overexpression of α2B-adrenergic receptor (Adra2b) (Kintsurashvili et al., 2009) was used. The Adra2b transgenic mice used a human platelet-derived growth factor-β promoter to target neurons in the cortex, hippocampus, hypothalamus, and cerebellum, and has shown a 1.8-fold increase of protein expression in the whole brain tissue compared with WT mice (Kintsurashvili et al., 2009). We had previously shown that administration of α2A-adrenergic agonist, guanfacine attenuated air pollution-induced increases in blood pressure (Liu et al., 2014), and α2A in the CNS inhibits the sympathetic nervous activity. We postulated that if air pollution-mediated increase in blood pressure involves central sympathetic activation, then transgenic overexpression of α2B receptors in the brain will augment blood pressure responses to PM2.5 exposure (Kanagy, 2005). Adra2b transgenic mice (Adra2bTg) and WT controls were exposed to FA or concentrated PM2.5 (PM), 6 h/day, 5 days/week for a total of 12 weeks. The average concentrations of PM2.5 (mean ± SD) in the exposure chamber and ambient air were 93.1 ± 37.1 and 12.2 ± 4.4 μg/m3 respectively (Figure 1I). We have previously demonstrated that these levels of exposure represent relevant doses of exposure routinely experienced in many cities in Asia (Rajagopalan and Brook, 2015). The XRF elemental composition analysis of the particles collected from filter membranes provides detailed chemical component characterization of the particulate matter during the exposure period (Table 1).
Table 1.
Elemental Concentrations (ng/m3) of Ambient Air or Concentrated Particles During the Exposure
| Ambient Air |
Exposure to PM2.5 |
|||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| S | 512.7 | 154.9 | 4658.8 | 1655.3 |
| Na | 149.9 | 27.1 | 990.0 | 227.4 |
| Fe | 79.3 | 25.8 | 932.3 | 404.0 |
| Si | 31.6 | 45.8 | 755.6 | 745.1 |
| Ca | 56.8 | 21.4 | 683.0 | 220.8 |
| Mg | 36.7 | 5.6 | 280.6 | 88.6 |
| K | 25.2 | 8.4 | 245.5 | 92.6 |
| Al | 8.9 | 16.4 | 220.7 | 339.3 |
| Zn | 25.8 | 11.0 | 173.2 | 78.9 |
| U | 12.0 | 18.6 | 110.1 | 150.2 |
| Br | 10.0 | 3.4 | 84.6 | 27.2 |
| Ba | 5.0 | 3.7 | 62.3 | 32.7 |
| Ti | 5.3 | 2.4 | 53.1 | 31.1 |
| Cu | 7.8 | 16.2 | 42.5 | 13.5 |
| Lu | 4.0 | 3.7 | 30.7 | 13.1 |
| Mn | 2.5 | 1.1 | 27.2 | 16.8 |
| P | 1.6 | 1.0 | 26.2 | 12.9 |
| Er | 2.0 | 0.9 | 22.7 | 10.4 |
| Te | 4.0 | 7.1 | 21.2 | 30.0 |
| Sb | 3.0 | 4.4 | 18.5 | 22.9 |
| Ni | 2.9 | 7.5 | 15.6 | 11.2 |
| Sr | 2.0 | 0.2 | 15.4 | 2.2 |
| Pb | 2.0 | 0.9 | 13.8 | 6.6 |
| W | 2.0 | 1.1 | 13.3 | 5.4 |
| Sn | 3.0 | 3.4 | 9.8 | 10.8 |
| Cr | 0.7 | 0.4 | 8.5 | 2.6 |
| V | 0.7 | 1.5 | 6.6 | 18.7 |
| In | 1.0 | 2.0 | 6.3 | 7.7 |
| As | 1.0 | 0.4 | 5.0 | 3.8 |
Effects of Adra2b on the Sensitivity to Air Pollution-induced Blood Pressure Elevation
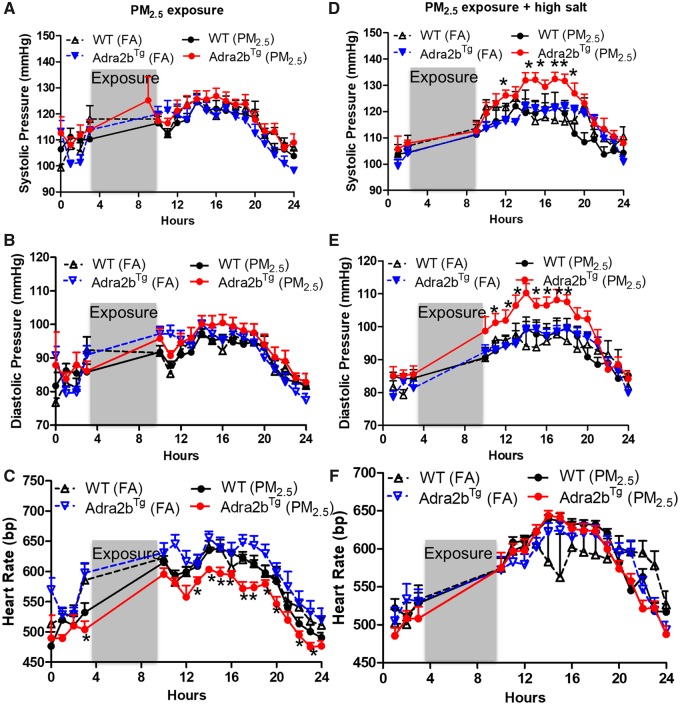
Implantable radio-telemetry devices allow continuous reliable measurement in freely moving animals while tail-cuff method has restraint stress. Therefore, we examined the blood pressure change using state-of-the-art radiotelemetry. After 11 weeks of air pollution exposure, blood pressure slightly increased in Adra2bTg mice compared with WT mice under normal chow diet condition, although not statistically different (Figs. 2A and 2B). Heart rate was reduced in Adra2bTg mice exposed to PM2.5 (Figure 2C). This may due to the baroreflex response to compensate for blood pressure change. Because FVB/N mice are more resistant to blood pressure changes (Hein et al., 1995) and we did not observe a difference in blood pressure under normal chow diet condition, we then fed all the mice with 4% high salt diet with concomitant exposure to corresponding FA or PM2.5 for 1 week (week 12). High salt intake is an acute risk factor for hypertension and can synergistically interact with other risk factors to increase blood pressure (Chen et al., 2018). It is able to desensitize baroreflexes in salt-sensitive animals (Andresen, 1989; Victor et al., 1986). Therefore, we used high salt diet as a stressor to examine if the combination of high salt diet and PM2.5 could induce blood pressure elevation. Both systolic (Figure 2D) and diastolic (Figure 2E) blood pressures were increased in the Adra2bTg mice exposed to PM2.5 and high salt diet, compared with WT mice or FA-exposed Adra2bTg mice on high salt diet. No significant difference in heart rate was seen among the 4 groups after combined treatment of high salt diet and FA/PM2.5 (Figure 2F).
Figure 2.
Effects of Adra2b on the sensitivity to air pollution-induced blood pressure elevation: A–F, WT and Adra2bTg mice were exposed to FA or PM2.5 for 11 weeks, then fed with 4% high salt diet simultaneously with exposure for 1 week (week 12). Systolic (A and D), diastolic (B and E) blood pressure, and heart rate (C and F) were examined before (A–C) and after (D–F) high salt diet feeding. Data are presented as means ± SE. N = 3–4 for each group. *p < .05 compared with FA within the same genotype.
Adra2b Enhances Anxiety in Open-field Test
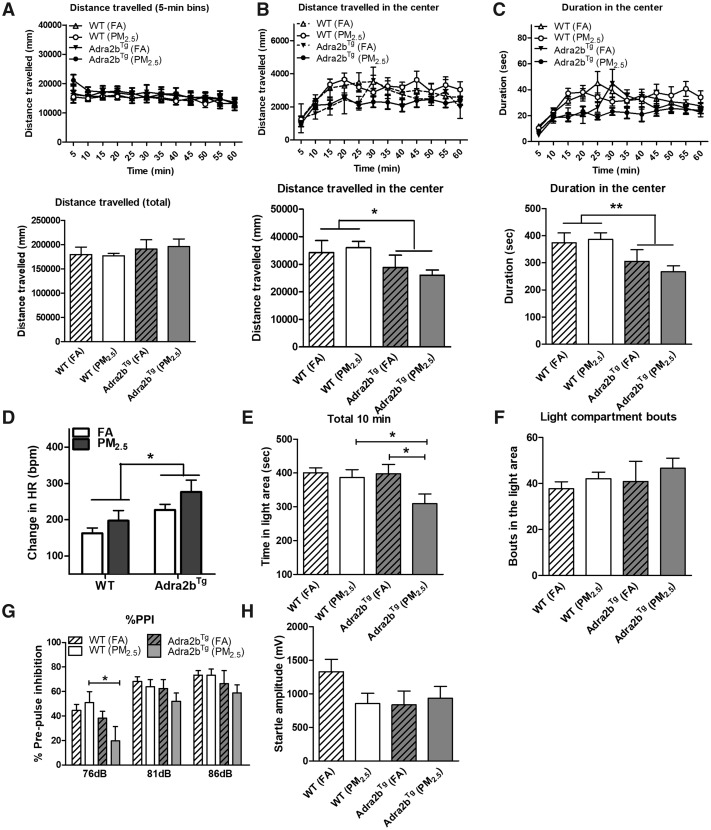
We next examined the behavior changes in Adra2bTg and WT mice exposed to air pollution. As shown in Figure 3A, there were no significant differences in distance traveled between FA- and PM2.5-exposed animals of either WT or Adra2bTg genotype under normal chow diet condition. However, overexpression of Adra2b in the brain reduced the distance traveled in the center and the duration in the center in both FA- and PM2.5-exposed Adra2bTg mice, compared with the WT mice (Figs. 3B and 3C), suggesting that Adra2bTg had an anxiety like behavior regardless the exposure of FA or PM2.5. We also observed a greater increase in heart rate (Figure 3D), but not blood pressure (Supplementary Figure 1), in Adra2bTg mice exposed to PM2.5 compared with FA, in open field, measured by the mouse radiotelemetry.
Figure 3.
Effects of Adra2b on anxiety-like behavior changes: A–C, FA- or PM2.5-exposed WT and Adra2bTg mice on a normal chow diet were subjected to open-field test. Total travel distance (A; upper panel, traveling speed [distance/min]; lower panel, cumulative distance traveled during the 60 min of detection period), travel distance in the center (B; upper panel, traveling speed [distance/min] in the center; lower panel, cumulative distance traveled in the center during the 60 min of detection period), and duration in the center (C; upper panel, the duration/minute stayed in the center; lower panel, cumulative duration stayed in the center during the 60 min of detection period) were measured. D, Heart rate changes in open-field test. Time in light area (E) and light compartment bouts (F) in the LDB test. % prepulse inhibition (G) and startle response (H) in the prepulse inhibition test. Data are presented as means ± SE. N = 5–7 for each group. *p < .05; **p < .01.
Effects of Adra2b on the Vulnerability to Air Pollution-induced Behavior Changes in Light-dark Box and Prepulse Inhibition Tests
To further examine anxiety-related behavior changes after air pollution exposure, exposed mice were further subjected to the LDB and prepulse inhibition tests. Exposure to PM2.5 significantly reduced time spent in light area in Adra2bTg mice but not WT mice (Figure 3E) consistent with anxiogenic effects, although bouts in the light area were not affected (Figure 3F). These results suggest that air pollution may enhance anxiety in susceptible individuals and Adra2b signaling is an important risk factor for air pollution-induced anxiety change. PM2.5 exposure reduced percentage of prepulse inhibition response following a 76 dB prepulse stimulation in Adra2bTg mice but not in WT mice. Similar trends were observed in 81 or 86 dB prepulse stimulation (Figure 3G). There was no significant difference in startle response among the 4 groups, although startle responses in the WT PM, Adra2bTg FA, and Adra2bTg PM groups were all slightly reduced compared with that in the WT FA group (Figure 3H).
Adra2b Activation in Air Pollution Exposure
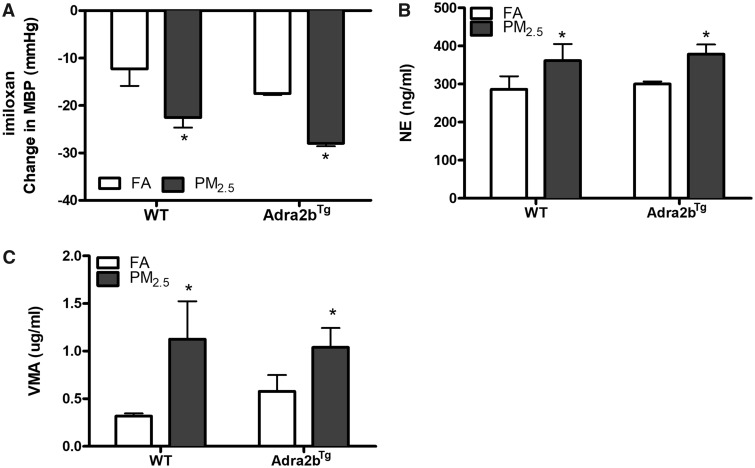
To confirm a pathophysiologic role of Adra2b in PM2.5-induced blood pressure elevation, blood pressure was monitored on FA/PM2.5-exposed WT and Adra2bTg mice on high salt diet after the intraperitoneal injection of the Adra2b-specific antagonist imiloxan. The blood pressure reductions were greater in PM2.5-exposed mice in both WT and Adra2bTg groups (Figure 4A), suggesting that Adra2b activation is involved in PM2.5-induced blood pressure elevation. The imiloxan-induced heart rate decrease was similar between WT FA and WT PM2.5, although PM2.5-exposed Adra2bTg mice had a smaller reduction of heart rate (Supplementary Figure 2). In addition, urine levels of NE and VMA, a metabolite of NE and epinephrine, were significantly increased in PM2.5-exposed mice in both WT and Adra2bTg groups (Figs. 4B and 4C).
Figure 4.
Sympathetic activation in PM2.5 exposure: A, FA- or PM2.5-exposed WT and Adra2bTg mice on a high salt diet were injected with the Adra2b-specific antagonist imiloxan and mean blood pressure (MBP) was monitor by telemetry. Changes in MBP were shown. N = 3–4 for each group. Norepinephrine (NE, B) and vanillylamandelic acid (VMA, C) levels were tested in the urine of exposed WT or Adra2bTg mice. Data are presented as means ± SE. *p < .05.
Effects of Adra2b Activation and Air Pollution Exposure on Inflammation
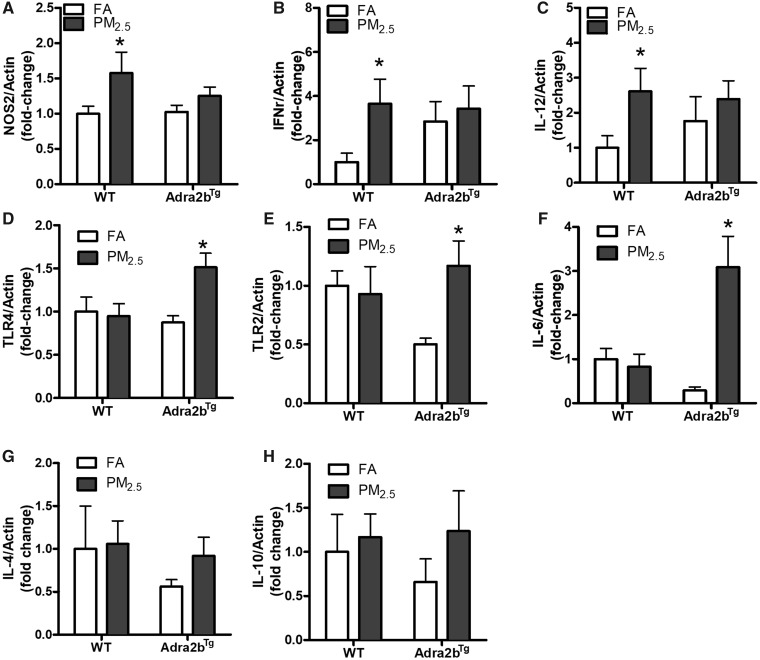
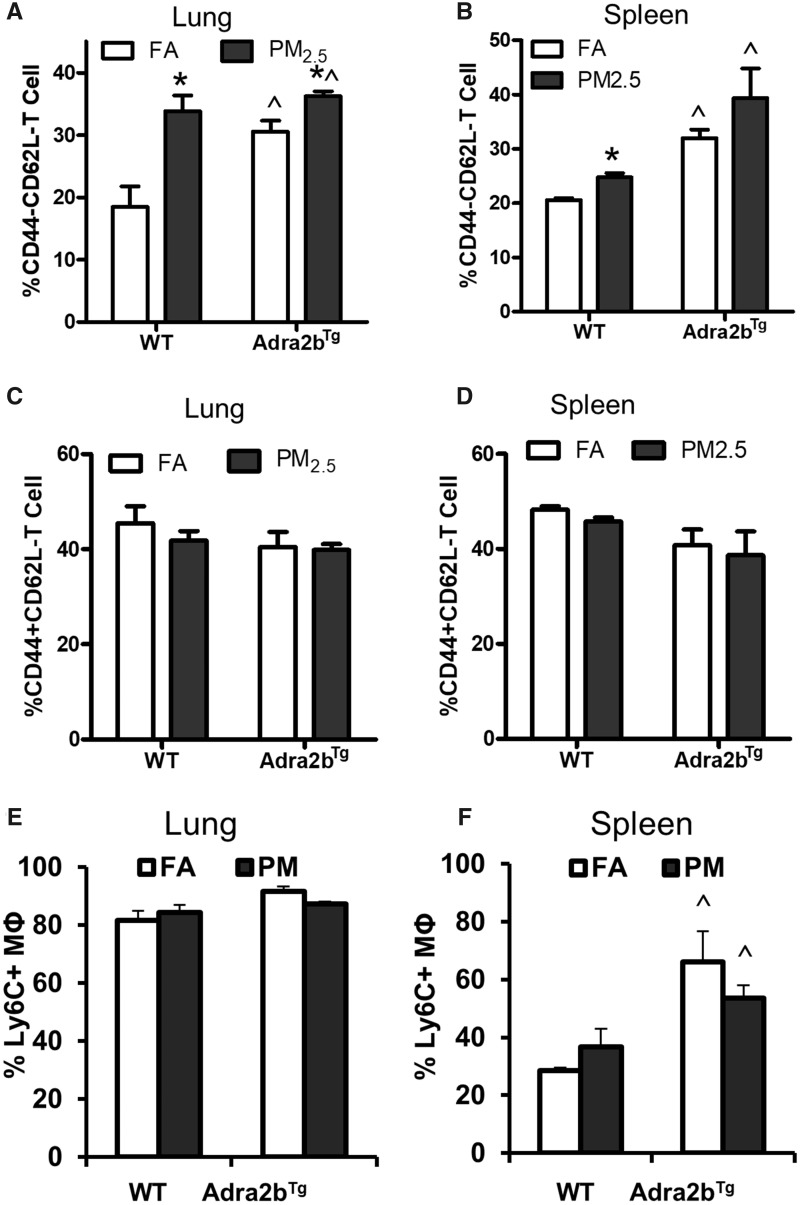
Inflammation has been confirmed as an important mechanism underlying air pollution-induced adverse health effects. Indeed, activation of the SNS has been shown to profoundly influence peripheral immune function (Chavan et al., 2017). To examine the involvement of Adra2b in PM2.5-induced inflammation, we detected the mRNA expression of inflammatory markers in the brain of PM2.5-exposed mice fed on a normal chow diet. As depicted in Figure 5, inflammation-associated genes, nitric oxide synthase 2 (NOS2), interferon gamma (IFNγ), and interleukin-12 (IL-12) increased in PM2.5-exposed WT mice. Overexpression of Adra2b also enhanced the gene expressions of IFNγ and IL-12, although PM2.5 exposure did not further enhance their expression in Adra2bTg mice (Figs. 5A–C). In addition, air pollution exposure increased the mRNA expression of Toll-like receptor 4 (TLR4), Toll-like receptor 2 (TLR2), and interleukin-6 (IL-6) in the brain of Adra2bTg but not WT mice, indicating that Adra2b overexpression enhanced the sensitivity to air pollution-induced inflammation (Figs. 5D–F). No significant changes in anti-inflammatory genes interleukin-4 (IL-4) and interleukin-10 (IL-10) were detected after PM2.5 exposure in both WT and Adra2bTg mice (Figs. 5G and 5H). Consistent with these results, flow cytometry also showed that the frequency of activated effector T cells (CD3+CD44–CD62L–), but not activated memory T cells (CD3+CD44+CD62L–), increased in both lung and spleen from PM2.5-exposed WT mice (Figs. 6A–D). No significant change in the amount of inflammatory macrophage (Ly6C+F4/80+) after PM2.5 exposure was observed in either lung or spleen from WT and Adra2bTg mice, although Ly6C+F4/80+ inflammatory macrophage increased in the spleen of Adra2bTg mice compared with WT mice (Figs. 6E and 6F).
Figure 5.
Adra2b activation in PM2.5-induced inflammatory gene expression: NOS2 (A), INFr (B), IL-12 (C), TLR4 (D), TLR2 (E), IL-6 (F), IL-4 (G), and IL-10 (H) expressions in the brain from FA- or PM2.5-exposed WT and Adra2bTg mice on a normal chow diet. Data are presented as means ± SE. N = 5–7 for each group. *p < .05 compared with FA.
Figure 6.
Adra2b activation in PM2.5-induced T cell activation: activated effector T cell (CD44–CD62L–, A and B) and activated memory T cell (CD44+CD62L–, C and D) in the lung and spleen from FA- or PM2.5-exposed WT and Adra2bTg mice on a normal chow diet. E and F, Percentage of inflammatory macrophage (Ly6C+ MΦ) in the lung and spleen from FA- or PM2.5-exposed WT and Adra2bTg mice. Data are presented as means ± SE. N = 5–7 for each group. *p < .05 compared with FA within the same genotype; ^p < .05 compared with WT exposed to the same aerosol.
Effects of Adra2b Activation and Air Pollution Exposure on CNS Oxidative Stress
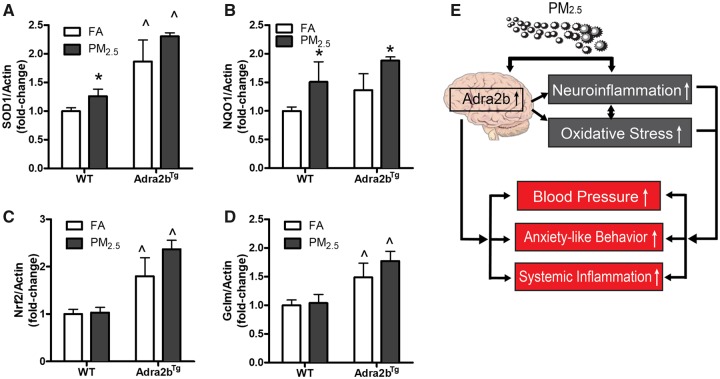
We and others have shown previously that air pollution exposure is associated with enhanced oxidative stress (Rao et al., 2018; Sorensen et al., 2003; Xu et al., 2011). To investigate if Adra2b activation is associated with oxidative stress following air pollution exposure, we examined the expression of oxidative stress-related genes in the brain tissue. Superoxide dismutase 1 (SOD1) and NAD(P)H quinone dehydrogenase 1 (NQO1) (Figs. 7A and 7B) but not nuclear factor erythroid 2-related factor 2 (Nrf2) and glutamate-cysteine ligase modifier subunit (Gclm) (Figs. 7C and 7D) were increased slightly in the brain of WT mice after PM2.5 exposure. The mRNA expressions of SOD1, Nrf2, and Gclm were increased in the brain of Adra2bTg, relative to WT mice (Figs. 7A–D). Furthermore, PM2.5 exposure increased the expression of NQO1, but not SOD1, Nrf2, and Gclm in the brain of Adra2bTg mice compared with FA-exposed Adra2bTg mice (Figure 7B).
Figure 7.
Adra2b activation in PM2.5-induced oxidative stress: Gene expression of SOD1 (A), NQO1 (B), Nrf2 (C), and Gclm (D) in the brain from FA- or PM2.5-exposed WT and Adra2bTg mice on a normal chow diet. E, Hypothetical mechanism. Data are presented as means ± SE. N = 5–7 for each group. *p < 0.05 compared with FA within the same genotype; ^p < .05 compared with WT exposed to the same aerosol.
DISCUSSION
Several previous studies, including our own, have indicated that central sympathetic activation is associated with air pollution-induced adverse health effects (de Hartog et al., 2009; Liu et al., 2014). Using a genetic approach of adrenergic receptor α2B overexpression in the brain as a model of central sympathetic activation, we provide direct evidence that sympathetic activation in response to air pollution results in behavioral and blood pressure changes to salt loading.
Ambient air pollution exposure has been associated with a number of human diseases such as hypertension, atherosclerosis, diabetes, stroke, etc. However, individuals may not be equally vulnerable to air pollution-induced adverse health effect. Age, behaviors, and underlying susceptibility are regarded as factors that can influence health effects of air pollution (Gent and Bell, 2010; Makri and Stilianakis, 2008). To identify if sympathetic activation could predispose individuals to air pollution, we used a mouse model that overexpresses Adra2b selectively in the brain. The Adra2b receptor plays a critical role in regulating noradrenaline signals and receptor responsiveness (Small et al., 2001). In the current study, we observed a selective elevation of Adra2b expression in the brain of PM2.5-exposed mice. Overexpression of Adra2b in the brain sensitized the blood pressure response of FVB/N mice to air pollution under high salt loading conditions. High salt intake and salt sensitivity have been associated with sympathetic activation (Fujita, 2014). We did not observe hypertensive response in Adra2b-expressing FVB/N mice after PM2.5 exposure under normal salt loading conditions. This may be consistent with previously noted findings that FVB/N strain may be more resistant to blood pressure changes (Hein et al., 1995). This may relate to the compensatory mechanisms to maintain blood pressure balance in vivo. For example, the hypertensive response of PM2.5 in Adra2bTg mice may reduce heart rate through baroreflex response to maintain blood pressure balance. High salt intake, conversely, may desensitize baroreflexes in salt-sensitive animals (Andresen, 1989; Victor et al., 1986). Therefore, Adra2bTg mice under high salt diet condition may have a disrupted baroreflex response to compensate for salt-induced pressor effects, resulting in a blood pressure elevation. Interestingly, it has also been shown that inhalation of PM2.5 may also affect cardiac vagal influence and baroreflex sensitivity when combined with modified diets (Carll et al., 2017), suggesting a more complicated relationship between high salt intake and air pollution exposure. In consistency with this, the difference in heart rate between Adra2bTg (PM2.5) group and other groups disappeared after treatment of a high salt diet, indicating that there might be a synergistic effect between high salt intake and air pollution exposure which disrupted the baroreflex compensatory mechanism(s), resulting in blood pressure elevation. In our study, imiloxan-induced heart rate reduction was smaller in Adra2bTg (PM2.5) group compared with Adra2bTg (FA) group, suggesting that there might be a higher level of baroreflex response and associated sympathetic inhibition although it may not be sufficient to maintain normal blood pressure. In contrast, Adra2b blockade by imiloxan had greater effects on the changes in blood pressure, NE and VMA in PM2.5-exposed animals. In combination with the findings that PM2.5 exposure enhanced Adra2b expression, these results suggest that Adra2b-associated sympathetic activation plays an important role in air pollution-induced elevations in blood pressure. Activation of the SNS relays stress interpretation from the brain to the immune system. This is pertinent as SNS activation centrally results in direct release of catecholamines and stimulation of adrenergic receptors in peripheral lymphoid cells resulting in alteration of their inflammatory potential and migration capacity (Chavan et al., 2017). Indeed, air pollution exposure resulted in robust evidence of sympathetic excess, together with increase in the expression of inflammation-related genes, an increase of T cell activation in both lung and spleen and CNS expression of TLR2, TLR4, and IL-6. Sympathetic activation could either directly regulate inflammatory response through adrenergic receptors, or indirectly via blood flow, immune cell distribution, and proinflammatory peptides (Pongratz and Straub, 2014). Immune cells express a variety of adrenergic receptors which have been directly associated with the regulation of immune activation (Bacou et al., 2017; Dasu et al., 2014; Scanzano and Cosentino, 2015). However, the exact role of Adra2b in direct regulation of immune function was not well understood (Scanzano and Cosentino, 2015). Similarly, Adra2b activation also enhanced the expression of antioxidant genes such as SOD1, NQO1, Nrf2, and Gclm in the brain. Recent studies have demonstrated an important homeostatic role of Nrf2 in the mitigation of blood pressure in response to hypertensinogenic stimuli (Gao et al., 2017). Ablation of Nrf2 selectively in areas of the brain resulted in potentiation of blood pressure in response to angiotensin II (Gao et al., 2017). Although we cannot determine if redox stress directly result in sympathetic activation in our experiments, prior observations have shown that air pollution exposure increases redox stress via nicotinamide adenine dinucleotide phosphate oxidase pathways (Rao et al., 2018).
In consistency with our previous report (Liu et al., 2014), chronic PM2.5 exposure resulted in sympathetic activation and peripheral inflammation. We have shown that chronic exposure to PM2.5 increased low-frequency blood pressure variability and urinary NE in C57BL/6 mice, whereas central inhibition of IKK/NF-κB reversed central inflammation and restored peripheral energy homeostasis and insulin sensitivity in PM2.5-exposed mice (Liu et al., 2014). In humans, exposure to PM2.5 and ozone has also been associated reduced heart rate variability, index of sympathetic activation (Park et al., 2005; Park et al., 2008). In addition, β blockers were able to reduce the low frequency heart rate variability in response to pollutants in human subjects (Park et al., 2005). It seems that older populations may be more sensitive to air pollution-induced alteration in sympathetic pathways (Creason et al., 2001; Holguin et al., 2003; Liao et al., 1999). Sympathetic pathways have also been associated with depression and anxiety disorders (Roth et al., 2008; Veith et al., 1994). We and others have indicated that chronic air pollution exposure may result in anxiety and depressive like behaviors in both humans and mice (Fonken et al., 2011; Hogan et al., 2015; Vert et al., 2017). However, whether the sympathetic signaling plays a role in air pollution-associated behavior changes is not clear. Several clinical and preclinical studies indicate that stress promotes the development of anxiety and depressive like behaviors (Gilman et al., 2013; Kendler et al., 1999; McLaughlin et al., 2010). In models of repetitive social defeat (RSD), anxiety like behavior in the open field and light-dark preference tests develop after RSD (3–6 cycles) and persists for days (Wohleb et al., 2014, 2013). Commonly used mouse models of anxiety include open field test, LDB test, and elevated plus/T maze. Each test may differ according to the feature of aversive signal, response, and sensitivity to different stresses (Bourin et al., 2007; Steimer, 2011). Open field test is sensitive to the anxiolytic-like effects of benzodiazepine receptor- and 5-HT1A receptor agonists but not to the effects of other compounds such as selective serotonin reuptake inhibitors and tricyclics (Prut and Belzung, 2003). Many models including open field test were developed and validated based on benzodiazepines, their sensibility on drugs acting on other system remains questionable (Bourin et al., 2007). LDB test, developed in 1980s, evaluates the innate aversion of rodents to brightly illuminated areas and their spontaneous exploratory behavior (Bourin et al., 2007). Prepulse inhibition test measures the inhibitory effect of a weak acoustic auditory stimulus on a subsequent startle response (Longenecker et al., 2016). Prepulse inhibition deficits are associated with psychosis and schizophrenia. In the open field test, we found that central overexpression of Adra2b significantly reduced distance traveled and time in the center, suggesting that SNS is involved in the anxiety response. In support of our findings, the deletion variant (Del301-303) of Adra2b that results in reduced agonist-promoted receptor desensitization and persistent receptor activation (Small et al., 2001) has been shown to be associated with vulnerability to emotional disorders such as anxiety and abnormal emotional response (de Quervain et al., 2007; Naudts et al., 2012). In the LDB test, PM2.5 exposure suppressed the willingness of transgenic mice to explore in a risky environment, suggesting there was a synergistic effect between air pollution and Adra2b overexpression on anxiety-like behavior. Prepulse inhibition deficits were identified in transgenic mice exposed to PM2.5. These results further support the conclusion that sympathetic activity controls the susceptibility of mice to air pollution-induced behavioral changes.
In contrast to our previous observation that air pollution exposure increases blood pressure in C57BL/6 mice (Liu et al., 2014), we did not observe a significant effect of air pollution exposure on baseline blood pressure in both WT and Adra2bTg mice after 12 weeks of PM2.5 exposure. This may be due to the differences in exposure duration and/or strain of mice. Indeed, FVB/N mice are salt-resistant and may be less vulnerable to air pollution exposure and environmental challenges (Hein et al., 1995; Masuzaki et al., 2003; Savov et al., 2004). However, the relative resistance to blood pressure changes in FVB/N mice also make it a good model to study exposure sensitivity to air pollution. This may also be the reason that PM2.5 exposure alone in WT FVB/N mice did not cause any behavioral changes in open field, LDB, and prepulse inhibition tests. We have shown that air pollution exposure alone provoked anxiety-like and depressive-like responses in C57BL/6 mice (Fonken et al., 2011) and C3H/HeNHsd mice (Hogan et al., 2015), although no significant behavioral changes were observed in FVB/N mice after exposure in this study. This study also shows that increasing the salt intake in PM2.5-exposed mice with genetic predisposition, ie, increased brain Adra2b expression, can lead to hypertension. Air pollution exposure has been recently linked to criminal activities (Kristiansson et al., 2015; Lu et al., 2018), suicide mortality (Lin et al., 2016), and depressive/anxiety symptoms (Pun et al., 2017) in epidemiologic studies. This study may provide a better understanding of the mechanisms underlying the link between air pollution exposure and psychologic/behavior changes. It may also contribute to the identification of vulnerable populations to air pollution exposure.
It should be noted that there are several limitations within this study. First, our study only focused on the Adra2b signaling and there might be other pathways regulating the SNS activation in air pollution exposure which requires further investigations. Second, the urinary levels of NE and VMA examined in our study were not normalized by creatinine and therefore could not exclude the possibility that PM2.5 exposure may affect urinary NE/VMA levels via altering kidney function. Third, this study used self-control for high salt diet treatment which results in small difference in PM2.5 exposure duration (11 weeks of PM2.5 exposure for normal chow diet vs. 12 weeks of PM2.5 exposure for high salt diet). Given the fact that the PM2.5 concentrations throughout the 12 weeks of exposure were consistent (Supplementary Figure 3), one more week of PM2.5 exposure would presumably have minimal added effect beyond 11 weeks.
In conclusion, our results provide support for an important and direct role for central sympathetic activation in behavioral (anxiety) and peripheral cardioimmune alterations (Figure 7E).
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
National Institute of Health (R01ES015146 and R01ES019616 to S.R., K99ES026241 to X.R., K01DK105108 to J.Z., HL126626 and HL141423 to G.H.M.) and American Heart Association (17GRNT33670485 to J.Z.).
AUTHORS’ CONTRIBUTIONS
X.R. contributed to the experimental design, performed experiments, collected samples, analyzed date, and drafted the manuscript. L.D.A. performed the radio-telemetry surgery. P.Z. performed the behavior tests. R.S.G., C.X., L.D., Y.M.C., and P.R. helped with the tissue collection and exposure. P.A.J., T.D.G., R.N., S.B., J.Z. provided critical comments on study design and manuscript. L.C.C. helped with element characterization of air particulate matters. S.R. conceived of and lead the study, drafted the manuscript.
Supplementary Material
REFERENCES
- Andresen M. C. (1989). High-salt diet elevates baroreceptor pressure thresholds in normal and Dahl rats. Circ. Res. 64, 695–702. [DOI] [PubMed] [Google Scholar]
- Bacou E., Haurogne K., Allard M., Mignot G., Bach J. M., Herve J., Lieubeau B. (2017). beta2-adrenoreceptor stimulation dampens the LPS-induced M1 polarization in pig macrophages. Dev. Comp. Immunol. 76, 169–176. [DOI] [PubMed] [Google Scholar]
- Bourin M., Petit-Demouliere B., Dhonnchadha B. N., Hascoet M. (2007). Animal models of anxiety in mice. Fundam. Clin. Pharmacol. 21, 567–574. [DOI] [PubMed] [Google Scholar]
- Carll A. P., Crespo S. M., Filho M. S., Zati D. H., Coull B. A., Diaz E. A., Raimundo R. D., Jaeger T. N. G., Ricci-Vitor A. L., Papapostolou V. (2017). Inhaled ambient-level traffic-derived particulates decrease cardiac vagal influence and baroreflexes and increase arrhythmia in a rat model of metabolic syndrome. Part. Fibre Toxicol. 14, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavan S. S., Pavlov V. A., Tracey K. J. (2017). Mechanisms and therapeutic relevance of neuro-immune communication. Immunity 46, 927–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M. L., Huang T. P., Chen T. W., Chan H. H., Hwang B. F. (2018). Interactions of genes and sodium intake on the development of hypertension: A cohort-based case-control study. Int. J. Environ. Res. Public Health 15, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creason J., Neas L., Walsh D., Williams R., Sheldon L., Liao D., Shy C. (2001). Particulate matter and heart rate variability among elderly retirees: The Baltimore 1998 PM study. J. Expo. Anal. Environ. Epidemiol. 11, 116–122. [DOI] [PubMed] [Google Scholar]
- Dasu M. R., Ramirez S. R., La T. D., Gorouhi F., Nguyen C., Lin B. R., Mashburn C., Stewart H., Peavy T. R., Nolta J. A. (2014). Crosstalk between adrenergic and toll-like receptors in human mesenchymal stem cells and keratinocytes: A recipe for impaired wound healing. Stem Cells Transl. Med. 3, 745–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hartog J. J., Lanki T., Timonen K. L., Hoek G., Janssen N. A., Ibald-Mulli A., Peters A., Heinrich J., Tarkiainen T. H., van Grieken R., et al. (2009). Associations between PM2.5 and heart rate variability are modified by particle composition and beta-blocker use in patients with coronary heart disease. Environ. Health Perspect. 117, 105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Quervain D. J., Kolassa I. T., Ertl V., Onyut P. L., Neuner F., Elbert T., Papassotiropoulos A. (2007). A deletion variant of the alpha2b-adrenoceptor is related to emotional memory in Europeans and Africans. Nat. Neurosci. 10, 1137–1139. [DOI] [PubMed] [Google Scholar]
- Fonken L. K., Xu X., Weil Z. M., Chen G., Sun Q., Rajagopalan S., Nelson R. J. (2011). Air pollution impairs cognition, provokes depressive-like behaviors and alters hippocampal cytokine expression and morphology. Mol. Psychiatry 16, 987–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T. (2014). Mechanism of salt-sensitive hypertension: Focus on adrenal and sympathetic nervous systems. J. Am. Soc. Nephrol. 25, 1148–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L., Zimmerman M. C., Biswal S., Zucker I. H. (2017). Selective Nrf2 gene deletion in the rostral ventrolateral medulla evokes hypertension and sympathoexcitation in mice. Hypertension 69, 1198–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gent J. F., Bell M. L. (2010). Air pollution, population vulnerability, and standards for ambient air quality. Am. J. Respir. Crit. Care Med. 182, 296–297. [DOI] [PubMed] [Google Scholar]
- Gilman S. E., Trinh N. H., Smoller J. W., Fava M., Murphy J. M., Breslau J. (2013). Psychosocial stressors and the prognosis of major depression: A test of Axis IV. Psychol. Med. 43, 303–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein L., Barsh G. S., Pratt R. E., Dzau V. J., Kobilka B. K. (1995). Behavioural and cardiovascular effects of disrupting the angiotensin II type-2 receptor in mice. Nature 377, 744–747. [DOI] [PubMed] [Google Scholar]
- Hogan M. K., Kovalycsik T., Sun Q., Rajagopalan S., Nelson R. J. (2015). Combined effects of exposure to dim light at night and fine particulate matter on C3H/HeNHsd mice. Behav. Brain Res. 294, 81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holguin F., Tellez-Rojo M. M., Hernandez M., Cortez M., Chow J. C., Watson J. G., Mannino D., Romieu I. (2003). Air pollution and heart rate variability among the elderly in Mexico City. Epidemiology 14, 521–527. [DOI] [PubMed] [Google Scholar]
- Kanagy N. L. (2005). Alpha(2)-adrenergic receptor signalling in hypertension. Clin. Sci. (Lond.) 109, 431–437. [DOI] [PubMed] [Google Scholar]
- Kendler K. S., Karkowski L. M., Prescott C. A. (1999). Causal relationship between stressful life events and the onset of major depression. Am. J. Psychiatry 156, 837–841. [DOI] [PubMed] [Google Scholar]
- Kintsurashvili E., Shenouda S., Ona D., Ona L., Ahmad S., Ravid K., Gavras I., Gavras H. (2009). Hypertension in transgenic mice with brain-selective overexpression of the alpha(2B)-adrenoceptor. Am. J. Hypertens. 22, 41–45. [DOI] [PubMed] [Google Scholar]
- Kioumourtzoglou M. A., Schwartz J. D., Weisskopf M. G., Melly S. J., Wang Y., Dominici F., Zanobetti A. (2016). Long-term PM2.5 exposure and neurological hospital admissions in the northeastern United States. Environ. Health Perspect. 124, 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansson M., Sorman K., Tekwe C., Calderon-Garciduenas L. (2015). Urban air pollution, poverty, violence and health–Neurological and immunological aspects as mediating factors. Environ. Res. 140, 511–513. [DOI] [PubMed] [Google Scholar]
- Liao D., Creason J., Shy C., Williams R., Watts R., Zweidinger R. (1999). Daily variation of particulate air pollution and poor cardiac autonomic control in the elderly. Environ. Health Perspect. 107, 521–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin G. Z., Li L., Song Y. F., Zhou Y. X., Shen S. Q., Ou C. Q. (2016). The impact of ambient air pollution on suicide mortality: A case-crossover study in Guangzhou, China. Environ. Health 15, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Fonken L. K., Wang A., Maiseyeu A., Bai Y., Wang T. Y., Maurya S., Ko Y. A., Periasamy M., Dvonch T., et al. (2014). Central IKKbeta inhibition prevents air pollution mediated peripheral inflammation and exaggeration of type II diabetes. Part. Fibre Toxicol. 11, 53.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Longenecker R. J., Alghamdi F., Rosen M. J., Galazyuk A. V. (2016). Prepulse inhibition of the acoustic startle reflex vs. auditory brainstem response for hearing assessment. Hear. Res. 339, 80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J. G., Lee J. J., Gino F., Galinsky A. D. (2018). Polluted morality: Air pollution predicts criminal activity and unethical behavior. Psychol. Sci. 29, 340–355. [DOI] [PubMed] [Google Scholar]
- Makri A., Stilianakis N. I. (2008). Vulnerability to air pollution health effects. Int. J. Hyg. Environ. Health 211, 326–336. [DOI] [PubMed] [Google Scholar]
- Masuzaki H., Yamamoto H., Kenyon C. J., Elmquist J. K., Morton N. M., Paterson J. M., Shinyama H., Sharp M. G., Fleming S., Mullins J. J., et al. (2003). Transgenic amplification of glucocorticoid action in adipose tissue causes high blood pressure in mice. J. Clin. Invest. 112, 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K. A., Conron K. J., Koenen K. C., Gilman S. E. (2010). Childhood adversity, adult stressful life events, and risk of past-year psychiatric disorder: A test of the stress sensitization hypothesis in a population-based sample of adults. Psychol. Med. 40, 1647–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munzel T., Sorensen M., Gori T., Schmidt F. P., Rao X., Brook J., Chen L. C., Brook R. D., Rajagopalan S. (2017). Environmental stressors and cardio-metabolic disease: Part I – epidemiologic evidence supporting a role for noise and air pollution and effects of mitigation strategies. Eur. Heart J. 38, 550–556. [DOI] [PubMed] [Google Scholar]
- Naudts K. H., Azevedo R. T., David A. S., van Heeringen K., Gibbs A. A. (2012). Epistasis between 5-HTTLPR and ADRA2B polymorphisms influences attentional bias for emotional information in healthy volunteers. Int. J. Neuropsychopharmacol. 15, 1027–1036. [DOI] [PubMed] [Google Scholar]
- Park S. K., O’Neill M. S., Vokonas P. S., Sparrow D., Schwartz J. (2005). Effects of air pollution on heart rate variability: The VA normative aging study. Environ. Health Perspect. 113, 304–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S. K., O‘Neill M. S., Vokonas P. S., Sparrow D., Wright R. O., Coull B., Nie H., Hu H., Schwartz J. (2008). Air pollution and heart rate variability: Effect modification by chronic lead exposure. Epidemiology 19, 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongratz G., Straub R. H. (2014). The sympathetic nervous response in inflammation. Arthritis Res. Ther. 16, 504.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prut L., Belzung C. (2003). The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur. J. Pharmacol. 463, 3–33. [DOI] [PubMed] [Google Scholar]
- Pun V. C., Manjourides J., Suh H. (2017). Association of ambient air pollution with depressive and anxiety symptoms in older adults: Results from the NSHAP study. Environ. Health Perspect. 125, 342–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan S., Brook R. D. (2015). Personalizing your airspace and your health. J. Am. Coll. Cardiol. 65, 2288–2290. [DOI] [PubMed] [Google Scholar]
- Rao X., Zhong J., Brook R. D., Rajagopalan S. (2018). Effect of particulate matter air pollution on cardiovascular oxidative stress pathways. Antioxid. Redox Signal. 28, 979–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao X., Zhong J., Maiseyeu A., Gopalakrishnan B., Villamena F. A., Chen L. C., Harkema J. R., Sun Q., Rajagopalan S. (2014). CD36-dependent 7-ketocholesterol accumulation in macrophages mediates progression of atherosclerosis in response to chronic air pollution exposure. Circ. Res. 115, 770–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth W. T., Doberenz S., Dietel A., Conrad A., Mueller A., Wollburg E., Meuret A. E., Barr Taylor C., Kim S. (2008). Sympathetic activation in broadly defined generalized anxiety disorder. J. Psychiatr. Res. 42, 205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savov J. D., Whitehead G. S., Wang J., Liao G., Usuka J., Peltz G., Foster W. M., Schwartz D. A. (2004). Ozone-induced acute pulmonary injury in inbred mouse strains. Am. J. Respir. Cell Mol. Biol. 31, 69–77. [DOI] [PubMed] [Google Scholar]
- Scanzano A., Cosentino M. (2015). Adrenergic regulation of innate immunity: A review. Front. Pharmacol. 6, 171.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small K. M., Brown K. M., Forbes S. L., Liggett S. B. (2001). Polymorphic deletion of three intracellular acidic residues of the alpha 2B-adrenergic receptor decreases G protein-coupled receptor kinase-mediated phosphorylation and desensitization. J. Biol. Chem. 276, 4917–4922. [DOI] [PubMed] [Google Scholar]
- Sorensen M., Daneshvar B., Hansen M., Dragsted L. O., Hertel O., Knudsen L., Loft S. (2003). Personal PM2.5 exposure and markers of oxidative stress in blood. Environ. Health Perspect. 111, 161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steimer T. (2011). Animal models of anxiety disorders in rats and mice: Some conceptual issues. Dialogues Clin. Neurosci. 13, 495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q., Yue P., Deiuliis J. A., Lumeng C. N., Kampfrath T., Mikolaj M. B., Cai Y., Ostrowski M. C., Lu B., Parthasarathy S., et al. (2009). Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation 119, 538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobian L. (1991). Salt and hypertension. Lessons from animal models that relate to human hypertension. Hypertension 17(Suppl. 1), I52–I58. [DOI] [PubMed] [Google Scholar]
- Veith R. C., Lewis N., Linares O. A., Barnes R. F., Raskind M. A., Villacres E. C., Murburg M. M., Ashleigh E. A., Castillo S., Peskind E. R. (1994). Sympathetic nervous system activity in major depression. Basal and desipramine-induced alterations in plasma norepinephrine kinetics. Arch. Gen. Psychiatry 51, 411–422. [DOI] [PubMed] [Google Scholar]
- Vert C., Sanchez-Benavides G., Martinez D., Gotsens X., Gramunt N., Cirach M., Molinuevo J. L., Sunyer J., Nieuwenhuijsen M. J., Crous-Bou M., et al. (2017). Effect of long-term exposure to air pollution on anxiety and depression in adults: A cross-sectional study. Int. J. Hyg. Environ. Health 220, 1074–1080. [DOI] [PubMed] [Google Scholar]
- Victor R. G., Morgan D. A., Thoren P., Mark A. L. (1986). High salt diet sensitizes cardiopulmonary baroreflexes in Dahl salt-resistant rats. Hypertension 8, II21–II27. [DOI] [PubMed] [Google Scholar]
- Wohleb E. S., McKim D. B., Shea D. T., Powell N. D., Tarr A. J., Sheridan J. F., Godbout J. P. (2014). Re-establishment of anxiety in stress-sensitized mice is caused by monocyte trafficking from the spleen to the brain. Biol. Psychiatry 75, 970–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb E. S., Powell N. D., Godbout J. P., Sheridan J. F. (2013). Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J. Neurosci. 33, 13820–13833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y. C., Lin Y. C., Yu H. L., Chen J. H., Chen T. F., Sun Y., Wen L. L., Yip P. K., Chu Y. M., Chen Y. C. (2015). Association between air pollutants and dementia risk in the elderly. Alzheimers Dement. (Amst.) 1, 220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Xu X., Zhong M., Hotchkiss I. P., Lewandowski R. P., Wagner J. G., Bramble L. A., Yang Y., Wang A., Harkema J. R., et al. (2011). Ambient particulate air pollution induces oxidative stress and alterations of mitochondria and gene expression in brown and white adipose tissues. Part. Fibre Toxicol. 8, 20.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P., Moaddel R., Morris P. J., Georgiou P., Fischell J., Elmer G. I., Alkondon M., Yuan P., Pribut H. J., Singh N. S., et al. (2016). NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533, 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.