Abstract
The adaptor protein CARD9 plays an important role in anti-fungal immunity responses, linking detection of fungi by surface receptors to activation of the transcription factor nuclear factor κB (NF-κB). Recent studies indicate that CARD9 also plays different but vital roles during the development of colitis-associated colorectal cancer (CAC). This review summarizes the current understanding of CARD9 functions in CAC, and we discuss its potentially carcinogenic mechanisms.
Keywords: CARD9, colitis-associated cancer, myeloid-derived suppressor cells, inflammasome activation, IL-22, STAT3
Main Text
It is well known that patients with inflammatory bowel disease (IBD) have an increased risk of developing colorectal cancer (CRC) as compared to healthy people, and this cancer is defined as colitis-associated CRC (CAC).1 CAC develops from a non-neoplastic inflammatory intestinal epithelium that progresses to cancer. Chronic inflammation enriches the colonic mucosa of reactive oxygen species and reactive nitrogen species, which can lead to DNA damage and genetic mutations (k-ras, p53, c-src, and β-catenin), and then induce cell transformation and the initiation of cancer.2 In addition, chronic inflammation also activates the signal transducer and activator (nuclear factor κB [NF-кB], signal transducers and activators of transcription [STAT]3, and β-catenin), promotes proliferation and remodeling of intestinal epithelial cells, and subsequently drives CAC initiation in IBD patients.3
CARD9 is classified as an inflammation-related protein, triggering the NF-κB and/or mitogen-activated protein kinase (MAPK) inflammatory signaling pathway.4 There is growing evidence to suggest that CARD9 maybe play a critical role in CAC.5, 6, 7 In these studies, CARD9 demonstrated a biphasic behavior in CAC, with anti-carcinogenic and pro-carcinogenic activities. Therefore, this review focuses on the dual role of CARD9 in CAC, and we discuss the potential molecular mechanisms.
CARD9 Protein
CARD9 is mainly expressed in myeloid cells, especially in macrophages and dendritic cells. It has disparate functions in these two pathways, linking Toll-like receptors to MAPK and C-type Lectins to activation of the transcription factor NF-κB.4 As a result, CARD9, a central integrator of innate and adaptive immunity, is involved in various inflammatory diseases.
CARD9 Protein in IBD
CARD9 is validated to play a pivotal role in the pathogenesis of IBD. In these recent studies, Card9 rs10870077, rs10781499, and rs4077515 variant in IBD patients showed a predisposing factor with an increase in the expression of CARD9 mRNA,8, 9 while Card9 c.IVS11+1G > C and rs200735402 variant had a protective factor with a loss of the biological function.10, 11
Next, animal studies were used to assess the susceptibility of Card9 to develop IBD. First, Card9-null mice showed an impaired intestinal mucosal immune response and defective expressions of interleukin (IL)-6, IL-17A, IL-22, regenerating islet-derived 3 gamma (RegIIIγ), and colonic T-helper (Th) 17 cells.12 It is widely known that these abnormal inflammatory factor and immune cells are strongly linked to IBD through the alteration of intestinal epithelial cell proliferation and apoptosis and the impairment of Th17 cell immune responses.13 Second, Card9−/− mice revealed a defective ecological effect in shaping the gut microbiota ecosystem. Numerous studies had confirmed a key role for the gut microbiota in the pathogenesis of IBD. The fecal fungal composition in Card9−/− mice was dominated by members of the phyla Ascomycota, Basidiomycota, and Zygomycota. However, the composition of the fecal bacterial microbiota was only found to have a slight alteration.14 Third, the gut bacteria of Card9−/− mice failed to metabolize tryptophan into aryl hydrocarbon receptor (AHR) ligands. AHR ligand production was found to normalize the presence of exogenous IL-22, effectively relieving IBD susceptibility.15 As predicted, Card9−/− mice with IBD could obviously inhibit the intestinal epithelial restitution and impair the gut recovery.14
CARD9 Protein in Intestinal Carcinoma
To date, the molecular mechanisms underlying the growth of intestinal carcinoma are still unknown. Of note, the role of CARD9 in intestinal carcinoma has been clearly described in some studies.16
A recent study reported a strong clinical correlation between CARD9 expression and human colon carcinoma.17 After carefully analyzing the clinic-pathologic features of 48 cases, CARD9 expression level was found to have a negative correlation with tumor differentiation and a positive correlation with tumor invasion depth and metastasis. It was important to note that aberrant CARD9 expression in tumor-infiltrating macrophages could contribute to liver metastasis of colon carcinoma cells through the following three molecular mechanisms: (1) CARD9 induced tumor metastasis via promoting macrophage function in the tumor microenvironment, independent of the number of infiltrating macrophages; (2) CARD9 was responsible for driving macrophage polarization via secreting tumor-promoting cytokines, such as IL-6, IL-12, IL-10, transforming growth factor β1 (TGF-β), and vascular endothelial growth factor (VEGF); and (3) CARD9 contributed to M2 macrophage polarization through activation of the NF-кB-signaling pathway.
Enteropathy-associated T cell lymphoma (EATL) is identified as a rare primary T cell lymphoma in the human small intestines. A study by Tomita et al.18 indicated that CARD9 was validated as a potential candidate gene for EATL in 20 patients. This study also reported a similarly frequent copy number change (about 75%) between Japanese and European EATL cases, without East and West ethnic differences.19
As is well known, the APCmin mouse model is used to mimic human familial adenomatous polyposis. CARD9 was found to promote tumorigenesis in sex-biased colon tumors, specifically in male mice.20 Sex-biased colon tumors may be attributed to less T cell and macrophage infiltration into colonic tumor tissues and decreased plasma cytokines (IL-6, G-CSF, and RANTES).21
Role of CARD9 in CAC
It is now recognized that inflammatory cytokines could stimulate epithelial cell proliferation, inhibit cell death, directly target neoplastic cells, and facilitate tumor progression.22 For instance, IKKβ-mediated NF-кB activation in myeloid cells stimulated the expression of pro-tumorigenic inflammatory factor, which promoted CAC induction and progression. Consistently, IKKβ inhibition or deletion was confirmed to restrain the cancer growth in CAC murine models.23, 24 Further work showed that IL-6, mainly produced by intestinal dendritic cells and macrophages, was a critical signaling pathway for CAC. The increased IL-6 expression could prompt STAT3 activation and greatly drive CAC tumorigenesis in mice, whereas IL-6 deletion suppressed the pro-carcinogenic effect.25, 26 IL-17A that was produced by Th17 cells directly promoted the progression of early non-neoplastic inflammatory epithelium into cancer, and the elevated expression of such cytokine, together with IL-23R, induced the development of CAC in colitis mice.27, 28, 29 On the other hand, cytotoxic T lymphocytes (CTLs) acted as tumor immunosurveillance, mounting vigorous immune responses against CAC tumor cells.30
In view of the close relationship of CARD9 pathways with the pathogenesis of IBD and intestinal carcinogenesis, it suggests that CARD9 could be playing a critical role in CAC as well. Although clinical evidence relating CARD9 protein to CAC is scarce, studies from animal models provide some insight. Since, in intestinal mucosa, CARD9 is overexpressed in tumor-infiltrating macrophages, but not in cancer cells, CARD9-expressing immune cells potentially influence tumor cell growth and survival in the development of CAC. Following the oral administration of dextran sodium sulfate (DSS) and azoxymethane (AOM), the susceptibility of such animals to develop DSS-driven CAC was assessed. However, to date, data available are limited to provide controversial results.
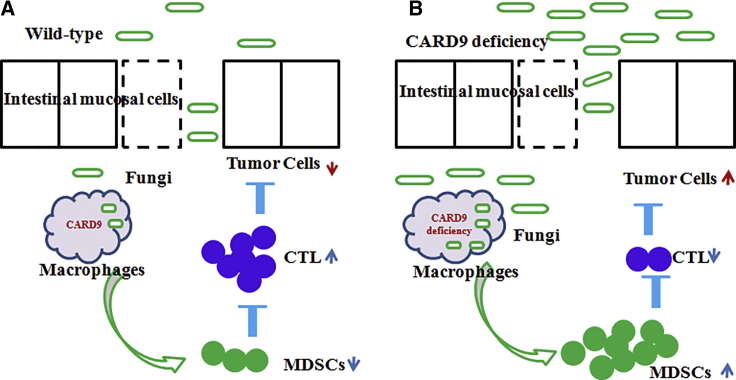
Wang and colleagues7 had observed that a high level of CARD9 expression was much less susceptible to CAC, exerting an anti-tumor immune response (Figure 1). Card9-knockout mice showed an increased tumor burden (size, number, and load) as compared to wild-type mice with AOM-DSS-induced CAC. Card9-deficient mice could alter the composition of the intestinal fungal microbiota, but not the composition of bacterial microbiota, exhibiting an impaired fungicidal ability, which led to increased fungi load and variation in the gut, with a notable increase in C. tropicalis. Fecal microbiota from tumor-bearing Card9−/− mice induced tumors, due to more total fungal burden and a greater proportion of C. tropicalis. Germ-free (GF) mice that received feces from tumor-bearing Card9−/− mice or C. tropicalis developed significantly more tumor burden, compared with those that received feces from tumor-bearing wild-type (WT) mice. These data suggested that the increased C. tropicalis in Card9−/− mice, instead of the lack of Card9 in recipient GF mice, was responsible for CAC development. Furthermore, anti-fungal fluconazole treatment significantly decreased fungal burden and ameliorated CAC in Card9−/− mice.
Figure 1.
CARD9 Function Inhibited CAC Growth through the Activation of Immune Cells
CARD9 showed a strong fungicidal ability, which led to decreased fungi load and MDSC accumulation, increased the frequency of cytotoxic lymphocytes (CTLs), and eventually suppressed CAC development. (A and B) WT (A) and Card9–/– (B) mice.
Next, this study investigated the potential mechanism whereby fungal dysbiosis increased the accumulation of myeloid-derived suppressor cells through modification of gut microbiota. This study emphasized that Card9-deficient macrophages exhibited impaired fungicidal abilities, resulting in a notable increase in C. tropicalis in the gut. The increased C. tropicalis induced the accumulation of intestinal myeloid-derived suppressor cells (MDSCs), eventually suppressed immunosurveillance, and facilitated tumor development.
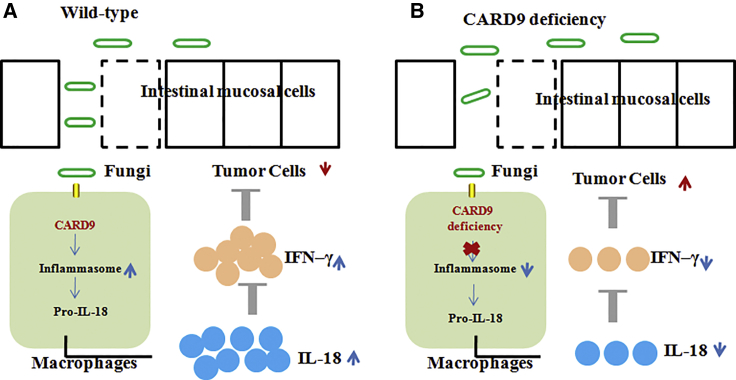
Malik et al.5 reported that CARD9 was a protective factor for CAC (Figure 2). In this study, Card9−/− mice exhibited increased number and size of tumors when compared to the WT mice with AOM-DSS administration. Card9 deletion changed the gut microbial landscape, especially commensal gut fungi. Further, anti-fungal amphotericin B treatment led to a significant decrease in commensal fungi but a distinct change in bacterial landscape. As a result, anti-fungal agents exacerbated colon tumorigenesis in Card9−/− mice, demonstrating an active role for commensal fungi in suppressing CAC. This study outlined a mechanism whereby commensal gut fungi are a critical contributor to inflammasome activation and IL-18 maturation via the CARD9-signaling axis. CARD9 is activated downstream of C-type lectin receptors (CLRs) and required for inflammasome activation during fungal infection. Inflammasomes are multimeric protein complexes, which could activate the cysteine protease caspase-1, leading to the proteolytic processing of IL-18.31 Early IL-18 maturation by inflammasome is known to promote epithelial barrier restitution and stimulate interferon gamma (IFN-γ) production from intestinal CD8+ T cells.32, 33 As expected, mice lacking Card9 with AOM-DSS-induced CAC were significantly defective in inflammasome activation and IL-18 maturation. What is more, after transferring Casp1−/− or WT bone marrow-derived myeloid cells (MCs) into Card9−/− mice, the decreased tumor burden was found in Card9−/− mice given WT MCs rather than Card9−/− mice given Casp1−/− MCs. Exogenous supplementation of IL-18 significantly increased IFN-γ production from T cells and exerted a positive anti-tumor effect. Thus, CARD9 signaling was required to ameliorate CAC in the AOM-DSS mouse model.
Figure 2.
CARD9 Function Inhibited CAC Growth through the Activation of Inflammasomes
Recognition of commensal gut fungi, sensed via a CARD9-dependent manner, promoted inflammasome activation and IL-18 maturation, and eventually it suppressed CAC development. (A and B) WT (A) and Card9–/– (B) mice.
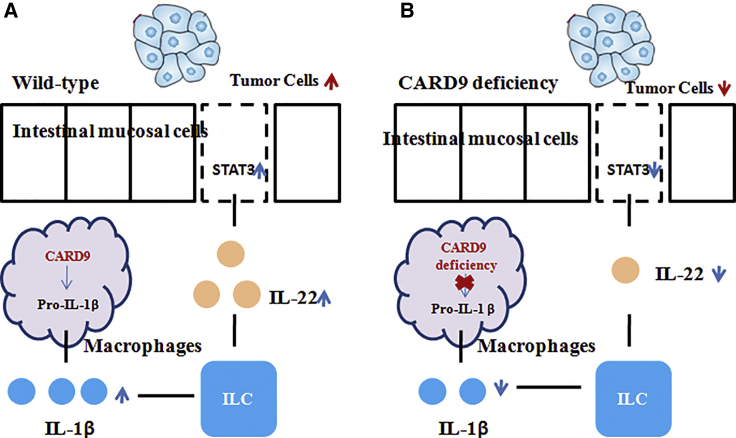
Bergmann et al.6 reported that CARD9 was a harmful factor for CAC (Figure 3). WT mice exhibited increased numbers of tumors in the gut that were also bigger in size when compared to the Card9−/− mice. Further, the pro-tumor function of CARD9-mediated CAC mainly contributed to regulating IL-22 production from intestinal group 3 innate lymphoid cells. It is clear that CARD9 is cooperative with the inflammasomes, which triggers the proteolytic processing of IL-1β.4 IL-1β by myeloid cells generally drives the IL-22-induced STAT3 phosphorylation that provides intestinal immunity.34, 35, 36 In this study, Card9 deletion in mice dramatically impaired IL-1β generation, subsequently controlled the production of IL-22 from group 3 innate lymphoid cells, and eventually blocked the tumor cell intrinsic STAT3 activation. Together, these results accounted for the induced tumor growth and malignant epithelial cell regeneration in WT mice.
Figure 3.
CARD9 Function Promoted CAC Growth through the Regulation of Type 3 Innate Lymphoid Cells
CARD9 specifically upregulated IL-1β production, leading to intrinsic IL-22/STAT3 activation in intestinal epithelial cells (IECs), and eventually it induced CAC development. (A and B) WT (A) and Card9–/– (B) mice.
Intestinal fungi were reported to modulate the development of CAC.5, 7 Compared to the WT mice, Card9 deletion mice exhibited an obvious change of the gut commensal fungal microbiota. Card9−/− mice showed a decreased occurrence of Ascomycota, including Claussenomyces of Leotiomycetes, Agaricomycetes, and Pseudocercospora cordiana of Dothideomycetes, along with a notable increase in Saccharomycetes, particularly Diutina catenulate and Cladosporium of Dothideomycetes.5 Due to the diversity of the gut fungi, it was difficult to define the healthy or diseased-related gut fungi, but increasing evidence suggested that C. tropicalis may contribute to a well-known inducer of CAC.7 Contrary to the above studies, colonic mucosa-associated fungal microbiota in CAC patients did not show any significant difference in comparison to the ones in sporadic cancer or in healthy subjects, dominating by two phyla, Basidiomycota and Ascomycota. As a result, commensal gut fungi maybe were not the potent risk factor for the development of CAC.37
CARD9 in CAC Immunoprevention
CAC is driven by a series of well-defined genetic and epigenetic alterations, and these typically take many years or decades to accumulate. This long development process provides a better opportunity for cancer immunoprevention.38 Immunoprevention is an effective approach to reduce cancer risk based on the stimulation of the immune system before intestinal tumor onset.39 Macrophages have emerged as a major player in CAC prevention, due to the increased appreciation of immunosurveillance, which trigger an anti-tumor immune response against early lesions.40, 41
The role of CARD9 in shaping the intestinal immune response needs to be defined. As reported, CARD9 was found in tumor-infiltrating macrophages, but not in intestinal cancer cells, suggesting that CARD9 was expressed by infiltrating macrophages in intestine tissue.17 It was important to note that CARD9 expression level was a clinical correlation with human colon carcinoma.17 Furthermore, CARD9 represented a vital role during the progression of CAC. The potential molecular mechanisms maybe contribute to sensing the intestinal fungi and regulating the immune cells through intestinal macrophages in a CARD9-dependent manner.5, 6, 7 Thus, the CARD9-signaling molecule in macrophages may inform the development of immunoprevention and immunotherapy strategies for CAC.
Conclusions
To date, the exact function of CARD9 in the development of CAC is still unknown, three aspects of which are particularly controversial. First, it is unclear whether CARD9 protects against CAC or not. One study showed the presence of CARD9 may facilitate CAC tumor progression. This pro-tumor function contributes to a Card9-controlled, IL-1β-mediated STAT3 activation mechanism. Accumulating evidence is defining the STAT3 activation as an important pathway for the transition from inflammation to cancer. However, the other two studies reported that the deficiency of CARD9 may be advantageous to CAC. The anti-tumor function contributes to a CARD9-controlled, anti-fungal immunity in the gastrointestinal environment. Likewise, intestinal immune responses generally link to a critical role in inflammatory-promoting tumor responses.
Second, how CARD9 contributes to inflammasome-mediated cytokine production is unclear. One study revealed that CARD9 exhibited the pathogenic effects of inflammasome-dependent IL-1β generation, but not inflammasome-mediated cytokine IL-18 in Card9−/− mice, resulting in a promoting role in CAC. Conversely, one study revealed that CARD9 induced inflammasome-mediated IL-18 production (not IL-1β data), ameliorating the development of CAC. As previously reported, NLRP3 inflammasome, the most intensely studied inflammasome, is triggered in a CARD9-dependent manner, which simultaneously activates the proteolytic processing of inflammatory cytokines IL-1β and IL-18. Thereby, it is confusing how CARD9-dependent inflammasome proteolytically activates IL-1β and IL-18.
Third, it is unclear whether gut commensal fungi are beneficial to CAC in Card9−/− mice or not. In a study, Amphotericin B treatment increased the colon tumorigenesis upon AOM-DSS administration, at least in part, by reducing exogenous IL-18 secretion through the inhibition of commensal fungi in the gut. In the other study, Amphotericin B treatment significantly ameliorated colitis-associated colon cancer, by controlling MDSC differentiation through modification of intestinal fungi. The molecular mechanism and biological function of gut commensal fungi require further exploration.
Author Contributions
X.Z., B.C., M.L., and Z.Y. co-wrote the manuscript.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
The authors are grateful to the National Natural Science Foundation of China (81960452).
References
- 1.Bye W.A., Ma C., Nguyen T.M., Parker C.E., Jairath V., East J.E. Strategies for Detecting Colorectal Cancer in Patients with Inflammatory Bowel Disease: A Cochrane Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2018;113:1801–1809. doi: 10.1038/s41395-018-0354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ritter B., Greten F.R. Modulating inflammation for cancer therapy. J. Exp. Med. 2019;216:1234–1243. doi: 10.1084/jem.20181739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fan Y., Mao R., Yang J. NF-κB and STAT3 signaling pathways collaboratively link inflammation to cancer. Protein Cell. 2013;4:176–185. doi: 10.1007/s13238-013-2084-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhong X., Chen B., Yang L., Yang Z. Molecular and physiological roles of the adaptor protein CARD9 in immunity. Cell Death Dis. 2018;9:52. doi: 10.1038/s41419-017-0084-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malik A., Sharma D., Malireddi R.K.S., Guy C.S., Chang T.C., Olsen S.R., Neale G., Vogel P., Kanneganti T.D. SYK-CARD9 Signaling Axis Promotes Gut Fungi-Mediated Inflammasome Activation to Restrict Colitis and Colon Cancer. Immunity. 2018;49:515–530.e5. doi: 10.1016/j.immuni.2018.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergmann H., Roth S., Pechloff K., Kiss E.A., Kuhn S., Heikenwälder M., Diefenbach A., Greten F.R., Ruland J. Card9-dependent IL-1β regulates IL-22 production from group 3 innate lymphoid cells and promotes colitis-associated cancer. Eur. J. Immunol. 2017;47:1342–1353. doi: 10.1002/eji.201646765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang T., Fan C., Yao A., Xu X., Zheng G., You Y., Jiang C., Zhao X., Hou Y., Hung M.C., Lin X. The Adaptor Protein CARD9 Protects against Colon Cancer by Restricting Mycobiota-Mediated Expansion of Myeloid-Derived Suppressor Cells. Immunity. 2018;49:504–514.e4. doi: 10.1016/j.immuni.2018.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhernakova A., Festen E.M., Franke L., Trynka G., van Diemen C.C., Monsuur A.J., Bevova M., Nijmeijer R.M., van ’t Slot R., Heijmans R. Genetic analysis of innate immunity in Crohn’s disease and ulcerative colitis identifies two susceptibility loci harboring CARD9 and IL18RAP. Am. J. Hum. Genet. 2008;82:1202–1210. doi: 10.1016/j.ajhg.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao Z., Conway K.L., Heath R.J., Rush J.S., Leshchiner E.S., Ramirez-Ortiz Z.G., Nedelsky N.B., Huang H., Ng A., Gardet A. Ubiquitin Ligase TRIM62 Regulates CARD9-Mediated Anti-fungal Immunity and Intestinal Inflammation. Immunity. 2015;43:715–726. doi: 10.1016/j.immuni.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong S.N., Park C., Park S.J., Lee C.K., Ye B.D., Kim Y.S., Lee S., Chae J., Kim J.I., Kim Y.H., IBD Study Group of the Korean Association for the Study of Intestinal Diseases (KASID) Deep resequencing of 131 Crohn’s disease associated genes in pooled DNA confirmed three reported variants and identified eight novel variants. Gut. 2016;65:788–796. doi: 10.1136/gutjnl-2014-308617. [DOI] [PubMed] [Google Scholar]
- 11.Beaudoin M., Goyette P., Boucher G., Lo K.S., Rivas M.A., Stevens C., Alikashani A., Ladouceur M., Ellinghaus D., Törkvist L., Quebec IBD Genetics Consortium. NIDDK IBD Genetics Consortium. International IBD Genetics Consortium Deep resequencing of GWAS loci identifies rare variants in CARD9, IL23R and RNF186 that are associated with ulcerative colitis. PLoS Genet. 2013;9:e1003723. doi: 10.1371/journal.pgen.1003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sokol H., Conway K.L., Zhang M., Choi M., Morin B., Cao Z., Villablanca E.J., Li C., Wijmenga C., Yun S.H. Card9 mediates intestinal epithelial cell restitution, T-helper 17 responses, and control of bacterial infection in mice. Gastroenterology. 2013;145:591–601.e3. doi: 10.1053/j.gastro.2013.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu W., He C., Liu C., Cao A.T., Xue X., Evans-Marin H.L., Sun M., Fang L., Yao S., Pinchuk I.V. miR-10a inhibits dendritic cell activation and Th1/Th17 cell immune responses in IBD. Gut. 2015;64:1755–1764. doi: 10.1136/gutjnl-2014-307980. [DOI] [PubMed] [Google Scholar]
- 14.Lamas B., Richard M.L., Leducq V., Pham H.P., Michel M.L., Da Costa G., Bridonneau C., Jegou S., Hoffmann T.W., Natividad J.M. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat. Med. 2016;22:598–605. doi: 10.1038/nm.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zelante T., Iannitti R.G., Cunha C., De Luca A., Giovannini G., Pieraccini G., Zecchi R., D’Angelo C., Massi-Benedetti C., Fallarino F. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39:372–385. doi: 10.1016/j.immuni.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Zhong X., Chen B., Yang L., Yang Z. Card9 as a critical regulator of tumor development. Cancer Lett. 2019;451:150–155. doi: 10.1016/j.canlet.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Yang M., Shao J.H., Miao Y.J., Cui W., Qi Y.F., Han J.H., Lin X., Du J. Tumor cell-activated CARD9 signaling contributes to metastasis-associated macrophage polarization. Cell Death Differ. 2014;21:1290–1302. doi: 10.1038/cdd.2014.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomita S., Kikuti Y.Y., Carreras J., Kojima M., Ando K., Takasaki H., Sakai R., Takata K., Yoshino T., Bea S. Genomic and immunohistochemical profiles of enteropathy-associated T-cell lymphoma in Japan. Mod. Pathol. 2015;28:1286–1296. doi: 10.1038/modpathol.2015.85. [DOI] [PubMed] [Google Scholar]
- 19.Deleeuw R.J., Zettl A., Klinker E., Haralambieva E., Trottier M., Chari R., Ge Y., Gascoyne R.D., Chott A., Müller-Hermelink H.K., Lam W.L. Whole-genome analysis and HLA genotyping of enteropathy-type T-cell lymphoma reveals 2 distinct lymphoma subtypes. Gastroenterology. 2007;132:1902–1911. doi: 10.1053/j.gastro.2007.03.036. [DOI] [PubMed] [Google Scholar]
- 20.Leo V.I., Tan S.H., Bergmann H., Cheah P.Y., Chew M.H., Lim K.H., Ruland J., Reilly P.T. CARD9 Promotes Sex-Biased Colon Tumors in the APCmin Mouse Model. Cancer Immunol. Res. 2015;3:721–726. doi: 10.1158/2326-6066.CIR-14-0148. [DOI] [PubMed] [Google Scholar]
- 21.Mehl K.A., Davis J.M., Clements J.M., Berger F.G., Pena M.M., Carson J.A. Decreased intestinal polyp multiplicity is related to exercise mode and gender in ApcMin/+ mice. J. Appl. Physiol. 2005;98:2219–2225. doi: 10.1152/japplphysiol.00975.2004. [DOI] [PubMed] [Google Scholar]
- 22.Reynders N., Abboud D., Baragli A., Noman M.Z., Rogister B., Niclou S.P., Heveker N., Janji B., Hanson J., Szpakowska M., Chevigné A. The Distinct Roles of CXCR3 Variants and Their Ligands in the Tumor Microenvironment. Cells. 2019;8:E613. doi: 10.3390/cells8060613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koliaraki V., Pasparakis M., Kollias G. IKKβ in intestinal mesenchymal cells promotes initiation of colitis-associated cancer. J. Exp. Med. 2015;212:2235–2251. doi: 10.1084/jem.20150542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pallangyo C.K., Ziegler P.K., Greten F.R. IKKβ acts as a tumor suppressor in cancer-associated fibroblasts during intestinal tumorigenesis. J. Exp. Med. 2015;212:2253–2266. doi: 10.1084/jem.20150576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ye X., Wu H., Sheng L., Liu Y.X., Ye F., Wang M., Zhou H., Su Y., Zhang X.K. Oncogenic potential of truncated RXRα during colitis-associated colorectal tumorigenesis by promoting IL-6-STAT3 signaling. Nat. Commun. 2019;10:1463. doi: 10.1038/s41467-019-09375-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wunderlich C.M., Ackermann P.J., Ostermann A.L., Adams-Quack P., Vogt M.C., Tran M.L., Nikolajev A., Waisman A., Garbers C., Theurich S. Obesity exacerbates colitis-associated cancer via IL-6-regulated macrophage polarisation and CCL-20/CCR-6-mediated lymphocyte recruitment. Nat. Commun. 2018;9:1646. doi: 10.1038/s41467-018-03773-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu Z.S., Zhang H.X., Li W.W., Ran Y., Liu T.T., Xiong M.G., Li Q.L., Wang S.Y., Wu M., Shu H.B. FAM64A positively regulates STAT3 activity to promote Th17 differentiation and colitis-associated carcinogenesis. Proc. Natl. Acad. Sci. USA. 2019;116:10447–10452. doi: 10.1073/pnas.1814336116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang R.J., Shen S.N., Zhao X.Y., Nie Y.Z., Xu Y.J., Ren J., Lv M.M., Hou Y.Y., Wang T.T. Mesenchymal stem cells-regulated Treg cells suppress colitis-associated colorectal cancer. Stem Cell Res. Ther. 2015;6:71. doi: 10.1186/s13287-015-0055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi C., Yang Y., Xia Y., Okugawa Y., Yang J., Liang Y., Chen H., Zhang P., Wang F., Han H. Novel evidence for an oncogenic role of microRNA-21 in colitis-associated colorectal cancer. Gut. 2016;65:1470–1481. doi: 10.1136/gutjnl-2014-308455. [DOI] [PubMed] [Google Scholar]
- 30.Bhattacharya N., Yuan R., Prestwood T.R., Penny H.L., DiMaio M.A., Reticker-Flynn N.E., Krois C.R., Kenkel J.A., Pham T.D., Carmi Y. Normalizing Microbiota-Induced Retinoic Acid Deficiency Stimulates Protective CD8(+) T Cell-Mediated Immunity in Colorectal Cancer. Immunity. 2016;45:641–655. doi: 10.1016/j.immuni.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gyongyosi B., Cho Y., Lowe P., Calenda C.D., Iracheta-Vellve A., Satishchandran A., Ambade A., Szabo G. Alcohol-induced IL-17A production in Paneth cells amplifies endoplasmic reticulum stress, apoptosis, and inflammasome-IL-18 activation in the proximal small intestine in mice. Mucosal Immunol. 2019;12:930–944. doi: 10.1038/s41385-019-0170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakanishi K. Unique Action of Interleukin-18 on T Cells and Other Immune Cells. Front. Immunol. 2018;9:763. doi: 10.3389/fimmu.2018.00763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li J., Qiu G., Fang B., Dai X., Cai J. Deficiency of IL-18 Aggravates Esophageal Carcinoma Through Inhibiting IFN-γ Production by CD8+T Cells and NK Cells. Inflammation. 2018;41:667–676. doi: 10.1007/s10753-017-0721-3. [DOI] [PubMed] [Google Scholar]
- 34.Coorens M., Rao A., Gräfe S.K., Unelius D., Lindforss U., Agerberth B., Mjösberg J., Bergman P. Innate lymphoid cell type 3-derived interleukin-22 boosts lipocalin-2 production in intestinal epithelial cells via synergy between STAT3 and NF-κB. J. Biol. Chem. 2019;294:6027–6041. doi: 10.1074/jbc.RA118.007290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ziesché E., Bachmann M., Kleinert H., Pfeilschifter J., Mühl H. The interleukin-22/STAT3 pathway potentiates expression of inducible nitric-oxide synthase in human colon carcinoma cells. J. Biol. Chem. 2007;282:16006–16015. doi: 10.1074/jbc.M611040200. [DOI] [PubMed] [Google Scholar]
- 36.Voigt C., May P., Gottschlich A., Markota A., Wenk D., Gerlach I., Voigt S., Stathopoulos G.T., Arendt K.A.M., Heise C. Cancer cells induce interleukin-22 production from memory CD4+ T cells via interleukin-1 to promote tumor growth. Proc. Natl. Acad. Sci. USA. 2017;114:12994–12999. doi: 10.1073/pnas.1705165114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richard M.L., Liguori G., Lamas B., Brandi G., da Costa G., Hoffmann T.W., Pierluigi Di Simone M., Calabrese C., Poggioli G., Langella P. Mucosa-associated microbiota dysbiosis in colitis associated cancer. Gut Microbes. 2018;9:131–142. doi: 10.1080/19490976.2017.1379637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song M., Nishihara R., Wang M., Chan A.T., Qian Z.R., Inamura K., Zhang X., Ng K., Kim S.A., Mima K. Plasma 25-hydroxyvitamin D and colorectal cancer risk according to tumour immunity status. Gut. 2016;65:296–304. doi: 10.1136/gutjnl-2014-308852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chu N.J., Armstrong T.D., Jaffee E.M. Nonviral oncogenic antigens and the inflammatory signals driving early cancer development as targets for cancer immunoprevention. Clin. Cancer Res. 2015;21:1549–1557. doi: 10.1158/1078-0432.CCR-14-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Finn O.J., Beatty P.L. Cancer immunoprevention. Curr. Opin. Immunol. 2016;39:52–58. doi: 10.1016/j.coi.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh K., Coburn L.A., Asim M., Barry D.P., Allaman M.M., Shi C., Washington M.K., Luis P.B., Schneider C., Delgado A.G. Ornithine Decarboxylase in Macrophages Exacerbates Colitis and Promotes Colitis-Associated Colon Carcinogenesis by Impairing M1 Immune Responses. Cancer Res. 2018;78:4303–4315. doi: 10.1158/0008-5472.CAN-18-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]