Abstract
Managing the dysregulated host response to infection remains a major challenge in sepsis care. Chinese treatment guideline recommends adding XueBiJing, a five-herb medicine, to antibiotic-based sepsis care. Although adding XueBiJing further reduced 28-day mortality via modulating the host response, pharmacokinetic herb–drug interaction is a widely recognized issue that needs to be studied. Building on our earlier systematic chemical and human pharmacokinetic investigations of XueBiJing, we evaluated the degree of pharmacokinetic compatibility for XueBiJing/antibiotic combination based on mechanistic evidence of interaction risk. Considering both XueBiJing‒antibiotic and antibiotic‒XueBiJing interaction potential, we integrated informatics-based approach with experimental approach and developed a compound pair-based method for data processing. To reflect clinical reality, we selected for study XueBiJing compounds bioavailable for drug interactions and 45 antibiotics commonly used in sepsis care in China. Based on the data of interacting with drug metabolizing enzymes and transporters, no XueBiJing compound could pair, as perpetrator, with the antibiotics. Although some antibiotics could, due to their inhibition of uridine 5′-diphosphoglucuronosyltransferase 2B15, organic anion transporters 1/2 and/or organic anion-transporting polypeptide 1B3, pair with senkyunolide I, tanshinol and salvianolic acid B, the potential interactions (resulting in increased exposure) are likely desirable due to these XueBiJing compounds' low baseline exposure levels. Inhibition of aldehyde dehydrogenase by 7 antibiotics probably results in undesirable reduction of exposure to protocatechuic acid from XueBiJing. Collectively, XueBiJing/antibiotic combination exhibited a high degree of pharmacokinetic compatibility at clinically relevant doses. The methodology developed can be applied to investigate other drug combinations.
Key Words: XueBiJing, Antibiotic, Combination drug therapy, Sepsis, Pharmacokinetic compatibility, Herb‒drug interaction
Abbreviations: ABC transporter, ATP-binding cassette transporter; ADR, adverse drug reaction; ALDH, aldehyde dehydrogenase; AMP, adenosine monophosphate; AQ, amodiaquine; ATP, adenosine triphosphate; BCRP, breast cancer resistance protein; BSEP, bile salt export pump; Cmax, maximum plasma concentration; CLR, renal clearance; CLtot,p, total plasma clearance; COMT, catechol-O-methyltransferase; DDI, drug‒drug interaction; DEAQ, desethylamodiaquine; E2, β-estradiol; E23βG, β-estradiol-3-β-d-glucuronide; E217βG, estradiol-17β-d-glucuronide; fe-U, fraction of dose excreted unchanged into urine; fu-p, unbound fraction in plasma; GF, glomerular filtration; GFR, glomerular filtration rate; HEK-293, human embryonic kidney 293 cell line; IC50, half-maximal inhibitory concentration; Km, Michaelis constant; MATE, multidrug and toxin extrusion protein; MDR1, multidrug resistance transporter 1; MRP, multidrug resistance protein; 4-MU, 4-methylumbelliferone; 4-MUG, 4-methylumbelliferyl-β-d-glucuronide; NAD+, nicotinamide adenine dinucleotide; OAT, organic anion transporter; OATP, organic anion-transporting polypeptide; OCT, organic cation transporter; PAH, para-aminohippuric acid; PK, pharmacokinetic; PKC, pharmacokinetic compatibility; SLC transporter, solute carrier transporter; t1/2, elimination half-life; TEA, tetraethylammonium; TFP, trifluoperazine; TFPG, trifluoperazine-N-β-d-glucuronide; TS, tubular secretion; UGT, uridine 5′-diphosphoglucuronosyltransferases; VSS, apparent volume of distribution at steady state
Graphical abstract
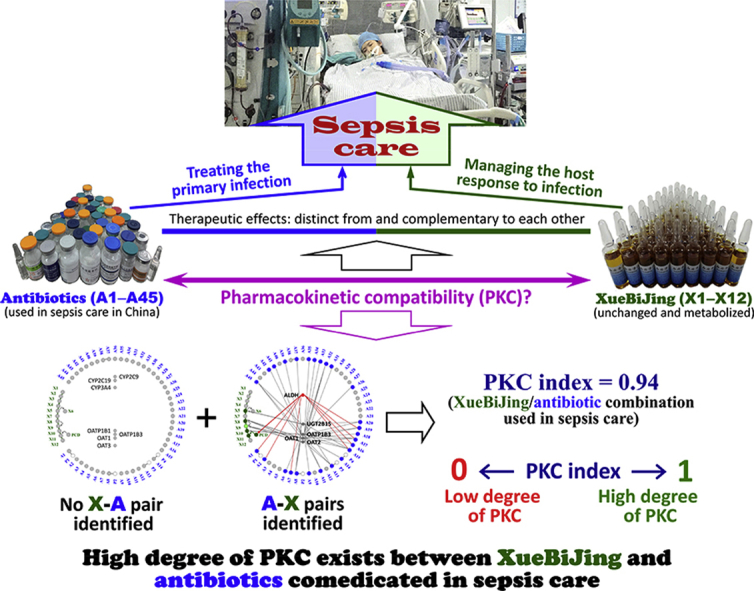
Managing the dysregulated host response to infection remains a major challenge in sepsis care. Chinese treatment guideline recommends adding XueBiJing, an intravenous five-herb injection, to antibiotic-based sepsis care. XueBiJing/antibiotic combination exhibited a high degree of pharmacokinetic compatibility at clinically relevant doses; this supports their concurrent use in sepsis care.
1. Introduction
Drugs in a combination drug therapy, particularly for multifactorial diseases, can have distinct mechanisms of action and exert enhanced pharmacodynamic effect1, 2, 3. To ensure such therapeutic benefit, a high degree of pharmacokinetic compatibility (PKC) is desired among the co-administered drugs; PKC is defined as absence of unintentional or unmanageable pharmacokinetic (PK) drug interaction that can lead to decreased drug efficacy or increased drug toxicity.
Sepsis is life-threatening organ dysfunction caused by a dysregulated host response to infection4. Because infection caused by pathogens is the triggering event in sepsis, prompt initiation of appropriate antibiotic therapies to eradicate the pathogens is a cornerstone of sepsis care5. However, even after successful treatment of the primary infection, the host response often remains dysregulated, and organ dysfunction and unwanted clinical outcome may occur. Hence, attenuating the host response is also important. Although substantial developments have been made in understanding the pathophysiology of the host response, which is characterized by overabundant innate immune, uncontrolled release of inflammatory mediators, inefficient use of the complement system, coagulation abnormalities, endothelial capillary leakage syndrome, immunosuppression and organ dysfunction6, 7, the search for pharmacotherapies to modulate the host response has been unsuccessful8, 9.
XueBiJing is the only medicine approved (2004) by the Chinese National Medical Products Administration (NMPA, formerly China Food and Drug Administration) specifically for treatment of sepsis and multiple organ dysfunction syndrome. It is a standardized intravenous injection, which is prepared from a five-herb combination, comprising Carthamus tinctorius flowers (Honghua in Chinese), Paeonia lactiflora roots (Chishao), Ligusticum chuanxiong rhizomes (Chuanxiong), Angelica sinensis roots (Danggui) and Salvia miltiorrhiza roots (Danshen); the manufacturing procedure for XueBiJing is described in Supporting Information. Chinese treatment guideline for sepsis care and expert consensuses on sepsis care recommend adding XueBiJing to antibiotic-based sepsis care10, 11, 12. It has been shown that adding XueBiJing to the conventional sepsis care further reduces patients' 28-day mortality and incidence of complications, improves their APCHE II scores and prognosis and shortens their stay in the ICU, with low incidence of side effects13, 14, 15, 16, 17. Clinical and experimental studies suggest that XueBiJing could inhibit the uncontrolled release of inflammatory mediators, relieve an early overabundant innate immune response and potentially cumulative immunosuppression, attenuate the crosstalk between inflammation and coagulation, protect endothelial cells and maintain physiological functions of vital organs18, 19, 20, 21, 22, 23, 24, 25. These effects are distinct from and complementary to the antibiotic therapy. Despite extensive use of XueBiJing in sepsis care (over 600,000 patients yearly in China), report on drug interaction with this herbal medicine is scarce; this may be attributed to under-recognition, under-reporting and under-researching of such interactions. Many antibiotics have high potential for drug interactions, particularly in critically ill patients who are predisposed to drug interactions due to multi-drug usage and who often present with organ dysfunction and multiple comorbidities26, 27, 28, 29, 30. Given these factors, as well as complex chemical composition of XueBiJing and its many bioactive constituents present31, 32, 33, 34, 35, it is important to investigate systematically the degree of PKC between XueBiJing and antibiotics, for the clinical success of the combination therapy.
Based on our earlier systematic chemical and PK investigations of XueBiJing33, 34, 35, a total of 12 unchanged and metabolized XueBiJing compounds (Supporting Information Fig. S1), from 104 constituents present in the injection (Supporting Information Table S1), are identified as bioavailable for drug interactions; their human PK and disposition data are summarized in Table 136, 37, 38, 39, 40, 41. In addition, 38 other XueBiJing compounds were also detected in systemic circulation, but may not be important for drug interaction due to their very low levels [maximum total (bound plus unbound) plasma concentration (total Cmax), <0.01 μmol/L]. These earlier studies enable us to investigate the degree of PKC between complex XueBiJing [with focus on the 12 herbal compounds, which exhibit various biological activities related to the therapeutic action (Supporting Information Table S2)] and various antibiotics used in sepsis care in China, based on mechanistic evidence of interaction risk. This study aimed to provide scientific evidence to guide clinical decision for XueBiJing/antibiotic combination in sepsis care. The study methodology developed here can be applied to investigate other drug combinations in management of multifactorial diseases.
Table 1.
Human pharmacokinetics of XueBiJing compounds, bioavailable for drug interaction, from the herbal injection (at 100 mL via 75-min intravenous infusion) and their interactions with drug metabolizing enzymes and transporters.
| XueBiJing compound (ID) | PK parameter |
Major elimination route | Related metabolism and transport data | LogD(7.4) [pKa] | Membrane permeability | ||||
|---|---|---|---|---|---|---|---|---|---|
| Cmax (μmol/L) | fu-p (%) | t1/2 (h) | VSS (L/kg) | CLtot,p (L/h/kg) | |||||
| Hydroxysafflor yellow A (X1, originating from Honghua)a | 3.8 | 30 | 4.0 | 0.34 | 0.08 | Renal excretion, GF-based, fe-U, 51% CLR/(GFR × fu-p), 1.2 |
Not the substrate of P450 enzymes, UGTs, SULTs, OATP1B1/1B3, OAT1/3, OCT2, MATE1/2-K, MDR1, or BCRP | −2.4 [4.5] | Poor |
| Paeoniflorin (X2, originating from Chishao)33 | 18.2 | 82 | 1.1 | 0.19 | 0.11 | Renal excretion, GF-based, fe-U, 63% CLR/(GFR × fu-p), 0.8 |
0.1 [11.5] | Poor | |
| Oxypaeoniflorin (X3, originating from Chishao)33 | 0.6 | 80 | 0.7 | 0.16 | 0.17 | Renal excretion, GF-based, fe-U, 84% CLR/(GFR × fu-p), 1.6 |
−0.2 [8.2] | Poor | |
| Albiflorin (X4, originating from Chishao)33 | 0.8 | 83 | 0.7 | 0.08 | 0.12 | Renal excretion, GF-based, fe-U, 62% CLR/(GFR × fu-p), 0.8 |
−0.9 [12.8] | Poor | |
| Senkyunolide I (X5, originating from Chuanxiong/Danggui)35 | 0.4 | 54 | 0.8 | 1.26 | 0.75 | Hepatic glucuronidation, to form senkyunolide I-7-O-β-glucuronide (X6) (This results in formation of a glutathione conjugate, suggesting formation of electrophilic metabolite.) |
Highly selective UGT2B15 substrate (Km, 18.4 μmol/L) | 0.8 [12.8] | Good |
| Senkyunolide G (X7, originating from Chuanxiong/Danggui)35 | 0.5 | 3 | 2.3 | 0.10 | 0.03 | Hepatic glucuronidation | Poor UGT substrate | 1.5 [10.7] | Good |
| Tanshinol (X8, originating from Danshen)34, 36, 37, 38 | 0.1 | 99 | 0.7 | 1.13 | 1.84 | Renal excretion, TS&GF-based, fe-U, 59% CLR/(GFR × fu-p), 10.0 Hepatic methylation, to form 3-O-methyltanshinol (X9) |
OAT1/2/3 substrate (Km, 121/859/1888 μmol/L) COMT substrate |
−3.3 [3.8] | Poor |
| 3-O-Methyltanshinol (X9, metabolite of X8)34, 36 | 0.01 | – | – | – | – | Renal excretion, TS&GF-based | OAT1/2/3 substrate (Km, 219/2854/871 μmol/L) | −3.3 [3.8] | Good |
| Salvianolic acid B (X10, originating from Danshen)34, 38 | 0.1 | 3 | 0.2 | 1.28 | 1.02 | Hepatobiliary excretion Hepatic methylation |
Highly selective OATP1B3 substrate (Km, 14.0 μmol/L)a COMT substrate |
−2.8 [2.8] | Poor |
| Protocatechuic acid [X11, the oxidized metabolite of protocatechuic aldehyde (PCD), originating from Danshen]34, 38 | 0.04 | 79 | 0.5 | – | – | Hepatic methylation and sulfation | PCD is metabolized by ALDH and COMT. Impaired ALDH activity can be compensated by COMT, but could result in decreased systemic exposure to X11a. | −1.9 [4.5] | Intermediate |
| Ferulic acid (X12, originating from Honghua/Chuanxiong/Danggui/Danshen)39, 40 | 0.12 | 70 | 0.7 | 0.93 | 3.02 | Renal excretion, fe-U, 4%–5% CLR/(GFR × fu-p), 1.8 Hepatic glucuronidation and sulfation |
UGT1A1/1A3/1A6/1A7/1A8/1A9/1A10/2B7 substrate (Km, 158/13000/5870/6150/4170/2470/1040/6320 μmol/L) SULT1A1/1E1 substrate (Km, 13.3/30.4 μmol/L) |
−1.4 [4.6] | Intermediate |
XueBiJing is prepared from a five-herb combination comprising Honghua (in Chinese; Carthamus tinctorius flowers), Chishao (Paeonia lactiflora roots), Chuanxiong (Ligusticum chuanxiong rhizomes), Danggui (Angelica sinensis roots) and Danshen (Salvia miltiorrhiza roots).
The data are pending publication elsewhere. Cmax, maximum plasma concentration; fu-p, unbound fraction in plasma; t1/2, elimination half-life; VSS, apparent volume of distribution at steady state; CLtot,p, total plasma clearance; fe-U, fraction of dose excreted unchanged into urine; CLR, renal clearance; GFR, glomerular filtration rate [115 ± 24 mL/min41]; TS, tubular secretion; GF, glomerular filtration; Km, Michaelis constant; UGT, uridine 5′-diphosphoglucuronosyltransferase; SULT, sulfotransferase; ALDH, aldehyde dehydrogenase; COMT, catechol-O-methyltransferase; OAT, organic anion transporter; OATP, organic anion-transporting polypeptide; OCT, organic cation transporter; MATE, multidrug and toxin extrusion protein; MDR1, multidrug resistance protein 1; BCRP, breast cancer resistance protein; LogD(7.4), logarithm of the distribution coefficients at pH 7.4; pKa, acid dissociation constants.
2. Materials and methods
A detailed description of materials and experimental procedures is provided in the Supporting Information Materials and methods.
2.1. Study design
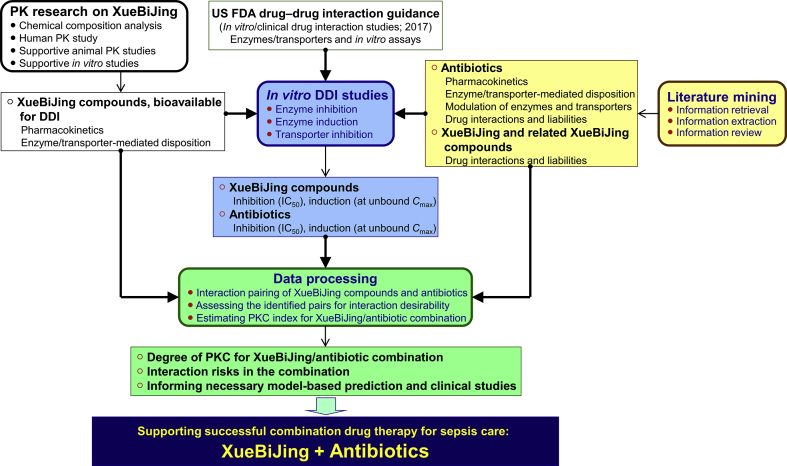
This study was designed to provide comprehensive insight into the degree of PKC between the five-herb medicine XueBiJing and various antibiotics used in sepsis care in China, based on mechanistic evidence of drug interaction risk. Evaluating PKC degree, considering both XueBiJing‒antibiotic (XueBiJing as perpetrator and the antibiotic as victim) and antibiotic‒XueBiJing (the antibiotic as perpetrator and XueBiJing as victim) interaction potential, requires information on the pharmacokinetics/disposition of XueBiJing and antibiotics and their related drug interaction liabilities. A data processing method is also needed to estimate the degree of PKC and to identify interaction risk in the combination. Data on human pharmacokinetics of compounds, bioavailable for drug interactions, from dosed XueBiJing were obtained from our earlier research on the herbal medicine. Literature mining was performed to identify antibiotics used for sepsis care in China and to obtain information regarding their pharmacokinetics/disposition and drug interaction liabilities, as well as information on interaction liabilities of XueBiJing and related herbal compounds. Due to literature-mined information alone being insufficient, in vitro studies were performed to obtain additional required information on interactions of the XueBiJing compounds, and of the identified antibiotics, with relevant drug metabolizing enzymes and transporters. Data processing was then performed in steps, i.e., pairing the compounds, assessing the desirability of the pair-associated interactions and estimating PKC index for the combination. Low interaction potential indicated by static-mechanistic-model-based investigation usually correlates with low clinical interaction potential, but high interaction potential indicated by such investigation does not necessarily translate to high clinical interaction potential. To this end, only the identified undesirable interactions with high potential would be further investigated by performing dynamic-model-based prediction and, if necessary, relevant clinical studies. Fig. 1 summarizes the study workflow.
Figure 1.
An overview of approach to evaluating PKC between XueBiJing and antibiotics in sepsis care. PK, pharmacokinetic; DDI, drug–drug interaction; IC50, half-maximal inhibitory concentration; Cmax, maximum plasma concentration; PKC, pharmacokinetic compatibility.
2.2. Literature mining
Literature mining, comprising retrieval, extraction and review of information, was conducted to obtain three types of information: (1) antibiotics commonly used in management of sepsis and septic shock in China, (2) human pharmacokinetics/disposition of these antibiotics (particularly in critically ill patients), their interactions with drug metabolizing enzymes or transporters, and clinical drug interactions and (3) drug interactions with XueBiJing and related herbal compounds.
2.3. Materials
Hydroxysafflor yellow A (X1), paeoniflorin (X2), oxypaeoniflorin (X3), albiflorin (X4), senkyunolides I (X5) and G (X7), tanshinol (X8), salvianolic acid B (X10), protocatechuic acid (X11), ferulic acid (X12) and 45 antibiotics (A1–A45) were obtained commercially. Senkyunolide I-7-O-β-glucuronide (X6) and 3-O-methyltanshinol (X9) were prepared in-house using the method described previously35, 36. Purity of the compounds was ≥98%.
cDNA-expressed human P450 enzymes, uridine 5′-diphosphoglucuronosyltransferases (UGT), pooled human liver microsomes and pooled human liver cytosols were obtained from Corning Gentest (Woburn, MA, USA). Cryopreserved primary human hepatocytes from four donors XSM, HVN, DQB and OMA (Supporting Information Table S3) were obtained from BioreclamationIVT (Baltimore, MD, USA) and human embryonic kidney 293 (HEK-293) cells were obtained from American Type Culture Collection (Manassas, VA, USA). Human solute carrier (SLC) transporter expression plasmids were constructed commercially. Inside-out membrane vesicles [prepared from insect cells expressing human ATP-binding cassette (ABC) transporters] were obtained commercially. Probe substrates and positive inhibitors of test enzymes and transporters and positive inducers of P450 enzymes were also obtained commercially.
2.4. In vitro assessment of P450 and UGT inhibition by XueBiJing compounds
Inhibition of the human enzymes by XueBiJing compounds (X1–X12) and MATI [a mixture of X1–X12 at concentrations similar to their unbound Cmax in humans after terminating the infusion of XueBiJing] was assessed using cDNA-expressed enzymes as described previously42. Half-maximal inhibitory concentration (IC50) was determined for those XueBiJing compounds (100 μmol/L) demonstrating >50% inhibition. Metabolism-dependent inhibition was examined using a single point inactivation method43, 44.
2.5. In vitro assessment of P450 induction by XueBiJing compounds
Induction of CYP1A2, CYP2B6 and CYP3A by XueBiJing compounds (X1–X12) and MATI was assessed using cryopreserved human hepatocytes from three donors XSM, HVN and DQB. Compounds exhibiting positive induction in the enzyme activity determinations were further assessed with respect to cellular enzyme mRNA levels.
2.6. In vitro assessment of drug transporter inhibition by XueBiJing compounds
Inhibition of the SLC transporters organic anion-transporting polypeptide (OATP) 1B1/1B3, organic anion transporter (OAT) 1/2/3, organic cation transporter (OCT) 2 and multidrug and toxin extrusion protein (MATE) 1 by XueBiJing compounds (X1–X12) and MATI was assessed using HEK293 cells expressing the transporters as described previously37, 45. IC50 was determined for those XueBiJing compounds (100 μmol/L) demonstrating > 50% inhibition.
Inhibition of the ABC transporters multidrug resistance transporter (MDR) 1 and breast cancer resistance protein (BCRP) by XueBiJing compounds (X1–X12) and MATI was assessed using inside-out membrane vesicles expressing the transporters as described previously37, 45. IC50 was determined for those XueBiJing compounds (100 μmol/L) demonstrating > 50% inhibition.
2.7. In vitro assessment of UGT2B15 and aldehyde dehydrogenase (ALDH) inhibition by antibiotics
Inhibition of UGT2B15 by antibiotics (A1–A45) was assessed using senkyunolides I as substrate and the cDNA-expressed UGT2B15 as described previously42. IC50 was determined for those antibiotics (initial test concentration, 100–5000 μmol/L) demonstrating > 50% inhibition.
Inhibition of ALDH by antibiotics (A1–A45) was assessed using protocatechuic aldehyde as substrate and human liver cytosol as described previously38. IC50 was determined for those antibiotics (initial test concentration, 100–5000 μmol/L) demonstrating > 50% inhibition.
2.8. In vitro assessment of OAT1/2 and OATP1B3 inhibition by antibiotics
Inhibition of OAT1/2 and OATP1B3 by antibiotics (A1–A45) was assessed using tanshinol and salvianolic acid B as substrates, respectively, and HEK293 cells expressing the transporters. IC50 was determined for those antibiotics (initial test concentration, 100–5000 μmol/L) demonstrating > 50% inhibition.
2.9. Bioanalytical assays
Validated liquid chromatography/mass spectrometry-based bioanalytical methods were used to determine the quantity of the metabolites formed and the probe substrates in samples.
2.10. Estimation of drug–drug interaction indices
Inhibitory drug–drug interaction index (DDI index) was estimated using Eq. (1):
| DDI index = (Cmax × fu-p × R)/IC50 | (1) |
where Cmax is the maximum plasma concentration of the perpetrator drug in μmol/L after dosing in critically ill patients or healthy subjects (if the patient concentration is not available), fu-p is its unbound fraction in human plasma, R is its accumulative factor and IC50 is its half-maximal inhibitory concentration. GraFit software (version 5.0; Erithacus Software, Surrey, UK) was used to determine the IC50 values.
2.11. Data processing
To evaluate PKC between XueBiJing and antibiotics in sepsis care, data processing was performed in three steps. First, compound pairs exhibiting XueBiJing‒antibiotic or antibiotic‒XueBiJing interaction potential were identified, based on literature-mined and experimental data obtained from this study and on data from our earlier PK research on XueBiJing. Factors influencing the potential of a compound to perpetrate drug interaction are the compound's exposure level (comprising unbound fraction, terminal half-life and in vivo reach) after dosing and its modulation potency on an interacting protein. A compound is deemed a potential perpetrator if it inhibits an interacting protein with a DDI index ≥0.1 or positively induces P450s at its unbound plasma Cmax. For intravenous administration, a compound is deemed a potential victim if the enzyme(s) or transporter(s) responsible for its elimination from systemic circulation are inhibited without any other elimination route(s) that can compensate the impaired route(s) or if such interacting protein(s) are positively induced.
Second, the identified pairs were assessed with respect to desirability of the associated potential interactions. Considering safety and efficacy of the antibiotics, alteration of their exposure by XueBiJing compounds was deemed undesirable. Given the low baseline exposure level of most XueBiJing compounds, only reduction of XueBiJing compounds' exposure by the antibiotics was considered undesirable. Also, enhanced glucuronidation of senkyunolide I (X5) was deemed undesirable because it is associated with increased formation of electrophilic metabolite.
Third, a PKC index was proposed to provide a measure of the degree of PKC between XueBiJing and antibiotics. Of all test XueBiJing compounds, percentage of those that could, as perpetrators, undesirably affect the antibiotics was defined as P1, while percentage of those that could, as victims, be undesirably affected by the antibiotics was defined as P2. Of all test antibiotics, percentage of those that could, as perpetrators, undesirably affect the XueBiJing compounds was defined as P3, while percentage of those that could, as victims, be undesirably affected by the XueBiJing compounds was defined as P4. PKC index was estimated using Eq. (2):
| PKC index = 1 − (P1 + P2 + P3 + P4)/4 | (2) |
A high PKC index (close to 1) represents a high degree of PKC for the XueBiJing/antibiotic combination (i.e., a low interaction risk), whereas a low PKC index indicates a low degree of PKC for the combination (i.e., a high interaction risk). The degree of PKC for the combination would be high when: (1) XueBiJing compounds and antibiotics could not pair on any interacting protein; (2) they could not pair due to perpetrator concentrations being too low to modulate the interacting proteins; (3) they could not pair due to limitedly altered victim exposure (caused by other elimination routes compensating the impaired route); (4) they could pair but result in desirable interactions and (5) they could pair but, due to victim's large therapeutic window, do not result in any undesirable interaction. PK incompatibility, if any, between XueBiJing and antibiotics results mainly from undesirable interactions.
All experiments were performed in triplicate repeats, with data expressed as mean ± SD. Statistical analyses were performed using PASW statistics 18 software (SPSS Inc., Chicago, IL, USA). Student's two-tailed t test was used for the comparison of two independent groups. P < 0.05 was considered statistically significant.
3. Results
3.1. Literature-mined information facilitating the PKC study
3.1.1. Antibiotics commonly used in sepsis care in China
A total of 45 antibiotics, all intravenously administered, were identified to be commonly used in management of sepsis and septic shock in China (Supporting Information Table S4). These antibiotics are all approved by the Chinese NMPA and appear in Guideline on Clinical Application of Antibiotics issued by the National Health Commission of China (2015), in research publications on antibiotic therapies of sepsis and septic shock by Chinese physicians, in reports on epidemiology of sepsis and septic shock in China, in the Sanford Guide to Antimicrobial Therapy (46th edition, 2016), and/or in Surviving Sepsis Campaign International Guidelines for Management of Sepsis and Septic Shock (2016). The antibiotics could be categorized as: hydrophilic antibacterials (A1‒A30), lipophilic antibacterials (A31–A39) and antifungals (A40–A45); detailed information is provided in Table S4.
3.1.2. PK drug interactions with identified antibiotics
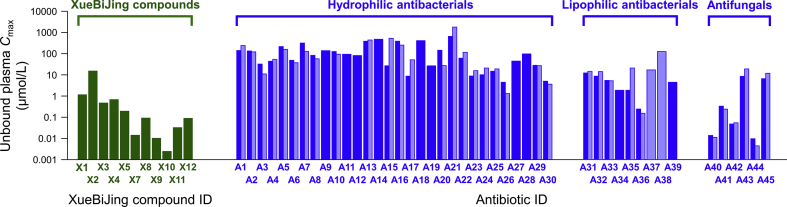
The literature-mined information on human pharmacokinetics of the identified antibiotics and their potential drug interactions is summarized in Supporting Information Table S5. Antibiotics can act as perpetrator and/or victim in drug interactions. Antibiotic-perpetrated drug interactions (involving P450 inhibition) have been reported for several lipophilic antibacterials, including CYP1A2-mediated ciprofloxacin (A32)-clozapine/theophylline/tizanidine interactions, CYP3A4-mediated erythromycin (A34)-midazolam/cyclosporine/tacrolimus interactions, CYP2C8-mediated trimethoprim (A37)-rosiglitazone/repaglinide interactions and CYP2C9-mediated trimethoprim-sulfamethoxazole (A37–A38)-warfarin/phenytoin interactions. Rifampin (A39) is reported to perpetrate drug interactions by inducing CYP3A or inhibiting OATP1B, e.g., CYP3A4-mediated A39-midazolam/simvastatin/atorvastatin interactions and OATP1B-mediated A39-glyburide interaction. Reported drug interactions with lipophilic antifungals via inhibiting P450s include: CYP2C9- and/or CYP3A4-mediated fluconazole (A43)-warfarin/fluvastatin/midazolam/diazepam interactions, CYP3A4-mediated itraconazole (A44)-midazolam/cyclosporine/tacrolimus/methylprednisolone/lovastatin/simvastatin/atorvastatin interactions and CYP2C9- and/or CYP3A4-mediated voriconazole (A45)-midazolam/diazepam/cyclosporine/tacrolimus interactions. Consistently, these antibiotics exhibited in vitro inhibitory or inductive activities against the respective drug metabolizing enzymes and/or transporter (Table S5). In addition, some hydrophilic antibacterials inhibit drug transporters in vitro, i.e., OATP1B3, inhibited by penicillin G (A15); OAT3, by cefamandole (A11), A15, metronidazole (A27) and linezolid (A29); bile salt export pump (BSEP), by piperacillin (A13) and A15; OATP2B1, by amikacin (A22); and multidrug resistance protein (MRP) 2, by ceftriaxone (A6), all with drug–drug interaction indices (DDI indices) ≥ 1.0 (estimated using reported plasma Cmax at clinically relevant doses and unbound fraction in plasma fu-p shown in Table S5). Therefore, despite no reported clinical PK drug interaction on the transporters, caution is advised in the use of these hydrophilic antibacterials. Most β-lactams (A1, A2, A4–A14, A16, A18 and A20), the phosphonic acid fosfomycin (A21), the aminoglycoside amikacin (A22) and the nitroimidazoles metronidazole (A27) and ornidazole (A28) administered at clinically relevant doses, exhibit considerably high unbound plasma Cmax (43–639 μmol/L; mean, 199 μmol/L), while the other hydrophilic antibacterials (A3, A15, A17, A19, A23–A26, A29 and A30) exhibit relatively low unbound Cmax (4–33 μmol/L; mean, 16 μmol/L). It is worth mentioning that, compared with these exposure levels, circulating XueBiJing compounds exhibit much lower unbound Cmax (0.003–1 μmol/L), except for paeoniflorin (X2; 15 μmol/L) (Fig. 2). Additional drug interaction-related information about these antibiotics, as perpetrators, is also provided in Table S5.
Figure 2.
Comparative unbound plasma Cmax values of XueBiJing compounds in healthy human subjects (green bars) after a 75-min intravenous infusion of XueBiJing at the label dose 100 mL injection/person and those of antibiotics in healthy human subjects (blue bars) and in critically ill patients (light blue bars) after an intravenous dosing of the antibiotics at their respective label doses. The Cmax values of XueBiJing compounds were measured by this laboratory (Table 1), while such data of antibiotics were obtained from literature (Supporting Information Table S5). X1, hydroxysafflor yellow A; X2, paeoniflorin; X3, oxypaeoniflorin; X4, albiflorin; X5, senkyunolide I; X7, senkyunolide G; X8, tanshinol; X9, 3-O-methyltanshinol; X10, salvianolic acid B; X11, protocatechuic acid; X12, ferulic acid; A1, imipenem; A2, meropenem; A3, ertapenem; A4, biapenem; A5, cefepime; A6, ceftriaxone; A7, ceftazidime; A8, cefoperazone; A9, cefotaxime; A10, cefuroxime; A11, cefamandole; A12, cefazolin; A13, piperacillin; A14, ticarcillin; A15, penicillin G; A16, ampicillin; A17, oxacillin; A18, carbenicillin; A19, flucloxacillin; A20, cefoxitin; A21, fosfomycin; A22, amikacin; A23, gentamicin; A24, tobramycin; A25, vancomycin; A26, teicoplanin; A27, metronidazole; A28, ornidazole; A29, linezolid; A30, daptomycin; A31, levofloxacin; A32, ciprofloxacin; A33, moxifloxacin; A34, erythromycin; A35, clindamycin; A36, tigecycline; A37, trimethoprim; A38, sulfamethoxazole; A39, rifampin; A40, micafungin; A41, caspofungin; A42, amphotericin B; A43, fluconazole; A44, itraconazole; A45, voriconazole.
Several hydrophilic antibacterials (β-lactams) were reported to be victims of drug interactions (due to transporter inhibition); they were OAT3-mediated probenecid-meropenem (A2)/cefotaxime (A9)/cefazolin (A12)/ticarcillin (A14)/ampicillin (A16)/flucloxacillin (A19) interactions and OAT1/3-mediated probenecid-piperacillin (A13)/penicillin G (A15) interactions. Besides perpetrating drug interactions, rifampin (A39) was also reported to be victim of drug interaction (due to dual inhibition of CYP3A4 and OATP1B by indinavir). In addition, several antifungals were reported to be victims of drug interactions (due to P450 induction), including CYP3A-mediated rifampin-caspofungin (A41) interactions, CYP3A-mediated rifampin/phenytoin/phenbarbital-itraconazole (A44) interactions and CYP2C19/2C9- and/or CYP3A-mediated rifampin/ritonavir/efavirenz-voriconazole (A45) interactions. Report for other antibiotics acting as victims in drug interactions is scarce, probably because their eliminations from systemic circulation involve more than one route, which could compensate for each other's impairment. Additional drug interaction-related information about these antibiotics, as victims, is provided in Table S5.
3.1.3. Drug interactions with XueBiJing
No report on drug interaction with XueBiJing and related herbal compounds was found from China National Knowledge Infrastructure (CNKI) (2004/01 to 2018/12), Medline (2004/01 to 2018/12), University of Washington Metabolism and Transport Drug Interaction Database (UW DIDB), National Adverse Drug Reaction (ADR) Information Bulletin [2008/06/26 (No. 1) to 2017/12/07 (No. 76); published by Chinese National Center for ADR Monitoring (Beijing, China)] or Shanghai ADR Information Bulletin [2017/11/06 to 2019/02/12; published by Shanghai Municipal Medical Products Administration (Shanghai, China)].
3.2. In vitro modulation of interacting proteins by XueBiJing compounds
To evaluate their potential for XueBiJing‒antibiotic interactions (XueBiJing as perpetrator and the antibiotic as victim), individual XueBiJing compounds (X1–X12) and MATI (a mixture of X1–X12 at concentrations similar to their unbound Cmax in humans after terminating the infusion of XueBiJing) were assessed for CYP2C9/2C19/3A4 inhibition, OAT1/3 inhibition, OATP1B1/1B3 inhibition and CYP2C/3A induction. Based on the literature-mined information, these enzymes and transporters are responsible for the systemic clearance of some antibiotics used in sepsis care. In addition, activities of the XueBiJing compounds and MATI on other drug metabolizing enzymes and transporters, highlighted in the US FDA drug–drug interaction guidance documents (2017), were also assessed.
3.2.1. Inhibition of P450 enzymes
The test XueBiJing compounds (100 μmol/L for each) and MATI exhibited < 50% inhibition of CYP1A2/2B6/2C8/2C9/2C19/2D6/3A4, except for oxypaeoniflorin (X3; 55% inhibition of CYP2C9) and salvianolic acid B (X10; 68%–80% inhibition of CYP2C9/2C19) (Supporting Information Table S6). Table 2 shows IC50 values of X3 and X10; time-dependent inhibition was found to be negligible for these XueBiJing compounds.
Table 2.
In vitro inhibition, by XueBiJing compounds, of human drug metabolizing enzymes and transporters.
| Drug metabolizing enzymes [substrate→metabolite] or transporters [substrate] | IC50 values of XueBiJing compounds (μmol/L) |
||||||
|---|---|---|---|---|---|---|---|
| X3 | X7 | X8 | X9 | X10 | X11 | X12 | |
| CYP2C9 [MFC→HFC] | 93 ± 18 | – | – | – | 75 ± 13 | – | – |
| CYP2C19 [CEC→CHC] | – | – | – | – | 19 ± 4 | – | – |
| UGT1A1 [E2→E23βG] | – | – | – | – | 10 ± 1 | – | – |
| UGT1A6 [4-MU→4-MUG] | – | – | – | – | 15 ± 1 | – | – |
| UGT1A9 [4-MU→4-MUG] | 74 ± 20 | – | – | – | 3 ± 1 | – | – |
| UGT2B15 [SENI→S7G] | – | – | – | – | 92 ± 25 | – | – |
| OATP1B1 [E217βG] | – | – | – | – | 38 ± 3 | – | – |
| OATP1B3 [E217βG] | – | – | – | – | 18 ± 4 | – | – |
| OAT1 [PAH] | – | 11 ± 3 | 78 ± 14 | 106 ± 26 | – | – | 2 ± 0 |
| OAT2 [prostaglandin F2α] | – | 51 ± 5 | – | – | – | – | 12 ± 1 |
| OAT3 [estrone-3-sulfate] | – | – | – | – | 31 ± 8 | 28 ± 7 | – |
Using pooled human liver microsomes, the XueBiJing compounds X3 and X10 (each at 100 μmol/L) exhibited the percentage inhibition of CYP2C9 60 ± 3% (by preincubation with NADPH)/56 ± 0% (by preincubation without NADPH) and 50 ± 3%/79 ± 2%, respectively. X10 (at 100 μmol/L) exhibited such percentage inhibition of CYP2C19 68 ± 16%/77 ± 11%. X3, oxypaeoniflorin; X7, senkyunolide G; X8, tanshinol; X9, 3-O-methyltanshinol; X10, salvianolic acid B; X11, protocatechuic acid; X12, ferulic acid; CEC: 3-cyano-7-ethoxycoumarin; CHC: 3-cyano-7-hydroxycoumarin; HFC: 7-hydroxytrifluoromethylcoumarin; MFC: 7-methoxy-4- trifluoromethylcoumarin; E2: β-estradiol; E23βG: β-estradiol-3-(β-d-glucuronide); 4-MU: 4-methylumbeliferone; 4-MUG: 4-methylumbelliferyl-β-d-glucuronide; SENI: senkyunolide I; S7G: senkyunolide I-7-O-β-glucuronide; E217βG, estradiol-17β-d-glucuronide; PAH, para-aminohippuric acid. Data are expressed as the mean ± SD (n = 3).
3.2.2. Induction of P450s
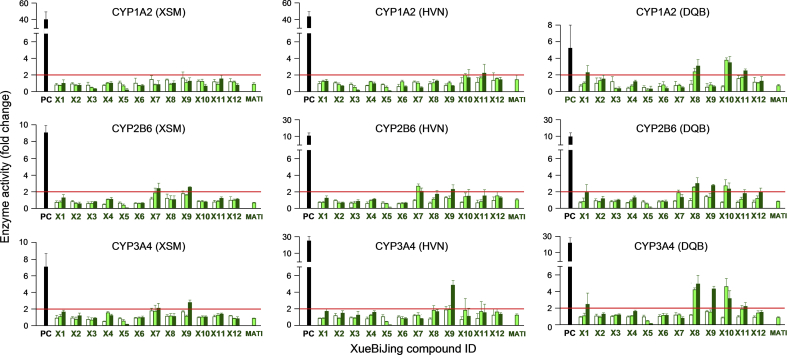
Cryopreserved human hepatocytes were treated with test XueBiJing compounds individually at three test concentrations (including their unbound Cmax) to assess their potential to induce CYP1A2, CYP2B6 and CYP3A4. None of the compounds exhibited P450 induction at their unbound Cmax, as indicated by treated-cell/untreated-cell ratios of enzyme activity (EA-T/U ratio) being < 2.0 (Fig. 3). Only at higher concentrations (10–100 times of their unbound Cmax), hydroxysafflor yellow A (X1), senkyunolide G (X7), tanshinol (X8), 3-O-methyltanshinol (X9), salvianolic acid B (X10) and protocatechuic acid (X11) moderately induced CYP1A2/3A4, CYP2B6/3A4, CYP1A2/2B6/3A4, CYP2B6/3A4, CYP1A2/2B6/3A4 and CYP1A2/3A4, respectively, in human hepatocytes from 1 to 3 donors (EA-T/U ratio, 2.1–4.9; P < 0.05). Consistent with these results, induction study measuring enzyme mRNA level suggested that these XueBiJing compounds (X1 and X7–X11) did not induce the enzymes at their unbound Cmax (mRNA-T/U ratio, < 4.0). Only at the higher concentrations, X1, X7, X8, X9, X10 and X11 shows moderate induction of CYP1A2/3A4, CYP2B6/3A4, CYP1A2/2B6/3A4, CYP2B6/3A4, CYP1A2/2B6/3A4 and CYP1A2/3A4, respectively, with mRNA-T/U ratios ranging from 4.0 to 9.6 (P < 0.05).
Figure 3.
Induction of P450s by XueBiJing compounds (X1‒X12) at three concentrations [unbound Cmax (open bars), 10 μmol/L (light green bars) and 100 μmol/L (green bars)] and MATI in cryopreserved human hepatocytes from three donors (XSM, HVN and DQB). Phenacetin, bupropion and midazolam were used as the probe substrates of CYP1A2, CYP2B6 and CYP3A, respectively. β-Naphthoflavone and rifampin were used as the positive controls (PC; 20 μmol/L) for CYP1A2 and CYP2B6/3A, respectively. X1, hydroxysafflor yellow A; X2, paeoniflorin; X3, oxypaeoniflorin; X4, albiflorin; X5, senkyunolide I; X6, senkyunolide I-7-O-β-glucuronide; X7, senkyunolide G; X8, tanshinol; X9, 3-O-methyltanshinol; X10, salvianolic acid B; X11, protocatechuic acid; X12, ferulic acid; MATI, a mixture of X1–X12 at concentrations similar to their unbound Cmax in humans after terminating the infusion of XueBiJing. Data are expressed as the mean ± SD (n = 3).
3.2.3. Inhibition of UGT enzymes
The test XueBiJing compounds (100 μmol/L for each) and MATI exhibited < 50% inhibition of UGT1A1/1A3/1A4/1A6/1A9/2B7/2B15, except for oxypaeoniflorin (X3; 57% inhibition of UGT1A9) and salvianolic acid B (X10; >90% inhibition of UGT1A1/1A6/1A9 and 55% inhibition of UGT2B15) (Table S6); Table 2 shows IC50 values of X3 and X10.
3.2.4. Inhibition of SLC transporters
Most test XueBiJing compounds (100 μmol/L) and MATI exhibited < 50% inhibition of OATP1B1/1B3, OAT1/2/3, OCT2 and MATE1 (Table S6). However, senkyunolide G (X7) inhibited OAT1/2; tanshinol (X8), OAT1; 3-O-methyltanshinol (X9), OAT1; salvianolic acid B (X10), OATP1B1/1B3 and OAT3; protocatechuic acid (X11), OAT3; and ferulic acid (X12), OAT1/2; with >50% inhibition (associated IC50 values shown in Table 2).
3.2.5. Inhibition of ABC transporters
The test XueBiJing compounds (100 μmol/L for each) and MATI exhibited < 50% inhibition for MDR1 and BCRP (Table S6).
3.3. In vitro modulation of interacting proteins by antibiotics
To evaluate their potential for antibiotic‒XueBiJing interactions (the antibiotic as perpetrator and XueBiJing compound as victim), 45 test antibiotics (A1–A45) were assessed individually for inhibition of UGT2B15, ALDH, OAT1/2 and OATP1B3. Based on our earlier PK research on XueBiJing, these enzymes and transporters are responsible for the systemic clearance of some XueBiJing compounds.
3.3.1. Inhibition of UGT2B15
Among the 45 antibiotics, cefoxitin (A20; test concentration, 1000 μmol/L), micafungin (A40; 100 μmol/L) and voriconazole (A45; 1000 μmol/L) exhibited ≥ 90% inhibition of UGT2B15, while cefoperazone (A8; 1000 μmol/L), piperacillin (A13; 5000 μmol/L), penicillin G (A15; 5000 μmol/L), flucloxacillin (A19; 1000 μmol/L) and caspofungin (A41; 100 μmol/L) exhibited 60%–88% inhibition of UGT2B15 (Supporting Information Table S7); Table 3 shows associated IC50 values.
Table 3.
In vitro inhibition, by antibiotics, of human drug metabolizing enzymes and transporters responsible for XueBiJing compounds' elimination.
| Antibiotics (ID) | IC50 values (μmol/L) |
||||
|---|---|---|---|---|---|
| UGT2B15 [X5→X6] | ALDH [PCD→X11] | OAT1 [X8] | OAT2 [X8] | OATP1B3 [X10] | |
| Imipenem (A1) | – | 120±30 | – | – | – |
| Meropenem (A2) | – | 164±39 | – | – | – |
| Ceftriaxone (A6) | – | – | – | – | 201±48 |
| Ceftazidime (A7) | – | 1034±155 | – | – | – |
| Cefoperazone (A8) | 971±130 | – | 49±11 | – | 27±9 |
| Cefotaxime (A9) | – | – | – | – | 68±20 |
| Cefamandole (A11) | – | – | 271±74 | – | 53±20 |
| Piperacillin (A13) | 797±142 | – | 591±154 | 1208±434 | – |
| Ticarcillin (A14) | – | – | 1833±572 | – | – |
| Penicillin G (A15) | 890±128 | 308±68 | 999±363 | – | 56±21 |
| Ampicillin (A16) | – | 2076±283 | – | – | 42±18 |
| Oxacillin (A17) | – | 465±72 | 99±31 | 467±97 | 11±2 |
| Carbenicillin (A18) | – | – | 311±90 | – | – |
| Flucloxacillin (A19) | 336±83 | 45±6 | 173±27 | 729±131 | 26±9 |
| Cefoxitin (A20) | 57±11 | 983±117 | – | – | 26±19 |
| Teicoplanin (A26) | – | – | – | 64±14 | 8±2 |
| Levofloxacin (A31) | – | – | – | 50±9 | – |
| Ciprofloxacin (A32) | – | – | 8±3 | 28±3 | 22±12 |
| Moxifloxacin (A33) | – | – | – | – | 17±2 |
| Erythromycin (A34) | – | – | – | – | 3±1 |
| Clindamycin (A35) | – | – | – | – | 12±2 |
| Trimethoprim (A37) | – | – | – | 667±146 | 378±109 |
| Rifamcin (A39) | – | – | – | – | 2±0 |
| Micafungin (A40) | 3±0 | – | 43±4 | 26±2 | 3±1 |
| Caspofungin (A41) | 39±2 | – | 7±2 | – | 1±0 |
| Amphotericin B (A42) | – | 87±9 | – | – | 1±0 |
| Itraconazole (A44) | – | – | – | – | 4±1 |
| Voriconazole (A45) | 55±6 | – | 189±75 | – | 19±8 |
X5, PCD, X8 and X10 were used as substrates for UGT2B15, ALDH, OAT1/2 and OATP1B3, respectively. X5, senkyunolide I; X6, senkyunolide I-7-O-β-glucuronide; X8, tanshinol; X10, salvianolic acid B; X11, protocatechuic acid; PCD, protocatechuic aldehyde. Data are expressed as the mean ± SD (n = 3).
3.3.2. Inhibition of ALDH
Several hydrophilic antibacterials, i.e., imipenem (A1; test concentration, 1000 μmol/L), meropenem (A2; 1000 μmol/L), ceftazidime (A7; 5000 μmol/L), penicillin G (A15; 5000 μmol/L), ampicillin (A16; 5000 μmol/L), oxacillin (A17; 1000 μmol/L), flucloxacillin (A19; 1000 μmol/L) and cefoxitin (A20; 1000 μmol/L), and the hydrophilic antifungal amphotericin B (A42; 100 μmol/L) exhibited 56%–88% inhibition of ALDH (Table S7); Table 3 shows associated IC50 values.
3.3.3. Inhibition of OAT1/2
Among the 45 antibiotics, carbenicillin (A18; test concentration, 5000 μmol/L), flucloxacillin (A19; 1000 μmol/L) and micafungin (A40; 100 μmol/L) exhibited ≥ 90% inhibition of OAT1, while cefoperazone (A8; 1000 μmol/L), cefamandole (A11; 1000 μmol/L), piperacillin (A13; 5000 μmol/L), ticarcillin (A14; 5000 μmol/L), penicillin G (A15; 5000 μmol/L), oxacillin (A17; 1000 μmol/L), ciprofloxacin (A32; 100 μmol/L), caspofungin (A41; 100 μmol/L) and voriconazole (A45; 1000 μmol/L) exhibited 63%–88% inhibition of the transporter. A40 (100 μmol/L) exhibited > 90% inhibition of OAT2, while A13 (5000 μmol/L), A17 (1000 μmol/L), A19 (1000 μmol/L), teicoplanin (A26; 100 μmol/L), levofloxacin (A31; 100 μmol/L), A32 (100 μmol/L) and trimethoprim (A37; 1000 μmol/L) exhibited 60%–84% inhibition of the transporter (Table S7). Notably, A13, A17, A19, A32 and A40 inhibited both OAT1 and OAT2 at their respective test concentrations. Table 3 shows associated IC50 values.
3.3.4. Inhibition of OATP1B3
Among the 45 antibiotics, cefamandole (A11; test concentration, 1000 μmol/L), penicillin G (A15; 5000 μmol/L), ampicillin (A16; 5000 μmol/L), oxacillin (A17; 1000 μmol/L), flucloxacillin (A19; 1000 μmol/L), cefoxitin (A20; 1000 μmol/L), teicoplanin (A26; 100 μmol/L), erythromycin (A34; 100 μmol/L), rifampin (A39; 100 μmol/L), micafungin (A40; 100 μmol/L) and caspofungin (A41; 100 μmol/L) exhibited > 90% inhibition of OATP1B3, while ceftriaxone (A6; 1000 μmol/L), cefoperazone (A8; 1000 μmol/L), cefotaxime (A9; 1000 μmol/L), ciprofloxacin (A32; 100 μmol/L), moxifloxacin (A33; 100 μmol/L), clindamycin (A35; 100 μmol/L), trimethoprim (A37; 1000 μmol/L), amphotericin B (A42; 100 μmol/L), itraconazole (A44; 100 μmol/L) and voriconazole (A45; 1000 μmol/L) exhibited 64%–88% inhibition of the transporter (Table S7). Table 3 shows associated IC50 values.
3.4. XueBiJing‒antibiotic and antibiotic‒XueBiJing pairs and overall PKC index for the combination
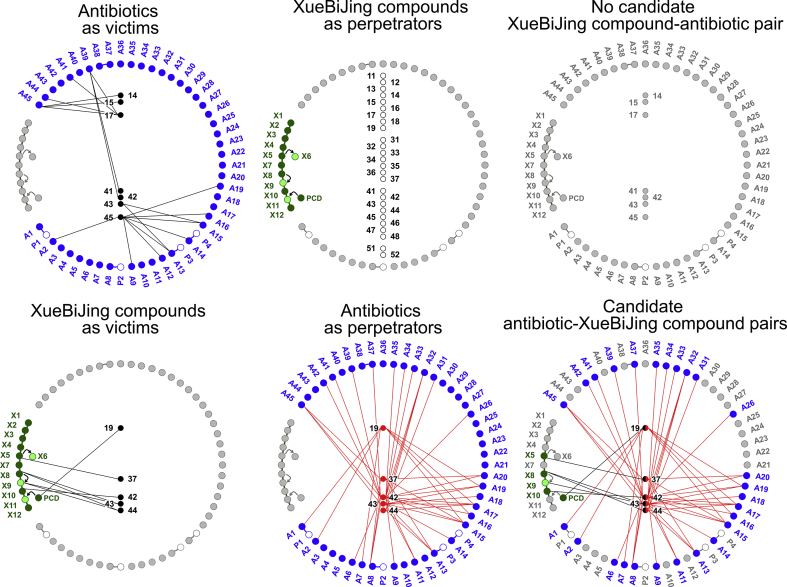
3.4.1. No XueBiJing‒antibiotic pair identified
Based on literature mining, CYP2C19/2C9/3A4 (for A45), CYP3A4 (for A41/A44), CYP3A4/OATP1B1/OATP1B3 (for A39), OAT3 (for A2/A9/A12/A14/A16/A19) and OAT1/3 (for A13/A15) were identified as the interacting proteins relevant for potential XueBiJing‒antibiotic interactions (Fig. 4). Most XueBiJing compounds exhibited no considerable in vitro inhibition or induction of P450 enzymes; they also exhibited no considerable in vitro inhibition of OATP1B1/1B3 and OAT1/3. Although oxypaeoniflorin (X3), X7‒X9, salvianolic acid B (X10), protocatechuic acid (X11) and ferulic acid (X12) inhibited CYP2C9, OAT1, CYP2C9/CYP2C19/OATP1B1/OATP1B3/OAT3, OAT3 and OAT1, respectively, to some extent in vitro (Table 2), their low unbound Cmax in humans receiving XueBiJing at label dose (Table 1) resulted in very low DDI indices of 0.00003–0.06. In addition, in vitro inhibition, by X3, senkyunolide G (X7), X10 and X12, of some enzymes and transporters [UGT1A1/1A6/1A9/2B15 and/or OAT2 (Table 2); only recommended in the US FDA drug–drug interaction guidance documents], is also negligible, with DDI indices of 0.00003–0.007. Collectively, no compound pair was identified for potential XueBiJing‒antibiotic interaction on CYP2C9, CYP2C19, CYP3A4, OATP1B1, OATP1B3, OAT1 and OAT3 (Fig. 4).
Figure 4.
Compound pairing of XueBiJing compounds and antibiotics. The definitions of XueBiJing compound IDs and those of antibiotic IDs are shown in Fig. 2 legend. P1, cilastatin; P2, sulbactam; P3, tazobactam; P4, clavulanic acid. 11, CYP1A2; 12, CYP2B6; 13, CYP2C8; 14, CYP2C9; 15, CYP2C19; 16, CYP2D6; 17, CYP3A4; 18, CYP3A5; 19, aldehyde dehydrogenase; 31, UGT1A1; 32, UGT1A3; 33, UGT1A4; 34, UGT1A6; 35, UGT1A9; 36, UGT2B7; 37, UGT2B15; 41, OATP1B1; 42, OATP1B3; 43, OAT1; 44, OAT2; 45, OAT3; 46, OCT2; 47, MATE1; 48, MATE2K; 51, MDR1; 52, BCRP.
3.4.2. Antibiotic‒XueBiJing pairs
Based on our earlier PK research on XueBiJing, UGT2B15 (for X5), ALDH (for protocatechuic aldehyde→X11), OAT1/2 (for X8/X9) and OATP1B3 (for X10) were identified as the relevant interacting proteins for assessing potential antibiotic‒XueBiJing interactions (Fig. 4). Among the 45 test antibiotics, 19 antibiotics (A3–A5, A10, A12, A21–A25, A27–A30, A36, A38, A40, A43 and A44) did not inhibit or negligibly inhibited (DDI indices < 0.1) these enzymes or transporters, suggesting that these antibiotics could not be paired with the XueBiJing compounds. As shown in Fig. 4, the other 26 antibiotics, inhibiting these enzymes and transporters, could be paired with the XueBiJing compounds senkyunolide I (X5), protocatechuic aldehyde, tanshinol (X8) and/or salvianolic acid B (X10), with DDI indices ≥ 0.1. Potential interaction outcomes and their desirability for these antibiotic‒XueBiJing pairs are summarized in Table 4.
Table 4.
Desirability of antibiotic‒XueBiJing compound pairs.
| Antibiotic‒XueBiJing pair | Interacting protein | Inhibition DDI index | Potential outcome | Remark |
|---|---|---|---|---|
| Cefoperazone (A8)‒senkyunolide I (X5) | UGT2B15 | 0.12 | Decreased glucuronidation of and increased systemic exposure to X5. For X5, its unbound Cmax 0.2 μmol/L (Table 1) is less than its effective concentration 10 μmol/L (Supporting Information Table S2), so inhibition of UGT2B15 by these antibiotics at the clinically relevant doses would lead to the desirable concentration. | Probably desirable due to the low baseline level of systemic exposure to X5. |
| Penicillin G (A15)‒senkyunolide I (X5) | 0.43–0.74 | |||
| Piperacillin (A13)‒senkyunolide I (X5) | 0.56 | |||
| Voriconazole (A45)‒senkyunolide I (X5) | 0.75 | |||
| Cefoxitin (A20)‒senkyunolide I (X5) | 1.28 | |||
| Oxacillin (A17)‒protocatechuic aldehyde | ALDH | 0.11 | Decreased systemic exposure to the metabolite X11 and increased methylated protocatechuic aldehyde. For X11, its unbound Cmax 0.03 μmol/L (Table 1) is low as compared with its effective concentrations 1–130 μmol/L (Supporting Information Table S2); inhibition of ALDH by these antibiotics at the clinically relevant doses would further decrease its concentration. | Undesirable but probably of limited therapeutic relevance due to the low baseline level of systemic exposure to X11. |
| Ampicillin (A16)‒protocatechuic aldehyde | 0.17 | |||
| Ceftazidime (A7)‒protocatechuic aldehyde | 0.19 | |||
| Flucloxacillin (A19)‒protocatechuic aldehyde | 0.62 | |||
| Meropenem (A2)‒protocatechuic aldehyde | 0.78 | |||
| Penicillin G (A15)‒protocatechuic aldehyde | 1.24–2.14 | |||
| Imipenem (A1)‒protocatechuic aldehyde | 2.14 | |||
| Flucloxacillin (A19)‒tanshinol (X8) | OAT1 | 0.16 | Decreased renal excretion of and increased systemic exposure to X8. For X8, its unbound Cmax 0.1 μmol/L (Table 1) is less than its effective concentrations 1–25 μmol/L (Supporting Information Table S2), so inhibition of OAT1/2 by these antibiotics at the clinically relevant doses would lead to the desirable concentrations. | Probably desirable due to the low baseline level of systemic exposure to X8. |
| Voriconazole (A45)‒tanshinol (X8) | 0.22 | |||
| Ticarcillin (A14)‒tanshinol (X8) | 0.26 | |||
| Cefamandole (A11)‒tanshinol (X8) | 0.33 | |||
| Penicillin G (A15)‒tanshinol (X8) | 0.38–0.66 | |||
| Cefoperazone (A8)‒tanshinol (X8) | 0.95–2.44 | |||
| Carbenicillin (A18)‒tanshinol (X8) | 1.31 | |||
| Levofloxacin (A31)‒tanshinol (X8) | OAT2 | 0.36 | ||
| Oxacillin (A17)‒tanshinol (X8) | OAT1/2 | 0.52/0.11 | ||
| Piperacillin (A13)‒tanshinol (X8) | 0.75/0.37 | |||
| Ciprofloxacin (A32)‒tanshinol (X8) | 1.96/0.55 | |||
| Trimethoprim (A37)‒salvianolic acid B (X10) | OATP1B3 | 0.12 | Decreased hepatobiliary excretion of and increased systemic exposure to X10. For X10, its unbound Cmax 0.003 μmol/L (Table 1) is less than its effective concentrations 0.1–1.4 μmol/L (Supporting Information Table S2), so inhibition of OATP1B3 by these antibiotics at the clinically relevant doses would lead to the desirable concentrations. | Probably desirable due to the low baseline level of systemic exposure to X10. |
| Amphotericin B (A42)‒salvianolic acid B (X10) | 0.22 | |||
| Moxifloxacin (A33)‒salvianolic acid B (X10) | 0.29–0.41 | |||
| Clindamycin (A35)‒salvianolic acid B (X10) | 0.30 | |||
| Caspofungin (A41)‒salvianolic acid B (X10) | 0.35 | |||
| Erythromycin (A34)‒salvianolic acid B (X10) | 0.35–1.04 | |||
| Teicoplanin (A26)‒salvianolic acid B (X10) | 0.60 | |||
| Ceftriaxone (A6)‒salvianolic acid B (X10) | 0.68 | |||
| Ciprofloxacin (A32)‒salvianolic acid B (X10) | 0.69 | |||
| Flucloxacillin (A19)‒salvianolic acid B (X10) | 1.07 | |||
| Cefamandole (A11)‒salvianolic acid B (X10) | 1.70 | |||
| Cefoperazone (A8)‒salvianolic acid B (X10) | 1.72–4.43 | |||
| Cefotaxime (A9)‒salvianolic acid B (X10) | 1.81–2.14 | |||
| Voriconazole (A45)‒salvianolic acid B (X10) | 2.16 | |||
| Rifamcin (A39)‒salvianolic acid B (X10) | 2.63 | |||
| Cefoxitin (A20)‒salvianolic acid B (X10) | 2.81 | |||
| Oxacillin (A17)‒salvianolic acid B (X10) | 4.64 | |||
| Penicillin G (A15)‒salvianolic acid B (X10) | 6.8–11.8 | |||
| Ampicillin (A16)‒salvianolic acid B (X10) | 8.55 |
3.4.3. Overall PKC index of XueBiJing/antibiotic combination
Interaction potential associated with ALDH inhibition by the antibiotics A1, A2, A7, A15–A17 and A19 was identified for XueBiJing/antibiotic combination; such inhibition may result in undesirable reduction in exposure to the XueBiJing compound protocatechuic acid (X11). Accordingly, the parameters P1 (percentage of perpetrators in XueBiJing compounds undesirably affecting the antibiotics), P2 (percentage of victims in XueBiJing compounds being undesirably affected by the antibiotics), P3 (percentage of perpetrators in antibiotics undesirably affecting XueBiJing compounds) and P4 (percentage of victims in antibiotics being undesirably affected by XueBiJing compounds), for calculating the PKC index, were estimated to be 0%, 8.3%, 15.6% and 0%, respectively. As a result, the estimated overall PKC index was 0.94 (close to 1), suggesting a high degree of PKC between XueBiJing and the antibiotics at clinically relevant doses. The identified PK interaction risk mainly involves reduced X11 exposure by coadministration of several antibiotics.
4. Discussion
Besides enhanced pharmacodynamic effects and/or reduced toxicity, clinical success of a combination drug therapy requires a high degree of PKC. Study of PKC within a synthetic medicine/herbal medicine combination that exhibits promising therapeutic benefit follows the same mechanistic basis of drug interaction as traditional evaluation of PK herb–drug interactions (traditional HDI study). However, these two types of studies differ in certain aspects. Regarding both types of medicine as therapeutic, PKC study seeks to define the degree of compatibility between an herbal medicine and co-administered synthetic medicines, by providing mechanistic evidence for interaction risks (evaluating the herbal medicine not only as perpetrator but also as victim), to ultimately provide evidence to guide clinical decision for the combination. Meanwhile, usually considering only the synthetic medicines as therapeutic, traditional HDI study aims to identify natural products (like St. John's wort and grapefruit juice) that can perpetrate therapeutic interactions with synthetic medicines, by assessing inhibition and induction of interacting proteins by herbal compounds in vitro and by performing clinical interaction studies of natural products (usually without considering the natural products as victims), to ultimately avoid or minimize therapeutic failure of synthetic medicines46, 47. In addition, to support a drug combination, PKC study involves all the enzymes and transporters that are related to the combination and highlighted for drug interactions, while most traditional HDI studies are focused on limited such proteins.
Multiple factors (such as patient therapeutic needs, inter-patient differences, drug preference ranking in hospital prescribing, drug commercial availability, etc.) make combination drug therapies complex; adding an herbal medicine even further complicates such therapies. Research on a drug combination needs a wealth of data related to the combination to assess its feasibility, to identify associated risks and, if necessary, to develop the risk management. In this study, informatics-based approach and experimental approach were integrated to obtain the data, which were processed using a newly developed compound pair-based method (Fig. 1). The PKC study first requires identifying both the relevant XueBiJing compounds and antibiotics for the investigation and understanding their pharmacokinetics/disposition and drug interaction liabilities. Although XueBiJing is a complex mixture of constituents, PK research can identify the herbal compounds bioavailable for drug interactions; substantial progresses have recently been made in PK research on such complex herbal medicines33, 34, 35, 38, 48, 49, 50, 51, 52, 53. Twelve herbal compounds (unchanged and metabolized) were tested in this study, the only ones identified in earlier human PK study (despite the high assay sensitivity) from the 104 constituents detected in XueBiJing. The notable difference in number between compounds detected in XueBiJing and those detected in systemic circulation after dosing might be due to: (1) the levels of different compounds in XueBiJing differ greatly (up to four orders of magnitude), (2) the post-administration distribution of the compounds in the body (dilution effect) and (3) their biotransformation in and rapid elimination from the body. Information on human pharmacokinetics/disposition of the identified 12 compounds was also obtained from the earlier PK research on the injection. In clinical practice, various antibiotics may be administered in septic patients who also receive XueBiJing. Identifying antibiotics commonly used for sepsis care in China and obtaining information on their pharmacokinetics/disposition and drug interaction liabilities were achieved via informatics-based approach. Meanwhile, information on drug interaction with XueBiJing and related herbal compounds was also carefully searched using the same approach. Because some literature-mined information was limited, biased, confusing and/or inconclusive, multiple in vitro studies were performed to supplement key information on interactions of the XueBiJing compounds, and of the antibiotics, with drug metabolizing enzymes and transporters. Selection of drug metabolizing enzymes and transporters for the in vitro studies was based on the mechanistic understanding of elimination of the antibiotics and the XueBiJing compounds. To assess potential for XueBiJing‒antibiotic interaction (XueBiJing as perpetrator and the antibiotic as victim), the enzymes and transporters that are known to be responsible for the systemic clearance of antibiotics were selected. In addition, a wider range of enzymes and transporters, which are highlighted in the US FDA drug–drug interaction guidance (2017), were also included. This is because better understanding is needed for some of the antibiotics regarding their elimination and drug interactions and because more antibiotics may later become available for sepsis care. To assess potential for antibiotic‒XueBiJing interaction (the antibiotic as perpetrator and XueBiJing as victim), only the enzymes and transporters that are responsible for systemic clearance of the 12 XueBiJing compounds were selected, because this injection is derived from a fixed five-herb formula with high batch-to-batch quality consistency (Table S1).
Data processing in PKC study started from identifying compound pairs that exhibited potential for interactions between the XueBiJing compounds and antibiotics. The identification was achieved by understanding the chemical basis (perpetrators and victims) of and mechanisms (interacting proteins and modulation modes) underlying potential interactions; pairs exhibiting DDI indices ≥ 0.1 for inhibition or positive induction at unbound Cmax were identified as candidates. Desirability of the pair-associated interaction was considered in further assessment, for which information on victim's therapeutic window and baseline concentration is useful. Since drug metabolizing enzymes and transporters may have multiple substrates or modulators, the modes of interactions between the XueBiJing compounds (X) and antibiotics (A) can be: a single X affecting a single A, a single X affecting multiple As, or multiple Xs affecting a single A, and vice versa. Hence, to indicate the degree of PKC, a PKC index (0–1) was calculated using the percentages of perpetrators (P1) and victims (P2) of all test XueBiJing compounds undesirably affecting or being affected by the antibiotics and those of perpetrators (P3) and victims (P4) of all test antibiotics undesirably affecting or being affected by the XueBiJing compounds. A low PKC index suggests that the drug combination is not recommended due to high drug interaction risk. For a high PKC index (close to 1), potential undesirable drug interactions in the recommended combination should still be considered by examining P1, P2, P3 and P4 individually; this is to ensure maximum PKC for the drug combination. Undesirable interactions with high potential should be further investigated by performing model-based prediction and, if necessary, relevant clinical studies.
XueBiJing/antibiotic combination for sepsis care exhibits a high degree of PKC. This could be understood from two perspectives. First, there is no XueBiJing‒antibiotic pair identified, suggesting that XueBiJing at clinically relevant dose has low propensity to perpetrate interactions with the antibiotics. Hydroxysafflor yellow A (X1) and paeoniflorin (X2) have levels of systemic exposure significantly higher than the other XueBiJing compounds (X3–X12), but they do not inhibit or induce any enzyme or transporter (CYP2C9/2C19/3A4, OAT1/3 or OATP1B1/1B3) that is responsible for the systemic clearance of those antibiotics reported as victims of PK drug interactions. Despite their in vitro inhibition of CYP2C9/2C19, OAT1/3 and/or OATP1B1/1B3, low unbound Cmax of oxypaeoniflorin (X3) and X7–X12 in humans receiving XueBiJing at label dose result in very low DDI indices (<0.1). Meanwhile, X1 and X7–X11 do not induce CYP3A at their unbound Cmax, albeit their moderate induction at much higher concentrations. In addition, inhibition, by X3, senkyunolide G (X7), salvianolic acid B (X10) and ferulic acid (X12), of some enzymes and transporters (only recommended in the US FDA drug–drug interaction guidance documents) is also negligible, with DDI indices of <0.1, while X1 and X7‒X11 do not induce CYP1A2/2B6 at their respective unbound Cmax (albeit their moderate induction at much higher concentrations). Second, there is no antibiotic‒XueBiJing pair identified for X1 and X2, because these XueBiJing compounds are mainly eliminated via glomerular-filtration-based renal excretion with high unbound fractions in human plasma (30% and 82%, respectively). Although the antibiotics A6, A8, A9, A11, A13–A20, A26, A31–A35, A37, A39, A41, A42 and A45 can pair with the XueBiJing compounds senkyunolide I (X5), tanshinol (X8) and/or X10 due to these antibiotics' inhibition of the enzymes and transporters UGT2B15, OAT1/2 and/or OATP1B3, the potential interactions with the XueBiJing compounds (associated with their increased systemic exposure) are likely desirable due to the herbal compounds' low baseline exposure levels. For the XueBiJing/antibiotic combination, inhibition of ALDH by the antibiotics A1, A2, A7, A15–A17 and A19 probably results in undesirable reduction of exposure to the XueBiJing compound X11; this explains the PKC index being 0.94 (<1). As the baseline unbound Cmax of X11 after dosing XueBiJing is low, these undesirable interactions might not be clinically significant. In addition, no antibiotics, at their unbound Cmax, were found to induce UGT2B15 for X5 (Supporting Information Fig. S2); this enzyme might be poorly inducible, because there is limited report about its potent positive inducer.
Although the high degree of PKC at clinically relevant doses supports XueBiJing/antibiotic combination in sepsis care, several factors should be considered in interpreting this result. First, the high degree of PKC reported here focused on XueBiJing and the antibiotics used in sepsis care at their clinically relevant doses, and does not account for the other types of medicine also used. Second, polypharmacy in sepsis care may result in a complex drug interaction environment in patients also receiving XueBiJing. As such, even a modest XueBiJing‒antibiotic interaction, particularly at an increased XueBiJing dose, might be the last straw in precipitating therapeutic failure or adverse effect. Therefore, potential interactions with XueBiJing should still be monitored, despite its high degree of PKC with the antibiotics. Third, this investigation focuses on traditional PK interactions involving inhibition or induction of drug metabolizing enzymes and transporters. Profound pathophysiological changes in patients with sepsis, such as renal and hepatic dysfunction, altered fluid status, microvascular failure, and altered serum albumin concentration, could alter antibiotics' pharmacokinetics and result in suboptimum outcomes54. Growing evidence has shown that inflammation and immune responses may alter the regulation of drug metabolizing enzymes and transporters55, 56, 57. Treatment with XueBiJing may considerably slow down such changes and may alter the influence of sepsis on antibiotics' pharmacokinetics; the antibiotics may also similarly affect XueBiJing. Such potential non-traditional PK drug interactions should also be considered.
This study has several limitations. While the inhibition studies involved various drug metabolizing enzymes and transporters, induction study mainly focused on P450s, because methods for evaluating the induction of phase II enzymes and transporters are still not well established and there are limited reports on such induction-based drug interactions58, 59. Although establishing PKC index provides a way to gauge degree of PKC for a drug combination, more PKC index values from studies of various drug combinations are required to set clearer index thresholds that will indicate recommendation for (unconditionally or conditionally) or against a drug combination. Estimating PKC index may also need differential weighting for all medicinal compounds, in the combination, according to their contributions to the herbal medicine's therapeutic action or their preference ranking in hospital prescribing.
Growing evidence shows that using Chinese herbal medicines and incorporating such medicines into synthetic medicine-based therapies for multifactorial diseases deliver therapeutic benefits13, 14, 15, 16, 17, 60, 61, 62, 63, 64. However, PK herb–drug interaction is a widely recognized issue that may counteract such benefit. Here, a PKC study was proposed and conducted to provide evidence to guide clinical decision for XueBiJing/antibiotic combination in sepsis care. The study suggests that this complex combination has a high degree of PKC at clinically relevant doses; the identified PK interaction risk mainly involves inhibition of ALDH by several antibiotics. The study was informed by clinical practice for patients with sepsis and based on comprehensive understanding of composition and pharmacokinetics of XueBiJing. Given the chemical complexity of XueBiJing and the clinical use of various antibiotics in sepsis care, this study used a wealth of data obtained by integrating informatics-based approach and experimental approach, and involved developing a compound pair-based method for data processing. A PKC index was proposed in the data processing to provide a pragmatic and concrete measure of the degree of PKC; this index will also provide an experimentally-accessible means of optimizing the combination therapy. The methodology established here can be applied to investigate other drug combinations. Although the early identification and treatment of sepsis is desirable, prompt administration of all the necessary medications (including XueBiJing) increases the drug interaction risk. For optimal sepsis care, a high degree of PKC is desirable not only between XueBiJing and antibiotics but also between XueBiJing and other medicines and among different types of synthetic medicine. Accordingly, more PKC studies are needed for medications in sepsis care and the information obtained should be made available to the health care providers.
Acknowledgments
We thank Professor Chung S. Yang from Rutgers, The State University of New Jersey, Professor Ge Lin from The Chinese University of Hong Kong (China), and Professor Su Zeng from Zhejiang University (China) for reviewing and helping improve the manuscript. This work was funded by grants from the National Science & Technology Major Project of China “Key New Drug Creation and Manufacturing Program” (2017ZX09301012006), the National Basic Research Program of China (2012CB518403), the National Natural Science Foundation of China (81503345), and the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA12050306).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2019.06.003.
Contributor Information
Chen Cheng, Email: chengchen@simm.ac.cn.
Chuan Li, Email: chli@simm.ac.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Keith C.T., Borisy A.A., Stockwell B.R. Multicomponent therapeutics for networked systems. Nat Rev Drug Discov. 2005;4:71–78. doi: 10.1038/nrd1609. [DOI] [PubMed] [Google Scholar]
- 2.Zimmermann G.R., Lehár J., Keith C.T. Multi-target therapeutics: when the whole is greater than the sum of the parts. Drug Discov Today. 2007;12:34–42. doi: 10.1016/j.drudis.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Medina-Franco J.L., Giulianotti M.A., Welmaker G.S., Houghten R.A. Shifting from the single to the multitarget paradigm in drug discovery. Drug Discov Today. 2013;18:495–501. doi: 10.1016/j.drudis.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M. The third international consensus definitions for sepsis and septic shock (sepsis-3) J Am Med Assoc. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rhodes A., Evans L.E., Alhazzani W., Levy M.M., Antonelli M., Ferrer R. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 6.Angus D.C., Van Der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–851. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 7.Van Der Poll T., Van De Veerdonk F.L., Scicluna B.P., Netea M.G. The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol. 2017;17:407–420. doi: 10.1038/nri.2017.36. [DOI] [PubMed] [Google Scholar]
- 8.Fink M.P., Warren H.S. Strategies to improve drug development for sepsis. Nat Rev Drug Discov. 2014;13:741–758. doi: 10.1038/nrd4368. [DOI] [PubMed] [Google Scholar]
- 9.Cohen J., Vincent J.L., Adhikari N.K., Machado F.R., Angus D.C., Calandra T. Sepsis: a roadmap for future research. Lancet Infect Dis. 2015;15:581–614. doi: 10.1016/S1473-3099(15)70112-X. [DOI] [PubMed] [Google Scholar]
- 10.Cao Y., Chai Y.F., Deng Y., Fang B.J., Liu M.H., Lu Z.Q. Chinese guidelines for emergency management of sepsis and septic shock 2018. J Clin Emerg (Chin) 2018;19:567–588. [Google Scholar]
- 11.Zhao G.Z., Chen R.B., Li B., Guo Y.H., Xie Y.M., Liao X. Clinical practice guideline on traditional Chinese medicine therapy alone or combined with antibiotics for sepsis. Ann Transl Med. 2019;7:122. doi: 10.21037/atm.2018.12.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chinese Society of Emergency Medicine Editorial board of Chinese critical care medicine, expert group of Chinese emergency medicine expert consensus on diagnosis and treatment of sepsis complicated with disseminated intravascular coagulation, wang L, chai Y. Chinese emergency medicine expert consensus on diagnosis and treatment of sepsis complicated with disseminated intravascular coagulation. Chin J Clin Pathol. 2017;9:129–132. [Google Scholar]
- 13.Chen G., Gao Y., Jiang Y., Yang F., Li S., Tan D. Efficacy and safety of Xuebijing injection combined with ulinastatin as adjunctive therapy on sepsis: a systematic review and meta-analysis. Front Pharmacol. 2018;9:743. doi: 10.3389/fphar.2018.00743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li C., Wang P., Zhang L., Li M., Lei X., Liu S. Efficacy and safety of Xuebijing injection (a Chinese patent) for sepsis: a meta-analysis of randomized controlled trials. J Ethnopharmacol. 2018;224:512–521. doi: 10.1016/j.jep.2018.05.043. [DOI] [PubMed] [Google Scholar]
- 15.Shi H., Hong Y., Qian J., Cai X., Chen S. Xuebijing in the treatment of patients with sepsis. Am J Emerg Med. 2017;35:285–291. doi: 10.1016/j.ajem.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y.X., Li C.S. The effectiveness of XueBiJing injection in therapy of sepsis: a multicenter clinical study. Chin J Emerg Med. 2013;22:130–135. [Google Scholar]
- 17.Gao J., Kong L., Liu S., Feng Z., Shen H., Liu Q. A prospective multicenter clinical study of Xuebijing injection in the treatment of sepsis and multiple organ dysfunction syndrome. Chin Crit Care Med. 2015;27:465–470. doi: 10.3760/cma.j.issn.2095-4352.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Yin Q., Li C. Treatment effects of Xuebijing injection in severe septic patients with disseminated intravascular coagulation. Evid Based Complement Alternat Med. 2014;2014:949254. doi: 10.1155/2014/949254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen X., Feng Y., Shen X., Pan G., Fan G., Gao X. Anti-sepsis protection of Xuebijing injection is mediated by differential regulation of pro- and anti-inflammatory Th17 and T regulatory cells in a murine model of polymicrobial sepsis. J Ethnopharmacol. 2018;211:358–365. doi: 10.1016/j.jep.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Wang L., Liu Z., Dong Z., Pan J., Ma X. Effects of Xuebijing injection on microcirculation in septic shock. J Surg Res. 2016;202:147–154. doi: 10.1016/j.jss.2015.12.041. [DOI] [PubMed] [Google Scholar]
- 21.Jiang M., Zhou M., Han Y., Xing L., Zhao H., Dong L. Identification of NF-κB Inhibitors in Xuebijing injection for sepsis treatment based on bioactivity-integrated UPLC-Q/TOF. J Ethnopharmacol. 2013;147:426–433. doi: 10.1016/j.jep.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 22.Dong T.H., Zhang G.P., Dong K., Liu S., Yao Y.M. Research progress on mechanism of action of Xuebijing injection in the treatment of sepsis. Chin J TCM WM Crit Care. 2016;23:554–557. [Google Scholar]
- 23.Wang Q., Wu X., Tong X., Zhang Z., Xu B., Zhou W. Xuebijing ameliorates sepsis-induced lung injury by downregulating HMGB1 and RAGE expressions in mice. Evid Based Complement Alternat Med. 2015;2015:860259. doi: 10.1155/2015/860259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zuo L., Zhou L., Xu T., Li Z., Liu L., Shi Y. Antiseptic activity of ethnomedicinal Xuebijing revealed by the metabolomics analysis using UHPLC‒Q-Orbitrap HRMS. Front Pharmacol. 2018;9:300. doi: 10.3389/fphar.2018.00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu T., Zhou L., Shi Y., Liu L., Zuo L., Jia Q. Metabolomics approach in lung tissue of septic rats and the interventional effects of Xuebijing injection using UHPLC-Q-Orbitrap-HRMS. J Biochem. 2018;164:427–435. doi: 10.1093/jb/mvy070. [DOI] [PubMed] [Google Scholar]
- 26.Pea F., Furlanut M. Pharmacokinetic aspects of treating infections in the intensive care unit: focus on drug interactions. Clin Pharmacokinet. 2001;40:833–868. doi: 10.2165/00003088-200140110-00004. [DOI] [PubMed] [Google Scholar]
- 27.Pai M.P., Momary K.M., Rodvold K.A. Antibiotic drug interactions. Med Clin N Am. 2006;90:1223–1255. doi: 10.1016/j.mcna.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 28.Pereira J.M., Paiva J.A. Antimicrobial drug interactions in the critically ill patients. Curr Clin Pharmacol. 2013;8:25–38. [PubMed] [Google Scholar]
- 29.Smithburger P.L., Kane-Gill S.L., Seybert A.L. Drug–drug interactions in the medical intensive care unit: an assessment of frequency, severity and the medications involved. Int J Pharm Pract. 2012;20:402–428. doi: 10.1111/j.2042-7174.2012.00221.x. [DOI] [PubMed] [Google Scholar]
- 30.Uijtendaal E.V., Van Harssel L.L., Hugenholtz G.W., Kuck E.M., Zwart-Van Rijkom J.E., Cremer O.L. Analysis of potential drug–drug interactions in medical intensive care unit patients. Pharmacotherapy. 2014;34:213–219. doi: 10.1002/phar.1395. [DOI] [PubMed] [Google Scholar]
- 31.Sun Z., Zuo L., Sun T., Tang J., Ding D., Zhou L. Chemical profiling and quantification of XueBiJing injection, a systematic quality control strategy using UHPLC-Q exactive hybrid quadrupole-orbitrap high-resolution mass spectrometry. Sci Rep. 2017;7:16921. doi: 10.1038/s41598-017-17170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zuo L., Sun Z., Hu Y., Sun Y., Xue W., Zhou L. Rapid determination of 30 bioactive constituents in XueBiJing injection using ultra high performance liquid chromatography-high resolution hybrid quadrupole-orbitrap mass spectrometry coupled with principal component analysis. J Pharm Biomed Anal. 2017;137:220–228. doi: 10.1016/j.jpba.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 33.Cheng C., Lin J.Z., Li L., Yang J.L., Jia W.W., Huang Y.H. Pharmacokinetics and disposition of monoterpene glycosides derived from Paeonia lactiflora roots (Chishao) after intravenous dosing of antiseptic XueBiJing injection in human subjects and rats. Acta Pharmacol Sin. 2016;37:530–544. doi: 10.1038/aps.2015.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X., Cheng C., Wang F., Huang Y., Jia W., Olaleye O.E. Pharmacokinetics of catechols in human subjects intravenously receiving XueBiJing injection, an emerging antiseptic herbal medicine. Drug Metab Pharmacokinet. 2016;31:95–98. doi: 10.1016/j.dmpk.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 35.Zhang N., Cheng C., Olaleye O.E., Sun Y., Li L., Huang Y. Pharmacokinetics-based identification of potential therapeutic phthalides from XueBiJing, a Chinese herbal injection used in sepsis management. Drug Metab Dispos. 2018;46:823–834. doi: 10.1124/dmd.117.079673. [DOI] [PubMed] [Google Scholar]
- 36.Tian D.D., Jia W.W., Liu X.W., Wang D.D., Liu J.H., Dong J.J. Methylation and its role in the disposition of tanshinol, a cardiovascular carboxylic catechol from Salvia miltiorrhiza roots (Danshen) Acta Pharmacol Sin. 2015;36:627–643. doi: 10.1038/aps.2015.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jia W., Du F., Liu X., Jiang R., Xu F., Yang J. Renal tubular secretion of tanshinol: molecular mechanisms, impact on its systemic exposure, and propensity for dose-related nephrotoxicity and for renal herb-drug interactions. Drug Metab Dispos. 2015;43:669–678. doi: 10.1124/dmd.114.062000. [DOI] [PubMed] [Google Scholar]
- 38.Li M., Wang F., Huang Y., Du F., Zhong C., Olaleye O.E. Systemic exposure to and disposition of catechols derived from Salvia miltiorrhiza roots (Danshen) after intravenous dosing DanHong injection in human subjects, rats, and dogs. Drug Metab Dispos. 2015;43:679–690. doi: 10.1124/dmd.114.061473. [DOI] [PubMed] [Google Scholar]
- 39.Wong C.C., Meinl W., Glatt H.R., Barron D., Stalmach A., Steiling H. In vitro and in vivo conjugation of dietary hydroxycinnamic acids by UDP-glucuronosyltransferases and sulfotransferases in humans. J Nutr Biochem. 2010;21:1060–1068. doi: 10.1016/j.jnutbio.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 40.Li X., Shang L., Wu Y., Abbas S., Li D., Netter P. Identification of the human UDP-glucuronosyltransferase isoforms involved in the glucuronidation of the phytochemical ferulic acid. Drug Metab Pharmacokinet. 2011;26:341–350. doi: 10.2133/dmpk.dmpk-10-rg-125. [DOI] [PubMed] [Google Scholar]
- 41.Denic A., Mathew J., Lerman L.O., Lieske J.C., Larson J.J., Alexander M.P. Single-nephron glomerular filtration rate in healthy adults. N Engl J Med. 2017;376:2349–2357. doi: 10.1056/NEJMoa1614329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhong C.C., Chen F., Yang J.L., Jia W.W., Li L., Cheng C. Pharmacokinetics and disposition of anlotinib, an oral tyrosine kinase inhibitor, in experimental animal species. Acta Pharmacol Sin. 2018;39:1048–1063. doi: 10.1038/aps.2017.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Obach R.S., Walsky R.L., Venkatakrishnan K. Mechanism-based inactivation of human cytochrome p450 enzymes and the prediction of drug‒drug interactions. Drug Metab Dispos. 2007;35:246–255. doi: 10.1124/dmd.106.012633. [DOI] [PubMed] [Google Scholar]
- 44.Grimm S.W., Einolf H.J., Hall S.D., He K., Lim H.K., Ling K.H. The conduct of in vitro studies to address time-dependent inhibition of drug-metabolizing enzymes: a perspective of the pharmaceutical research and manufacturers of America. Drug Metab Dispos. 2009;37:1355–1370. doi: 10.1124/dmd.109.026716. [DOI] [PubMed] [Google Scholar]
- 45.Jiang R., Dong J., Li X., Du F., Jia W., Xu F. Molecular mechanisms governing different pharmacokinetics of ginsenosides and potential for ginsenoside-perpetrated herb‒drug interactions on OATP1B3. Br J Pharmacol. 2015;172:1059–1073. doi: 10.1111/bph.12971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hermann R., Von Richter O. Clinical evidence of herbal drugs as perpetrators of pharmacokinetic drug interactions. Planta Med. 2012;78:1458–1477. doi: 10.1055/s-0032-1315117. [DOI] [PubMed] [Google Scholar]
- 47.Venkataramanan R., Komoroski B., Strom S. In vitro and in vivo assessment of herb drug interactions. Life Sci. 2006;78:2105–2115. doi: 10.1016/j.lfs.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 48.Lu T., Yang J., Gao X., Chen P., Du F., Sun Y. Plasma and urinary tanshinol from Salvia miltiorrhiza (Danshen) can be used as pharmacokinetic markers for cardiotonic pills, a cardiovascular herbal medicine. Drug Metab Dispos. 2008;36:1578–1586. doi: 10.1124/dmd.108.021592. [DOI] [PubMed] [Google Scholar]
- 49.Chen F., Li L., Xu F., Sun Y., Du F., Ma X. Systemic and cerebral exposure to and pharmacokinetics of flavonols and terpene lactones after dosing standardized Ginkgo biloba leaf extracts to rats via different routes of administration. Br J Pharmacol. 2013;170:440–457. doi: 10.1111/bph.12285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Olaleye O.E., Niu W., Du F.F., Wang F.Q., Xu F., Pintusophon S. Multiple circulating saponins from intravenous ShenMai inhibit OATP1Bs in vitro: potential joint precipitants of drug interactions. Acta Pharmacol Sin. 2018 doi: 10.1038/s41401-018-0173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu X.W., Yang J.L., Niu W., Jia W.W., Olaleye O.E., Wen Q. Human pharmacokinetics of ginkgo terpene lactones and impact of carboxylation in blood on their platelet-activating factor antagonistic activity. Acta Pharmacol Sin. 2018;39:1935–1946. doi: 10.1038/s41401-018-0086-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xie G., Wang S., Zhang H., Zhao A., Liu J., Ma Y. Poly-pharmacokinetic study of a multicomponent herbal medicine in healthy Chinese volunteers. Clin Pharmacol Ther. 2018;103:692–702. doi: 10.1002/cpt.784. [DOI] [PubMed] [Google Scholar]
- 53.Yan R., Yang Y., Chen Y. Pharmacokinetics of Chinese medicines: strategies and perspectives. Chin Med. 2018;13:24. doi: 10.1186/s13020-018-0183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roberts J.A., Abdul-Aziz M.H., Lipman J., Mouton J.W., Vinks A.A., Felton T.W. Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis. 2014;14:498–509. doi: 10.1016/S1473-3099(14)70036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Congiu M., Mashford M.L., Slavin J.L., Desmond P.V. UDP glucuronosyltransferase mRNA levels in human liver disease. Drug Metab Dispos. 2002;30:129–134. doi: 10.1124/dmd.30.2.129. [DOI] [PubMed] [Google Scholar]
- 56.Aitken A.E., Richardson T.A., Morgan E.T. Regulation of drug-metabolizing enzymes and transporters in inflammation. Annu Rev Pharmacol Toxicol. 2006;46:123–149. doi: 10.1146/annurev.pharmtox.46.120604.141059. [DOI] [PubMed] [Google Scholar]
- 57.Harvey R.D., Morgan E.T. Cancer, inflammation, and therapy: effects on cytochrome P450–mediated drug metabolism and implications for novel immunotherapeutic agents. Clin Pharmacol Ther. 2014;96:449–457. doi: 10.1038/clpt.2014.143. [DOI] [PubMed] [Google Scholar]
- 58.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research. In vitro metabolism- and transporter-mediated drug–drug interaction studies guidance for industry guidance for industry. [updated 2017 Oct 24]. Available from: <https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM581965.pdf>.
- 59.König J., Müller F., Fromm M.F. Transporters and drug–drug interactions: important determinants of drug disposition and effects. Pharmacol Rev. 2013;65:944–966. doi: 10.1124/pr.113.007518. [DOI] [PubMed] [Google Scholar]
- 60.Wang L., Zhou G.B., Liu P., Song J.H., Liang Y., Yan X.J. Dissection of mechanisms of Chinese medicinal formula Realgar-Indigo naturalis as an effective treatment for promyelocytic leukemia. Proc Natl Acad Sci U S A. 2008;105:4826–4831. doi: 10.1073/pnas.0712365105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lam W., Bussom S., Guan F., Jiang Z., Zhang W., Gullen E.A. The four-herb Chinese medicine PHY906 reduces chemotherapy-induced gastrointestinal toxicity. Sci Transl Med. 2010;2:45ra59. doi: 10.1126/scitranslmed.3001270. [DOI] [PubMed] [Google Scholar]
- 62.Wang C., Cao B., Liu Q.Q., Zou Z.Q., Liang Z.A., Gu L. Oseltamivir compared with the Chinese traditional therapy Maxingshigan-Yinqiaosan in the treatment of H1N1 influenza: a randomized trial. Ann Intern Med. 2011;155:217–225. doi: 10.7326/0003-4819-155-4-201108160-00005. [DOI] [PubMed] [Google Scholar]
- 63.Li X., Zhang J., Huang J., Ma A., Yang J., Li W. A multicenter, randomized, double-blind, parallel-group, placebo-controlled study of the effects of qili qiangxin capsules in patients with chronic heart failure. J Am Coll Cardiol. 2013;62:1065–1072. doi: 10.1016/j.jacc.2013.05.035. [DOI] [PubMed] [Google Scholar]
- 64.Zhang L., Li P., Xing C.Y., Zhao J.Y., He Y.N., Wang J.Q. Efficacy and safety of Abelmoschus manihot for primary glomerular disease: a prospective, multicenter randomized controlled clinical trial. Am J Kidney Dis. 2014;64:57–65. doi: 10.1053/j.ajkd.2014.01.431. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.