Abstract
We have previously shown that high expression of the nucleic acid binding factor YB-1 is strongly associated with poor prognosis in a variety of cancer types. The 3-dimensional protein structure of YB-1 has yet to be determined and its role in transcriptional regulation remains elusive. Drug targeting of transcription factors is often thought to be difficult and there are very few published high-throughput screening approaches. YB-1 predominantly binds to single-stranded nucleic acids, adding further difficulty to drug discovery. Therefore, we have developed two novel screening assays to detect compounds that interfere with the transcriptional activation properties of YB-1, both of which may be generalizable to screen for inhibitors of other nucleic acid binding molecules. The first approach is a cell-based luciferase reporter gene assay that measures the level of activation of a fragment of the E2F1 promoter by YB-1. The second approach is a novel application of the AlphaScreen system, to detect interference of YB-1 interaction with a single-stranded DNA binding site. These complementary assays examine YB-1 binding to two discrete nucleic acid sequences using two different luminescent signal outputs and were employed sequentially to screen 7360 small molecule compounds leading to the identification of three putative YB-1 inhibitors.
Abbreviations: cDNA, complementary DNA; CSD, cold shock domain; CTD, C-terminal domain; DMSO, dimethylsulfoxide; dsDNA, double-stranded DNA; E2F1, E2F transcription factor 1; EGR1, early growth response 1; HTS, high-throughput screening; NTD, N-terminal domain; shRNA, short-hairpin RNA; siRNA, small-interfering RNA; ssDNA, single-stranded DNA; YB-1, Y-box binding protein-1; YBX1, Y-box binding protein gene 1
Key words: Cancer, YB-1, Luciferase, AlphaScreen, Transcription factor, Single-stranded DNA
Graphical abstract
Novel AlphaScreen and luciferase reporter gene assays for the discovery of novel small-molecule inhibitors of the transcription factor YB-1 were developed and applied in a collection of 7360 compounds. Finally, three putative YB-1 inhibitors were yielded.
1. Introduction
Y-box binding protein-1 (YB-1) is a multifunctional nucleic acid binding protein that preferentially binds single-stranded DNA (ssDNA) and RNA. It also binds double stranded DNA (dsDNA). YB-1 regulates gene expression at both transcriptional and translational levels1., 2., 3. and is involved in the splicing, packaging and stabilization of mRNA, as well as DNA replication and repair4. These diverse functions appear to share a common theme of direct or indirect nucleic acid binding, through which YB-1 can influence a multitude of cellular processes that are disturbed during cancer5.
YB-1 appears to be a driver of many cancer types including tumors of the breast, ovary, intestine, lung, liver, prostate, skin and blood5., 6.. In breast cancer, the highest expression levels of Y-box binding protein gene 1 (YBX1), the RNA that encodes YB-1, are found in the most aggressive and rapidly proliferating tumor subtypes7. YBX1 RNA levels provide a significant indicator of breast cancer patient prognosis8., 9., 10. and in a rapidly proliferating breast cancer cell line, YB-1 promotes resistance to paclitaxel via its downstream target early growth response 1 (EGR1)11. In addition to breast cancer, small-interfering (si)RNA-mediated knockdown of YB-1 inhibits the growth of tumor cell lines of several other histological types7., 12.. For example, in vitro short-hairpin (sh)RNA-mediated knockdown of YB-1 reduces melanoma cell proliferation, migration and invasion, decreases drug resistance, and increases apoptosis13. Conversely, increased expression of YB-1 correlates with melanoma progression13., 14. and epithelial-to-mesenchymal transition15.
YB-1 has been shown to preferentially transactivate genes encoding proteins involved in cellular proliferation16, including cyclins17, E2F transcription factor 1 (E2F1) targets and E2F family members7, and is highly expressed in tumors with a high mitotic index7 or resistant to chemotherapy18.
YB-1 consists of a short, 51-residue N-terminal domain (NTD), a 78-residue cold shock domain (CSD), and a large, 195-residue C-terminal domain (CTD)19. The CSD is evolutionarily conserved with homologues found across mammalian species like primates, rodents, rabbits, bats and cats4. While a prediction of the YB-1 structure was recently made4, only the CSD structure has been determined using NMR20. The 3-dimentional (3-D) structure of the NTD and CTD are still unknown, possibly because they are usually disordered, only becoming rigid upon ligand binding, and may vary when bound to different ligands. This lack of a rigid structure may enhance YB-1׳s capacity to interact specifically with a variety of ligands6. However, without 3-D structures of the NTD and CTD, it is not possible to conduct rational and structure-based drug design21. Therefore, we developed functional assays to identify compounds that inhibit YB-1 activity.
2. Materials and methods
2.1. Cell culture
HCT116 (colon cancer; American Type Culture Collection (ATCC), Manassas, VI, USA) and MDA-MB-231 (breast cancer; ATCC) cells were cultured in RPMI 1640 (ThermoFisher, Waltham, MA, USA) supplemented with 5% (v/v) fetal bovine serum (FBS) and 1% (v/v) penicillin-streptomycin-glutamine (PSG; ThermoFisher). A375 (melanoma; ATCC) cells were cultured in complete Dulbecco׳s modified Eagle׳s medium (DMEM; ThermoFisher) supplemented with 5% (v/v) FBS (ThermoFisher) and 1% (v/v) PSG. Cells were grown in a humidified incubator at 37 °C with 5% (v/v) CO2.
2.2. Reporter gene assay
A 728 base-pair E2F1 promoter fragment22 was cloned into the pGL4.17 vector (Promega, Fitchburg, WI, USA) upstream of a firefly luciferase reporter gene to create the pGL4.17-E2F1-728 plasmid. Cloning was confirmed by restriction digest and sequencing.
To establish a cell-based luminescence assay capable of measuring the activity of YB-1, HCT116 cells were transfected with the pGL4.17-E2F1-728 plasmid. Through this promoter fragment, endogenous YB-1 activates transcription of the luciferase reporter gene7. In addition to YB-1, the transcription factor E2F1 autonomously binds and increases the activity of the promoter of its encoding gene E2F122. Increased transcription of the luciferase gene leads to a greater amount of luciferase protein, which is proportional to the amount of luminescence produced as a result of bioluminescent reactions catalyzed by the activity of luciferase upon addition of its substrate.
An inhibitor of YB-1 activity was required as a control to validate this E2F1 promoter: luciferase reporter gene assay. YB-1 has previously been shown to strongly bind (Kd~4 nmol/L) to a promoter fragment of the human γ-globin genes either in cells or cell-free systems23., 24.. This sequence has been used to isolate YB-1 from cellular extracts by affinity purification23. A decoy oligonucleotide containing the same sequence (5′-CCTCCCACCCTCCCCACCCTCCCCACCCTCCCC-3′) was constructed and used in excess molar amounts to be bound by YB-1, thereby mimicking or modeling inhibition of YB-1 binding to other nucleic acids.
HCT116 cells were seeded into 100 mm cell culture dishes 12–18 h prior to transfection with 8 µg of pGL4.17-E2F1–728 plasmid DNA by Lipofectamine 3000 (ThermoFisher). A parallel transfection was performed with this plasmid and 5 nmol of decoy oligonucleotide. After incubation for 6 h at 37 °C, cells were re-suspended and dispensed into 384-well plates at 8000 cells/well. Eight hours thereafter, screening compounds in dimethylsulfoxide (DMSO) were dispensed by robot [final concentration of DMSO was 0.5% (v/v)]. An equivalent amount of DMSO without compound was added to control wells containing transfected cells with or without decoy oligonucleotide. Thirty-six hours after transfection, each well received 30 µL of SteadyGlo luciferase Substrate (Promega), incubated at room temperature for 20 min and measured for luminescence using an EnSpire® Multimode Plate Reader (PerkinElmer, Boston, MA, USA). IC50 concentrations were calculated by fitting data to dose-response equations, and then calculating concentrations at which the relative luminescent signal is 50% of that of the control wells.
2.3. AlphaScreen assay
An AlphaScreen assay system was adapted to screen compounds that inhibit YB-1 binding to ssDNA, which is a biotinylated oligonucleotide containing a 3× repeat of the promoter fragment of human γ-globin genes.
AlphaScreen acceptor beads (PerkinElmer) were conjugated, according to the manufacturer׳s instructions, to a polyclonal sheep anti-YB-1 antibody generated as previously described23. Fifty µL AlphaScreen reactions were performed in 96-well OptiPlates (PerkinElmer) using PBS with 0.2% (w/v) bovine serum albumin (MilliporeSigma, Burlington, MA, USA) buffer. The reactions were set up as follows, with final reaction concentrations given in parentheses. Dispensed into each well was 20 µL of buffer containing purified YB-1 protein23 (40 fmol/L), with control wells also receiving decoy oligonucleotide (1 pmol/L). After 30 min incubation at room temperature, each well received 10 µL of buffer containing antibody-conjugated AlphaScreen acceptor beads (20 μg/mL) and the biotinylated 3× repeat oligonucleotide (2.5 fmol/L). Plates were then incubated in darkness for 60 min at room temperature before addition of 20 µL of buffer containing streptavidin-coated AlphaScreen donor beads (20 μg/mL; PerkinElmer). Following another 60 min incubation in the dark, plates were read on the Enspire® Multimode Plate Reader, with excitation and emission detection wavelengths of 680 and 570 nm, respectively. IC50 concentrations were calculated by fitting data to dose-response equations, and then calculating concentrations at 50% of the maximal response. As a complementary screen to eliminate false positives, an AlphaScreen TruHits Kit (PerkinElmer) was used according to the manufacturer׳s instructions. This kit consists of acceptor beads and donor beads that form a complex via a streptavidin to biotin interaction which results in emission of a luminescent signal. This signal can be reduced by compounds capable of interfering with these fundamental AlphaScreen assay system components.
2.4. Screening compounds
For the primary screening, 7360 small molecule compounds from the Chinese National Compound Library in Shanghai (http://en.cncl.org.cn/) were used. For compounds of interest that were re-ordered for evaluation experiments, compound identity and purity were confirmed by nuclear magnetic resonance (NMR) and mass spectra.
2.5. Computational filtering
Computational filtering was performed on compound structures to remove samples possessing specified traits or containing certain substructures. Filters were applied using the SYBYL-X 2.11 software (Certara, Princeton, NJ, USA) with compound structure inputted in Structure Data File (SDF) format. The first filter eliminated compounds containing substructures identified as pan-assay interference compounds (PAINS)25. Five increasingly stringent filters were applied to eliminate groups unfavorable for drug development, such as groups with toxicity, poor pharmacokinetic behavior or that are highly electrophilic. The filters applied, from least stringent to most stringent, were: WEHI_93K, Baell 2013 Filters 1, 2 and 3, and the CTX filter26.
2.6. Cell enumeration assay
Cell lines A375, MDA-MB-231 or HCT116 were seeded at approximately 2000 cells/well into 96-well plates. After allowing 2 h for adhesion, cells were treated with a range of concentrations of three putative YB-1 inhibitors identified during the high-throughput screening (HTS) campaign. Each of these was diluted in DMSO, with DMSO without compound added to control cells [final concentration of DMSO was 0.5% (v/v)]. DNA content was measured, as a surrogate for adherent live cell numbers, using a SYBR Green I-based fluorimetric assay as described previously11. In brief, cells were incubated with diluted compound or DMSO only for 24, 48 or 72 h at 37 °C, medium was then removed and plates frozen at –80 °C. Plates were thawed before each well received SYBR Green I Nucleic Acid Gel Stain (ThermoFisher) diluted 1:4000 (v/v) in lysis buffer [10 mmol/L Tris–HCl pH 8.0, 140 mmol/L NaCl, 1 mmol/L ethylenediaminetetraacetic acid (EDTA), 1% (v/v) Triton X-100, 0.1% (w/v) sodium deoxycholate, 0.1% (w/v) sodium dodecyl sulfate (SDS)]. After 8 h of incubation at room temperature, fluorescence was measured with excitation/emission wavelengths of 485 nm/535 nm to derive a signal proportional to cell number for each well. IC50 concentrations were calculated by fitting data to dose-response equations, and then calculating compound concentrations at 50% of the control cell signal.
2.7. Reverse transcription-quantitative PCR
Six-well tissue culture plates were seeded with 160,000 MDA-MB231 cells. After allowing 2 h for adhesion, cells were treated with three putative YB-1 inhibitors (20–160 µmol/L) before incubation at 37 °C. Equivalent volumes of DMSO were added to control wells to a final concentration of 0.5% (v/v). Ten h after addition of compounds, medium was removed and plates were frozen at −80 °C. Cellular RNA was extracted using TRIzol reagent (ThermoFisher) according to the manufacturer׳s instructions.
cDNA was synthesized in reverse transcription reactions using SuperScript IV (ThermoFisher) according to the manufacturer׳s instructions. Upon completion of reverse transcription, reactions were diluted 1:3 prior to 1 μL being used in each 10 μL reverse transcription-quantitative polymerase chain reaction (RT-qPCR). Primers were used to amplify EGR1 and LAMIN transcripts as previously described11. RT-qPCR was performed using SYBR Select Master Mix (ThermoFisher) according to the manufacturer׳s instructions on a QuantStudio 12 K Flex Real-Time PCR system (ThermoFisher). Data were analyzed by normalizing to the reference transcript (LAMIN) and the level of expression relative to the DMSO-treated control cells was calculated using the 2ΔddCt method27.
3. Results
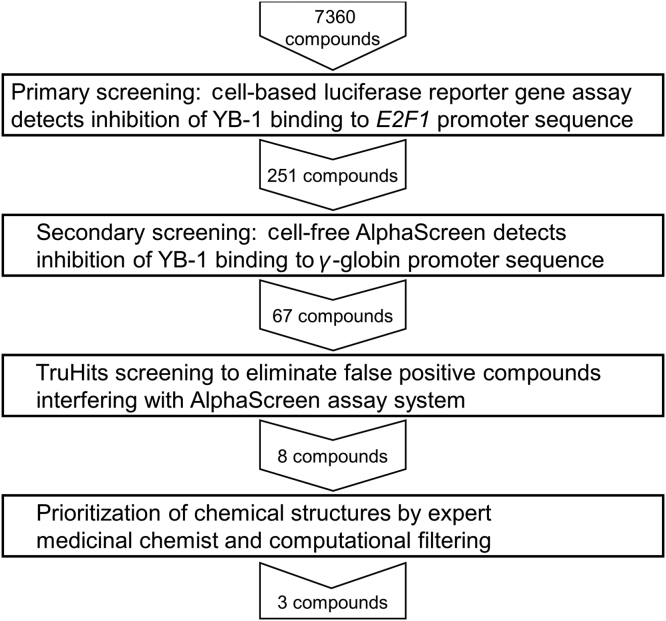
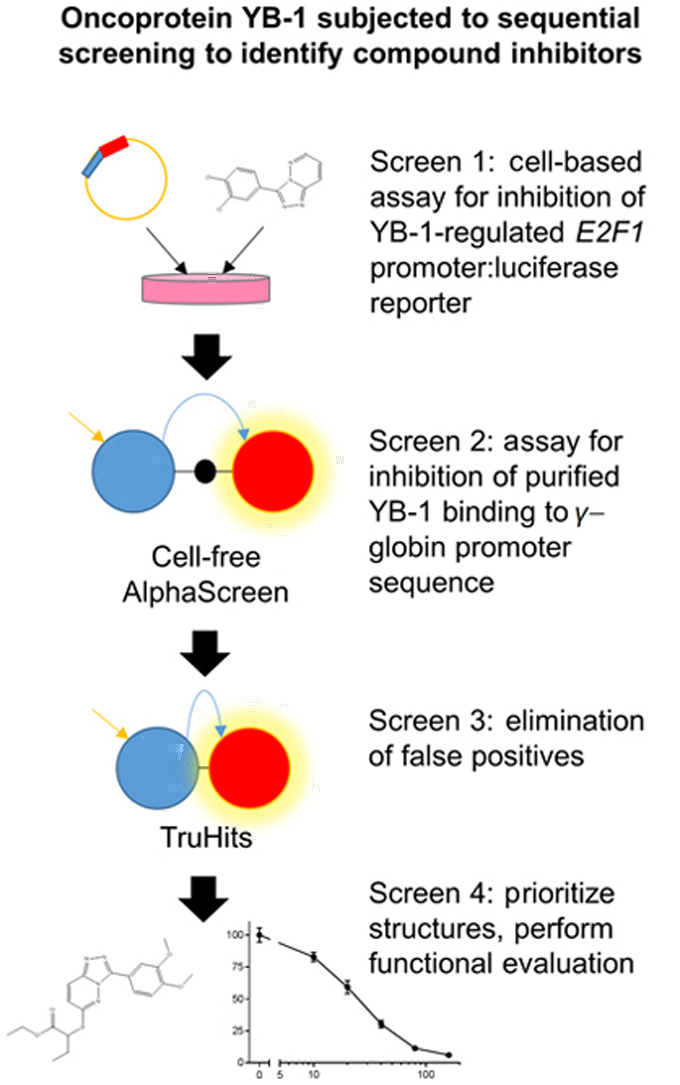
In order to discover potential YB-1 inhibitors, two complementary assays were developed to detect modulation of its transcription factor activity or inhibition of its binding to a specific nucleic acid sequence. The first assay was based on the transcriptional activation of the E2F1 promoter by YB-17 and employed a cell-based luciferase reporter gene system to screen for inhibitors. The compounds identified by this method were then tested in a novel AlphaScreen assay, using a single-stranded oligonucleotide which YB-1 is known to bind with high affinity23., 24., in order to identify compounds that also interfere with YB-1 binding to this sequence. The sequential HTS process involving 7360 compounds is shown in Fig. 1.
Figure 1.
Process of screening for compounds that inhibit YB-1 nucleic acid binding. A sequential screening approach was used to reduce 7360 starting compounds to three putative YB-1 inhibitors.
3.1. Primary screening
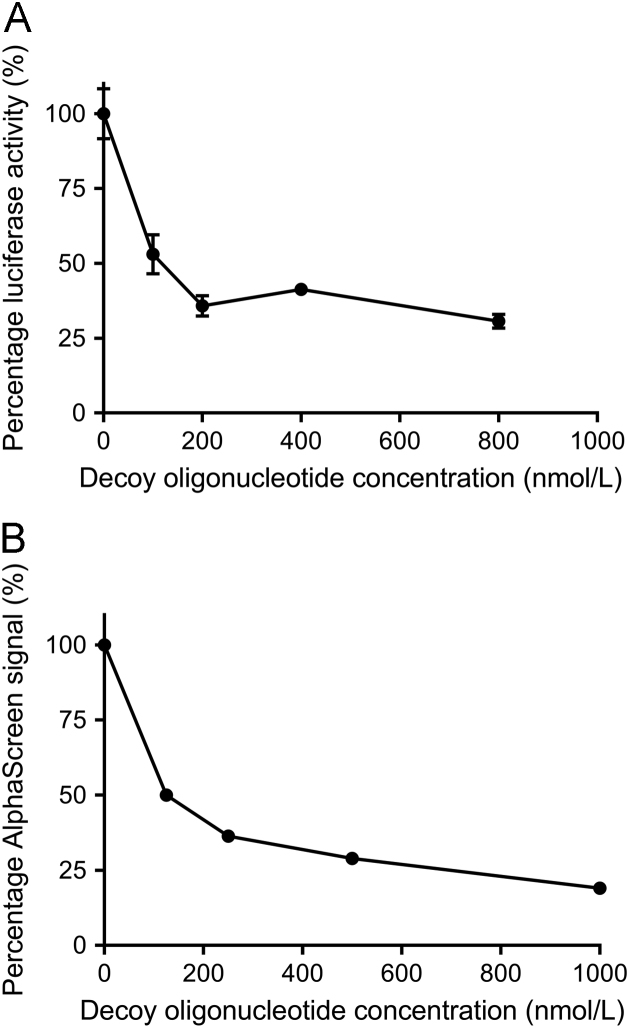
In HCT116 cells transfected with the pGL4.17-E2F1-728 plasmid, we observed that co-transfection of increasing concentrations of the decoy oligonucleotide reduced the level of E2F1 promoter-driven luciferase activity (Fig. 2A). As the decoy oligonucleotide concentration increased, the luciferase activity decreased, but at a diminishing rate. This, along with the saturation of this effect at the highest oligonucleotide concentrations, suggests that the decoy oligonucleotide competed with the E2F1 promoter for binding by YB-1, and thus decreased transcriptional activation of the E2F1 promoter by YB-1. Maximal inhibition was observed with 800 nmol/L of decoy oligonucleotide, so subsequent screening experiments used 1 µmol/L of decoy oligonucleotide, exceeding the concentration of maximal inhibition, as a control to generate a luminescent signal that was designated “100% inhibited”. To exhibit luminescent signal representing reporter gene activity that has not been inhibited, i.e., 0% inhibition, we used cells transfected with only the pGL4.17-E2F1-728 plasmid without compound treatment. The decrease in luminescent signal effected by each compound was mapped as a percentage inhibition between these two controls representing 0% and 100% inhibition.
Figure 2.
Competitive inhibition of E2F1 promoter activation (as measured by luciferase activity) and YB-1 binding to oligonucleotide (as measured by AlphaScreen signal) by the decoy oligonucleotide. In the luciferase and AlphaScreen assays, the decoy oligonucleotide competes with other nucleic acid sequences for binding by YB-1. (A) YB-1 activation of the E2F1 promoter decreases with increasing concentrations of decoy oligonucleotide. Data shown are the mean of three replicates at each decoy oligonucleotide concentration within one experiment, with error bars indicating standard error of the mean (SEM). Results shown here are representative of three independent experiments. (B) YB-1 interaction with the binding oligonucleotide decreases with increasing concentrations of the decoy oligonucleotide, resulting in decreasing AlphaScreen signal. Data shown are the mean of two replicates at each decoy oligonucleotide concentration within one experiment. Results shown here are representative of five independent experiments.
In total, 7360 compounds were screened on twenty-three 384-well plates (Fig. 1 and Supporting Information Table S1) at concentrations of 10 µmol/L. The Z′ factor defined by Zhang et al.28 was used to evaluate assay quality29. The Z′ factors for the HTS campaign involving 23 plates were within the range of 0 to 0.5 and transfection was performed in six batches. There was strong evidence that the Z′ factor was significantly affected by transfection batch (***P=0.000191), and was negatively correlated to the standard deviation of the 0% inhibited control well signal (correlation coefficient=−0.94).
Compounds were prioritized for advancement to the secondary screening if their reduction of luciferase signal was at least 90% of that achieved by 1 μmol/L of the decoy oligonucleotide. For plates where five or fewer compounds were identified, the luminescent signal threshold was lowered to 80%. Each 384-well plate containing 320 compounds identified up to 26 hits, to give a total of 272 hit compounds that were prioritized for advancement. They were then re-tested to confirm inhibition of the E2F1 promoter activity in this assay, from which, 251 compounds proceeded into the secondary screening (Table S1).
3.2. Secondary screening
An in vitro AlphaScreen assay was developed and used as an orthogonal, secondary screening for 251 initial hits. In contrast to the cell-based assay which used endogenous YB-1, this method utilized purified YB-1 incubated with an oligonucleotide concatemer containing three repeat sequences of the “decoy” oligonucleotide. In South-Western dot blot-based analysis, this concatemer is capable of increasing signal by stabilizing the interaction between protein and DNA30. It was confirmed in our study that the concatemer bound by YB-1 protein produces a higher signal than that of the single-repeat decoy oligonucleotide (data not shown).
We observed that the AlphaScreen signal decreased with addition of an increasing concentration of a decoy oligonucleotide (Fig. 2B), suggesting that this sequence competes with the oligonucleotide concatemer for binding by YB-1. A maximal level of signal inhibition occurred with 1 μmol/L decoy oligonucleotide and so this concentration was used in subsequent screening experiments as a “100% inhibition” control. The 251 initial hits were then tested in three sets (Fig. 1), each set contained eight 0% inhibition and four 100% inhibition control reactions. The decrease in signal caused by each compound was calculated as a percentage inhibition between these two controls. The Z′ factor was calculated from the controls in each set and found to be 0.20, 0.37 and 0.56, respectively. The calculated percentage of signal reduction for the three sets was 33.2%. Sixty-seven compounds that reduced the luminescent signal by >50% were selected for further assessment. They were screened in the TruHits assay to eliminate false positives (Fig. 1) resulting in 8 confirmed hits (Table S1).
3.3. Computational filtering
To prioritize the 8 compounds identified above for suitability for drug development, their structures were subjected to computational filters (Fig. 1). The first filter eliminated compounds containing substructures identified as PAINS25, which would indicate they are likely false positives. All eight compounds passed this PAINS filter. Then five increasingly stringent filters were applied to eliminate groups unfavorable for drug development, such as groups with toxicity, poor pharmacokinetic behavior or are highly electrophilic. These filters, from least stringent to most stringent, were: WEHI_93K, Baell 2013 Filters 1, 2 and 3, and the CTX filter26. The three least stringent filters passed 7 of the 8 structures, eliminating one compound as overly chemically reactive. None of the compounds passed the two most stringent filters, Baell 2013 Filter 3 and CTX, however this did not exclude them from further investigation (see Section discussions).
Next, medicinal chemistry expertise was utilized and as a result, a further three compounds were recognized as belonging to classes known to undergo colloidal aggregation, suggesting that they may still be false positives despite passing the TruHits assay and the PAINS filter. A further compound was identified as containing a bond vulnerable to hydrolysis, requiring modification if the compound was to progress as a drug lead. The remaining three compounds (RUS0207-A006, RUS0202-G005, and JK0395-B007, structures shown in Table 1) were assessed as being unlikely to be residual false positives, with structures sufficiently drug-like to warrant continued investigation as putative drug leads.
Table 1.
Chemical structures and bioactivities of eight hit compounds identified by screening.
| Compd. | CAS registry number | Structure | Chemical formula | Molecular weight | IC50 Reporter gene assay (µmol/L) | IC50 Alpha Screen assay (µmol/L) | Percentage inhibition (%) in primary screening (reporter gene assay) | Percentage inhibition (%) in secondary screening (Alpha Screen) | Percentage non-specific inhibition (%) in TruHits screening to eliminate false positives | Medicinal chemist׳s annotation |
|---|---|---|---|---|---|---|---|---|---|---|
| RUS0207-A006 | 497917-11-0 | 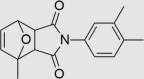 |
C17H17NO3 | 283.322 | 73 | 41 | 76 | 71 | 13 | Related to compound BMS-641988, a novel androgen receptor antagonist for the treatment of prostate cancer Possible co-polymer |
| RUS0202-G005 | 602283-51-2 | 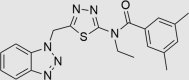 |
C20H20N6OS | 392.477 | 59 | 27 | 99 | 65 | −24 | Class known to have antifungal/antimicrobial activity |
| JK0395-B007 | 852437-97-9 | 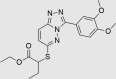 |
C19H22N4O4S | 402.467 | 30 | 25 | 80 | 59 | 13 | Triazolopyridazines patented as protein kinase inhibitors, both broadly, and specifically for inhibition of LRRK2. Inhibition of GABA-A also published |
| RUS0016-B003 | 460073-26-1 | 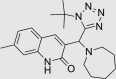 |
C22H30N6O | 394.513 | – | – | 103 | 90 | −9 | Class known to undergo colloidal aggregation (dominant mechanism for artifactual inhibition of proteins*), patented for altering eukaryote lifespan |
| RUS0020-D011 | 460070-80-8 | 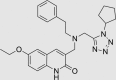 |
C27H32N6O2 | 472.582 | – | – | 103 | 58 | 16 | Class known to undergo colloidal aggregation (dominant mechanism for artifactual inhibition of proteins*), patented for altering eukaryote lifespan |
| RUS0028-G002 | 516450-12-7 | 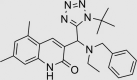 |
C26H32N6O | 444.572 | – | – | 104 | 81 | 13 | Predicted to aggregate by Aggregator Advisor Database*, patented as inhibitors of lymphoid tyrosine phosphatise and urea channel protein |
| RUS0116-H006 | 1164455-04-2 | 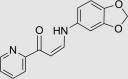 |
C15H12N2O3 | 268.267 | – | – | 96 | 50 | 6 | Patented for altering eukaryote lifespan, related to compounds as antitumor agents against human cancer cell lines. May not be stable in vivo, as it contains an easily hydrolyzed bond |
| RUS0116-G009 | 23576-89-8 | 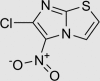 |
C5H2ClN3O2S | 203.606 | – | – | 101 | 148 | 10 | Nitro group too chemically reactive, positive in Ames mutagenicity assay |
The three putative YB-1 inhibitors (RUS0207-A006, RUS0202-G005 and JK0395-B007) are shown in the top three rows with estimated IC50 values. *www.advisor.bkslab.org. –Not applicable.
3.4. Functionality evaluation
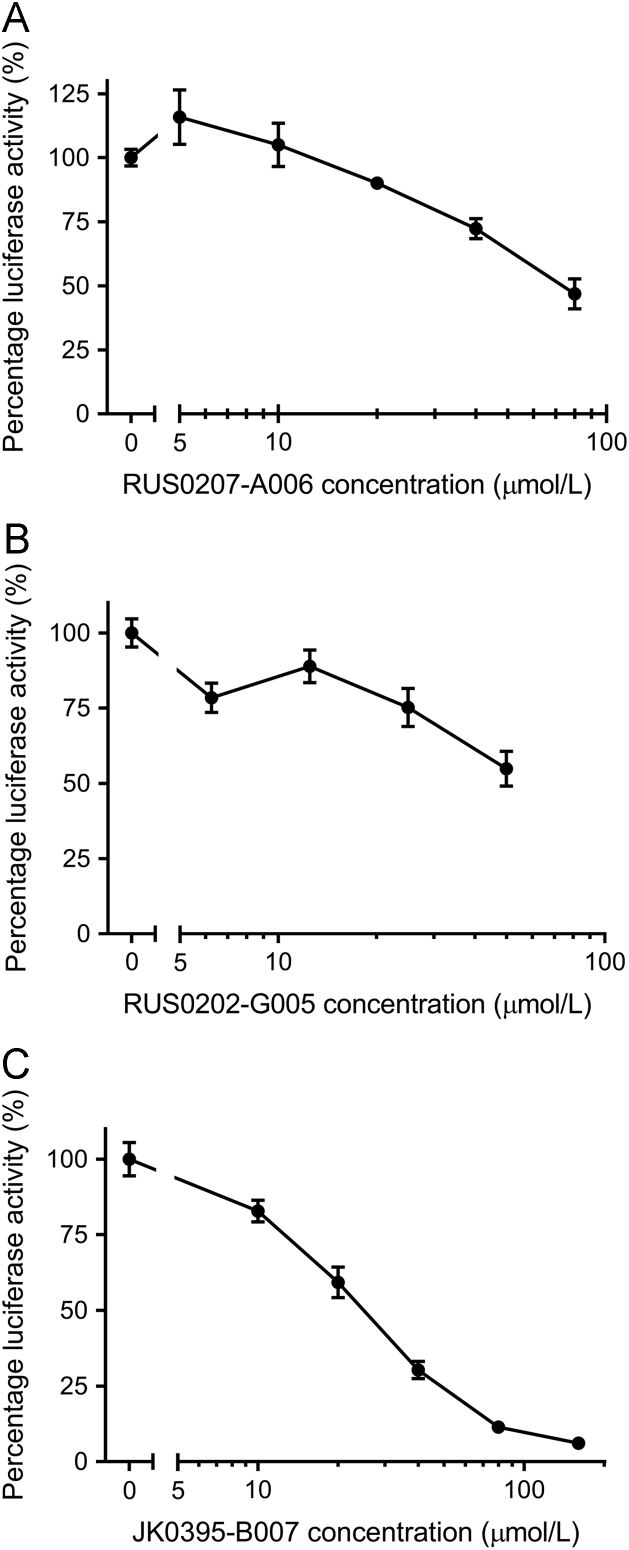
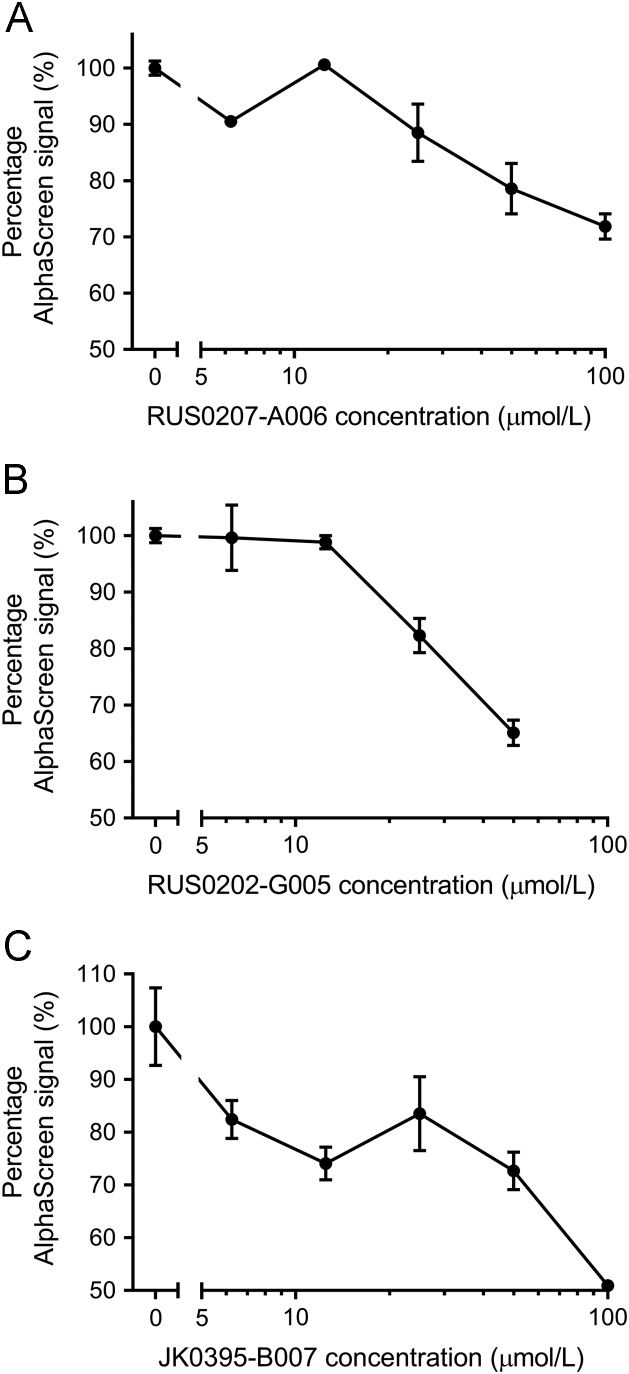
In order to confirm the bioactivity of these three putative YB-1 inhibitors, they were independently re-synthesized and then tested in the same E2F1 promoter:luciferase reporter gene and AlphaScreen assays at a range of concentrations to generate dose-response curves. It was shown that both luciferase activity (Fig. 3) and AlphaScreen signal (Fig. 4) were decreased with increasing concentrations of the compounds. In both assays, JK0395-B007 elicited the greatest signal reduction and AlphaScreen was more sensitive to low concentrations (6.25 and 12.5 µmol/L) of this compound than either of the other two.
Figure 3.
Effect of the three putative YB-1 inhibitors on E2F1 promoter:luciferase reporter gene activity in cancer cell lines. Results shown here are representative of two independent experiments. (A) Effect of RUS0207-A006 on luciferase activity in A375 cells (IC50=73 µmol/L). Data shown are the mean of four replicates at each compound concentration within one experiment, with error bars indicating SEM. (B) Effect of RUS0202-G005 on luciferase activity in HCT116 cells (IC50=59 µmol/L). Data shown are the mean of three replicates at each compound concentration within one experiment, with error bars indicating SEM. (C) Effect of JK0395-B007 on luciferase activity in HCT116 cells (IC50=30 µmol/L). Data shown are the mean of three replicates at each compound concentration within one experiment, with error bars indicating SEM.
Figure 4.
Effects of the three putative YB-1 inhibitors on YB-1 binding to decoy oligonucleotide, tested at a range of concentrations by AlphaScreen assay. Data shown are the mean of two replicates at each compound concentration within single experiment, with error bars indicating range. Results shown here are representative of two independent experiments. (A) RUS0207-A006 (IC50=41 µmol/L). (B) RUS0202-G005 (IC50=27 µmol/L). (C) JK0395-B007 (IC50=25 µmol/L).
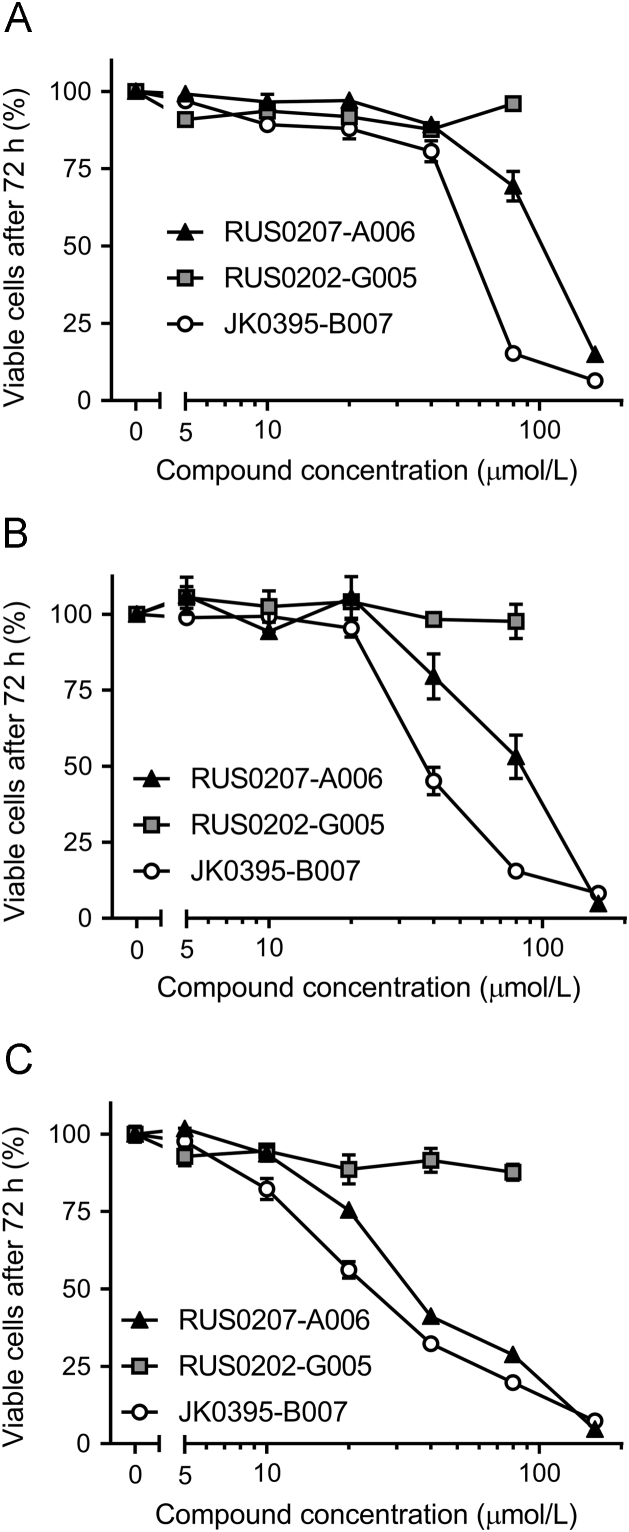
Next, we studied the effect of these compounds on cancer cell proliferation to verify if they exert action consistent with reduction of YB-1 activity in vitro7., 11.. A375, HCT116 and MDA-MB-231 cells were grown for 72 h in the presence of the three compounds and the results (Fig. 5) showed that RUS0207-A006 and JK0395-B007 inhibited the proliferation of all three cell lines, with MDA-MB-231 cells appearing most sensitive. RUS0202-G005 did not display any significant inhibitory effect on these cell lines.
Figure 5.
Effects of the three putative YB-1 inhibitors on the growth of three cancer cell lines. Measurement of percentage viable cells, based on DNA content, was performed after treatment with a concentration range of each compound for 72 h. Data were normalized and plotted relative to DMSO-treated control (no compound) cells and expressed as means±standard error of at least three replicates within a single experiment. Results shown here are representative of two independent experiments. (A) A375 cells. RUS0207-A006 IC50=102 µmol/L, JK0395-B007 IC50=50 µmol/L. (B) HCT116 cells. RUS0207-A006 IC50=85 µmol/L, JK0395-B007 IC50=38 µmol/L. (C) MDA-MB-231 cells. RUS0207-A006 IC50=38 µmol/L, JK0395-B007 IC50=30 µmol/L.
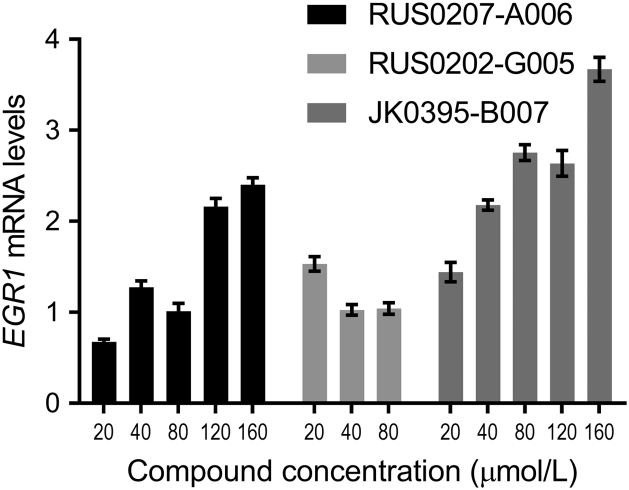
In order to determine whether these compounds were acting on-target, we further analyzed their effect on the transcription of a downstream mRNA target of YB-1. EGR1 was selected for this purpose since previous studies have demonstrated that EGR1 mRNA levels increase with reduction of YB-1 and/or YBX1 mRNA in MDA-MB-231 cells, with EGR1 postulated as a downstream target of YB-111. The three compounds, at 20–160 µmol/L concentrations, were incubated with MDA-MB-231 cells and the levels of EGR1 mRNA were found to increase in proportion to increasing concentrations of RUS0207-A006 and JK0395-B007 (Fig. 6). These effects were consistent with the hypothesis that these two compounds were acting as YB-1 inhibitors in cells.
Figure 6.
EGR1 mRNA levels, a downstream target of YB-1, following incubation of MDA-MB-231 cells for 10 h with the three putative YB-1 inhibitors at a range of concentrations from 20 µmol/L to 160 µmol/L. Data were quantitated relative to EGR1 mRNA levels in untreated cells and expressed as means ± standard error of three replicates within one experiment. For 20 µmol/L concentrations of RUS0207-A006 and JK0395-B007, the EGR1 mRNA levels are significantly lower than EGR1 mRNA levels at all higher concentrations (P < 0.05, unpaired Student׳s t-test). Results shown here are representative of two independent experiments. Note: RUS0202-G005 was used at 20 to 80 µmol/L only as it precipitates into solution at concentrations above.
4. Discussions
The present study aimed at discovering sequential orthogonal assays to identify potential inhibitors of YB-1 via a pilot screening of a compound library. A novel AlphaScreen assay for protein:ssDNA interaction was developed and could be further adapted to detect binding of ssDNA or RNA by other nucleic acid binding proteins. As an ssDNA- and RNA-binding transcription factor with a dynamic and flexible protein structure, YB-1 is an unusual and potentially difficult target. Therefore, we screened a collection of diverse small molecules that cover a broader chemical space than some more focused libraries31. Of the 7360 compounds screened in the primary assay, 251 proceeded to the secondary AlphaScreen followed by the supplementary TruHits assay to eliminate false positive hits (Fig. 1). Prioritization of the remaining compounds identified three putative YB-1 inhibitors that were further evaluated for their effect on tumor cell growth in vitro and on the mRNA level of an YB-1 target. Two of them caused a dose-dependent increase in the expression of EGR1, a downstream target of YB-1.
The E2F1 promoter:luciferase reporter gene assay was used as the primary screening due to its relatively low cost per reaction and its ability to identify compounds that not only inhibit YB-1 binding to E2F1 promoter, but may also perturb the interaction between YB-1 and E2F1 or other protein co-factors. Theoretically, this cell-based assay has the advantage of discovering compounds that are biologically relevant for future in vivo studies. For example, only compounds that passed the cell membrane would be active. We also observed that Z′ factors were significantly affected by each batch of transfected cells, suggesting variabilities in transfection efficiency. While the percentage inhibition of signal exhibited by each compound was calculated relative to the control wells on each screening plate, there may be some difficulty comparing these values across batches. However, within a single plate the values can be expected to be relative and comparable. Therefore, the threshold for identifying hit compounds was lowered when five or fewer compounds were identified in a single plate in order to capture compounds that show the greatest signal inhibition relative to the other compounds on the same plate. This use of a lower selection threshold in the primary luciferase screen when ≤5 compounds were identified in a single plate was justified by the fact that all selected compounds would be subjected to secondary screens using the AlphaScreen system.
While E2F1 promoter activity and cell proliferation rate are dependent upon E2F1 and YB-1 protein activity, many other factors also exert influences. Compounds that impact cell proliferation or other cellular processes, such as translation, will affect luminescent signal. Before the primary screening was run, it was expected that some compounds would inhibit luminescent signal independent of E2F1 or YB-1 protein inhibition. For this reason, the YB-1-specific AlphaScreen assay was designed as an orthogonal secondary screening to filter out compounds not associated with YB-1 (including E2F1 inhibitors). Key components differed between the two assays, creating complementation that improved the ability to identify YB-1 inhibitors. Each assay used a different YB-1 binding site sequence: a single-stranded oligonucleotide sequence derived from a promoter of human γ-globin genes was used in the AlphaScreen assay while a fragment of the human E2F1 promoter was used in the luciferase reporter gene assay. Additionally, the primary and secondary screenings employed different sources of YB-1: the AlphaScreen assay utilized purified YB-1, while the luciferase reporter gene assay used endogenous YB-1 within the HCT116 cells.
An advantage of our AlphaScreen assay is its adaptability. AlphaScreen is based on a signal emitted when a series of binding interactions brings two types of beads into close proximity and was originally developed for detecting protein to protein interactions32. We adapted this system to screen potential YB-1 inhibitors. While AlphaScreen was used to detect protein binding to dsDNA or RNA33., 34., 35., 36., only very recently it has been adapted to detect protein binding to ssDNA37. We initially used a similar approach but made some modifications during the process of iterative experimental development. We observed a high background noise when using protein A-coated acceptor beads and addressed this by chemically conjugating YB-1 antibody directly to Acceptor beads. Background noise was further reduced by addition of bovine serum albumin to our assay buffer. Additionally, to increase signal, a concatemer oligonucleotide containing three repeats of an YB-1 binding site was used rather than an oligonucleotide containing a single binding site. These improvements may have been required because of the flexible protein structure of YB-1, a trait that may be shared by other ssDNA-binding transcription factors. The human γ-globin promoter fragment ssDNA oligonucleotide was chosen due to the high affinity of its binding to YB-123., 24.. However, other ssDNA oligonucleotide sequences were tried in this assay during development, as well as an RNA oligonucleotide. The oligonucleotide sequence can be changed to specifically focus on YB-1 binding. Therefore, this adaptable assay may have utility for the screening with other ssDNA binding proteins, or even with RNA binding proteins, thereby expanding the use of this powerful technology37.
The present AlphaScreen assay had two uses, i.e., counter-screening as well as selectivity screening. As a counter-screening, it removed false positives. Clearly, our application of two separate, orthogonal screening systems significantly reduced the number of false positives to only those that disrupt both assay systems, such as light-absorbing compound aggregates. As a selectivity screening, the AlphaScreen assay discriminated compounds that decreased signal in the primary screening via inhibition of YB-1 binding to DNA from compounds that reduced activation of the E2F1 promoter via interaction with other proteins, such as E2F1.
The TruHits kit should be considered as an essential supplement for identifying false positives that interfere with the assay system, such as light scatterers, color quenchers, singlet oxygen quenchers and biotin mimetics. Despite the primary and secondary screenings, some of such compounds did pass through both screenings before being picked up by the TruHits kit. It should be noted that other classes of false positives exist that are not identified by the TruHits kit, such as compounds that compete with the AlphaScreen acceptor bead to bind YB-1, or those that undergo colloidal aggregation to impede assay signal. The former would not be expected to pass the primary screening. To eliminate the latter, we relied upon medicinal chemistry expertise.
None of the eight confirmed hits identified in the secondary screening passed the two most stringent computational filters, Baell 2013 Filter 3 and CTX. Baell 2013 Filter 3 represents a progressive tightening of the criteria used in Filters 1 and 2. The high stringency of Filter 3 and CTX means that they should not absolutely exclude compounds from development into drug leads. While a compound that does pass these filters would be an especially good candidate for drug development, compounds that do not pass could still be suitable for further investigation. For example, it may be possible to find more drug-like, but still active, analogues of these compounds.
Computational filtering was supplemented with the medicinal chemistry expertise that selected three putative YB-1 inhibitors. When tested in cancer cell lines, two of them inhibited proliferation and increased EGR1 mRNA levels—both activities consistent with suppression of YB-1 activity. It is possible that the third compound inhibited YB-1 via a mechanism distinct and perhaps more subtle than that of the other two compounds, such as inhibition of YB-1 polymerization38 rather than nucleic acid binding. It may also be possible that signal outputs of the assays used during this round of in vitro experiments were not as sensitive as the two screening assays originally used to identify this compound. It may be possible to investigate the inhibition of YB-1 polymerization by performing AlphaScreen experiments using variable concentrations of YB-1, which may suggest mechanisms of inhibition particular to compound RUS0202-G005, or the seemingly biphasic inhibitor profile of compound JK0395-B007 in Fig. 4C. The signal reduction caused in AlphaScreen assays by compounds that inhibit YB-1 polymerization would be expected to be more sensitive to decreasing concentrations of YB-1.
At present, the cold shock domain is the only domain of YB-1 with a determined structure20. Computational docking simulations may be able to dock the compound structures to this domain. A flexible structure may underlie the capacity of YB-1 to interact specifically with a variety of ligands6, and the structure or folding of YB-1 may be altered by these three putative YB-1 inhibitors.
5. Conclusions
We report here the development of novel AlphaScreen and luciferase reporter gene assays for the discovery of novel small-molecule inhibitors of the transcription factor YB-1. Applying these assays to screen a collection of 7360 compounds yielded three putative YB-1 inhibitors. Consistent with YB-1 inhibition, two of them reduced growth of three cancer cell lines in vitro and also increased expression of EGR1, a downstream target of YB-1. Follow-up studies are required to verify the present findings using additional experimental techniques, including animal models.
Acknowledgments
We thank Yang Feng, Xiaoqing Cai, Margareta Sutija, Sandra Fitzgerald, Puja Bhatia and Ji Wu for technical assistance. This work was partially supported by grants from Health Research Council of New Zealand (IRF213-China, 13-1019 to PS, CP, AL and AB), the Ministry of Science and Technology of China (2014DFG32200 to M-WW), Shanghai Science and Technology Development Fund (15DZ2291600 to M-WW, China) and the Thousand Talents Program in China ([2011]166 to M-WW).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Supporting data associated with this article can be found in the online version at https://doi.org/10.1016/j.apsb.2018.12.011.
Contributor Information
Cristin G. Print, Email: c.print@auckland.ac.nz.
Ming-Wei Wang, Email: mwwang@simm.ac.cn.
Annette Lasham, Email: a.lasham@auckland.ac.nz.
Appendix A. Supplementary material
Supplementary material
.
Supplementary material
.
References
- 1.MacDonald G.H., Itoh-Lindstrom Y., Ting J.P. The transcriptional regulatory protein, YB-1, promotes single-stranded regions in the DRA promoter. J Biol Chem. 1995;270:3527–3533. doi: 10.1074/jbc.270.8.3527. [DOI] [PubMed] [Google Scholar]
- 2.Evdokimova V., Ruzanov P., Imataka H., Raught B., Svitkin Y., Ovchinnikov L.P. The major mRNA-associated protein YB-1 is a potent 5′ cap-dependent mRNA stabilizer. EMBO J. 2001;20:5491–5502. doi: 10.1093/emboj/20.19.5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Svitkin Y.V., Evdokimova V.M., Brasey A., Pestova T.V., Fantus D., Yanagiya A. General RNA-binding proteins have a function in poly(A)-binding protein-dependent translation. EMBO J. 2009;28:58–68. doi: 10.1038/emboj.2008.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yadav B.S., Singh S., Shaw A.K., Mani A. Structure prediction and docking-based molecular insights of human YB-1 and nucleic acid interaction. J Biomol Struct Dyn. 2016;34:2561–2580. doi: 10.1080/07391102.2015.1124050. [DOI] [PubMed] [Google Scholar]
- 5.Lasham A., Print C.G., Woolley A.G., Dunn S.E., Braithwaite A.W. YB-1: oncoprotein, prognostic marker and therapeutic target? Biochem J. 2013;449:11–23. doi: 10.1042/BJ20121323. [DOI] [PubMed] [Google Scholar]
- 6.Eliseeva I.A., Kim E.R., Guryanov S.G., Ovchinnikov L.P., Lyabin D.N. Y-box-binding protein 1 (YB-1) and its functions. Biochemistry. 2011;76:1402–1433. doi: 10.1134/S0006297911130049. [DOI] [PubMed] [Google Scholar]
- 7.Lasham A., Samuel W., Cao H., Patel R., Mehta R., Stern J.L. YB-1, the E2F pathway, and regulation of tumor cell growth. J Natl Cancer Inst. 2012;104:133–146. doi: 10.1093/jnci/djr512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janz M., Harbeck N., Dettmar P., Berger U., Schmidt A., Jurchott K. Y-box factor YB-1 predicts drug resistance and patient outcome in breast cancer independent of clinically relevant tumor biologic factors HER2, uPA and PAI-1. Int J Cancer. 2002;97:278–282. doi: 10.1002/ijc.1610. [DOI] [PubMed] [Google Scholar]
- 9.Yoshimatsu T., Uramoto H., Oyama T., Yashima Y., Gu C., Morita M. Y-box-binding protein-1 expression is not correlated with p53 expression but with proliferating cell nuclear antigen expression in non-small cell lung cancer. Anticancer Res. 2005;25:3437–3443. [PubMed] [Google Scholar]
- 10.Chatterjee M., Rancso C., Stuhmer T., Eckstein N., Andrulis M., Gerecke C. The Y-box binding protein YB-1 is associated with progressive disease and mediates survival and drug resistance in multiple myeloma. Blood. 2008;111:3714–3722. doi: 10.1182/blood-2007-05-089151. [DOI] [PubMed] [Google Scholar]
- 11.Lasham A., Mehta S.Y., Fitzgerald S.J., Woolley A.G., Hearn J.I., Hurley D.G. A novel EGR-1 dependent mechanism for YB-1 modulation of paclitaxel response in a triple negative breast cancer cell line. Int J Cancer. 2016;139:1157–1170. doi: 10.1002/ijc.30137. [DOI] [PubMed] [Google Scholar]
- 12.Lee C., Dhillon J., Wang M.Y., Gao Y., Hu K., Park E. Targeting YB-1 in HER-2 overexpressing breast cancer cells induces apoptosis via the mTOR/STAT3 pathway and suppresses tumor growth in mice. Cancer Res. 2008;68:8661–8666. doi: 10.1158/0008-5472.CAN-08-1082. [DOI] [PubMed] [Google Scholar]
- 13.Schittek B., Psenner K., Sauer B., Meier F., Iftner T., Garbe C. The increased expression of Y box-binding protein 1 in melanoma stimulates proliferation and tumor invasion, antagonizes apoptosis and enhances chemoresistance. Int J Cancer. 2007;120:2110–2118. doi: 10.1002/ijc.22512. [DOI] [PubMed] [Google Scholar]
- 14.Sinnberg T., Sauer B., Holm P., Spangler B., Kuphal S., Bosserhoff A. MAPK and PI3K/AKT mediated YB-1 activation promotes melanoma cell proliferation which is counteracted by an autoregulatory loop. Exp Dermatol. 2012;21:265–270. doi: 10.1111/j.1600-0625.2012.01448.x. [DOI] [PubMed] [Google Scholar]
- 15.Kosnopfel C., Sinnberg T., Sauer B., Busch C., Niessner H., Schmitt A. YB-1 expression and phosphorylation regulate tumorigenicity and invasiveness in melanoma by influencing EMT. Mol Cancer Res. 2018;16:1149–1160. doi: 10.1158/1541-7786.MCR-17-0528. [DOI] [PubMed] [Google Scholar]
- 16.Ladomery M., Sommerville J. A role for Y-box proteins in cell proliferation. Bioessays. 1995;17:9–11. doi: 10.1002/bies.950170104. [DOI] [PubMed] [Google Scholar]
- 17.Jurchott K., Bergmann S., Stein U., Walther W., Janz M., Manni I. YB-1 as a cell cycle-regulated transcription factor facilitating cyclin A and cyclin B1 gene expression. J Biol Chem. 2003;278:27988–27996. doi: 10.1074/jbc.M212966200. [DOI] [PubMed] [Google Scholar]
- 18.Alemasova E.E., Naumenko K.N., Kurgina T.A., Anarbaev R.O., Lavrik O.I. The multifunctional protein YB-1 potentiates PARP1 activity and decreases the efficiency of PARP1 inhibitors. Oncotarget. 2018;9:23349–23365. doi: 10.18632/oncotarget.25158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guryanov S.G., Filimonov V.V., Timchenko A.A., Melnik B.S., Kihara H., Kutyshenko V.P. The major mRNP protein YB-1: structural and association properties in solution. Biochim Biophys Acta. 2013;1834:559–567. doi: 10.1016/j.bbapap.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Kloks C.P., Spronk C.A., Lasonder E., Hoffmann A., Vuister G.W., Grzesiek S. The solution structure and DNA-binding properties of the cold-shock domain of the human Y-box protein YB-1. J Mol Biol. 2002;316:317–326. doi: 10.1006/jmbi.2001.5334. [DOI] [PubMed] [Google Scholar]
- 21.Wu X.N., Huang Y.D., Li J.X., Yu Y.F., Qian Z., Zhang C. Structure-based design, synthesis, and biological evaluation of novel pyrimidinone derivatives as PDE9 inhibitors. Acta Pharm Sin B. 2018;8:615–628. doi: 10.1016/j.apsb.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson D.G., Ohtani K., Nevins J.R. Autoregulatory control of E2F1 expression in response to positive and negative regulators of cell cycle progression. Genes Dev. 1994;8:1514–1525. doi: 10.1101/gad.8.13.1514. [DOI] [PubMed] [Google Scholar]
- 23.Cohen S.B., Ma W., Valova V.A., Algie M., Harfoot R., Woolley A.G. Genotoxic stress-induced nuclear localization of oncoprotein YB-1 in the absence of proteolytic processing. Oncogene. 2010;29:403–410. doi: 10.1038/onc.2009.321. [DOI] [PubMed] [Google Scholar]
- 24.Horwitz E.M., Maloney K.A., Ley T.J. A human protein containing a “cold shock” domain binds specifically to H-DNA upstream from the human γ-globin genes. J Biol Chem. 1994;269:14130–14139. [PubMed] [Google Scholar]
- 25.Baell J.B., Holloway G.A. New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays. J Med Chem. 2010;53:2719–2740. doi: 10.1021/jm901137j. [DOI] [PubMed] [Google Scholar]
- 26.Baell J.B. Broad coverage of commercially available lead-like screening space with fewer than 350,000 compounds. J Chem Inf Model. 2013;53:39–55. doi: 10.1021/ci300461a. [DOI] [PubMed] [Google Scholar]
- 27.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J.H., Chung T.D., Oldenburg K.R. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 29.Xiao T., Liu R., Proud C.G., Wang M.W. A high-throughput screening assay for eukaryotic elongation factor 2 kinase inhibitors. Acta Pharm Sin B. 2016;6:557–563. doi: 10.1016/j.apsb.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh H., Clerc R.G., LeBowitz J.H. Molecular cloning of sequence-specific DNA binding proteins using recognition site probes. BioTechniques. 1989;7:252–261. [PubMed] [Google Scholar]
- 31.Gong Z., Hu G., Li Q., Liu Z., Wang F., Zhang X. Compound libraries: recent advances and their applications in drug discovery. Curr Drug Discov Technol. 2017;14:216–228. doi: 10.2174/1570163814666170425155154. [DOI] [PubMed] [Google Scholar]
- 32.Eglen R.M., Reisine T., Roby P., Rouleau N., Illy C., Bosse R. The use of AlphaScreen technology in HTS: current status. Curr Chem Genom. 2008;1:2–10. doi: 10.2174/1875397300801010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mukherjee S., Hanson A.M., Shadrick W.R., Ndjomou J., Sweeney N.L., Hernandez J.J. Identification and analysis of hepatitis C virus NS3 helicase inhibitors using nucleic acid binding assays. Nucleic Acids Res. 2012;40:8607–8621. doi: 10.1093/nar/gks623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen C.Z., Sobczak K., Hoskins J., Southall N., Marugan J.J., Zheng W. Two high-throughput screening assays for aberrant RNA–protein interactions in myotonic dystrophy type 1. Anal Bioanal Chem. 2012;402:1889–1898. doi: 10.1007/s00216-011-5604-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cassel J.A., Blass B.E., Reitz A.B., Pawlyk A.C. Development of a novel nonradiometric assay for nucleic acid binding to TDP-43 suitable for high-throughput screening using AlphaScreen technology. J Biomol Screen. 2010;15:1099–1106. doi: 10.1177/1087057110382778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mills N.L., Shelat A.A., Guy R.K. Assay Optimization and screening of RNA–Protein interactions by AlphaScreen. J Biomol Screen. 2007;12:946–955. doi: 10.1177/1087057107306128. [DOI] [PubMed] [Google Scholar]
- 37.Khageh Hosseini S., Kolterer S., Steiner M., von Manstein V., Gerlach K., Trojan J. Camptothecin and its analog SN-38, the active metabolite of irinotecan, inhibit binding of the transcriptional regulator and oncoprotein FUBP1 to its DNA target sequence FUSE. Biochem Pharmacol. 2017;146:53–62. doi: 10.1016/j.bcp.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 38.Kretov D.A., Curmi P.A., Hamon L., Abrakhi S., Desforges B., Ovchinnikov L.P. mRNA and DNA selection via protein multimerization: YB-1 as a case study. Nucleic Acids Res. 2015;43:9457–9473. doi: 10.1093/nar/gkv822. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material