Introduction
Bromhidrosis is known as foul-smelling perspiration with or without hyperhidrosis. Apocrine bromhidrosis is the result of apocrine sweat being degraded by cutaneous bacteria, leading to ammonia and short-chain fatty acids.1 The odor from apocrine bromhidrosis will resemble typical body odor. In contrast, eccrine bromhidrosis results in various distinguishing odors unique to its primary cause: bacterial degradation of the stratum corneum, metabolic disorders, or the ingestion of odorgenic foods such as garlic and asparagus.1
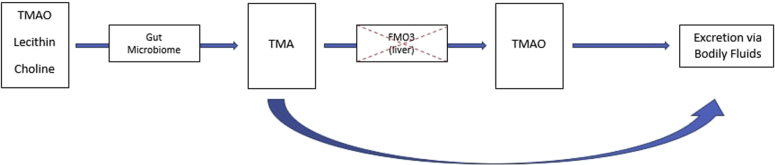
Trimethylaminuria (TMAU) is a rare autosomal recessive metabolic disorder hallmarked by the loss of the hepatic enzyme, flavin mono-oxygenase 3 (FMO3). This results in the inability to properly metabolize trimethylamine (TMA) to trimethylamine-N-oxide. Consequently, a build-up of excessive TMA, which has a fishy odor, is eventually excreted through bodily fluids such as perspiration, giving the sweat a characteristic foul odor (Fig 1).2
Fig 1.
Pathway for the metabolism of trimethylamine-N-oxide (TMAO), lecithin, and choline. In trimethylaminuria (TMA), there is a loss of function of the enzyme flavin mono-oxygenase 3 (FMO3), resulting in the inability to properly metabolize TMA. The overabundance of TMA is eventually excreted through body fluids including urine and sweat, resulting in a foul fish-like odor.
TMAU can have a significant impact on an individual's quality of life and predisposes those affected to self-isolation, depression, and psychosocial stress. Patients are often forced to avoid foods that contain precursors of TMA or inhibitors of FMO3, such as eggs, seafood, legumes, and soy, in an attempt to reduce their bromhidrosis, but this can be difficult to do and minimally effective.3
MiraDry (Sientra, Santa Clara, CA) is a technology approved by the US Food and Drug Administration in 2011 and has been used to treat hyperhidrosis. The treatment is a noninvasive procedure that uses controlled microwave-generated thermal energy to create heat within the reticular dermis and subcutaneous tissue, thus destroying adnexal structures, including apocrine and eccrine sweat glands. The results are permanent and have been clinically proven to show an 82% reduction in sweat and 89% reduction in odor.4, 5 Exuberant swelling is the most common posttreatment reaction. Two treatments, 2 to 3 months apart, are generally required to achieve satisfying results in the average patient.
We present the case of a 31-year-old woman in whom trimethylaminuria was diagnosed at the age of 19 years who received MiraDry to nonselectively destroy her sweat glands, significantly reducing her bromhidrosis with satisfying results.
Case report
A 31-year-old woman presented to our clinic with the chief complaint of severe bromhidrosis secondary to a metabolic disorder, TMAU, involving the axilla and groin. She reported that around the age of 16 years, she began experiencing hyperhidrosis with a foul odor, which was diagnosed when she was 19 years old as TMAU. The patient found her condition, particularly the axillary bromhidrosis, to be severely socially debilitating because she was very self-conscious about the odor and whether others around her could perceive it. As a result, she restricted herself from public areas and would go out only immediately after showering. She became ashamed of her body and felt like she was unable to participate in normal activities such as going to the gym, dating, and socializing with friends. She had joined multiple support groups and was seeing a psychologist regularly because her self-isolation resulted in her becoming increasingly depressed.
Before presenting to us, she had tried topical aluminum chloride, which was ineffective. She did not want to try oral anticholinergics because of possible adverse effects. We assessed her quality of life using the Dermatology Life Quality Index (DLQI), on which she scored 28 out of 30, which is interpreted as her disease having an “extremely large effect on [the] patient's life.”6 Because MiraDry is a permanent method of nonselectively destroying apocrine and eccrine glands, we offered the treatment as an option. After careful review of all of the possible adverse effects associated with the treatment, the patient believed that this would be her only permanent option for improvement.
On the treatment day, the patient received tumescent anesthesia, and both axillae were gridded with a template, according to the manufacturer's recommended protocol, indicating where the treatment points would be. The device was set to a setting of 5 for both axilla. She tolerated the procedure and experienced mild pinpoint bleeding from the anesthesia insertion points. For about 1 week after the procedure, she was markedly swollen, which is an expected response. She did not experience any prolonged numbness, tingling, or paresthesia.
Six weeks after her first treatment, the patient reported that her perspiration had been reduced by approximately 75%, but her repeat DLQI was relatively unchanged (score of 24). A second treatment was offered at that time, and the patient returned 10 weeks later. At that visit, she reported that her symptoms had resolved by 80% to 85%, and her final DLQI reflected that improvement (score of 10).
Discussion
Although TMAU and bromhidrosis are not life-threatening conditions, the resulting symptoms may have considerable negative psychosocial consequences. As evident with the patient described in this case report, affected individuals may suffer from anxiety, depression, social isolation, and low self-esteem.7 Several treatment options for axillary hyperhidrosis exist: topical antiperspirants, oral medications, injectable agents (ie, botulinum toxin), surgery, and laser procedures. However, noninvasive treatments such as botulinum toxin injections and topicals offer only temporary solutions, and invasive treatments such as thoracic sympathectomy, subcutaneous shaving, and tumescent liposuction may lead to adverse effects, including compensatory hyperhidrosis, significant scarring, infection, and restricted arm movement.8, 9
MiraDry, a microwave-based device, provides an alternative, more permanent solution to hyperhidrosis. This particular patient experienced improvements in her DLQI score after each of her 2 treatments—a dramatic 18-point total difference characterized by increased comfort in social settings and less interference with daily activities—and noted an 80% to 85% improvement in sweat and odor. As a treatment that offers the possibility of long-term efficacy with mild adverse effects, this microwave-based technology is a favorable option for axillary bromhidrosis secondary to TMAU and, possibly, other metabolic or genetic disorders that result in various forms of hyperhidrosis or bromhidrosis.
Footnotes
Funding sources: None.
Disclosure: Dr Chapas is an investigator and advisory board member for Candela, has been previously an investigator for Endymed, and is a consultant for Solta and Merz. Dr Patal, Ms Tu, and Ms Fernandes have no conflicts of interest to declare.
References
- 1.Semkova K., Gergovska M., Kazandjieva J., Tsankov N. Hyperhidrosis, bromhidrosis, and chromhidrosis: fold (intertriginous) dermatoses. Clin Dermatol. 2015;33(4):483–491. doi: 10.1016/j.clindermatol.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 2.Phillips I.R., Shephard E.A. Primary trimethylaminuria. In: Adam M.P., Ardinger H.H., Pagon R.A., editors. GeneReviews. University of Washington, Seattle; Seattle, WA: 1993. [Google Scholar]
- 3.Sabir N., Jones E.A., Padmakumar B. Trimethylaminuria. BMJ Case Rep. 2016;2016 doi: 10.1136/bcr-2015-213742. bcr2015213742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lupin M., Hong H.C., O'Shaughnessy K.F. Long-term efficacy and quality of life assessment for treatment of axillary hyperhidrosis with a microwave device. Dermatol Surg. 2014;40(7):805–807. doi: 10.1111/DSU.0000000000000041. [DOI] [PubMed] [Google Scholar]
- 5.Hong H.C., Lupin M., O'Shaughnessy K.F. Clinical evaluation of a microwave device for treating axillary hyperhidrosis. Dermatol Surg. 2012;38(5):728–735. doi: 10.1111/j.1524-4725.2012.02375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finlay A.Y., Khan G.K. The Dermatology Life Quality Index: A simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–216. doi: 10.1111/j.1365-2230.1994.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 7.Oliveira A., Faria A., Oliva M. Fish malodour syndrome in a child. BMJ Case Rep. 2015;2015 doi: 10.1136/bcr-2014-207002. bcr2014207002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh S., Davis H., Wilson P. Axillary hyperhidrosis: a review of the extent of the problem and treatment modalities. Surgeon. 2015;13(5):279–285. doi: 10.1016/j.surge.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Hsu T.H., Chen Y.T., Tu Y.K., Li C.N. A systematic review of microwave-based therapy for axillary hyperhidrosis. J Cosmet Laser Ther. 2017;19(5):275–282. doi: 10.1080/14764172.2017.1303168. [DOI] [PubMed] [Google Scholar]