Abstract
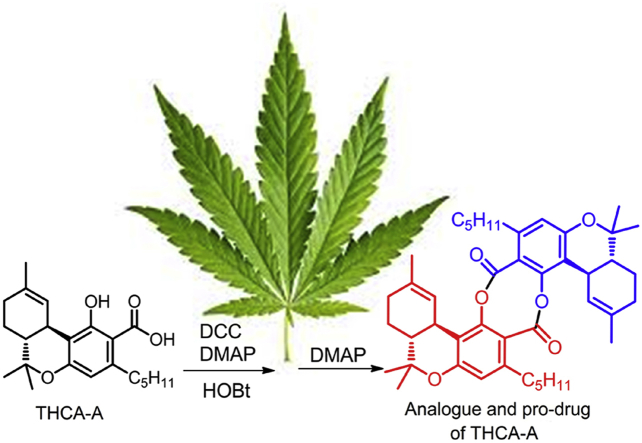
The renewed interest in dimeric salicylates as broad-spectrum anti-inflammatory and anti-diabetic agents provided a rationale to investigate the dimerization of the substituted salicylate Δ9-tetrahydrocannabinolic acid (THCA-A, 3a) as a strategy to solve its instability to decarboxylation and to generate analogues and/or pro-drugs of this native pre-cannabinoid. Activation of the carboxylic group with the DCC-HOBt-DMAP protocol afforded a high yield of the OBt ester 4, that was next converted into the highly crystalline di-depsidic dimer 5 upon treatment with DMAP. The mono-depsidic dimer 6 was also formed when the reaction was carried out with partially decarboxylated THCA-A samples. The structure of the depsidic dimers was established by spectroscopic methods and by aminolysis of 5 into the pre-cannabinoid amide 7. Both dimers showed excellent shelf stability and did not generate significant amounts of Δ9-THC upon heating. However, only the didepsidic dimer 5 activated PPAR-γ, the major target of pre-cannabinoids, but strong binding to serum proteins abolished this activity, also shielding it from the action of esterases.
Key words: Phytocannabinoids, Dimerization, Δ9-Tetrahydrocannabinolic acid A, Δ9-Tetrahydrocannabinol, PPAR-γ
Graphical abstract
THCA-A was dimerized to a highly crystalline bis-depsidic analogue by carboxylate activation as HOBt ester followed by treatment with DMAP. The dimer was stable toward decarboxylation and partially retained the PPAR-γ-activating properties of THCA-A.
1. Introduction
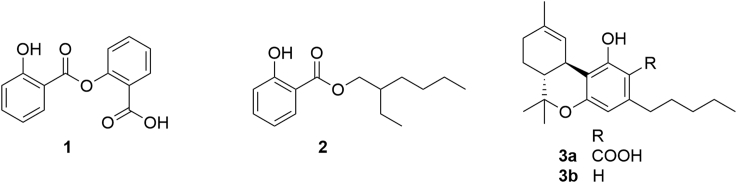
Over the past years, there has been a renewed interest for salicyl esters, a very old class of medicines whose pharmacological investigation pre-dates the one of Aspirin, the best known salicylate1. The renaissance of biomedical interest for these compounds is associated to the clinical efficacy and low toxicity of salicylsalicilate (salsalate, 1), an anti-inflammatory2 and anti-diabetic multi-target agent3, 4. The surprising and serendipitous discovery that the popular sun-screen agent octyl salicylate (octyl salate, 2) can prevent auto-immune neurodegenerative diseases, has extended the interest also to non depsidic salicyl esters5. Aspirin and salsalate are both phenolic esters, but the former is a covalent enzymatic inhibitor of the inflammatory cascade, while the latter acts non-covalently at a genomic level, powerfully inhibiting IκB kinase and down-regulating the activity of NF-κB genes5.
Neurodegenerative diseases, inflammation, and diabetes are major investigation areas for the clinical potential of cannabis meroterpenoids (phytocannabinoids) and their non-narcotic acidic precursors6. Since acidic phytocannabinoids (pre-cannabinoids) can be conceived as substituted salicylates, we have investigated the possibility to dimerize them to the corresponding salsalate-type analogues. This maneuver is expected to solve the issue of decarboxylative instability7, a major drawback for their clinical development6, and to afford analogues worth investigating for their pharmacological properties and/or for their capacity to act as prodrugs of their native precursors. Δ9-Tetrahydrocannabinolic acid (THCA-A, 3a), the best investigated acidic-cannabinoid in terms of molecular targets8, was used as a benchmark substrate for this study.
2. Results and discussion
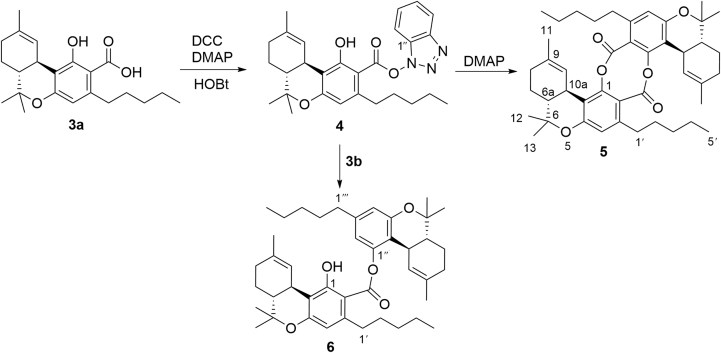
The synthesis of dimeric salicylates requires protection/deprotection steps to avoid oligomerization9. Since Δ9-THC and its derivatives have a limited range of pH stability and rapidly undergo aromatization to the corresponding cannabinol analogues under aerobic conditions6, 7, a direct dimerization strategy mediated by the formation of an intermediate activated ester seemed worth exploring. Various coupling protocols [DCC(dicyclohexylcarbodiimide)-DMAP (4-dimetthylaminopyridine), carbonyldiimidazole, P3P (propylphosphonic anhydride), Yamaguchi coupling]10 were investigated, obtaining, however, complex reaction mixtures. On the other hand, treatment of 3a with dicyclohexylcarbodiimide (DCC), 4-dimethylaminopyridine (DMAP) and hydroxybenzotriazole (HOBt) afforded, in a spot-to-spot fashion, the stable hydroxybenzotriazolyl ester 4 (Fig. 2), showing that the activation and the coupling steps could not be carried out in a one-pot operational step. Accordingly, stand-alone coupling reagents (BOP and HATU) gave poorer yields, as did N-hydroxysuccinimide, a less reactive active ester precursor. Dichloromethane was the solvent of choice, presumably because precipitation of dicyclohexylurea (DCU) drove the reaction to completion, securing a rapid (30 min) conversion of 3a into 4. The activate ester 4 could be purified by silica gel chromatography and was then cleanly converted into the highly crystalline di-depsidic dimer 5 by treatment with DMAP in dichloromethane in overall 80% yield from 4 (Fig. 2). DMAP seemingly activates the complementary “adhesive” terminations of 4 by acting as a nucleophile toward the activated ester carbonyl, and a base toward the phenolic hydroxyl.
Figure 2.
Preparation scheme for didepsidic (5) and monodepsidic (6) dimers of THCA-A.
Attempt to affect the dimerization by using a larger amount of DMAP at the stage of formation of the active ester 4 gave unsatisfactory results, presumably due to interference by the excess of DCC and HOBt necessary to drive to completion the formation of the activated ester. With partially decarboxylated samples of THCA-A, the mono-depsidic dimer 6 was also obtained as a result of the reaction of Δ9-THC (3b) with the activated ester 4 (Fig. 1). The amount formed depended on the purity of the starting sample, suggesting that 6 is produced by an in situ trapping that required a non-intramolecularly-bonded phenolic hydroxyl. This observation also explained the absence of higher salicyloyl oligomers in the reaction mixture.
Figure 1.
Important salicylate esters [salsalate (1), octylsalate (2), THCA-A (3a)] and Δ9-THC (3b).
The 1H and 13C NMR spectra of dimers 5 and 6 (Supporting Information Figs. S1‒S6) were fully assigned by means of 2D NMR experiments (COSY, HSQC and HMBC). The NMR spectra of 5 clearly indicated the symmetric nature of this dimer and closely paralleled those reported for THCA-A in the same solvent11. The only expected exceptions were the upfield shifts observed for the resonances of H2-1′ (ΔH 2.32 and 2.57 instead of ΔH 2.78 and 2.94), C-1 (ΔC 149.2 instead of 164.7), and COO- (ΔC 164.9 instead of 176.2). The diastereotopic nature of the protons H2-1′, already reported for THCA-A11, is worthy of note and it is most likely due to the steric hindrance induced by the bulky substituent at C-2. To investigate this point, we have registered 1H NMR spectra of 5 at different temperatures; however, even at 55 °C no significant differences were observed.
On the other hand, the NMR spectra of the mono-depside 6 showed an almost complete duplication of signals, thus indicating the non-symmetric nature of this dimer. Given the crowded nature of these spectra (especially 1H NMR), and the consequent overlapping of many signals, the complete resonance assignment reported in Experimental Section required an extensive use of 2D NMR spectroscopy. As expected, also in the case of 6, the diastereotopic H2-1′ appeared as couple of signals (ΔH 3.27 and 2.75) while H2-1‴ resonated as triplet at ΔH 2.53. Interestingly, for 6 also protons assigned to H2-2′ were slightly splitted (ΔH 1.75 and 1.64).
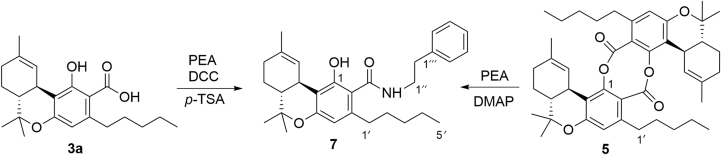
The structure of the bis-depsidic dimer 5 was confirmed by aminolysis to the pre-cannabinoid amide 7 by reaction with β-phenethylamine (PEA) and DMAP. The amide 7 was identical to the product obtained by condensing Δ9-THCA-A (3a) with the same amine (Fig. 3).
Figure 3.
Preparation of the phenethylamide of THCA-A.
Taken together, these observations showed that activation of 3a with HOBT gave the activated ester 4 that, after purification, undergoes smooth dimerization to the bis-depside 5 upon treatment with DMAP. Interestingly, the reaction of salicylic acid with the DCC-HOBT-DMAP protocol afforded salicyloylurea12 as the major reaction product, suggesting a rapid isourea-to-urea rearrangement. This rearrangement hampered the direct dimerization of simple salicylates, as exemplified by the synthesis of salsalate from salicylic acid, which required benzyl protection at the phenolic hydroxyl for the acylating electrophilic unit and at the carboxylic group of the nucleophilic phenolic unit13. In the isourea obtained from 3a, isourea-to-urea rearrangement was apparently slowed by the presence of multiple aryl substituents, thus explaining the success of the direct dimerization strategy.
The depsides 5 and 6 were stable under laboratory conditions, with no sign of degradation after 8-month storage at room temperature. Conversely, their precursor THCA-A (3a) needed cold storage to avoid decarboxylation and aromatization7, 13. The decarboxylation of non-narcotic THCA-A (3a) generates narcotic Δ9-THC (3b), and THCA-A is, indeed, popular as “crystal THC” in the illegal drug market, where it is (ab)used to boost the potency of marijuana. On the other hand, when heated under standard decarboxylation conditions for acidic cannabinoids (110 °C, 1 h), 5 and 6, though partially degraded, did not produce detectable (1H NMR) amounts of Δ9-THC.
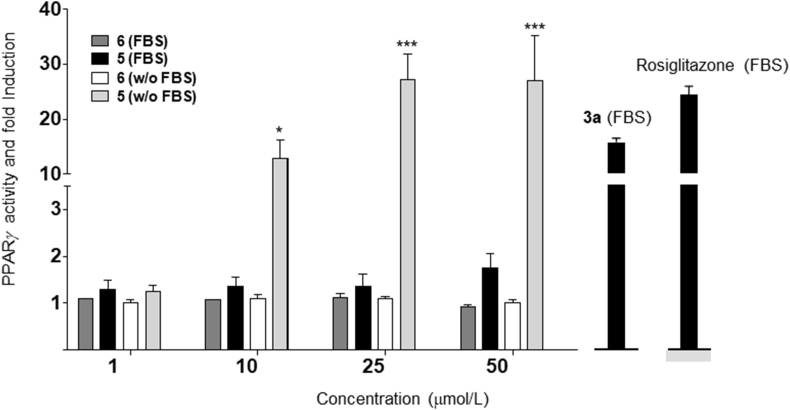
Acidic cannabinoids do not bind CB1, the narcotic target of phytocannabinoids, but show potent affinity for PPAR-γ8, the key end-point for their neuroprotective and anti-diabetic properties8. The mono-depsidic dimer 6 was devoid of PPAR-γ-activating properties, but the bis-depsidic dimer 5 significantly activated this transcription factor, albeit with lower potency (ca. 50%) compared to THCA-A (3a, Fig. 4).
Figure 4.
PPARγ transcriptional activity of depsides 5 and 6. HEK-293T cells were transiently co-transfected with PPARγ-GAL4 plus GAL-Luc plasmids and incubated with increasing concentrations of the indicated dimers for 6 h in the presence or absence of fetal bovine serum (FBS). Rosiglitazone (1 μmol/L) and 3a (5 μmol/L) were tested in parallel. Results are expressed as mean±SEM of three independent experiments. Statistical significance was determined by two-way ANOVA Bonferroni's multiple comparison test. *P<0.05; ***P<0.001.
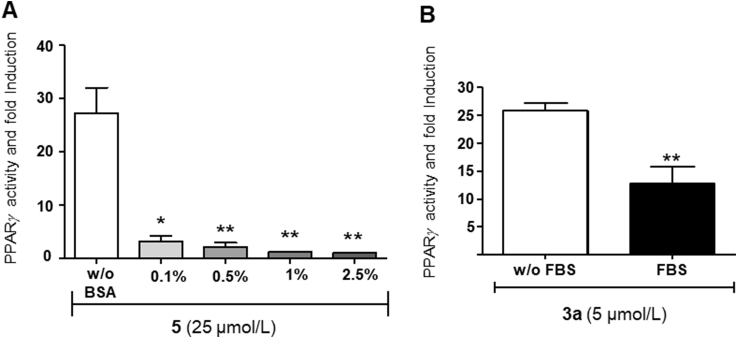
Since phenolic esters are rapidly cleaved in vivo14, we expected that, by promoting the enzymatic hydrolysis of 5 to 3a, the inclusion of serum in the assay could boost the potency of 3a. Surprisingly, serum completely abolished instead activity, and a similar effect was seen with bovine serum albumin (BSA, Fig. 5A), while THCA-A (3a) substantially retained activity in these assay conditions (Fig. 5B). These observations suggest that, presumably because of its lipophilicity, compound 5 strongly binds to plasma proteins, and this binding prevents both interaction with PPAR-γ and hydrolysis by serum esterases.
Figure 5.
(A) PPARγ transcriptional activity of dimer 5. HEK293T-GAL4-PPAR gamma-GAL4-luc transiently transfected cells were treated with compound 5 (25 μmol/L) in presence of increasing concentrations or absence of bovine serum albumin (BSA). Results are expressed as mean±SEM of three independent experiments. Statistical significance was determined by one-way ANOVA Bonferroni's multiple comparison test. *P<0.05; **P<0.01. (B) PPARγ transcriptional activity of THCA-A (3a) (5 μmol/L) in the presence or absence of FBS. Results are expressed as mean±SEM of three independent experiments. Statistical significance was determined by unpaired t test. **P<0.01.
3. Conclusions
THCA-A (3a) can be directly dimerized in high yield to the highly crystalline bis-depsidic dimer 5 without the protection/deprotection steps required for the dimerization of more simple salicylic acid derivatives. The dimer 5 is chemically stable toward decarboxylation, and retains strong affinity for PPAR-γ, potentially qualifying both as an analogue and as a pro-drug of THCA-A. On the other hand, a strong binding to serum proteins fully prevented the interaction of 5 with PPAR-γ, also shielding it from the activity of esterases, at least under the conditions of cellular assays. The relevance of albumin binding for in vivo bioactivity has been questioned15, but in vivo studies will be necessary to advance the bis-depsidic dimer 5 for further development.
4. Experimental
4.1. General experimental procedures
1H (500 or 400 MHz) and 13C (125 or 100 MHz) NMR spectra were measured on a Varian INOVA spectrometer. Chemical shifts were referenced to the residual solvent signal (CDCl3: ΔH=7.26, ΔC=77.0; DMSO-d6: ΔH=2.50). Low- and high-resolution ESI-MS spectra were obtained on an LTQ OrbitrapXL (Thermo Scientific) mass spectrometer. Silica gel 60 (70‒230 mesh) for gravity column chromatography (GCC), and Merck 60 F254 (0.25 mm) TLC plates were purchased from Merck (Germany). Work-up solutions were dried with Na2SO4 before evaporation.
4.2. THCA-A hydroxybenzotriazolide (4)
To a solution of 200 mg THCA-A (0.56 mmol) in CH2Cl2 (10 mL), dicyclohexylcarbodiimide (DCC, 231 mg, 1.12 mmol, 2 mol equiv.), hydroxybenzotriazole (HOBt, 171 mg, 1.12 mmol, 2 mol equiv.) and 4-dimethylaminopyridine (DMAP, 7 mg, 0.056 mmol, 0.10 mol equiv.) were sequentially added. A copious precipitate of dicyclohexylurea formed in a few minutes, and the reaction course was followed by TLC (petroleum ether‒EtOAc=9:1; RfTHCA-A=0.13; Rf4=0.31), continuing stirring at room temperature for 30 min. The reaction was next worked up by dilution with 15 mL petroleum ether and filtration over Celite, and the filtrate was sequentially washed with 1 mol/L H2SO4 and brine, and then evaporated. The gummy residue was purified by GCC (10 mL silica gel, petroleum ether‒EtOAc=98:2 as eluant) to afford 213 mg (80%) 4 as a gum.
1H NMR (CDCl3, 400 MHz) ΔH 7.96 (2H, d, J=7.2 Hz, H-2″, H-5″), 7.31 (2H, t, J=7.2 Hz, H-3″, H-4″), 6.38 (1H, s, H-4), 5.81 (1H, bs, H-10), 3.18 (1H, d, J=10.7 Hz, H-10a), 2.57 (1H, m, H-1′a), 2.32 (1H, m, H-1′b) 2.15(2H, d, J=5.8 Hz, H-8), 1.89 (2H, m, H-7), 1.67 (3H, s, H-11), 1.61 (1H, m, H-6a), 1.58 (2H, m, H-2′), 1.37 (3H, s, H-12), 1.29 (4H, m, H-3′-H-4′), 1.08 (3H, s, H-13), 0.87 (3H, t, J=6.8 Hz, H-5′); ESI-MS m/z 476 [M+H]+; HR-ESI-MS m/z [M+H]+ 476.2554, Calcd. for C28H34N3O4, 476.2549.
4.3. Dimerization of the THCA-A hydroxybenzotriazolide (4) to the bis-depside (5)
To a stirred solution of 4 (400 mg, 0.81 mmol) in CH2Cl2 (20 mL), DMAP (80 mg, 0.38 mmol, 0.47 mol equiv.) was added. Stirring was continued at room temp., following the reaction course by TLC (petroleum ether‒EtOAc=9:1, Rf4=0.31, Rf5=0.47). After 45 min the reaction was worked up by dilution with 1 mol/L H2SO4 (20 mL) and petroleum ether (20 mL), and the organic phase was dried and evaporated. The residue was crystallized by heptane to afford 218 mg (32% on molar basis, 64% when calculated on the sum of the two identical starting materials) 5 as fine needles. Additional amounts of 5 (55 mg) were obtained by GCC (petroleum ether‒EtOAc=9:1) of the mother liquors, with an overall molar yield of 40%.
When the reaction was carried out with non-crystalline and partially decarboxylated amorphous samples of THCAA, variable amounts of the monodepsidic dimer 6 were also obtained.
4.4. Bis-depside of THCA-A (5)
=‒353 (c 0.5 in CHCl3). 1H NMR (CDCl3, 400 MHz) Δ 6.40 (1H, s, H-4), 6.37 (1H, bs, H-10), 3.05 (1H, d, J=10.7 Hz, H-10a), 2.57 (1H, m, H-1′a), 2.32 (1H, m, H-1′b), 2.16 (2H, d, J=5.8 Hz, H-8), 1.89 (2H, m, H-7), 1.72 (3H, s, H-11), 1.66 (1H, m, H-6a), 1.60 (2H, m, H-2′), 1.37 (3H, s, H-12), 1.29 (4H, m, H-3′-H-4′), 0.87 (6H, overlapped, H-13, H-5′); 13C NMR (CDCl3, 100 MHz) Δ 164.9 (COO-), 156.7 (C-4a), 149.2 (C-1), 140.8 (C-3), 135.3 (C-9), 122.0 (C-10), 115.9 (C-4), 115.6 (C-1a), 115.1 (C-2), 78.7 (C-6), 45.2 (C-6a), 34.0 (C-1′), 32.6 (C-10a), 31.5 (C-4′), 31.1 (C-8), 30.4 (C-2′), 27.3 (C-12), 24.9 (C-7), 23.4 (C-11), 22.5 (C-3′), 18.9 (C-13), 13.9 (C-5′); ESI-MS m/z 681 [M+H]+; HR-ESI-MS m/z [M+H]+ 681.4153, Calcd. for C44H57O6, 681.4155.
4.5. Monodepside of THCA-A (6)
=‒191.7 (c 0.6, CHCl3); 1H NMR (CDCl3, 500 MHz) Δ 6.62 (1H, s, H-4″), 6.45 (1H, s, H-2″), 6.40 (1H, bs, H-10″), 6.30 (1H, s, H-4), 5.91 (1H, bs, H-10), 3.27 (1H, overlapped, H-1′a), 3.24 (1H, overlapped, H-10″a), 3.19 (1H, bd, J=10.1 Hz, H-10a), 2.75 (1H, m, H-1′b), 2.53 (2H, t, J=7.6 Hz, H2-1‴), 2.17 (2H, bs, H-8″), 2.08 (2H, bd, J=6.4 Hz, H-8, 1.91 (2H, m, H-7″), 1.89 (2H, m, H-7), 1.75 (1H, m, H-2′a), 1.69 (1H, overlapped, H-6a), 1.68 (3H, bs, H-11″), 1.66 (1H, overlapped, H-6″a), 1.64 (1H, overlapped, H-2′b), 1.60 (2H, overlapped, H-2‴), 1.54 (3H, s, H-11), 1.46 (3H, s, H-12″), 1.43 (3H, s, H-12), 1.35 (4H, overlapped, H-4‴-4′), 1.33 (4H, overlapped, H-3‴-3′), 1.14 (3H, s, H-13), 1.12 (3H, s, H-13″), 0.89 (6H, overlapped, H-5‴-5′); 13C NMR (CDCl3, 125 MHz) Δ 170.9 (C-2′a), 164.5 (C-1), 159.5 (C-4a), 155.2 (C-4″a), 148.9 (C-1″), 146.3 (C-3), 143.5 (C-3″), 134.8 (C-9), 134.3 (C-9″), 123.8 (C-10″), 123.4 (C-10), 116.0 (C-1″a), 115.7 (C-4″), 114.4 (C-2), 112.8 (C-4), 110.6 (C-1a), 104.2 (C-2), 79.0 (C-6), 77.6 (C-6″), 45.6 (C-6″a), 45.5 (C-6a), 36.9 (C-1′), 35.6 (C-1‴), 34.3 (C-10a), 33.8 (C-10″a), 31.9 (C-2′-4′), 31.5 (C-4‴), 31.3 (C-8″), 30.9 (C-8), 30.6 (C-2‴), 27.6 (C-12), 27.5 (C-12″), 25.0 (C-7″), 24.8 (C-7), 23.6 (C-11), 23.5 (C-11″), 23.1 (C-3′-3‴), 19.5 (C-13″-13), 14.4 (C-5′-5‴); ESI-MS m/z 677 [M+Na]+; HR-ESI-MS m/z [M+Na]+ 677.4186 (Calcd. for C43H58O5Na, 677.4182).
4.6. Preparation of tetrahydrocannabinolic acid phenethylamide (7)
4.6.1. From the amidation of 3a
To a stirred solution of 3a (100 mg, 0.28 mmol) in CH2Cl2 (2.5 mL), phenethylamine (35 μL, 33.7 mg, 0.56 mmol, 2 mol equiv.), DCC (69 mg, 0.33 mmol, 1.2 mol equiv.) and p-toluensulfonic acid monohydrate (10 mg, 0.050 mmol, 0.18 mol equiv.) were sequentially added. The advancement of the reaction was followed by TLC (petroleum ether‒EtOAc=9:1, Rf3a=0.13; Rf7=0.21), and after stirring 40 min at room temperature, the reaction was worked up by evaporation of the solvent. The residue was taken up in toluene, and the solution was cooled at 4 °C for 2 h. The white precipitate of DCU was removed by filtration over Celite, and the filtrate was purified by GCC on silica gel (5 mL, petroleum ether–EtOAc as eluant) to afford 102 mg (82%) 7 as an amorphous gum.
4.6.2. From the aminolysis of 5
To a stirred solution of 5 (50 mg, 0.073 mmol) in CH2Cl2 (2.5 mL), phenethylamine (110 μL, 106 mg, 0.87 mmol, 12 mol equiv.) and DMAP (18 mg, 0.14 mmol, 2 mol equiv.) were added. The reaction course was followed by TLC (petroleum ether‒EtOAc=9:1, Rf6=0,47; Rf7=0,21). After stirred at room temperature for 24 h, the reaction was worked up by dilution with 1mol/L H2SO4 (5 mL) and petroleum ether (10 mL). The organic phase was dried and evaporated, and the residue was purified by GCC on silica gel (petroleum ether‒EtOAc=9:1) to afford 17 mg (26%) 7 as an amorphous gum.
4.7. Tetrahydrocannabinolic acid phenethylamide (7)
=‒81 (c 1.2 in CHCl3). 1H NMR (CDCl3, 400 MHz) ΔH 7.33–7.25 (5H, overlapped, H-2‴-H-6‴), 6.41 (1H, s, H-4), 5.91 (1H, bs, H-10), 3.75 (2H, m, H2-1″), 3.17 (1H, d, J=10.7 Hz, H-10a), 2.92 (2H, m, H2-2″), 2.44 (2H, m, H2-1′), 2.16 (2H, d, J=5.8 Hz, H-8), 1.89 (2H, m, H-7), 1.67 (3H, s, H-11), 1.59 (1H, m, H-6a), 1.60 (2H, m, H-2′), 1.37 (3H, s, H-12), 1.29 (4H, m, H-3′-H-4′), 1.02 (3H, s, H-13), 0.87 (3H, t, J=6.8 Hz, H-5′); 13C NMR (CDCl3, 100 MHz) ΔC 171.1, 160.3, 156.7, 139.4, 138.4, 133.8, 128.8, 128.6, 126.8, 123.7, 110.7, 78.1, 45.6, 40.9, 35.2, 34.5, 33.6, 31.6, 31.2, 30.8, 27.4, 25.0, 23.4, 22.4, 19.4, 14.0; ESI-MS m/z 462 [M+H]+; HR-ESI-MS m/z [M+H]+ 462.3010, Calcd. for C30H39NO3, 462.3008.
4.8. PPAR-γ activity assay
Human embryonic kidney epithelial cells 293T cells were obtained from the American Type Culture Collection (CRL-3216) and cultured in DMEM supplemented with 10% FCS and antibiotics. To analyze PPAR-γ transcriptional activity HEK-293T cells were cultured in 24-well plates (2 × 104 cells/well) and transiently co-transfected with GAL4-PPAR-γ (50 ng) GAL4-luc (firefly luciferase, 50 ng) vectors using Roti-Fect (Carl Roth, Karlsruhe, Germany). Twenty hours after transfection the cells were stimulated with increasing concentrations of the compounds for 6 h and luciferase activities were quantified using Dual-Luciferase Assay (Promega, Madison, WI, USA). Rosiglitazone (1 μmol/L, Cayman Chemical, MI, USA), was used as a positive control for PPAR-γ activation (50-fold induction over basal activity). Test compounds and controls stocks were prepared in DMSO and the final concentration of the solvent was always less than 0.5% v/v. The plasmid GAL4-PPAR-γ was obtained from Prof. Christopher Sinal (Dalhousie University, Canada).
Acknowledgments
We are grateful to MIUR (Ministero Universita' e Ricerca) for financial support to the groups in Novara and Naples (PRIN2017, Project 2017WN73PL, bioactivity-directed exploration of the phytocannabinoid chemical space, Italy). Eduardo Muñoz, Juan D. Unciti-Broceta and Giovanni Appendino were also supported by Emerald Health Biotechnology España (Spain).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2019.06.007.
Contributor Information
Orazio Taglialatela-Scafati, Email: scatagli@unina.it.
Giovanni Appendino, Email: giovanni.appendino@uniupo.it.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Montinari M.R., Minelli S., De Caterina R. The first 3500 years of aspirin history from its roots—a concise summary. Vasc Pharmacol. 2019;113:1–8. doi: 10.1016/j.vph.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Bombardier C., Peloso P.M., Goldsmith C.H. Salsalate, a nonacetylated salicylate, is as efficacious as diclofenac in patients with rheumatoid arthritis. Salsalate-diclofenac study group. J Rheumatol. 1995;22:617–624. [PubMed] [Google Scholar]
- 3.Anderson K., Wherle L., Park M., Nelson K., Nguyen L. Salsalate, an old, inexpensive drug with potential new indications: a review of the evidence from 3 recent studies. Am Health Drug Benefits. 2014;7:231–235. [PMC free article] [PubMed] [Google Scholar]
- 4.Salastekar N., Desai T., Hauser T., Schaefer E.J., Fowler K., Joseph S. Salsalate improves glycaemia in overweight persons with diabetes risk factors of stable statin-treated cardiovascular disease: a 30-month randomized placebo-controlled trial. Diabetes Obes Metab. 2017;19:1458–1462. doi: 10.1111/dom.12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y.P., Marling S.J., Plum L.A., DeLuca H.F. Salate derivatives found in sunscreens block experimental autoimmune encephalomyelitis in mice. Proc Natl Acad Sci U S A. 2017;114:8528–8531. doi: 10.1073/pnas.1703995114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanuš L.O., Meyer S.M., Muñoz E., Taglialatela-Scafati O., Appendino G. Phytocannabinoids: a unified critical inventory. Nat Prod Rep. 2016;33:1357–1392. doi: 10.1039/c6np00074f. [DOI] [PubMed] [Google Scholar]
- 7.Lindholst C. Long term stability of cannabis resin and cannabis extracts. Aust J Forensic Sci. 2010;42:181–190. [Google Scholar]
- 8.Nadal X., Del Río C., Casano S., Palomares B., Ferreiro-Vera C., Navarrete C. Tetrahydrocannabinolic acid is a potent PPARγ agonist with neuroprotective activity. Br J Pharmacol. 2017;174:4263–4276. doi: 10.1111/bph.14019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cavallito C.J., Buck J.S. Synthesis of phenolic acid esters. I. Depsides. J Am Chem Soc. 1943;65:2140–2142. [Google Scholar]
- 10.El-Faham A., Albericio F. Peptide coupling reagents, more than a letter soup. Chem Rev. 2011;111:6557–6602. doi: 10.1021/cr100048w. [DOI] [PubMed] [Google Scholar]
- 11.Choi Y.H., Hazekamp A., Peltenburg-Looman A.M., Frédérich M., Erkelens C., Lefeber A.W. NMR assignments of the major cannabinoids and cannabiflavonoids isolated from flowers of Cannabis sativa. Phytochem Anal. 2004;15:345–354. doi: 10.1002/pca.787. [DOI] [PubMed] [Google Scholar]
- 12.Mucheli M.V., Kudav N.A. ChemInform abstract: o-hydroxybenzoyl ureas in the synthesis of 1-hydroxyxanthones. Chem Inform. 1985;16:31–32. [Google Scholar]
- 13.McPartland J.M., MacDonald C., Young M., Grant P.S., Furkert D.P., Glass M. Affinity and efficacy studies of tetrahydrocannabinolic acid A at cannabinoid receptor types one and two. Cannabis Cannabinoid Res. 2017;2:87–95. doi: 10.1089/can.2016.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mounter L.A., Whittaker V.P. The hydrolysis of esters of phenol by cholinesterases and other esterases. Biochem J. 1953;54:551–559. doi: 10.1042/bj0540551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu X., Wright M., Hop C.E. Rational use of plasma protein and tissue binding data in drug design. J Med Chem. 2014;57:8238–8248. doi: 10.1021/jm5007935. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.