Introduction
Bullous pemphigoid (BP) is a rare autoimmune blistering disease that classically presents with pruritic, tense bullae on an erythematous base. Approximately 20% of patients with BP never develop bullae.1 In nonbullous pemphigoid (NBP), patients often experience severe pruritus with eczematous patches or urticarial plaques. The diagnosis of NBP represents a diagnostic challenge given its morphologic ambiguity. Clinical, histopathologic, serologic, and immunologic studies are necessary to establish a diagnosis.2
Programmed cell death 1 (PD-1) inhibitors block inhibitory T-cell signals to stimulate an effective antitumor response. This medication class is commonly associated with cutaneous immune-related adverse events, including BP-like eruptions.3, 4 Although sporadic NBP is well described in the dermatologic literature, to our knowledge, NBP developing in the setting of immune checkpoint inhibitors (ICI) has not been described.5 We report 4 cases of NBP in patients receiving PD-1 inhibitors for advanced malignancy.
Case reports
Case 1
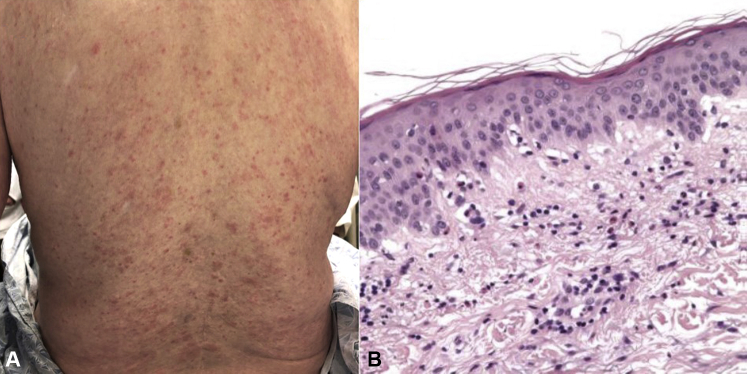
A 78-year-old man with metastatic melanoma and a complete tumor response when receiving nivolumab developed a diffuse, pruritic eruption before initiation of cycle 21. Oral corticosteroids and betamethasone 0.05% ointment were prescribed by his oncologist, with complete resolution of his symptoms. The eruption recurred once steroids were tapered. Given his profound pruritus and the complete response of his cancer, nivolumab was stopped. The patient was referred to the Skin Toxicity Program 6 months after developing the eruption. Examination showed urticarial plaques, erythematous macules, and papules on the trunk and lower part of the extremities (Fig 1, A) with desquamative gingivitis.
Fig 1.
A, Clinical presentation of case 1. Erythematous papules coalescing into plaques on the lower portion of the back. B, Histopathology of case 1. Eosinophilic spongiosis with tagging of eosinophils at dermal-epidermal junction.
Histopathologic analysis showed eosinophilic spongiosis with eosinophil tagging of the dermal-epidermal junction (Fig 1, B). Direct immunofluorescence (DIF) showed linear deposition of IgG and complement C3 at the basement membrane zone. Indirect immunofluorescence (IIF) results were positive for IgG antibodies that localized to the epidermal roof of salt-split skin. Enzyme-linked immunosorbent assay (ELISA) results for anti–bullous pemphigoid 180 (BP180), anti–bullous pemphigoid 230 (BP230), anti–desmoglein 1, and anti–desmoglein 3 were negative.
The patient was prescribed triamcinolone 0.1% cream, 100 mg doxycycline, and 500 mg nicotinamide, each twice daily. Despite treatment, his symptoms persisted with intermittent flares. He self-discontinued doxycycline and began prednisone 10 mg daily, which was tapered over 2 months. In the setting of elevated immunoglobulin E, omalizumab 300 mg monthly was started. He achieved complete resolution of the eruption and significant resolution in itch following 4 doses of omalizumab. Though the patient has not received nivolumab for over 15 months, his metastatic melanoma remains in complete remission.
Case 2
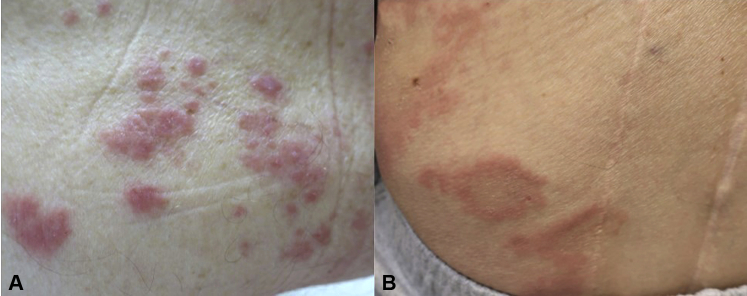
A 78-year-old man with metastatic esophageal adenocarcinoma and no relevant dermatologic history presented with a new-onset pruritic, erythematous eruption on the trunk after his first cycle of pembrolizumab. He was prescribed a topical steroid by his oncologist, but the eruption worsened with each infusion. He presented to the Skin Toxicities Program 6 months after rash onset with eczematous papules and thin urticarial plaques scattered over the neck, trunk, and bilateral upper and lower portions of the extremities (Fig 2, A). Examination did not reveal bullae or mucosal involvement (Table I).
Fig 2.
Other clinical images of nonbullous pemphigoid secondary to programmed cell death 1 inhibitor therapy. A, Pink edematous papules on the trunk in case 2. B, Urticarial plaques with central scale on the lower portion of the back in case 3.
Table I.
Summary of clinical characteristics and treatment course
| Patient no. | Age (y) and sex | Cancer diagnosis | Metastatic sites | PD-1 inhibitor | Best tumor response to PD-1 | Time (mo) to onset of eruption after PD-1 | Clinical features on physical examination | Primary clinical morphologies | PD-1 interrupted or discontinued because of NBP? | Oral steroid course and time | Treatment course of NBP | Treatment response of NBP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 78, male | Melanoma | Lung | Nivolumab | Complete response | 7 | Pruritic, erythematous, polymorphic macules and papules | Eczematous and urticarial | Discontinued | 10 mg qd for 1 mo, subsequent taper over 1 mo |
|
Complete resolution after omalizumab |
| 2 | 78, male | Esophageal adenocarcinoma | Lung and pleura | Pembrolizumab | Stable disease initially, but ultimately progressed | <1 | Pruritic, erythematous eruption with urticarial and eczematous features on trunk, extremities, and neck | Urticarial | Not interrupted or discontinued | 4-mo steroid taper, maximum dose: 60 mg × 5 d |
|
Complete resolution after omalizumab |
| 3 | 62, male | Lung adenocarcinoma | Brain, adrenal gland | Pembrolizumab | Stable disease initially, but ultimately progressed | 12 | Urticarial plaques with central scale on trunk (Fig 2, B) | Urticarial | Discontinued because of disease progression | Steroid taper, maximum dose: 10 mg |
|
Complete resolution after rituximab |
| 4 | 58, male | Melanoma | Lung, skin | Pembrolizumab | Decreased tumor burden | 7 | Diffuse erythematous, pruritic eruption on neck, trunk, and extremities | Urticarial | Discontinued | 14-day steroid taper, maximum dose: 10 mg |
|
Complete resolution after rituximab |
bid, Twice daily; NBP, nonbullous pemphigoid; PD-1, programmed cell death 1; prn, as needed; q4w, every 4 weeks; qd, every day; qw, every week; subq, subcutaneous.
Histopathologic and immunologic studies confirmed a diagnosis of NBP (Table II). Because of substantial sleep disturbance from itch, prednisone 60 mg daily was started with triamcinolone 0.1% cream. With the clinical, histologic, and laboratory findings noted, a diagnosis of NBP was made, and doxycycline 100 mg and nicotinamide 500 mg, each twice daily, were added. The patient's itched worsened with prednisone dose below 30 mg daily, and he was noted to have an elevated immunoglobulin E level. Monthly omalizumab 300 mg was added. Prednisone was tapered and permanently discontinued less than 3 months after initiation of omalizumab, of which he received 8 doses, with complete resolution of the eruption and itch. Pembrolizumab therapy was continued throughout the course of treating his NBP but was ultimately discontinued 15 months after its initiation because of progression of oncologic disease.
Table II.
Summary of histopathologic, immunologic, and serologic testing results
| Patient no. | Histopathology | Direct immunofluorescence | Indirect immunofluorescence |
Antigen-specific serologic testing |
IgE level∗ (IU/mL) | ||
|---|---|---|---|---|---|---|---|
| Monkey esophagus basement membrane zone | Salt-split skin, epidermal binding | BP180 antibody level (U/mL) |
BP230 antibody level (U/mL) |
||||
| 1 | Eosinophilic spongiosis with tagging of eosinophils at dermal-epidermal junction (Fig 2, A) | Linear deposition of IgG and C3 (2-3+) at the BMZ (Fig 2, B and C) |
IgG: + (40) IgG4: + (160) |
IgG: + (trace 20) IgG4: + (≥20) |
− (<5.00) |
− (<5.00) |
+ (1316) |
| 2 | Mixed spongiotic, micropustular, and interface dermatitis with numerous eosinophils (Fig 2, D) | 1-2+ granular C3 deposits at dermal-epidermal junction and patchy epidermal fibrin deposition | IgG: − (weak 10) IgG4: + (80) |
IgG: − <5 IgG4: + (≥10) |
− (<5.00) |
− (<5.00) |
+ (371) |
| 3 | Subacute spongiosis and papillary dermal chronic inflammation with numerous eosinophils | Strong linear C3 and IgG at the dermal-epidermal junction | N/A | N/A | + (42.2) |
− (<5.00) |
− (80.8) |
| 4 | Acute and chronic inflammation suggestive of component of hypersensitivity reaction | N/A |
IgG: + (20) IgG4: + (80) |
IgG: + (≥20) IgG4: + (≥20) |
− (<5.00) |
− (<5.00) |
− (41.0) |
Bold indicates a positive test result.
BP180, Antibody targeting BP180 antigen (collagen XVII); BP230, antibody targeting BP230 antigen (dystonin); C3, complement protein 3; Ig, immunoglobulin; N/A, not applicable.
Normal range for serum IgE was defined as 0-100 IU/mL.
Discussion
Checkpoint inhibitors have been approved across diverse malignancies and are associated with numerous cutaneous immune-related adverse events, including BP-like eruptions.3 It is believed that PD-1 inhibitor–associated BP has a similar pathogenic mechanism to classic BP, with autoantibody production against hemidesmosomal proteins, but the exact mechanism is not known.6 It is possible that inhibition of PD-1 dysregulates B-cell regulatory T cells, resulting in nonspecific production of both pathogenic and nonpathogenic autoantibodies.7 Furthermore, it has been hypothesized that tumor expression of BP180 may contribute to the pathogenesis of BP in some cases by stimulating production of anti-BP180 antibodies.6 Although there are multiple reports of anti–PD-1–induced BP with bullae, diagnosis of the nonbullous variant requires knowledge of this entity and a high index of suspicion.
Diagnosing NBP associated with anti–PD-1 therapy presents a diagnostic challenge. In our series, all 4 patients were male; 3 were receiving pembrolizumab, and 1 was receiving nivolumab. There was significant variation in both the clinical morphology and time to onset of disease. Although also presenting with eczematous and papular lesions, all 4 had predominantly urticarial plaques on examination. Of interest, 1 of our patients reported mucosal symptoms. Mucosal involvement in BP occurs in 10% to 30% of adult patients, often in drug-induced cases.2, 8
Given the lack of accepted criteria for diagnosing BP and the high rate of pruritus among patients treated with PD-1 inhibitors, clinicians must take clinical, histopathologic, serologic, and immunologic data into account. Histopathologic analysis for 3 of our patients showed eosinophilic spongiosis, which is a hallmark of BP. Of the 3 patients who had DIF performed, all showed linear deposition of C3 and/or IgG at the dermal-epidermal junction, consistent with BP. Additionally, in the 3 patients in whom IIF was performed, the test result was positive for antibodies to the epidermal roof of normal human salt-split skin, which has been shown to have a very high positive predictive value for BP.9 Only 1 of our patients tested positive for BP180 antibodies, and none were positive for BP230 antibodies, which may indicate targeting of other hemidesmosomal antigens. When available, more specialized testing, such ELISA or Western blot analysis for recombinant or cell-derived antigens,10 can be considered; these methods may detect circulating autoantibodies that target less common hemidesmosomal antigens. Finally, 2 of our 4 patients had elevated immunoglobulin E levels, which may be seen in BP11 and can serve as a therapeutic target.
In patients with low body surface area involvement, we recommend starting treatment with high-potency topical steroids. We initiated, and would continue to consider, oral doxycycline with nicotinamide because this has up to 74% response rates in classic BP.12 However, none of our patients responded to doxycycline and nicotinamide, and this regimen is of particular interest in light of recent evidence that antibiotics may dampen response to immunotherapy via reduced gut microbiome diversity.13 All 4 of our patients were treated with oral corticosteroids by their oncologists, but steroids were discontinued once symptoms were well managed with steroid-sparing agents. All patients achieved complete resolution of cutaneous symptoms with either omalizumab or rituximab, both of which have been shown to be effective in controlling classic BP14 and have been used successfully for ICI-induced BP.3, 4 We selected these biologics over T-cell–depleting agents such as azathioprine and mycophenolate mofetil because of greater concern of dampening the antitumor T-cell–mediated immune response. In the setting of recent data showing that high-dose systemic steroids may reduce overall survival and time to treatment failure in patients on ICI therapy,15 we advocate for minimizing broad immunosuppression whenever possible.
Conclusions
Because of the protean presentation of NBP, we encourage consideration of histopathology, DIF, IIF, anti-hemidesmosomal antibody ELISAs, and immunoglobulin E levels in patients receiving ICI with refractory pruritic cutaneous eruptions. Once a diagnosis of NBP is confirmed, we advocate for optimizing topical steroids and, when indicated, early introduction of systemic steroid-sparing therapy. By uncoupling the cutaneous toxicity from the therapeutic effect of ICIs, the goal remains to effectively treat the immune-related adverse event while maintaining the therapeutic benefit of the patient's anticancer regimen.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Lamberts A., Meijer J.M., Jonkman M.F. Nonbullous pemphigoid: A systematic review. J Am Acad Dermatol. 2018;78(5):989–995. doi: 10.1016/j.jaad.2017.10.035. [DOI] [PubMed] [Google Scholar]
- 2.Cozzani E., Gasparini G., Burlando M., Drago F., Parodi A. Atypical presentations of bullous pemphigoid: clinical and immunopathological aspects. Autoimmun Rev. 2015;14(5):438–445. doi: 10.1016/j.autrev.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Damsky W., Kole L., Tomayko M.M. Development of bullous pemphigoid during nivolumab therapy. JAAD Case Rep. 2016;2(6):442–444. doi: 10.1016/j.jdcr.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel J., Totonchy M., Damsky W. Bullous disorders associated with anti–PD-1 and anti–PD-L1 therapy: a retrospective analysis evaluating the clinical and histopathologic features, frequency, and impact on cancer therapy. J Am Acad Dermatol. 2018;79(6):1081–1088. doi: 10.1016/j.jaad.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Lopez A.T., Khanna T., Antonov N., Audrey-Bayan C., Geskin L. A review of bullous pemphigoid associated with PD-1 and PD-L1 inhibitors. Int J Dermatol. 2018;57(6):664–669. doi: 10.1111/ijd.13984. [DOI] [PubMed] [Google Scholar]
- 6.Naidoo J., Schindler K., Querfeld C. Autoimmune bullous skin disorders with immune checkpoint inhibitors targeting PD-1 and PD-L1. Cancer Immunol Res. 2016;4(5):383–389. doi: 10.1158/2326-6066.CIR-15-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sage P.T., Sharpe A.H. T follicular regulatory cells in the regulation of B cell responses. Trends Immunol. 2015;36(7):410–418. doi: 10.1016/j.it.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kridin K., Bergman R. Assessment of the prevalence of mucosal involvement in bullous pemphigoid. JAMA Dermatol. 2019;155(2):166–171. doi: 10.1001/jamadermatol.2018.5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sárdy M., Kostaki D., Varga R., Peris K., Ruzicka T. Comparative study of direct and indirect immunofluorescence and of bullous pemphigoid 180 and 230 enzyme-linked immunosorbent assays for diagnosis of bullous pemphigoid. J Am Acad Dermatol. 2013;69(5):748–753. doi: 10.1016/j.jaad.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Griffiths C., Barker J., Bleiker T., Chalmers R., Creamer D. Wiley; Hoboken, NJ: 2016. Rook's Textbook of Dermatology. [Google Scholar]
- 11.Freire P., Munoz C., Stingl G. IgE autoreactivity in bullous pemphigoid: eosinophils and mast cells as major targets of pathogenic immune reactants. Br J Dermatol. 2017;177(6):1644–1653. doi: 10.1111/bjd.15924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams H.C., Wojnarowska F., Kirtschig G. Doxycycline versus prednisolone as an initial treatment strategy for bullous pemphigoid: a pragmatic, non-inferiority, randomised controlled trial. Lancet. 2017;389(10079):1630–1638. doi: 10.1016/S0140-6736(17)30560-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Routy B., Le Chatelier E., Derosa L. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 14.Kremer N., Snast I., Cohen E.S. Rituximab and omalizumab for the treatment of bullous pemphigoid: a systematic review of the literature. Am J Clin Dermatol. 2019;20(2):209–216. doi: 10.1007/s40257-018-0401-6. [DOI] [PubMed] [Google Scholar]
- 15.Faje A.T., Lawrence D., Flaherty K. High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer. 2018;124(18):3706–3714. doi: 10.1002/cncr.31629. [DOI] [PubMed] [Google Scholar]