Highlights
-
•
High rates of PIF are detected 3 years after VMAT based CRT for rectal cancer.
-
•
Patients with PIFs received non-significantly higher V30 Gy to sacroiliac joints.
-
•
3 arc VMAT techniques can be optimized for bone.
-
•
Proton beam therapy has the potential for further optimization for bone.
Keywords: Rectal cancer, Radiotherapy, Insufficiency fracture, VMAT, IMRT, Proton
Abstract
Pelvic insufficiency fractures (PIF) is a known but under-acknowledged late effect of pelvic radiotherapy. In rectal cancer, studies describing incidence of PIF and relation to dose volume relationships are lacking. The aim of this study was (i) to analyse dose volume histograms (DVH) from pelvic bones in patients with and without PIF, and (ii) to determine bone sparing capacity of 2 and 3 arc volumetric arc therapy (VMAT), intensity modulated radiotherapy (IMRT) and proton beam therapy (PBT), in rectal cancer patients treated with chemoradiotherapy (CRT).
Material and methods
Patients treated with CRT for primary rectal cancer underwent a 3-year pelvic MRI for identification of PIFs. Bone structures were retrospectively delineated, and DVHs were re-calculated. Comparative planning was done with 2 (original) and 3 arc VMAT, fixed field IMRT and PBT plans.
Results
27 patients (18 men, mean age 64 years) were included and PIFs were identified in 9 (33%), most (n = 6) had multiple fracture sites. In general, patients with PIFs received higher doses to pelvic bones, and V30 Gy to the sacroiliac joint was non-significantly higher in patients with PIF 68.5% (60.1–69.3 IQR) vs. 56% (54.1–66.6 IQR), p = 0.064. Comparative planning showed that especially 3 arc VMAT and proton beam therapy could be optimized for bone.
Conclusions
Patients, treated with VMAT based CRT for rectal cancer, have high rates of PIFs after 3 years. Patients with PIFs tended to have received higher doses to sacroiliac joints. Comparative planning demonstrated most pronounced bone sparing capacity of 3 arc VMAT and with PBT having the potential to further lower doses. These results should be validated in larger and preferably prospective cohorts.
1. Introduction
Neo-adjuvant chemoradiotherapy (CRT) is an integrated part of the standard treatment for advanced primary rectal cancers, as it decreases the local recurrence rate [1], [2].
Pelvic radiotherapy is associated with both acute and late toxicities including intestinal, urogenital and bone marrow affection [3]. Furthermore, it alters and weakens bone structure over time by affection of both osteoblasts/clasts and small vasculature [4], [5]. Insufficiency fracture is a type of stress fracture that occurs when physiological stress is applied to weakened bone. Pelvic insufficiency fracture (PIF) is a well-known late complication to pelvic radiotherapy and can be misinterpreted clinically as local recurrence causing pain and decreased mobility. In patients with rectal cancer, the incidence of PIF is reported from 3.3% to 7.1% and recently up to 33%, depending on detection method, follow-up timing and definition [4], [6], [7]. In other cancer types such as cervical cancer the incidence has been described as high as 90% up to 3-years after radiotherapy [8], [4], [9], [10].
Radiotherapy techniques have changed from 3D to Intensity modulated radiation therapy (IMRT) or Volumetric modulated arc therapy (VMAT) with the potential to decrease doses to organs at risk (OAR) and subsequent toxicity [11], [12]. However, studies describing dose volume relationships for pelvic bones are lacking for rectal cancer, and only a few studies describe dose volume relationships for pelvic bones in cervical cancer [13], [14].
Consequently, in daily treatment planning there are no validated constraints to the bony structures, and in general, sparing of the bowel cavity and bladder wall is prioritized compared to doses to the pelvic bones.
The aim of this study was (i) to analyse dose volume histograms (DVH) to pelvic bones in patients with and without PIF, and (ii) in patients with PIF to make a comparative planning study determining bone sparing capacity of 2 and 3 arc VMAT, IMRT and proton beam therapy (PBT) , in primary rectal cancer patients treated with neo-adjuvant CRT.
2. Materials and methods
2.1. Patients
Consecutive patients with primary rectal adenocarcinoma (located within 15 cm from the anal verge) were invited to participate in a nationwide project with the aim of detecting local recurrence by MRI scan 3 years after curative surgery. Patients were identified from a national database (Danish Colorectal Cancer Group database). All patients underwent rectal resection (partial mesorectal excision (PME), total mesorectal excision (TME) or abdominoperineal excision (APE)) with curative intent from April 2011 to August 2012. The inclusion procedure and characteristics of the total cohort is previously described in detail by Jørgensen et al. 2018 [7]. This study includes a sub-group of the above-mentioned nationwide study, namely all patients treated with neoadjuvant CRT at one of the participating institutions, Aarhus University Hospital.
2.2. Identification of PIFs
PIFs were identified on 3-year post-operative MRIs. The MRIs were performed at 1.5T platforms with a standardised scan-protocol including a 4 mm sagittal short T1 inversion recovery (STIR) sequence of the bony pelvis combined with sagittal, axial and coronal 4 mm T2-weighted turbo spin echo sequences as well. All MRI examinations were re-evaluated by a consultant radiologist, with more than 8 years of sub-specialisation in pelvic MRI, blinded to all clinical data, with the exception of the preoperative MRI examination.
High signal intensity changes in the bone marrow at the STIR sequence, indicating bone marrow oedema, with accompanying subtle linear, low signal intensity changes at T2 weighted images were regarded as suggestive of the presence PIF. STIR is the most sensitive sequence for the detection of bone marrow oedema while the T2 images ensured precise anatomical mapping.
As most patients had multiple fractures, fracture sites were divided into following areas: pubic rami, acetabulum, iliac bone near joint, alae of the sacrum, and midline of sacral bone.
2.3. CRT Treatment
Twenty-five patients received 52 Gy to tumour (clinical target volume-tumour: gross tumour volume and rectal circumference at tumour level, plus an isometric margin of 0.5 mm except 1 cm craniocaudally and an individualized internal target volume (ITV) margin) and 46 Gy to elective nodal target in 26 fractions (two patients received 45 Gy in 25 fractions, with one dose level). Twenty-five treatment plans were based on 2 arc VMAT technology with 15 MV (Varian Eclipse planning system). One patient received fixed 5-field IMRT and one 6 MV. Patients received concomitant Capecitabin (1700 mg/m2) on radiotherapy treatment days.
2.4. Delineation
Pelvic bones were delineated retrospectively on the treatment planning CT-scans including separate delineation of the sacral bone (including the outer contour of the bone from S1 to S5) and sacroiliac joints (Including the joint space between the sacral and ileum bones and outer contour of the bones 1 cm to each side of the sacroiliacal joint) and DVHs were re-calculated (three different structure sets were included 1: Pelvic bones, total (os ischium, os ileum, os sacrum, os pubis)), 2: the sacral bone 3: sacroiliac joint).
Dose volume relationships (V45 Gy, V30 Gy, and V20 Gy), mean and max doses of the pelvic bones (total), sacral bone and sacroiliac joints were compared between the patients with and without PIFs and in the comparative planning setting. V20 Gy, V30 Gy and V45 Gy were chosen to represent low, mid and high dose ranges.
2.5. Comparative planning
In the Patients with PIFs, alternative treatment plans were generated using 6 field IMRT and 3 arc VMAT (also Varian Eclipse planning system). These plans were optimized by lowering V30 Gy to the sacroiliac joints. In the comparative plan setting target coverage and homogeneity had to be comparable or better than the original treatment plan. Constraints to bowel and bladder also had to be comparable or better than the original treatment plan when generating alternate plans. Two pencil beam proton therapy plans were generated in the case with the highest V30Gy to the sacroiliac joints. We compared a 2 field (lateral opposing) and a 3 field (lateral opposing and posterior/anterior) technique on Eclipse optimized with Multi Field Optimization (IMPT).
2.6. Statistical analyses
For comparison between patients with and without PIF and comparative plans Wilcoxon rank-sum and signed-rank test were used. For testing of variable distribution between groups Chi2 or Fischeŕs exact test (n < 5) was used, and p-values < 0.05 considered statistically significant.
The STATA statistical software (ver. 15.1) was used for statistical analyses.
3. Results
Twenty-seven patients (18 men, median age 64 years), treated at a single institution, were included from the original prospective cohort. Baseline characteristics were comparable between groups, despite smoking habits, which were unequally distributed (p = 0.029), Table 1.
Table 1.
Patient characteristics.
| All patients (n = 27) | Patients with PIF (n = 9) | Patients w/o PIF (n = 18) | p-values | |
|---|---|---|---|---|
| Age (median, range) | 64 (35–81) | 69 (35–81) | 61.5 (44–73 | p = 0.25 |
| Gender (Male, %) | 67% | 56% | 72% | p = 0.42 |
| Clinical T category (n, %) | ||||
| cT1-cT2: | 1 (3.7) | 0 (0.0) | 1 (5.6) | p = 0.51 |
| cT3: | 20 (74.1) | 6 (66.7) | 14(77.8) | |
| cT4: | 6 (22.2) | 3 (33.3) | 3 (16.7) | |
| Clinical N category (n, %) | p = 0.22 | |||
| cN0 | 18 (66.7) | 5 (55.6) | 13 (72.2) | |
| cN1 | 7 (26) | 4 (44.4) | 3 (16.7) | |
| cN2 | 2 (7.4) | 2 (11.1) | ||
| Timing of MRI in years after rectal resection (median, range) | 3.4 (2.9–4.1) | 3.4 (2.9–3.6) | 3.4 (3.3–4.1) | P = 0.1 |
| Risk factors (n, %) | ||||
| Smoker | ||||
| Yes | 10 (37) | 2 (22.2) | 8 (44.4) | |
| Former (>8 weeks) | 6 (22.2) | 5 (55.6) | 1 (5.6) | |
| Never | 9 (33.3) | 2 (22.2) | 7 (38.9) | |
| NA | 2 (2 (74) | 2 (11.1) | p = 0.029 | |
| BMI | ||||
| (Median, IQR) | 25.6 (23–28.7) | 4.5 (23–26.2) | 26.8 (23–29.1) | p = 0.27 |
Pelvic insufficiency fractures were identified in 9 (33%) patients, 5 (56%) men, median age 69 years. Six of these patients had fractures in multiple locations, including: pubic rami (4 patients), acetabulum (6 patients), iliac bone (near joint) (6 patients), sacral bone (alae) (8 patients), and sacral bone (midline) (2 patients).
Planning target volume (PTV) receiving 52 Gy and PTV-46 Gy were comparable between the two groups, Table 2.
Table 2.
Treatment and dose-volume characteristics.
| All patients (n = 27) | Patients with PIF (n = 9) | Patients w/o PIF (n = 18) | p-Values | |
|---|---|---|---|---|
| PTV46 Gy (ccm) (median, IQR) | 1380 (1262–1640) | 1499 (1309–1967) | 1332 (1171–1568) | P = 0.12 |
| PTV52 Gy (ccm) (median, IQR) | 406 (363–575) | 430 (378–789) | 397 (347–529) | P = 0.33 |
| Mean dose pelvic bones, Gy (median, IQR) | 28.9 (27.1–30.6) | 28.8 (25.9–32.4) | 28.9 (27.5–29.7) | P = 0.96 |
| Mean dose sacrum, Gy (median, IQR) | 38.9 (36.5–39.8) | 39.4 (38.3–39.8) | 38.4 (36.5–39.8) | p = 0.61 |
| Mean dose sacroiliac joint, Gy (median, IQR) | 32.9 (31–34.5) | 33.8 (32.9–34) | 32.7 (31–34.5) | p = 0.63 |
| V45Gy pelvic bones (%) (median, IQR) | 11 (10–14.1) | 12.4 (11–13.6) | 10.6 (10–14.1) | P = 0.5 |
| V45Gy sacrum (%) (median, IQR) | 30.6 (27.5–35.1) | 31.1 (29–35) | 30.4 (27.5–35.1) | p = 0.94 |
| V45Gy sacroiliac joint (%) (median, IQR) | 11.3 (7.9–13.4) | 11.6 (7–12.2) | 11.1 (8.7–18.4) | p = 0.59 |
| V30Gy pelvic bones (%) (median, IQR) | 51.3 (46–57.4) | 55 (50.1–66.7) | 50.7 (43.6–57) | P = 0.18 |
| V30Gy sacrum (%) (median, IQR) | 83.9 (75.5–89.4) | 85.6 (83.9–90) | 80.7 (73.8–87.7) | p = 0.14 |
| V30Gy sacroiliac joint (%) (median, IQR) | 62.5 (54.6–69.3) | 68.5 (60.1–69.3) | 56 (54.1–66.6) | p = 0.064 |
We compared dose volume relationships (V45 Gy, V30Gy, and V20 Gy), mean and max doses of the pelvic bones (total), sacral bone and sacroiliac joints between patients with and without PIFs. In general, patients with PIFs received higher doses to pelvic bones, and V30 Gy to the sacroiliac joint was non-significantly higher in patients with PIF 68.5% (60.1–69.3 IQR) compared to those without PIF 56% (54.1–66.6 IQR), p = 0.064, Table 2.
We compared 2 arc (the original plan, not optimized for bone) and 3 arc VMAT and fixed 6-field IMRT (both optimized for bone). Overall 3 arc VMAT performed better, on most bone DVHs, than both 2 arc VMAT and fixed 6-field IMRT. Max doses, however, were comparable, as were mean dose and V45 Gy to sacroiliac joints when comparing 3 arcs with IMRT. Comparing 2 arcs with 6-field IMRT, IMRT performed better, but with comparable max doses and V20 Gy to the sacrum and sacroiliac joint, Table 3.
Table 3.
Comparative planning on PIF cases. *3 fields: lateral opposing and PA.
| 2 ARC VMAT | 3 ARC VMAT | Fixed field IMRT | 3-field PBT* | |
|---|---|---|---|---|
| N = 9, median (IQR) | N = 9, median (IQR) | N = 9, median (IQR) | N = 1 | |
| Pelvic bones, total | ||||
| Mean dose (Gy) | 30,8 (28,3–32,4)1,2 | 26,8 (25–28,3)3 | 28,7 (27,2–30) | 19,7 |
| Max dose (Gy) | 53,4 (52,3–53,8)2 | 55,1 (53,1–55,5) | 55 (53–55,2) | 52 |
| V20 Gy (%) | 82,6 (72,6–85,3)1,2 | 69 (63–74)3 | 76.6 (70–82) | 46,7 |
| V30 Gy (%) | 55 (50,1–66,7)1,2 | 40 (33,9–41)3 | 49 (47–56,9) | 31,3 |
| V45 Gy (%) | 12,4 (11–13.6)1,2 | 7,3 (5,3–8,9)3 | 9.9 (9,1–11,4) | 10,7 |
| Sacral bone | ||||
| Mean dose (Gy) | 39,7 (38.9–39.8)1,2 | 33,3 (32,6–34,9)3 | 37,9 (36,6–38,4) | 30,2 |
| Max dose (Gy) | 52,6 (52,1–53,7) | 55 (52–55,4) | 55 (51–55,1) | 51,7 |
| V20 Gy (%) | 96,8 (94,1–99,6)1 | 83,9 (79,2–85)3 | 96 (89,3–100) | 67,6 |
| V30 Gy (%) | 85,6 (83,9–90)1,2 | 63 (61,3–63,6)3 | 81 (70–84) | 55,3 |
| V45 Gy (%) | 31,1 (29–35)1,2 | 21 (16–25,8)3 | 25,3 (24,7–27,1) | 30,6 |
| Sacroiliac joints | ||||
| Mean dose (Gy) | 33,9 (33,3–34,5)1,2 | 26 (25,3–28,2) | 29,3 (27,7–31) | 17,6 |
| Max dose (Gy) | 48,7 (47,6–50,5) | 48,7 (48–49,8) | 47,9 (47,3–49) | 50 |
| V20 Gy (%) | 95,5 (90,2–98)1 | 66 (63,4–67)3 | 86 (80,5–92) | 36,9 |
| V30 Gy (%) | 68,5 (60,1–69,3)1,2 | 32,7 (31–37,6)3 | 40,3 (34,1–48,8) | 24,5 |
| V45 Gy (%) | 5,5 (4,4–7) | 7 (4,5–8,4) | 6,7 | |
Statistically significant differences between: 2 arc vs. 3 arc1, 2 arc vs. IMRT2, 3 arc vs. IMRT3.
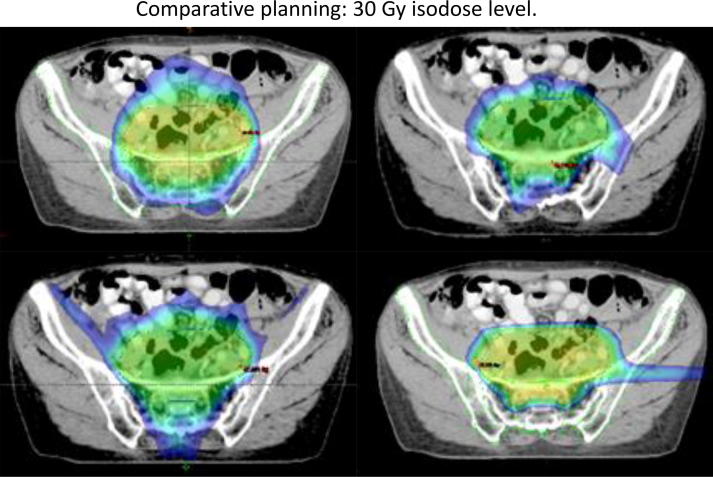
The two proton beam plans demonstrated sparing on all bone parameters except V45 Gy, but most pronounced sparing on low dose (V20 and V30 Gy) volumes, exemplified by the 3 beam PBT technique in Table 3. An example of 2 and 3 arc VMAT, 6 field IMRT and 3 field PBT dose distribution is depicted in Fig. 1 at 30 Gy isodose level.
Fig. 1.
Comparative planning and sparing of sacroiliac joints, showing 30 Gy isodose levels. Top left: 2 arc VMAT Top right: 3 arc VMAT Bottom left: 6 field IMRT Bottom right: Three beam proton plan. Two lateral opposing and one posterior-anterior field.
4. Discussion
This study describes PIF in relation to dose volume parameters in patients treated with 2 arc VMAT for primary rectal cancer. No previous studies have correlated dose volume parameters for pelvic bones or sub structures to PIF after CRT for rectal cancer. Furthermore, we show, by comparative planning, that alternate treatment techniques can be optimized for bone and have bone sparing potential.
Three years after CRT for rectal cancer 33% had detectable PIFs on pelvic MRI, which was in line with the findings from the nationwide study (33.6%) that this population is derived from [7]. It is much higher than previously reported after radiotherapy for rectal cancer. The incidence of PIFs after rectal cancer treatment has previously been reported in 3.3%–7.1% of patients up to 3-years after radiotherapy [4], [9], [10], whereas the incidence after cervical cancer treatment has been described as high as 90% [8]. Several reasons can contribute to the observed discrepancies in incidence: the timing of imaging, method of detection, radiological review, radiotherapy technique and dosage and selection of patients as described below.
The median time to development of PIFs reportedly varies from 3 to 44 months. One study describes 1-, 2- and 3-year cumulative incidences of 22%, 41% and 49% in a variety of pelvic cancers, thus the majority developed within 2 years [9], [10]. Similar findings have been reported by others [6]. The patients in present study were evaluated after median of 3.4 years (range 2.9–4 years), which would then have contributed to a high cumulative incidence, but also a possibility of PIFs having already resolved [8].
The method of detection has varied over time, but MRI has been described as the most sensitive method with a sensitivity of 100% and estimated specificity of 85% [15], [16]. MRI shows abnormal bone marrow signal with fracture lines most often in the weight bearing parts of the pelvic ring, which also explains the frequent involvement of the alae of the sacral bone. Most PIFs are identified as diffuse and poorly delineated areas of bone marrow oedema with typical locations and accompanying sclerotic lines. MRI can often distinguish between PIFs and metastatic bone lesions, the latter being with more local well circumscribed (peri-focal) oedema and often also more intensely signalling. Focus on insufficiency fractures in the radiological review increases the reported incidence as studies show that several PIFs were not found in the initial radiology report [6], [13]. Some studies only include symptomatic patients yielding, creating an enriched population and a higher incidence of PIFs [4]. The ratio of symptomatic patients differs, but is generally around 50% [9]. Results from Stockholm I and II trials (patients receiving 25 Gy in 5 fractions) reported fractures in 5.3% of irradiated patients, but determined only by ICD-10 coding, resulting in a high likelihood of under-estimation [17].
Risk factors for developing PIF includes age, gender (women), osteoporosis, menopause, radiation dose, chemotherapy, and body weight [6], [9], [14]. Our data did not include baseline factors such as osteoporosis and menopause status. Moreover, the sample size and events in this study were too small to include these variables in the analysis. In the total nationwide cohort [7], beside CRT, gender (women) and age >65 years were found to increase risk of PIF.
Radiation dose and techniques have varied over recent years, from 3-D techniques to IMRT. The significance of changes in technique is not well described. However, for patients with gynaecological cancers, Shih et al. found no difference in PIF incidence in patients treated with either 3-D, 4-field box technique or IMRT [18]. Bazire et al (2017) [19] reported higher max doses to fracture sites in patients with PIF treated for cervical or anal cancer compared to no-PIF. Others [9] found doses above 50.4 Gy associated with increased risk of PIF in multivariate analyses in patients treated for cervical cancer. As described above, result from Stockholm studies receiving hypofractionated schedule reported relatively low incidence of PIF, which, however, could be because of under reporting.
Patients treated with VMAT generally receive radiation dose to high volumes of pelvic bones, which could also be a factor in the high number of PIFs in this study. Ramlov et al. found only minor overlaps between bone hot spots and PIFs suggesting no relation of high doses to minor parts of bone, rather increased dose to entire bone seemed of importance [13]. Also, many studies [8], [13] report multiple fracture sites in each patient also suggestive of a generalised weakened bone structure. To date, no dose constraint to pelvic bones or substructures has been validated and implemented into general clinical practice.
Treated volumes and baseline characteristics were comparable between patients with and without PIFs in this study. Most dose volume parameters were comparable between the two groups, but especially V30 Gy to the sacroiliac joint was higher in patients with PIFs. Others have found that 74% of fractures were located in joint area, suggesting that dose could be especially important in the weight bearing part of the pelvic bones.
The comparative planning part of the study, showed that 3 arc VMAT had better bone sparing capacity than fixed field IMRT, while maintaining doses to other OARs and target coverage at levels similar to the original plan. Thus, when evaluating the parameters selected in this study, 3 arc VMAT can be a valid treatment option sparing pelvic bones and with short delivery times, still baring in mind the significance of the low dose bath provided by arc therapies is not fully elucidated. However, as a first step, contouring pelvic bones as an organ of risk and incorporating this into the planning procedure could lower the dose to pelvic bones regardless of the applied technique. A further reduction in bone doses could be obtained with PBT planning especially for low to intermediate doses, which could be an objective when planning proton therapy for pelvic cancers.
5. Conclusion
Patients treated with VMAT based CRT for primary rectal cancer have a high rate of PIFs detected by MRI 3 years after treatment, most with multiple fracture sites. Patients with PIFs have received non-significantly higher doses (V30 Gy) to sacroiliac joints than patients without fractures. These data should be validated in larger and preferably prospective cohorts. The results, however, signify the importance of clinicians informing their patients of this risk and for future studies to include prospective details on patient reported pelvic pain and impact on quality of life. Comparative planning demonstrated most pronounced bone sparing capacity of 3 arc VMAT and with PBT having the potential to further lower doses to pelvic bones.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
Funding from The Danish Cancer Society.
References
- 1.Glimelius B. Neo-adjuvant radiotherapy in rectal cancer. World J Gastroenterol. 2013;19(46):8489–8501. doi: 10.3748/wjg.v19.i46.8489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCarthy K., Pearson K., Fulton R., Hewitt J. Pre-operative chemoradiation for non-metastatic locally advanced rectal cancer. Cochrane Database Syst Rev. 2012;12:CD008368. doi: 10.1002/14651858.CD008368.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joye I., Haustermans K. Early and late toxicity of radiotherapy for rectal cancer. Recent Results Cancer Res. 2014;203:189–201. doi: 10.1007/978-3-319-08060-4_13. [DOI] [PubMed] [Google Scholar]
- 4.Higham C.E., Faithfull S. Bone health and pelvic radiotherapy. Clin Oncol (R Coll Radiol) 2015 Nov;27(11):668–678. doi: 10.1016/j.clon.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Pacheco R., Stock H. Effects of radiation on bone. Curr Osteoporos Rep. 2013;11(4):299–304. doi: 10.1007/s11914-013-0174-z. [DOI] [PubMed] [Google Scholar]
- 6.Kim H.J., Boland P.J., Meredith D.S., Lis E., Zhang Z., Shi W. Fractures of the sacrum after chemoradiation for rectal carcinoma: incidence, risk factors, and radiographic evaluation. Int J Radiat Oncol Biol Phys. 2012;84(3):694–699. doi: 10.1016/j.ijrobp.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 7.Jorgensen J.B., Bondeven P., Iversen L.H., Laurberg S., Pedersen B.G. Pelvic insufficiency fractures frequently occur following preoperative chemo-radiotherapy for rectal cancer - a nationwide MRI study. Colorectal Dis. 2018;20(10):873–880. doi: 10.1111/codi.14224. [DOI] [PubMed] [Google Scholar]
- 8.Blomlie V., Rofstad E.K., Talle K., Sundfor K., Winderen M., Lien H.H. Incidence of radiation-induced insufficiency fractures of the female pelvis: evaluation with MR imaging. AJR Am J Roentgenol. 1996;167(5):1205–1210. doi: 10.2214/ajr.167.5.8911181. [DOI] [PubMed] [Google Scholar]
- 9.Oh D., Huh S.J. Insufficiency fracture after radiation therapy. Radiat Oncol J. 2014;32(4):213–220. doi: 10.3857/roj.2014.32.4.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ugurluer G., Akbas T., Arpaci T., Ozcan N., Serin M. Bone complications after pelvic radiation therapy: evaluation with MRI. J Med Imaging Radiat Oncol. 2014;58(3):334–340. doi: 10.1111/1754-9485.12176. [DOI] [PubMed] [Google Scholar]
- 11.Appelt A.L., Sebag-Montefiore D. Technological advances in radiotherapy of rectal cancer: opportunities and challenges. Curr Opin Oncol. 2016;28(4):353–358. doi: 10.1097/CCO.0000000000000306. [DOI] [PubMed] [Google Scholar]
- 12.Arbea L., Ramos L.I., Martinez-Monge R., Moreno M., Aristu J. Intensity-modulated radiation therapy (IMRT) vs. 3D conformal radiotherapy (3DCRT) in locally advanced rectal cancer (LARC): dosimetric comparison and clinical implications. Radiat Oncol. 2010;5 doi: 10.1186/1748-717X-5-17. 17-717X-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramlov A., Pedersen E.M., Rohl L., Worm E., Fokdal L., Lindegaard J.C. Risk factors for pelvic insufficiency fractures in locally advanced cervical cancer following intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2017;97(5):1032–1039. doi: 10.1016/j.ijrobp.2017.01.026. [DOI] [PubMed] [Google Scholar]
- 14.Sakaguchi M., Maebayashi T., Aizawa T., Ishibashi N. Risk factors for sacral insufficiency fractures in cervical cancer after whole pelvic radiation therapy. Anticancer Res. 2019;39(1):361–367. doi: 10.21873/anticanres.13120. [DOI] [PubMed] [Google Scholar]
- 15.Matcuk G.R., Jr, Mahanty S.R., Skalski M.R., Patel D.B., White E.A., Gottsegen C.J. Stress fractures: pathophysiology, clinical presentation, imaging features, and treatment options. Emerg Radiol. 2016;23(4):365–375. doi: 10.1007/s10140-016-1390-5. [DOI] [PubMed] [Google Scholar]
- 16.Herman M.P., Kopetz S., Bhosale P.R., Eng C., Skibber J.M., Rodriguez-Bigas M.A. Sacral insufficiency fractures after preoperative chemoradiation for rectal cancer: incidence, risk factors, and clinical course. Int J Radiat Oncol Biol Phys. 2009;74(3):818–823. doi: 10.1016/j.ijrobp.2008.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Birgisson H., Pahlman L., Gunnarsson U., Glimelius B. Swedish Rectal Cancer Trial Group. Adverse effects of preoperative radiation therapy for rectal cancer: long-term follow-up of the Swedish Rectal Cancer Trial. J Clin Oncol. 2005;23(34):8697–8705. doi: 10.1200/JCO.2005.02.9017. [DOI] [PubMed] [Google Scholar]
- 18.Shih K.K., Folkert M.R., Kollmeier M.A., Abu-Rustum N.R., Sonoda Y., Leitao M.M., Jr Pelvic insufficiency fractures in patients with cervical and endometrial cancer treated with postoperative pelvic radiation. Gynecol Oncol. 2013;128(3):540–543. doi: 10.1016/j.ygyno.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 19.Bazire L., Xu H., Foy J.P., Amessis M., Malhaire C., Cao K. Pelvic insufficiency fracture (PIF) incidence in patients treated with intensity-modulated radiation therapy (IMRT) for gynaecological or anal cancer: single-institution experience and review of the literature. Br J Radiol. 2017;90(1073):20160885. doi: 10.1259/bjr.20160885. [DOI] [PMC free article] [PubMed] [Google Scholar]