Abstract
Background
Human papillomavirus (HPV) infects and propagates in the cervical mucosal epithelium. Hence, in addition to assessing systemic immunity, the accurate measurement of cervical immunity is important to evaluate local immune responses to HPV infection and vaccination. This review discusses studies that investigated the presence of infection and vaccine-induced HPV-specific antibodies in cervicovaginal secretions (CVS).
Methods
We searched the two main health sciences databases, PubMed and the ISI Web of Science, from the earliest dates available to March 2019. From the eligible publications, information was extracted regarding: (i) study design, (ii) the reported HPV-specific antibody concentrations in CVS (and the associated serum levels, when provided), (iii) the CVS collection method, and (iv) the immunoassays used.
Results
The systematic search and selection process yielded 44 articles. The evidence of HPV-specific antibodies in CVS after natural infection (26/44) and HPV vaccination (18/44) is discussed. Many studies indicate that HPV-specific antibody detection in CVS is variable but feasible with a variety of collection methods and immunoassays. Most CVS samples were collected by cervicovaginal washing or wicks, and antibody presence was mostly determined by VLP-based ELISAs. The moderate to strong correlation between vaccine-induced antibody levels in serum and in CVS indicates that HPV vaccines generate antibodies that transudate through the cervical mucosal epithelium.
Conclusion
Although HPV-specific antibodies have lower titres in CVS than in serum samples, studies have shown that their detection in CVS is feasible. Nevertheless, the high variability of published observations and the lack of a strictly uniform, well-validated method for the collection, isolation and quantification of antibodies indicates a need for specific methods to improve and standardize the detection of HPV-specific antibodies in CVS.
Keywords: Human papillomavirus, Mucosal immunity, Antibodies, Cervicovaginal secretions
1. Introduction
Persistent infection with an oncogenic human papillomavirus (HPV) type is the major risk factor for the development of cervical carcinoma [1], which is the fourth most common cancer in women worldwide [2]. HPV infections may persist due to a variety of viral immune evasion mechanisms (reviewed in Refs. [3,4]). However, despite the mechanisms that are in place that allow HPV to evade host defences, at least 80–90% of genital HPV infections are cleared in approximately 12–24 months, indicating that the immune system is mostly able to eliminate these infections [5].
1.1. Humoral immune response to natural HPV infection
Neutralizing antibodies are believed to be the main effectors of protection against HPV infection by preventing the initial entry of the virus into basal epithelial cells [6]. Although only small numbers of virions are presumably exposed to the immune system, both mucosal and systemic antibodies against selected HPV antigens have been detected in infected women. However, the titres of these infection-induced (IgA and IgG) antibodies are low, and seroconversion occurs months or even years after infection. In addition, in some women, antibodies are never detected, as only 50–70% of women eventually seroconvert. Furthermore, the detected antibodies do not necessarily protect against subsequent infection, and it is unknown whether natural immunity can persist throughout life [7]. Although HPV infects and propagates in the cervical mucosal epithelium and has almost no viremic phase, the ensuing humoral responses are most frequently detected in sera. Immune responses in cervicovaginal secretions (CVS) are usually not investigated.
1.2. Humoral immune response to HPV vaccination
The discovery that the major capsid antigen, L1, of HPV could self-assemble into virus-like particles (VLPs) that are highly immunogenic led to the development and licensure of three VLP-based HPV vaccines (the bivalent HPV16/18 vaccine, the quadrivalent HPV6/11/16/18 vaccine, and the nonavalent HPV 6/11/16/18/31/33/45/52/58 vaccine) [[8], [9], [10]]. The current HPV vaccines are delivered intramuscularly, circumventing intra-epithelial immune evasion strategies. In contrast to natural infection, HPV vaccination induces high-quality and sustained serum (mostly IgG) antibody titres against HPV L1, conferring protection against persistent incident infections and pre-malignant neoplasias [11]. In fact, HPV vaccination generates 10- to 100-fold higher titres of L1-specific serum neutralizing antibodies than natural infection [4].
Since HPV requires disruption of the epithelial barrier to infect, direct exudation of capillary and interstitial vaccine-induced type-specific HPV antibodies at these sites occur, neutralizing the virions. This mechanism is probably elucidating why the quadri- and nonavalent vaccines are highly protective against infections of cutaneous epithelia (e.g. external genital warts), which are not routinely bathed in mucus. However, also significant transudation of systemic antibodies (e.g. via the neonatal Fn receptor [12,13]) in the female genital mucus takes place. The impact on infection and transmission of these transudated anti-HPV antibodies also warrants further investigation [4,14,15].
Genital HPV infections are rapidly acquired after the initiation of sexual activity, hence, prophylactic HPV vaccination programmes generally target preadolescents prior to their initiation into sexual activity. However, infection can occur throughout the lifetime of a sexually active person [16,17]. HPV vaccines are therefore required to elicit potent, long-lasting HPV-specific antibodies in serum that can exudate and transudate into the genital mucosa, where the virus is first encountered.
1.3. Importance of humoral immune responses in female genital secretions
The evaluation of immunogenicity in HPV vaccine trials has relied largely on serology. In the absence of a correlate of protection, it is generally accepted that the presence of high concentrations of vaccine-induced neutralizing antibodies in serum that are greater than those elicited by natural infection are the best indicator of long-term protection against HPV infection. Nevertheless, as cervical cancers typically occur at the cervical transformation zone, HPV-specific antibodies need to exudate/transudate from serum in mucosal secretions to prevent new infections, to block re-infection at another site in the cervical, vaginal or vulvar region, and to stop possible transmission [18].
The purpose of this exhaustive review is to provide an overview of the published studies that have reported the detection of HPV-specific antibodies in CVS, whether or not these are linked to the detection of serological HPV-specific antibody titres. In addition, we have assessed how different settings and methodologies contributed to various outcomes and discussed the potential relevance of mucosal HPV-specific antibody detection. In this review, we want to assess the current knowledge to aid future studies investigating mucosal HPV-specific antibodies to evaluate the immunogenicity of current HPV vaccines and the impact of altered dosing schemes or new vaccine formulations.
2. Methods
2.1. Search strategy and selection criteria
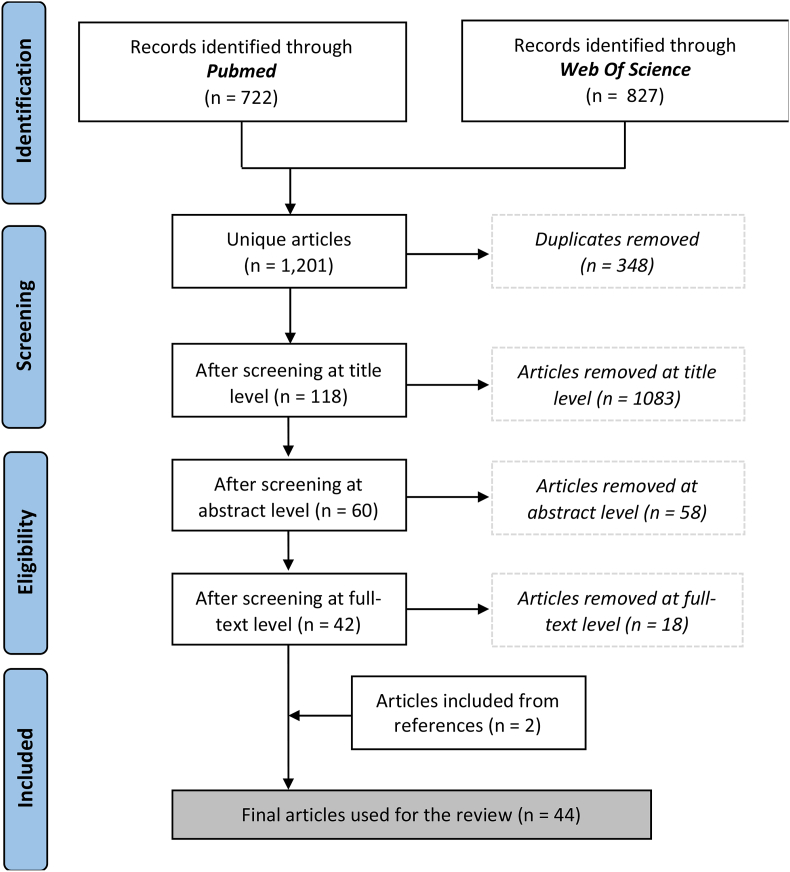
A review of the literature was performed on March 15, 2019 using the two main health sciences databases: PubMed (‘All Fields Contain’) and ISI Web of Science (‘Title/Keywords/Abstracts’) (Fig. 1). In each database, the following search terms were used: ‘(HPV* OR human papilloma virus* OR human papillomavirus* OR cervical cancer OR cervical cancers OR cervical carcinoma* OR cancer of the cervix OR cancers of the cervix) AND (mucosal OR cervicovaginal OR cervical secretion* OR vaginal secretion*) AND (antibod* OR immune globulin* OR immunoglobulin* OR IgG OR IgA OR immune OR immunity OR immunologic)’. A prospective protocol was registered on PROSPERO (identification number CRD42018104963). Studies were eligible for inclusion if they reported on the detection of HPV-specific antibodies in female genital mucosal secretions, whether or not these were compared with the detection of HPV-specific antibodies in serum. The search was restricted to English language studies that involved human subjects. The databases were searched starting from the earliest dates. The reference lists of the included articles were explored to identify other relevant publications. Nevertheless, as with any systematic review, the search string used for this review strikes a balance between completeness and feasibility. It is inevitable that we missed some relevant research.
Fig. 1.
Flow diagram of the literature search.
2.2. Data extraction, synthesis and presentation of the results
From each eligible article, data related to: (i) study population characteristics, (ii) the study context, (iii) the CVS collection method, (iv) the immunoassays used, (v) the HPV-specific antibody concentration in CVS (and when provided, the associated serum titres) and (vi) the normalization methods used were obtained. When applicable, other interesting information that could had an influence on the detection of HPV-specific antibodies in CVS was also collected. The extracted data were heterogeneous, and therefore meta-analysis was not feasible. Narrative reporting was used to describe the data from the studies.
3. Results and discussion
3.1. Search results
The search query identified a total of 1,549 records from PubMed and the ISI Web of Science (Fig. 1). Within the total records, 348 were duplicates and were therefore excluded. The remaining 1,201 records were screened based on the title, abstract, keywords and full-text, if necessary. Studies were excluded if they mostly concerned: (i) animal research, (ii) other pathology, (iii) the detection of non-specific HPV antibodies and (iv) language (to a lesser extent). Ultimately, 44 articles were included in the full-text analysis. The selected papers were published between 1989 and 2018. Evidence regarding HPV-specific antibodies in CVS after natural HPV infection (26/44) [[19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44]] and HPV vaccination (18/44) [[45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62]] was discussed. Most papers reported paired CVS and serum data (36/44).
3.2. Evaluation of naturally induced HPV-specific antibodies in CVS
Twenty-six articles were selected that investigated naturally induced HPV-specific antibodies in CVS. In 18 articles, paired CVS and serum data was reported. A summary of the articles and their main outcomes are given in Table 1. Two papers that specifically investigated the reliability and validity of different CVS collection methods used for the detection of HPV-specific antibodies are discussed in more detail in section 3.4.1 [27,39].
Table 1.
Details of papers describing detection of mucosal HPV-specific antibodies after HPV infection.
| First author (year of publication) [ref] | Country of execution | Number of participants | Mean age (total age range) | Study population | Methodology antibody analysed in CVS (class) | CVS collection method Storage | Serum samples collected | Main study outcome |
|---|---|---|---|---|---|---|---|---|
| Dillner (1989) [21] | Sweden | 42 | Not reported (20–50 y) | women with genital condylomas or CIN |
BPV-based ELISA anti-PV (IgA) |
Cytobrush Samples were placed in a glass tube filled with PBS and mixed on a vortex mixer. Specimens were stored at -20 °C until used |
Yes | 8/9 women with CIN and 3/9 with koilocytosis and condylomas but no CIN had IgA antibodies to PV. 6/24 with normal pap smear and colposcopy also had IgA antibodies against PV. The proportion of IgA-positive CVS was significantly higher in the CIN group than in the normal group |
| Snyder (1991) [40] | USA | 30 | 24 y (16–36y) | women with abnormal pap smear and controls |
Fusion protein based ELISA anti-HPV16 L1/E4 (not reported) |
CVL Samples were placed in saline with 1% BSA. Samples were centrifuged and the supernatants were sonicated and stored with 0.01% sodiumazide at 4 °C |
No | Antibodies in CVS showed reactivity to HPV type 16 E4 or L1 or both, with highest binding in patients with CIN |
| Dillner (1993) [22] | Sweden | 60 | 25.1 y (17–39 y) | women with genital condylomas and controls |
1/Synthetic peptide based ELISA anti-HPV16 L1/L2/E2/E7 (IgA) 2/BPV-based ELISA anti-PV (IgA) |
Cytobrush Samples were placed in a test tube containing PBS, EDTA, penicillin and streptomycin and the test tubes were frozen until use (storage temperature not reported) |
Yes | Both the local antibodies to E2 (peptide 245) and E7 antigens were associated with a diagnosis of condyloma. However, there was no significant correlation between the presence of antibodies and the detection of HPV DNA. No difference between patients and controls in their antibody responses to L1 and L2 was observed |
| Veress (1994) [43] | Hungary | 163 | 28.4 y (17–51 y) | cytologically healthy women |
Synthetic peptide based ELISA anti-HPV16 E2/E7/L1/L2 (sIgA) anti-HPV11 L2 (sIgA) |
Cytobrush Samples were placed in PBS. The specimens were briefly vortexed and the suspensions were centrifuged at 3,000 g for 10 min. The supernatant was used for antibody detection (storage temperature not reported) |
No | 34 secretions (20.9%) were found to react with at least one of the oligopeptides. Correlation between HPV DNA and anti-HPV IgA detection was rather weak: anti-peptide IgA positivity was 34.3% (12 of 35) among HPV DNA positive patients compared to 17.2% (22 of 128) among HPV DNA negative women |
| Dreyfus (1995) [23] | France | 61 | 31.8 y (19–53 y) | women with histological diagnosis of HPV infection and controls |
Synthetic peptide based ELISA anti-HPV16 E2 (IgG/A) |
CVL Samples were centrifuged at 1000 g. The resulting supernatant was stored at -20 °C |
Yes | The proportion of IgA positive secretions (48.8% in the case-group vs. 15.0% in the control-group) was significantly higher in women with HPV infection and seemed to increase with the severity of the cervical lesion. No difference was found for specific IgG |
| Elfgren (1996) [25] | Sweden | 23 | 35.8 y (20–51 y) | women with conization for CIN2/3 or cancer in situ |
1/VLP-based ELISA anti-HPV16 (IgA) 2/Synthetic peptide based ELISA anti-HPV16 E2/E7/L1/L2; HPV6 L1; HPV16/18 peptide 245 (IgA) |
Cytobrush Samples were placed in a plastic tube with PBS solution containing 5 mmol/L EDTA and was stored at -20 °C |
Yes | HPV antibody levels, especially local IgA, declined after efficient treatment |
| Wang (1996) [44] | Sweden | 359 | 38.4 y (18–74 y) | women with abnormal pap smear, referred to a colposcopy clinic |
Capsid-based ELISA anti-HPV16/18/33 (IgA) |
Cytobrush Samples were swirled in test tubes containing saline. The specimens were centrifuged at 4,400 g for 5 min. The cell pellets and the supernatants were kept at -20 °C until analysis |
No | Among subjects with at least one cervical sample positive for HPV16, 28.1% also had at least one HPV16 IgA-positive cervical sample. IgA to HPV18 was also more common among HPV18 DNA-positive subjects and IgA to HPV33 was more common among HPV33 DNA-positive subjects. Cervical IgA antibodies to HPV16 were more common among patients with CIN |
| Bontkes (1999) [20] | The Netherlands | 125 | 31.1–36.9 y (16–53 y) | women with abnormal pap smear |
VLP-based ELISA anti-HPV16 L1/L2 (IgG/A) |
Cytobrush Samples were collected in PBS/Merthiolate. After centrifugation, the supernatants were stored at -20 °C for antibody testing |
Yes | Local IgG and IgA HPV16 VLP-specific antibodies do not correlate with virus clearance. There was no significant difference in the proportion of cervical IgG positivity between HPV16-infected women with normal and abnormal cytology |
| Hagensee (2000) [26] | USA | 292 | 19.2 y (18–24 y) | women enrolled in a longitudinal study of the natural history of HPV infection |
Luminex immunoassay (LIA) anti-HPV16 (IgG/(s)IgA) |
Wick After collection, the samples were frozen until processed (storage temperature not reported) |
Yes | IgG, IgA, and sIgA to HPV16 were detected in 12%, 6%, and 8%, respectively, of samples tested. Cervical IgG antibodies were most strongly associated with HPV16 DNA detected within the previous 12 months. Secretory IgA was most strongly associated with detection of a squamous intraepithelial lesions 4–8 months earlier |
| Marais (2000) [30] | South Africa | 112 | 26 y (16–48 y) | HIV+ seropositive and HIV- female sex workers |
VLP-based ELISA anti-HPV16 (IgG/A) |
CVL Samples were collected with saline and stored at -70 °C until required |
Yes | Both HIV+ (27/40) and HIV- (30/43) sex workers displayed a high seroprevalence rate for anti-VLP-16 IgG. Significantly more HIV+ (16/49) women than HIV- (6/63) women had cervical anti-VLP-16 IgG, but not IgA antibodies |
| Tjiong (2000) [41] | The Netherlands | 82 |
Median: 34–38 y (18–67 y) |
women with CIN, CxC and controls |
Radioactive immunoprecipitation assay (RIPA) anti-HPV16 E7 (IgG) |
CVL Samples were collected with PBS. The fluid was centrifuged for 10 min at 1000 g at 4 °C. The supernatant was stored in aliquots at –80 °C until analysis |
Yes | HPV16 E7 specific IgG antibodies seem to be locally produced in a number of patients with HPV16 positive (pre)malignant cervical lesions |
| Tjiong (2001) [42] | 81 |
Synthetic peptide based ELISA anti-HPV16/18 E6/E7 (IgG) |
Yes | Antibodies against the native HPV16 and HPV18 E6/E7 proteins were detectable in CVS (48%) and sera (29%) from patients with CxC (n = 21). In 7 of 11 patients with antibody reactivity against HPV16 or HPV18 E6 and/or E7 proteins a higher level of antibody reactivity in CVS than in the paired serum samples was found at similar inputs of total IgG. This suggests that the antibodies in CVS against the investigated HPV proteins in these patients were locally produced | ||||
| Onda (2003) [34] | USA | 24 | Study population: Hagensee (2000) [26] |
Capsid-based ELISA anti-HPV16 L1 (IgA) |
Wick Samples were placed into a vial containing PBS, and the samples were kept frozen at –20 °C until use |
Yes | The median time to antibody detection from the first detection of HPV 16 DNA was 10.5 months for IgA in cervical secretions and 19.1 months for serum IgA. The duration of IgA in cervical secretions and sera was shorter than the duration of serum IgG | |
| Rocha-Zavaleta (2003) [37] | Mexico | 797 | 30.8–31.6 y (19–49 y) | HPV16 infected women with and without detectable pathology and controls |
VLP-based ELISA anti-HPV 16 (sIgA/G) |
CVL Samples were collected by PBS. Cell debris was eliminated by centrifugation at 13,000 rpm for 5 min. Mucus samples were stored at –70 °C until tested |
No | sIgA and IgG antibodies were found in a significantly higher proportion of infected patients compared with uninfected women. Both sIgA and IgG are found in patients without pathological signs of infection, however, the response increases significantly in patients with pathological evidence |
| Sasagawa (2003) [38] | Japan | 627 | Not reported (16–70 y) | women who visited clinics for various gynecologic problems or routine cancer screening |
VLP-based ELISA anti-HPV16/18/31/45 (IgA/G) |
Cytobrush The remaining cell samples with mucosal secretion on the brush were suspended PBS and stored at –30 °C until examination |
No | Mucosal IgA response reflects current HPV infection, whereas an IgG response may be induced with the development of cervical lesions. The longitudinal study demonstrated that the IgA response was elicited earlier than the IgG response, and the IgG response was barely induced in the preclinical HPV infection. However, once an IgG response was induced, it persisted longer after HPV clearance |
| Rocha-Zavaleta (2004) [36] | Mexico | 704 | 31.3–49.9 y (16–81 y) | women with HPV positive LSIL or CxC |
Synthetic peptide based ELISA anti-HPV16 L1 (IgA) |
CVL Samples were collected with sterile PBS. Cell debris was eliminated by centrifugation at 9000 g for 5 min. Cervical mucus were stored at –70 °C until use |
Yes | Cervical IgA antibodies were detected in a significantly high proportion of women with high-risk HPV-associated LSIL compared with controls. However, the proportion of IgA-positive patients was lower than the proportion of IgG seropositives |
| Bierl (2005) [19] | USA | 540 | 26.6–26.9 y (18–60 y) | women with newly diagnosed CIN and controls |
VLP-based ELISA anti-HPV16 L1 (IgG/A) |
Cytobrush The frozen samples were thawed in PBS and EDTA and vortexed to dislodge cells. Following centrifugation, the supernatant was collected and stored at –70 °C until use |
Yes | Cervical anti-HPV16 IgA and IgG inversely correlated with HPV DNA, HPV16 DNA, and cervical disease. These findings suggest that mucosal antibodies may protect against HPV infection and cervical disease |
| Nguyen (2005) [33] | USA | 55 | Not reported (22–81 y) | women who underwent hysterectomy for CxC (HCC) or for diseases unrelated to CxC (HNN) or underwent loop excisions due to dysplasia (LOOP) |
Synthetic peptide based ELISA anti-HPV16 E7 (IgG/A) |
CVL Samples were collected using PBS. A protease inhibitor cocktail was added. The supernatants were aliquoted and stored at –70 °C until use |
Yes | While levels of HPV16 E7-specific IgG in vaginal wash were significantly higher in women undergoing HCC and HNN, the levels of the HPV16 E7-specific IgA in vaginal wash of women with CxC and cervical dysplasia were lower as compared to patients in HNN. These results suggest a selective down-regulation of local HPV-specific IgA responses in women with CxC |
| Passmore (2007) [35] | South Africa | 103 | 33.3–36.4 y (18–40 y) | women with varying grades of CIN |
VLP-based ELISA anti-HPV16 (IgG/A) |
Cytobrush Samples were stored on ice and processed within 4 hr after sampling. Specimens were centrifuged at 1,300 rpm for 10 min to pellet cells and the supernatant fraction was collected and stored at –20 °C until use |
Yes | Both the frequency and level of HPV16-specific cervical IgA was significantly elevated in women with CIN 2/3 compared with women with CIN1. An HPV16-specific antibody response in one mucosal compartment in women with CIN was not found to be predictive of a response to another |
| Lopez (2008) [28] | Mexico | 312 | 27.4–44.2 y (15–47 y) | women with LSIL and CxC with HPV16 infection and antibodies to HPV16-VLP and controls | 1/Inhibition assay, using HPV16 VLP and heparan sulfate proteoglycan-coated plates 2/Pseudo infection systems using HPV16 pseudovirions anti-HPV16 (IgG/A) |
CVL Samples from Rocha-Zavaleta (2004) [36] |
No | Mucosal antibodies inhibiting binding of VLP to heparan sulfate are developed in most LSIL patients, but are hardly present in CxC patients. However LSIL and CxC cases, showed similar levels of HPV16 L1-specific antibodies |
| Mbulawa (2008) [31] | South Africa | 84 | 35 y (22–62y) | women with/without HPV and associated cervical disease, 27 HIV+ |
1/VLP-based ELISA anti-HPV16 (IgG/A) 2/Neutralizing assay nAb-HPV-16 |
CVL Not reported |
Yes | Cervical neutralizing antibodies were detected in 38% of women with HPV16 infection and in 17% of women infected with the HPV16-related type HPV31. Cervical neutralizing antibodies correlated with HPV16 infection, but not with cervical disease. Serum and cervical HPV16 antibody responses were not affected significantly by HIV1 infection |
| Marais (2009) [29] | South Africa | 104 | 26 y (16–45y) | female sex workers participating in nonoxynol -9 efficacy trial; 40 HIV+ |
VLP-based ELISA anti-HPV16 (IgG/A) |
CVL The samples were obtained by the insertion of saline (storage temperature not reported) |
Yes | HIV1 seroconversion resulted in a reduced prevalence of serum HPV16 IgA and cervico-vaginal IgA and IgG but an increased prevalence of serum HPV16 IgG |
| Monroy (2010) [32] | Mexico | 511 | 28.9–33.3 y (15–51y) | women with cervical ectopy, LSIL and controls |
VLP-based ELISA anti-HPV16/18 (IgA) |
CVL Samples were collected by PBS. Cell debris was eliminated by centrifugation at 13,000 rpm for 5 min and supernatant was collected for analysis. Samples were subsequently stored at −70 °C until tested. |
No | HPV infection in cervical ectopy patients was accompanied by a mucosal IgA-antibody response. Antibody reactivity to HPV18 was significantly higher than the response to HPV16 |
| Ekalaksananan (2014) [24] | Thailand | 100 |
Not reported >30y |
women with LSIL and planned cryosurgery or pap smear |
Western blotting anti-HPV16 L1 (IgG/A) |
CVL Not reported |
No | When individuals were compared between first recruitment and after abrasion for 6 months, anti-HPV16 L1 IgA antibodies were significantly increased in the cryotherapy group |
|
Studies investigating the reliability and validity of the various CVS collection method | ||||||||
| Snowhite et al. (2002) [39] | USA | 15 |
Not reported 21–45y |
HPV infected women (9 HIV+) | Capture ELISA anti-HPV6/11/16/31/45 L1 (IgG/A) | CVL and Wick | Yes | The total protein (2-fold) and IgG concentration (10-fold) were higher in the Sno-strip samples, were reproducible (%CV < 3) and these levels correlated with their paired cervicovaginal sample |
| Kemp et al. (2008a) [27] | Costa Rica | Not reported | women in a natural history study of HPV and cervical neoplasia (spike-recovery experiment) |
Neutralizing assay Mouse anti-HPV16 (V5) |
Wicks Merocel vs. Ultracell sponges |
Yes | V5 recovery from sterile Merocel sponges was complete, yet that from Ultracell sponges was null. The mean V5 recoveries from participant Ultracell and Merocel sponges were 61.2% and 93.5%, respectively, suggesting that Merocel sponges are more appropriate for specimen collection | |
Abbreviations: CVS, cervicovaginal secretions; CIN, Cervical intraepithelial neoplasia; (B)PV, (Bovine) papillomavirus; sIgA, secretory Immunoglobuline A; PBS, Phosphate buffered saline; BSA, Bovine Serum Albumine; CVL, cervicovaginal lavage; EDTA, Ethylenediaminetetraacetic acid; CxC, cervical cancer; LSIL, Low-grade squamous intraepithelial lesion; nAb, neutralizing antibodies.
The included articles reported on antibodies in CVS from patients with cervical neoplasia and cancer, patients with condylomata, women with cervical ectopy, and women with normal cervical cytology. Two studies were included in this review that investigated immune modulation in CVS after treatment [24,25], and three studies investigated HPV-specific antibodies in CVS from HIV-infected women [[29], [30], [31]].
3.2.1. Mucosal antibodies against HPV proteins and their impact on HPV-associated disease
Although small numbers of virions are presumably exposed to the immune system, local antibodies against different HPV proteins have been detected in CVS from HPV-infected women. The reported naturally induced HPV-specific antibodies were predominantly type-specific and directed against the late viral capsid proteins (L1/L2) but also against 5 non-structural early proteins commonly expressed in disease-associated HPV infections (E2/E4/E5/E6 and E7). Most studies were limited only to the measurement of cervical antibodies against HPV16.
It has been suggested that mucosal antibodies may protect against HPV16 infection and cervical disease [19]. However, although cervical HPV-specific antibodies from women with active HPV16 infections have been shown to have some virus neutralizing capabilities [28], in general, local HPV-specific IgA and IgG presence do not correlate with viral clearance [43] and are not effective in inducing the regression of established lesions [20]. In contrast, detectable mucosal HPV-specific antibodies reflect current infection and HPV-associated lesions by concordant HPV types, respectively [21,23,40,44]. The same results were found for HPV18, although such results were based on only a few studies [32,38,42,44].
As is the case for HPV-specific antibodies in serum, it seems that there is also a several-month delay between initial cervical HPV infection and the detection of cervical antibodies [26,34]. Persistence of the IgG over time and the transient nature of the IgA was observed [38]. Most of the local IgA preceded the decline in the systemic presence.
Since the E6 and E7 oncoproteins are involved in tumour growth, local antibodies against these oncoproteins have been thought to play an important role in the monitoring of HPV-related cervical cancer development. However, E6/E7 antibodies were inconsistently detected and found to be unspecific for the diagnosis and/or prognosis of cervical cancer [25,33,[41], [42], [43]].
In general, if they were detectable, the concentrations of naturally induced antibodies collected from CVS were low, transient and extremely variable. For example, although the study of Hagensee et al. (2000) was longitudinal, the infrequent detection of cervical antibodies (10%) meant that the kinetics of the cervical humoral immune response against HPV16 could not be analysed in detail [26]. Local HPV-specific IgA and IgG levels did not correlate with virus clearance, nor did they strongly correlate with disease course. The results suggest that the measurement of local antibody production against selected HPV antigens is somewhat useful in the study of HPV immunology but not in the diagnosis or prognostic prediction of HPV infection. However, all observations should be interpreted with caution, as the CVS methods used for the collection, processing and measurement of HPV-specific antibody concentrations are not standardized and are relatively insensitive due to low signal-to-noise ratios [63].
3.2.2. Low concordance between antibody presence in CVS and serum after natural infection
Eighteen out of the 26 included studies that investigated naturally induced antibodies in CVS also provided results regarding serum HPV-specific antibody titres. However, not all studies tested both types of samples with the same substrates or assays. Furthermore, it must be noted that comparison between different studies was difficult because of the few numbers of HPV-infected women whose samples had converted (both serum and CVS).
Bontkes et al. (1999) were the first to investigate in more detail the association between local and systemic antibody prevalence (both IgA and IgG) against HPV16 VLP. Systemic but not local IgA correlated with the clearance of HPV16. As the systemic IgA prevalence was not accompanied by local IgA, the systemic IgA responses in patients with HPV16 clearance were suggested to be a by-product of an effective cellular immune response induced in the local lymph nodes that was mediated by cytokines. A relatively strong correlation between local and systemic IgG titres was found [20]. VLP-specific IgG antibodies detected locally had likely entered the mucosal surface by transudation from the serum. IgG was often found both locally and systemically at the same time in HPV16 patients. In some cases, local but not systemic IgG was detected, suggesting that some local IgG-producing B-cells must have been present in these individuals. Systemic and local cervical neutralizing-antibody prevalence was further directly compared by Mbulawa and colleagues (2008) and were found to not correlate significantly within individuals, similar results were obtained by Dreyfus et al. (1995) [23,31]. Passmore and colleagues (2007) further observed poor concordance and correlation between cervicovaginal, oral, and serum IgG and IgA responses to HPV16 [35]. The observations of the latter studies do not support the idea of a “common mucosal immune system” or a link between naturally induced HPV-specific antibodies in the genital tract and the systemic circulation. The variations observed between the different studies might be due to differences in study population characteristics, the collection or analysis methods used.
Overall, the immune profiles did not correlate well between serum and CVS, necessitating the direct collection of CVS to study cervix-specific immunity to HPV in natural history studies. In addition, more studies are needed to determine whether the mucosal immune response may be more important than the systemic response in determining the outcome for infections such as HPV.
3.3. Evaluation of vaccine-induced HPV-specific antibodies in CVS
Eighteen publications that reported on 11 studies investigated vaccine-induced HPV-specific antibodies in CVS [[46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61]]. All papers reported paired CVS and serum data. Three papers reported cervical mucosal immune responses after the administration of HPV11 and HPV16 VLPs [50,54,55], nine articles reported responses to the bivalent HPV16/18 vaccine (Cervarix®, GlaxoSmithKline Biologicals) [51,53,[56], [57], [58], [59], [60], [61], [62]], one article reported responses to the quadrivalent HPV6/11/16/18 vaccine (Gardasil®, Merck) [52], and four articles compared the latter two vaccines [[46], [47], [48], [49]]. In addition, one letter to the editor was included [45]. A summary of the papers and their main outcomes is given in Table 2. All studies were performed on healthy women.
Table 2.
Details of papers describing detection of mucosal HPV-specific antibodies after HPV vaccination.
| First author (year of publication) [ref] | Country of execution | Study Vaccine Vaccine schedule Vaccine administration |
Number of participants | Mean age (total age range) | Total number of collected CVS samples (collection time (month (M)) from 1st vaccine dose)e | Methodology and antibodies analysed in CVS (class) | CVS collection method Storage | Main study outcome |
|---|---|---|---|---|---|---|---|---|
| 1/Experimental HPV immunization | ||||||||
| Nardelli-Haefliger et al. (2003) [55] | Switzerland |
HPV16 L1 VLP vaccine different immunization/dosing schedules no adjuvant i.m. |
18; (n = 7, taking oral contraceptives; n = 11, ovulating) |
Not reported 18–45 y |
216 (M0-M2/M5, followed by twice weekly for 5 weeks) |
VLP-based ELISA anti-HPV16 L1 (IgG) |
Wick Samples were placed on ice, and PBS containing protease inhibitors were added. The liquid fraction (i.e., diluted cervical secretion) was frozen at –70 °C until analysis |
The cervical titers among participants in the contraceptive group were relatively constant throughout the contraceptive cycle. In contrast, the cervical titers among participants in the ovulatory group varied during the menstrual cycle |
| Fife et al. (2004) [50] | USA |
HPV11 or 16 L1 VLP vaccine different immunization/dosing schedules i.m. |
249 |
Median: 20 y 18–25 y |
182 (M7) |
VLP-based ELISA anti-HPV11/16 L1 (IgG/A) |
CVL Not reported |
Only about one half of the study participants who received the three highest experimental HPV11 vaccine doses had in their CVL detectable anti-HPV11 by month 7. The proportion was even lower in the HPV16 vaccine study |
| Nardelli-Haefliger et al. (2005) [54] | Switzerland |
HPV16 L1 VLP vaccine different immunization/dosing schedules no adjuvant nasal spray/bronchial aerosol or combination i.m./aerosol |
32 |
Not reported 18–45 y |
64 (M0-M2) |
VLP-based ELISA anti-HPV16 L1 (sIgA, IgG/A) |
Conducted as Nardelli-Haefliger et al. (2003) [55] | Data suggest that aerosol administration of HPV VLPs may represent a potential alternative to parenteral injection. IgA was detected at the cervix in a subset of these vaccines |
| 2/HPV-6/11/16/18 (Gardasil®) immunization | ||||||||
| Huo et al. (2012) [52] | England |
HPV-6/11/16/18 vaccine 0,1,4 month schedule Sublingual (SL) drops/i.m. |
18 | 24.2–26.3 y 19–31 y |
81 (M0-M1-M2-M4-M5) |
1/VLP-based ELISA anti-HPV6/16/18 L1 (IgG/A) 2/Neutralizing assay nAb-HPV16 |
Wick Samples were snipped into the top chamber of a Spin-X tube containing 300 mL sterile filtered extraction buffer and centrifuged at 4 °C for 15 min at 13,000 g. A repeat extraction was performed by adding additional extraction buffer to the top chamber, and then 8 mL heat inactivated FBS added to pooled secretions from each sample site, prior to separation into 200 mL aliquots and freezing at –80 °C until analysis |
SL antigens induced 38-fold lower serum and 2-fold lower cervical/vaginal IgG than i.m. delivery, and induced or boosted serum virus neutralizing antibody in only 3/12 subjects. Neither route reproducibly induced HPV-specific mucosal IgA. The observation that SL immunization could boost pre-existing serum neutralizing activity points to the possible use of i.m. prime/SL boost schedules |
| 3/HPV-16/18 (Cervarix®) immunization | ||||||||
| Kemp et al. (2008b) [53] | Costa Rica |
HPV-16/18 vaccine 0,1,6 month schedule i.m. |
50 |
Not reported 18–25 y |
47 (M0 (n = 5) -M12) |
1/Neutralizing assay nAb-HPV16/18 2/VLP-based ELISA anti-HPV16/18 L1 (IgG) |
Wick Samples were placed into a 10 mL tube for storage in liquid nitrogen. After shipment the samples were stored at −70 °C. Sponge were extracted in a buffer containing PBS, NaCl and Aprotinin. 300 μl of cervical extraction buffer was slowly added to the top of the sponge. 4 μl was added FBS. The extracts were aliquoted and frozen at −70 °C until further testing |
Strong correlations between SEAP-NA and ELISA were observed. Systemic and cervical antibody measures also correlated well except at mid-cycle. Correlations between antibody levels at one and twelve months following the start of vaccination were poor |
| Schwarz et al. (2010) [60],a | Germany, The Netherlands, Finland, USA, Poland, Denmark |
HPV-16/18 vaccine 0,1,6 month schedule i.m. |
350 |
Not reported 10–65 y |
553 (M7-M12-M18-M24-M36) |
VLP-based ELISA anti-HPV16/18 L1 (IgG) |
Wick Conducted as Kemp et al. (2008b) [53]. Samples were stored at -20 °C or -70 °C until antibody extraction |
Good correlation was seen between HPV16/18 antibody levels at all time-points. The strong correlation between levels of HPV16/18 antibodies in serum and CVS up to 36 months post-vaccination supports transudation of serum antibodies as the mechanism by which antibodies are introduced into CVS |
| Petäjä et al. (2011) [56] | Denmark, Estonia, Finland |
HPV-16/18 vaccine 0,1,6 month schedule i.m. |
321 | 24.2 y (Group 15–25y) 10–25 y |
69 (only from the age 15–25 group; M24-M36-M48) |
VLP-based ELISA anti-HPV16/18 L1 (IgG) |
Wick Conducted as Schwarz et al. (2009) [61] |
Anti-HPV16/18 antibodies in CVS were detectable for subjects aged 15–25 years (84% and 70%, respectively). There was a strong correlation between serum and CVS anti-HPV16/18 antibodies levels |
| Scherpenisse et al. (2013) [57] | The Netherlands |
HPV-16/18 vaccine 0,1,6 month schedule i.m. |
1,151 | 15.1 y 14–16 y |
649 (M0-M12-M24) |
VLP-based multiplex immunoassay Anti-HPV16/18/31/33 /45/52/58 L1 (IgG/A) |
Wick Samples were collected from the tampons by addition of PBS containing complete protease inhibitor cocktail and subsequent centrifugation for 30 min, 3,200 g at 4 °C. CVS samples were stored at -80 °C until analysis |
Post-vaccination, HPV16/18 IgG and IgA are detectable in CVS. The correlation of HPV16/18 IgG antibody levels between serum and CVS suggests that vaccine-induced HPV antibodies transudate and/or exudate from the systemic circulation to the cervical mucosa to provide protection against HPV infections |
| Gonçalves et al. (2016) [51],b | Brazil |
HPV-16/18 vaccine 0,1,6 month schedule i.m. |
60 | 27.2 y 19–43 y |
60 (M0-M1-M6-M7) |
VLP-based ELISA anti-HPV 16/18 L1 (IgG/A) |
CVL Samples were obtained by PBS (No storage temperature reported) |
After the third vaccination, there is a strong agreement between cervical and systemic IgG antibody responses and a weak agreement between cervical and systemic IgA antibody responses. The induction of IgA antibodies seems to be secondary to that of IgG antibodies in response to HPV i.m. vaccination |
| Ferreira Costa et al. (2018) [62],b | Brazil |
HPV-16/18 vaccine 0,1,6 month schedule i.m. |
35 | Same population as Gonçalves et al. (2016) [51]; age not specified | 70 (M7-M18) |
VLP-based ELISA anti-HPV/VLP (IgA/G); HPV type not specified |
CVL Not reported |
Cervical samples were positive for both IgG and IgA antibodies at 7 months and decreased after 1 year to 33% and 29%. The median absorbance in serum and the cervix for IgG and IgA anti-HPV-VLP antibodies was significantly higher at month 7 after vaccination when compared to 1 year post-vaccination |
| Schwarz et al. (2009) [47],c | Germany, Poland |
HPV-16/18 vaccine 0,1,6 month schedule i.m. |
531 | 35 y 15–55 y |
149 (M18-M24) |
VLP-based ELISA anti-HPV16/18 L1 (IgG) |
Wick Samples were collected per woman and stored at −20 °C until antibody extraction. The extraction protocol was conducted as Castle et al. (2004) [71] |
There was a high correlation between HPV16 and HPV18 antibody levels (IgG) in CVS and sera, regardless of age |
| Schwarz et al. (2015) [58],c | 488 |
Not reported 15–55 y |
190 (M60-M72) | A strong correlation between anti-HPV16/18 levels in serum and CVS samples 6 years after vaccination indicates a long-lasting transudation of serum antibodies across the cervical epithelium | ||||
| Schwarz et al. (2017)[59],c | 470 |
Not reported 15–55 y |
107 (M120) | Correlation coefficients for antibody titers in serum and CVS were 0.64 (anti-HPV16) and 0.38 (anti-HPV18) | ||||
| 4/HPV-16/18 (Cervarix®) vs HPV-6/11/16/18 (Gardasil®) immunization | ||||||||
| Draper et al. (2013) [46] | UK |
HPV-16/18 vaccine HPV-6/11/16/18 vaccine Both 0,1,6 month schedule i.m. |
198 |
Not reported 12–15 y |
50 (M7) |
VLP-based ELISA anti- HPV16/18/31/45 L1 (IgG) |
Cytobrush Samples were rehydrated with PBS and subjected to centrifugation within a Amicon tube for 5 min at 2,500 g. Two such extractions were performed and the eluted material pooled and subjected to centrifugation at 13,000 g to remove cellular debris. The clarified supernatant was aliquoted and stored at -80 °C |
Levels of neutralizing and binding antibodies in genital secretions were closely associated with those found in the serum, with Cervarix® having a median 2.5 fold higher level of HPV-specific IgG ratio in serum and genital samples than Gardasil® |
| Einstein et al. (2009) [47],d | USA |
HPV-16/18 vaccine 0,1,6 month schedule i.m. HPV-6/11/16/18 vaccine 0,2,6 month schedule i.m. |
920 |
30.2-30.7 y 18–45 y |
165 (M0-M7) |
1/Neutralizing assay nAb-HPV16/18 2/VLP-based ELISA anti-HPV16/18 L1 (IgG) |
Wick Conducted as Schwarz (2009) [61] |
Positivity rates for anti-HPV16/18 nAb in CVS and circulating HPV16/18-specific memory B-cell frequencies were higher after vaccination with Cervarix® compared with Gardasil® |
| Einstein et al. (2011) [48],d | 799 | 31 y 18–45 y |
222 (M12-M18-M24) |
VLP-based ELISA anti-HPV16/18 L1 (IgG) |
Positivity rates and levels of antigen-specific IgG antibodies in CVS were not significantly different between vaccines | |||
| Einstein et al. (2014) [49] d | 524 | 31 y 18–45 y |
170 (M36-M48) | Limited CVS samples were available. Positivity rates of anti-HPV16/18 IgG antibodies in CVS appeared higher in the Cervarix® group compared with the Gardasil® group, while CVS antibody levels were of similar level. Antibody levels in serum and CVS are poorly correlated, especially for HPV18 | ||||
| Barr, Koutsky (2004) [45] | Letter to Nardelli-Haefliger et al. (2003) [55] | |||||||
This pooled analysis from four separate phase III clinical trials includes data from Einstein et al. (2009) [47] and Schwarz et al. (2009) [61].
Ferreira Costa et al. (2018) is a follow-up study of Gonçalves et al.(2016) reporting 7–18 months after HPV-16/18 vaccination.
Schwarz et al. (2009/2012/2017) is a follow-up study with reports 24-72-120 months after HPV-16/18 vaccination.d Einstein et al. (2009/2011/2014) is a follow-up study comparing HPV-16/18 and -6/11/16/18 vaccination after 7-24-48 months.
Several CVS samples were still excluded because of weight, blood contamination, etc. Abbreviation: VLP, virus-like-particles; i.m., intramuscular; OC, oral contraceptives; nAb, neutralizing antibodies; FBS, Fetal bovine serum; PBS, Phosphate buffered saline; SEAP-NA, secreted alkaline phosphatase neutralization assay.
3.3.1. Vaccine-induced antibody assessment in CVS
The presence of HPV-specific vaccine-induced antibodies at the cervical epithelium was first observed after experimental HPV vaccination [50,55]. Nardelli-Haefliger et al. (2003) detected high titres of anti-HPV16-specific IgG antibodies in the cervical secretions of women immunized with the experimental HPV16 L1 VLP vaccine [55]. All participants had detectable titres of anti-HPV16 IgG in their cervical secretions, which showed promise in terms of vaccine efficacy. In addition, Fife et al. (2004) investigated two candidate HPV vaccines (HPV16 L1 VLP and HPV11 L1 VLP vaccine) in a dose-escalation trial. In this study, only half of the study participants who received the three highest experimental HPV11 vaccine doses had detectable anti-HPV11 titres in their CVS. The proportion of study participants who had detectable antibody titres in CVS was even lower than that in the HPV16 vaccine study [50]. The dilution factor resulting from the lavage procedure used in this study could have reduced the number of study participants with genital tract antibodies compared with that found in the study by Nardelli-Haefliger et al. [55]. Additionally, two studies were identified that investigated CVS antibody titres after mucosal vaccination (nasal/aerosol/sublingual), which was used as an alternative to intramuscular immunization [52,54]. However, in contrast to results in mice [64,65], mucosal vaccination in women was found to be poorly immunogenic to induce antibodies in CVS.
The majority of identified vaccine studies explored the presence of HPV-specific vaccine-induced antibodies at the cervix after bivalent HPV16/18 vaccination. Kemp and colleagues (2008) detected anti-HPV antibodies at the cervix in women (aged 18–25 years) for up to two years after the first vaccine dose of the bivalent HPV vaccine. Presence indicated the efficacy of site-specific vaccine-induced immunity [53]. The same results were found by Scherpenisse et al. (2013) in a younger cohort (aged 14–16 years) [57]. Nevertheless, in 10% and 30% of the girls, Scherpenisse and colleagues were not able to detect HPV16 and 18 antibodies, respectively, in CVS, although the HPV16 and 18 antibody titres in serum were high. In addition, reduced cervicopositivity compared to seropositivity was shown in other studies after bivalent HPV vaccination [51,56,[58], [59], [60], [61]]. Briefly, a pooled analysis of data from four clinical trials showed that the positivity rates for anti-HPV16 and 18 antibodies in CVS were 95% and 92%, respectively, at month seven and ranged between 71–100% and 55–100%, respectively, from month 12 through 36 [60]. The study by Petäjä et al. (2011) reported data after 48 months and showed that anti-HPV16/18 antibodies in CVS were still detectable in subjects aged 15–25 years (in 84% and 70% of subjects for HPV16 and 18, respectively) [56]. The follow-up study by Schwarz et al. (2015) showed that after six years, serum anti-HPV16/18 titres were still consistently higher in women who had detectable anti-HPV16/18 antibodies in CVS [58]. Anti-HPV16 and anti-HPV18 antibodies were detected in approximately 72% and 54–69% of the CVS samples, respectively. After ten years of follow-up, anti-HPV16 antibodies were detected in 54%–70% of CVS samples from women, and anti-HPV-18 antibodies were detected in 35–45% of CVS samples [59]. A downside was that, only a few CVS samples were collected which were evaluable at the end of the study by Schwarz et al. (2017). Nevertheless, it is reasonable to conclude that when antibody titres are reduced or can no longer be detected in serum following vaccination, fewer antibodies will transudate into CVS to provide protection against HPV at the site of infection.
After natural infection, HPV-specific IgA antibodies were detectable at the cervix, mainly in women with persistent HPV infections [32,38]. Data regarding IgA antibody responses after vaccination are limited [51,57] since IgG is the principal antibody class present in the female genital tract and systemic immunization is known to induce mainly IgG production [66]. However, HPV16/18-specific IgA responses, although considerably lower than IgG responses, were reported in CVS after HPV vaccination in two studies [51,57].
3.3.2. Differences in CVS antibody levels induced by different HPV vaccines
Direct comparison of the clinical trial data for bivalent and quadrivalent HPV vaccines from different studies is often not feasible given the differences in study design and the methodologies used to evaluate HPV16/18-specific immune responses. Four papers that reported on two head-to-head studies were identified that investigated the mucosal immune responses to quadrivalent and bivalent vaccines using the same methodology for the assessment of immune responses and reactogenicity [[46], [47], [48], [49]].
Einstein et al. (2009) conducted a randomized, observer-blind study to compare the two vaccines in a single, well-defined population of healthy women aged 18–45 years [[47], [48], [49]]. The study showed at month 7 and month 48 that a greater proportion of the women who received the bivalent vaccine had detectable HPV type-specific neutralizing antibodies in CVS than those who received the quadrivalent vaccine. These findings are consistent with the results found by Draper et al. (2013) [46]. Nevertheless, further long-term studies are needed to understand whether the differences in the levels of local immune responses produced by the vaccines have clinical importance.
To the best of our knowledge, no studies have yet reported the detection of mucosal HPV-specific antibodies: (i) after the current recommended 2-dose schedule (0, 6–12 month schedule) in preadolescents, (ii) in one-dose HPV vaccine trials, (iii) after immunization with the recently licensed nonavalent vaccine (Gardasil9®, Merck), or (iv) after HPV vaccination administered before or immediately after surgical treatment. The last case is of particular interest since vaccination before or immediately after treatment could produce a massive number of local antibodies within the basal membrane of the cervical surface. During the regeneration of the removed tissue, these antibodies could prevent self-reinfection of the surgical site by blocking virus entry into the basal layers of uninfected cells, hence preventing disease relapse [67].
3.3.3. Vaccine-induced HPV-specific antibody CVS levels show correlation with serum levels
Several studies were performed to assess whether serum antibody levels are correlated with levels in the mucosa. In general, studies reported a moderate to strong correlation between the HPV16 and 18 antibody levels in serum and CVS, suggesting that vaccine-induced HPV antibodies transudate from the systemic circulation to the cervical mucosa to provide, together with exudated HPV antibodies, protection against HPV infection (the correlations are shown in Table 3). This hypothesis is supported by the strong correlation between the levels of antibodies specific for diphtheria and tetanus toxoid in serum and CVS because these vaccine-induced antibodies are not produced in the genital matrix [57,68].
Table 3.
Correlations between antibody levels in pairs of CVS and serum samples after HPV vaccination.
| Study vaccine | Study (publication year) [ref] | Number of participants (total age range) | Measured anti-HPV antibody response | Correlation between antibody levels in paired CVS and serum samples |
|---|---|---|---|---|
| HPV-16 VLPs | Nardelli-Haefliger (2003) [55] | N = 18 (18–45 y) | HPV-16 (contraceptive group) | ρ = .86 |
| HPV-16 (ovulatory group) | ρ = .27 | |||
| HPV-11 or 16 VLPs | Fife (2004) [45] | N = 249 (18–25 y) | HPV-11 | τ = . 27 |
| HPV-16 | Not reported | |||
| HPV-16/18 vaccine (2HPV) | Kemp (2008b) [53] | N = 50 (18–25 y) | HPV-16 (ELISA) | ρ = .73 |
| HPV-16 (SEAP) | ρ = .74 | |||
| HPV-18 (ELISA) | ρ = .75 | |||
| HPV-18 (SEAP) | ρ = .64 | |||
| Schwarz (2010) [60] | N = 350 (10–65 y) | HPV-16 | r = .84 - .92 | |
| HPV-18 | r = .90 - .91 | |||
| Petäjä (2011) [56] | N = 321 (10–25 y) | HPV-16 | r = .84 | |
| HPV-18 | r = .90 | |||
| Scherpenisse (2013) [57] | N = 1151 (14–16 y) | HPV-16 IgG | ρ = .58 | |
| HPV-18 IgG | ρ = .50 | |||
| HPV-16 IgA | ρ = .54 | |||
| HPV-18 IgA | ρ = .55 | |||
| Schwarz (2009) [61] | N = 531 (15–55 y) | HPV-16 | r = .73 - .90 | |
| HPV-18 | r = .82 - .93 | |||
| Schwarz (2015) [58] | N = 488 (15–55 y) | HPV-16 | r = .81 - .96 | |
| HPV-18 | r = .69 - .84 | |||
| Schwarz (2017) [59] | N = 470 (15–55 y) | HPV-16 | r = .64 | |
| HPV-18 | r = .38 | |||
| HPV-16/18 vaccine (2HPV) HPV-6/11/16/18 vaccine (4HPV) | Einstein (2009) [47],a | N = 920 (18–45 y) | HPV-16 (ELISA) | r = .74 |
| HPV-16 (PBNA) | r = .64 | |||
| HPV-18 (ELISA) | r = .83 | |||
| HPV-18 (PBNA) | r = .46 | |||
| Einstein (2011) [48],a | N = 799 (18–45 y) | HPV-16 (2HPV) | r = .91 | |
| HPV-16 (4HPV) | r = .97 | |||
| HPV-18 (2HPV) | r = .96 | |||
| HPV-18 (4HPV) | r = .90 | |||
| Einstein (2014)[49],a | N = 524 (18–45 y) | HPV-16 (2HPV) | r = .88 | |
| HPV-16 (4HPV) | r = .82 | |||
| HPV-18 (2HPV) | r = .47 | |||
| HPV-18 (4HPV) | r = .66 | |||
| Draper (2009) [46],a | N = 198 (12–15 y) | HPV-16 (ELISA) | r = .80 | |
| HPV-16 (PBNA) | r = .73 | |||
| HPV-18 (ELISA) | r = .69 | |||
| HPV-18 (PBNA) | r = .74 | |||
| HPV31 (ELISA) | r = .84 | |||
| HPV45 (ELISA) | r = .71 |
Since the mechanism of transudation of serum antibodies into the CVS is expected to be the same regardless of the vaccine eliciting the immune response, overall Pearson correlation coefficients were calculated for each antigen using data for Cervarix® (2HPV) and Gardasil® (4HPV) combined. Abbreviations: ρ, Spearman correlation coefficient; τ, Kendall's τ value; r, Pearson correlation coefficient; SEAP-NA, secreted alkaline phosphatase neutralization assay; PBNA, pseudovirion-based neutralization assay.
3.3.4. Detectable vaccine-induced antibody levels in CVS are lower than serum levels
The study performed by Nardelli-Haefliger et al. (2003) found that the levels of cervical HPV16 type-specific antibodies were 0.5–50% of the systemic levels [55]. In a study by Kemp (2008), cervical antibody levels were found to be 2–3 logs lower than serum levels [53]. IgG antibody concentrations detected by Scherpenisse et al. (2013) in CVS were approximately 2% of the IgG serum antibody concentrations for both HPV16 and HPV18 [57]. Generally, it is difficult to estimate the mucosal concentration of HPV-specific antibodies because the method of recovery mostly leads to a dilution of the original secretion. In addition, a gradient of decreasing exudate antibody concentration would be expected with increasing distance from the site of trauma or infection [69], it is plausible that the concentration of HPV-specific antibodies at the site of initial virus deposition, the basement membrane, might approach that of systemic antibodies [53].
Whether the low CVS antibody titres observed in these studies are sufficient to offer protection at the site of infection is unknown, as the immune correlate of protection remains to be determined. However, Longet et al. (2011) showed by in vivo genital HPV pseudovirion challenge in mice that very low vaccine-derived HPV-specific antibody levels could protect against HPV infection and that these protective antibody levels are far below the detection limits of pseudovirion-based neutralization assays [69].
3.3.5. Cross-reactive antibodies detectable in CVS
The studies of Draper (2013) [46] and Scherpenisse (2013) [57] sought to detect antibodies against non-vaccine types elicited by bi- and quadrivalent HPV vaccination in CVS from vaccinated individuals. Draper investigated CVS from young women (aged 12–15 years) to detect antibodies against the closely related non-vaccine types HPV31 and HPV45. All serum samples and the majority of the genital samples were positive for antibodies against HPV31 and 45 VLPs, and a strong relationship between the levels of HPV31- or HPV45-specific IgG in serum and in genital samples was demonstrated (Table 3). The titres of serum antibodies against non-vaccine types were 1% of the titres of antibodies against vaccine types. Although it is difficult to understand how low levels of cross-neutralizing antibodies in serum could result in sufficient levels for protection at the site of infection, the data from Draper et al. (2013) do at least indicate that such antibodies against HPV31 and HPV45 are present in CVS. In addition, in the study by Scherpenisse et al. (2013), a significant increase in the concentrations of IgG antibodies against five cross-reactive HPV types (HPV31/33/45/52 and 58) in CVS 12 months after vaccination was observed. For the cross-reactive types, no IgA responses could be detected in CVS by both studies. Notably, all observations should be interpreted with caution, as both studies indicated that the levels of cross-reactive antibodies detected in CVS were close to or below the limits of detection of the assays used.
3.4. Methods used for HPV-specific antibody detection in CVS
The accurate collection and processing of CVS and the use of appropriate immunoassays are of paramount importance for the reliable evaluation of local humoral immune responses to HPV infections or HPV vaccinations. Several different methods were used in the analysed papers to collect the mucosal samples, extract the antibodies, and measure HPV antibodies.
3.4.1. CVS collection methods
Measurements of local immune responses at the cervix have been hampered by the difficulty in the reliable collection of female genital secretions for use in immunological evaluation and the absence of an absolute method of sampling. In Table 4, an overview is given of the different methods used to collect CVS. Most CVS samples were collected by cervicovaginal lavage, during which the vaginal vault is rinsed with washing buffer (mostly PBS, ranging from 1 to 20 mL) that is collected, and cervicovaginal wicks, which are Sno-strip, Ultracell, Merocel or Weck-cell sponges that are inserted in the female genital tract and passively absorb CVS.
Table 4.
Overview of different methods used by the selected papers to collected CVS.
| CVS collection method | Studies | Remarks |
|---|---|---|
| 1/Wick techniques | ||
| Tampon (self-collected) | Scherpenisse et al. (2013) [57] | |
| Ultracell sponges | Kemp et al. (2008a) [27] | |
| Merocel sponges | Kemp et al. (2008a,b) [27,53]; Einstein et al.(2009/2011/2014) [[47], [48], [49]]; Schwarz et al. (2009/2010/2015/2017) [[58], [59], [60], [61]]; Petäjä et al.(2011) [56] | |
| Weck-cel sponges | Nardelli-Haefliger et al. (2003/2005) [54,55] | |
| Huo et al. (2012) [52] | ||
| Sno-strips | Snowhite et al. (2002) [39]; Onda et al. (2003) [34] | |
| Not reported | Hagensee et al. (2000) [26] | |
| 2/Aspiration, cervical-vaginal lavage techniques | Volume | |
| Collected by PBS | Rocha-Zavaleta et al. (2003/2004) [36,37]; Lopez et al. (2008) [28]; Monroy et al. (2010) [32] | 1 mL |
| Gonçalves et al. (2016) [51] | 2 mL | |
| Snowhite et al. (2002) [39]; Nguyen et al. (2005) [33] | 5 mL | |
| Tjiong et al. (2000/2001) [41,42] | 20 mL | |
| Collected by physiologic serum | Dreyfus et al. (1995) [23] | Not reported |
| Collected by saline | Marais et al. (2000/2009) [29,30] | 5 mL |
| Snyder et al. (1991) [40] | Not reported | |
| Not reported | Fife et al. (2004) [50]; Mbulawa et al. (2008) [31]; Ekalaksananan et al. (2014) [24]; Ferreira Costa (2018) [62] | Not reported |
| 3/Cytobrush/swab | Device | |
| Swabbing the endocervix | Bierl et al. (2005) [19]; Passmore et al. (2007) [35] | Digene sampler, Maryland, USA |
| Swabbing ectocervix | Dillner et al. (1993) [22]; Wang et al. (1996) [44] | Medscand, Mahnö, Sweden |
| Swabbing endo and ectocervix | Elfgren et al. (1996) [25] | |
| Veress et al. (1994) [43] | Not reported | |
| Swabbing endo/ectocervices posterior fornix of the vagina | Sasagawa et al. (2003) [38] | |
| Vaginal swab (self-collected) | Draper et al. (2013) [38] | Netcell Slimpack™ Polyvinyl acetate media; Network Medical Products, UK |
| Not reported | Dillner et al. (1989) [21]; Bontkes et al. (1999) [20] | Not reported |
Despite the fact that there are numerous studies examining local HPV-specific immunological responses in the female reproductive tract, only two studies were found that have systematically examined the reliability and validity of the various CVS collection methods used to detect HPV-specific antibodies (Table 1) [27,39]. The first study, by Snowhite et al. (2002), compared cervicovaginal lavage (CVL) with the use of Sno-strips (SS). Unlike CVL, which collects a diluted admixture of the secretions of the cervix and vagina, SS collects undiluted cervical secretions. The comparison of paired SS–CVL samples suggested that either collection method allowed for the reproducible measurement of local antibodies. However, in general, the immunoglobulin concentrations were 10-fold higher when using SS, while the average eluted volume was approximately 10-fold smaller than the average volume recovered from CVL. Additionally, the relative IgA/IgG ratio was higher for SS than for CVL, which is reflected in the lack of correlation in IgA levels between CVL and SS. These findings could reflect local variations at the different sample sites in the female genital tract in antibody-producing cells, epithelial receptor-mediated IgA secretion, and the transudation of serum immunoglobulins [70]. Secondly, Kemp et al. (2008) [27] evaluated antibody recovery using a cellulose-based Ultracell ophthalmic sponge (Ultracell Medical Technologies, Inc., North Stonington, CT) and a polyvinyl acetate-based Merocel ophthalmic sponge (Medtronic Ophthalmics, Jacksonville, FL). They demonstrated full neutralizing antibody recovery from Merocel sponges, while the recoveries from Ultracell sponges were low and more variable. This difference was explained by the chemical compositions of the sponges. The poor recovery from the Ultracell sponges was in contrast with that found during a previous study that analysed the recovery of cytokines and antibodies from different sponge materials [71]. However, the Ultracell sponges evaluated in the study by Castle et al. (2002) were made of polyvinyl alcohol, in contrast to the cellulose-based Ultracell sponges used in the study by Kemp et al. (2008).
In general, it appears that when dilution of the sample may hamper antibody detection, wicks would provide a valuable alternative collection method. However, the use of such devices should in no way damage the cervical mucosa. Although reported as non-invasive, the application of sponges at the cervix may induce microtraumas. Hildesheim et al. (1998), for example, reported visible bleeding in 25% of cases [72,73]. Likewise, almost half of all CVS samples collected in the study by Schwarz (2010) were not suitable for analysis, mainly due to blood contamination [60]. The characteristics of the different collection methods used are summarized in Table 5.
Table 5.
Characteristics of different methods used by the different papers to collected CVS.
| Characteristics | Cervical wick | Cervicovaginal lavages | Cytobrush/swab |
|---|---|---|---|
| Minimal trauma | +/- | + | |
| Known dilution of secretions | +/- | +/- | |
| Minimal dilution of secretions | + | + | |
| Sufficient material collected | + | ||
| Ease of collection | + | +/- | + |
| Self-insertion / self-collection possible | + | + |
Abbreviations: +, good; +/-, moderate.
In two studies, CVS was self-sampled (by a tampon [57] and a vaginal swab [46]). The subjects in the study by Scherpenisse et al. (2013) reported that the use of a tampon as a collection method was easy and comfortable. To the best of our knowledge, no studies have yet explored the use of a Softcup, although in other research areas, compared to lavage, the cervical Softcup method resulted in a 13-fold higher level of total IgG [74,80]. Furthermore, no studies were found that investigated HPV-specific antibodies in (first-void) urine. However, our research group recently published that vaccine-induced HPV-type restricted antibodies can be detected in first-void urine and correlated with paired serum levels [75]. Although the inclusion of pre-teenage girls and young adolescents was considered, this was not reasonable in several studies that involved a speculum exam for CVS collection. Because of this, self-sampling and non-invasive urine collection, which is feasible for (young) girls, could provide a good alternative for cervicovaginal sampling. In addition, this could increase study participation rates and guarantee the avoidance of microtrauma.
3.4.2. Immunoassays used for HPV-specific antibody detection in CVS
Several assays have been developed to monitor antibodies to HPV (reviewed in Ref. [76]). In Table 6, an overview is given of the immunoassays used within the included papers. Early studies of the mucosal responses to HPV infection used either bovine papillomavirus or a denatured HPV protein as an antigen, which made the interpretation of the results of these studies difficult. Currently, the pseudovirion-based neutralization assay (PBNA) is the “gold standard” for the detection of antibody responses to HPV. Nevertheless, most mucosal antibody responses in the selected studies were determined by VLP-based ELISAs. Neutralizing and non-neutralizing antibodies detected in VLP-based assays are mainly type-restricted and directed against conformational epitopes. In contrast to VLP-based ELISA, the pseudovirion-based neutralizing assay used in seven of the included papers detects neutralization activity, which may be a more relevant measurement of titers in prophylactic vaccine studies [27,31,46,47,49,52,53]. However, Kemp et al. (2008) observed a high correlation in women vaccinated with the HPV16/18 vaccine when CVS anti-HPV16/18 antibodies were measured by direct ELISA and PBNA. These results, combined with the higher reproducibility and simplicity of ELISA, suggest that ELISA is a good surrogate assay to measure neutralizing activity [53]. The variations in the VLP-ELISA assay in the different studies could be accounted for by the origin of the produced VLP and the different purification systems used.
Table 6.
Immunoassays used in selected papers to detected HPV-specific antibodies in CVS.
| Detection method | Studies | Cervical HPV-specific antibodies detected (reported antibody class) |
|---|---|---|
| Neutralizing assays | ||
| SEAP-NA | Kemp et al. (2008a) [27] | nAb-HPV16/18 |
| Kemp et al. (2008b) [53] | nAb-HPV16 V5 mouse | |
| Mbulawa et al. (2008) [31] | nAb-HPV16 (IgG/A) | |
| PBNA | Einstein et al. (2009/2014) [47,49] | nAb -HPV16/18 |
| Huo et al. (2012) [52] | nAb-HPV16 | |
| Draper et al. (2013) [46] | nAb-HPV31/45 | |
| VLP-based assays | ||
| HPV VLP ELISA | Nardelli-Haefliger et al. (2003) [55] | anti-HPV16 (IgG) |
| Fife et al. (2004) [50] | anti-HPV11/16 (IgG/A) | |
| Nardelli-Haefliger et al. (2005) | anti-HPV16 (sIgA, IgG/A) | |
| Kemp et al. (2008a) | anti-HPV16/18 (IgG) | |
| Einstein et al. (2009/2011/2014) | anti-HPV16/18 (IgG) | |
| Petäjä et al. (2011) | anti-HPV16/18 (IgG) | |
| Rocha-Zavaleta et al. (2003) | anti-HPV16 (sIgA/G) | |
| Passmore et al. (2007) | anti-HPV16 (IgG/A) | |
| Mbulawa et al. (2008) | anti-HPV16 (IgG/A) | |
| Huo et al. (2012) | anti-HPV6/16/18 (IgG/A) | |
| Draper et al. (2013) | anti-HPV16/18/31/45 | |
| Schwarz et al. (2009/2010/2015/2017) | anti-HPV16/18 (IgG) | |
| Monroy et al. (2010) | anti-HPV16/18 (IgA) | |
| Bontkes et al. (1999) | anti-HPV16 (IgG/A) | |
| Marais et al. (2000,2009) | anti-HPV16 (IgG/A) | |
| Elfgren et al. (1996) | anti-HPV16 (IgA) | |
| Bierl et al. (2005) | anti-HPV16 (IgG/A) | |
| Sasagawa et al. (2003) | anti-HPV16/18/31/45 (IgA/G) | |
| Gonçalves et al. (2016) | anti-HPV 16/18 (IgG/A) | |
| Ferreira Costa et al. (2018) | anti-HPV VLP (IgG/A) | |
| VLP-based multiplex immunoassay | Scherpenisse et al. (2013) | anti-HPV16/18/31/33/45/52/58 (IgG/A) |
| Oligopeptide ELISA | ||
| Synthetic peptide based | Snyder et al. (1991) | anti-HPV16 L1/E4 |
| Dillner et al. (1993) | anti-HPV16 L1/L2/E2/E7 (IgA/G) | |
| Dreyfus et al. (1995) | anti-HPV16 E2 (IgG/A) | |
| Elfgren et al. (1996) | anti-HPV16 E2/E7/L1/L2; HPV6 L1; HPV16/18 peptide 245 (IgA/G) | |
| Veress et al. (1994) | anti-HPV16 E2/E7/L1/L2 HPV11 L2 (sIgA) | |
| Rocha-Zavaleta et al. (2004) | anti-HPV16 L1 (IgA) | |
| Nguyen et al. (2005) | anti-HPV16 E7 (IgG/A) | |
| Tjiong et al. (2001) | anti-HPV16/18 E6/E7 (IgG) | |
| Wang et al. (1996) | anti-HPV16/18/33 L1 (IgA) | |
| Snowhite et al. (2002) | anti-HPV6/11/16/31/45 L1 (IgG/A) | |
| Onda et al. (2003) | anti-HPV16 L1 (IgA) | |
| Purified BPV-ELISA | Dillner et al. (1989;1993) | anti-PV (IgA/G) |
| Competitive Luminex immunoassay | ||
| HPV16 luminescence immunoassay (LIA) | Hagensee et al. (2000) | anti-HPV16 (IgG/(s)IgA) |
| Other | ||
| Radioactive immunoprecipitation assay (RIPA) | Tjiong et al. (2000) | anti-HPV16 E7 (IgG) |
| Inhibition assay, using HPV16 VLP and heparan sulfate proteoglycan-coated plates and pseudo infection systems, using HPV16 pseudovirions | Lopez et al. (2008) | anti-HPV16 (IgG/A) |
| Western blotting using HPV16 L1 protein from VLP16 (Kim et al., 2010) | Ekalaksananan et al. (2014) | anti-HPV16 L1 (IgG/A) |
Abbreviations: SEAP-NA, Secreted Alkaline Phosphatase Neutralization Assay; PBNA, Pseudovirion-based Neutralization Assay; VLP, virus-like particles; BPV, Bovine papillomavirus; RIPA, Radioactive immunoprecipitation assay.
Most studies used only centrifugation as a purification step before antibody detection. This is remarkable given the low levels of HPV-specific antibodies measured in CVS. However, three studies were identified that explored the effect of an extra purification step on HPV-specific antibody detection [28,41,42].
To distinguish a specific antibody response from background noise, several studies compared the results to those obtained in HPV-naïve persons without prior HPV infection. Sera from young children (virgin girls) could be used for such a study, yet CVS collection from young children is unethical. Therefore, different arbitrary cut-offs were established.
Inherent differences in the types of antibody responses measured, the lack of standardized reagents and the lack of uniform methods to establish cut-off values have made comparisons between assays difficult. Although an international initiative has been launched for standardizing and harmonizing serological assays used for HPV antibody testing [76], until now, no accessible, uniform, and standardized assays, reagents, and procedures to measure HPV-specific antibodies in serum and CVS have been available. However, these are essential to ensure data quality and allow the comparison of results from different studies and vaccines.
3.5. Possible factors affecting antibody detection in CVS
Mucosal samples are challenging for the measurement of antibodies because of the low levels of antibodies present in genital secretions as well as the presence of interfering components. In sharp contrast to serum, CVS exhibit several characteristic features that must be considered in the collection and measurement of HPV-specific antibodies. Various factors were found to significantly interfere with immunoglobulin measurement at the cervix.
3.5.1. Influence of the menstrual cycle and oral contraceptives
In contrast to IgA in other mucosal departments, IgG is the dominant mucosal antibody within the female genital tract. However, the female genital tract also differs as it is affected by the menstrual cycle, which creates variations in the amounts of mucosal antibodies and cervical secretions produced [77,78]. Nardelli-Haefliger et al. (2003) found that the cervical titres of HPV-specific IgG and total human IgG and IgA in an ovulating group varied during the menstrual cycle and decreased approximately nine-fold during ovulation. Kemp et al. (2008) found similar lower CVS IgG measurements mid-cycle based on a small number of observations [53]. The decrease in vaccine-specific antibody concentration during ovulation might represent a protective mechanism that reduces the levels of anti-sperm antibodies in the female genital tract at a time when conception is most likely to occur [79].
Although the evidence indicates there are hormone-dependent variations in antibody levels, most studies did not take the menstrual cycle into account when sampling. However, most studies did not sample during menses to avoid blood contamination. Hence, the proportion of positive CVS samples may be underestimated if secretions are collected at random points in the cycle.
Nardelli-Haefliger et al. (2003) also examined whether contraceptives affected the immune response in women vaccinated with the HPV16 VLP vaccine. After vaccination, the cervical titres of HPV-specific IgG and total human IgG and IgA in the contraceptive group were relatively constant throughout the menstrual cycle. It is therefore suggested that endogenous sex hormones are primarily responsible for the antibody concentration in the female genital tract [55]. Scherpenisse et al. (2013) did not find an difference between non-oral contraceptive (OC) users and OC users in vaccine-derived HPV16 and 18 antibody levels one year after the first HPV vaccination, even when the levels were normalized to the total amount of IgG [57]. Kemp et al. (2008) also observed that OC use was not an important modifier of the correlation observed between serum and CVS measurements, which appears contract the data obtained by Nardelli-Haefliger et al. [53] (Table 3). Consequently, the influence of oral contraceptives on HPV-specific antibodies in CVS is still debatable, and further studies are needed.
3.5.2. Influence of blood contamination
As high antibody levels are present in serum, even small traces of blood could contribute considerably to the antibody levels in CVS. CVS samples in most studies were collected regardless of the day of the menstrual cycle, but samples were generally not collected during menstruation to avoid blood contamination. The levels of blood were measured by a Haemastix™ test, although less stringent exclusion criteria were also used, such as visible blood staining in CVS samples [29,30].
Blood contamination in CVS could be due to many factors: infection, lesion (HPV-related or not), menstruation, or the sampling method itself. Most studies ensured that contamination with serum of systemic origin was minimized to ensure that true mucosal immune responses were measured. It should be noted, however, that it is important to discern between the sources of blood in secretion collections, since induced bleeding should be avoided, while bleeding that is not induced by the collection instrument is a natural phenomenon at the cervix and therefore an important route for the exposure of the cervix to immune-related factors [72,73].
3.5.3. Influence of age
It was expected that post-menopausal women would produce less CVS in their genital tracts because of decreased oestrogen production [55,61]. However, the results of Schwarz et al. (2010) confirm that the correlation between serum and CVS anti-HPV antibody levels is independent of age, with high correlations observed in all age groups after vaccination. A high correlation between CVS and serum antibodies in the 46- to 55-year-old age group was also found by Kemp et al. (2008) [53]. These results indicate that serum IgG antibodies likely transudate into the cervical epithelium regardless of age to confer site-specific immunity at the cervix. However, further studies are required to confirm these findings.
3.5.4. Normalization methods for measuring HPV-specific antibodies in CVS
In this review, several studies have presented the levels of HPV-specific antibodies in a normalized form to correct for differences in the techniques used to obtain the mucosal samples and subtle changes due to hormonal or environmental influences. The most frequently used method of normalization was to calculate the ratio of the HPV-specific IgG titre divided by the total human IgG concentration in CVS. In addition, normalization was also performed based on the amount of total protein [26,29,30,32,38,39] and according to differences in specimen weight/volume pre- and post-sampling to estimate the dilution factor [46,53,55,61]. However, in several studies, normalized and unnormalized values resulted in similar response rates, so the uncorrected values were used in the final analysis, and the normalization procedure was not performed in further studies based on prior results [20,26,34,53,57].
In the study by Elfgren and colleagues (1996), in addition to the levels of total immunoglobulin, the cervical mucus samples were also analysed for the presence of IgA against Epstein-Barr virus, which is a ubiquitous virus that can also infect the cervix [25].
4. Conclusion
HPV infects and propagates in cervical mucosal epithelium. Hence, it is important to have appropriate tools to monitor and measure mucosal antibodies in addition to systemic antibodies against HPV infection and vaccination. The aim of this review was to provide an overview of published studies reporting the detection of HPV-specific antibodies in CVS whether or not this was linked to the detection of HPV-specific antibodies in serum. Although considerably lower in quantity than systemic antibodies, studies have shown that HPV-specific antibody detection in CVS is feasible. However, the high variability of published observations and the lack of a strictly uniform, well-validated method for the collection, isolation and quantification of HPV-specific antibodies at the cervix indicates the need for specific methods that can improve and standardize detection. Furthermore, heterogeneity in absorbed and extracted sample volumes requires normalization to allow valid inter-individual comparisons.
The levels of naturally induced HPV-specific antibodies in CVS were very low and near the detection limits, indicating that antibody measurements in CVS in a non-vaccinated population might be challenging. The extreme variation observed may reflect differences in collection techniques or the study populations. This creates difficulties for interpreting published results to elucidate the local immune responses to HPV. Further examinations of naturally developed antibodies with more sensitive assays may provide additional insight into the strengths and limitations of the natural immune response to HPV.
Intramuscular immunization with HPV vaccines was shown to be highly effective at inducing both serum and mucosal antibodies. Furthermore, the moderate to strong correlation of HPV-specific antibody levels in serum and CVS suggests that vaccine-induced HPV antibodies exude/transudate from the systemic circulation to the cervical mucosa to provide protection against HPV infection. However, more studies are needed to reveal how the correlations among mucosal antibodies, serological antibodies, and HPV infection and disease are involved with physiological disease progression and immune prevention mechanisms. Furthermore, it is not known whether or how local immunity at the cervix affects the long-term duration of HPV vaccine efficacy. Additional longer-term efficacy studies should help to clarify whether serum antibody levels and/or cervical antibody levels are the primary correlate of vaccine efficacy.
Funding
J. Pattyn is supported by a Ph. D. fellowship from the Royal Belgian Academy of Medicine and S. Van Keer is supported by the Research Foundation, Flanders (FWO), Belgium [1120816N].
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pvr.2019.100185.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Walboomers J.M., Jacobs M.V., Manos M.M., Bosch F.X., Kummer J.A., Shah K.V. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 1999;189(1):12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 2.Bray F.F.J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. CA Cancer J Clin; 2018. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin in. [DOI] [PubMed] [Google Scholar]
- 3.Frazer I.H. Interaction of human papillomaviruses with the host immune system: a well evolved relationship. Virology. 2009;384(2):410–414. doi: 10.1016/j.virol.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Stanley M. HPV - immune response to infection and vaccination. Infect. Agents Cancer. 2010;5:19. doi: 10.1186/1750-9378-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez A.C., Schiffman M., Herrero R., Wacholder S., Hildesheim A., Castle P.E. Rapid clearance of human papillomavirus and implications for clinical focus on persistent infections. J. Natl. Cancer Inst. 2008;100(7):513–517. doi: 10.1093/jnci/djn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stanley M. Immune responses to human papillomavirus. Vaccine. 2006;24(Suppl 1):S16–22. doi: 10.1016/j.vaccine.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Carter J.J., Koutsky L.A., Hughes J.P., Lee S.K., Kuypers J., Kiviat N. Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. J. Infect. Dis. 2000;181(6):1911–1919. doi: 10.1086/315498. [DOI] [PubMed] [Google Scholar]
- 8.Frazer I.H. Prevention of cervical cancer through papillomavirus vaccination. Nat. Rev. Immunol. 2004;4(1):46–54. doi: 10.1038/nri1260. [DOI] [PubMed] [Google Scholar]
- 9.Schiller J.T., Hidesheim A. Developing HPV virus-like particle vaccines to prevent cervical cancer: a progress report. J. Clin. Virol.: Off. Publ. Pan Am. Soc. Clin. Virol. 2000;19(1–2):67–74. doi: 10.1016/s1386-6532(00)00091-3. [DOI] [PubMed] [Google Scholar]
- 10.Zhou J., Sun X.Y., Stenzel D.J., Frazer I.H. Expression of vaccinia recombinant HPV 16 L1 and L2 ORF proteins in epithelial cells is sufficient for assembly of HPV virion-like particles. Virology. 1991;185(1):251–257. doi: 10.1016/0042-6822(91)90772-4. [DOI] [PubMed] [Google Scholar]
- 11.Paavonen J., Jenkins D., Bosch F.X., Naud P., Salmeron J., Wheeler C.M. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet (London, England) 2007;369(9580):2161–2170. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- 12.Bordon Y. Antibody responses: FcRn-not just for the kids. Nat. Rev. Immunol. 2011;11(4):234–235. doi: 10.1038/nri2967. [DOI] [PubMed] [Google Scholar]
- 13.Li Z., Palaniyandi S., Zeng R., Tuo W., Roopenian D.C., Zhu X. Transfer of IgG in the female genital tract by MHC class I-related neonatal Fc receptor (FcRn) confers protective immunity to vaginal infection. Proc. Natl. Acad. Sci. U. S. A. 2011;108(11):4388–4393. doi: 10.1073/pnas.1012861108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stanley M., Lowy D.R., Frazer I. Chapter 12: prophylactic HPV vaccines: underlying mechanisms. Vaccine. 2006;24(Suppl 3):106–113. doi: 10.1016/j.vaccine.2006.05.110. S3/ [DOI] [PubMed] [Google Scholar]
- 15.Stanley M., Pinto L.A., Trimble C. Human papillomavirus vaccines--immune responses. Vaccine. 2012;30(Suppl 5):F83–87. doi: 10.1016/j.vaccine.2012.04.106. [DOI] [PubMed] [Google Scholar]
- 16.Schiffman M., Doorbar J., Wentzensen N., de Sanjose S., Fakhry C., Monk B.J. Carcinogenic human papillomavirus infection. Nat. Rev. Dis. Prim. 2016;2:16086. doi: 10.1038/nrdp.2016.86. [DOI] [PubMed] [Google Scholar]
- 17.Trottier H., Franco E.L. The epidemiology of genital human papillomavirus infection. Vaccine. 2006;24(Suppl 1):S1–15. doi: 10.1016/j.vaccine.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 18.Schwarz T.F., Leo O. Immune response to human papillomavirus after prophylactic vaccination with AS04-adjuvanted HPV-16/18 vaccine: improving upon nature. Gynecol. Oncol. 2008;110(3 Suppl 1):S1–10. doi: 10.1016/j.ygyno.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 19.Bierl C., Karem K., Poon A.C., Swan D., Tortolero-Luna G., Follen M. Correlates of cervical mucosal antibodies to human papillomavirus 16: results from a case control study. Gynecol. Oncol. 2005;99(3 Suppl 1):S262–268. doi: 10.1016/j.ygyno.2005.07.100. [DOI] [PubMed] [Google Scholar]
- 20.Bontkes H.J., de Gruijl T.D., Walboomers J.M., Schiller J.T., Dillner J., Helmerhorst T.J. Immune responses against human papillomavirus (HPV) type 16 virus-like particles in a cohort study of women with cervical intraepithelial neoplasia. II. Systemic but not local IgA responses correlate with clearance of HPV-16. J. Gen. Virol. 1999;80(Pt 2):409–417. doi: 10.1099/0022-1317-80-2-409. [DOI] [PubMed] [Google Scholar]
- 21.Dillner L., Bekassy Z., Jonsson N., Moreno-Lopez J., Blomberg J. Detection of IgA antibodies against human papillomavirus in cervical secretions from patients with cervical intraepithelial neoplasia. Int. J. Cancer. 1989;43(1):36–40. doi: 10.1002/ijc.2910430109. [DOI] [PubMed] [Google Scholar]
- 22.Dillner L., Fredriksson A., Persson E., Forslund O., Hansson B.G., Dillner J. Antibodies against papillomavirus antigens in cervical secretions from condyloma patients. J. Clin. Microbiol. 1993;31(2):192–197. doi: 10.1128/jcm.31.2.192-197.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dreyfus M., Baldauf J.J., Ritter J., Obert G. Seric and local antibodies against a synthetic peptide of HPV16. Eur. J. Obstet. Gynecol. Reprod. Biol. 1995;59(2):187–191. doi: 10.1016/0028-2243(95)02049-x. [DOI] [PubMed] [Google Scholar]
- 24.Ekalaksananan T., Malat P., Pientong C., Kongyingyoes B., Chumworathayi B., Kleebkaow P. Local cervical immunity in women with low-grade squamous intraepithelial lesions and immune responses after abrasion. Asian Pac. J. Cancer Prev. APJCP. 2014;15(10):4197–4201. doi: 10.7314/apjcp.2014.15.10.4197. [DOI] [PubMed] [Google Scholar]
- 25.Elfgren K., Bistoletti P., Dillner L., Walboomers J.M., Meijer C.J., Dillner J. Conization for cervical intraepithelial neoplasia is followed by disappearance of human papillomavirus deoxyribonucleic acid and a decline in serum and cervical mucus antibodies against human papillomavirus antigens. Am. J. Obstet. Gynecol. 1996;174(3):937–942. doi: 10.1016/s0002-9378(96)70330-7. [DOI] [PubMed] [Google Scholar]
- 26.Hagensee M.E., Koutsky L.A., Lee S.K., Grubert T., Kuypers J., Kiviat N.B. Detection of cervical antibodies to human papillomavirus type 16 (HPV-P6) capsid antigens in relation to detection of HPV-16 DNA and cervical lesions. JID (J. Infect. Dis.) 2000;181(4):1234–1239. doi: 10.1086/315364. [DOI] [PubMed] [Google Scholar]
- 27.Kemp T.J., Hildesheim A., Falk R.T., Schiller J.T., Lowy D.R., Rodriguez A.C. Evaluation of two types of sponges used to collect cervical secretions and assessment of antibody extraction protocols for recovery of neutralizing anti-human papillomavirus type 16 antibodies. Clin. Vaccine Immunol. : CVI. 2008;15(1):60–64. doi: 10.1128/CVI.00118-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez T.V., Cancio C., Cruz-Talonia F., Ruiz B., Sapp M., Rocha-Zavaleta L. Binding of human papillomavirus type 16 to heparan sulfate is inhibited by mucosal antibodies from patients with low-grade squamous intraepithelial lesions but not from cervical cancer patients. FEMS Immunol. Med. Microbiol. 2008;54(2):167–176. doi: 10.1111/j.1574-695X.2008.00484.x. [DOI] [PubMed] [Google Scholar]
- 29.Marais D.J., Carrara H., Ramjee G., Kay P., Williamson A.L. HIV-1 seroconversion promotes rapid changes in cervical human papillomavirus (HPV) prevalence and HPV-16 antibodies in female sex workers. J. Med. Virol. 2009;81(2):203–210. doi: 10.1002/jmv.21343. [DOI] [PubMed] [Google Scholar]
- 30.Marais D.J., Vardas E., Ramjee G., Allan B., Kay P., Rose R.C. The impact of human immunodeficiency virus type 1 status on human papillomavirus (HPV) prevalence and HPV antibodies in serum and cervical secretions. J. Infect. Dis. 2000;182(4):1239–1242. doi: 10.1086/315815. [DOI] [PubMed] [Google Scholar]
- 31.Mbulawa Z.Z.A., Williamson A.L., Stewart D., Passmore J.A.S., Denny L., Allan B. Association of serum and mucosal neutralizing antibodies to human papillomavirus type 16 (HPV-16) with HPV-16 infection and cervical disease. J. Gen. Virol. 2008;89:910–914. doi: 10.1099/vir.0.83458-0. [DOI] [PubMed] [Google Scholar]
- 32.Monroy O.L., Aguilar C., Lizano M., Cruz-Talonia F., Cruz R.M., Rocha-Zavaleta L. Prevalence of human papillomavirus genotypes, and mucosal IgA anti-viral responses in women with cervical ectopy. J. Clin. Virol.: Off. Publ. Pan Am. Soc. Clin. Virol. 2010;47(1):43–48. doi: 10.1016/j.jcv.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen H.H., Broker T.R., Chow L.T., Alvarez R.D., Vu H.L., Andrasi J. Immune responses to human papillomavirus in genital tract of women with cervical cancer. Gynecol. Oncol. 2005;96(2):452–461. doi: 10.1016/j.ygyno.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 34.Onda T., Carter J.J., Koutsky L.A., Hughes J.P., Lee S.K., Kuypers J. Characterization of IgA response among women with incident HPV 16 infection. Virology. 2003;312(1):213–221. doi: 10.1016/s0042-6822(03)00196-x. [DOI] [PubMed] [Google Scholar]
- 35.Passmore J.A.S., Marais D.J., Sampson C., Allan B., Parker N., Milner M. Cervicovaginal, oral, and serum IgG and IgA responses to human papillomavirus type 16 in women with cervical intraepithelial neoplasia. J. Med. Virol. 2007;79(9):1375–1380. doi: 10.1002/jmv.20901. [DOI] [PubMed] [Google Scholar]
- 36.Rocha-Zavaleta L., Ambrosio J.P., Mora-Garcia Mde L., Cruz-Talonia F., Hernandez-Montes J., Weiss-Steider B. Detection of antibodies against a human papillomavirus (HPV) type 16 peptide that differentiate high-risk from low-risk HPV-associated low-grade squamous intraepithelial lesions. J. Gen. Virol. 2004;85(Pt 9):2643–2650. doi: 10.1099/vir.0.80077-0. [DOI] [PubMed] [Google Scholar]
- 37.Rocha-Zavaleta L., Pereira-Suarez A.L., Yescas G., Cruz-Mimiaga R.M., Garcia-Carranca A., Cruz-Talonia F. Mucosal IgG and IgA responses to human papillomavirus type 16 capsid proteins in HPV16-infected women without visible pathology. Viral Immunol. 2003;16(2):159–168. doi: 10.1089/088282403322017893. [DOI] [PubMed] [Google Scholar]
- 38.Sasagawa T., Rose R.C., Azar K.K., Sakai A., Inoue M. Mucosal immunoglobulin-A and -G responses to oncogenic human papilloma virus capsids. Int. J. Cancer. 2003;104(3):328–335. doi: 10.1002/ijc.10939. [DOI] [PubMed] [Google Scholar]
- 39.Snowhite I.V., Jones W.E., Dumestre J., Dunlap K., Braly P.S., Hagensee M.E. Comparative analysis of methods for collection and measurement of cytokines and immunoglobulins in cervical and vaginal secretions of HIV and HPV infected women. J. Immunol. Methods. 2002;263(1–2):85–95. doi: 10.1016/s0022-1759(02)00038-8. [DOI] [PubMed] [Google Scholar]
- 40.Snyder K.A., Barber S.R., Symbula M., Taylor P.T., Crum C.P., Roche J.K. Binding by immunoglobulin to the HPV-16-derived proteins L1 and E4 in cervical secretions of women with HPV-related cervical disease. Cancer Res. 1991;51(16):4423–4429. [PubMed] [Google Scholar]
- 41.Tjiong M.Y., Ter Schegget J., Tjong-A-Hung S.P., Out T.A., van der Vange N., Burger M.P.M. IgG antibodies against human papillomavirus type 16 E7 proteins in cervicovaginal washing fluid from patients with cervical neoplasia. Int. J. Gynecol. Cancer. 2000;10(4):296–304. doi: 10.1046/j.1525-1438.2000.010004296.x. [DOI] [PubMed] [Google Scholar]
- 42.Tjiong M.Y., Zumbach K., Schegget J.T., van der Vange N., Out T.A., Pawlita M. Antibodies against human papillomavirus type 16 and 18 E6 and E7 proteins in cervicovaginal washings and serum of patients with cervical neoplasia. Viral Immunol. 2001;14(4):415–424. doi: 10.1089/08828240152716655. [DOI] [PubMed] [Google Scholar]
- 43.Veress G., Konya J., Csiky-Meszaros T., Czegledy J., Gergely L. Human papillomavirus DNA and anti-HPV secretory IgA antibodies in cytologically normal cervical specimens. J. Med. Virol. 1994;43(2):201–207. doi: 10.1002/jmv.1890430219. [DOI] [PubMed] [Google Scholar]
- 44.Wang Z., Hansson B.G., Forslund O., Dillner L., Sapp M., Schiller J.T. Cervical mucus antibodies against human papillomavirus type 16, 18, and 33 capsids in relation to presence of viral DNA. J. Clin. Microbiol. 1996;34(12):3056–3062. doi: 10.1128/jcm.34.12.3056-3062.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barr E., Koutsky L.A. Re: specific antibody levels at the cervix during the menstrual cycle of women vaccinated with human papillomavirus 16 virus-like particles. J. Natl. Cancer Inst. 2004;96(5):412–413. doi: 10.1093/jnci/djh071. author reply 413-414. [DOI] [PubMed] [Google Scholar]
- 46.Draper E., Bissett S.L., Howell-Jones R., Waight P., Soldan K., Jit M. A randomized, observer-blinded immunogenicity trial of Cervarix((R)) and Gardasil((R)) Human Papillomavirus vaccines in 12-15 year old girls. PLoS One. 2013;8(5) doi: 10.1371/journal.pone.0061825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Einstein M.H., Baron M., Levin M.J., Chatterjee A., Edwards R.P., Zepp F. Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18-45 years. Hum. Vaccine. 2009;5(10):705–719. doi: 10.4161/hv.5.10.9518. [DOI] [PubMed] [Google Scholar]
- 48.Einstein M.H., Baron M., Levin M.J., Chatterjee A., Fox B., Scholar S. Comparative immunogenicity and safety of human papillomavirus (HPV)-16/18 vaccine and HPV-6/11/16/18 vaccine: follow-up from months 12-24 in a Phase III randomized study of healthy women aged 18-45 years. Hum. Vaccine. 2011;7(12):1343–1358. doi: 10.4161/hv.7.12.18281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Einstein M.H., Levin M.J., Chatterjee A., Chakhtoura N., Takacs P., Catteau G. Comparative humoral and cellular immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and HPV-6/11/16/18 vaccine in healthy women aged 18-45 years: follow-up through Month 48 in a Phase III randomized study. Hum. Vaccines Immunother. 2014;10(12):3455–3465. doi: 10.4161/hv.36117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fife K.H., Wheeler C.M., Koutsky L.A., Barr E., Brown D.R., Schiff M.A. Dose-ranging studies of the safety and immunogenicity of human papillomavirus Type 11 and Type 16 virus-like particle candidate vaccines in young healthy women. Vaccine. 2004;22(21–22):2943–2952. doi: 10.1016/j.vaccine.2003.11.058. [DOI] [PubMed] [Google Scholar]
- 51.Goncalves A.K., Giraldo P.C., Farias K.J., Machado P.R., Costa A.P., de Souza L.C. Characterization of immunoglobulin A/G responses during 3 doses of the human papillomavirus-16/18 ASO4-adjuvanted vaccine. Sex. Transm. Dis. 2016;43(5):335–339. doi: 10.1097/OLQ.0000000000000429. [DOI] [PubMed] [Google Scholar]
- 52.Huo Z.M., Bissett S.L., Giemza R., Beddows S., Oeser C., Lewis D.J.M. Systemic and mucosal immune responses to sublingual or intramuscular human papilloma virus antigens in healthy female volunteers. PLoS One. 2012;7(3) doi: 10.1371/journal.pone.0033736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kemp T.J., Garcia-Pineres A., Falk R.T., Poncelet S., Dessy F., Giannini S.L. Evaluation of systemic and mucosal anti-HPV16 and anti-HPV18 antibody responses from vaccinated women. Vaccine. 2008;26(29–30):3608–3616. doi: 10.1016/j.vaccine.2008.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nardelli-Haefliger D., Lurati F., Wirthner D., Spertini F., Schiller J.T., Lowy D.R. Immune responses induced by lower airway mucosal immunisation with a human papillomavirus type 16 virus-like particle vaccine. Vaccine. 2005;23(28):3634–3641. doi: 10.1016/j.vaccine.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 55.Nardelli-Haefliger D., Wirthner D., Schiller J.T., Lowy D.R., Hildesheim A., Ponci F. Specific antibody levels at the cervix during the menstrual cycle of women vaccinated with human papillomavirus 16 virus-like particles. J. Natl. Cancer Inst. 2003;95(15):1128–1137. doi: 10.1093/jnci/djg018. [DOI] [PubMed] [Google Scholar]
- 56.Petaja T., Pedersen C., Poder A., Strauss G., Catteau G., Thomas F. Long-term persistence of systemic and mucosal immune response to HPV-16/18 AS04-adjuvanted vaccine in preteen/adolescent girls and young women. Int. J. Cancer. 2011;129(9):2147–2157. doi: 10.1002/ijc.25887. [DOI] [PubMed] [Google Scholar]
- 57.Scherpenisse M., Mollers M., Schepp R.M., Meijer C.J., de Melker H.E., Berbers G.A. Detection of systemic and mucosal HPV-specific IgG and IgA antibodies in adolescent girls one and two years after HPV vaccination. Hum. Vaccines Immunother. 2013;9(2):314–321. doi: 10.4161/hv.22693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schwarz T., Spaczynski M., Kaufmann A., Wysocki J., Galaj A., Schulze K. Persistence of immune responses to the HPV-16/18 AS04-adjuvanted vaccine in women aged 15-55 years and first-time modelling of antibody responses in mature women: results from an open-label 6-year follow-up study. BJOG An Int. J. Obstet. Gynaecol. 2015;122(1):107–118. doi: 10.1111/1471-0528.13070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schwarz T.F., Galaj A., Spaczynski M., Wysocki J., Kaufmann A.M., Poncelet S. Ten-year immune persistence and safety of the HPV-16/18 AS04-adjuvanted vaccine in females vaccinated at 15-55 years of age. Cancer medicine. 2017;6(11):2723–2731. doi: 10.1002/cam4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schwarz T.F., Kocken M., Petaja T., Einstein M.H., Spaczynski M., Louwers J.A. Correlation between levels of human papillomavirus (HPV)-16 and 18 antibodies in serum and cervicovaginal secretions in girls and women vaccinated with the HPV-16/18 AS04-adjuvanted vaccine. Hum. Vaccine. 2010;6(12):1054–1061. doi: 10.4161/hv.6.12.13399. [DOI] [PubMed] [Google Scholar]
- 61.Schwarz T.F., Spaczynski M., Schneider A., Wysocki J., Galaj A., Perona P. Immunogenicity and tolerability of an HPV-16/18 AS04-adjuvanted prophylactic cervical cancer vaccine in women aged 15-55 years. Vaccine. 2009;27(4):581–587. doi: 10.1016/j.vaccine.2008.10.088. [DOI] [PubMed] [Google Scholar]
- 62.Ferreira Costa A.P., Goncalves A.K., Machado P.R.L., Souza L., Sarmento A., Cobucci R.N.O. Immune response to human papillomavirus one year after prophylactic vaccination with AS04-adjuvanted HPV-16/18 vaccine: HPV-specific IgG and IgA antibodies in the circulation and the cervix. Asian Pac. J. Cancer Prev. APJCP. 2018;19(8):2313–2317. doi: 10.22034/APJCP.2018.19.8.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stanley M.A. Epithelial cell responses to infection with human papillomavirus. Clin. Microbiol. Rev. 2012;25(2):215–222. doi: 10.1128/CMR.05028-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Balmelli C., Roden R., Potts A., Schiller J., De Grandi P., Nardelli-Haefliger D. Nasal immunization of mice with human papillomavirus type 16 virus-like particles elicits neutralizing antibodies in mucosal secretions. J. Virol. 1998;72(10):8220–8229. doi: 10.1128/jvi.72.10.8220-8229.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cho H.J., Kim J.Y., Lee Y., Kim J.M., Kim Y.B., Chun T. Enhanced humoral and cellular immune responses after sublingual immunization against human papillomavirus 16 L1 protein with adjuvants. Vaccine. 2010;28(14):2598–2606. doi: 10.1016/j.vaccine.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 66.Scherpenisse M., Schepp R.M., Mollers M., Meijer C.J., Berbers G.A., van der Klis F.R. Characteristics of HPV-specific antibody responses induced by infection and vaccination: cross-reactivity, neutralizing activity, avidity and IgG subclasses. PLoS One. 2013;8(9) doi: 10.1371/journal.pone.0074797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ghelardi A., Parazzini F., Martella F., Pieralli A., Bay P., Tonetti A. SPERANZA project: HPV vaccination after treatment for CIN2. Gynecol. Oncol. 2018;151(2):229–234. doi: 10.1016/j.ygyno.2018.08.033. [DOI] [PubMed] [Google Scholar]
- 68.Bouvet J.P., Belec L., Pires R., Pillot J. Immunoglobulin G antibodies in human vaginal secretions after parenteral vaccination. Infect. Immun. 1994;62(9):3957–3961. doi: 10.1128/iai.62.9.3957-3961.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Longet S., Schiller J.T., Bobst M., Jichlinski P., Nardelli-Haefliger D. A murine genital-challenge model is a sensitive measure of protective antibodies against human papillomavirus infection. J. Virol. 2011;85(24):13253–13259. doi: 10.1128/JVI.06093-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Quesnel A., Cu-Uvin S., Murphy D., Ashley R.L., Flanigan T., Neutra M.R. Comparative analysis of methods for collection and measurement of immunoglobulins in cervical and vaginal secretions of women. J. Immunol. Methods. 1997;202(2):153–161. doi: 10.1016/s0022-1759(97)00003-3. [DOI] [PubMed] [Google Scholar]
- 71.Castle P.E., Rodriguez A.C., Bowman F.P., Herrero R., Schiffman M., Bratti M.C. Comparison of ophthalmic sponges for measurements of immune markers from cervical secretions. Clin. Diagn. Lab. Immunol. 2004;11(2):399–405. doi: 10.1128/CDLI.11.2.399-405.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hildesheim A., Bratti M.C., Edwards R.P., Schiffman M., Rodriguez A.C., Herrero R. Collection of cervical secretions does not adversely affect Pap smears taken immediately afterward. Clin. Diagn. Lab. Immunol. 1998;5(4):491–493. doi: 10.1128/cdli.5.4.491-493.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hildesheim A., McShane L.M., Schiffman M., Bratti M.C., Rodriguez A.C., Herrero R. Cytokine and immunoglobulin concentrations in cervical secretions: reproducibility of the Weck-cel collection instrument and correlates of immune measures. J. Immunol. Methods. 1999;225(1–2):131–143. doi: 10.1016/s0022-1759(99)00037-x. [DOI] [PubMed] [Google Scholar]
- 74.Mkhize N.N., Durgiah R., Ashley V., Archary D., Garrett N.J., Karim Q.A. Broadly neutralizing antibody specificities detected in the genital tract of HIV-1 infected women. AIDS (London, England) 2016;30(7):1005–1014. doi: 10.1097/QAD.0000000000001038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Van Keer S., Willhauck-Fleckenstein M., Pattyn J., Butt J., Tjalma W.A.A., Van Ostade X. First-void urine as a non-invasive liquid biopsy source to detect vaccine-induced human papillomavirus antibodies originating from cervicovaginal secretions. J. Clin. Virol.: Off. publ. Pan Am. Soc. Clin. Virol. 2019;117:11–18. doi: 10.1016/j.jcv.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 76.Pinto L.A., Dillner J., Beddows S., Unger E.R. Immunogenicity of HPV prophylactic vaccines: serology assays and their use in HPV vaccine evaluation and development. Vaccine. 2018;36(32 Pt A):4792–4799. doi: 10.1016/j.vaccine.2017.11.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mestecky J., Fultz P.N. Mucosal immune system of the human genital tract. J. Infect. Dis. 1999;179(Suppl 3):S470–474. doi: 10.1086/314806. [DOI] [PubMed] [Google Scholar]
- 78.Mestecky J., Moldoveanu Z., Russell M.W. Immunologic uniqueness of the genital tract: challenge for vaccine development. Am. J. Reprod. Immunol. 2005;53(5):208–214. doi: 10.1111/j.1600-0897.2005.00267.x. (New York, NY : 1989) [DOI] [PubMed] [Google Scholar]
- 79.Brazdova A., Senechal H., Peltre G., Poncet P. Immune aspects of female infertility. Int J Fertil Steril. 2016;10(1):1–10. doi: 10.22074/ijfs.2016.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Abraham S., Juel H.B., Bang P., Cheeseman H.M., Dohn R.B., Cole T., Kristiansen M.P., Korsholm K.S., Lewis D., Olsen A.W. Safety and immunogenicity of the chlamydia vaccine candidate CTH522 adjuvanted with CAF01 liposomes or aluminium hydroxide: a first-in-human, randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Infect Dis. 2019 doi: 10.1016/S1473-3099(19)30279-8. PMID: 31416692. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.