Abstract
Introduction
Visceral aneurysms are rare and a life threatening condition in the case of rupture.
Report
A 78 year old woman presented with sudden brief loss of consciousness and complained of abdominal tenderness on examination. Computed tomography revealed a gigantic 100 × 130 × 200 mm ruptured true aneurysm of the gastroduodenal artery, which was successfully treated by endovascular coiling. Post-operative observation was uneventful and the six week follow up duplex ultrasound confirmed absence of luminal flow in the aneurysm.
Discussion
The treatment threshold of visceral aneurysms and treatment modalities are reviewed.
Keywords: Embolisation, Gastroduodenal, Rupture, Treatment, Visceral aneurysm
Highlights
-
•
Giant ruptured visceral aneurysm successfully treated endovascularly.
-
•
Visceral aneurysms are rare and mostly asymptomatic.
-
•
Rupture is associated with high mortality.
-
•
Endovascular approach for treatment is fast and safe.
-
•
Wide consensus on treatment threshold is lacking.
Introduction
Visceral aneurysms are aneurysms of the coeliac trunk, superior mesenteric and inferior mesenteric arteries and their branches; renal artery aneurysms are sometimes but not always, included in the group. They are rare, with an estimated incidence of 0.01–1%,1, 2 but as symptoms are uncommon, most visceral aneurysms are detected incidentally on computed tomography (CT) or ultrasound scans performed in other contexts and the actual prevalence of visceral aneurysms is therefore presumably much higher than realised. The pathogenesis of true aneurysms is usually associated with atherosclerosis, fibrodysplasia (e.g. Ehlers–Danlos or Marfan syndrome) or due to changed haemodynamics (portal hypertension, distal to a stenosis with increased collateral flow). The underlying cause of false or pseudo-aneurysms can include infection, inflammation, trauma or iatrogenic lesions.3 The vast majority of the acknowledged true visceral aneurysms are localised to the splenic artery (40 %–60%) and the hepatic artery (10%–30 %).2, 3, 4 Symptoms, when present, are primarily caused by pressure on neighbouring organs, e.g. abdominal pain, vomiting or cholestasis.5, 6 Rupture can present as acute abdominal pain, reduced haemoglobin and haemodynamic shock, and is associated with a reported mortality as high as 76%.1
Treatment is either open surgery or endovascular intervention. Open surgery involves excision or occlusion by ligation; revascularisation can be secured either by bypass graft or transposition of the artery. Endovascular occluding options include direct embolisation of the aneurysm, and indirect aneurysm occlusion by embolisation of all afferent and efferent arteries. To maintain immediate patency of the artery, it is possible to exclude the aneurysm with a covered stent or by embolisation through a bare stent or around an inflated balloon, although these strategies have higher risk of aneurysm recanalisation.4
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Case report
A 78 year old female patient with a history of non-ischaemic heart disease, non-insulin dependent diabetes mellitus, and no history of smoking was admitted to the emergency room following sudden loss of consciousness. Alzheimer's dementia and multiple episodes of mental absences were described by family members but only one episode of loss of consciousness about four years prior to admission had occurred. There was no history of abdominal trauma or surgery. Hepatomegaly had been noted in the patient's medical records, but had not been investigated further when blood work for liver disease was negative. Six years previously, in another context, a CT scan without contrast enhancement had been performed in which a large aneurysm was seen and interpreted as being a benign chronic intrahepatic haematoma (77 × 107 × 140 mm). No further follow up was performed.
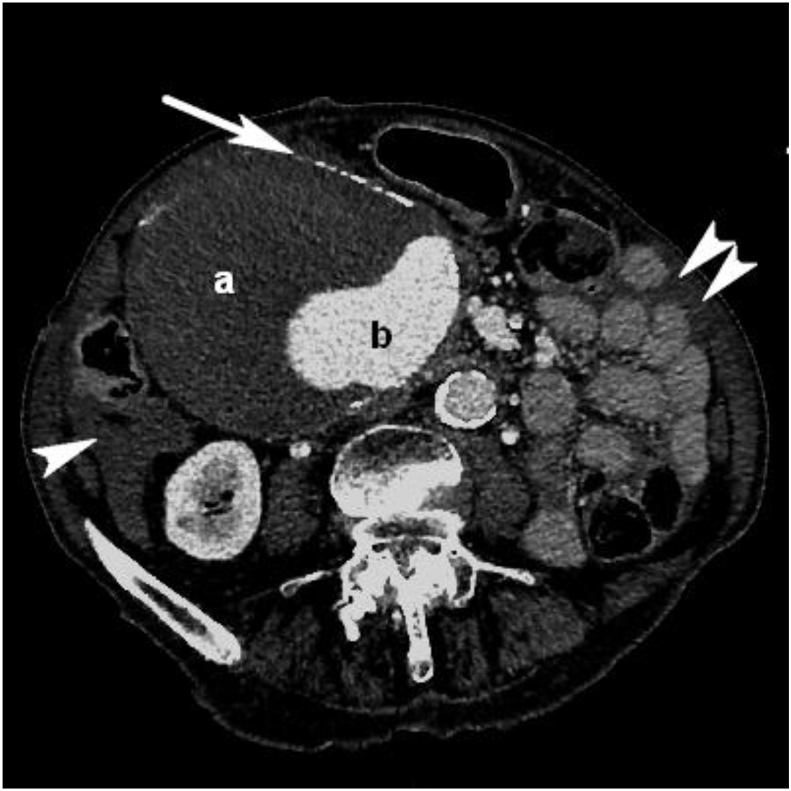
In the emergency room, the patient was conscious and hypotensive with a blood pressure of 89/59 mmHg, did not complain of pain, but on examination had left flank abdominal tenderness. During preliminary observation the patient had a declining haemoglobin, from 6.7 g/dL on admission to 5.2 g/dL four hours later. A focused assessment with sonography for trauma (FAST) scan was negative, but contrast enhanced CT revealed free fluid, attenuating at Hounsfield units indicating blood, in the abdominal cavity and a ruptured, extremely large (100 × 130 × 200 mm) aneurysm arising from a branch from the coeliac trunk and primarily localised in the right side of the abdomen (Fig. 1). The aorta was atherosclerotic, as was the superior mesenteric artery and the coeliac trunk. A smaller aneurysm (15 mm) (Fig. 2) of the left gastric artery was also present, but otherwise all other vessels were non-aneurysmal. Calcification in the aneurysm wall and mural thrombus (Fig. 3) in the large aneurysm suggested a slow growing true aneurysm. The aneurysm began just distal to the splenic artery and the arteries to the right liver lobe, suggesting the aneurysm feeding artery was the common hepatic or gastroduodenal artery; later angiography confirmed the latter.
Figure 1.
Computed tomography angiography axial image through the aneurysm with calcified walls (white arrow), large mural thrombus with slightly non-homogeneous attenuation (a) and a contrast filled lumen (b). Note free pericolic and perijejunal fluid (single and double white arrowhead respectively).
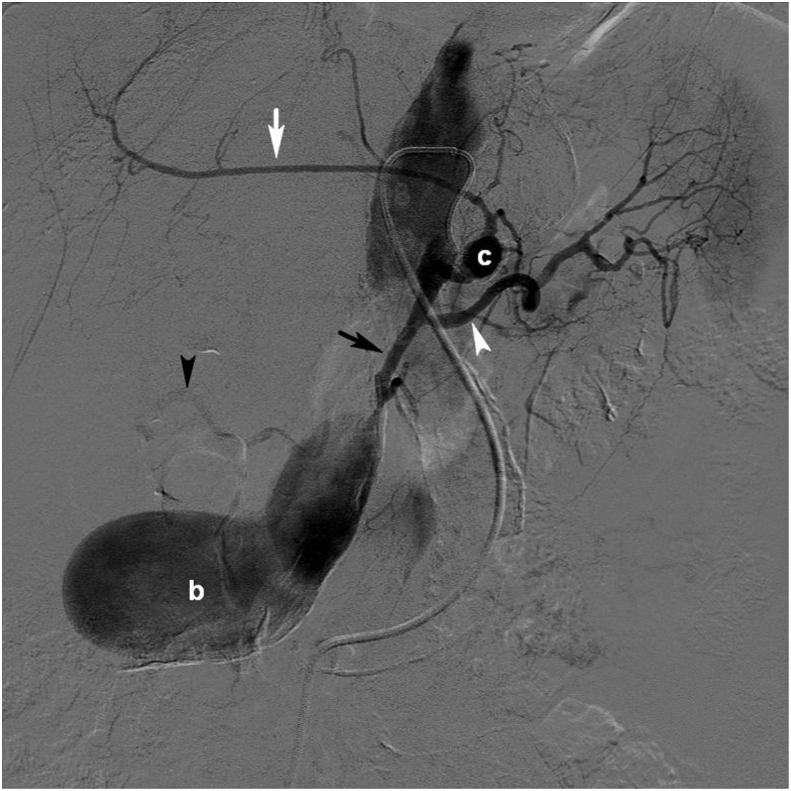
Figure 2.
Selective angiography in the coeliac trunk showing the left gastric artery with a minor spherical aneurysm (c) also feeding left hepatic artery (white arrow). The splenic artery is normal (white arrowhead). The inflow to the aneurysm (b) comes from the hepatic artery (black arrow). Branches to the right hepatic artery just before the inlet to the aneurysm, seem to be collaterals (black arrowhead).
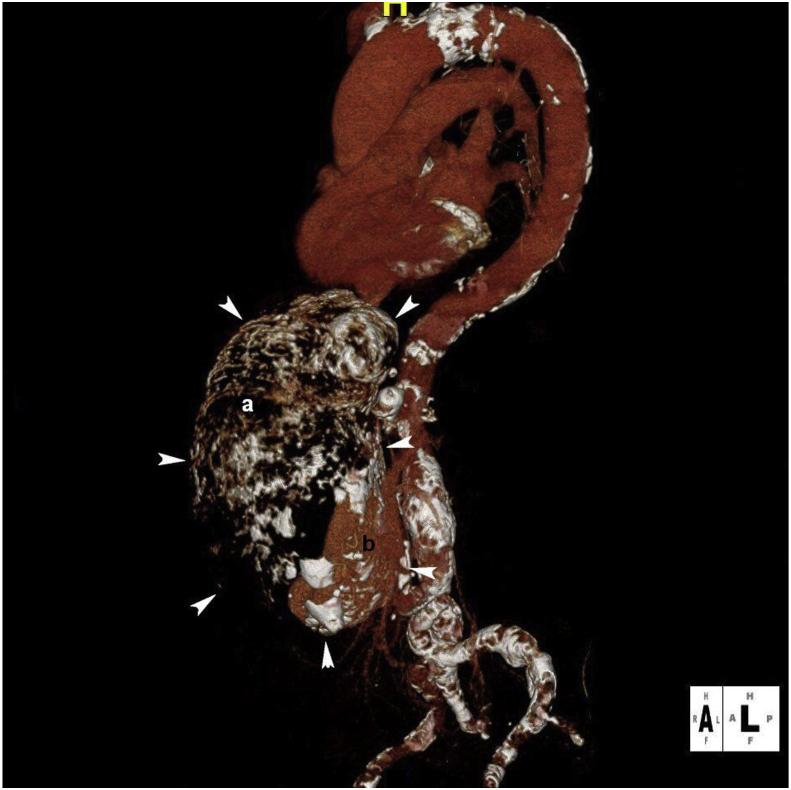
Figure 3.
Three dimensional angiogram shows the giant aneurysm located in front of aorta. Calcification in the aneurysm wall outlines the contour of the sack (white arrowheads). Note mural thrombus in the upper ventral part (a) and a minor contrast filled lumen in the lower dorsal part of the aneurysm (b).
The patient was immediately transferred to a hybrid operating room equipped for endovascular intervention. Initial angiography revealed no apparent contrast extravasation. Initially embolisation of three distal flow contributing arteries (Fig. 4) was performed with micro coils (Nester no. 3, size range 18-7-2 to 18-14-10 and Tornado no. 8 size range 18-5/2 to 18-10/4, Cook Medical, Bloomington, IN, USA) followed by macro coils (Nester no. 3, size range 35-14-4 to 35-14-6, Cook Medical) in the proximal supplying artery. Angiography immediately after embolisation showed no flow in the aneurysm (Fig. 5). About 15 minutes into the procedure the patient developed nausea, tachycardia and systolic hypertension of 185 mmHg. Blood pressure and pulse normalised on cessation of the sedative remifentanil infusion and administration of clonidine and ondansetron. The procedure duration was 80 minutes, and other than the brief hypertensive episode the patient remained completely haemodynamically stabile before, during and after the procedure. A total of three units of blood were transfused, two during transfer and one peri-operatively. Post-operatively, the patient was well, had stabile a haemoglobin of 5.9 g/dL and was discharged after two days of uneventful observation. The six week post-procedural duplex ultrasound scan revealed no luminal flow in the aneurysm. The patient was scheduled for CT after one year but cancelled for unknown reasons.
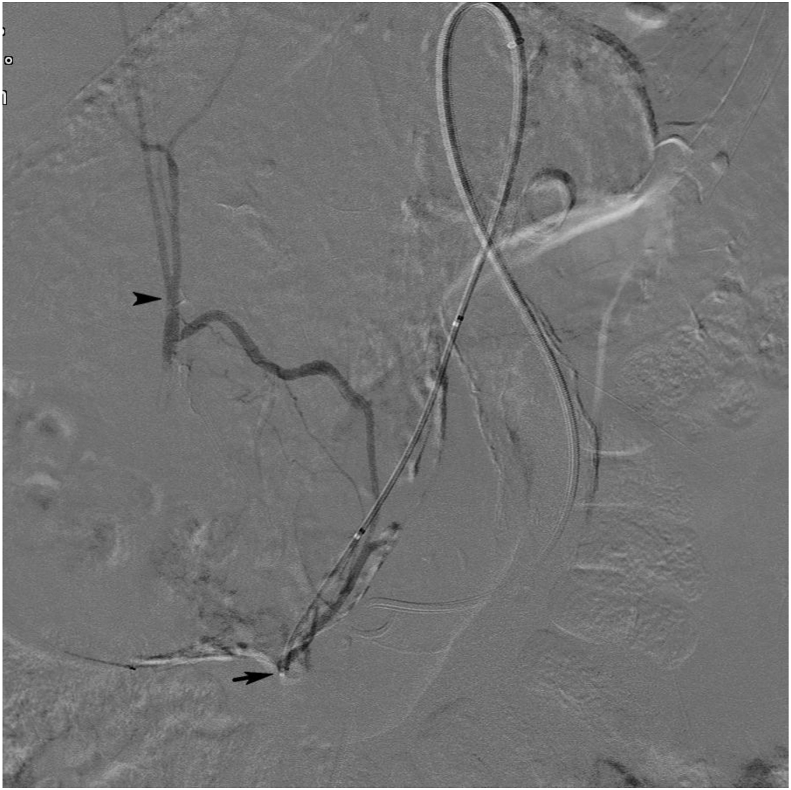
Figure 4.
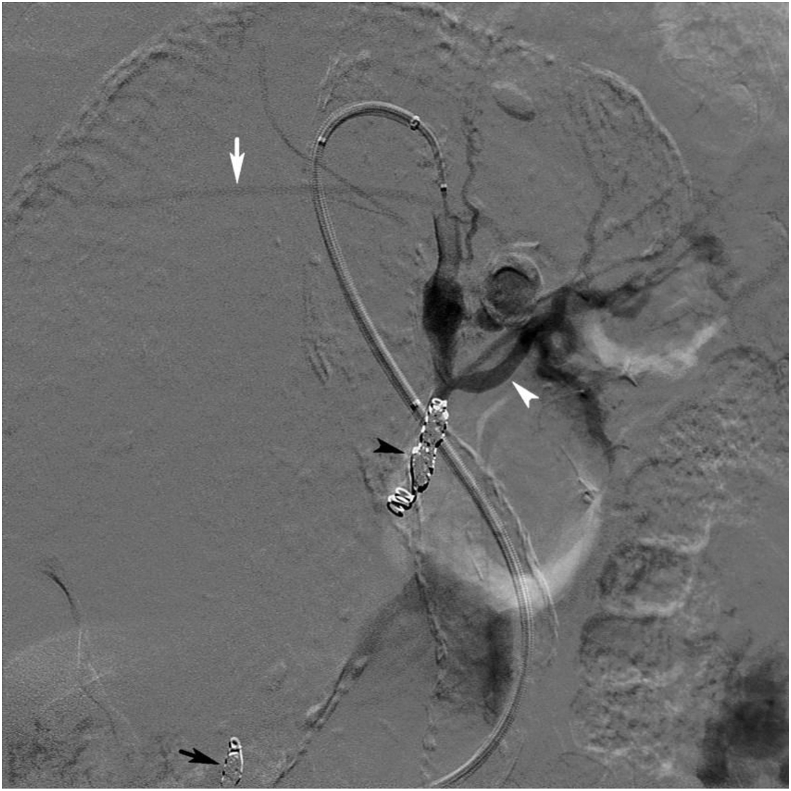
Pre-embolisation angiogram with selective catheterisation with a 4 F catheter (Navicross, Terumo) (black arrow) of one of the outflow branches from the aneurysm with connection to the right hepatic artery (black arrowhead).
Figure 5.
Selective post-embolisation angiogram in the coeliac trunk. The outflow arteries (black arrow) and the inflow artery (black arrowhead) were embolised using microcoils. Flow is preserved in the left hepatic (white arrow) and splenic artery (white arrowhead), to some degree blurred by artefact from gastric peristalsis.
Discussion
A case of successful endovascular treatment of an extremely large visceral aneurysm of 100 × 130 × 200 mm, which was found on contrast enhanced CT scan in a 78 year old female following brief loss of consciousness, is presented.
A true aneurysm of the gastroduodenal artery is a rare finding in the small subgroup of visceral aneurysms. As most visceral aneurysms are asymptomatic and remain undetected until incidentally found, reported sizes vary. The sheer size of the aneurysm in this case is highly exceptional and no other visceral aneurysm close to this case has ever been reported. The aneurysm presumably had a very slow growth rate allowing it to become this large before spontaneous rupture.
True visceral aneurysms are less likely to be symptomatic and have a much lower risk of presenting with rupture (3%–11 %) than false aneurysms (60–70 %).3, 5 Consequently treatment is recommended for all pseudo-aneurysms and all symptomatic true aneurysms. For the most common visceral aneurysm, the splenic, Trastek et al.7 suggested a treatment threshold of 2 cm in 1985. There is however no wide consensus on the threshold for treatment of asymptomatic true aneurysms and the decision to monitor or treat seems to be based on the surgeon's preference.8 Rupture has however been seen in aneurysms <10 mm,9 which has led some centres to treat all acknowledged visceral aneurysms.
Studies describing visceral aneurysms are scarce and usually have very small cohorts. Subsequently, reported mean asymptomatic aneurysm size, rupture rate and mean rupture size vary widely.
A recent larger retrospective cohort of 112 patients with visceral aneurysms5 found that except for aneurysms correlated with stenosis of the coeliac trunk, rupture was seen in sizes over 27 mm and represented 6% of all true aneurysms. True aneurysms associated with coeliac stenosis however had a mean rupture size of 9 mm. Corey et al.8 pooled data from 166 patients over two decades and suggested treatment of aneurysms over 25 mm and all aneurysms of the pancreaticoduodenal and gastroduodenal arteries regardless of size.
The endovascular approach to the treatment of visceral aneurysms is efficient, has lower peri- and post-operative risk, is minimally invasive and is therefore preferred. However, severe haemodynamic instability does not usually allow time for imaging or image guided interventions. Local availability may also limit the endovascular approach. Moreover, anatomical circumstances may warrant open surgery for instance when access and deployment by catherisation is difficult or when the risk of distal embolisation and organ ischaemia is unacceptable. However no randomised controlled trials exist to confirm the superiority of endovascular or open surgery.1
This case report confirms that the endovascular coiling approach is effective even in very large aneurysms, and more than sufficiently fast and safe to use in the event of a ruptured visceral aneurysm and subtle blood pressure drop. The extraordinary size of the aneurysm and great volume of solidified blood may however have helped in containing the rupture. Unfortunately, its peri-hepatic location led the large aneurysm to be misdiagnosed and dismissed as first hepatomegaly and then a hepatic haematoma in previous CT scans, which might otherwise have prompted observation and probably earlier intervention to prevent rupture. Unnecessary scans with unnecessary contrast enhancement are of course not recommended to investigate all incidental findings of the ever increasing usage of scans, but, as this albeit rare case illustrates, should be considered when these are very large.
Conflicts of interest
None.
Funding
None.
References
- 1.Juntermanns B., Bernheim J., Karaindros K., Walensi M., Hoffmann J.N. Visceral artery aneurysms. Gefasschirurgie. 2018;23(Suppl 1):19–22. doi: 10.1007/s00772-018-0384-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Panayiotopoulos Y.P., Assadourian R., Taylor P.R. Aneurysms of the visceral and renal arteries. Ann R Coll Surg Engl. 1996;78:412–419. [PMC free article] [PubMed] [Google Scholar]
- 3.Pitton M.B., Dappa E., Jungmann F., Kloeckner R., Schotten S., Wirth G.M. Visceral artery aneurysms: incidence, management, and outcome analysis in a tertiary care center over one decade. Eur Radiol. 2015;25:2004–2014. doi: 10.1007/s00330-015-3599-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiaradia M., Novelli L., Deux J.-F., Tacher V., Mayer J., You K. Ruptured visceral artery aneurysms. Diagn Interv Imaging. 2015;96:797–806. doi: 10.1016/j.diii.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Tetreau R., Beji H., Henry L., Valette P.-J., Pilleul F. Arterial splanchnic aneurysms: presentation, treatment and outcome in 112 patients. Diagn Interv Imaging. 2016;97:81–90. doi: 10.1016/j.diii.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 6.Kok H.K., Asadi H., Sheehan M., Given M.F., Lee M.J. Systematic review and single-center experience for endovascular management of visceral and renal artery aneurysms. J Vasc Interv Radiol. 2016;27:1630–1641. doi: 10.1016/j.jvir.2016.07.030. [DOI] [PubMed] [Google Scholar]
- 7.Trastek V.F., Pairolero P.C., Bernatz P.E. Splenic artery aneurysms. World J Surg. 1985 Jun;9:378–383. doi: 10.1007/BF01655271. [DOI] [PubMed] [Google Scholar]
- 8.Corey M.R., Ergul E.A., Cambria R.P., English S.J., Patel V.I., Lancaster R.T. The natural history of splanchnic artery aneurysms and outcomes after operative intervention. J Vasc Surg. 2016;63:949–957. doi: 10.1016/j.jvs.2015.10.066. [DOI] [PubMed] [Google Scholar]
- 9.Loffroy R., Guiu B., Cercueil J.-P., Lepage C., Cheynel N., Steinmetz E. Transcatheter arterial embolization of splenic artery aneurysms and pseudoaneurysms: short- and long-term results. Ann Vasc Surg. 2008;22:618–626. doi: 10.1016/j.avsg.2008.02.018. [DOI] [PubMed] [Google Scholar]