Abstract
Imipenem is a carbapenem antibiotic. However, Imipenem could not be marketed owing to its instability and nephrotoxicity until cilastatin, an inhibitor of renal dehydropeptidase-I (DHP-I), was developed. In present study, the potential roles of renal organic anion transporters (OATs) in alleviating the nephrotoxicity of imipenem by cilastatin were investigated in vitro and in rabbits. Our results indicated that imipenem and cilastatin were substrates of hOAT1 and hOAT3. Cilastatin inhibited hOAT1/3-mediated transport of imipenem with IC50 values comparable to the clinical concentration, suggesting the potential to cause a clinical drug–drug interaction (DDI). Moreover, imipenem exhibited hOAT1/3-dependent cytotoxicity, which was alleviated by cilastatin and probenecid. Furthermore, cilastatin and probenecid ameliorated imipenem-induced rabbit acute kidney injury, and reduced the renal secretion of imipenem. Cilastatin and probenecid inhibited intracellular accumulation of imipenem and sequentially decreased the nephrocyte toxicity in rabbit primary proximal tubule cells. Renal OATs, besides DHP-I, was also the target of interaction between imipenem and cilastatin, and contributed to the nephrotoxicity of imipenem. This therefore gives in part the explanation about the mechanism by which cilastatin protected against imipenem-induced nephrotoxicity. Thus, OATs can potentially be used as a therapeutic target to avoid the renal adverse reaction of imipenem in clinic.
Abbreviations: BUN, blood urea nitrogen; Cil, cilastatin; CKD, chronic kidney disease; CLp, plasma clearance; CLr, renal clearance; CRE, creatinine; DDIs, drug-drug interactions; DHP-I, renal dehydropeptidase-I; ES, estrone-3-sulfate; GSH, glutathione; hOAT, human OAT; Imp, imipenem; MDA, malonaldehyde; OATs, renal organic anion transporters; PAH, p-aminophenol acid; Prb, probenecid; raOAT, rabbit OAT; rOAT, rat OAT; rPTCs, rabbit primary proximal tubule cells; SNP, single nucleotide polymorphism; t1/2, half life
KEY WORDS: Imipenem, Cilastatin, Probenecid, OATs, hOAT1, hOAT3, Nephrotoxicity
Graphical abstract
Renal OATs, for the first time, were identified to facilitate the transport and nephrotoxicity of imipenem, which could be abolished by cilastatin partly through OATs inhibition.
1. Introduction
Imipenem (Imp) is the first approved carbapenem antibiotic with a broad antimicrobial activity. Imp is highly resistant to the β-lactamase enzymes produced by many multiple drug-resistant Gram-negative bacteria, thus represents a new generation of β-lactam antibiotic and play a key role in the treatment of infections not readily treated with other antibiotics1., 2.. However, Imp was found to be susceptible to deactivation by renal dehydropeptidase I (DHP-I) in vivo, resulting low recovery of Imp in the urine, which is one of the major hindrances to the clinical application3., 4.. Besides, Imp generally demonstrates nephrotoxicity in experimental animals like rhesus monkeys, rabbits, and rats5., 6., 7.. Imp blocked mitochondrial substrate uptake and respiration, induced oxidative stress and lipid peroxidative injury by depletion of glutathione (GSH)5., 6.. In order to improve the stability, cilastatin (Cil), a potent inhibitor of DHP-I, was developed and successfully applied to increase the urinary recovery of Imp. At an Imp/Cil ratio of 1:1, the urinary recovery of Imp in healthy subjects was increased to 72% from 7.7%–43% when Imp was given alone8. Currently, Imp is coadministered with Cil (1:1) to avoid its inactivation by human DHP-I. Nephrotoxicity induced by Imp in monkeys and rabbits, unexpectedly, was prevented completely by coadministration of Cil9. That means that Cil not only inhibits DHP-I-mediated metabolism toward Imp, but also abolishes Imp-induced nephrotoxicity. Renal adverse reactions are rare after dosing with Cil; however, the nephrotoxicity of Imp should not be ignored. Drug labels of PRIMAXIN® i.v., the trade name of Imp/Cil for injection, indicate that there is the risk of acute renal failure, oliguria/anuria, polyuria, and urine discoloration, which are more common in pediatric patients with the incidence rate >1%. In a number of clinical and retrospective analysis, renal adverse reactions accounted for 1%–4.17% of total adverse events, which was increased to 5% in pediatric patients10., 11.. In order to promote the clinical safety and rational application of Imp/Cil, more attention should be paid to the risk of renal injury induced by Imp.
Recently, Cil was found to be a potential therapeutic agent to protect against nephrotoxicity induced by vancomycin, cisplatin, and cyclosporin A12., 13., 14.. The protective mechanism of Cil involved anti-apoptotic effect, anti-inflammatory and antioxidation activity, and reduction of renal transport and accumulation of toxins15., 16., 17.. Kidney is the main organ responsible for the elimination of Imp. Imp undergoes predominantly renal excretion close to 70%1. Therefore, we hypothesized that Cil interfered with the renal disposition of Imp and consequently reduced its nephrotoxicity.
Organic anion transporters (OATs) are located on the basolateral membrane of renal proximal tubule cells and facilitate substrates entry into tubule cells from blood18., 19.. OATs recognize endogenous substances (uric acids, indoxyl-sulfate, and anionic uremic toxins) and many drugs (methotrexate, non-steroidal anti-inflammatory drugs, and angiotensin-converting enzyme inhibitors) as substrates18., 20.. Due to the broad substrate spectrum, OATs-mediated accumulation of toxic metabolites can induce nephrotoxicity and lead unexpected clinically outcome. Recently, a case control study revealed that patients with chronic kidney disease (CKD) had a higher frequency of the single nucleotide polymorphism (SNP) (−475T>T/G) which induced OAT1 expression, resulting in increased transportation of organic anion toxins into cells and increased risk of CKD21. In our previous studies, OAT inhibitors (puerarin, resveratrol and rhein) decrease methotrexate renal toxicity in rats and cytotoxicity in human OAT1/3 (hOAT1/3) overexpressed cells through inhibiting the function of rat OATs (rOATs) and hOAT1/322., 23., 24.. Therefore, OAT1/3 plays a critical role in kidney injury by mediating accumulation of organic anionic toxins in the kidney. OATs can potentially be used as a therapeutic target to avoid the renal damage induced by toxic substrates. In present study, we hypothesized that drug–drug interactions (DDIs) between Imp and Cil were mediated not only by DHP-I, but also by OAT1/3, which might contribute to the renal protective effect of Cil against Imp-induced nephrotoxicity. To test the hypothesis, intracellular accumulation and cytotoxicity of Imp were investigated using hOAT1/3 overexpressed cell models and rabbit primary proximal tubule cells (rPTCs) in vitro, nephrotoxicity and urinary excretion of Imp were further evaluated in rabbits in vivo. Finally, we found that Imp and Cil were substrates of hOAT1/3 and rabbit OATs (raOATs) and inhibition of hOATs/raOATs by Cil protected against Imp-induced renal toxicity.
2. Materials and methods
2.1. Materials
Imp and Cil were purchased from Merck Sharp & Dohme Corp. (VA, USA). p-Aminophenol acid (PAH) and probenecid (Prb) were purchased from Dalian Meilun Biology Technology Co., Ltd. (Dalian, China). Estrone-3-sulfate (ES) was purchased from Sigma–Aldrich (St. Louis, MO, USA). All other chemicals were of analytical grade and were available from commercial sources.
2.2. Animals
New Zealand white male rabbits (2.0–3.0 kg) were obtained from the Experimental Animal Center of Dalian Medical University (Dalian, China; permit number SCXK 2013-0003). All animal experiments were performed according to the guidelines for the care and use of laboratory animals of the National Institutes of Health. Rabbits were fasted overnight, allowed free access to water prior to pharmacokinetic experiments.
2.3. Cell culture and rPTCs isolation
Mock-, hOAT1-, and hOAT3-HEK293 cells were routinely maintained in Dulbecco׳s modified Eagle׳s medium (DMEM; Invitrogen, USA) supplemented with 10% fetal bovine serum (FBS) (heat-inactivated), 1% non-essential amino acid solution, 100 U/mL penicillin and 0.1 mg/mL streptomycin and kept at 37 °C in a 95% relative humidity atmosphere containing 5% CO2.
rPTCs were isolated from male rabbits by the previous method with minor modifications25., 26.. Briefly, rabbits were anesthetized with diethyl ether, and kidneys were perfused with Ca2+ free Hank׳s buffer (pH 7.4) containing 25 mmol/L HEPES (HBSS) to remove blood, and then kidneys were removed and minced in a sterile cell culture dish on ice. The minced tissue was washed repeatedly in HBSS solution containing 1% penicillin/streptomycin and digested in HBSS solution containing 1 mg/mL collagenase IV by shaking for 40 min at 37 °C. The renal proximal tubular fragments were then isolated mechanically by sequential filtration through a cell strainer of 80 μm (BD Falcon, Bedford, MA, USA). The isolated fragments were resuspended in culture medium DMEM/F12 supplemented with 10% FBS, 1% penicillin/streptomycin and 1% insulin–transferrin–selenium and then seeded into collagen I coated 96- or 24-well culture plates (Costar Corning Inc., Corning, NY, USA) and incubated in a humidified air/CO2 incubator (5%, v/v) at 37 °C. Fragments of tubules adhered to the culture dish, in which the medium was changed every 2 days, and cells were observed to form confluent monolayers over a 4–5-day period.
2.4. Uptake studies in transporter-transfected cells and rPTCs
hOAT1/3-HEK293 and Mock cells were seeded in 24-well culture plates for 48 h before uptake studies27. After washing 3 times with transport buffer (containing 118 mmol/L NaCl, 23.8 mmol/L NaHCO3, 4.8 mmol/L KCl, 1.0 mmol/L KH2PO4, 1.2 mmol/L MgSO4, 12.5 mmol/L HEPES, 5.0 mmol/L glucose, 1.5 mmol/L CaCl2, pH 7.4), monolayers were preincubated in transport buffer for 15 min at 37 °C. Uptake studies were initiated by the addition of 1 mL of buffer containing substrates. PAH (10 μmol/L) and ES (10 μmol/L) were used as positive control for OAT1 and OAT3, respectively. Following incubation for 10 min at 37 °C with gentle shaking, the medium was removed to stop uptake and cells were washed three times with 1 mL of ice-cold buffer. For inhibition assays, various concentrations of Imp (0–800 μmol/L) were added simultaneously to the buffer and the uptake was examined after incubation. Cell monolayers were subsequently lysed with 0.3 mL of 0.1% Triton X-100® for 2 h. Concentrations of substrates in cell lysates were determined using LC–MS/MS. Protein concentrations were measured by the bicinchoninic acid procedure using bovine serum albumin as the standard (BCA; Solarbio, Beijing, China).
2.5. Cytotoxicity assay
HEK293 and rPTCs cells were seeded at 4×103 cells/well in 96-well plates and cultured for 24 h. Fresh medium containing Imp with or without Cil (200 μmol/L) and Prb (200 μmol/L) was subsequently added, and the cells were incubated for an additional 24 h. Cell viability was determined by a CCK-8 assay (Solarbio).
2.6. Toxicity and pharmacokinetic study in rabbits
Rabbits were divided randomly into four groups: (1) vehicle group, (2) Imp (200 mg/kg) group, (3) Imp (200 mg/kg) + Cil (200 mg/kg), and Imp (200 mg/kg) + Prb (50 mg/kg). The doses used in in vivo studies were set according to previous research1., 28., 29.. Imp, Cil and Prb were dissolved in saline. Imp with or without Cil and Prb was injected intravenously (i.v.) through the ear vein to rabbits at a rate of 5 mL/min.
At 0, 30, 60, 120, 180, 240, and 360 min after administration, blood samples (0.1 mL) were collected via the ear vein in heparin tubes and then centrifuged at 1000×g for 10 min to obtain plasma. Urine was collected directly from the intraurethral cannula at 2, 4, and 6 h following administration. Plasma and urine samples were stored at –20 °C until analytical determination.
After pharmacokinetic studies, rabbits were maintained individually in the metabolism cage for 2 days. Body weight was recorded and blood and urine were collected every day for the determination of biochemical indicators. After 2 days, rabbits were decapitated and kidneys were immediately excised and weighed. Then kidneys were fixed in neutral 10% buffered formalin. Histopathological examination was conducted through routine paraffin embedding. Tissue samples were sectioned, stained with hematoxylin–eosin (HE) or periodic acid–Schiff (PAS).
2.7. siRNA design and transfection
siRNAs targeting DHP-I expressions were purchased from ShangHai GenePharma Co. (Shanghai, China). The sequence of DHP-I siRNA is as following: 5′-GCUGAACAAGUUCAACAAUdTdT-3′; 5′-AUUGUUGUUCUUGUUCAGCdTdT-3′. The negative control siRNA sequences are 5′-UUCUCCGAACGUGUCACGUTT-3′; 5′-ACGUGACACGUUCGGAGAATT-3′. rPTCs cells were transiently transfected with these siRNAs using Lipofectamine 2000 Reagent (Invitrogen). Forty-eight hours after transfection, cells were used for uptake studies and cytotoxicity assays. Effect of siRNA was evaluated by the stability of Imp incubated with rPTCs cells. After transfected, the metabolic activity of rPTCs toward Imp was significantly inhibited, indicating that DHP-I was downregulated by siRNA (Supporting Information Fig. S1).
2.8. LC–MS/MS analysis
The concentrations of the test compounds in the plasma, urine, and cell lysate were determined using an AB Sciex Qtrap® 5500 LC–MS/MS system (Foster City, CA, USA). Chromatographic separation was performed on a Hypersil BDS-C18 column (150 mm×4.6 mm, 5 μm; Dalian Elite Analytical Instruments Company Ltd., Dalian, China) at ambient temperature. The mobile phase consisted of acetonitrile and water with 0.1% (v/v) formic acid at a flow rate of 0.6 mL/min. The detection was performed by multiple reaction monitoring (MRM). The selected m/z transitions were 300.1→126.1 for Imp, 318.1→103 for Imp-M, 193.0→149.0 for PAH, 348.9 →268.9 for ES30. Analyst 1.6.1 software (Applied Biosystems) was used to control the equipment and for data acquisition and analysis.
2.9. Data analysis
All results were obtained from three independent experiments run in duplicate. Each experimental point represents the mean ± standard error of mean (SEM). All statistical analyses were conducted with SPSS 17.0 software. Two-tailed Student׳s t-tests were used to identify significant differences between the test and control groups for a specific parameter. P < 0.05 was considered a significant difference.
3. Results
3.1. Inhibitory effect of Cil on Imp uptake by hOAT1/3- HEK293 cells
Uptake studies using hOAT1/3 transfected cells were conducted to identify whether OAT1/3 were involved in the renal transport of Imp and Cil. Intracellular concentration of Imp or Cil was determined after incubation of the substrates with Mock-, hOAT1-, and hOAT3-HEK293 cells. Compared with Mock-HEK293 cells, hOAT1- and hOAT3-HEK293 cells showed an elevated intracellular concentration of Imp or Cil that could be inhibited by Prb, an inhibitor of OAT1/3 (Fig. 1A and B), suggesting that Imp and Cil were substrates of hOAT1 and hOAT3. PAH and ES, the specific substrate of OAT1 and OAT3, were selectively concentrated into hOAT1 and hOAT3 transfected cells, respectively, which were inhibited by Prb, Imp and Cil (Fig. 1C), further indicating that hOAT1/3 facilitated the transport of Imp and Cil. Due to the similar transport profile, an interaction mediated by OAT between Imp and Cil may occur when dosing together. Indeed, Imp uptake by hOAT1 and hOAT3 transfected cells were inhibited by Cil in a concentration-dependent manner, with IC50 values of 652 ± 29 μmol/L and 639 ± 36 μmol/L toward hOAT1 and hOAT3, respectively (Fig. 1D). These findings indicated that Imp and Cil could be recognized by hOAT1/3. The intracellular accumulation of Imp mediated by OAT1/3 might contribute to the toxicity of Imp, which could be improved by the competitive inhibition of Cil.
Figure 1.
hOAT1/3-induced interaction between Imp and Cil in Mock-, hOAT1-, and hOAT3-HEK293 cells. (A) Uptake of Imp (100 μmol/L) with or without Prb (200 μmol/L) for 10 min. (B) Uptake of Cil (100 μmol/L) with or without Prb (200 μmol/L) for 10 min. *P < 0.05 vs Mock cells; #P < 0.05 vs control group. (C) PAH (10 μmol/L) and ES (10 μmol/L) uptake with or without Prb (200 μmol/L), Imp (100 μmol/L) and Cil (200 μmol/L) for 10 min. (D) Concentration-dependent inhibition of Cil (0–800 μmol/L) on Imp (100 μmol/L) uptake *P < 0.05 vs PAH or ES alone group. Data are expressed as mean±SEM, n = 3.
3.2. Protective effect of Cil on Imp-induced cytotoxicity in hOAT1/3-HEK293 cells
In order to explore the contribution of hOAT1/3 to Imp-induced cytotoxicity and the effect of Cil on the cytotoxicity of Imp, cell viability was accessed by CCk-8 assays after incubation of Imp for 24 h in the absence or presence of Cil or Prb. When incubated with Imp alone, the survival rate of hOAT1/3-HEK293 cells was significantly lower than that of Mock-HEK293 cells (Fig. 2A–C). It was 85.8%, 47.5% or 27.2% for Mock-, hOAT1-, and hOAT3-HEK293 cells with Imp at the concentration of 1 mmol/L, respectively. The results suggested that hOAT1/3-mediated transmembrane transport increased the cytotoxicity of Imp. Furthermore, co-incubation with Cil or Prb, the inhibitors of OAT1/3, significantly decreased the cytotoxicity induced by Imp (Fig. 2A–C). Compared with Imp alone, the survival rate with Imp at the concentration of 1 mmol/L in the presence of Cil or Prb increased to 74.6% and 55.4% for hOAT1-HEK293 cells, and 66.3% and 76.2% for hOAT3-HEK293 cells, respectively (Fig. 2B and C). On the contrary, Cil and Prb had little effect on the survival rate of Mock cells (Fig. 2A). Due to the limitation of the clinical concentration range and drug solubility, the survival rate at high levels of Imp was not studied and the exact IC50 was not obtained. The findings indicated that inhibition of hOAT1/3 by Cil and Prb protected against Imp-induced cytotoxicity in vitro.
Figure 2.
Effects of Cil and Prb on the cytotoxicity of Imp on Mock-, hOAT1/3-HEK293 cells. (A) Cytotoxicity of Imp (0–1 mmol/L) in Mock-HEK293 cells in the absence or presence of Prb (200 μmol/L) and Cil (200 μmol/L). (B) Cytotoxicity of Imp (0–1 mmol/L) in hOAT1-HEK293 cells in the absence or presence of Prb (200 μmol/L) and Cil (200 μmol/L). (C) Cytotoxicity of Imp (0–1 mmol/L) in hOAT3-HEK293 cells in the absence or presence of Prb (200 μmol/L) and Cil (200 μmol/L). *P < 0.05, Imp+Prb group vs Imp alone group. #P < 0.05, Imp+Cil group vs Mock cells. Data are expressed as mean±SEM, n = 3.
3.3. Protective effect of Cil on Imp-induced nephrotoxicity in rabbits
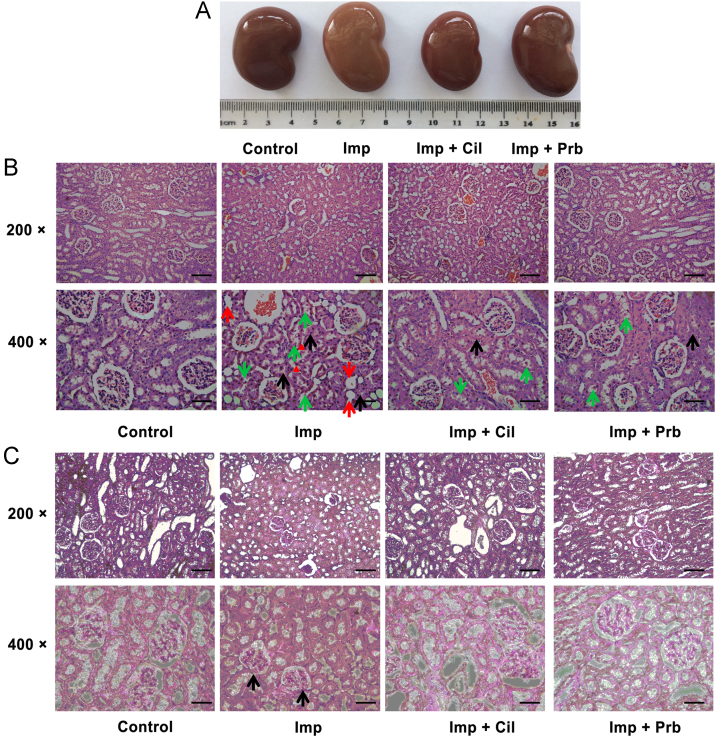
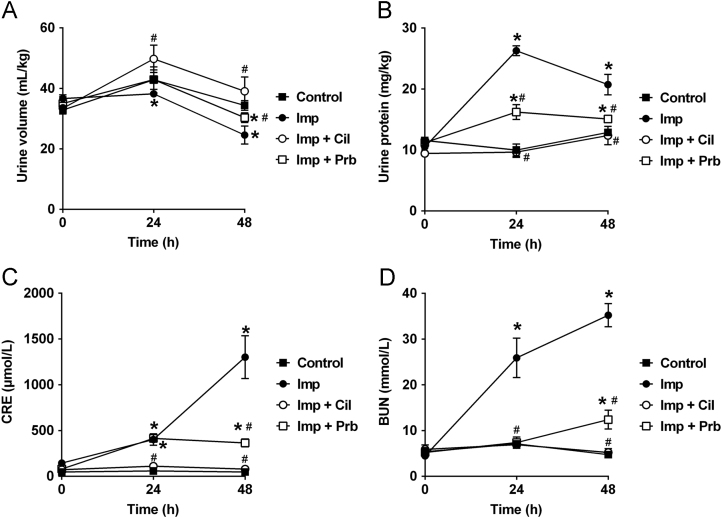
Nephrotoxicity of Imp was further evaluated in rabbits in vivo to elucidate the role of OATs in the protective effect of Cil. Imp was administered to rabbits via an ear vein in the absence or presence of Cil or Prb and nephrotoxicity was determined through histopathological examinations and biochemical indicators of renal injury. Two days after treatment, Imp reduced body weight gain compared with the control animals. Cil and Prb significantly prevented, but did not normalize, Imp-induced weight loss (Table 1). Contrary to weight loss, Imp treatment increased the weight of kidney and the ratio of kidney weight to body weight compared with the control group. Coadministration of Cil reduced the ration to the normal level, while Prb treatment did not modify kidney weight (Table 1). Gross examination of the kidneys showed normal shape and appearance except the kidneys from Imp group, which were significantly swelling and greyish-yellow appearance (Fig. 3A). Further histopathological examinations also confirmed the nephrotoxicity of Imp. While the kidney slices of control rabbits exhibited normal renal tissue morphology after HE staining and PAS staining, Imp-treated animals demonstrated severe renal damage characterized by renal tubular epithelial cell swelling, necrosis, and tubular dilation, decreased glomerular volume, mesangial cell proliferation, and the basement membrane thickening (Fig. 3B and C). After coadministration of Cil or Prb, Imp-induced acute structural damage of rabbit kidney was significantly reduced (Fig. 3B and C), indicating that Cil and Prb protected against the nephrotoxicity induced by Imp. Besides the histology change, Imp treatment induced renal dysfunction. Urinary volume was decreased and protein excretion was increased in the Imp group compared with control group (Fig. 4A and B). Imp also increased the serum creatinine (CRE) and blood urea nitrogen (BUN) levels compared with control group (Fig. 4C and D) levels. Coadministration of Cil or Prb totally or partially prevented the changes induced by Imp, respectively (Fig. 4A and B). These findings suggested that both of Cil and Prb, the inhibitors of hOATs, ameliorates Imp-induced renal injury.
Table 1.
Effects of Cil and Prb on Imp-induced changes in body and kidney weight of rabbits.
| Group | Body weight (kg) |
ΔWeight (g) | Kidney (g) | Kidney/body (g/kg) | |
|---|---|---|---|---|---|
| 0 h | 48 h | ||||
| Control | 2.80 ± 0.33 | 2.85 ± 0.34 | 35 ± 7 | 11.0 ± 2.0 | 3.79 ± 0.25 |
| Imp | 2.87 ± 0.24 | 2.69 ± 0.24 | –177 ± 7a | 13.2 ± 1.5 | 4.81 ± 0.29a |
| Imp+Cil | 2.97 ± 0.31 | 2.97 ± 0.30 | 10 ± 3a,b | 11.4 ± 1.6 | 3.74 ± 0.16b |
| Imp+Prb | 2.42 ± 0.34 | 2.43 ± 0.29 | 5 ± 1a,b | 11.1 ± 2.2 | 4.39 ± 0.31a |
Data are mean ± SEM, n = 3.
P < 0.05 vs control group.
P < 0.05 vs imipenem group.
Figure 3.
Protective effect of Cil and Prb on renal histology in Imp-treated rabbits. Imp (200 mg/kg) was injected intravenously (i.v.) through the ear vein to rabbits with or without Cil (200 mg/kg) or Prb (50 mg/kg). Kidney was collected at 48 h after administration of Imp for Gross observation (A), HE staining (B) and PAS staining (C). Renal tubular epithelial cell swelling (black arrows), tubule dilatation (red arrows) and necrosis and shedding of cell (green arrows) were indicated in representative images of HE staining. Glomerular atrophy and matrix increase were indicated by black arrows in PAS staining. Bars = 100 μm at 200×; bars = 50 μm at 400×. Data are expressed as mean±SEM, n = 3.
Figure 4.
Effects of Cil and Prb on Imp-induced nephrotoxicity in rabbits. Imp (200 mg/kg) was injected intravenously (i.v.) through the ear vein to rabbits with or without Cil (200 mg/kg) or Prb (50 mg/kg). Urine and blood were collected at 0, 24 and 48 h after administration of Imp for the determination of urine protein, CRE and BUN. *P < 0.05 vs control group. #P < 0.05 vs Imp group. Data are expressed as mean±SEM, n = 3.
3.4. Effect of Cil on the pharmacokinetics of Imp in rabbits
In order to explore the protective mechanisms of Cil and Prb preliminary, effect of Cil and Prb on the plasma concentration and urinary excretion of Imp were determined by LC–MS/MS. After intravenous administration, Imp was rapidly eliminated from blood and primarily recovered from urine as prototype and its metabolite (Fig. 5A–C and Table 2). Concurrent use of Cil and Prb increased the plasma concentration and AUC of Imp significantly, reduced the plasma clearance (CLp), while had no effect on half life (t1/2) (Fig. 5A–C and Table 2). Interestingly, Prb had little effect on the urinary excretion and decreased the renal clearance (CLr) of Imp (Fig. 5B and Table 2), while Cil, the DHP-I inhibitor, enhanced the urinary recovery and increased CLr of Imp (Fig. 5B and Table 2). In order to exclude interference by DHP-I, urinary excretion of Imp-M, the major metabolite of Imp produced by DHP-I, was also determined. Extensive Imp-M was found in the urine from Imp group, which was significantly reduced by combined Cil (Fig. 5C). After adding the amount of Imp and Imp-M, the calculated CLr(Imp + Imp-M) in Imp group was significantly higher than these in Imp+Cil group and Imp+Prb group (Table 2), suggesting that Cil and Prb inhibited the renal excretion of Imp. Combination with Cil or Prb reduced renal exposure of Imp which might consequently contribute to their renal protection effect.
Figure 5.
Effects of Cil and Prb on the plasma concentration and urinary excretion of Imp in rabbits. Imp (200 mg/kg) was injected intravenously (i.v.) through the ear vein to rabbits with or without Cil (200 mg/kg) or Prb (50 mg/kg). Plasma and urine were collected for the determination of Imp and Imp-M by LC–MS/MS. *P < 0.05, Imp+Cil vs Imp group; #P < 0.05, Imp+Prb vs Imp group. Data are expressed as mean±SEM, n = 3.
Table 2.
Pharmacokinetic parameters of Imp after intravenous administration of Imp (200 mg/kg) with or without Cil (200 mg/kg) or Prb (50 mg/kg) in rabbits.
| Parameter | Unit | Imp | Imp+Cil | Imp+Prb |
|---|---|---|---|---|
| AUC(0–6) | μg/mL·h | 289 ± 48.7 | 374 ± 70.3a | 379 ± 81.0a |
| t1/2 | min | 58 ± 4.2 | 64 ± 6.7 | 69 ± 8.9 |
| CLp | L/h/kg | 0.712 ± 0.119 | 0.554 ± 0.104a | 0.553 ± 0.107a |
| CLr(Imp) | L/h/kg | 0.167 ± 0.0258 | 0.296 ± 0.0641a | 0.0992 ± 0.0126a |
| CLr(Imp+Imp-M) | L/h/kg | 0.497 ± 0.0804 | 0.325 ± 0.0524a | 0.248 ± 0.0330a |
Data are mean ± SEM, n = 3.
P < 0.05 vs Imp only group.
3.5. Inhibition of intracellular accumulation of Imp by Cil in rPTCs
To further reveal the molecular mechanism underlying the renal interaction between Imp and Cil or Prb, intracellular accumulation and cytotoxicity of Imp were evaluated in the absence or presence of Cil and Prb using rPTCs. OATs expression and function of rPTCs were first evaluated by uptake assays using PAH and ES as probe substrates. rPTCs showed temperature-dependent uptake of PAH and ES, which was inhibited by the adding of Prb, Imp and Cil (Fig. 6A). The results demonstrated that isolated rPTCs were capable to be used as an in vitro model to evaluate uptake processes mediated by raOATs. Like PAH and ES, Imp uptake by rPTCs also exhibited a temperature-dependent manner and was inhibited by Prb and Cil (Fig. 6B), indicating that renal interaction between Imp and Cil or Prb was mediated by raOATs. But the need to pay attention to is that Cil showed slightly inhibitory potential (Fig. 6B). Cil is a potent inhibitor of DHP-I that is responsible for the metabolism of Imp. The inhibition capacity of Cil on raOATs might be covered by the increased stability of Imp. To verify this hypothesis, siRNA of rabbit DHP-I was transfected into rPTCs and uptake assays using rPTCs with low level of DHP-I was conducted. After knockdown of DHP-I, uptake of PAH or ES was not changed compared with cells transfected with si-Control (Fig. 6C), indicating that knockdown of DHP-I did not change the function of raOATs. On the contrary, rPTCs transfected with si-DHP-I had a 30% increase in intracellular level of Imp (Fig. 6D), resulting in a lower residual activity which reflected the true inhibition capacity of Cil on Imp uptake mediated by raOATs in rPTCs. Indeed, IC50 values of Cil toward si-Control and si-DHP-I cells were 257 ± 36.2 μmol/L and 52.9 ± 10.8 μmol/L, respectively (Fig. 6E). The findings suggested that Cil not only blocked DHP-I to stabilize Imp, but also inhibited raOATs to decrease the intracellular accumulation of Imp.
Figure 6.
Effects of Cil and Prb on the Intracellular accumulation of Imp in rPTCs. (A) PAH (10 μmol/L) and ES (10 μmol/L) uptake by rPTCs at 37 or 4 °C with or without Prb (200 μmol/L) and Cil (200 μmol/L) for 10 min. (B) Imp (100 μmol/L) uptake by rPTCs at 37 or 4 °C with or without Prb (200 μmol/L) and Cil (200 μmol/L) for 10 min. (C) PAH (10 μmol/L) and ES (10 μmol/L) uptake by rPTCs transfected with siRNA of DHP-I (si-DHP-I) at 37 or 4 °C with or without Prb (200 μmol/L) and Cil (200 μmol/L) for 10 min. (D) Imp (100 μmol/L) uptake by rPTCs transfected with si-DHP-I at 37 or 4 °C with or without Prb (200 μmol/L) and Cil (200 μmol/L) for 10 min. (E) Concentration-dependent inhibition of Cil (0–800 μmol/L) on Imp (100 μmol/L) uptake by rPTCs transfected with si-DHP-I at 37 °C for 10 min. IC50 was determined after the deduction of uptake at 4 °C. *P < 0.05 vs control or si-DHP-I group. Data are expressed as mean±SEM, n = 3.
3.6. Protective effect of Cil on Imp-induced cytotoxicity in rPTCs
Cytotoxicity of Imp in rPTCs was also investigated after incubation with Imp in the absence or presence of Prb or Cil for 24 h. In the presence of Prb or Cil, the survival rates of rPTCs treated with Imp were significantly increased (Fig. 7A). The results confirmed that inhibition of raOATs protected against Imp-induced cytotoxicity. Interestingly, knockdown of DHP-I increased the survival rates of rPTCs compared with si-Control cells (Fig. 7B). And presence of Cil and Prb made further efforts to decrease the cytotoxicity of Imp (Fig. 7B). These findings indicated that DHP-I and raOATs were targets of interaction between Imp and Cil. The inhibitory potential of Cil on DHP-I and raOATs provided protection for the kidney to avoid the renal injury induced by Imp.
Figure 7.
Effects of Cil and Prb on the cytotoxicity of Imp in rPTCs. (A) Cytotoxicity of Imp in rPTCs in the absence or presence of Prb (200 μmol/L) and Cil (200 μmol/L). *P < 0.05 vs control. (B) Cytotoxicity of Imp in rPTCs transfected with si-DHP-I in the absence or presence of Prb (200 μmol/L) and Cil (200 μmol/L). *P < 0.05 vs si-DHP-I group. Data are expressed as mean±SEM, n = 3.
4. Discussion
Although Imp/Cil is generally well tolerated, adverse reaction is still a problem that cannot be ignored in the clinical application of Imp/Cil. In addition to the nervous system (seizures) and digestive system (nausea, diarrhea, and gastroenteritis), renal injury is also a common adverse reaction of Imp/Cil, including elevated creatinine, urea nitrogen, urine discoloration, and oliguria/anuria31., 32.. In the present study, we found Imp and Cil were substrates of hOAT1 and hOAT3. Intracellular accumulation and renal transport of Imp mediated by OATs contributed to its cytotoxicity and nephrotoxicity. Inhibition of OATs by Cil reduced renal exposure of Imp and consequently protected against Imp-induced renal injury in vitro and in vivo. OATs played a vital role in the renal disposition of Imp and thus could be used as a potential therapeutic target to avoid the nephrotoxicity of Imp and other nephrotoxics.
4.1. DDIs between Imp and Cil mediated by OATs
The combination of Imp and Cil is used widely in clinical for 30 years. While Imp has a broad spectrum of antibacterial activity, Cil has no pharmacological activity but has been developed as DHP-I inhibitor to effectively inhibit the metabolism of Imp and thus increase the urinary recovery1., 8.. Cil is used as a pharmacoenhancer. Traditionally, DHP-I is considered as the target of DDIs between Imp and Cil. A similar example is tazobactam which inhibits β-lactamase to maintain the plasma concentration of piperacillin33. Recently, we found that piperacillin and tazobactam were the substrates of hOAT1/3 which were the second target of DDI between piperacillin and tazobactam34. With the deepening of research , roles of drug transporters in DDIs and progression of diseases have been revealed constantly. Another pharmacoenhancer cobicistat, a inhibitor of CYP3As, was also been found to enhance the effect of combined drugs via interactions with P-gp and OAT235., 36.. These findings encouraged us to explore whether a transporter based DDI existed between Imp and Cil.
OATs are involved in the renal elimination of a wide array of metabolites, nutrients, toxins, xenobiotics, and drugs, which contributes to the normal renal function18., 19.. Considering that renal excretion was the major elimination pathway of Imp and Cil1, together with the findings that Cil was an inhibitor of hOATs37., 38., we hypothesized that OATs might involved in the DDIs between Imp and Cil. Our results finally demonstrated that both Imp and Cil were substrates of human OAT1 and OAT3 and Cil concentration-dependently inhibited Imp uptake in vitro (Fig. 1). The maximum clinical plasma concentrations of Cil are documented as 88 μg/mL1., 8.. Taking into account the plasma protein binding of approximately 40%, the peak level of free Cil in human was calculated as about 140 μmol/L, which was high enough to induce an in vivo DDI mediated by OATs based on the criteria of FDA39. In present study, an in vivo DDI in rabbits was indeed observed. Cil, as well as Prb, a common OATs inhibitor, inhibited the renal excretion of Imp (Fig. 5 and Table 2). Coupled with the findings of uptake assays using rPTCs (Fig. 6), we could conclude that renal OATs were the target of DDIs between Imp and Cil. For the first time, OAT1/3-mediated DDIs were identified to modulate the pharmacokinetics of Imp.
Besides OATs, transporters located in apical membrane of renal tubular epithelial cells, including efflux transporters (MRP2, P-gp, and MATE1/2K), and reabsorption transporters (URATs, PEPT2, and OCTN), are also prone to be targets of DDI40. However, predictable consequences of a DDI mediated by an efflux transporter are reduction of urinary excretion or enhanced intracellular accumulation40, and inhibition of transporters responsible for reabsorption leads to an increased urinary excretion40, which is contrary to our results that Cil and Prb decreased the urinary excretion of IMP (Fig. 5) and decreased the intracellular accumulation of IMP in rPTCs (Fig. 6B). These findings suggested that transporters expressed on apical membrane of renal tubular epithelial cells may be not involved in the transport of IMP or play a minor role in the renal disposition of IMP.
4.2. OATs in nephrotoxicity of Imp
Medications, especially for the drugs eliminated by kidney, are a common cause of kidney injury, which accounts for approximately 19%–26% of cases of acute kidney injury in hospitalized patients41., 42.. In fact, 32% of the top 200 prescribed drugs in 2010 are cleared by renal mechanisms40. OATs-mediated renal transport facilitates substrates entry into kidney, which in turn results in higher exposure and increases the risk of toxicity19., 43., 44.. Carbapenem antibiotics, such as Imp and panipenem, are well known for their nephrotoxicity2., 45.. When coadministered with Cil and betamipron respectively; however, Imp and panipenem are well tolerated46. While betamipron was found to reduce the nephrotoxicity of panipenem through inhibiting the active transport of carbapenems in the renal cortex46, the mechanism of protection effect of Cil on Imp-induced renal injury was not fully understood. It was reported that Imp induced mitochondrial dysfunction by inhibition of mitochondrial substrate uptake and respiration5., 6., suggesting that the cytotoxicity of Imp predominantly depended on intracellular interactions. That means that Imp must enter the cells before induction of cytotoxicity. In present study, we found that Cil reduced intracellular accumulation of Imp in nephrocytes via inhibition of OATs. DDIs mediated by OATs between Imp and Cil might contribute to the protective role of Cil. Our results indicated that Imp exhibited OAT1/3-dependent cytotoxicity in HEK293 cells and rPTCs in vitro (Figure 2, Figure 7). Coadministration of Prb and Cil ameliorated Imp-induced nephrotoxicity in rabbits (Figure 3, Figure 4 and Table 1), which could be explained by the decreased renal excretion of Imp (Fig. 5 and Table 2). Good correlations were observed between intracellular accumulation and cytotoxicity in vitro, as well as renal excretion and nephrotoxicity in vivo, suggesting that Cil inhibited OATs-mediated renal transport and then protected against its nephrotoxicity. In present study, rabbit was used to evaluated the nephrotoxicity of Imp and protective effect of Cil because rabbits are susceptible to the nephrotoxicity of Imp9. The sensibility of experimental animals to the nephrotoxicity of Imp was in the order of rabbits > monkeys > rats9, suggesting significant species differences. Imp was not marketed as a single-ingredient medication, which resulted in lack of cases of nephrotoxicity in human. Imp/Cil, at concentrations more than 10-fold higher as peak plasma concentrations achieved in humans, induced marked malonaldehyde (MDA) production after 3 h in human renal cortical slices47, suggesting nephrotoxic and peroxidative potential of Imp/Cil in human. Indeed, Imp/Cil have been reported to cause increases in serum creatinine and serum urea with an incidence less than 1%10., 11., which may be underestimated because factors predisposing to pre-renal azotemia or to impaired renal function usually have been present. Cannella and Wilkinson48 reported a case of nephrotoxicity, in which acute renal failure requiring dialysis occurred in a high-risk patient receiving Imp/Cil and inhaled tobramycin, another nephrotoxic agent. This illustrates that concomitant administration of the nephroprotective agent Cil cannot completely remove the nephrotoxicity of Imp. According to the results in present study, inhibition of OAT contributed to the protective effect of Cil. This provided a new insight on the pharmacoenhancement of Cil. It is expected that a more potent OAT inhibitor with low toxicity would exhibit extra protective effect on Imp-induced nephrotoxicity when coadministered with Imp/Cil.
In addition, it must be noted that, knockdown of DHP-I by siRNA made a higher intracellular level of Imp and a lower susceptibility to cytotoxicity of Imp (Figure 6, Figure 7), indicating that DHP-I level had an effect on the cytotoxicity of Imp and the metabolites of Imp might have more potent cytotoxicity than Imp. According to the results of preliminary experiment, a number of metabolites were obtained by incubation of rabbit kidney homogenate with Imp (Supporting Information Fig. S2A). Several of them exhibited stronger cytotoxicity than Imp (Fig. S2B). Unfortunately, the metabolites were unstable and were not obtained. These findings suggested that Cil protected against Imp-induce nephrotoxicity through not only OATs inhibition but also DHP-I inhibition. According to Fig. 7, the survival rate was increased by 7.8%, 14.7% and 25.6% in the presence of Prb, si-DHP-I and Cil, respectively (Fig. 7), which represented the contribution rates of two protective pathways at a level of rough estimate. And of course, as a promising agent for renal protection, other mechanisms may be involved in protective effect of Cil on Imp-induced renal injury, which will be studied in the future research.
5. Conclusions
In conclusion, OATs-mediated renal transport of Imp contributed to Imp nephrotoxicity, which could be abolished by Cil, at least in part, through inhibition of OATs. OATs can potentially be used as a therapeutic target to avoid the renal adverse reaction of Imp in clinic.
Acknowledgments
The work was supported by a grant from the National Natural Science Foundation of China, China (Nos. 81874324, 81473280, and U1608283) and Dalian Science and technology innovation found, China (No. 2018J12SN065). The authors thank Prof. Yuichi Sugiyama (Sugiyama Laboratory, RIKEN, Japan) for kindly providing Mock/ hOAT1/3-HEK293 cells.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Supporting data associated with this article can be found in the online version at doi:10.1016/j.apsb.2019.02.005.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Balfour J.A., Bryson H.M., Brogden R.N. Imipenem/cilastatin: an update of its antibacterial activity, pharmacokinetics and therapeutic efficacy in the treatment of serious infections. Drugs. 1996;51:99–136. doi: 10.2165/00003495-199651010-00008. [DOI] [PubMed] [Google Scholar]
- 2.Papp-Wallace K.M., Endimiani A., Taracila M.A., Bonomo R.A. Carbapenems: past, present, and future. Antimicrob Agents Chemother. 2011;55:4943–4960. doi: 10.1128/AAC.00296-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kropp H., Sundelof J.G., Hajdu R., Kahan F.M. Metabolism of thienamycin and related carbapenem antibiotics by the renal dipeptidase, dehydropeptidase. Antimicrob Agents Chemother. 1982;22:62–70. doi: 10.1128/aac.22.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Graham D.W., Ashton W.T., Barash L., Brown J.E., Brown R.D., Canning L.F. Inhibition of the mammalian β-lactamase renal dipeptidase (dehydropeptidase-i) by (z)-2-(acylamino)-3-substituted-propenoic acids. J Med Chem. 1987;30:1074–1090. doi: 10.1021/jm00389a018. [DOI] [PubMed] [Google Scholar]
- 5.Tune B.M., Hsu C.Y. The renal mitochondrial toxicity of β-lactam antibiotics: in vitro effects of cephaloglycin and imipenem. J Am Soc Nephrol. 1990;1:815–821. doi: 10.1681/ASN.V15815. [DOI] [PubMed] [Google Scholar]
- 6.Tune B.M., Fravert D., Hsu C.Y. Thienamycin nephrotoxicity. Mitochondrial injury and oxidative effects of imipenem in the rabbit kidney. Biochem Pharmacol. 1989;38:3779–3783. doi: 10.1016/0006-2952(89)90585-6. [DOI] [PubMed] [Google Scholar]
- 7.Sack K., Herhahn J., Marre R., Schulz E. Renal tolerance of imipenem/cilastatin and other β-lactam antibiotics in rats. Infection. 1985;13 Suppl 1:S156–S160. doi: 10.1007/BF01644239. [DOI] [PubMed] [Google Scholar]
- 8.Norrby S.R., Alestig K., Bjornegard B., Burman L.A., Ferber F., Huber J.L. Urinary recovery of n-formimidoyl thienamycin (MK0787) as affected by coadministration of n-formimidoyl thienamycin dehydropeptidase inhibitors. Antimicrob Agents Chemother. 1983;23:300–307. doi: 10.1128/aac.23.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birnbaum J., Kahan F.M., Kropp H., MacDonald J.S. Carbapenems. a new class of β-lactam antibiotics. Discovery and development of imipenem/cilastatin. Am J Med. 1985;78:3–21. doi: 10.1016/0002-9343(85)90097-x. [DOI] [PubMed] [Google Scholar]
- 10.Zhanel G.G., Simor A.E., Vercaigne L., Mandell L. Imipenem and meropenem: comparison of in vitro activity, pharmacokinetics, clinical trials and adverse effects. Can J Infect Dis. 1998;9:215–228. doi: 10.1155/1998/831425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hornik C.P., Herring A.H., Benjamin D.K., Jr., Capparelli E.V., Kearns G.L., van den Anker J. Adverse events associated with meropenem versus imipenem/cilastatin therapy in a large retrospective cohort of hospitalized infants. Pediatr Infect Dis J. 2013;32:748–753. doi: 10.1097/INF.0b013e31828be70b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tejedor A., Torres A.M., Castilla M., Lazaro J.A., de Lucas C., Caramelo C. Cilastatin protection against cyclosporin A-induced nephrotoxicity: clinical evidence. Curr Med Res Opin. 2007;23:505–513. doi: 10.1185/030079906X167633. [DOI] [PubMed] [Google Scholar]
- 13.Humanes B., Lazaro A., Camano S., Moreno-Gordaliza E., Lazaro J.A., Blanco-Codesido M. Cilastatin protects against cisplatin-induced nephrotoxicity without compromising its anticancer efficiency in rats. Kidney Int. 2012;82:652–663. doi: 10.1038/ki.2012.199. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura T., Hashimoto Y., Kokuryo T., Inui K.I. Effects of fosfomycin and imipenem/cilastatin on nephrotoxicity and renal excretion of vancomycin in rats. Pharm Res. 1998;15:734–738. doi: 10.1023/a:1011971019868. [DOI] [PubMed] [Google Scholar]
- 15.Camano S., Lazaro A., Moreno-Gordaliza E., Torres A.M., de Lucas C., Humanes B. Cilastatin attenuates cisplatin-induced proximal tubular cell damage. J Pharmacol Exp Ther. 2010;334:419–429. doi: 10.1124/jpet.110.165779. [DOI] [PubMed] [Google Scholar]
- 16.Perez M., Castilla M., Torres A.M., Lazaro J.A., Sarmiento E., Tejedor A. Inhibition of brush border dipeptidase with cilastatin reduces toxic accumulation of cyclosporin a in kidney proximal tubule epithelial cells. Nephrol Dial Transplant. 2004;19:2445–2455. doi: 10.1093/ndt/gfh397. [DOI] [PubMed] [Google Scholar]
- 17.Moreno-Gordaliza E., Giesen C., Lazaro A., Esteban-Fernandez D., Humanes B., Canas B. Elemental bioimaging in kidney by LA-ICP-MS as a tool to study nephrotoxicity and renal protective strategies in cisplatin therapies. Anal Chem. 2011;83:7933–7940. doi: 10.1021/ac201933x. [DOI] [PubMed] [Google Scholar]
- 18.Yin J., Wang J. Renal drug transporters and their significance in drug–drug interactions. Acta Pharm Sin B. 2016;6:363–373. doi: 10.1016/j.apsb.2016.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huo X., Liu K. Renal organic anion transporters in drug–drug interactions and diseases. Eur J Pharm Sci. 2018;112:8–19. doi: 10.1016/j.ejps.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Hsueh C.H., Yoshida K., Zhao P., Meyer T.W., Zhang L., Huang S.M. Identification and quantitative assessment of uremic solutes as inhibitors of renal organic anion transporters, OAT1 and OAT3. Mol Pharm. 2016;13:3130–3140. doi: 10.1021/acs.molpharmaceut.6b00332. [DOI] [PubMed] [Google Scholar]
- 21.Sun C.Y., Wu M.S., Lee C.C., Chen S.H., Lo K.C., Chen Y.H. A novel SNP in the 5′ regulatory region of organic anion transporter 1 is associated with chronic kidney disease. Sci Rep. 2018;8:8085. doi: 10.1038/s41598-018-26460-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jia Y., Liu Z., Wang C., Meng Q., Huo X., Liu Q. P-gp, MRP2 and OAT1/OAT3 mediate the drug–drug interaction between resveratrol and methotrexate. Toxicol Appl Pharmacol. 2016;306:27–35. doi: 10.1016/j.taap.2016.06.030. [DOI] [PubMed] [Google Scholar]
- 23.Liu Q., Wang C., Meng Q., Huo X., Sun H., Peng J. Mdr1 and OAT1/OAT3 mediate the drug–drug interaction between puerarin and methotrexate. Pharm Res. 2014;31:1120–1132. doi: 10.1007/s11095-013-1235-9. [DOI] [PubMed] [Google Scholar]
- 24.Liu Z., Jia Y., Wang C., Meng Q., Huo X., Sun H. Organic anion transporters 1 (OAT1) and OAT3 meditated the protective effect of rhein on methotrexate-induced nephrotoxicity. RSC Adv. 2017;7:25461–25468. [Google Scholar]
- 25.Alexander L.D., Alagarsamy S., Douglas J.G. Cyclic stretch-induced cPLA2 mediates ERK 1/2 signaling in rabbit proximal tubule cells. Kidney Int. 2004;65:551–563. doi: 10.1111/j.1523-1755.2004.00405.x. [DOI] [PubMed] [Google Scholar]
- 26.Li L.P., Song F.F., Weng Y.Y., Yang X., Wang K., Lei H.M. Role of OCT2 and MATE1 in renal disposition and toxicity of nitidine chloride. Br J Pharmacol. 2016;173:2543–2554. doi: 10.1111/bph.13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huo X., Wang C., Yu Z., Peng Y., Wang S., Feng S. Human transporters, PEPT1/2, facilitate melatonin transportation into mitochondria of cancer cells: an implication of the therapeutic potential. J Pineal Res. 2017;62:12390. doi: 10.1111/jpi.12390. [DOI] [PubMed] [Google Scholar]
- 28.Shiba K., Saito A., Shimada J., Hori S., Kaji M., Miyahara T. Renal handling of fleroxacin in rabbits, dogs, and humans. Antimicrob Agents Chemother. 1990;34:58–64. doi: 10.1128/aac.34.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim J.M., Ha J.R., Oh S.W., Kim H.G., Lee J.M., Kim B.O. Comparison of in vivo nephrotoxicity in the rabbit by a pyrrolidinyl-thio carbapenem CW-270031. J Microbiol Biotechnol. 2008;18:1768–1772. doi: 10.4014/jmb.0800.106. [DOI] [PubMed] [Google Scholar]
- 30.Kulkarni M.V., Zurita A.N., Pyka J.S., Murray T.S., Hodsdon M.E., Peaper D.R. Use of imipenem to detect KPC, NDM, OXA, IMP, and VIM carbapenemase activity from Gram-negative rods in 75 minutes using liquid chromatography-tandem mass spectrometry. J Clin Microbiol. 2014;52:2500–2505. doi: 10.1128/JCM.00547-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verwaest C. Meropenem versus imipenem/cilastatin as empirical monotherapy for serious bacterial infections in the intensive care unit. Clin Microbiol Infect. 2000;6:294–302. doi: 10.1046/j.1469-0691.2000.00082.x. [DOI] [PubMed] [Google Scholar]
- 32.Vazquez J.A., Gonzalez Patzan L.D., Stricklin D., Duttaroy D.D., Kreidly Z., Lipka J. Efficacy and safety of ceftazidime–avibactam versus imipenem–cilastatin in the treatment of complicated urinary tract infections, including acute pyelonephritis, in hospitalized adults: results of a prospective, investigator-blinded, randomized study. Curr Med Res Opin. 2012;28:1921–1931. doi: 10.1185/03007995.2012.748653. [DOI] [PubMed] [Google Scholar]
- 33.Hayashi Y., Roberts J.A., Paterson D.L., Lipman J. Pharmacokinetic evaluation of piperacillin–tazobactam. Expert Opin Drug Metab Toxicol. 2010;6:1017–1031. doi: 10.1517/17425255.2010.506187. [DOI] [PubMed] [Google Scholar]
- 34.Wen S., Wang C., Duan Y., Huo X., Meng Q., Liu Z. OAT1 and OAT3 also mediate the drug–drug interaction between piperacillin and tazobactam. Int J Pharm. 2018;537:172–182. doi: 10.1016/j.ijpharm.2017.12.037. [DOI] [PubMed] [Google Scholar]
- 35.Lepist E.I., Zhang X., Hao J., Huang J., Kosaka A., Birkus G. Contribution of the organic anion transporter OAT2 to the renal active tubular secretion of creatinine and mechanism for serum creatinine elevations caused by cobicistat. Kidney Int. 2014;86:350–357. doi: 10.1038/ki.2014.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gordon L.A., Kumar P., Brooks K.M., Kellogg A., McManus M., Alfaro R.M. Antiretroviral boosting agent cobicistat increases the pharmacokinetic exposure and anticoagulant effect of dabigatran in HIV-negative healthy volunteers. Circulation. 2016;134:1909–1911. doi: 10.1161/CIRCULATIONAHA.116.025257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takeda M., Narikawa S., Hosoyamada M., Cha S.H., Sekine T., Endou H. Characterization of organic anion transport inhibitors using cells stably expressing human organic anion transporters. Eur J Pharmacol. 2001;419:113–120. doi: 10.1016/s0014-2999(01)00962-1. [DOI] [PubMed] [Google Scholar]
- 38.Enomoto A., Takeda M., Tojo A., Sekine T., Cha S.H., Khamdang S. Role of organic anion transporters in the tubular transport of indoxyl sulfate and the induction of its nephrotoxicity. J Am Soc Nephrol. 2002;13:1711–1720. doi: 10.1097/01.asn.0000022017.96399.b2. [DOI] [PubMed] [Google Scholar]
- 39.Feng B, Varma MV. Evaluation and quantitative prediction of renal transporter-mediated drug–drug interactions. J Clin Pharmacol 2016;56 Suppl 7:S110–21 [DOI] [PubMed]
- 40.Morrissey K.M., Stocker S.L., Wittwer M.B., Xu L., Giacomini K.M. Renal transporters in drug development. Annu Rev Pharmacol Toxicol. 2013;53:503–529. doi: 10.1146/annurev-pharmtox-011112-140317. [DOI] [PubMed] [Google Scholar]
- 41.Markowitz G.S., Bomback A.S., Perazella M.A. Drug-induced glomerular disease: direct cellular injury. Clin J Am Soc Nephrol. 2015;10:1291–1299. doi: 10.2215/CJN.00860115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mehta R.L., Pascual M.T., Soroko S., Savage B.R., Himmelfarb J., Ikizler T.A. Spectrum of acute renal failure in the intensive care unit: the PICARD experience. Kidney Int. 2004;66:1613–1621. doi: 10.1111/j.1523-1755.2004.00927.x. [DOI] [PubMed] [Google Scholar]
- 43.Nigam S.K. What do drug transporters really do? Nat Rev Drug Discov. 2015;14:29–44. doi: 10.1038/nrd4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang L., Sweet D.H. Renal organic anion transporters (SLC22 family): expression, regulation, roles in toxicity, and impact on injury and disease. AAPS J. 2013;15:53–69. doi: 10.1208/s12248-012-9413-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hirouchi Y., Naganuma H., Kawahara Y., Okada R., Kamiya A., Inui K. Preventive effect of betamipron on nephrotoxicity and uptake of carbapenems in rabbit renal cortex. Jpn J Pharmacol. 1994;66:1–6. doi: 10.1254/jjp.66.1. [DOI] [PubMed] [Google Scholar]
- 46.Bassetti M., Nicolini L., Esposito S., Righi E., Viscoli C. Current status of newer carbapenems. Curr Med Chem. 2009;16:564–575. doi: 10.2174/092986709787458498. [DOI] [PubMed] [Google Scholar]
- 47.Yousif T., Pooyeh S., Hannemann J., Baumann J., Tauber R., Baumann K. Nephrotoxic and peroxidative potential of meropenem and imipenem/cilastatin in rat and human renal cortical slices and microsomes. Int J Clin Pharmacol Ther. 1999;37:475–486. [PubMed] [Google Scholar]
- 48.Cannella C.A., Wilkinson S.T. Acute renal failure associated with inhaled tobramycin. Am J Health Syst Pharm. 2006;63:1858–1861. doi: 10.2146/ajhp060196. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material