Abstract
Polycystic ovary syndrome (PCOS) is a common condition of reproductive-aged women. In a well-validated sheep model of PCOS, testosterone (T) treatment of pregnant ewes culminated in placental insufficiency and intrauterine growth restriction of offspring. The purpose of this study was to explore specific mechanisms by which T excess compromises placental function in early, mid, and late gestation. Pregnant Suffolk sheep received T propionate 100 mg intramuscularly or control vehicle twice weekly from gestational days (GD) 30 to 90 (term = 147 days). Placental harvest occurred at GD 65, 90, and 140. Real-time RT-PCR was used to assess transcript levels of proinflammatory (TNF, IL1B, IL6, IL8, monocyte chemoattractant protein-1/chemokine ligand 2, cluster of differentiation 68), antioxidant (glutathione reductase and superoxide dismutase 1 and 2), and angiogenic [vascular endothelial growth factor (VEGF) and hypoxia-inducible factor 1α (HIF1A)] genes. Lipid accumulation was assessed using triglyceride assays and Oil Red O staining. Placental measures of oxidative and nitrative stress included the thiobarbituric acid reactive substance assay and high-pressure liquid chromatography. Tissue fibrosis was assessed with Picrosirius Red staining. Student t tests and Cohen effect-size analyses were used for statistical analysis. At GD 65, T-treated placentomes showed increased lipid accumulation and collagen deposition. Notable findings at GD 90 were a significant increase in HIF1A expression and a large effect increase in VEGF expression. At GD 140, T-treated placentomes displayed large effect increases in expression of hypoxia and inflammatory markers. In summary, T treatment during early pregnancy induces distinct gestational age-specific effects on the placental milieu, which may underlie the previously observed phenotype of placental insufficiency.
Polycystic ovary syndrome (PCOS) is an endocrinopathy affecting up to 15% of reproductive-aged women (1, 2). Characterized by androgen excess, anovulation, polycystic ovarian morphology, and insulin resistance, PCOS has reproductive, gynecologic, and metabolic implications for women throughout the lifespan (3). Women with PCOS may experience infertility and higher rates of pregnancy complications, including hypertension, preeclampsia, gestational diabetes, and preterm labor (4, 5). Maternal PCOS is also associated with fetal and neonatal morbidity, including complications of prematurity, intrauterine growth restriction (IUGR), extremes of birth weight, and higher neonatal admission rates to the Intensive Care Unit (6, 7).
The placenta is an important mediator of pregnancy complications, birth outcomes, and developmental programming (8–10). It is intuitive, therefore, that maternal PCOS may compromise placental physiology, which may predispose to poor outcomes, such as IUGR and low birth weight. Evidence from a small set of human studies suggests that maternal PCOS may be associated with altered placental morphologic measures, such as weight, and increased frequency of placental histologic lesions, which may reflect ischemic or inflammatory insults (11, 12). However, a major limitation to human placental research is in determining the timing of onset of placental pathology, as most human placental samples are obtained at the time of delivery. Animal models, therefore, are of high translational value for placental research.
In a well-validated sheep model of PCOS, early gestational exposure to testosterone (T) treatment induces low birth weight in female offspring, in addition to the programming of metabolic and reproductive disorders in adulthood (13, 14). Prior work using this translational model of PCOS demonstrated that gestational T treatment compromises placental efficiency in sheep, suggesting that placental insufficiency is the mechanism by which IUGR occurs in this model and that this phenomenon is mediated by the androgenic actions of T (15). However, the placental pathways that may be modulated by gestational androgen excess are not currently known.
Placental mechanisms that have been linked to poor pregnancy and birth outcomes in both humans and animal models include impaired placental oxygen and nutrient transport, oxidative and nitrative stress, inflammation, lipid accumulation, and accelerated placental aging (16–19). In this study, we explored various placental mechanisms by which early gestational T treatment may alter the placental milieu and lead to placental insufficiency in pregnant sheep. Placental changes were assessed at three gestational time points (early, mid, and late gestation) to elucidate the timing of onset and progression of placental dysfunction.
Materials and Methods
Animal care and treatment
All procedures were approved by the University of Michigan Institutional Animal Care and Use Committee, consistent with the National Research Council’s Guide for the Care and Use of Laboratory Animals. Animals were housed at the University of Michigan Research Facility (Ann Arbor, MI). Detailed descriptions of breeding and gestational treatment have been previously published (15). In brief, multiparous Suffolk ewes were pair matched for weight and body condition scores, mated with pure-bred Suffolk rams, and randomly assigned to control (C) or T treatment groups (13, 20). Body weight of C and T treatment groups averaged 81.81 ± 2.48 kg and 80.31 ± 3.24 kg, respectively, and did not differ. There was no difference in body condition scores between C and T groups (3.0 ± 0.1 vs 2.9 ± 0.1, respectively). After mating, the C group received vehicle (cottonseed oil), and the T group received 100 mg T propionate in cottonseed oil (∼1.2 mg/kg; Sigma Chemical Co., St. Louis, MO) by intramuscular injections twice weekly from gestational days (GD) 30 through 90. Both groups were maintained until euthanasia at GD 65, 90, or 140 (term = GD 147).
Experimental design
Uteri and fetuses were removed at the designated pregnancy stages, and placentomes (cotyledonary-caruncular units) were dissected from the uterine wall. Individual placentomes of various morphologic types (A, B, C, and D) were cryopreserved in −80°C (15). Detailed descriptions of the four distinct placentome types have been previously published (15). For the experiments described herein, as a result of the progression of placental differentiation with advancing pregnancy (for example, type A predominates in early pregnancy) and the focus on early pathology, type A placentomes were used for placental studies at GD 65 (C n = 13, T n = 14); types A and B for GD 90 (C n = 7, T n = 7); and type B for GD 140 (C n = 3, T n = 4). For each animal, a single placentome was analyzed.
Measures of placental inflammation
Placentome histology was compared between C and T groups after hematoxylin and eosin staining of placental tissue sections to assess for inflammatory changes and differences in overall morphology. Six proinflammatory genes were selected, as a result of their relevance to obstetrical outcomes: IL1B, IL6, IL8, TNF-α, monocyte chemoattractant protein-1/chemokine ligand 2 (CCL2), and cluster of differentiation 68 (CD68). Gene expression was determined using real-time quantitative RT-PCR (QRT-PCR). RNA was isolated with Trizol reagent (Invitrogen, Carlsbad, CA), treated with DNAse, and purified using the RNAeasy kit (Qiagen, Germantown, MD), per the manufacturers’ instructions. RNA was reverse transcribed into cDNA using the SuperScript VILO cDNA synthesis kit (Invitrogen). A SYBR Green-based QRT-PCR assay was used to determine mRNA levels. Oligonucleotide primers were designed using Primer Express software (Life Technologies, Carlsbad, CA). Primer sequences are shown in Table 1. The relative amount of each transcript was calculated using the ΔΔCT method and normalized to ribosomal protein L19, a reference gene.
Table 1.
Ovine Oligonucleotide Primers
| Primer Name | Primer Length | Sequence (5′ to 3′) |
|---|---|---|
| RPL19 F | 15 | CCTTGGCTCGCCGGA |
| RPL19 R | 22 | CATGTGGCGGTCAATCTTCTTA |
| TNF F | 20 | ACACCATGAGCACCAAAAGC |
| TNF R | 20 | AGGCACAAGCAACTTCTGGA |
| IL1B F | 20 | CGAACATGTCTTCCGTGATG |
| IL1B R | 20 | GAAGCTCATGCAGAACACCA |
| CCL2 F | 21 | CCAGCAGCAAGTGTCCTAAAG |
| CCL2 R | 21 | GGCTTTGGAGTTTGGTTTTTC |
| IL6 F | 24 | ACATCGTCGACAAAATCTCTGCAA |
| IL6 R | 21 | GCCAGTGTCTCCTTGCTGTTT |
| IL8 F | 27 | CACCATGACTTCCAAGCTGGCTGTTGC |
| IL8 R | 25 | TCATGGATCTTGCTTCTCAGCTCTC |
| CD68 F | 19 | CAGGGGACAGGGAATGACT |
| CD68 R | 19 | CCAAGTGGTGGTTCTGTGG |
| GSR F | 19 | CTGGAAGAGTTGCCTCGCC |
| GSR R | 26 | TCATTATTGATGTCTTAGAACCCAGG |
| SOD1 F | 19 | ATCATGGGTTCCACGTCCA |
| SOD1 R | 22 | CATGCCTCTCTTCATCCTTTGG |
| SOD2 F | 20 | CGCTGGAGAAGGGTGATGTC |
| SOD2 R | 24 | CAGATTTGTCCAGAAGATGCTGTG |
| VEGF F | 20 | CGAAAGTCTGGAGTGTGTGC |
| VEGF R | 20 | TATGTGCTGGCTTTGGTGAG |
| HIF1A F | 23 | CGCATCTTGATAAGCTTCTGTT |
| HIF1A R | 22 | CACCAGCATCCAGAAGTTTCCT |
Abbreviations: F, forward; GSR, glutathione disulfide reductase; HIF1A , hypoxia-inducible factor 1α; R, reverse; VEGF, vascular endothelial growth factor.
Measures of placental oxidative stress, nitrative stress, and antioxidants
Thiobarbituric acid reactive substance (TBARS) assay and high-pressure liquid chromatography were used to assess placental oxidative and nitrative stress. TBARS assay was performed using a commercially available kit (TBARS assay kit, item no. 10009055; Cayman Chemical, Ann Arbor, MI), per the manufacturer’s instructions. In brief, 25 mg tissue was homogenized in 250 µL radioimmunoprecipitation assay buffer and centrifuged and the supernatant used for analysis. For TBARS, the assay range was 0.0625 to 50 µM, and the intra-assay coefficient of variation was 1.7%. In addition, with the use of 100 mg tissue, levels of three protein-bound oxidized tyrosine moieties [3-nitrotyrosine (NY), o,o′-dityrosine (DY), and 3-chlorotyrosine (ClY)] were quantified using isotope dilution liquid chromatography electrospray ionization tandem mass spectrometry (21). The detection limits for NY, ClY, and DY measures were 0.025, 0.05, and 0.05 μM, respectively. For these same measures, intra-assay coefficients of variation were 9.0%, 10.6%, and 16.0%. To assess expression levels of antioxidant genes, QRT-PCR was performed for glutathione disulfide reductase (GSR), and superoxide dismutase (SOD)1 and -2. Primer sequences are included in Table 1. The relative amount of each transcript was calculated using the ΔΔCT method and normalized to reference gene, ribosomal protein L19.
Measures of placental markers of angiogenesis
QRT-PCR was performed for two genes: vascular endothelial growth factor (VEGF) and hypoxia-inducible factor 1α (HIF1A). Gene expression was normalized to ribosomal protein L19. Primer sequences are shown in Table 1.
Measures of placental lipid accumulation
Lipid accumulation was assessed using Oil Red O staining of placental cryosections, and triglyceride content was measured using a commercially available assay kit. For Oil Red O staining, frozen placental sections (5 μm; Leica CM3050S cryostat; Leica Microsystems, Wetzlar, Germany) were fixed for 20 minutes in neutral-buffered formalin and washed with tap water (5 minutes), followed by 60% isopropanol (5 minutes). For each animal (C n = 5, T n = 4 at GD 65; C n = 4, T n = 3 at GD 90), three tissue cryosections, 50 μm apart, were stained with Oil Red O for 15 minutes (Catalog no. O-0625 FW 408.5; Sigma Chemical Co.). Each slide was imaged in five regions, corresponding to the four corners and the center of each tissue section. The percentage of positive Oil Red O staining (relative to total tissue area analyzed) was determined using Image Pro-Plus (Media Cybernetics, Rockville, MD) with a previously validated densitometrical method (22).
To assess placental triglyceride content, 100 mg tissue was homogenized in 1 mL PBS. Lipid extraction was performed using a modified Bligh and Dyer method (23). Briefly, 3.75 mL 1:2 (v/v) chloroform/methanol, 1.25 mL chloroform, and 1.25 mL distilled water were added to the homogenized tissue in a borosilicate glass tube, vortexed, and centrifuged at room temperature for 5 minutes. The bottom phase was recovered, air dried overnight, reconstituted with 200 µL isopropyl alcohol, and then assessed for triglyceride content using the L-Type TG M kit (Wako Diagnostics, Mountain View, CA). Triglyceride content was assessed using a colorimetric assay kit (Cayman Chemical), following the manufacturer’s instructions, and each sample was assayed in duplicates. The triglyceride assay range was 25 to 800 mg/mL, and the intra- and interassay coefficients of variance were 5.5% and 6.0%, respectively.
Measures of placental collagen
Placental collagen deposition was assessed using Picrosirius Red staining. Placental tissue was fixed overnight in a neutral-buffered formaldehyde solution. Paraffin blocks were sectioned (5 μm thickness), and sections were de-waxed and hydrated. For each animal (C n = 4, T n = 5 at GD 65; C n = 7, T n = 5 at GD 90), three tissue samples, sectioned 50 μm apart, were stained with Picrosirius Red for 1 hour, washed twice with acidified water, dehydrated three times with 100% ethanol, cleared with xylene, and mounted. Each slide was imaged in five regions, corresponding to the four corners and the center of each tissue section. The percent of Picrosirius Red staining (relative to the total tissue area analyzed) was determined using ImageJ software (v. 1.47; National Institutes of Health. Bethesda, MD).
Statistical methods
Outliers, defined by a cutoff of >3 SD, were identified and eliminated. Student t test was used to assess differences for all measured parameters between C and T groups. Statistical significance was determined at P < 0.05. Cohen effect-size analysis, a statistical method that can be useful in biologic studies with small sample sizes (24, 25), was also used to assess the magnitude of difference between C and T groups. A computed Cohen d value ≥ 0.8 indicates large effect-size differences.
Results
Impact of T on placental inflammation
Gestational T treatment resulted in large-magnitude increases in TNF expression at GD 90 (d = 1.3) and GD 140 (d = 1.0; Fig. 1) and large-magnitude increases in expression of IL1B (d = 2.0) and CCL2 (d = 0.8) in GD 140 placenta (Fig. 1). However, these did not achieve statistical significance. There were no differences in IL6, IL8, or CD68 mRNA levels between C and T groups at GD 65, 90, or 140, either by t test or effect-size analyses (Fig. 1). Histologic review of GD 65 and GD 90 placentomes did not reveal any inflammatory changes in T animals compared with C (Fig. 1).
Figure 1.
(A) Inflammatory cytokine mRNA expression of C vs T sheep at GD 65, 90, and 140. Data are presented as the mean ± SEM of the fold change in mRNA levels. Large-magnitude differences in C vs T groups by Cohen effect-size analysis are marked with gray circles. (B) Hematoxylin and eosin histology of C vs T sheep at GD 65 and 90. Scale bars, 200 μm.
Impact of T on placental redox status
At GD 65, gestational T treatment had no effect on placental lipid peroxidation, as measured by malondialdehyde content (TBARS assay; Fig. 2). However, T treatment was associated with a large-magnitude (d = 0.9) nonsignificant increase in NY expression at GD 65 compared with C (Fig. 2). Gestational T treatment had no effect on DY or ClY content either by effect-size analysis or t test (DY data shown in Fig. 2; ClY data not shown).
Figure 2.
(Top left) GD 65 placental lipid peroxidation of C vs T sheep, as measured by malondialdehyde content using the TBARS assay. (Middle left) GD 65 placental NY content of C vs T sheep. (Bottom left) GD 65 placental DY content of C vs T sheep. (Top, middle, and bottom right) Antioxidant mRNA expression of C vs T sheep at GD 65, GD 90, and GD 140. Data are presented as the mean ± SEM of the fold change in mRNA levels. Large-magnitude differences in C vs T groups by Cohen effect-size analysis are shown with gray circles. *P < 0.05 statistical significance.
With regard to antioxidant genes, gestational T treatment had a nonsignificant large-effect increase in levels of GSR (d = 1.4) and SOD1 (d = 1.1) mRNAs at GD 140 (Fig. 2). T treatment significantly increased SOD2 gene expression at GD 90 (P < 0.05, d=1.3) and produced a large-magnitude increase at GD 140 (d = 1.2) that did not achieve statistical significance (Fig. 2).
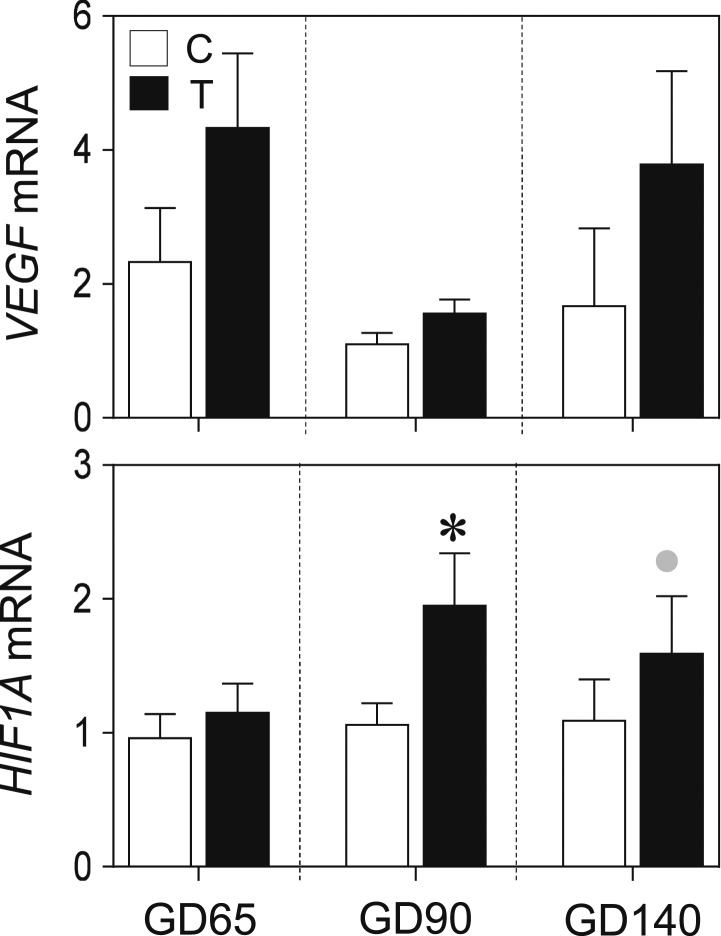
Impact of T on placental markers of angiogenesis
There was a large effect increase observed in placental expression of VEGF in the gestational T group, relative to the C group, at the two later pregnancy time points examined (GD 90 d = 1.0, GD 140 d = 0.9; Fig. 3), although these differences did not achieve statistical significance. Gestational T treatment resulted in a significant increase in HIF1A mRNA levels at GD 90 (P < 0.05, d = 1.2) and a nonsignificant large-effect increase at GD 140 (d = 0.9; Fig. 3). Histologic analysis did not reveal noticeable differences in vascular structure of C vs T placentomes at GD 65 or GD 90 (data not shown). Fixed tissue sections were not available for GD 140 samples; however, intact placentomes in T-treated animals appeared grossly more vascular than C animals at this time point (15), suggesting that obvious vascular changes may arise later in gestation.
Figure 3.
VEGF and HIF1A gene expression of C vs T sheep at GD 65, 90, and 140. Data are presented as the mean ± SEM of the fold change in mRNA levels. Large-magnitude differences in C vs T groups by Cohen effect-size analysis are shown with gray circles. *P < 0.05 statistical significance.
Impact of T on placental lipid accumulation
At GD 65, T-treated ewes demonstrated higher placental Oil Red O staining than C ewes (P < 0.05; Fig. 4). No difference in Oil Red O staining was observed between C and T animals at GD 90. With regard to triglyceride content, no differences were observed between C and T groups at GD 65 or GD 90 (Fig. 4).
Figure 4.
Lipid content of C vs T sheep. (Left) Images of Oil Red O staining of C vs T sheep at GD 65 and 90. (Top right) Percentage of Oil Red O stain vs total area, mean ± SEM. (Bottom right) Placental triglyceride content of C vs T sheep. *P < 0.05 statistical significance. Scale bars, 25 μm.
Impact of T on placental collagen deposition
At both GD 65 and GD 90, T treatment induced a statistically significant large-magnitude increase in placental collagen content compared with the C group (P < 0.05; d = 1.0 and d = 2.2, respectively; Fig. 5).
Figure 5.
Placental collagen content in C vs T sheep. (Left) Images of Picrosirius Red (PSR) staining of C vs T sheep at GD 65 and 90. (Right) Percentage of Picrosirius Red stain vs total area, mean ± SEM. *P < 0.05 statistical significance. Scale bars, 100 μm.
Sex-specific impact of T on GD 65 placenta
Interestingly, gestational T treatment revealed several sex-specific effects on mRNA expression of proinflammatory, antioxidant, and angiogenic genes at GD 65, with some achieving statistical significance (Fig. 6). For instance, in pregnancies with only female offspring, gestational T treatment resulted in a large-magnitude increase in placental VEGF and HIF1A mRNA expression, with only HIF1A achieving statistical significance (VEGF: P = 0.07, d = 1.4; HIF1A: P = 0.01, d = 2.3). A large-magnitude increase (d = 1.1) in antioxidant enzyme SOD1 was also evident in pregnancies with female but not male offspring (Fig. 6). Additionally, whereas there was a trend toward decreased IL1B and IL8 expression in pregnancies with male offspring (d = 0.8 and d = 1.1, respectively), there was a trend toward decreased IL6 in pregnancies with female offspring (d = 1.0; Fig. 6). CCL2 demonstrated particularly notable sex-specific findings, as gestational T had opposing effects depending on whether the pregnancies were with male or female offspring. Specifically, CCL2 expression was significantly increased in pregnancies with female offspring (P = 0.001, d = 1.9) and significantly decreased in pregnancies with male offspring (P = 0.02, d = 1.4; Fig. 6).
Figure 6.
Sex-specific differences in placental mRNA expression of inflammatory, antioxidant, and angiogenic genes at GD 65. Large-magnitude differences in C vs T groups by Cohen effect-size analysis are shown with gray circles. *P < 0.05 statistical significance. F, female; M, male.
Discussion
Findings from this study demonstrate that gestational T treatment in sheep induces distinct, stage-specific changes in the placental milieu. The translational relevance of these observations is detailed below.
In this sheep model of PCOS, T treatment, starting at GD 30, induced a variety of placental changes, as early as GD 65. The significantly altered parameters include lipotoxicity and collagen deposition, suggesting a sequence of events in which androgen-induced lipid accumulation can lead to tissue damage and fibrosis. Placental lipotoxicity, defined as lipid accumulation in nonadipose tissue (26), has been linked to maternal obesity, insulin resistance, and gestational diabetes in humans (18, 27, 28). To our knowledge, the specific link between T excess and placental lipotoxicity has not been explored, and the specific mechanisms by which T excess leads to lipid accumulation in placenta are presently unknown. In swine, placentas from obese sows demonstrated increased mRNA expression of lipogenic genes and higher lipid content in addition to decreased antioxidant capacity and greater inflammatory gene expression (29). In our study, none of the ewes were obese, and there was no difference in body composition scores between C and T groups. However, gestational T-treated sheep did demonstrate insulin resistance (30) and decreased medium chain acylcarnitines (31), which may partially underlie the placental lipotoxic changes that were observed in our model (32).
Normal pregnancy is characterized by physiologic oxidative and nitrative stress, as the metabolic activity of the developing fetus and placenta may lead to increased reactive oxidative and nitrative species (33, 34). Placental nitrative stress, a pathologic imbalance that favors reactive nitrative species over antioxidants, has been observed in gestational diabetic and pre-eclamptic pregnancies (35, 36) and is also linked to preterm labor and IUGR (37). In this study, we found that T treatment did not significantly alter lipid peroxidation or markers of nitrative stress in early pregnancy. However, we observed significantly increased placental mRNA expression of antioxidant gene SOD2 in T-treated sheep, particularly at midgestation, which may represent a compensatory effort to overcome early oxidative or nitrative insults.
Collagen deposition is a normal physiologic response to tissue injury. In pathologic states, however, deposition of excess collagen can result in fibrosis and loss of tissue function (38). Fibrotic lesions within the placenta have been linked to preeclampsia (39, 40). The present study demonstrating increase in collagen deposition due to androgen excess offers a potential mechanism for development of such fibrotic lesions in conditions of placental deficiency such as preeclampsia. As a result of the lack of tissue-sample availability, Picrosirius Red staining was not performed on day 140 placentomes. However, the statistically significant differences in collagen content observed at GD 65 and GD 90 suggest that gestational T induces placental tissue injury within a relatively short frame after onset of treatment.
Even after cessation of T treatment, distinct changes were noted in GD 90 and GD 140 placentas. Notably, T treatment significantly increased placental expression of HIF1A at midgestation. In the obstetric literature, HIF1A has been used as a marker for placental hypoxia, particularly when associated with preeclampsia (41, 42). Interestingly, T treatment was also associated with a trend toward increased VEGF expression; however, the fact that T-treated sheep manifested a phenotype of placental insufficiency suggests that VEGF expression was unable to compensate for early T-induced hypoxic insults. Meanwhile, vascular growth within the placenta is regulated by a complex balance of pro- and antiangiogenic factors, and impaired placental angiogenic mechanisms have well-established associations with pregnancy loss, preeclampsia, and fetal growth restriction (43, 44). VEGF is a protein secreted in response to tissue hypoxia and endothelial damage, and T treatment has been shown to significantly increase placental VEGF expression in other studies (45). In the literature, androgens have been implicated in placental vasculopathies, possibly by increasing the vascular tone of the uterine spiral arteries (46, 47).
Additionally, although inflammatory cytokine expression was generally unchanged at GD 65 with T treatment, there was a trend toward increased TNF, IL1B, and CCL2 expression at GD 140. Although these findings did not achieve statistical significance, these large-magnitude differences may result from the accumulation of the early T-induced insults previously mentioned. Similar to oxidative and nitrative stress, a physiologic increase in systemic maternal inflammation is observed in normal pregnancy (48, 49). Within the placenta is a complex network of inflammatory cytokines and adipokines, which are important regulators of maternal-fetal metabolism, fetal development, and onset of labor (49). However, aberrant inflammation within the placenta has been linked to adverse outcomes, including preeclampsia and spontaneous preterm birth (50–52).
In summary, in this sheep model of PCOS, T treatment, starting at GD 30, is associated with a variety of changes in the placental milieu throughout gestation, highlighting the plasticity of the placental environment to external insults. Notably, within 60 days after initiation of T treatment (GD 90), T-treated placentomes are characterized by increased lipid accumulation, an increase in tissue collagen content, and an increase in markers of hypoxia. Toward late gestation (near term), T-treated placentomes are characterized by a trend toward increased HIF1A and inflammatory cytokine expression. These gestational age-specific findings, in addition to proposed mechanistic associations among them, are illustrated in Fig. 7. In the context of previously published work from our laboratory, which demonstrated that T treatment in this sheep model culminates in placental insufficiency as a mechanism for fetal growth restriction (15), these findings suggest that a variety of placental pathways may be involved.
Figure 7.
Schematic showing proposed gestational T-induced changes in sheep placentomes. We propose that gestational T treatment during early pregnancy may induce lipotoxic changes that may drive a vicious cycle by increasing oxidative stress, hypoxia, fibrosis, and inflammation, which may advance placental differentiation and induce placental insufficiency. Findings that achieved statistical significance are shown in dark blue circles. Findings that demonstrated large-magnitude changes by Cohen effect-size analysis but failed to achieve statistical significance are shown in light blue circles. RNS, reactive nitrogen species.
Importantly, several interesting observations were noted with regard to fetal sex. As a result of sample-size limitations, male and female gestations were grouped together for all aforementioned analyses at all three time points. However, sample availability for QRT-PCR experiments was adequate to perform preliminary subgroup analyses by fetal sex (female C vs T, male C vs T) at GD 65. Sex differences were particularly notable for CCL2, as T treatment significantly increased mRNA expression in pregnancies with female offspring but decreased mRNA expression in pregnancies with male offspring (P < 0.05). Meanwhile, regarding HIF1A, T treatment significantly increased HIF1A expression in pregnancies with female offspring (P < 0.05) but had no effect on HIF1A expression in pregnancies with males. These findings align with emerging evidence that the sex of the fetus impacts placental growth, development, and placental adaptation to insults (53, 54) and that a better understanding of these sex-specific mechanisms is needed to optimize maternal and fetal well-being.
One of the major benefits of animal models is that early changes in the placental milieu can be assessed. In humans, early placental mechanisms are difficult to investigate for both technical and ethical reasons. In experimental animal cohorts, assessment of placental phenotype at various time points can help identify the gestational age at which placental dysfunction is apparent. This, in turn, can help develop appropriately timed preventive strategies to optimize pregnancy outcomes. The findings from this study suggest that early exposure to T excess can negatively impact placental development as early as midgestation and that the development of placental insufficiency progresses even after T treatment is discontinued. PCOS is the most common endocrinopathy of reproductive-aged women, and although not all women with PCOS have androgen excess, findings from this sheep model may have translational implications for women with other hyperandrogenic conditions, including obesity, congenital adrenal hyperplasia, preeclampsia, luteoma of pregnancy, adrenal or ovarian neoplasm, and exposure to endocrine-disrupting chemicals (55).
One important distinction between human and sheep placentation is that humans demonstrate “hemochorial” placentation, wherein extravillous trophoblasts directly interface and invade maternal vasculature. In contrast, sheep exhibit epitheliochorial placentation, where chorionic villi interface with the uterine epithelium in discrete units called placentomes (56, 57). Nevertheless, the villous architecture of the placenta with stem, intermediate, and terminal blood vessels is similar between humans and sheep (58, 59) and allows sheep to be a useful model for placental research. Furthermore, sheep are a precocial species like humans, with a similar developmental timeline that offers additional translational value (60, 61).
Strengths of our study include the use of a well-validated animal model of PCOS and the ability to assess placental changes at varying gestational time points. Limitations include a small sample size, particularly near term, which may have limited the achievement of statistical significance in spite of large effect-size differences. Additionally, the complex structure of the placentome, with an increasing fetal contribution to placental mass as gestation advances, may also have provided a technical limitation to our experimental analyses.
Conclusions
In a sheep model of PCOS, T treatment in early pregnancy induces changes in the placental milieu as early as GD 65. Possible mechanisms for T-induced placental insufficiency warrant further investigation but may include T-induced placental lipotoxicity, nitrative stress, collagen deposition, hypoxia, and inflammation. These findings may have important translational value for pregnant women with PCOS and their offspring.
Acknowledgments
We acknowledge Douglas Doop and Gary McCalla for animal care and Dr. Almudena Veiga-Lopez, Dr. Bachir Abi Salloum, Evan Beckett, Carol Herkimer, and students in the University of Michigan Undergraduate Research Opportunity Program for assistance with administration of treatments and tissue collection.
Financial Support: This work was supported by the National Institutes of Health Grant P01 HD44232 (to V.P.).
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
Glossary
Abbreviations:
- C
control
- CCL2
monocyte chemoattractant protein-1/chemokine ligand 2
- CD68
cluster of differentiation 68
- ClY
3-chlorotyrosine
- DY
o,o′-dityrosine
- GD
gestational day
- GSR
glutathione disulfide reductase
- HIF1A
hypoxia-inducible factor 1α
- IUGR
intrauterine growth restriction
- NY
3-nitrotyrosine
- PCOS
polycystic ovary syndrome
- QRT-PCR
quantitative RT-PCR
- SOD
superoxide dismutase
- T
testosterone
- TBARS
thiobarbituric acid reactive substance
- VEGF
vascular endothelial growth factor
References and Notes
- 1. Fauser BCJM, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, Carmina E, Chang J, Yildiz BO, Laven JS, Boivin J, Petraglia F, Wijeyeratne CN, Norman RJ, Dunaif A, Franks S, Wild RA, Dumesic D, Barnhart K. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97(1):28–38.e25. [DOI] [PubMed] [Google Scholar]
- 2. Azziz R. Polycystic ovary syndrome. Obstet Gynecol. 2018;132(2):321–336. [DOI] [PubMed] [Google Scholar]
- 3. Goodman NF, Cobin RH, Futterweit W, Glueck JS, Legro RS, Carmina E, American Association of Clinical Endocrinologists (AACE)American College of Endocrinology (ACE)Androgen Excess and PCOS Society. American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society Disease State Clinical Review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—part 2. Endocr Pract. 2015;21(12):1415–1426. [DOI] [PubMed] [Google Scholar]
- 4. de Wilde MA, Lamain-de Ruiter M, Veltman-Verhulst SM, Kwee A, Laven JS, Lambalk CB, Eijkemans MJC, Franx A, Fauser BCJM, Koster MPH. Increased rates of complications in singleton pregnancies of women previously diagnosed with polycystic ovary syndrome predominantly in the hyperandrogenic phenotype. Fertil Steril. 2017;108(2):333–340. [DOI] [PubMed] [Google Scholar]
- 5. Kjerulff LE, Sanchez-Ramos L, Duffy D. Pregnancy outcomes in women with polycystic ovary syndrome: a metaanalysis. Am J Obstet Gynecol. 2011;204(6):558.e1–558.e6. [DOI] [PubMed] [Google Scholar]
- 6. Boomsma CM, Eijkemans MJC, Hughes EG, Visser GHA, Fauser BCJM, Macklon NS. A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum Reprod Update. 2006;12(6):673–683. [DOI] [PubMed] [Google Scholar]
- 7. Qin JZ, Pang LH, Li MJ, Fan XJ, Huang RD, Chen HY. Obstetric complications in women with polycystic ovary syndrome: a systematic review and meta-analysis. Reprod Biol Endocrinol. 2013;11(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Godfrey KM. The role of the placenta in fetal programming—a review. Placenta. 2002;23(Suppl A):S20–S27. [DOI] [PubMed] [Google Scholar]
- 9. Brosens I, Pijnenborg R, Vercruysse L, Romero R. The “great obstetrical syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol. 2011;204(3):193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burton GJ, Fowden AL, Thornburg KL. Placental origins of chronic disease. Physiol Rev. 2016;96(4):1509–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Palomba S, Russo T, Falbo A, Di Cello A, Tolino A, Tucci L, La Sala GB, Zullo F. Macroscopic and microscopic findings of the placenta in women with polycystic ovary syndrome. Hum Reprod. 2013;28(10):2838–2847. [DOI] [PubMed] [Google Scholar]
- 12. Koster MPH, de Wilde MA, Veltman-Verhulst SM, Houben ML, Nikkels PG, van Rijn BB, Fauser BC. Placental characteristics in women with polycystic ovary syndrome. Hum Reprod. 2015;30(12):2829–2837. [DOI] [PubMed] [Google Scholar]
- 13. Manikkam M, Crespi EJ, Doop DD, Herkimer C, Lee JS, Yu S, Brown MB, Foster DL, Padmanabhan V. Fetal programming: prenatal testosterone excess leads to fetal growth retardation and postnatal catch-up growth in sheep. Endocrinology. 2004;145(2):790–798. [DOI] [PubMed] [Google Scholar]
- 14. Veiga-Lopez A, Steckler TL, Abbott DH, Welch KB, MohanKumar PS, Phillips DJ, Refsal K, Padmanabhan V. Developmental programming: impact of excess prenatal testosterone on intrauterine fetal endocrine milieu and growth in sheep. Biol Reprod. 2011;84(1):87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Beckett EM, Astapova O, Steckler TL, Veiga-Lopez A, Padmanabhan V. Developmental programing: impact of testosterone on placental differentiation. Reproduction. 2014;148(2):199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Burton GJ, Yung H-W, Cindrova-Davies T, Charnock-Jones DS. Placental endoplasmic reticulum stress and oxidative stress in the pathophysiology of unexplained intrauterine growth restriction and early onset preeclampsia. Placenta. 2009;30(Suppl A):S43–S48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim CJ, Romero R, Chaemsaithong P, Kim J-S. Chronic inflammation of the placenta: definition, classification, pathogenesis, and clinical significance. Am J Obstet Gynecol. 2015;213(4 Suppl):S53–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Saben J, Lindsey F, Zhong Y, Thakali K, Badger TM, Andres A, Gomez-Acevedo H, Shankar K. Maternal obesity is associated with a lipotoxic placental environment. Placenta. 2014;35(3):171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sultana Z, Maiti K, Dedman L, Smith R. Is there a role for placental senescence in the genesis of obstetric complications and fetal growth restriction? Am J Obstet Gynecol. 2018;218(2S):S762–S773. [DOI] [PubMed] [Google Scholar]
- 20. Steckler T, Manikkam M, Inskeep EK, Padmanabhan V. Developmental programming: follicular persistence in prenatal testosterone-treated sheep is not programmed by androgenic actions of testosterone. Endocrinology. 2007;148(7):3532–3540. [DOI] [PubMed] [Google Scholar]
- 21. Vivekanandan-Giri A, Byun J, Pennathur S. Quantitative analysis of amino acid oxidation markers by tandem mass spectrometry. Methods Enzymol. 2011;491:73–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lejeune M, Jaén J, Pons L, López C, Salvadó MT, Bosch R, García M, Escrivà P, Baucells J, Cugat X, Alvaro T. Quantification of diverse subcellular immunohistochemical markers with clinicobiological relevancies: validation of a new computer-assisted image analysis procedure. J Anat. 2008;212(6):868–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37(8):911–917. [DOI] [PubMed] [Google Scholar]
- 24. Cohen J. A power primer. Psychol Bull. 1992;112(1):155–159. [DOI] [PubMed] [Google Scholar]
- 25. Nakagawa S, Cuthill IC. Effect size, confidence interval and statistical significance: a practical guide for biologists [published correction appears in Biol Rev Camb Philos Soc. 2009;84(3):515]. Biol Rev Camb Philos Soc. 2007;82(4):591–605. [DOI] [PubMed] [Google Scholar]
- 26. Engin AB. What is lipotoxicity? Adv Exp Med Biol. 2017;960:197–220. [DOI] [PubMed] [Google Scholar]
- 27. Diamant YZ, Metzger BE, Freinkel N, Shafrir E. Placental lipid and glycogen content in human and experimental diabetes mellitus. Am J Obstet Gynecol. 1982;144(1):5–11. [DOI] [PubMed] [Google Scholar]
- 28. Visiedo F, Bugatto F, Sánchez V, Cózar-Castellano I, Bartha JL, Perdomo G. High glucose levels reduce fatty acid oxidation and increase triglyceride accumulation in human placenta. Am J Physiol Endocrinol Metab. 2013;305(2):E205–E212. [DOI] [PubMed] [Google Scholar]
- 29. Liang T, Jinglong X, Shusheng D, Aiyou W. Maternal obesity stimulates lipotoxicity and up-regulates inflammatory signaling pathways in the full-term swine placenta. Anim Sci J. 2018;89(9):1310–1322. [DOI] [PubMed] [Google Scholar]
- 30. Puttabyatappa M, Padmanabhan V. Prenatal testosterone programming of insulin resistance in the female sheep. Adv Exp Med Biol. 2017;1043:575–596. [DOI] [PubMed] [Google Scholar]
- 31. Abi Salloum B, Veiga-Lopez A, Abbott DH, Burant CF, Padmanabhan V. Developmental programming: exposure to testosterone excess disrupts steroidal and metabolic environment in pregnant sheep. Endocrinology. 2015;156(6):2323–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Trouwborst I, Bowser SM, Goossens GH, Blaak EE. Ectopic fat accumulation in distinct insulin resistant phenotypes; targets for personalized nutritional interventions. Front Nutr. 2018;5:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wu F, Tian F-J, Lin Y, Xu W-M. Oxidative stress: placenta function and dysfunction. Am J Reprod Immunol. 2016;76(4):258–271. [DOI] [PubMed] [Google Scholar]
- 34. Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 2005;3(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Taysi S, Tascan AS, Ugur MG, Demir M. Radicals, oxidative/nitrosative stress and preeclampsia. Mini Rev Med Chem. 2019;19(3):178–193. [DOI] [PubMed] [Google Scholar]
- 36. Horváth EM, Magenheim R, Kugler E, Vácz G, Szigethy A, Lévárdi F, Kollai M, Szabo C, Lacza Z. Nitrative stress and poly(ADP-ribose) polymerase activation in healthy and gestational diabetic pregnancies. Diabetologia. 2009;52(9):1935–1943. [DOI] [PubMed] [Google Scholar]
- 37. Pereira AC, Martel F. Oxidative stress in pregnancy and fertility pathologies. Cell Biol Toxicol. 2014;30(5):301–312. [DOI] [PubMed] [Google Scholar]
- 38. Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18(7):1028–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fedorova OV, Ishkaraeva VV, Grigorova YN, Reznik VA, Kolodkin NI, Zazerskaya IE, Zernetkina V, Agalakova NI, Tapilskaya NI, Adair CD, Lakatta EG, Bagrov AY. Antibody to marinobufagenin reverses placenta-induced fibrosis of umbilical arteries in preeclampsia. Int J Mol Sci. 2018;19(8):E2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ohmaru-Nakanishi T, Asanoma K, Fujikawa M, Fujita Y, Yagi H, Onoyama I, Hidaka N, Sonoda K, Kato K. Fibrosis in preeclamptic placentas is associated with stromal fibroblasts activated by the transforming growth factor-β1 signaling pathway. Am J Pathol. 2018;188(3):683–695. [DOI] [PubMed] [Google Scholar]
- 41. Kimura C, Watanabe K, Iwasaki A, Mori T, Matsushita H, Shinohara K, Wakatsuki A. The severity of hypoxic changes and oxidative DNA damage in the placenta of early-onset preeclamptic women and fetal growth restriction. J Matern Fetal Neonatal Med. 2013;26(5):491–496. [DOI] [PubMed] [Google Scholar]
- 42. Verma S, Pillay P, Naicker T, Moodley J, Mackraj I. Placental hypoxia inducible factor -1α & CHOP immuno-histochemical expression relative to maternal circulatory syncytiotrophoblast micro-vesicles in preeclamptic and normotensive pregnancies. Eur J Obstet Gynecol Reprod Biol. 2018;220:18–24. [DOI] [PubMed] [Google Scholar]
- 43. Alfaidy N, Hoffmann P, Boufettal H, Samouh N, Aboussaouira T, Benharouga M, Feige JJ, Brouillet S. The multiple roles of EG-VEGF/PROK1 in normal and pathological placental angiogenesis. BioMed Res Int. 2014;2014:451906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Soto E, Romero R, Kusanovic JP, Ogge G, Hussein Y, Yeo L, Hassan SS, Kim CJ, Chaiworapongsa T. Late-onset preeclampsia is associated with an imbalance of angiogenic and anti-angiogenic factors in patients with and without placental lesions consistent with maternal underperfusion. J Matern Fetal Neonatal Med. 2012;25(5):498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cleys ER, Halleran JL, Enriquez VA, da Silveira JC, West RC, Winger QA, Anthony RV, Bruemmer JE, Clay CM, Bouma GJ. Androgen receptor and histone lysine demethylases in ovine placenta. PLoS One. 2015;10(2):e0117472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Maliqueo M, Echiburú B, Crisosto N. Sex steroids modulate uterine-placental vasculature: implications for obstetrics and neonatal outcomes. Front Physiol. 2016;7:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kumar S, Gordon GH, Abbott DH, Mishra JS. Androgens in maternal vascular and placental function: implications for preeclampsia pathogenesis. Reproduction. 2018;156(5):R155–R167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bowen JM, Chamley L, Keelan JA, Mitchell MD. Cytokines of the placenta and extra-placental membranes: roles and regulation during human pregnancy and parturition. Placenta. 2002;23(4):257–273. [DOI] [PubMed] [Google Scholar]
- 49. Hauguel-de Mouzon S, Guerre-Millo M. The placenta cytokine network and inflammatory signals. Placenta. 2006;27(8):794–798. [DOI] [PubMed] [Google Scholar]
- 50. Wei S-Q, Fraser W, Luo Z-C. Inflammatory cytokines and spontaneous preterm birth in asymptomatic women: a systematic review. Obstet Gynecol. 2010;116(2 Pt 1):393–401. [DOI] [PubMed] [Google Scholar]
- 51. Boyle AK, Rinaldi SF, Norman JE, Stock SJ. Preterm birth: inflammation, fetal injury and treatment strategies. J Reprod Immunol. 2017;119:62–66. [DOI] [PubMed] [Google Scholar]
- 52. Lopez-Jaramillo P, Barajas J, Rueda-Quijano SM, Lopez-Lopez C, Felix C. Obesity and preeclampsia: common pathophysiological mechanisms. Front Physiol. 2018;9:1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Clifton VL. Review: Sex and the human placenta: mediating differential strategies of fetal growth and survival. Placenta. 2010;31(Suppl):S33–S39. [DOI] [PubMed] [Google Scholar]
- 54. Kalisch-Smith JI, Simmons DG, Dickinson H, Moritz KM. Review: Sexual dimorphism in the formation, function and adaptation of the placenta. Placenta. 2017;54:10–16. [DOI] [PubMed] [Google Scholar]
- 55. Hakim C, Padmanabhan V, Vyas AK. Gestational hyperandrogenism in developmental programming. Endocrinology. 2017;158(2):199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Carter AM, Enders AC. Comparative aspects of trophoblast development and placentation. Reprod Biol Endocrinol. 2004;2(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Furukawa S, Kuroda Y, Sugiyama A. A comparison of the histological structure of the placenta in experimental animals. J Toxicol Pathol. 2014;27(1):11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Leiser R, Krebs C, Ebert B, Dantzer V. Placental vascular corrosion cast studies: a comparison between ruminants and humans. Microsc Res Tech. 1997;38(1-2):76–87. [DOI] [PubMed] [Google Scholar]
- 59. Kaufmann P, Mayhew TM, Charnock-Jones DS. Aspects of human fetoplacental vasculogenesis and angiogenesis. II. Changes during normal pregnancy. Placenta. 2004;25(2-3):114–126. [DOI] [PubMed] [Google Scholar]
- 60. Padmanabhan V, Veiga-Lopez A. Sheep models of polycystic ovary syndrome phenotype. Mol Cell Endocrinol. 2013;373(1-2):8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Carter AM. Animal models of human placentation—a review. Placenta. 2007;28(Suppl A):S41–S47. [DOI] [PubMed] [Google Scholar]