Abstract
Human prostate stem and progenitor cells express estrogen receptor (ER)α and ERβ and exhibit proliferative responses to estrogens. In this study, membrane-initiated estrogen signaling was interrogated in human prostate stem/progenitor cells enriched from primary epithelial cultures and stem-like cell lines from benign and cancerous prostates. Subcellular fractionation and proximity ligation assays localized ERα and ERβ to the cell membrane with caveolin-1 interactions. Exposure to 17β-estradiol (E2) for 15 to 60 minutes led to sequential phosphorylation of signaling molecules in MAPK and AKT pathways, IGF1 receptor, epidermal growth factor receptor, and ERα, thus documenting an intact membrane signalosome that activates diverse downstream cascades. Treatment with an E2–dendrimer conjugate or ICI 182,870 validated E2-mediated actions through membrane ERs. Overexpression and knockdown of ERα or ERβ in stem/progenitor cells identified pathway selectivity; ERα preferentially activated AKT, whereas ERβ selectively activated MAPK cascades. Furthermore, prostate cancer stem-like cells expressed only ERβ, and brief E2 exposure activated MAPK but not AKT cascades. A gene subset selectively regulated by nongenomic E2 signaling was identified in normal prostate progenitor cells that includes BGN, FOSB, FOXQ1, and MAF. Membrane-initiated E2 signaling rapidly modified histone methyltransferases, with MLL1 cleavage observed downstream of phosphorylated AKT and EZH2 phosphorylation downstream of MAPK signaling, which may jointly modify histones to permit rapid gene transcription. Taken together, the present findings document ERα and ERβ membrane-initiated signaling in normal and cancerous human prostate stem/progenitor cells with differential engagement of downstream effectors. These signaling pathways influence normal prostate stem/progenitor cell homeostasis and provide novel therapeutic sites to target the elusive prostate cancer stem cell population.
Prostate cancer is the leading cause of noncutaneous cancer-related mortality in men in the United States and many countries worldwide. Despite being studied for decades, prostate cancer still poses elusive questions, including the origin of cancer stem cells and their role in hormonal carcinogenesis. Various hypotheses have been proposed regarding the cellular differentiation process in the origin of cancer stem cells in a variety of tissues. Within the prostate, transforming mutations with dedifferentiation of the basal or luminal cells are suggested to result in the generation of prostate cancer stem cells (1). Other hypotheses propose that any cell within the stem cell hierarchy is capable of accumulating mutations and transforming into a cancer stem cell (2). Identifying the hierarchy, biology, and regulation of normal stem and progenitor cells may be a critical step toward understanding how prostate cancer stem cells arise and are regulated.
The prostate gland is a ductal system made up of epithelial, stromal, and endothelial components with the epithelium comprised of luminal, basal, and a very rare fraction of neuroendocrine cell types. The origin of these epithelial cells in the human prostate has been traced to a common precursor stem cell using lineage tracing techniques involving the study of mitochondrial mutations (3–5). More recently, our laboratory has defined and characterized human prostate stem and progenitor cells using long-term label retention in prostaspheres cultured from disease-free primary cells (6), complementing earlier studies on prostate stem cell characterization (7–9).
Hormonal control of prostate cancer has largely been focused on androgen-mediated actions. However, accumulating evidence has shed light on the role of estrogens in prostate carcinogenesis and progression. Estrogen action in the prostate gland has been shown to be mediated via estrogen receptors (ERs) within differentiated basal, luminal, and stromal cell populations (10, 11). Although past studies provide excellent insights into ER signaling within these prostate cells, the signaling mechanisms at play within prostate stem and progenitor cells are yet to be uncovered.
Recently, our laboratory discovered that normal human prostate stem and progenitor cells, albeit androgen receptor negative and resistant to androgen exposures, express ERα and ERβ (12) that transduce signals when exposed to 17β-estradiol (E2) or endocrine-disrupting agents that mimic estrogens (13). Using a three-dimensional culture system that permits survival of primary prostate epithelial stem cells that form spheroids of proliferating daughter progenitor cells (6), we found that exposure to E2 resulted in significant increases in prostasphere numbers and size, implicating estrogenic roles in stem cell self-renewal and progenitor cell proliferation. Furthermore, brief estrogenic exposures altered the spheroid transcriptome, epigenetically reprogramed the prostate stem and progenitor cells, and, when grafted in vivo to form prostate-like structures, predisposed them to estrogen-driven carcinogenesis (14, 15). Similar evidence indicates a role for steroids such as E2 and progesterone in the control of normal mammary stem cell function (16, 17) and implicates stem cells as key targets during hormonal carcinogenesis. It is therefore imperative to develop a thorough understanding of the signaling mechanisms governed by estrogen in stem cell homeostasis and disease.
The nature of E2-mediated signaling within differentiated cells and stem cells in various tissues has typically been studied in the context of ligand-dependent nuclear genomic signaling. However, expanding evidence suggests that membrane-initiated, nongenomic rapid signaling occurs in various cell types upon exposure to steroids mediated through membrane-localized steroid receptors (18, 19). Following exposure to E2, dimerization of ERs occurs at the membrane that generates ultra-rapid signals (e.g., cAMP, cGMP, Ca2+) and/or initiates activation of kinase cascades that, in turn, may influence functions of nuclear transcription factors (20). A few examples include the MCF7 breast cell line in which ER and progesterone receptor crosstalk in a rapid nongenomic manner and activate pathways that impinge on the regulation of progesterone receptor gene expression (21). Likewise, in the PC3 prostate cancer cell line, activation of the proto-oncogene SRC occurs within 20 minutes of exposure to the androgenic steroid R1881 (22).
In this context, our initial studies on E2 action in prostate stem and progenitor cells identified nuclear genomic signaling as well as rapid activation of membrane-initiated nongenomic pathways via AKT and ERK1/2 (13). These nongenomic pathways, however, are complex and their full characterization remains to be determined. As the prostate stem and progenitor cells differentially express ERα and ERβ proteins (23), it is important to uncover whether they are both localized to the membrane and whether they cross-talk at that location or activate separate signaling cascades. Additionally, it is critical to elucidate how these nongenomic pathways impinge on gene expression modulation and cellular function within the prostate stem and progenitor populations. Of further interest, recent studies in the rodent prostate and MCF7 cells showed that activation of the nongenomic pathways by E2 have a downstream effect on histone methyltransferase (HMT) MLL1 cleavage and its subsequent activation (24). This HMT, which lays down activating H3K4me3 marks, represents an important example of how rapid nongenomic signaling pathways may be critical in modulating epigenetic marks and gene expression. It is presently unknown whether these pathways are operational in prostate progenitor cell populations.
The aims of the present studies were to molecularly characterize membrane-initiated signaling via ERs within prostate stem and progenitor cells, to decipher the functional differences between ERα and ERβ activities initiated at the membrane, and to identify the downstream actions of specific ER signaling pathways in regulating prostate stem and progenitor cell gene expression and homeostasis. We examine this in the context of normal prostate stem and progenitor cell types as well as in prostate cancer stem-like cells to identify pathways that may be used in future studies to control prostate cancer stem cell repopulation of tumors.
Materials and Methods
Cells and sphere culture
Primary prostate epithelial cells (PrEC) from disease-free organ donors were cultured in a three-dimensional Matrigel culture as described previously (12, 13). Briefly, two-dimensional cultured cells were resuspended in 1:1 Matrigel (Corning, Corning, NY)/ProstaLife culture medium (Lifeline Cell Technology, Frederick, MD), plated in 12-well clear-bottom tissue culture plates (Falcon, Corning, NY) covered with 1 mL of ProstaLife culture medium and cultured at 37°C and 5% CO2. Resulting prostaspheres grown for 5 to 7 days were exposed to 10 nM E2 (Sigma-Aldrich, St. Louis, MO) in the presence or absence of inhibitors described below. Prostasphere number and size were counted using automated image-processing software (13). Human prostate cancer stem-like cells (HuSLCs), spontaneously immortalized from primary cells of a Gleason grade 9 tumor in Dr. Susan Kasper’s laboratory, and early-passage cultures were used (25). HuSLCs express high levels of SOX2, OCT4, and NANOG, are negative for p63 and cytokeratins 8/18, and fully reestablish Gleason grade 9 tumors when grafted with urogenital sinus mesenchyme in SCID mice. WPE-stem cells (American Type Culture Collection, Manassas, VA), a human papillomavirus–immortalized stem-like cell line derived from RWPE benign prostate cells, were used to overexpress ERα or ERβ. Stem and progenitor cells from the DU145 prostate cancer cell line (American Type Culture Collection) were grown using three-dimensional culture conditions as described for PrEC prostaspheres and cultured for 3 weeks before harvesting. To interrogate nongenomic E2 actions, cells and spheres were subjected to various reagents, including 10 µM ICI 182,870 (ICI), an ERα/ERβ antagonist (Santa Cruz Biotechnology, Dallas, TX); 10 µM LY294002, a phosphatidylinositol 3-kinase (PI3K) inhibitor (Selleckchem, Houston, TX); 1 µM U0126, a MEK inhibitor (Selleckchem); and an E2 dendrimer (26) at a molar equivalent of 10 nM E2.
Flow cytometry
Human prostaspheres were grown for 7 days and dispersed into single cells using dispase (Stem Cell Technologies, Cambridge, MA) and collagenase (Stem Cell Technologies) treatment as described (13). The single cells were stained with anti-human/mouse CD49f (α6 integrin) (27) and anti-human Trop2 (EGP-1) Alexa Fluor 488 (28) (eBioscience, San Diego, CA) antibodies. Cells were permeabilized overnight using a FoxP3 kit (eBioscience) as per the manufacturer’s instructions. Following permeabilization, cells were incubated with either ERα (29), ERβ (30), or IgG (31, 32) antibodies. Detection was by secondary antibody-linked fluorescent probes: goat anti-rabbit (H+L) Alexa Fluor 488 (33), goat anti-rabbit (H+L) Alexa Fluor 586 (34), goat anti- mouse IgG (H+L) Alexa Fluor 488 (35), or goat anti-mouse IgG (H+L) Alexa Fluor 586 (36). Cells were counted using the Beckman Coulter CyAn™ ADP analyzer and analyzed using Summit Software v4.3 (Beckman Coulter, Indianapolis, IN). Live cells were gated by propidium iodide staining. Mouse IgG2a κ isotype control Alexa Fluor 488 (31) and rat IgG2a κ isotype control–allophycocyanin (32) (eBioscience) antibodies were used as negative controls. Triple-positive stem-like cells exhibiting CD49fhigh/Trop2high/ERα+ or ERβ+ were gated and quantified using the polygon tool as described (13) with the addition of a third gate for ERα+ or ERβ+ stem-like cells.
Subcellular protein isolation
A stepwise preparation of different cell compartments (cytoplasmic, membrane, soluble nuclear, chromatin-bound nuclear, and cytoskeletal fraction) was conducted using a subcellular protein fractionation kit (Thermo Fisher Scientific, Waltham, MA) as per the manufacturer’s instructions. Briefly, day 7 prostaspheres were isolated from Matrigel using dispase and pelleted. The sphere pellet was subjected to cell lysis followed by sequential resuspension and centrifugation using different kit buffers to isolate proteins of specific cellular compartments. The protein concentration for each fraction was measured using the Pierce BCA assay (Thermo Fisher Scientific). Samples were prepared for western blotting using 4× sample buffer and thermal denaturation of proteins.
Lipid raft fractionation
HuSLC pellets were suspended in detergent-free Tricine buffer [250 mM sucrose, 1 mM EDTA, 20 mM Tricine (pH 7.4)]. Cellular material was homogenized and centrifuged (1400g for 5 minutes at 4°C) to precipitate nuclear material. The resulting supernatant (homogenate) was collected, mixed with 30% Percoll in Tricine buffer, and subjected to ultracentrifugation for 25 minutes (Beckmann MLS50 rotor, 77,000g at 4°C) to collect the plasma membrane fraction. Plasma membrane fractions were collected and sonicated (three times, 3-second bursts). The sonicated material was mixed with 60% sucrose (to a final concentration of 40%), overlaid with a 35% to 5% step sucrose gradient, and subjected to overnight ultracentrifugation (Beckman MLS50 rotor, 87,400g at 4°C). Fractions were collected every 400 µL from the top sucrose layer and proteins were precipitated using 0.25 volume trichloracetic acid/deoxycholic acid [100% (w/v)]. The collected membrane fractions (1 to 10), cell homogenate, and isolated plasma membrane were prepared for SDS-PAGE, transferred to membranes, and immunoblotted for caveolin-1 (37) and ERβ (30).
Lentiviral overexpression
Human ESR1 (ERα) and ESR2 (ERβ) protein-coding sequences were cloned by long-range PCR using PrEC cDNA as a template. pLVX-ESR1 and pLVX-ESR2 vectors were constructed by cloning protein-coding sequences into pLVX-puro (Clontech, Fremont, CA) with a Gibson Assembly cloning kit (New England BioLabs, Ipswich, MA), and vectors were validated by sequencing. ERα and ERβ overexpression plasmids were cotransfected with lentiviral packaging plasmid psPAX2 (gift from Didier Trono, plasmid no. 12260, Addgene, Watertown, MA) (38) and envelope plasmid pMD2.G (gift from Didier Trono, plasmid no. 12259, Addgene) (39) into HEK293FT cells by Lipofectamine 2000 transfection reagent. Lentivirus-containing supernatants were collected 48 hours after transfection and used to infect cells in the presence of 8 μg/mL polybrene (Sigma-Aldrich). Positive cells were selected with 1 μg/mL puromycin for 1 week.
RNA interference
Primary prostate epithelial cells were plated in six-well culture plates until 70% to 80% confluent. Small interfering RNA (siRNA) against ERα and ERβ were delivered to the cells using Lipofectamine 2000 (Thermo Fisher Scientific) transfection reagent. Sequences used were: ERα, sense, 5′-ACCUUGCAGAUAUGUUUAACCAAGC-3′, antisense, 5′-GCUUGGUUAACAUAUCUGCAAGGUUA-3′; ERβ, sense, 5′-CUACAAAUCAGUGUACAAUCGAUAA-3′, antisense, 5′-UUAUCGAUUGUACACUGAUUUGUAGCU-3′. Cells were incubated with the siRNA–Lipofectamine 2000 mixture for 6 hours and then replaced with normal growth media overnight. Following recovery, cells were seeded in Matrigel to isolate stem/progenitor cells via spheroid culture. Prostaspheres were recovered after 96 hours and subjected to cell lysis for protein isolation or RNA isolation. Knockdown was validated by quantitative RT-PCR (qRT-PCR) on RNA isolates and western blot analysis of protein isolates.
Western blotting
Prostaspheres, HuSLCs, and WPE-stem cells with overexpressed ERα or ERβ were interrogated for rapid actions in response to 10 nM E2 for 15, 30, or 60 minutes. Day 7 prostaspheres were isolated using dispase followed by resuspension in ProstaLife media. Following exposure to either inhibitors or vehicle in the presence and absence of E2, prostaspheres, HuSLCs, and WPE-stem cells were lysed using RIPA buffer (Cell Signaling Technology, Danvers, MA) for 30 minutes followed by sonication (three times for 10 seconds each). Lysates were centrifuged (14,000g, 15 minutes), and protein levels were determined using the Pierce BCA method. Samples were denatured by boiling in the presence of 2-mercaptoethanol, and 30 or 40 μg of protein was loaded and separated using 4% to 20% gradient gels (Genscript, Piscataway, NJ). Separated proteins were transferred onto a 0.2-μm polyvinylidene difluoride membrane (Bio-Rad Laboratories, Hercules, CA) and probed with the appropriate primary 5-bromo-2′-deoxyuridine 1:500 (40), ERβ 1:500 (30), caveolin-1 1:1000 (37), ERα 1:1000 (29), phosphorylated (p‐)AKT 1:1000 (41), AKT 1:1000 (42), p‐ERK1/2 1:1000 (43), ERK1/2 1:1000 (44), p‐p38 1:1000 (45), p38 1:1000 (46), p–c-Jun N-terminal kinase (JNK) 1:1000 (47), JNK 1:1000 (48), p‐p65 1:1000 (49), p65 1:1000 (50), p‐IGF1R 1:500 (51), IGF1R 1:1000 (52), p–epidermal growth factor receptor (EGFR) 1:1000 (53), EGFR 1:1000 (54), p‐SRC 1:1000 (55), SRC 1:1000 (56), EZH2 1:1000 (57), GAPDH 1:10,000 (58), MLL‐C 1:1000 (59), p‐EZH2 1:1000 (60), ERβ2 1:200 (61), PELP1 1:1000 (62), and secondary antibodies. Horseradish peroxidase–conjugated anti-mouse (63) or anti-rabbit antibodies (64) (Cell Signaling Technology) plus enhanced chemiluminescence (SuperSignal West Dura Extended, Thermo Fisher Scientific) were used to visualize proteins. ImageJ software (National Institutes of Health, Bethesda, MD) was used to quantify pixel density of bands for quantitative analysis.
Immunocytochemistry
HuSLCs were grown on four- or eight-well chamber slides and fixed using 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA). Cells were lightly permeabilized using 0.1% Triton X-100 and blocked with 5% normal goat serum. Cells were incubated with anti-ERβ primary antibody followed by anti-rabbit secondary antibody conjugated to Alexa Fluor 488 (Invitrogen, Carlsbad, CA) for detection. Cells were mounted using Vectashield with 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA) and visualized by a Zeiss 710 Meta confocal microscope.
Proximity ligation assay
Untreated day 5 prostaspheres were seeded on eight-well chamber slides overnight and fixed with 4% paraformaldehyde at 4°C. A Duolink proximity ligation assay (PLA) kit (Sigma-Aldrich) was used as per the manufacturer’s recommendations. Briefly, following fixation, spheres were subjected to mild permeabilization with 0.05% Triton X-100 for 10 minutes and blocked using a proprietary blocking buffer. Spheres were incubated with primary antibodies against either ERα (29) or ERβ (30) along with antibody against caveolin-1 (37) overnight at 4°C. Spheres were next incubated with kit-provided PLA probes at 37°C for 1 hour. A ligation reaction followed by an amplification reaction was conducted using the appropriate kit reagents according to instructions. Spheres were next incubated with Alexa Fluor 488 phalloidin (Invitrogen) to stain membrane actin, and slides were mounted using mounting media containing DAPI (Vector Laboratories) as a counterstain. Resulting fluorescence was visualized using a Zeiss 710 Meta confocal microscope.
Phosphorylated kinase array
A human phosphorylated kinase array (R&D Systems, Minneapolis, MN) was used to screen for the phosphorylation status of 42 kinases within prostaspheres following E2 exposure. Day 7 prostaspheres were treated with vehicle or E2 for 15, 30, and 60 minutes, rapidly lysed, and processed as per the manufacturer’s instructions. Briefly, array membranes were exposed to 600 μg of lysate protein overnight at 4°C, washed repeatedly, and membranes with biotinylated detection antibodies were followed by streptavidin-conjugated secondary antibodies. Proteins were visually detected by membrane exposure to horseradish peroxidase–conjugated enhanced chemiluminescence and X-ray films, and resulting spots were quantified by measuring pixel density using ImageJ software (National Institutes of Health). Measurements for duplicate spots were averaged and background signal was subtracted from each raw measurement.
qRT-PCR
Total RNA was isolated using either an RNeasy Mini kit (Qiagen, Hilden, Germany) or a Direct-zol RNA Miniprep kit (Zymo Research, Irvine, CA). cDNA was synthesized using iScript cDNA synthesis (Bio-Rad Laboratories). PCR was performed using SsoAdvanced SYBR Green supermix (Bio-Rad Laboratories) or 2× SYBR Green quantitative PCR master mix (Bimake.com, Houston, TX) on a CFX96 real-time system (Bio-Rad Laboratories). Cycling conditions of 95°C for 5 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute were used. Primers used were as follows: ALDH3A1, forward, 5′-ATCACCTTGCACTCTCTGCC-3′, reverse, 5′-TCAGTGCTGGGTCATCTTGG-3′; TXNIP, forward, 5′-GACCAGCCAACAGGTGAGAA-3′, reverse, 5′-CTGAGGAAGCTCAAAGCCGA-3′; MAF, forward, 5′-GGAGTCGGAGAAGAACCAGC-3′, reverse, 5′-CGAGTGGGCTCAGTTATGAA-3′; FOSB, forward, 5′-CTCACCCCAGAGGAAGAGGA-3′, reverse, 5′-CAACTGATCTGTCTCCGCCT-3′; S100P, forward, 5′-GGCTTCCTGCAGAGTGGAAA-3′, reverse, 5′-AGCCACGAACACGATGAACT-3′; DAPL1, forward, 5′-CCTGCAGTAAAAGCTGGAGGA-3′, reverse, 5′-CGTCATTCAGGGCATCCAGT-3′; biglycan (BGN), forward, 5′-GGGAACCCACTGGAGAACAG-3′, reverse, 5′-TCAGGGAGGTCTTTGGGGAT-3′; FOXQ1, forward, 5′-GCACGCAGCAAGCCATATAC-3′, reverse, 5′-CGCGGAAAAAGGGGAACTTG-3′; C10orf99, forward, 5′-AGTCCCTAGCCCCAACTCAA-3′, reverse, 5′-CTCCGCTGTCTGGAGTCTTG-3′; CCNA1, forward, 5′-CGTCACTTGGGATGGAGACC, reverse, 5′-GTAGCCAGCACAACTCCACT-3′; CCND1, forward, 5′-GGCGGAGGAGAACAAACAGA-3′, reverse, 5′-TGTGAGGCGGTAGTAGGACA-3′. The 2−ΔΔCT method of data analysis was used to determine fold changes in RNA expression, and individual mRNA levels were normalized to the housekeeping gene RPL13.
DNA microarray
Day 7 prostaspheres were treated with either vehicle or 10 nM E2 for 3, 6, and 18 hours in triplicate. Prostasphere RNA was extracted using Qiagen RNeasy. RNA integrity was analyzed on an Agilent Bioanalyzer, and RNA integrity number scores were 10 for all microarray samples used. Illumina HT-12 BeadArray microarrays were performed by the University of Chicago Genomics Core Facility. Microarray data were analyzed in the R statistical environment using the lumi package as downloaded from Bioconductor (www.bioconductor.org). The BeadStudio output file was loaded into lumi and background corrected with the lumiB function. Variance-stabilizing transformation was performed with the lumiT function. Based on its performance on the bead array data, robust spline normalization was chosen and implemented via the lumiN function. Quality control was performed before filtering for probes with a positive signal (P ≤ 0.01) in ≥80% of sample arrays. After using the limma function to fit a linear model, differentially expressed genes were compiled using an adjusted P value threshold of P ≤ 0.01. Conversion of Illumina nuIDs to HUGO gene symbols was performed with subsequent annotation with the lumiHumanAll.db database. Hierarchical clustering of arrays was performed using the embedded heat map function. Background-corrected, variance-stabilized, and robust spline normalization data were output to tab-delimited files for additional analyses using the BRB-ArrayTools program developed by Dr. Richard Simon at the National Institutes of Health.
Statistical analysis
One- or two-way ANOVA was used to determine significance depending on the experimental conditions. Multiple comparisons were made using ANOVA followed by Tukey–Kramer or Student–Newman–Keuls post hoc tests to determine significance between groups. Significance was accepted at P < 0.05. A minimum of three independent trials was considered for statistical analyses.
Results
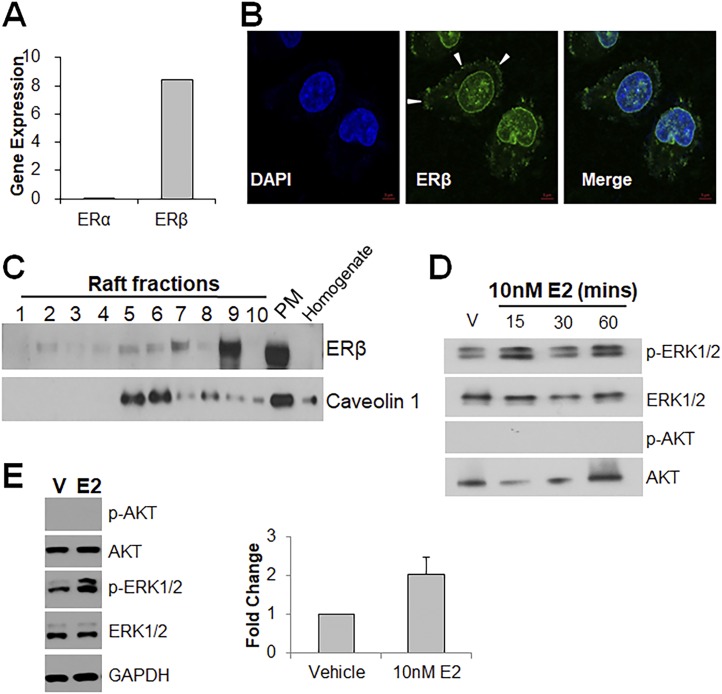
ERα and ERβ localize to the membrane and interact with caveolin-1 in normal human prostate stem/progenitor cells
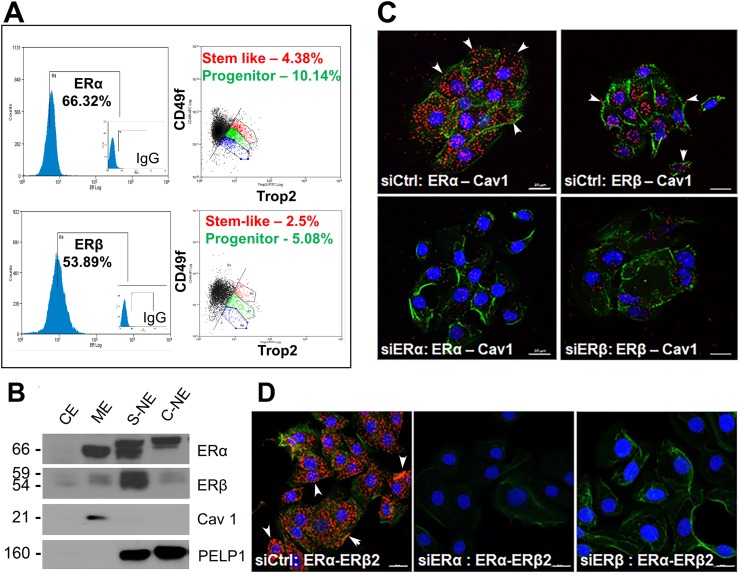
We previously reported that normal human prostate stem and progenitor cells express ERα and ERβ mRNA and protein using gene expression and immunofluorescence analysis of prostaspheres (12, 23). In this study, we extend this evidence using triple-channel flow cytometry analysis of day 7 prostasphere cells labeled for ERα or ERβ, which revealed ∼66% of spheroid cells as ERα+ and ∼55% as ERβ+ (Fig. 1A). Among ERα+ or ERβ+ prostasphere cells, between 2.5% and 4.5% were Trop2+/CD49fhigh (stem-like cells) and between 5% and 12% were Trop2+/CD49fmedium (early stage progenitor cells) whereas the remainder of ER+ cells were progenitors at different stages of lineage commitment (6).
Figure 1.
Localization of ERα and ERβ at the membrane of three-dimensional prostaspheres (PSs). (A) PSs cultured for 7 d were dispersed, triple stained for stem markers CD49f, Trop2, and either ERα or ERβ, and sorted by flow cytometry. Triple-stained subpopulations of cells comprising the stem cell and early-stage progenitor subpopulation expressing either ERα or ERβ were identified using the Summit software analysis package (n = 4). (B) Subcellular protein fractionation of day 7 PSs revealed the presence of ERα and ERβ in the membrane extract (ME), the soluble nuclear extract (S-NE), and chromatin-bound nuclear extract (C-NE), with limited protein found in the soluble cytoplasmic extract (CE). Caveolin-1 (Cav1) was used as a membrane marker, and PELP1 was used as a nuclear marker (n = 3). Differential ER migration between fractions is likely due to use of different extraction buffers and/or posttranslational modifications. (C) PLA on day 5 PSs revealed ERα and ERβ interactions with Cav1 at the cell membrane (arrowheads point to examples) and in cytoplasm, presumably during transport or turnover. Loss of most signal with ERα or ERβ knockdown by siRNA (bottom panels) documents signal specificity. (D) PLA on day 5 PSs between ERα and ERβ, depicted by the red punctate staining, indicates ERα–ERβ heterodimerization, including at the membrane (arrowheads). Loss of signals with siRNA for either ER documents specificity. For both (C) and (D), Alexa Fluor 488–phalloidin stain was used to visualize the membrane and demarcate cells. Nuclei are demarcated by DAPI counterstain (blue). Scale bars, 20 µm. Each PLA was repeated for n = 3.
To assess the cellular localization of the ERs, subcellular protein isolation was performed on day 7 spheroids, and resulting extracts from different cellular compartments were analyzed via western blots (Fig. 1B). ERα was identified in nuclear extracts (soluble and chromatin bound) and in the membrane fraction, which was confirmed by caveolin-1, a lipid raft membrane marker. Although most ERβ was found in the soluble nuclear extract, a minor portion also localized within the membrane extract. Membrane localization was further confirmed using a PLA that identified direct interactions of ERα and ERβ with caveolin-1 at cell membranes (Fig. 1C, arrowheads). The loss of PLA interactions with caveolin-1 upon siRNA for ERα or ERβ documented the specificity of the ER antibodies. Furthermore, interaction between ERα and ERβ was noted via the PLA, including signals at the membrane (Fig. 1D, arrowheads), which suggests the possibility of a heterodimeric interactome to facilitate the onset of nongenomic signaling. Taken together, these data support our previous studies that human prostate stem and progenitor cells are ERα+ and ERβ+ and demonstrate that a portion of each resides at the cell membrane. Furthermore, the findings provide evidence that the cellular machinery necessary for ER transport to the membrane is functional in prostate stem/progenitor cells as previously demonstrated for other cell types (65).
Human prostate stem and progenitor cells phosphorylate multiple kinases upon short-term E2 exposure
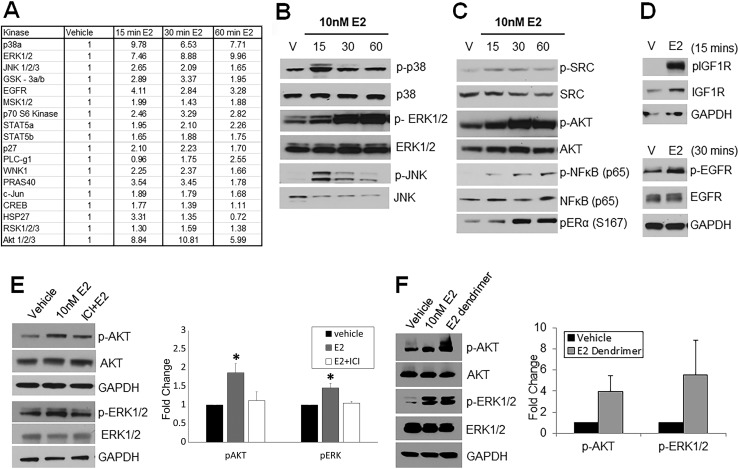
To identify membrane-initiated signaling cascades activated by brief E2 exposure, a phosphokinase array was used to screen for rapid phosphorylation of intracellular kinases. Eighteen of 43 different kinases showed a more than twofold phosphorylation increase across 15, 30, and 60 minutes of exposure of prostaspheres to 10 nM E2 (Fig. 2A). Among them, increased phosphorylation of AKT pathway proteins, including AKT at ser473, PRAS 40, and p70S6 kinase, and MAPK pathway proteins, including ERK1/2, JNK, and p38, predominated. Activation of these proteins during a time course, in addition to proteins not on the array (e.g., SRC and p65), was further confirmed by western blot (Fig. 2B and 2C). Modest differences in phosphorylation peaks at 15, 30, and 60 minutes of E2 exposure suggest possible temporal patterns in the ensuing signaling cascades. Of note, phosphorylation of ERα (S167) as early as 30 minutes implicates that the nongenomic ER signals may directly influence genomic ER actions, as described for other systems (66). Additionally, membrane receptors IGF1R and EGFR exhibited phosphorylation at 15 and 30 minutes, respectively (Fig. 2D), indicating that ER crosstalk with growth factor receptors may be involved in downstream signaling cascades.
Figure 2.
Membrane-bound ER initiates phosphorylation of several kinases downstream upon E2 exposure. (A) Day 7 prostaspheres (PSs) treated with vehicle or 10 nM E2 for 15, 30, and 60 min were subjected to phosphokinase arrays spotted with 43 different kinase proteins. The table shows 18 proteins that exhibited >50% increased phosphorylation levels over vehicle with E2 exposure during the course of 60 min. Values are an average of n = 3 repeats relative to vehicle (normalized to 1) using ImageJ image analysis software. (B–D) Western blot analysis shows (B) phosphorylation MAPK pathway molecules p38, ERK1/2, and JNK, (C) AKT pathway cascades including SRC, AKT, NF-κB, and ERα S167, and (D) membrane-bound receptors IGF1R and EGFR during a 15-, 30-, and 60-min time course following E2 exposure to day 7 PSs (n = 3). (E) Western blot analysis of day 7 PSs treated with vehicle and 10 nM E2 with/without 1 μM ICI for 30 min shows an attenuation of E2-mediated phosphorylation of both AKT and ERK with ER antagonism. Graphic representation is shown at right for n = 4. *P < 0.05 vs vehicle and E2 plus ICI. (F) Day 7 PSs treated with 10 µM E2 dendrimer (impermeable to nuclear membrane) show a marked increase in phosphorylation of AKT and ERK1/2 by the E2 dendrimer, comparable to 10 nM E2. Graphic representation at right for n = 4.
Exposure to E2 for 30 minutes in the presence of ICI, an ERα/ERβ-specific antagonist, resulted in attenuation of AKT and ERK1/2 phosphorylation as compared with E2 alone, confirming that activation of these cascades was mediated through ERα or ERβ (Fig. 2E). Furthermore, addition of an estrogen dendrimer, which is membrane-impenetrable and potentiates only nongenomic signaling (26), led to robust activation of both AKT and ERK1/2 (Fig. 2F), corroborating these as nongenomic E2 actions in the normal prostate stem/progenitor cells.
AKT and ERK1/2 signaling cascades are essential for prostate stem/progenitor cell homeostasis
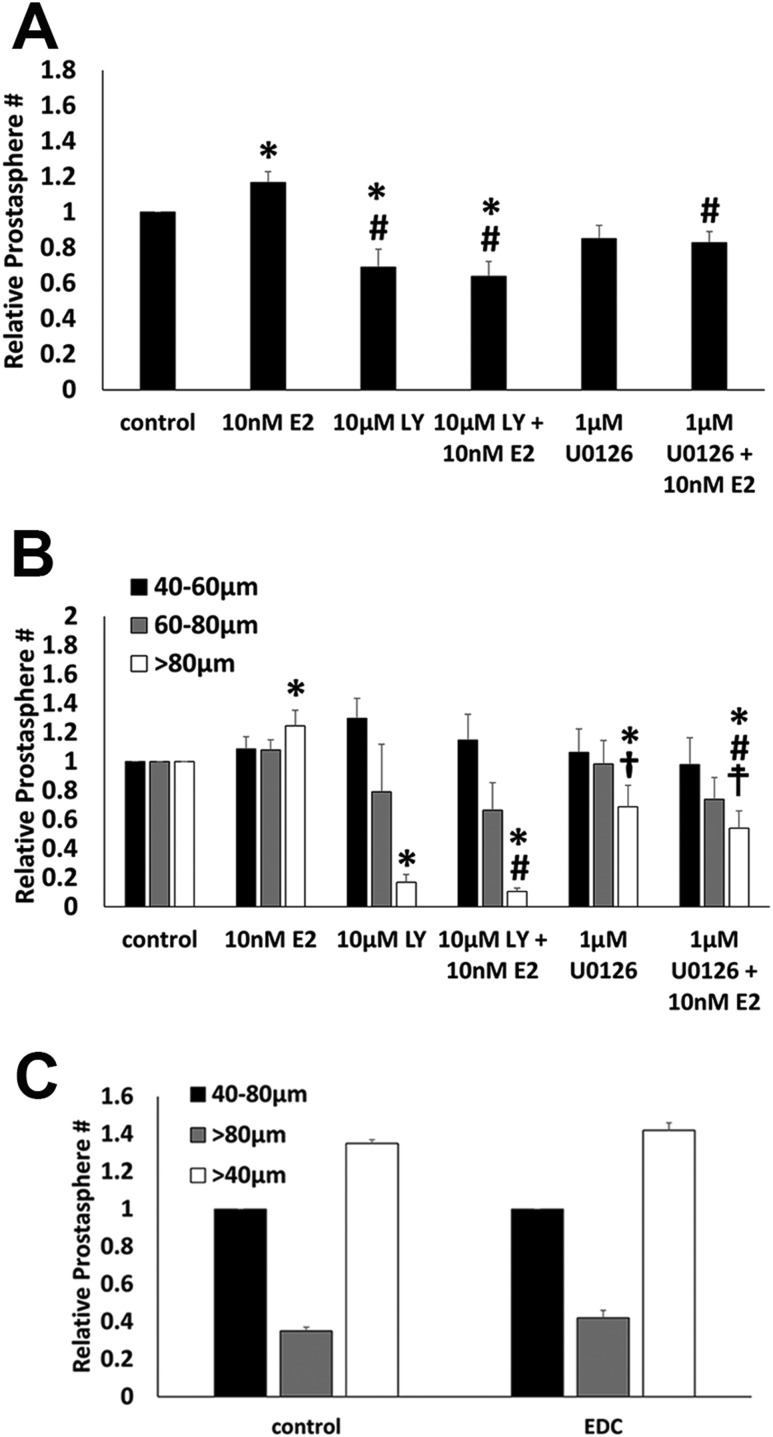
To examine the role of AKT and MAPK signaling pathways in prostate stem/progenitor cells, LY294002, a PI3K inhibitor, was administered to block activation of AKT whereas U0126, a MEK1/2 inhibitor, was used to block activation of ERK1/2 over the course of 7 days during prostasphere establishment and growth. There was a marked decrease in the total number of prostaspheres (Fig. 3A) and spheroids >80 µm in size (Fig. 3B) when treated with either inhibitor alone as compared with vehicle controls, which implies that both AKT and MAPK pathways play essential roles in stem cell asymmetric self-renewal during spheroid establishment as well as progenitor cell proliferation during spheroid growth. Although E2 alone increased prostasphere numbers and spheroids >80 µm, as previously shown (12), it was insufficient to overcome the inhibition of AKT or ERK signaling pathways. To assess whether membrane-initiated E2 signaling alone was sufficient to increase sphere numbers and size, prostaspheres were cultured in the absence or presence of an E2-dendrimer compound that engages membrane ERs but cannot enter the cells and activate nuclear ERs (26). After 7 days, there was no difference in total sphere numbers or spheres >80 µm in size between control and E2-dendrimer compound–exposed prostaspheres (Fig. 3C). Collectively, these findings imply that both AKT and MAPK pathways play essential roles in stem cell asymmetric self-renewal during spheroid establishment as well as progenitor cell proliferation during spheroid growth. Furthermore, although E2 increases spheroid growth, the findings indicate that membrane-initiated signaling or nuclear ER signaling alone is not sufficient to enhance a growth response in prostate stem and progenitor cells and suggest that cooperativity between the two pathways may be required.
Figure 3.
AKT and MAPK signaling pathways are critical for prostate stem cell self-renewal and progenitor cell proliferation. (A and B) Prostaspheres (PSs) were cultured for 7 d in the absence or presence of PI3K–AKT pathway inhibition [LY294002 (LY)], MAPK (ERK1/2) pathway inhibition [U0126 (UO)], without and with concomitant 10 nM E2 exposure. (A) Exposure to 10 nM E2 increased the total spheroid numbers (relative to vehicle control, set as 1.0), whereas the presence of LY294002 or U0126 decreased the number of spheres as compared with vehicle controls or E2-exposed PSs. The addition of E2 to either the LY294002- or the U0126-exposed cultures was unable to mitigate the loss in spheroid numbers (n = 7). *P < 0.05 vs control; #P < 0.05 vs E2. (B) Spheroid size, measured between 40 and 60 µm, 60 and 80 µm, and >80 µm, was assessed in cultures exposed to LY294002 and U0126 with/without 10 nM E2. Exposure to E2 increased spheroids >80 µm in size, indicating a proliferative effect on daughter progenitor cells. Inhibition of PI3K–AKT or ERK1/2 pathways markedly reduced the numbers of large-sized spheres, indicating their essential role in progenitor cell proliferation. E2 treatment with LY294002 or U0126 could not mitigate this effect, suggesting a requirement of membrane-initiated pathways in E2-induced progenitor cell proliferation. n = 7. *P < 0.05 vs control; #P < 0.05 vs E2; †P < 0.05 vs LY294002; ‡P < 0.05 vs LY294002 plus E2. (C) Shown are total sphere numbers (open bar) and sphere numbers between 40 and 80 µm and >80 µm in PSs grown in the absence or presence of E2-dendrimer compound (EDC) for 7 d.
Membrane ERα and ERβ differentially activate AKT and ERK signaling cascades
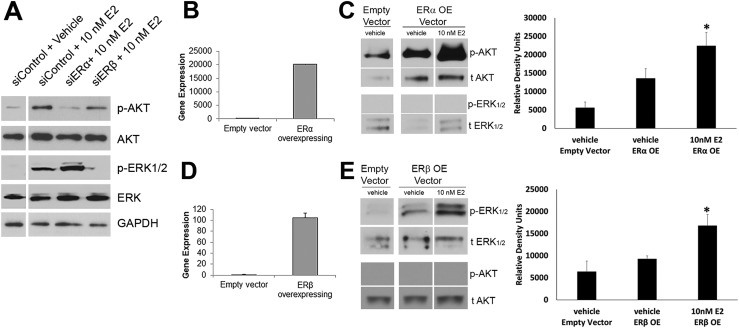
We next sought to determine whether the nongenomic signaling cascades were receptor subtype specific. ERα or ERβ was knocked down using siRNA in two-dimensional cultured PrECs and transferred to three-dimensional Matrigel culture. Day 5 prostaspheres were exposed to 10 nM E2 or vehicle for 30 minutes and harvested for western blot analysis. ERα silencing led to an attenuation of AKT phosphorylation at S473 compared with control cells, whereas ERβ silencing had no effect at this site (Fig. 4A). In contrast, ERβ silencing blocked phosphorylation of ERK1/2 at Tyr202/204 whereas ERα knockdown did not influence ERK activation (Fig. 4A). To corroborate these results, ERα and ERβ were overexpressed using lentiviral vectors in WPE-stem cells, a benign human prostate stem cell line exhibiting stemness characteristics and low to no constitutive ERα or ERβ expression, respectively. Overexpression of ERα (Fig. 4B) increased basal AKT phosphorylation compared with empty vector and further increased upon treatment with 10 nM E2 for 15 to 30 minutes, whereas ERK1/2 remained unphosphorylated (Fig. 4C). Conversely, ERβ overexpression in WPE cells (Fig. 4D) elevated ERK1/2 phosphorylation upon E2 exposure for 30 to 60 minutes whereas AKT phosphorylation was undetected during this time (Fig. 4E). Taken together, the results indicate that activation of AKT and MAPK pathways are receptor selective in prostate progenitor cells; that is, AKT activation is primarily reliant on membrane ERα signaling, whereas membrane ERβ signals predominantly through the MAPK pathway.
Figure 4.
Receptor subtype–specific signaling cascades are elicited via membrane ERs. (A) Day 5 prostaspheres with ERα or ERβ knocked down by siRNA were exposed to 10 nM E2 for 30 min followed by western blot analysis of AKT and ERK1/2 phosphorylation. Spheroid cells with reduced ERα showed no p-AKT increase upon E2 exposure whereas p-ERK1/2 was robust. In contrast, spheres with reduced ERβ exhibited increased p-AKT with E2 treatment but no phosphorylation of ERK1/2. Images are representative of three to four separate experiments. (B–E) ERα (ERαOE) or ERβ (ERβOE) were overexpressed in WPE-stem cells, and phosphorylation of AKT and ERK1/2 was examined by western blot analysis. (B) qRT-PCR confirms overexpression of ERα in ERαOE cells. (C) Western blots of ERαOE WPE-cells show increased basal p-AKT compared with control vector, which further increased after 30 min of exposure to 10 nM E2. There was no ERK1/2 phosphorylation in ERαOE cells with/without 10 nM E2. Bar graph shows p-AKT in vehicle and with 15 to 30 min of 10 nM E2 (n = 5). *P < 0.05 vs empty vector control. (D) qRT-PCR confirms overexpression of ERβ in ERβOE cells. (E) Western blots of ERβOE WPE cells show increased basal p-ERK1/2 after 60 min of 10 nM E2 exposure but no AKT activation by E2 compared with control. Bar graph shows p-ERK1/2 in vehicles and with 30 to 60 min of 10 nM E2 (n = 3). *P < 0.05 vs empty vector control.
Prostate cancer stem cells express ERβ on their membranes and signal through the MAPK pathway
To determine whether prostate cancer stem cells possess similar nongenomic signaling pathways as normal prostate stem/progenitor cells, HuSLCs (25) were interrogated for rapid E2 responses. HuSLCs expressed high levels of ERβ but no ERα (Fig. 5A). Immunofluorescence validated the presence of punctate ERβ staining at the cell membrane (Fig. 5B, arrowheads) as well as in the nucleus. Density gradient ultracentrifugation of the cell membrane fraction followed by western blots revealed ERβ presence in membrane fractions with highest levels in lipid-raft regions, as denoted by caveolin-1 localization (Fig. 5C). Exposure to E2 for 15, 30, and 60 minutes led to robust phosphorylation of ERK1/2 but minimal to no phosphorylation of AKT (Fig. 5D), substantiating that ERβ signals predominantly via the MAPK axis. To corroborate these results, prostaspheres with enrichment for cancer stem-like cells were grown from the DU145 cancer cell line (ERα−, ERβ+), and exposure to 10 nM E2 for 30 minutes resulted in increased phosphorylation of ERK1/2 with no activation of AKT (Fig. 5E).
Figure 5.
Prostate cancer stem-like cells express ERβ on their membranes and signal through the MAPK pathway. (A) qRT-PCR shows high expression of ERβ mRNA and an absence of ERα mRNA in HuSLCs. (B) Immunocytochemistry of HuSLCs for ERβ shows intracellular, nuclear, and membrane-bound (arrowheads) ERβ protein (green). Nuclei were stained with DAPI (blue). Magnification ×40. (C) Membrane fractions of HuSLCs were separated by density gradient centrifugation followed by western blot analysis for ERβ protein. ERβ was found at high levels in the whole plasma membrane fraction (PM) and the lipid raft fractions (5–8) marked by caveolin-1 expression. (D) HuSLCs treated with 10 nM E2 for 15, 30, and 60 min showed AKT phosphorylation but no activation of ERK1/2. (E) Prostate cancer stem/progenitor cells were isolated from DU145 cultures by transferring to three-dimensional Matrigel culture for 3 wk. Harvested DU145 spheres were exposed to 10 nM E2 for 30 min. Western blot analysis showed an increased ERK1/2 phosphorylation but no AKT activation as compared with vehicle-treated controls. Graphic representation of p-ERK1/2 signal at right for n =3.
Nongenomic ER signaling activates unique gene expression profiles
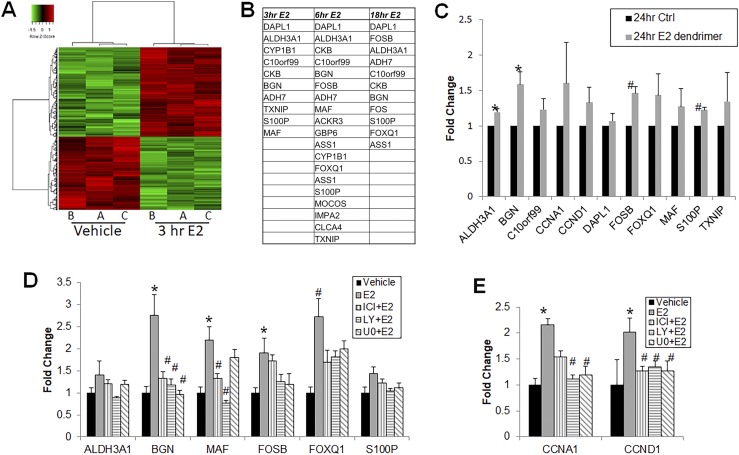
Phosphorylation of protein kinases, including ERK1/2, JNK, p38, and AKT, leads to transcriptional control of several genes through activation of downstream transcription factors. To investigate which genes are transcriptionally regulated via these kinases through nongenomic ER signaling, we performed gene microarray analysis after 3, 6, or 18 hours of exposure to 10 nM E2. A subset of genes was more than twofold upregulated at 3 hours (Fig. 6A), with differential expression also noted at 6 and 18 hours (not shown). Because the 3-hour treatment carried a smaller likelihood of nuclear ER genomic transcription, we focused subsequent studies on genes upregulated at the 3-hour time point.
Figure 6.
Microarray profiling of prostate stem/progenitor cells following short-term E2 treatment. (A) Microarray heat map of day 7 prostaspheres (PSs) treated with vehicle or 10 nM E2 for 3 h revealed a set of genes modulated by short-term E2 exposure compared with controls. Results are shown from three biologic repeats. (B) Illumina microarray genes showing more than twofold upregulation in day 7 PSs following treatment with 10 nM E2 for 3, 6, or 18 h relative to control. Numbers represent average of n = 3. (C) PSs were exposed to 10 nM E2 dendrimer (impermeable to nuclear membrane) for 24 h and qRT-PCR validated increased expression of several genes that were upregulated more than twofold in the gene expression microarray experiment (n = 3) *P < 0.05; #P < 0.005. (D) A subset of the microarray genes upregulated more than twofold by 3 h E2 exposure were validated by qRT-PCR in day 7 PSs treated with 10 nM E2 for 3 h with BGN, MAF, FOSB, and FOXQ1 significantly greater than vehicle controls. Increased BGN, MAF, and FOXQ1 expression was attenuated by ICI pretreatment. Elevated BGN, FOSB, and FOXQ1 expression was also blocked by LY294002 (LY) or U0126 (UO) pretreatment, whereas increased MAF expression was only blocked by LY294002 (n = 7). *P < 0.005 vs vehicle; #P < 0.05 vs E2. (E) qRT-PCR showed that cyclin gene expression (CCND1, CCNA1) was significantly increased by 3 h E2 exposure compared with vehicle, and the effect was blocked upon pretreatment with ICI, LY294002, or U0126 (n = 7). *P < 0.05; #P < 0.005.
To eliminate ambiguity of genomic transcription, two approaches were used. First, day 7 prostaspheres were treated with E2 dendrimer for 24 hours to ensure penetrance throughout the spheroids. RT-PCR revealed that the E2 dendrimer largely mimicked E2 responses with upregulation of several genes that exhibited more than a twofold increase in the 3-hour microarray experiment (Fig. 6B). Next, day 7 prostaspheres were pretreated with ICI to block ER action or with kinase inhibitors LY294002 or U0126 to target genes activated by AKT or ERK1/2 signaling, respectively. E2 exposure for 3 hours led to significant increases in expression of BGN, MAF, FOSB, and FOXQ1, with minor increases in ALD3A1 and S100P. Treatment with 1 µM ICI verified that increased gene expression was mediated by ERs and not a result of other receptors. Blockade of AKT activation attenuated the E2-stimulated increase in ALDH3A1, BGN, MAF, FOSB, FOXQ1, and S100P, although blocking the ERK pathway similarly reduced BGN, FOSB, FOXQ1, and S100P expression, suggesting cross-talk between AKT and ERK1/2 pathways or commonality of downstream targets in response to membrane ER activation. Of note, MAF showed a pathway-specific response, with only LY294002 blocking its expression (Fig. 6C). Cell cycle genes such as CCND1 and CCNA1 were also downstream of both pathways; blocking either pathway resulted in decreased E2-mediated expression of these genes (Fig. 6D). Taken together, these experiments confirm the role of ER nongenomic signaling in inducing transcription on a subset of genes distinct from genomic activation through nuclear ERs.
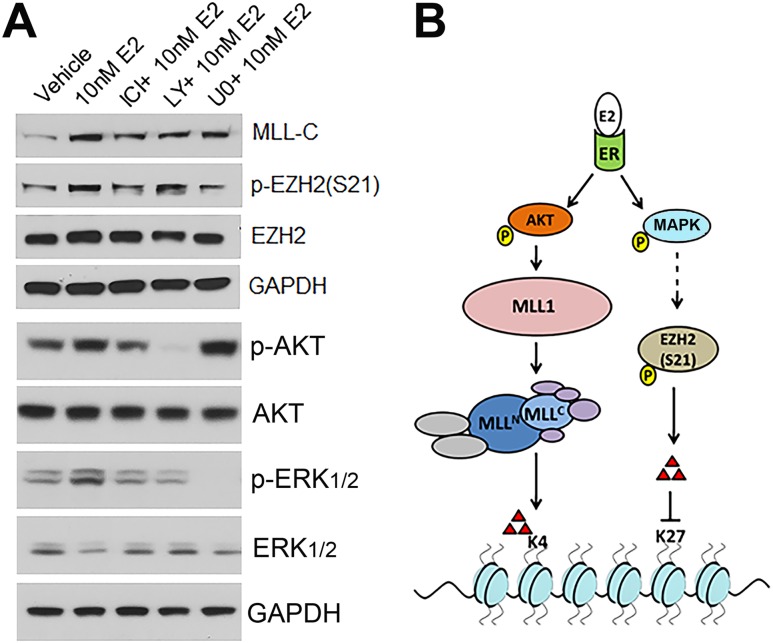
Nongenomic ER signaling modulates HMTs
Recent work has shown that membrane-initiated ER actions can influence gene transcription through rapid activation of HMTs that lay down activational or repressive marks on H3 lysines (24, 67). Therefore, the potential for epigenetic control of gene transcription by rapid E2 actions in prostate stem/progenitor cells was investigated by examining the activation or repression of specific HMTs following short-term E2 exposure to day 7 prostaspheres. Cleavage of MLL1, an HMT component of the COMPASS complex, to MLL-C and MLL-N denotes its activation with cleavage products responsible for laying down activational trimethyl marks on H3K4. MLL-C was detected within 30 minutes after E2 addition (Fig. 7A), and the effect was abrogated by pretreatment with ICI or LY294002, but not U0126, suggesting that MLL-1 cleavage was downstream of membrane ER activation of AKT (Fig. 7A). Serine 21 phosphorylation of EZH2, an HMT responsible for laying down repressive trimethylation marks on H3K27, leads to silencing of its activity (68). Prostasphere cells treated with E2 for 30 minutes exhibited increased phosphorylation of EZH2 at S21, which was blocked by ICI (Fig. 7A). Although pretreatment with LY294002 did not influence the E2-initiated EZH2 phosphorylation, pretreatment with U0126 blocked p-EZH2 (S21) by E2, suggesting a role for an MAPK-activated pathway in this activity. The differential activation of HMTs by E2 utilizing distinct membrane-initiated pathways is shown schematically in Fig. 7B. Taken together, these findings provide mechanistic evidence that rapid membrane-initiated ER signaling can modify epigenetic writers that establish epigenetic marks that may in turn influence gene transcription in cells.
Figure 7.
Rapid activation of HMTs in prostaspheres by short-term E2 exposure. (A) Day 7 prostaspheres were harvested, treated with vehicle, 10 nM E2, and E2 plus pathway inhibitors for 30 min and analyzed by western blots. MLL activation was monitored by immunoblotting for MLL-C protein, which increased by E2 exposure. MLL activation was attenuated by pretreatment with ICI and LY294002 (LY), which reduced p-AKT, but not by U0126 (UO), indicating that ER-mediated p-AKT activates MLL. EZH2 was phosphorylated at S21 by 30-min E2 exposure. This was blocked when spheres were pretreated with ICI and U0126, which blocked p-ERK1/2, but not by LY294002, indicating that ER-mediated p-ERK1/2 phosphorylates EZH2. AKT and ERK phosphorylation were monitored as controls for the inhibitors on a duplicate blot. The GAPDH loading control is shown for both blots. Representative images are from three independent trials. (B) Model depicting possible mechanism of epigenetic control of gene transcription via MLL activation and EZH2 phosphorylation via short-term E2 action.
Discussion
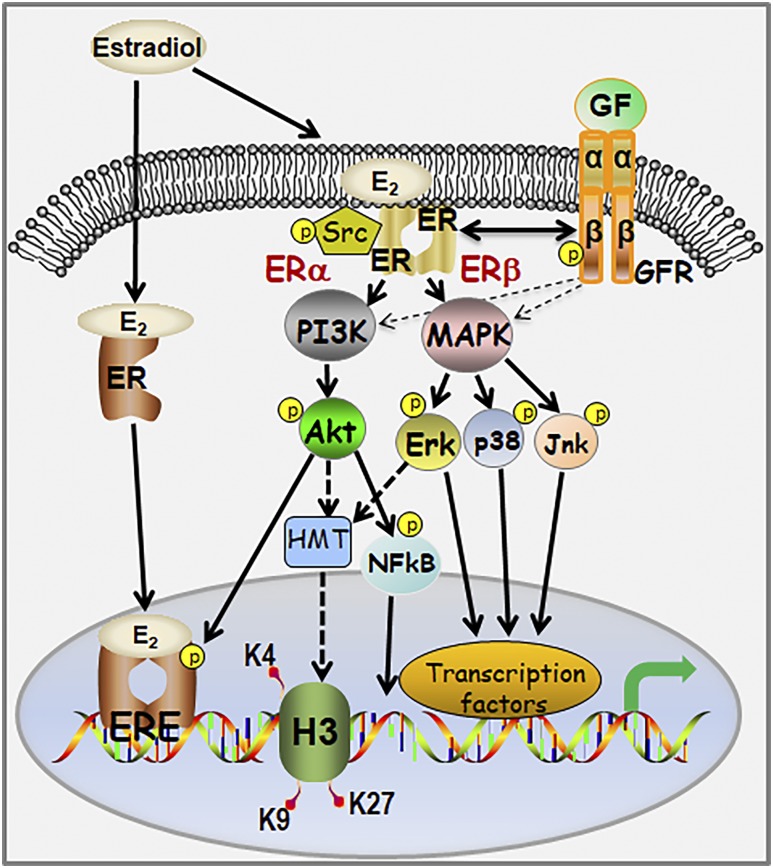
ERs have been localized to the cell membrane in multiple cell types where they engage in membrane-initiated estrogen signaling (19, 20, 69). Although past studies have identified steroid actions within various stem and progenitor cell populations (16, 17, 70–73), there remains a knowledge gap regarding nongenomic signaling via membrane-associated steroid receptors within this cell niche. The present studies build on our previous discovery of ER expression and estrogenic actions in prostate stem and progenitor cells by delineating nongenomic actions of estrogens mediated through membrane-associated ERα and ERβ, as summarized in Fig. 8. In brief, we provide definitive proof that both ERα and ERβ localize to the membrane, interact with caveolin-1, and initiate downstream signaling cascades that result in expression of a distinct set of genes. Although multiple kinases were rapidly phosphorylated upon E2 exposure, activation of AKT and MAPK signaling cascades predominated. Additionally, E2 initiated rapid phosphorylation of IGFR, EGFR, and ERα in stem/progenitor cells, implicating complex crosstalk between membrane-localized ERs, growth factor signaling cascades, and nuclear (genomic) ER actions. Of particular note, we observed differential engagement of downstream effectors for the two ERs, with ERα preferentially activating AKT signaling pathways whereas ERβ selectively activated MAPK signaling cascades in the stem/progenitor cells. Furthermore, membrane-initiated ER signaling modulated HMTs, with AKT activation involved in MLL1 cleavage and an MAPK pathway activation playing a role in EZH2 phosphorylation. Finally, these findings are applicable to prostate cancer stem-like cells where membrane-associated ERβ initiated MAPK signaling cascades in response to E2, suggesting a role for rapid estrogen action in regulating this unique cancer cell population.
Figure 8.
Schematic representation of the estrogen–ER signaling pathways operational in human prostate stem/progenitor cells. ERE, estrogen response element; GF, growth factor; GFR, growth factor receptor.
Membrane ER localization and activation in prostate stem and progenitor cells
We previously ascertained that human prostaspheres, enriched in stem and progenitor cells, express ERα and ERβ and exhibit proliferative responses to estrogens (12, 13). Additionally, a long-term label retention assay, which identifies spheroid stem-like cells, combined with immunohistochemistry detected relatively higher levels of ERβ in the prostate stem cells and elevated ERα levels in the progenitor population (23). The present studies expand this evidence using triple FACS labeling of prostasphere cells for Trop2, CD49f, and either ERα or ERβ to demonstrate that a portion of sorted stem-like (Trop2+/CD49fhigh) and early-stage progenitor (Trop2+/CD49fmedium) cell populations express both ERs. Furthermore, western blots of cell fractions confirmed not only that ERα and ERβ proteins are present at the expected molecular masses for full-length receptors, but also that a component fraction of both receptors localizes to the caveolin-1–rich membrane extract. Although a greater fraction of ERα was found in the membrane extract than ERβ when compared with the nuclear extracts, this may be a function of the mixed cell populations within prostaspheres where most are progenitor cells (6). Alternatively, ERα may be the predominant membrane-localized ER in prostate stem/progenitor cells as was similarly noted in endothelial populations (19).
Following palmitoylation within the E domain, a portion of ERs is transported to the cell membrane, with ER–caveolin-1 interaction facilitating ER trafficking to caveolae on the cell surface (74–77). The direct association of caveolin-1 with ERα and ERβ in prostate progenitor cells, as shown in the current study by PLA, provides a mechanistic basis for its transport to the membrane. Using the PLA, ERα and ERβ were also found to closely interact at the cell membrane, which supports ER heterodimerization at this location, as has been previously shown in endothelial and breast cancer cells (78). The PLA requires utilization of two primary antibodies raised in different species to generate a signal that precludes use of this assay to monitor ERα or ERβ homodimerization at the membrane. Nonetheless, because homodimerization and heterodimerization of ERs occur at the membrane and are essential for membrane-initiated E2 signaling (78), the identification of E2-initiated ER dimerization at the membrane in prostasphere cells implicates their functional activation at this site. The species-specific antibody available for the PLA in the current study was raised against ERβ2, which in addition to ERβ1, was identified in prostaspheres by RNA sequencing analysis (6). Interestingly, a recent study found that ERβ2 initiates noncanonical E2-mediated signaling in castrate-resistant prostate cancer cells, activating the SRC–IGF1R complex and the downstream nuclear factor κB (NF-κB) axis (79), which supports a potential similar role for membrane-localized ERβ2 in the prostate progenitor cells.
Kinase cascade activation by E2 plays a key role in stem/progenitor cell homeostasis
Receptor-mediated activation of MAPK and AKT signaling pathways is present across all cell types and plays critical roles in cellular physiology. The MAPK pathway is a mitogenic pathway that controls cellular proliferation (80–83), whereas the AKT pathway plays a role in cell survival, proliferation, and differentiation (84–86). A hallmark of stem cells is their ability to self-renew through symmetric or asymmetric cell divisions, and pathways such as AKT and MAPK play a critical role in these processes (87–92). In this study, we found that inhibition of either of these pathways yielded lower prostasphere numbers and size, a readout for stem cell asymmetric self-renewal and progenitor cell proliferation, respectively, emphasizing their crucial roles within the prostate stem/progenitor populations. That E2 exposure rapidly activated these essential signaling pathways through membrane-associated ERs signifies a key role for estrogens in maintaining prostate stem/progenitor cell homeostasis. Within minutes of E2 exposure, multiple proteins downstream of p-SRC were phosphorylated with most as participants in the MAPK (JNK, p38, ERK1/2, EGFR, MSK1/2, c-Jun, RSK1/2/3) and AKT (AKT1/2/3, PRAS 40, p70 S6 kinase, p27, GSK3a/b, NF-κB, WNK1) signaling cascades. ICI blockade of E2 activation for both pathways indicates that they are downstream of ERα and ERβ and not a function of other steroid receptors such as GPER. In addition to MAPK and AKT pathways, rapid activation of CREB and STAT5a/b was also observed, which underscores the complex nongenomic component of estrogen signaling in these cells. Future studies are aimed at identifying specific roles for the diverse downstream kinase cascades activated by E2 in the prostate stem/progenitor cell pool. Gene set enrichment analysis of RNA sequencing data from sorted prostasphere stem and progenitor cells previously identified “membrane ER signaling” as a top enriched pathway in stem cells, whereas NF-κB signaling predominated in the progenitors (6). In this study, phosphorylation of p65, a component of NF-κB whose activation is downstream of p-AKT, was observed within 15 minutes following E2 activation of membrane ER, thus linking both key pathways. Furthermore, inhibition of NF-κB signaling led to cell cycle blockade and reduced progenitor cell proliferation (6), similar to AKT pathway inhibition shown in the present study. Taken together, the evidence indicates that MAPK and AKT signaling are essential to prostate stem and progenitor cell maintenance and that their activation by estrogens through membrane ERs contributes to this homeostasis.
Levin and Hammes (18) and Levin (93) have described in detail a membrane complex comprised of ERs, SRC, and G-protein–coupled receptors that act together as a signalosome that also involves membrane-localized growth factor receptors IGFR and EGFR (18, 93). Indeed, we show in the present study that IGF1R and EGFR are phosphorylated within minutes of E2 addition to prostaspheres, and separate studies from our laboratory are dissecting this interplay in greater detail (94). The present data also identified the rapid phosphorylation of ERα at the S167 residue upon E2 exposure to prostasphere cells. ERα phosphorylation at this site has been shown to be downstream of the AKT activation in other cell systems (95) and is an example of a feed-forward mechanism that enhances the transactivational capacity of nuclear ER. As such, an intersection of nongenomic and genomic ER pathways in prostate stem/progenitor cells is likely whereby phosphorylated ERα may activate a unique subset of genes not typically under the control of the traditional (i.e., nonphosphorylated) nuclear signaling pathway (18).
Membrane-associated ERα and ERβ differentially engage downstream signaling cascades
We further investigated whether the membrane ER-mediated pathways are receptor subtype specific. Using both ERα/ERβ siRNAs in prostate stem/progenitor cells as well as stable overexpression in WPE-stem cells, the present studies determined that the AKT pathway primarily operates downstream of ERα, whereas the MAPK pathway is preferentially activated by ERβ. To our knowledge, this is the first report of membrane ER subtype selectivity for activation of downstream signaling pathways when both ERα and ERβ are operational at the membrane. Prior studies have identified pathway specificity for single ERs localized to the membrane, such as ERβ interaction with ERK pathways in cardiac cells (96) and MAPK activation via membrane-bound ERα in MCF7 cells (97). Although considerable overlap and crosstalk seem to occur between these separate signaling cascades, the possibility of receptor specificity in activating downstream players, which could include selective nuclear transcription factor activation and/or specific phosphorylation sites on steroid receptors, may help to unravel the complex nature of estrogen actions in prostate growth and disease (98, 99). Intriguingly, in the two prostate cancer stem-like cells examined, HuSLCs and DU145 spheroids, only ERβ expression was observed with exclusive activation of the MAPK pathway in response to membrane-initiated E2 signaling. Whether this is consistent across clinical specimens and the disease spectrum remains to be determined. However, a recent transcriptomic analysis of high-impact responses to androgen-deprivation therapy (ADT) for advanced prostate cancer identified a significant enrichment of upregulated genes in the estrogen signaling and MAPK pathways, suggesting compensatory estrogen/MAPK signaling to bypass ADT (100). In this context, the present findings open up possibilities for identifying novel druggable targets for effective control of prostate cancer at the stem cell level including utilization of selective ER modulators (99, 101).
Membrane-initiated E2 signaling induces expression of a unique subset of genes in prostate progenitor cells
The results in the present study identify a unique subset of genes selectively regulated via nongenomic E2 signaling through MAPK and AKT signaling cascades. Microarray analysis with subsequent RT-PCR validation pinpointed several genes whose expression (i) increased upon short-term E2 treatment, (ii) increased following exposure to a nonpenetrable E2 dendrimer, and (iii) was blocked by selective AKT or MAPK pathway inhibitors. The extracellular proteoglycan BGN and transcription factors FOSB, FOXQ1, and MAF fit these three criteria for membrane ER-regulated genes. Although their roles in prostate stem cells have not been described, the functions of these in other stem cells and in prostate cancer deserve mention. BGN is overexpressed in colon cancer stem cells where it induces activation of NF-κB and contributes to chemoresistance (102). Furthermore, early-life E2 exposure increased BGN expression in the pig prostate (103), and its elevated expression in prostate cancer is associated with poor prognosis (104). FOSB, a member of the MAPK signaling pathway, is required for migration and invasion of prostate cancer cells (105) and is one of 33 transcription factors associated with ADT response and metastatic progression (100). Furthermore, FOSB is one of four transcription factors required to reprogram mouse endothelial cells to hematopoietic stem cells (106). FOXQ1 is a key regulator of several stem cell populations, increases breast cancer stem-like cells (107), and influences apoptosis and invasion in prostate cancer (108). The MAF transcription factor controls a gene network crucial for epidermal progenitor cell repression and activation of differentiation (109) whereas enhanced MAF expression is a biomarker for metastatic breast and prostate cancer (110, 111). Interestingly, MAF exhibited pathway specificity in the current study with expression blocked by AKT but not MAPK–ERK pathway inhibition, suggesting that MAF transcription may be ERα mediated in prostate stem/progenitor cells. We hypothesize that rapid membrane-initiated regulation of these genes by E2 contributes to unique estrogenic actions in prostate stem and progenitor cells and potentially to prostate cancer stem cell behaviors.
One identified mechanism for gene transcriptional control downstream of membrane ERs is rapid modifications of HMTs (24, 112). The current study explored this possibility in prostate stem/progenitor cells and demonstrated activation of MLL1 and phosphorylation of EZH2 at S21 within 30 minutes of E2 exposure. Because MLL1 activation controls trimethylation of H3K4 (113) and EZH2 S21 phosphorylation blocks its ability to trimethylate H3K27 (68), both of these events can effectively activate gene transcription. Interestingly, these activities were differentially regulated by AKT and MAPK pathways in prostate progenitor cells, with the AKT pathway controlling MLL1 activation and the MAPK pathway involved in EZH2 S21 phosphorylation, suggesting that they may be independently triggered by ERα and ERβ, respectively, in this cell population. Nongenomic activation of MLL1 by membrane ERα mediated through the AKT pathway was previously shown within 6 hours of E2 exposure in MCF7 cells and neonatal rat prostates (24), and the current study expands this functional pathway to the programmable prostate progenitor population. Although it is well established that p-AKT phosphorylates EZH2 at S21 (68) and this is downstream of the membrane ERα–PI3K–AKT cascade in uterine cells (67), inhibition of PI3K-AKT by LY294022 failed to block E2-induced EZH2 phosphorylation in prostate progenitors. Unexpectedly, we observed that MAPK pathway inhibition by U0126 blocked this action, suggesting an alternate pathway in the prostate progenitor cells. Although previous studies have not identified MAPK-mediated phosphorylation of EZH2 at S21, PhosphoNET Kinase Predictor (www.phosphonet.ca) predicts recognition of this site by several MAPK-activated protein kinases that are phosphorylated by ERK1/2 and p38, including MSK1, MK2, and MK3 at predictive scores higher than AKT1/2 and RSKs at a somewhat lower values. Because U0126, the MAPK inhibitor used in these studies, inhibits MEK1 and MEK2 at high affinity and MEK3 with weaker activity, the predicted MAPK-activated protein kinase effectors of EZH2 phosphorylation at S21 would likely have been be blocked in our experiments. Future direct studies will be necessary to determine the specific MAPK family members involved in EZH2 phosphorylation in the prostate progenitor cells.
In summary, we have demonstrated the presence of membrane ERs in prostate stem and progenitor cells and show that they are actively involved in eliciting nongenomic actions by activating downstream signaling pathways. This ultimately impinges on genomic as well as epigenetic control of gene expression important in stem cell amplification and homeostasis as well as prostate cancer stem-like cell activity.
Acknowledgments
Financial Support: This work was supported by National Institutes of Health Grants R01-CA172220 (to G.S.P., S.K., and W.-Y.H.), R01-ES02207 (to G.S.P. and L.X.), P30-ES027792 (to G.S.P.), and R01-DK015556 (to J.A.K.), as well as by funding from the Michael Reese Research and Education Foundation (to G.S.P.).
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
Glossary
Abbreviations:
- ADT
androgen-deprivation therapy
- BGN
biglycan
- DAPI
4′,6-diamidino-2-phenylindole
- E2
17β-estradiol
- EGFR
epidermal growth factor receptor
- ER
estrogen receptor
- HMT
histone methyltransferase
- HuSLC
human prostate cancer stem-like cell
- ICI
ICI 182,870
- IGF1R
IGF1 receptor
- JNK
c-Jun N-terminal kinase
- NF-κB
nuclear factor κB
- p-
phosphorylated
- PI3K
phosphatidylinositol 3-kinase
- PLA
proximity ligation assay
- PrEC
primary prostate epithelial cell
- qRT-PCR
quantitative RT-PCR
- siRNA
small interfering RNA
References and Notes
- 1. Rycaj K, Tang DG. Cell-of-origin of cancer versus cancer stem cells: assays and interpretations. Cancer Res. 2015;75(19):4003–4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kasper S. Exploring the origins of the normal prostate and prostate cancer stem cell. Stem Cell Rev. 2008;4(3):193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blackwood JK, Williamson SC, Greaves LC, Wilson L, Rigas AC, Sandher R, Pickard RS, Robson CN, Turnbull DM, Taylor RW, Heer R. In situ lineage tracking of human prostatic epithelial stem cell fate reveals a common clonal origin for basal and luminal cells. J Pathol. 2011;225(2):181–188. [DOI] [PubMed] [Google Scholar]
- 4. Gaisa NT, Graham TA, McDonald SA, Poulsom R, Heidenreich A, Jakse G, Knuechel R, Wright NA. Clonal architecture of human prostatic epithelium in benign and malignant conditions. J Pathol. 2011;225(2):172–180. [DOI] [PubMed] [Google Scholar]
- 5. Moad M, Hannezo E, Buczacki SJ, Wilson L, El-Sherif A, Sims D, Pickard R, Wright NA, Williamson SC, Turnbull DM, Taylor RW, Greaves L, Robson CN, Simons BD, Heer R. Multipotent basal stem cells, maintained in localized proximal niches, support directed long-ranging epithelial flows in human prostates. Cell Reports. 2017;20(7):1609–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hu WY, Hu DP, Xie L, Li Y, Majumdar S, Nonn L, Hu H, Shioda T, Prins GS. Isolation and functional interrogation of adult human prostate epithelial stem cells at single cell resolution. Stem Cell Res (Amst). 2017;23:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lukacs RU, Lawson DA, Xin L, Zong Y, Garraway I, Goldstein AS, Memarzadeh S, Witte ON. Epithelial stem cells of the prostate and their role in cancer progression. Cold Spring Harb Symp Quant Biol. 2008;73(0):491–502. [DOI] [PubMed] [Google Scholar]
- 8. Guo C, Zhang B, Garraway IP. Isolation and characterization of human prostate stem/progenitor cells. Methods Mol Biol. 2012;879:315–326. [DOI] [PubMed] [Google Scholar]
- 9. Zhang D, Park D, Zhong Y, Lu Y, Rycaj K, Gong S, Chen X, Liu X, Chao HP, Whitney P, Calhoun-Davis T, Takata Y, Shen J, Iyer VR, Tang DG. Stem cell and neurogenic gene-expression profiles link prostate basal cells to aggressive prostate cancer. Nat Commun. 2016;7(1):10798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nelles JL, Hu WY, Prins GS. Estrogen action and prostate cancer. Expert Rev Endocrinol Metab. 2011;6(3):437–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cooke PS, Nanjappa MK, Ko C, Prins GS, Hess RA. Estrogens in male physiology. Physiol Rev. 2017;97(3):995–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hu WY, Shi GB, Lam HM, Hu DP, Ho SM, Madueke IC, Kajdacsy-Balla A, Prins GS. Estrogen-initiated transformation of prostate epithelium derived from normal human prostate stem-progenitor cells. Endocrinology. 2011;152(6):2150–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Prins GS, Hu WY, Shi GB, Hu DP, Majumdar S, Li G, Huang K, Nelles JL, Ho SM, Walker CL, Kajdacsy-Balla A, van Breemen RB. Bisphenol A promotes human prostate stem-progenitor cell self-renewal and increases in vivo carcinogenesis in human prostate epithelium. Endocrinology. 2014;155(3):805–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cheong A, Zhang X, Cheung YY, Tang WY, Chen J, Ye SH, Medvedovic M, Leung YK, Prins GS, Ho SM. DNA methylome changes by estradiol benzoate and bisphenol A links early-life environmental exposures to prostate cancer risk. Epigenetics. 2016;11(9):674–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Prins GS, Ye SH, Birch L, Zhang X, Cheong A, Lin H, Calderon-Gierszal E, Groen J, Hu WY, Ho SM, van Breemen RB. Prostate cancer risk and DNA methylation signatures in aging rats following developmental BPA exposure: a dose-response analysis. Environ Health Perspect. 2017;125(7):077007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Asselin-Labat ML, Vaillant F, Sheridan JM, Pal B, Wu D, Simpson ER, Yasuda H, Smyth GK, Martin TJ, Lindeman GJ, Visvader JE. Control of mammary stem cell function by steroid hormone signalling. Nature. 2010;465(7299):798–802. [DOI] [PubMed] [Google Scholar]
- 17. Joshi PA, Jackson HW, Beristain AG, Di Grappa MA, Mote PA, Clarke CL, Stingl J, Waterhouse PD, Khokha R. Progesterone induces adult mammary stem cell expansion. Nature. 2010;465(7299):803–807. [DOI] [PubMed] [Google Scholar]
- 18. Levin ER, Hammes SR. Nuclear receptors outside the nucleus: extranuclear signalling by steroid receptors. Nat Rev Mol Cell Biol. 2016;17(12):783–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arnal JF, Lenfant F, Metivier R, Flouriot G, Henrion D, Adlanmerini M, Fontaine C, Gourdy P, Chambon P, Katzenellenbogen B, Katzenellenbogen J. Membrane and nuclear estrogen receptor alpha actions: from tissue specificity to medical implications. Physiol Rev. 2017;97(3):1045–1087. [DOI] [PubMed] [Google Scholar]
- 20. Levin ER. Membrane estrogen receptors signal to determine transcription factor function. Steroids. 2018;132:1–4. [DOI] [PubMed] [Google Scholar]
- 21. Diep CH, Ahrendt H, Lange CA. Progesterone induces progesterone receptor gene (PGR) expression via rapid activation of protein kinase pathways required for cooperative estrogen receptor alpha (ER) and progesterone receptor (PR) genomic action at ER/PR target genes. Steroids. 2016;114:48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zarif JC, Lamb LE, Schulz VV, Nollet EA, Miranti CK. Androgen receptor non-nuclear regulation of prostate cancer cell invasion mediated by Src and matriptase. Oncotarget. 2015;6(9):6862–6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Prins GS, Calderon-Gierszal EL, Hu WY. Stem cells as hormone targets that lead to increased cancer susceptibility. Endocrinology. 2015;156(10):3451–3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang Q, Trevino LS, Wong RL, Medvedovic M, Chen J, Ho SM, Shen J, Foulds CE, Coarfa C, O’Malley BW, Shilatifard A, Walker CL. Reprogramming of the epigenome by MLL1 links early-life environmental exposures to prostate cancer risk. Mol Endocrinol. 2016;30(8):856–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vummidi Giridhar P, Williams K, VonHandorf AP, Deford PL, Kasper S. Constant degradation of the androgen receptor by MDM2 conserves prostate cancer stem cell integrity. Cancer Res. 2019;79(6):1124–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harrington WR, Kim SH, Funk CC, Madak-Erdogan Z, Schiff R, Katzenellenbogen JA, Katzenellenbogen BS. Estrogen dendrimer conjugates that preferentially activate extranuclear, nongenomic versus genomic pathways of estrogen action. Mol Endocrinol. 2006;20(3):491–502. [DOI] [PubMed] [Google Scholar]
- 27. RRID:AB_891482, https://scicrunch.org/resolver/AB_891482.
- 28. RRID:AB_10852561, https://scicrunch.org/resolver/AB_10852561.
- 29. RRID:AB_2617128, https://scicrunch.org/resolver/AB_2617128.
- 30. RRID:AB_2049623, https://scicrunch.org/resolver/AB_2049623.
- 31. RRID:AB_470233, https://scicrunch.org/resolver/AB_470233.
- 32. RRID:AB_470181, https://scicrunch.org/resolver/AB_470181.
- 33. RRID:AB_2576217, https://scicrunch.org/resolver/AB_2576217.
- 34. RRID:AB_10563566, https://scicrunch.org/resolver/AB_10563566.
- 35. RRID:AB_2534088, https://scicrunch.org/resolver/AB_2534088.
- 36. RRID:AB_144696, https://scicrunch.org/resolver/AB_144696.
- 37. RRID:AB_397788, https://scicrunch.org/resolver/AB_397788.
- 38. RRID:Addgene_12260, http://n2t.net/addgene:12260.
- 39. RRID:Addgene_12259, http://n2t.net/addgene:12259.
- 40. RRID:AB_476793, https://scicrunch.org/resolver/AB_476793.
- 41. RRID:AB_2315049, https://scicrunch.org/resolver/AB_2315049.
- 42. RRID:AB_915783, https://scicrunch.org/resolver/AB_915783.
- 43. RRID:AB_2315112, https://scicrunch.org/resolver/AB_2315112.
- 44. RRID:AB_390779, https://scicrunch.org/resolver/AB_390779.
- 45. RRID:AB_2139682, https://scicrunch.org/resolver/AB_2139682.
- 46. RRID:AB_10999090, https://scicrunch.org/resolver/AB_10999090.
- 47. RRID:AB_823588, https://scicrunch.org/resolver/AB_823588.
- 48. RRID:AB_2141027, https://scicrunch.org/resolver/AB_2141027.
- 49. RRID:AB_331284, https://scicrunch.org/resolver/AB_331284.
- 50. RRID:AB_10859369, https://scicrunch.org/resolver/AB_10859369.
- 51. RRID:AB_331253, https://scicrunch.org/resolver/AB_331253.
- 52. RRID:AB_2122378, https://scicrunch.org/resolver/AB_2122378.
- 53. RRID:AB_2096270, https://scicrunch.org/resolver/AB_2096270.
- 54. RRID:AB_2246311, https://scicrunch.org/resolver/AB_2246311.
- 55. RRID:AB_331034, https://scicrunch.org/resolver/AB_331034.
- 56. RRID:AB_2106047, https://scicrunch.org/resolver/AB_2106047.
- 57. RRID:AB_10694683, https://scicrunch.org/resolver/AB_10694683.
- 58. RRID:AB_1642205, https://scicrunch.org/resolver/AB_1642205.
- 59. RRID:AB_10948142, https://scicrunch.org/resolver/AB_10948142.
- 60. RRID:AB_1860363, https://scicrunch.org/resolver/AB_1860363.
- 61. RRID:AB_2262592, https://scicrunch.org/resolver/AB_2262592.
- 62. RRID:AB_2810832, https://scicrunch.org/resolver/AB_2810832.
- 63. RRID:AB_330924, https://scicrunch.org/resolver/AB_330924.
- 64. RRID:AB_2099233, https://scicrunch.org/resolver/AB_2099233.
- 65. Razandi M, Pedram A, Rosen EM, Levin ER. BRCA1 inhibits membrane estrogen and growth factor receptor signaling to cell proliferation in breast cancer. Mol Cell Biol. 2004;24(13):5900–5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Anbalagan M, Rowan BG. Estrogen receptor alpha phosphorylation and its functional impact in human breast cancer. Mol Cell Endocrinol. 2015;418(Pt 3):264–272. [DOI] [PubMed] [Google Scholar]
- 67. Bredfeldt TG, Greathouse KL, Safe SH, Hung MC, Bedford MT, Walker CL. Xenoestrogen-induced regulation of EZH2 and histone methylation via estrogen receptor signaling to PI3K/AKT. Mol Endocrinol. 2010;24(5):993–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cha TL, Zhou BP, Xia W, Wu Y, Yang CC, Chen CT, Ping B, Otte AP, Hung MC. Akt-mediated phosphorylation of EZH2 suppresses methylation of lysine 27 in histone H3. Science. 2005;310(5746):306–310. [DOI] [PubMed] [Google Scholar]
- 69. Wu Q, Chambliss K, Umetani M, Mineo C, Shaul PW. Non-nuclear estrogen receptor signaling in the endothelium. J Biol Chem. 2011;286(17):14737–14743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Alferez DG, Simões BM, Howell SJ, Clarke RB. The role of steroid hormones in breast and effects on cancer stem cells. Curr Stem Cell Rep. 2018;4(1):81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Nakada D, Oguro H, Levi BP, Ryan N, Kitano A, Saitoh Y, Takeichi M, Wendt GR, Morrison SJ. Oestrogen increases haematopoietic stem-cell self-renewal in females and during pregnancy. Nature. 2014;505(7484):555–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Pal B, Bouras T, Shi W, Vaillant F, Sheridan JM, Fu N, Breslin K, Jiang K, Ritchie ME, Young M, Lindeman GJ, Smyth GK, Visvader JE. Global changes in the mammary epigenome are induced by hormonal cues and coordinated by Ezh2. Cell Reports. 2013;3(2):411–426. [DOI] [PubMed] [Google Scholar]
- 73. Clarke RB, Spence K, Anderson E, Howell A, Okano H, Potten CS. A putative human breast stem cell population is enriched for steroid receptor-positive cells. Dev Biol. 2005;277(2):443–456. [DOI] [PubMed] [Google Scholar]
- 74. Chambliss KL, Yuhanna IS, Mineo C, Liu P, German Z, Sherman TS, Mendelsohn ME, Anderson RG, Shaul PW. Estrogen receptor α and endothelial nitric oxide synthase are organized into a functional signaling module in caveolae. Circ Res. 2000;87(11):E44–E52. [DOI] [PubMed] [Google Scholar]
- 75. Chambliss KL, Yuhanna IS, Anderson RG, Mendelsohn ME, Shaul PW. ERβ has nongenomic action in caveolae. Mol Endocrinol. 2002;16(5):938–946. [DOI] [PubMed] [Google Scholar]
- 76. Razandi M, Alton G, Pedram A, Ghonshani S, Webb P, Levin ER. Identification of a structural determinant necessary for the localization and function of estrogen receptor α at the plasma membrane. Mol Cell Biol. 2003;23(5):1633–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Pedram A, Razandi M, Sainson RC, Kim JK, Hughes CC, Levin ER. A conserved mechanism for steroid receptor translocation to the plasma membrane. J Biol Chem. 2007;282(31):22278–22288. [DOI] [PubMed] [Google Scholar]
- 78. Razandi M, Pedram A, Merchenthaler I, Greene GL, Levin ER. Plasma membrane estrogen receptors exist and functions as dimers. Mol Endocrinol. 2004;18(12):2854–2865. [DOI] [PubMed] [Google Scholar]
- 79. Kim H, Datta A, Talwar S, Saleem SN, Mondal D, Abdel-Mageed AB. Estradiol-ERβ2 signaling axis confers growth and migration of CRPC cells through TMPRSS2-ETV5 gene fusion. Oncotarget. 2016;8(38):62820–62833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002;12(1):9–18. [DOI] [PubMed] [Google Scholar]
- 81. Pagès G, Lenormand P, L’Allemain G, Chambard JC, Meloche S, Pouysségur J. Mitogen-activated protein kinases p42mapk and p44mapk are required for fibroblast proliferation. Proc Natl Acad Sci USA. 1993;90(18):8319–8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pedram A, Razandi M, Levin ER. Extracellular signal-regulated protein kinase/Jun kinase cross-talk underlies vascular endothelial cell growth factor-induced endothelial cell proliferation. J Biol Chem. 1998;273(41):26722–26728. [DOI] [PubMed] [Google Scholar]
- 83. Takenaka K, Moriguchi T, Nishida E. Activation of the protein kinase p38 in the spindle assembly checkpoint and mitotic arrest. Science. 1998;280(5363):599–602. [DOI] [PubMed] [Google Scholar]
- 84. Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 2005;9(1):59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Yu JS, Cui W. Proliferation, survival and metabolism: the role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development. 2016;143(17):3050–3060. [DOI] [PubMed] [Google Scholar]
- 86. Graff JR, Konicek BW, McNulty AM, Wang Z, Houck K, Allen S, Paul JD, Hbaiu A, Goode RG, Sandusky GE, Vessella RL, Neubauer BL. Increased AKT activity contributes to prostate cancer progression by dramatically accelerating prostate tumor growth and diminishing p27Kip1 expression. J Biol Chem. 2000;275(32):24500–24505. [DOI] [PubMed] [Google Scholar]
- 87. Rivera-Gonzalez GC, Shook BA, Andrae J, Holtrup B, Bollag K, Betsholtz C, Rodeheffer MS, Horsley V. Skin adipocyte stem cell self-renewal is regulated by a PDGFA/AKT-signaling axis. Cell Stem Cell. 2016;19(6):738–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Paling NR, Wheadon H, Bone HK, Welham MJ. Regulation of embryonic stem cell self-renewal by phosphoinositide 3-kinase-dependent signaling. J Biol Chem. 2004;279(46):48063–48070. [DOI] [PubMed] [Google Scholar]
- 89. Perry JM, He XC, Sugimura R, Grindley JC, Haug JS, Ding S, Li L. Cooperation between both Wnt/β-catenin and PTEN/PI3K/Akt signaling promotes primitive hematopoietic stem cell self-renewal and expansion. Genes Dev. 2011;25(18):1928–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sabri A, Ziaee AA, Ostad SN, Alimoghadam K, Ghahremani MH. Crosstalk of EGF-directed MAPK signalling pathways and its potential role on EGF-induced cell proliferation and COX-2 expression in human mesenchymal stem cells. Cell Biochem Funct. 2011;29(1):64–70. [DOI] [PubMed] [Google Scholar]
- 91. Li J, Wang G, Wang C, Zhao Y, Zhang H, Tan Z, Song Z, Ding M, Deng H.. MEK/ERK signaling contributes to the maintenance of human embryonic stem cell self-renewal. Differentiation. 2007;75(4):299–307. [DOI] [PubMed] [Google Scholar]
- 92. Niu Z, Mu H, Zhu H, Wu J, Hua J. p38 MAPK pathway is essential for self-renewal of mouse male germline stem cells (mGSCs). Cell Prolif. 2017;50(1):e12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Levin ER. Minireview: extranuclear steroid receptors: roles in modulation of cell functions. Mol Endocrinol. 2011;25(3):377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Rinaldi JC, Hu W, Majundar S, Hu D, Prins GS, Justulin LA, Felisbino SL. Cross-talk between estrogen receptors and insulin-like growth factor type-1 receptor modulates human prostate stem/progenitor cell amplification. J Investig Med. 2016;64(4):929. [Google Scholar]
- 95. Likhite VS, Stossi F, Kim K, Katzenellenbogen BS, Katzenellenbogen JA. Kinase-specific phosphorylation of the estrogen receptor changes receptor interactions with ligand, deoxyribonucleic acid, and coregulators associated with alterations in estrogen and tamoxifen activity. Mol Endocrinol. 2006;20(12):3120–3132. [DOI] [PubMed] [Google Scholar]
- 96. Pedram A, Razandi M, Lubahn D, Liu J, Vannan M, Levin ER. Estrogen inhibits cardiac hypertrophy: role of estrogen receptor-β to inhibit calcineurin. Endocrinology. 2008;149(7):3361–3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Zhang Z, Maier B, Santen RJ, Song RX. Membrane association of estrogen receptor α mediates estrogen effect on MAPK activation. Biochem Biophys Res Commun. 2002;294(5):926–933. [DOI] [PubMed] [Google Scholar]
- 98. Prins GS, Korach KS. The role of estrogens and estrogen receptors in normal prostate growth and disease. Steroids. 2008;73(3):233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Dobbs RW, Malhotra NR, Greenwald DT, Wang AY, Prins GS, Abern MR. Estrogens and prostate cancer. Prostate Cancer Prostatic Dis. 2019;22(2):185–194. [DOI] [PubMed] [Google Scholar]
- 100. Sharma NV, Pellegrini KL, Ouellet V, Giuste FO, Ramalingam S, Watanabe K, Adam-Granger E, Fossouo L, You S, Freeman MR, Vertino P, Conneely K, Osunkoya AO, Trudel D, Mes-Masson AM, Petros JA, Saad F, Moreno CS. Identification of the transcription factor relationships associated with androgen deprivation therapy response and metastatic progression in prostate cancer. Cancers (Basel). 2018;10(10):E379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Fujimura T, Takayama K, Takahashi S, Inoue S. Estrogen and androgen blockade for advanced prostate cancer in the era of precision medicine. Cancers (Basel). 2018;10(2):E29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Liu B, Xu T, Xu X, Cui Y, Xing X. Biglycan promotes the chemotherapy resistance of colon cancer by activating NF-κB signal transduction. Mol Cell Biochem. 2018;449(1–2):285–294. [DOI] [PubMed] [Google Scholar]
- 103. Kradolfer D, Flöter VL, Bick JT, Fürst RW, Rode K, Brehm R, Henning H, Waberski D, Bauersachs S, Ulbrich SE. Epigenetic effects of prenatal estradiol-17β exposure on the reproductive system of pigs. Mol Cell Endocrinol. 2016;430:125–137. [DOI] [PubMed] [Google Scholar]
- 104. Jacobsen F, Kraft J, Schroeder C, Hube-Magg C, Kluth M, Lang DS, Simon R, Sauter G, Izbicki JR, Clauditz TS, Luebke AM, Hinsch A, Wilczak W, Wittmer C, Büscheck F, Höflmayer D, Minner S, Tsourlakis MC, Huland H, Graefen M, Budäus L, Thederan I, Salomon G, Schlomm T, Melling N. Up-regulation of biglycan is associated with poor prognosis and PTEN deletion in patients with prostate cancer. Neoplasia. 2017;19(9):707–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Barrett CS, Millena AC, Khan SA. TGF-β effects on prostate cancer cell migration and invasion require FosB. Prostate. 2017;77(1):72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lis R, Karrasch CC, Poulos MG, Kunar B, Redmond D, Duran JGB, Badwe CR, Schachterle W, Ginsberg M, Xiang J, Tabrizi AR, Shido K, Rosenwaks Z, Elemento O, Speck NA, Butler JM, Scandura JM, Rafii S. Conversion of adult endothelium to immunocompetent haematopoietic stem cells. Nature. 2017;545(7655):439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Kim SH, Kaschula CH, Priedigkeit N, Lee AV, Singh SV. Forkhead box Q1 is a novel target of breast cancer stem cell inhibition by diallyl trisulfide. J Biol Chem. 2016;291(26):13495–13508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Zhang X, Wang L, Wang Y, Shi S, Zhu H, Xiao F, Yang J, Yang A, Hao X. Inhibition of FOXQ1 induces apoptosis and suppresses proliferation in prostate cancer cells by controlling BCL11A/MDM2 expression. Oncol Rep. 2016;36(4):2349–2356. [DOI] [PubMed] [Google Scholar]
- 109. Labott AT, Lopez-Pajares V. Epidermal differentiation gene regulatory networks controlled by MAF and MAFB. Cell Cycle. 2016;15(11):1405–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Pavlovic M, Arnal-Estapé A, Rojo F, Bellmunt A, Tarragona M, Guiu M, Planet E, Garcia-Albéniz X, Morales M, Urosevic J, Gawrzak S, Rovira A, Prat A, Nonell L, Lluch A, Jean-Mairet J, Coleman R, Albanell J, Gomis RR. Enhanced MAF oncogene expression and breast cancer bone metastasis. J Natl Cancer Inst. 2015;107(12):djv256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. D’Oronzo S, Brown J, Coleman R. The role of biomarkers in the management of bone-homing malignancies. J Bone Oncol. 2017;9:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Greathouse KL, Bredfeldt T, Everitt JI, Lin K, Berry T, Kannan K, Mittelstadt ML, Ho SM, Walker CL. Environmental estrogens differentially engage the histone methyltransferase EZH2 to increase risk of uterine tumorigenesis. Mol Cancer Res. 2012;10(4):546–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Eissenberg JC, Shilatifard A. Histone H3 lysine 4 (H3K4) methylation in development and differentiation. Dev Biol. 2010;339(2):240–249. [DOI] [PMC free article] [PubMed] [Google Scholar]