Abstract
Oxidative stress is reportedly associated with many diseases such as cancer, arteriosclerosis, diabetes and aging, but no practical biomarkers are currently available in actual clinical practice. Human mercaptoalbumin (HMA) and human non-mercaptoalbumin (HNA) are expected to become markers of oxidative stress, but the stability of HMA/HNA has been problematic. We investigated the conditions for stabilizing HMA/HNA and found that HMA/HNA was stable at room temperature for 25 h if whole blood samples were mixed with a citrate buffer so that the citric acid concentration after mixing was 70 mM or higher and the pH of the added buffer was less than pH 6.0. Whole blood samples were then collected under the above conditions, and the reference range for HNA was set at 21.8% ± 7.4% (HMA, 78.2% ± 7.4%) based on samples from 65 volunteers (28 males and 37 females; average age, 55.0 ± 13.8 years). The clinical usefulness of HMA/HNA as an oxidative stress marker should be clarified for specific pathological conditions using the previously reported, highly accurate measurement method under the conditions required for HMA/HNA stability.
Keywords: Oxidative stress, Albumin, HMA/HNA, Stabilization method, Reference range
Highlights
-
•
We identified the conditions under which human mercaptoalbumin and non-mercaptoalbumin were stable.
-
•
The reference range for non-mercaptoalbumin was defined under the above conditions.
-
•
An accurate measurement method and specimen stabilization are important for evaluating the clinical usefulness of measuring oxidized albumin levels.
-
•
The clinical usefulness of HMA/HNA will be clarified using highly accurate measurement method under stable conditions.
1. Introduction
Living organisms are capable of detoxifying substances (e.g., active oxygen and free radicals) that are harmful to their cells. This function enables them to repair damage caused by toxic substances. Usually, pro- and anti-oxidation activities are balanced in living organisms. In 1958, H. Sies defined oxidative stress as “a disturbance in the prooxidant-antioxidant balance in favor of the former” [1]. Since then, oxidative stress has been reported to be associated with many diseases such as cancer, arteriosclerosis, and diabetes as well as aging itself [[2], [3], [4]]. Various biomarkers have been used to evaluate oxidative stress; however, no practical biomarker has been identified. Therefore, there is an urgent demand to address this unmet medical need.
Human serum albumin is produced in the liver and is rapidly secreted to the extracellular space. Albumin reportedly has many important functions and was recently shown to be involved in the control of the redox state in the body [5]. Albumin is present in the form of either reduced albumin (HMA) with a 34Cys free SH group or oxidative albumin (HNA), which is produced by binding with cysteine and glutathione in the blood. Reportedly, approximately 75% and 25% of all albumin molecules are in the forms of HMA and HNA, respectively [6]. HNA (or HMA) has been considered to be a practical marker of oxidative stress, and studies using clinical specimens have been reported [[6], [7], [8]]. However, the conventional measurement methods of HNA or HMA have been complicated and requires a substantial amount of time. Furthermore, there was a problem in measurement accuracy for use in routine examination. Therefore, HNA measurements are not typically performed in daily clinical practice.
We recently reported a simple HPLC measurement system that enabled us to measure HMA/HNA precisely within a short period of time [9]. This system overcomes the above problems related to HNA measurement. However, the stability of HNA and HMA after blood sampling remains problematic [10]. HNA gradually increases over time (while HMA decreases) after blood sampling. Consequently, serum specimens must be promptly separated and stored at −80 °C. However, such practices are often difficult to achieve in daily clinical practice.
Kubota et al. have been studying the stabilization of clinical specimens [11] and reported that their samples remained stable at refrigeration temperature (4 °C). During routine clinical examinations, however, it is usually difficult to preserve samples at 4 °C immediately after sampling. Therefore, the development of a practical sample collection method to prevent changes in HMA/HNA levels is needed. In the current study, we aimed to establish a specimen collection method that would stabilize HMA/HNA values at room temperature immediately after blood collection; we then examined the reference ranges for these values.
2. Methods
2.1. Specimen stabilization
First, the stability of HMA/HNA under conventional sampling conditions was investigated. Three mL of whole blood was collected from one volunteer, the serum was immediately separated by centrifugation at 3000 rpm for 5 min at 4 °C and ice-cooled. Measurement of HMA/HNA was started immediately with the HPLC device, which was on standby. The HMA/HNA value in the same sample was measured continuously 100 times (the measurement time was 20 h). In a high-performance liquid chromatography (HPLC) (LabSolutions system; Shimadzu Co. Ltd, Kyoto, Japan) set at 15 °C, which were the same conditions as those previously reported [9]. An anion exchange column (50 × 7.6 mm ID) packed with a polyvinyl alcohol gel containing diethyl amine was used under the following conditions. Eluent A was a solution of 25 mM phosphate buffer containing 60 mM sodium sulfate (pH6.0), and eluent B was a 250mM magnesium chloride solution. The flow rate was 1 mL/min after equilibrating the column for 4.5 min with eluent A. We programed the linear gradient time from eluent A (100%) to eluent B (100%) for 7.5 min. The total measurement time was 12 min per sample. The sample size was 3 μL, and the temperature was 40 °C. The excitation and emission wavelengths were 280 nm and 340 nm, respectively. The repeatability (within-day variability) and reproducibility (day-to-day variability) were 0.3% and 0.27% (CV), respectively. The values were expressed as HMA% = HMA area/Total Alb area × 100 or HNA% = HNA area/Total Alb area × 100 using the area value (μV × seconds) of each chromatogram peak obtained using HPLC.
Secondly, we investigated the differences in the time-dependent changes in HNA/HMA values according to differences in the blood collection tubes. Blood samples from one volunteer were collected simultaneously in each of the blood collection tubes mentioned below. A vacuum blood tube for serum (Insepack, Tokuyama Sekisui, Japan), an EDTA-NaF tube (InsepackⅡ, Tokuyama Sekisui, Japan), an EDTA-2K tube (Neotube, Nipuro, Japan), a 3.13% citrate Na tube (Neotube, Nipuro, Japan), an EDTA-2Na tube (Neotube, Nipuro, Japan), and a heparin-Na tube (Venoject, Terumo, Japan) were used. The HMA/HNA values measured immediately after collection into each tube and after storage at 4 °C for 22 and 48 h after the sample collection were compared. The measurement of HMA/HNA was performed twice and the average value was used.
We then compared the HMA/HNA values measured immediately after collection and those measured at 22 h after storage at 4 °C and 37 °C. The samples were collected from a single volunteer and the HMA measurements were performed in duplicate; the average value was used.
We also investigated the appropriate composition of the citrate buffer solution (in terms of concentration and pH). In the citrate buffer concentration study, the concentration of citrate when the buffer and sample were mixed at a 1:1 ratio was adjusted to the range of 30 mM–150 mM in 20 mM–30 mM steps. In the pH study, the pH of the added citrate buffer (140 mM) was adjusted to the range of 5.0–7.0 in 0.5-increments and mixed 1:1 with the sample. The average value of duplicate measurements was considered as the measurement result. The results in Table 2 are from samples obtained from the same volunteer. The measurement of HMA was performed twice and the average value was used.
Table 2.
Changes in HMA (%) immediately after drawing and after 22 h of storage. (percent changes are denoted in parentheses).
| Immediately after drawing | After 22 h | |
|---|---|---|
| Stored at 4 °C | 78.6 | 75.6 (−3.0%) |
| Stored at 37 °C | 78.6 | 56.5 (−22.1%) |
2.2. Reference ranges for HNA/HMA
From April to October 2017, we enrolled a total of 106 participants who had received a medical checkup at the Center for Epidemiology and Preventive Medicine or were volunteers from the Department of Clinical Laboratory of the University of Tokyo Hospital. Among them, 65 subjects (28 male and 37 female) who fulfilled the criteria shown in Table 1 were selected as the reference individuals and their data were used as the reference range.
Table 1.
Criteria for reference individuals.
| Age | Body mass index (kg/m2) | Systolic blood pressure (mmHg) | Diastolic blood pressure (mmHg) | Red blood cells ( × 1012/L) | White blood cells ( × 109/L) | |
|---|---|---|---|---|---|---|
| male | >30 | 18–25 | <140 | <90 | 435–555 | 3.3–8.6 |
| female | 370–490 | |||||
| Platelets ( × 109/L) | Hemoglobin (g/dL) | Hematocrit (%) | Total protein (g/dL) | Albumin (g/dL) | Aspartate transaminase (IU/L) | |
| male | 15.8–34.8 | 13.7–16.8 | 40.7–50.1 | 6.5–8.6 | 3.9–5.1 | 13–31 |
| female | 11.0–14.8 | 34.0–44.0 | ||||
| Alanine transaminase (IU/L) | γ-glutamyl transferase (U/L) | Creatinine (mg/dL) | Plasma glucose (mg/dL) | Hemoglobin A1c (%) | Total cholesterol (mg/dL) | |
| male | 10–42 | 13–64 | 0.65–1.07 | 140> | 4.9–6.0 | 130–250 |
| female | 7–26 | 9–32 | 0.46–0.88 | |||
| LDL cholesterol (mg/dL) | HDL-cholesterol (mg/dL) | Triglyceride (mg/dL) | Uric acid (mg/dL) | |||
| male | 58–163 | 38–91 | 29–250 | 3.3–7.8 | ||
| female | 37–107 | 2.6–5.9 |
We added citrate buffer (pH6.0) to the blood collection tubes. The amount of citrate buffer was adjusted to a final concentration of 70 mM after blood sampling. Approximately 2 mL of blood was collected using the current tube, and the sample was centrifuged and stored at −80 °C until measurement. The measurement of HMA/HNA was performed under the HPLC conditions mentioned above.
The reference range was expressed as the mean ± 2 SD. Also, regression equations and correlations between HNA and the other parameters were determined using Spearman’s rank correlation coefficient.
2.3. Feasibility of HNA measurements using serum after routine examination
We selected the first 30 volunteers out of the 106 volunteers for this feasibility study. The 30 serum samples on which the routine examinations had been completed were collected as quickly as possible, and the HMA/HNA levels were measured in the residual serum samples. Blood samples were collected simultaneously from the same volunteers using a stabilizing agent, and the measured HMA/HMA values were compared with the HMA/HNA levels in the residual serum samples.
3. Results
3.1. Specimen stability after blood drawing
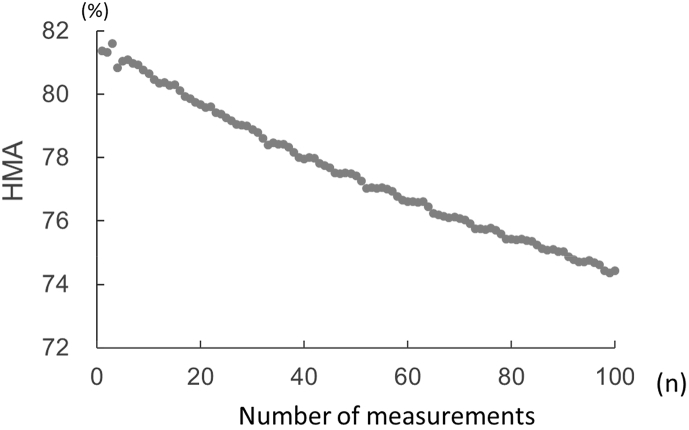
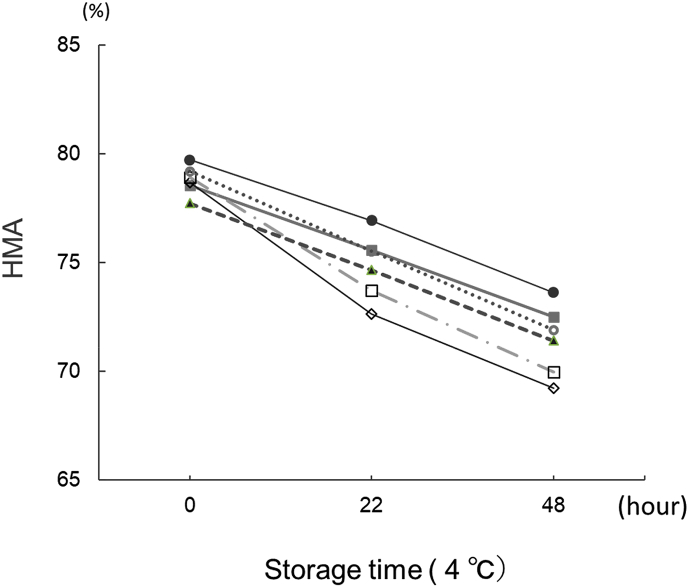
Fig. 1 shows the time course of HMA measurements performed using 100 samples every 12 min for 20 h. The HMA value decreased by about 8% (8% increase in HNA) over the 20-h period. Next, we collected blood samples using various commercially available collection tubes. Fig. 2 shows the HMA values obtained immediately after blood collection and at 22 and 48 h after blood collection and storage at 4 °C. HMA decreased over time in a linear manner in all the blood collection tubes that were used.
Fig. 1.
Changes in HMA values (%) when measured 100 consecutive times immediately after blood collection. The serum samples were placed in an HPLC autosampler set at 15 °C and using previously reported HPLC measurement conditions [9].
Fig. 2.
Change in HMA values (%) over time in serum and plasma samples collected using various commercially available blood collection tubes. The HPLC measurement conditions were the same as those used in Fig. 1. .
.
When collection tubes containing citric acid were used, the HMA values tended to be higher throughout the time course, compared with the values for other collection tubes. Also, the reductions in values were more remarkable for collection tubes containing sodium fluoride. Table 2 shows the decreases in the HMA levels when the serum samples were stored at 4 °C or 37 °C for 22 h. The HMA values decreased at both temperatures, but the reduction was more obvious for the latter condition.
Finally, we examined the appropriate composition of the citrate buffer solution. Table 3a shows the changes in HMA values for mixtures of citrate buffer and whole blood (1:1 ratio), and Table 3b show the changes in the HMA values according to different pH values for the citrate buffer solution. The HMA/HNA level did not decrease even after storage at room temperature for 25 h when a citrate buffer with a concentration of 70 mM or higher and a pH of pH6.0 or less was used.
Table 3a.
Time course of HMA according to citrate buffer concentration (concentration of total citrate buffer sample in addition to total blood sample).
| Citric acid concentration after addition (mM) | HMA (%) |
|
|---|---|---|
| Immediately after drawing | After 25 h | |
| 30 | 77.4 | 74.6 |
| 50 | 78.1 | 76.5 |
| 70 | 77.9 | 77.2 |
| 90 | 77.7 | 77.3 |
| 120 | 77.3 | 77.0 |
| 150 | 77.6 | 77.4 |
Note: The pH of each buffer sample was adjusted to pH5.0.
Table 3b.
Changes in HMA according to pH of added citrate buffer.
| pH | HMA(%) |
|
|---|---|---|
| Immediately after drawing | After 25 h | |
| 5.00 | 81.2 | 80.7 |
| 5.50 | 81.6 | 80.6 |
| 6.00 | 81.8 | 80.6 |
| 6.50 | 81.8 | 74.4 |
| 7.00 | 81.7 | 75.0 |
| 7.92 | 81.6 | 73.2 |
Note: The citric acid concentration of each added buffer was 140 mM (mixed citrate buffer and whole blood mixed at 1:1 ratio).
3.2. Reference ranges for HMA/HNA
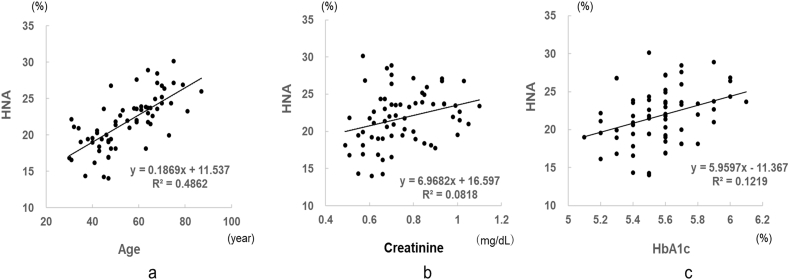
We set the reference ranges for HMA/HNA using blood samples collected into tubes containing citrate buffer under conditions at which HMA/HNA was shown to be stable (70 mM, pH6.0). The reference range for HNA was determined to be 21.8% ± 7.4% (HMA, 78.2% ± 7.4%) (mean age, 55.0 ± 13.8 years). The HNA values were normally distributed, with a 99% confidence interval of 1.19 and a 95% confidence interval of 0.89. The HNA level increased with age (Fig. 3), but sex differences were not observed. Table 4 shows the relationships between HNA and other parameters. HNA was positively correlated with age and the creatinine and HbA1c levels in healthy subjects.
Fig. 3.
Relationship between clinical parameters and HNA values (%) in reference individuals. The HPLC measurement conditions were the same as those used in Fig. 1 a; age, b; creatinine, c; HbA1c.
Table 4.
Spearman’s rank correlation coefficients between HNA and other parameters.
| β | α | RS | P value | |
|---|---|---|---|---|
| Age | 0.187 | 11.537 | 0.697 | 0.000 |
| Body mass index (kg/m2) | 0.355 | 14.235 | 0.217 | 0.082 |
| Systolic blood pressure (mmHg) | 0.056 | 15.401 | 0.218 | 0.081 |
| Diastolic blood pressure (mmHg) | 0.031 | 19.629 | 0.068 | 0.589 |
| Pulse pressure (mmHg) | 0.080 | 18.272 | 0.238 | 0.056 |
| Red blood cells ( × 1012/L) | −0.004 | 23.687 | 0.044 | 0.727 |
| White blood cells ( × 109/L) | −0.716 | 25.330 | 0.217 | 0.082 |
| Platelets ( × 109/L) | −0.057 | 23.109 | 0.058 | 0.648 |
| Hemoglobin (g/dL) | 0.324 | 17.454 | 0.098 | 0.436 |
| Hematocrit (%) | 0.160 | 15.255 | 0.137 | 0.275 |
| Total protein (g/dL) | 0.922 | 15.414 | 0.094 | 0.458 |
| Albumin (g/dL) | −0.125 | 22.352 | 0.008 | 0.949 |
| Aspartate transaminase (IU/L) | 0.949 | 18.380 | 0.200 | 0.111 |
| Alanine transaminase (IU/L) | 0.058 | 20.889 | 0.086 | 0.494 |
| γ-glutamyl transferase (U/L) | 0.110 | 19.603 | 0.231 | 0.064 |
| Creatinine (mg/dL) | 6.968 | 16.597 | 0.286 | 0.021 |
| Plasma glucose (mg/dL) | −0.020 | 23.708 | 0.058 | 0.647 |
| Hemoglobin A1c (%) | 5.960 | −11.367 | 0.349 | 0.004 |
| Total cholesterol (mg/dL) | 0.016 | 18.695 | 0.131 | 0.300 |
| LDL cholesterol (mg/dL) | 0.026 | 18.791 | 0.192 | 0.124 |
| HDL cholesterol (mg/dL) | −0.037 | 24.309 | 0.143 | 0.254 |
| Triglyceride (mg/dL) | −0.006 | 22.333 | 0.061 | 0.627 |
| Uric acid (mg/dL) | 0.761 | 19.014 | 0.280 | 0.054 |
3.3. Feasibility of HNA measurements using serum after routine examination
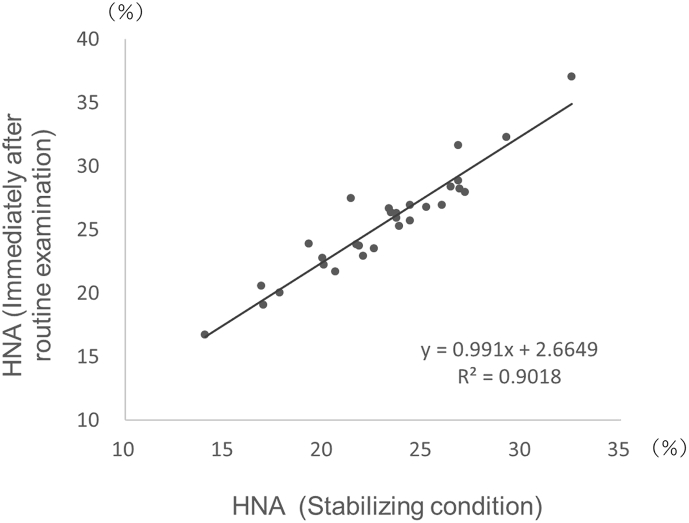
The residual blood remaining after routine examination can be collected quickly and is often used for subsequent evaluations. We examined whether residual blood can be used for the clinical evaluation of HNA levels. Residual serum was collected every hour for 1–4 h after routine biochemical testing using samples obtained from 30 volunteers. The HNA values in the residual blood after routine examination were 2.5% (range, 0.8%–6.1%) higher than those obtained in stable samples (Fig. 4).
Fig. 4.
Comparison of HNA values (%) of stabilized specimens and HNA values (%) in residual blood after routine testing. The HPLC measurement conditions were the same as those used in Fig. 1.
4. Discussion
In the 1980s, an HMA/HNA measurement method using HPLC was developed by Sogami et al. [6], and this method was subsequently improved by Era et al. [12]. Although relationships between HMA/HNA and several diseases have been reported, HMA/HNA measurements were complicated to perform and were considered to have a poor accuracy.
Because 1 h was required to measure one sample, use in daily clinical practice was difficult. The stability of the specimens was also problematic [10], and clinical applications did not progress. The principal aim of the current study was to overcome these problems.
In the recent study, we developed a simple and accurate HPLC measurement method. Also, in the current study we examined the stability of HMA/HNA, which is important for routine use in clinical practice. We found that HMA/HNA was stable at room temperature for 25 h when a high concentration citrate buffer solution with a pH of pH6.0 or less had been previously added to the blood collection tube. The current method may enable more accurate evaluations of HMA/HNA and may lead to widespread measurement at clinical laboratories.
The stability of HMA/HNA in blood specimens has been studied by Kubota et al. [11]. Samples, such as plasma, were diluted between 50 and 100000 times using a buffer solution with a pH of pH4-9. By diluting the samples, the concentration of low molecular weight molecules, which promote the change from HMA to HNA, was reduced. As a result, the HMA/HNA levels did not change and remained stable for 48 h at 4 °C. Furthermore, it was found that HMA remained stable at 4 °C for 78 h after the addition of 0.5 M sodium citrate to whole blood at a ratio of 1:9. While this method seemed to be an effective means of stabilizing HMA/HNA, storage at 4 °C during and after blood collection is difficult to perform in daily clinical practice. According to the results of the current study, a higher concentration of citric acid than that used in the study by Kubota et al. is needed to stabilize HMA/HNA and lowering the pH of the citrate buffer increases the stabilizing effect.
We also set the reference range for HNA, which is necessary for the clinical use of HNA as a disease marker. In this study, the reference range of HNA was set using samples collected under conditions at which HMA/HNA remained stable and the previously developed high-precision HPLC method [9]. In comparison with the previously reported values [6], the HNA value was 3% lower than the conventional value (while the HMA value was about 3% higher). The reason for this discrepancy might be the effect of the stabilization method used to collect the specimens in the present study. As shown in Fig. 1, the HMA/HNA value varies immediately after blood sampling. Specimens were placed in the HPLC apparatus for measurement, but the HMA/HNA levels continued to change inside the HPLC autosampler. Using the conventional method, the measurement process requires 1 h for each sample, during which time the HMA/HNA levels would continue to change.
The results of the current study showed that the differences in the HMA/HNA values caused by the conditions of sample collection were considerably large. The HNA values in residual blood after routine examination were 2.5% (range, 0.8%–6.1%) higher than those in fresh samples (Fig. 4). In some cases, this difference may be larger than the difference in HNA values between normal subjects and patients with targeted diseases that are being examined using the current biomarker. Therefore, the sample collection conditions are very important not only for HMA/HNA, but also for many oxidative stress markers. When measuring oxidative stress markers, it is necessary to consider fully the influence of the sample collection method on the measured values as well as the accuracy of the measurement method itself.
The relationships between the HNA level and other parameters have been previously studied using our HPLC method [13,14]. In patients with CKD and diabetes, HNA was strongly and positively associated with age, creatinine, and hemoglobin, but not with HbA1c. On the other hand, in the presently reported study using normal subjects, HNA was positively correlated with age and HbA1c but was not correlated with the blood glucose level.
According to the present and previous reports, the HNA value increased with age (while the HMA value decreased) [9,10,13,14]. For the clinical use of HMA/HNA, further study to identity the proper method of correcting HMA values according to age is likely needed, since the number of subjects in the present study was too small to examine such matters. In the future, the collection of accurate data using a larger cohort is needed.
Currently, the relationship between the levels of HMA/HNA and various pathological conditions is being studied using our measurement method under the sample stabilization conditions described. The HMA/HNA levels are being found to be well correlated with the development of oxidative stress-related diseases.
5. Conclusion
We previously reported an accurate method for measuring HMA/HNA that can be applied to multiple specimens. In the current study, we identified the appropriate conditions for sample collection necessary to stabilize HMA/HNA at room temperature. Also, we set the reference range for HNA. Future studies are expected to clarify the clinical significance of HMA/HNA.
Declarations of interest
None.
Funding acknowledgement
This research was conducted by the Nakatani Medical Instrumentation Technology Promotion Foundation (Japan) and Scientific Research Fee A(25253040) (Japan). We thank the people concerned.
References
- 1.Sies H. Academic Press; London: 1985. Oxidative Stress. [Google Scholar]
- 2.Poprac P., Jomova K., Simunkova M., Kollar V., Rhodes C.J., Valko M. Targeting free radicals in oxidative stress-related human diseases. Trends Pharmacol. Sci. 2017;38:592–607. doi: 10.1016/j.tips.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Kattoor A.J., Pothineni N.V.K., Palagiri D., Mehta J.L. Oxidative stress in atherosclerosis. Curr. Atheroscler. Rep. 2017;19 doi: 10.1007/s11883-017-0678-6. [DOI] [PubMed] [Google Scholar]
- 4.Ewald C. Redox signaling of NADPH oxidases regulates oxidative stress responses. Immun. Aging Antioxid. 2018;7:130. doi: 10.3390/antiox7100130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anraku M., Chuang V.T.G., Maruyama T., Otagiri M. Redox properties of serum albumin. Biochim. Biophys. Acta. 2013;1830:5465–5472. doi: 10.1016/j.bbagen.2013.04.036. [DOI] [PubMed] [Google Scholar]
- 6.Sogami M., Era S., Nagaoka S., Kuwata K., Kida K., Sigemi J. High-performance liquid chromatographic studies on non-mercapt ⇆ mercapt conversion of human serum albumin. II. J. Chromatogr. 1985;332:19–27. doi: 10.1016/s0021-9673(01)83283-0. [DOI] [PubMed] [Google Scholar]
- 7.Oettl K., Birner-Gruenberger R., Spindelboeck W., Stueger H.P., Dorn L., Stadlbauer V., Putz-Bankuti C., Krisper P., Graziadei I., Vogel W., Lackner C., Stauber R.E. Oxidative albumin damage in chronic liver failure: relation to albumin binding capacity, liver dysfunction and survival. J. Hepatol. 2013;59:978–983. doi: 10.1016/j.jhep.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 8.Terawaki H., Yoshimura K., Hasegawa T., Matsuyama Y., Negawa T., Yamada K., Matsushima M., Nakayama M., Hosoya T., Era S. Oxidative stress is enhanced in correlation with renal dysfunction: examination with the redox state of albumin. Kidney Int. 2004;66:1988–1993. doi: 10.1111/j.1523-1755.2004.00969.x. [DOI] [PubMed] [Google Scholar]
- 9.Yasukawa K., Shimosawa T., Okubo S., Yatomi Y. A simple, rapid and validated high-performance liquid chromatography method suitable for clinical measurements of human mercaptalbumin and non-mercaptalbumin. Ann. Clin. Biochem. 2018;55 doi: 10.1177/0004563217693257. [DOI] [PubMed] [Google Scholar]
- 10.Era S., Kuwata K., Imai H., Nakamura K., Hayashi T., Sogami M. Age-related change in redox state of human serum albumin. Biochim. Biophys. Acta. 1995;1247:12–16. doi: 10.1016/0167-4838(94)00166-e. [DOI] [PubMed] [Google Scholar]
- 11.Kubota K., Nakayama A., Takehana K., Kawakami A., Yamada N., Suzuki E.I. A simple stabilization method of reduced albumin in blood and plasma for the reduced/oxidized albumin ratio measurement. Int. J. Biomed. Sci. 2009;5:293–301. [PMC free article] [PubMed] [Google Scholar]
- 12.Era S., Hamaguchi T., Sogami M., Kuwata K., Suzuki E., Miura K., Kawai K., Kitazawa Y., Okabe H., Noma A., Miyata S. Further studies on the resolution of human mercapt- and nonmercaptalbumin and on human serum albumin in the elderly by high-performance liquid chromatography. Int. J. Pept. Protein Res. 2009;31:435–442. doi: 10.1111/j.1399-3011.1988.tb00900.x. [DOI] [PubMed] [Google Scholar]
- 13.Masudo R., Yasukawa K., Nojiri T., Yoshikawa N., Shimosaka H., Sone S., Oike Y., Ugawa A., Yamazaki T., Shimokado K., Yatomi Y., Ikeda H. Evaluation of human nonmercaptalbumin as a marker for oxidative stress and its association with various parameters in blood. J. Clin. Biochem. Nutr. 2017;61 doi: 10.3164/jcbn.17-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakatani S., Yasukawa K., Ishimura E., Nakatani A., Toi N., Uedono H., Tsuda A., Yamada S., Ikeda H., Mori K., Emoto M., Yatomi Y., Inaba M. Non-mercaptalbumin, oxidized form of serum albumin, significantly associated with renal function and anemia in chronic kidney disease patients. Sci. Rep. 2018;8:1. doi: 10.1038/s41598-018-35177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]