Abstract
Half of individuals with a whiplash injury experience ongoing pain and disability. Many are insufficiently active for good health, increasing their risk of preventable morbidity and mortality, and compounding the effects of the whiplash injury. This paper describes a protocol for evaluating the efficacy of a physical activity promotion intervention in adults with whiplash associated disorders. A multiple-baseline, single case experimental design will be used to evaluate the effects of a physical activity (PA) intervention that includes evidence-based behaviour change activities and relapse prevention strategies for six adults with chronic whiplash. A structured visual analysis supplemented with statistical analysis will be used to analyse: accelerometer-measured PA, confidence completing PA in the presence of neck pain, and pain interference.
Keywords: Whiplash associated disorders, Physical activity, Health promotion
Abbreviations used in text
- AEP
Accredited Exercise Physiologist
- APAP
Adapted Physical Activity Program
- DASS-21
The Depression, Anxiety and Stress Scale Short Version
- IER-S
The Impact of Event Scale – Revised;
- MVC
motor vehicle crash
- PA
physical activity
- PCS
Pain Catastrophizing Scale
- PSEQ
Pain Self-Efficacy Questionnaire;
- RCI
Reliability Change Index
- SCED
single case experimental design
- SF-12
The Medical Outcomes Survey Short Form
- WAD
whiplash associated disorders
1. Background
Whiplash associated disorders (WAD) is a term used to describe a syndrome of symptoms, including neck pain and disability that may result from an acceleration/deceleration injury of the neck following a motor vehicle crash (MVC). WAD are the most common non-hospitalised injuries resulting from a MVC, accounting for approximately 75% of all survivable MVC injuries [4]. The consequent pain and disability experienced incurs substantial socioeconomic costs; for example, these costs exceeded $350 million in Queensland, Australia from 2011 to 2012 [5]. Research evidence consistently reports that only 50% of individuals with WAD experience full recovery; approximately 25% continue to experience persistent moderate/severe pain and disability; and 25% have milder levels of pain and disability [6,7], with the moderate/severe disability group incurring the majority of the associated costs.
Emerging evidence has shown that individuals with chronic WAD may have lower levels of aerobic capacity and isometric strength compared with age-matched individuals with no neck pain [8], and higher levels of self-reported pain and disability when compared to individuals with non-traumatic neck pain [9]. It is not known if this reduced physical fitness is associated with an increased risk of on-going pain and disability. Previous research has shown that compared with healthy individuals, individuals with chronic non-traumatic neck pain have lower levels of leisure-time PA, with reduced PA associated with heightened risk of on-going neck pain [10,11]. It may be that individuals with chronic WAD are insufficiently active for good health, increasing their risk of preventable morbidity and mortality, and compounding the effects of the WAD.
To date, interventions for individuals with WAD have been based on a deficit model focussed on impairment or remediation in a rehabilitation setting with the aim of improving pain and disability, however the results of these trials are equivocal and identifying the optimal treatment for these individuals continues to be a challenge [[12], [13], [14]]. Previous interventions have not addressed the need for promoting sustained PA participation to improve physical and psychological health [13,15]. Moreover, many intervention trials used a group-level design and analysis which has limited the ability to characterise individual effects in heterogeneous samples [13,16,17]. To our knowledge, no study has evaluated the effects of an intervention specifically focussed on increasing habitual PA in individuals with chronic WAD using a single-subject experimental design. Due to heterogeneous nature of the WAD population, the use of a single-case experimental design is advantageous as it allows for the analysis of the intervention effect at an individual level and ensures that average effects that would be generated by a randomised controlled trial do not mask a heterogeneous response.
A treatment approach that has previously been shown to effectively increase PA adoption in other populations is the Adapted Physical Activity Program (APAP) [18,19]. This paper presents a protocol to evaluate the impact of participation in APAP on target behaviours comprising: accelerometer-measured PA participation; perceived confidence in participating in PA in the presence of neck disability and pain; and pain interference. Measures associated with the adoption and maintenance of PA and factors associated with on-going pain and disability in WAD will be evaluated to determine the generalised impact of the intervention. Acceptability of the intervention program will be evaluated using a semi-structured interview.
2. Methods
2.1. Study design
The efficacy of the intervention will be evaluated using a single-case experimental design, specifically a concurrent multiple-baseline design across participants with replication (A-B + maintenance), where A is the baseline, B is the intervention period and maintenance is the follow up period [20].
The study will be conducted and reported as per the Single Case Reporting Guidelines In BEhavioural Interventions (SCRIBE) 2016 Statement [21]. A minimum of three participants, and at least five data collection points within each phase, is recommended to meet design standards [22].
2.2. Participants
Six participants who meet the following inclusion criteria will be recruited for this study: aged 18–60 years; grade II whiplash of at least 3 months duration; living in a community setting in the Brisbane, Gold Coast or Northern New South Wales region; a Neck Disability Index Scale score >30% [23]; deemed medically safe to participate in moderate to vigorous intensity PA as per Exercise and Sports Science Australia's Adult Pre-Exercise Screening System [24] and the American College of Sports Medicine Guidelines [25]; currently not enrolled in structured sport or training for physical fitness; and not completing 30 min of moderate PA on 5 or more days per week. Exclusion criteria for the study will be: known or suspected serious spinal pathology; nerve root compromise; confirmed fracture or dislocation at time of injury; or spinal surgery in the past 12 months.
Individuals with chronic WAD who have previously contacted Recover Injury Research Centre, Brisbane, and expressed interest in participating in experimental studies will be contacted by a Research Assistant (RA) and screened for eligibility for the study. Individuals who are eligible and interested will be provided with information regarding the study and invited to participate. Following an opportunity for questions, individuals will be asked to provide written, informed consent.
2.3. Procedure
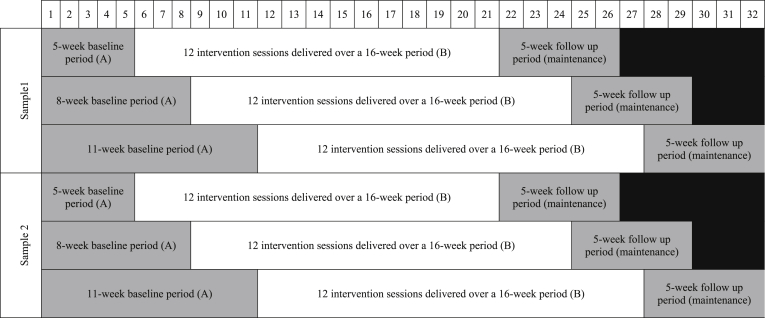
The six participants will be recruited in two samples of three participants (Study 1 initial evaluation; and Study 2 direct replication), represented schematically in Fig. 1. Each sample will comprise three participants who receive the same 16-week intervention (B) and 5-week follow up (maintenance), but have different lengths of the baseline period (A). The participants will be randomly allocated to one of the two samples, then subsequently randomly allocated to a baseline of either 5, 8 or 11 weeks. All participants will begin the baseline phase during the same week. Concurrent enrolment will minimize environmental influences and enhance internal validity [20].
Fig. 1.
Participants will be randomly allocated to one of two samples of three participants. Within each sample participants will be randomly allocated to either a 5, 8 or 11 week baseline data collection period (A). The baseline phase will be followed by a 16-week intervention period (B); comprised of 12 1 h sessions with an Accredited Exercise Physiologist completed in their home and community environment. The intervention phase will be followed by a 5-week follow up phase (maintenance) where participants will have no contact with the intervention personnel.
Experimental control is demonstrated by using the staggered multiple-baseline design across the participants, which controls for threats to internal validity (e.g., history, maturation) [20]. The added feature of randomisation strengthens the scientific rigor of the study [26]. The design also allows for three demonstrations of the experimental effect (i.e., increased PA following the introduction of the intervention, but not before), and replication in a second sample of participants [20]. Replication of the experimental design allows for improved clarity regarding the demonstration of intervention effect [27].
During the baseline data collection period (A) participants will be encouraged to undertake their usual behaviour. Target behaviour measures will be collected weekly during the baseline phase. Individual variability is addressed by repeated measurement of the target behaviour, and specifically, five data collection points within each phase is recommended to effectively evaluate a SCED intervention [22].
The 16-week intervention period (B) comprises 12 one-hour intervention sessions (described below). An Accredited Exercise Physiologist (AEP) with experience in exercise prescription for individuals with a disability and behaviour change strategies associated with increasing PA will deliver the intervention. During the intervention period, the target behaviour measures will be collected fortnightly.
The intervention phase will be followed by a 5-week follow up phase where participants will have no contact with the AEP and target behaviour measures will be collected weekly. The maintenance component allows the target behaviour to be monitored after the completion of the intervention, with the expectation that the target behaviour will not revert to baseline levels after the intervention completion [28].
Generalisation measures will be collected at 4 sampling points throughout the study duration: 1) at the commencement of the baseline data collection period; 2) at the finish of the baseline data collection period (which coincides with the intervention start point); 3) at the end of the intervention period (which coincides with the commencement of the maintenance period); and 4) at the end of the maintenance period.
Ethical approval for this study was received from Griffith University's Human Research Ethics Committee (Approval Number 2017/743) and the University of Queensland's Human Research Ethics Committee (Approval Number 2018000349/HREC/2017/743). The protocol of this study is registered through the Australia and New Zealand Clinical Trials Registry (ACTRN: ACTRN12617001261303p) and ClinicalTrials.gov (Protocol Number: 2018000349/2017/743).
2.4. Intervention
APAP is a theory-based PA promotion intervention for people with chronic and complex conditions delivered in the participant's home and community [18,19]. APAP is comprised of four steps described below and presented schematically in Fig. 2. A detailed description of the program, including the theoretical framework and a worked case study has been published elsewhere [18].
Fig. 2.
The Adapted Physical Activity Program. All participants receive Step 1, 2, 3 and 4 of the intervention program. Post completion of the pre-participation activities (Step 1), individuals receive individualized behaviour change strategies tailored to their Stage of Change (Step 2). Based on the discussions undertaken during Step 2, individuals participate in two main types of physical activity: structured exercise (Step 3a); and/or lifestyle physical activity (Step 3b). Participants will also receive tailored relapse prevention strategies in order to promote the sustainability of the program outcomes (Step 4). Adapted from: Clanchy (2018) [[1], [2], [3]].
All participants receive the four program steps during 12 sessions conducted over a 16-week period. Each session is approximately 1 h in duration and the inter-session time varies depending on the needs of the individual. Typically more sessions are completed in the first 6 weeks as discussions regarding values and motivations relating to PA participation evolve, community access is arranged, skills relating to PA participation are learned and habits for increased participation established. Session frequency diminishes as a greater emphasis is placed on fostering independence and self-management of PA. An example of a home visit schedule for 12 home visits over 16 weeks that would promote the adoption of physical activity participation and the evaluation of the maintenance of the activity undertaken would be: twice-weekly sessions for weeks 1–2; weekly sessions during weeks 3–6; and once every two to four weeks for weeks 7–16. The gradual increase in time between face to face sessions permits the practitioner to evaluate the likelihood of physical activity maintenance and the identification of potential barriers with the aim of providing tailored strategies as solutions to these barriers, prior to the intervention concluding.
Step 1 involves a comprehensive preparticipation assessment undertaken to determine key factors that would impede or enhance PA adoption and maintenance for the individual participant. Factors specific to WAD include: mechanism and injury history; previous treatment received; primary effects of the injury; activities that aggravate current injury symptoms; pain levels and pain management strategies; and medication use.
Step 2 entails the application of evidence based strategies for the promotion of PA participation tailored to the participant's motivational readiness. The included strategies specifically target factors related to increased PA participation including: knowledge about the behaviour; value of the behaviour; perceived costs and benefits of the behaviour; barriers to change; and beliefs regarding the individual's ability to perform the behaviour [29]. In addition to the participant's motivational readiness, selection of an appropriate strategy is dependent on the participant's intended goals or activities of interest, the results of their preparticipation assessment and the outcomes from the previous session.
-
Step 3 involves participants self-selecting and undertaking an individually tailored combination of structured exercise (Step 3a) and/or lifestyle PA (Step 3b) dependant on earlier discussions (Step 2). The focus of Step 3a is to implement a safe, effective and enjoyable exercise program for the individual tailored to the individual's current fitness and physical functioning, health goals, and the outcomes of the pre-participation screening. Program development focuses on movements and activities that the participant can complete safely and confidently, with the inclusion of education regarding the maintenance and progression of the exercise program post completion of the program.
Lifestyle PA prescription (Step 3b) includes the promotion of PA achieved through participation in leisure, occupational, household or sport related activities. The process of sourcing and evaluating suitable and sustainable options for lifestyle PA participation is achieved through collaboration with the participant and the AEP, with a focus on developing the skills required for the participant to complete these tasks independently beyond the program duration.
Step 4 entails the delivery of individually tailored relapse prevention strategies designed to help participants identify potential situations in which their PA routine may be disrupted or stopped (e.g., illness, injury or pain, reduction in social support, weather changes) and to formulate appropriate strategies to restart or continue with their PA program [30].
The features of APAP are well situated to help promote PA and positive health outcomes in adults with WAD. The focus of the intervention is to promote intrinsically enjoyable activity that has a demonstrated influence on the risk of chronic disease. Additionally, the intervention described may also have the secondary benefits of improving symptoms of chronic WAD including pain, and psychological measures. Step 3a relates to active exercise that can involve functional exercises (stretching and isometric), range of motion exercises and general strengthening exercises which have been determined to be an important component of rehabilitation for patients with WAD [31]. The exercise program incorporates pacing of activity participation including a slow progression of the duration and intensity of activity with a focus on increasing general physical fitness and reducing fear of movement [32]. Step 3b promotes the adoption and maintenance of normal recreational and occupational activities with a focus on improving function and community integration. Tailoring of APAP will be undertaken to include strategies known to be associated with increased PA participation and effective self-management in adults with WAD including setting specific, realistic and relevant goals, the use of positive encouragement, promotion of positive attitudes and beliefs and barrier identification and resolution [31,33,34].
Training of the AEP will be provided by two of the investigators (KC and ST). Four 3-hour training sessions will be undertaken comprising presentations of the intervention protocols, case studies and role play. Supervision of the intervention delivery will be conducted through regular meetings between the AEP and research staff. Intervention fidelity will be evaluated through a review of the activities undertaken during individual intervention sessions to ensure that the activities completed are consistent with the intervention protocol.
3. Measures
Outcome measures will be collected through three means: objectively through the use of a PA monitor; self-reported through the use of online surveys; and a face-to-face semi-structured interview. Compliance with the PA monitor will be monitored by the AEP during the intervention period and a research assistant, independent to the intervention delivery, during the baseline and maintenance periods. Compliance to the self-report measures will be monitored by an investigator external to the study. The influence of bias is reduced through collecting both the target behaviours and generalisation measures via machine or computerised methods [28]. The semi-structured interview will be conducted by a researcher external to the delivery of the intervention to gain an in-depth understanding of the participant's experience with the intervention.
4. Target behaviours
The three target behaviours are: PA participation; confidence for completing daily tasks in the presence of neck pain and disability; and pain interference relating to participation in day to day tasks and home and social activities. The primary outcome is physical activity participation and the secondary outcomes related to increased physical activity participation are confidence for completing daily tasks in the presence of neck pain and disability; and pain interference relating to participation in day to day tasks and home and social activities.
Habitual PA will be measured using the ActiGraph GT9X Link wrist worn accelerometer (ActiGraph, USA). The ActiGraph is a triaxial accelerometer that measures vertical acceleration 25 times each second, and these data are integrated over a user defined period, or epoch, to give a number of “counts.” The monitor will be programmed to display the time and date only to the participant. An epoch of 1 min will be used in this study.
A custom, single-item question to assess confidence for completing daily tasks will be assessed at the start of each ActiGraph monitoring period. This question will ask participants to identify “how confident are you in your ability to perform your daily tasks in the presence of your neck pain or disability?” with 1 indicating not all confident; 2 - slightly confident; 3 - moderately confident; 4 - very confident; and 5 - extremely confident.
To assess pain interference relating to participation in day to day tasks, and home and social activities, participants will complete 3 questions from the PROMIS – Pain Interference Scale [35,36]: In the past seven days, how much did pain interfere with your a) day to day activities, b) work around the home, and c) ability to participate in social activities. Responses are made on a 5 point Likert-type scale with 1 indicating not at all, 2 - a little bit, 3 - somewhat, 4 - quite a bit and 5 - very much.
4.1. Generalisation measures
Generalisation measures are dependent variables that are measured in addition to the target behaviours to evaluate whether the effects of the intervention generalises to other settings, behaviours or outcomes of interest proximal to the target behaviours. Generalisation measures are proposed to strengthen the external validity of the research outcomes [28].
Generalisation measures will assess changes in measures associated with the adoption and maintenance of PA. Stage of Change or motivational readiness will be assessed using Marcus’ (1992) Stage of Change Questionnaire [37]. This measure aims to quantify motivational readiness for change and current engagement in PA participation. Evidence indicates significant differences in PA participation across stages of change [38]. Social support for PA participation will be measured using the 13-item scale developed by Sallis et al. (1987). The measure has demonstrated acceptable levels of reliability, internal consistency and concurrent criterion validity with self-reported PA participation [39]. Decisional balance for PA participation will be measured using the 16-item scale developed by Marcus et al. (1992a) which included 6 items representing the avoidance of exercise (cons) and 10 items representing the positive perceptions of exercise (pros). Analysis of variance indicates that total decisional balance, the pro items and con items are significantly associated with stage of change [40].
Generalisation measures will also assess changes in several factors shown to be associated with on-going pain and disability in WAD. Neck disability will be assessed using the Neck Disability Index, a valid, reliable and responsive measure of neck pain related disability [23]. The questionnaire has 10 items concerning pain and activities of daily living including personal care, lifting, reading, headaches, concentration, work status, driving, sleeping and recreation. Perceived health related quality of life will be assessed using The Medical Outcomes Survey Short Form (SF-12) measure. The SF-12 contains 12 questions relating to physical functioning and mental health. The SF-12 has demonstrated evidence of validity and reliability for use in individuals with chronic disease [41,42]. A Numeric Pain Rating Scale will be used to assess neck pain [43]. Evidence indicates that Numeric Pain Rating Scales have a sufficient level of discriminative validity to differentiate pain intensity in chronic pain patients [44]. The Pain Catastrophizing Scale (PCS) will be used to assess catastrophizing thoughts associated with pain [45]. The PCS has been shown to have adequate internal consistency [46]. The Pain Self-Efficacy Questionnaire (PSEQ) will be used to assess an individual's confidence to perform specific tasks in the presence of pain. The PSEQ has been shown to have excellent internal consistency, a high test-retest reliability [47] and strong correlations with measures relating to pain related disability, coping strategies, and activity-specific measure of self-efficacy beliefs [48]. Avoidance of PA participation will be assessed using the Avoidance Subscale of the Negative Responsivity to Pain measure [49]. The Negative Responsivity to Pain Measure has been shown to have excellent internal consistency and adequate test-retest reliability [49]. The Depression, Anxiety and Stress Scale Short Version (DASS-21) will be used to assess symptoms of three negative emotional states: depression, anxiety and stress [50]. The DASS‐21 subscales have been demonstrated to have acceptable levels of validity and reliability [51]. The Impact of Event Scale-Revised (IES-R) is a 22-item survey developed to assess an individuals' subjective distress to a specific traumatic event [52]. The IES-R has been demonstrated to have adequate internal consistency and concurrent and discriminative validity [53].
4.2. Semi-structured interview
At the conclusion of the intervention, participants will be asked to participate in a semi-structured interview regarding their perceptions of the intervention's efficacy and acceptability. Interview questions will relate to: participant expectations; positive and negative experiences; PA, health and lifestyle outcomes associated with their participation in APAP; and the broad benefits of the intervention for individuals with WAD.
5. Data analysis
Data for each participant across each target behaviour will be graphed and analysed separately using a structured visual analysis supplemented with statistical analysis. Data will be screened for serial dependency using the recommended delta-recursive estimator [54], and if present, the statistical significance of the autocorrelation will be tested using the formula recommended by Huitema and colleagues [55]. Visual analysis is used to determine whether a functional relationship exists between the introduction of an intervention and a change in the target behaviours. Furthermore, evaluation of the structured visual analyses across participants helps to determine whether these changes are reliably and consistently replicated across multiple participants [20,56]. Visual analysis examines the data within each phase of the study to assess the within-phase pattern; and compares the data from each phase with data in the adjacent phase to assess potential changes in the target behaviours resulting from the introduction of the intervention [22]. Visual analysis is an accepted method for SCED analysis, with the assumption that only clear and potent intervention effects produce dramatic, replicable changes in behaviour that can be easily identified in a well-designed graphic display [56]. Following the recommendation of Kratochwill et al. [22] within-phase data patterns are evaluated using four features: 1) level; 2) trend; 3) variability; and 4) immediacy of effect. The between-phase patterns will be evaluated through evaluation of the data overlap between phases and review of the consistency of data patterns across similar phases in different participants. The results of these analytic processes will be assessed individually to determine whether any change in each outcome measure can be attributed to participation in the intervention program.
Level is the discontinuity or shift of performance from the end of one phase to the beginning of the next phase (e.g., change in the target behaviours between the baseline phase and the intervention phase) [20]. Due to the potential latency of the intervention effect in this study, the median values for the target behaviours in each phase will be used to determine changes in level [56].
Trend is the tendency of data to show a systematic increase or decrease over time within- and between-each phase, indicated by the slope of the best-fitting straight line for the target behaviour within a phase. A split-middle approach will be applied to estimate trend direction within each phase using the median values for the target outcomes, and regression coefficients will be calculated using ordinary least squares estimation to evaluate trend [20,56]. The split-middle approach is recommended as it does not rely upon independence of the data, being robust to the effects of serial dependency or autocorrelation of the data [56].
Variability refers to the range, variance or standard deviation of the data points around the best-fitting line. Excessive variability or scatter in the data is expected to reduce the strength of the inferences that can be made regarding the intervention effect. The variability of the data in each phase will be calculated and compared to subsequent phases. Considerable overlap in the variability (e.g., range) between phases will be noted as a potential confounder of the intervention effect [22]. A principle requirement for demonstrating the intervention effect, is the change in the target behaviours after the introduction of the intervention, not before, therefore a stable baseline phase is required [20]. The variability within the baseline values for the target behaviours will be evaluated by applying a stability criterion of 80% of the data points being within ±25% of the median value for the phase [56].
Immediacy of effect is the period of time between the change in phase and the subsequent change in the target behaviours (e.g., the time between completion of the baseline phase and the subsequent changes in target behaviours after the intervention begins). It is proposed that the closer the change in the target behaviours occurs after the start of the intervention, the clearer the intervention effect [20]. However, as this intervention aims to promote increased PA participation in individuals who are predominately sedentary, a latency of change is expected as the motivation and values that determine the change in behaviour are established. Consequently, the immediacy of effect will be noted, however it will not be used as a key factor to determine intervention efficacy [20].
Overlap is the proportion of data from one phase that overlaps with data from the previous phase [57]. The percentage of non-overlapping data will be determined, with a higher percentage of non-overlapping data indicates a larger intervention effect [56]. Tau-U will be used to determine the overlap of data points between baseline and intervention phases [58]. Tau-U is a non-parametric statistical method that combines non-overlap between phases with trends from within phases including corrections for possible trends during the baseline phase or during the intervention phase [59,60]. This technique provides a result for each individual tier of the multiple-baseline design, as well as a weighted average across the tiers as an estimate of the intervention effect. This statistical technique will also be applied separately to the second sample. Tau U is derived from Kendall's Rank Correlation (Tau) and the Mann-Whitney U test, and provides an intervention effect size (ES) and a weighted average for the three tiers [27,[58], [59], [60]].
Consistency is defined as the extent to which data patterns are the same across phases within the same condition and the replication of the demonstration of intervention effect between participants [61]. A higher consistency in the data patterns is associated with a greater likelihood that the changes in the target behaviour can be attributed to the introduction of the intervention [22]. The CONsistency of Data Patterns (CONDAP) approach will be applied in order to assess consistency in the data across the AB phase with the same condition (e.g., consistency in baseline data between participants). The CONsistency of the EFFects (CONEFF) approach will be applied in order to assess the consistency of data patterns for level, trend, variability, overlap and immediacy of effect when changing from the baseline to experimental phase (i.e., AB sequence in each tier) [61]. Data that present minimal variability within phases and show consistent patterns in means, levels or trends that are replicated across subjects are proposed to demonstrate intervention efficacy [20].
Generalisation measures will be evaluated using the Reliable Change Index (RCI) [62].
All post-intervention, semi-structured interviews will be audio-taped and transcribed verbatim. Thematic analysis will be used to explore the responses to the open-ended interview questions. NVivo data analysis software will be used to manage the qualitative data and two researchers will be involved in the process of open selective coding to create themes for further inquiry. Responses from participants will be coded broadly in the first instance to provide an overview of the response, with a secondary hierarchal analysis applied to ascertain key themes. Differences of opinions relating to the deconstruction, interpretation and reconstruction of data will be resolved through discussion and consensus with all researchers [63].
The combination of the above analyses will be used to determine the intervention efficacy.
6. Discussion
This paper describes a study protocol for the evaluation of a community-based, individually-tailored intervention for the promotion of PA participation in adults with chronic WAD using a SCED. WAD are the most common non-hospitalised injury resulting from a motor vehicle crash, with approximately 50% of whiplash injured individuals experiencing some level of on-going pain and disability. In addition to pain and disability, individuals with chronic WAD are frequently insufficiently active for good health, increasing their risk of preventable morbidity and mortality, and compounding the effects of the WAD. To date, studies evaluating interventions for this population have used group-level design and analysis, and findings have been equivocal.
The study protocol described in this paper will evaluate an intervention with two novel features. Firstly, to our knowledge, no study has evaluated the effects of an intervention specifically focused on increasing habitual PA in individuals with chronic WAD. Previous interventions have been clinic based and focused on remediation, rather than focused on promotion of intrinsically enjoyable PA that has a demonstrated influence on the risk of chronic disease. The Adapted Physical Activity Program combines evidence-based behaviour change activities, individualized exercise and lifestyle physical activity prescription and relapse prevention strategies with principles of community-based rehabilitation, and has been shown to be effective in promoting PA adoption in adults with brain impairment [19]. Secondly, SCED enables individual level analysis that is not possible with typical group level designs, including identification of characteristics of responders and non-responders. An increased emphasis on evidence-based practice, requires research models that allow for the assessment of individual change and the alteration of interventions to produce clinically-significant changes. SCED studies allow for the evaluation of participants performance session to session, and the adaption and modification of a program based on individual participants responses [56]. SCED is advantageous for the development of interventions in applied settings with heterogeneous populations, such as WAD. Our hypothesis is that the intervention will not only increase participation in health enhancing physical activity, but through that engagement, patients will gain increased confidence to engage in activity in the presence of neck pain, thereby reducing pain-related disability.
Funding
This work is supported by a Collaboration Seeding Grant, Faculty of Health and Behavioural Sciences, The University of Queensland. Dr Kelly Clanchy's work is supported by the Menzies Health Institute of Queensland's Early Researcher Mentorship Program. The funding sources specified had no role in determining the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Declaration of competing interest
The Authors declare that there is no conflict of interest.
Acknowledgements
Not applicable.
Contributor Information
Kelly M. Clanchy, Email: k.clanchy@griffith.edu.au.
Sean M. Tweedy, Email: s.tweedy@uq.edu.au.
Robyn L. Tate, Email: rtate@med.usyd.edu.au.
Michele Sterling, Email: m.sterling@uq.edu.au.
Melissa A. Day, Email: m.day@uq.edu.au.
Jane Nikles, Email: catherine.nikles@uq.edu.au.
Carrie Ritchie, Email: c.ritchie@uq.edu.au.
References
- 1.Casperson C.J., Powell K.E., Christenson G.M. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126. [PMC free article] [PubMed] [Google Scholar]
- 2.Dunn A.L., Andersen R.E., Jakicic J.M. Lifestyle physical activity interventions: history, short- and long-term effects, and recommendations. Am. J. Prev. Med. 1998;15(4):398–412. doi: 10.1016/s0749-3797(98)00084-1. [DOI] [PubMed] [Google Scholar]
- 3.American College of Sports Medicine . General principles of exercise prescription. In: Thompson W.R., Gordon N.F., Pescatello L.S., editors. ACSM's Guidelines for Exercise Testing and Prescription. Lippincott Williams & Wilkins; Philadelphia: 2010. [Google Scholar]
- 4.Connelly L.B., Supangan R. The economic costs of road traffic crashes: Australia, states and territories. Accid. Anal. Prev. 2006;38(6):1087–1093. doi: 10.1016/j.aap.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 5.Motor Accident Insurance Commission . Brisbane; Queensland: 2012. Motor Accident Insurance Commission Annual Report 2011-2012. [Google Scholar]
- 6.Sterling M., Hendrikz J., Knardy J. Compensation claim lodgement and health outcome developmental trajectories following whiplash injury: a prospective study. Pain. 2010;150(1):22–28. doi: 10.1016/j.pain.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Kamper S.J. Course and prognostic factors of whiplash: a systematic review and meta-analysis. Pain. 2008;138(3):617–629. doi: 10.1016/j.pain.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 8.Smith A. Exercise induced hypoalgesia is elicited by isometric, but not aerobic exercise in individuals with chronic whiplash associated disorders. Scand. J. Pain. 2017;15(Supplement C):14–21. doi: 10.1016/j.sjpain.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Anstey R. Are people with whiplash-associated neck pain different from people with nonspecific neck pain? J. Orthop. Sport. Phys. Ther. 2016;46(10):894–901. doi: 10.2519/jospt.2016.6588. [DOI] [PubMed] [Google Scholar]
- 10.Hallman D.M., Ekman A.H., Lyskov E. Changes in physical activity and heart rate variability in chronic neck-shoulder pain: monitoring during work and leisure time. Int. Arch. Occup. Environ. Health. 2014;87(7):735–744. doi: 10.1007/s00420-013-0917-2. [DOI] [PubMed] [Google Scholar]
- 11.Cheung J., Kajaks T., MacDermid J.C. The relationship between neck pain and physical activity. Open Orthop. J. 2013;7(4):521–529. doi: 10.2174/1874325001307010521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoving J.L. Manual therapy, physical therapy, or continued care by the general practitioner for patients with neck pain: long-term results from a pragmatic randomized clinical trial. Clin. J. Pain. 2006;22:370–377. doi: 10.1097/01.ajp.0000180185.79382.3f. [DOI] [PubMed] [Google Scholar]
- 13.Michaleff Z.A. Comprehensive physiotherapy exercise programme or advice for chronic whiplash (PROMISE): a pragmatic randomised controlled trial. Lancet. 2014;384(9938):133–141. doi: 10.1016/S0140-6736(14)60457-8. [DOI] [PubMed] [Google Scholar]
- 14.Peeters G.G.M. The efficacy of conservative treatment in patients with whiplash injury. Spine. 2001;26(4):E64–E73. doi: 10.1097/00007632-200102150-00006. [DOI] [PubMed] [Google Scholar]
- 15.Ludvigsson M.L. The effect of neck-specific exercise with, or without a behavioral approach, on pain, disability, and self-efficacy in chronic whiplash-associated disorders: a randomized clinical trial. Clin. J. Pain. 2015;31(4):294–303. doi: 10.1097/AJP.0000000000000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jull G. Does the presence of sensory hypersensitivity influence outcomes of physical rehabilitation for chronic whiplash? – A preliminary RCT. Pain. 2007;129(1–2):28–34. doi: 10.1016/j.pain.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 17.Stewart M.J. Randomized controlled trial of exercise for chronic whiplash-associated disorders. Pain. 2007;128(1–2):59–68. doi: 10.1016/j.pain.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 18.Clanchy K., Trost S., Tweedy S. Brain Impairment; 2018. The Adapted Physical Activity Program: a Theory-Driven, Evidence Based Physical Activity Intervention for People with Brain Impairment; pp. 1–15. [Google Scholar]
- 19.Clanchy K., Tweedy S.M., Trost S.G. Evaluation of a physical activity intervention for adults with brain impairment: a controlled clinical trial. Neurorehabilitation Neural Repair. 2016;30(9):854–865. doi: 10.1177/1545968316632059. [DOI] [PubMed] [Google Scholar]
- 20.Kazdin A.E. second ed. Oxford University Press; New York: 2011. Single-Case Research Designs: Methods for Clinical and Applied Settings. [Google Scholar]
- 21.Tate R.L. The single-case reporting guideline in BEhavioural interventions (SCRIBE) 2016 statement. Evidence-Based Commun. Assess. Interv. 2016;10(1):44–58. doi: 10.1080/17489539.2016.1190525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kratochwill T.R. Single-case intervention research design standards. Remedial Special Educ. 2013;34(1):26–38. [Google Scholar]
- 23.MacDermid J.C. Measurement properties of the neck disability Index: a systematic review. J. Orthop. Sport. Phys. Ther. 2009;39(5):400–412. doi: 10.2519/jospt.2009.2930. [DOI] [PubMed] [Google Scholar]
- 24.Exercise, Sports Science Australia . 2001. Adult Pre-exercise Screening Tool. Version 1. [Google Scholar]
- 25.American College of Sports Medicine . Eight ed. Lippincott Williams and Wilkins; Philadelphia: 2010. ACSM's Guidelines for Exercise Testing and Prescription. [Google Scholar]
- 26.Kratochwill T.R., Levin J.R. Enhancing the scientific credibility of single-case intervention research: randomization to the rescue. Psychol. Methods. 2010;15:122–144. doi: 10.1037/a0017736. [DOI] [PubMed] [Google Scholar]
- 27.Heyvaert M. Randomization and data-analysis items in quality standards for single-case experimental studies. J. Spec. Educ. 2015;49(3):146–156. [Google Scholar]
- 28.Tate R.L. Author; Sydney, Australia: 2015. The Risk of Bias in N-Of-1 Trials (RoBiNT) Scale: an Expanded Manual for the Critical Appraisal of Single-Case Reports. [Google Scholar]
- 29.Bundy C. Changing behaviour: using motivational interviewing techniques. J. R. Soc. Med. 2004;97(44):43–47. [PMC free article] [PubMed] [Google Scholar]
- 30.Blair S.N. Human Kinetics; Champaign, IL: 2001. Active Living Every Day. [Google Scholar]
- 31.Motor Accident Commission and South Australian Centre for Trauma and Injury Recovery, Clinical Guidelines For Best Practice Management Of Acute And Chronic Whiplash Associated Disorders: Clinical Resource Guide. TRACsa: Trauma and Injury Recovery; South Australia: 2008. [Google Scholar]
- 32.Gill J.R., Brown C.A. A structured review of the evidence for pacing as a chronic pain intervention. Eur. J. Pain. 2009;13(2):214–216. doi: 10.1016/j.ejpain.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 33.Soderlund A., Lindberg P. Cognitive behavioural components in physiotherapy management of chronic whiplash associated disorders (WAD)- a randomised group study. Supplemento A, Psicologia. 2007;29(1):A5–A11. [PubMed] [Google Scholar]
- 34.McClune T., Burton A., Waddell G. Whiplash associated disorders: a review of the literature to guide patient information and advice. Emerg. Med. J. 2002;19(6):499–506. doi: 10.1136/emj.19.6.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amtmann D. Development of a PROMIS item bank to measure pain interference. Pain. 2010;150(1):173–182. doi: 10.1016/j.pain.2010.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cella D. The patient-reported outcomes measurement information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J. Clin. Epidemiol. 2010;63(11):1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marcus B.H. Self-efficacy and the stages of exercise behaviour change. Res. Q. Exerc. Sport. 1992;63:60–66. doi: 10.1080/02701367.1992.10607557. [DOI] [PubMed] [Google Scholar]
- 38.Marcus B.H., Simkin L.R. The stages of exercise behaviour. J. Sport. Med. Phys. Fit. 1993;33:83–88. [PubMed] [Google Scholar]
- 39.Sallis J. The development of scales to measure social support for diet and exercise behaviours. Prev. Med. 1987;16:825–836. doi: 10.1016/0091-7435(87)90022-3. [DOI] [PubMed] [Google Scholar]
- 40.Marcus B.H., Rakowski W., Rossi J.S. Assessing motivational readiness and decision making for exercise. Health Psychol. 1992;11:257–261. doi: 10.1037//0278-6133.11.4.257. [DOI] [PubMed] [Google Scholar]
- 41.Cheak-Zamora N.C., Wyrwich K.W. Reliability and validity of the SF-12v2 in the medical expenditure panel survey. Qual. Life Res. 2009;18(6):727–735. doi: 10.1007/s11136-009-9483-1. [DOI] [PubMed] [Google Scholar]
- 42.Lee C., Jones D. Measuring health of patients with cervical spinal disorder: is the SF-12 health survey a valid alternative for the SF-36. J. Pain. 2007;8(4):s22. doi: 10.1016/j.apmr.2007.09.056. [DOI] [PubMed] [Google Scholar]
- 43.McCaffery M., Beebe A. Mosby; St. Louis, MO: 1989. Pain: Clinical Manual for Nursing Practice. [Google Scholar]
- 44.Jensen M.P., Turner J.A., Romano J.M. What is the maximum number of levels needed in pain intensity measurement? Pain. 1994;58:387–392. doi: 10.1016/0304-3959(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 45.Sullivan M.J.L. Theoretical perspectives on the relation between catastrophizing and pain. Clin. J. Pain. 2001;17:52–64. doi: 10.1097/00002508-200103000-00008. [DOI] [PubMed] [Google Scholar]
- 46.Sullivan M.J.L., Bishop S., Pivik J. The pain catastrophizing scale: development and validation. Psychol. Assess. 1995;7:524–532. [Google Scholar]
- 47.Asghari A., Nicholas M.K. Pain self-efficacy beliefs and pain behaviour. A prospective study. Pain. 2001;94(1):85–100. doi: 10.1016/S0304-3959(01)00344-X. [DOI] [PubMed] [Google Scholar]
- 48.Kaivanto K.K. Isokinetic performance in low back pain patients: the predictive power of the self-efficacy scale. J. Occup. Rehabil. 1995;5(2):87–100. doi: 10.1007/BF02109912. [DOI] [PubMed] [Google Scholar]
- 49.Jensen M. Measuring the cognitions, emotions, and motivation associated with avoidance behaviors in the context of pain. Clin. J. Pain. 2017;33(4):325–344. doi: 10.1097/AJP.0000000000000407. [DOI] [PubMed] [Google Scholar]
- 50.Parkitny L., McAuley J. The depression anxiety stress scale (DASS) J. Physiother. 2010;56:204. doi: 10.1016/s1836-9553(10)70030-8. [DOI] [PubMed] [Google Scholar]
- 51.Henry J.D., Crawford J.R. The short-form version of the depression anxiety stress scales (DASS-21): construct validity and normative data in a large non-clinical sample. Br. J. Clin. Psychol. 2005;44(2):227–239. doi: 10.1348/014466505X29657. [DOI] [PubMed] [Google Scholar]
- 52.Weiss D. Assessing Psychological Trauma and PTSD: a Practitioner's Handbook. Guilford Press; New York: 1999. The impact of event scale-revised; pp. 399–411. [Google Scholar]
- 53.Beck G.J. The impact of event scale –revised: psychometric properties in a sample of motor vehicle accident survivors. J. Anxiety Disord. 2008;22(2):187–198. doi: 10.1016/j.janxdis.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Solanas A., Manolov R., Onghena P. Estimating slope and level change in N = 1 designs. Behav. Modif. 2010;34:195–218. doi: 10.1177/0145445510363306. [DOI] [PubMed] [Google Scholar]
- 55.Huitema B.E., McKean J.W. Design specification issues in time-series intervention models. Educ. Psychol. Meas. 2000;60:38–58. [Google Scholar]
- 56.Lane J.D., Gast D.L. Visual analysis in single case experimental design studies: brief review and Guidelines. Neuropsychol. Rehabil.: Int. J. 2014;24(3–4):445–463. doi: 10.1080/09602011.2013.815636. [DOI] [PubMed] [Google Scholar]
- 57.Kratochwill T.R. What Works Clearinghouse; 2010. Single-case Designs Technical Documentation.http://ies.ed.gov/ncee/wwc/pdf/wwc_scd.pdf [cited 2016 30/8/2016]; Available from: Retrieved from website: [Google Scholar]
- 58.Parker R.I., Vannest K.J., Davis J.L. Effect size in single-case research: a review of nine nonoverlap techniques. Behav. Modif. 2011;35:303–322. doi: 10.1177/0145445511399147. [DOI] [PubMed] [Google Scholar]
- 59.Brossart D.F., Laird V.C., Armstrong T.W. Interpreting Kendall's Tau and Tau-U for single-case experimental designs. Cogent Psychol. 2018;5 1518687. [Google Scholar]
- 60.Parker R.I. Combining nonoverlap and trend for single-case research: tau-U. Behav. Ther. 2011;42:284–299. doi: 10.1016/j.beth.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 61.Tanious R. 2019. Assessing Consistency in Single-Case A-B-A-B Phase Designs; pp. 1–34. Behavior Modification. [DOI] [PubMed] [Google Scholar]
- 62.Jacobson N.S., Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J. Consult. Clin. Psychol. 1991;59:12–19. doi: 10.1037//0022-006x.59.1.12. [DOI] [PubMed] [Google Scholar]
- 63.Braun V., Clarke V. Using thematic analysis in psychology. Qual. Res. Psychol. 2006;3(2):77–101. [Google Scholar]