Highlights
-
•
First UHPLC-MS/MS assay for screening of 15 emerging SARMs in muscle tissue.
-
•
Method fully validated according to relevant EU food control legislation.
-
•
Analyte detection capability (CCβ) determined in the range of 0.5–5 ng g−1.
-
•
Assay amenable for use within routine residue control programmes.
Keywords: Selective androgen receptor modulators, SARMs, UHPLC-MS/MS, Muscle tissue, Residue screening, Food safety
Abstract
Selective androgen receptor modulators (SARMs) are non-steroidal compounds widely reported as drugs of abuse in human and animal sports, with potential for misuse as growth promoters in animal-based food production. In this study, a first analytical methodology to simultaneous screen for a panel of emerging SARMs in bovine muscle was developed, validated (CCβ values from 0.5–5 ng g−1), and applied to detect 15 structurally diverse compounds from nine SARM families. Muscle samples (200 mg) were homogenised in extraction solvent (MeCN:H2O, 4:1, v/v) before clean-up (end-capped C18 dSPE), defatting (n-hexane pre-saturated with MeCN partitioning) and concentration prior to UHPLC-MS/MS analysis. In the absence of incurred bovine muscle, method applicability was demonstrated by the analysis of rodent muscle tissue. The developed screening assay serves as a rapid, simple and cost-effective tool for surveillance monitoring of SARM abuse in livestock production systems as a pre-emptive measure ensuring food safety.
1. Introduction
Selective androgen receptor modulators (SARMs) represent a new generation of non-steroidal compounds reported (Dalton, Mukherjee, Zhu, Kirkovsky, & Miller, 1998) to separate anabolic effects from androgenic activity in target tissues. These structurally diverse molecules primarily act as full androgen receptor (AR) agonists in anabolic tissue (muscle and bone), whilst exhibiting only partial agonist activity on the androgenic tissue located AR (e.g. prostate and seminal vesicle) (Narayanan et al., 2018, Zhang and Sui, 2013). SARMs are widely recognised as significant drugs of abuse in both human and animal (e.g. equine) sports due to simplicity of use (oral bioavailability), advantageous biological effects, as well as their ease of availability through black- and grey-market sources (Cawley et al., 2016, Thevis et al., 2018, Thevis and Schänzer, 2018, Thevis and Volmer, 2018). With regards to veterinary practice SARMs also have a potential for illicit application in food livestock production (i.e. cattle) through animal feed incorporation, to improve meat production by increasing lean muscle mass, reducing meat fat, improving feed efficiency and carcass grading (Dalton, Miller, & Kearbey, 2016). For these reasons, SARM use is banned by the World Anti-Doping Agency (WADA) (WADA, 2019), the International Agreement on Breeding, Racing and Wagering (IABRW) (IABRW, 2019) and the Fédération Equestre Internationale (International Equestrian Federation, FEI) (FEI, 2019). Additionally, substances such as SARMs exhibiting hormonal action have been banned in livestock farming within the EU since 1988 (EC, 1988) based on the precautionary principle (EC, 2002b) aimed at avoidance of risk to consumers’ health. Therefore, methodologies capable of detecting potential misuse of emerging SARMs in stock animal farming are urgently required to enable the proactive and effective enforcement of their prohibition (EC, 2017).
To date, a number of LC-MS/MS applications have been reported with respect to the analysis of SARM residues in bovine species including urine (Beucher et al., 2017, Cesbron et al., 2017, de Rijke et al., 2013, Rojas et al., 2017, Schmidt and Mankertz, 2018, Ventura et al., 2019), blood (Ventura et al., 2018a, Ventura et al., 2018b) and faeces (Cesbron et al., 2017, Rojas et al., 2017) matrices, some of which have been validated in accordance with current regulatory legislation (EC, 2002a). However, in the case of surveillance at food retail level and for products imported into the EU, it is necessary to have methods applicable to the analysis of meat-based matrices. The objective of the current study was therefore to develop and validate a reliable and effective UHPLC-MS/MS-based assay with the capability to screen for a wide range of emerging SARM residues in muscle tissue that could be adopted in control programmes for residue monitoring in foods of bovine origin. SARM compounds identified as priority for inclusion in residue screening were selected based upon their reported use in human and animal sports and/or availability as certified analytical standards. This study thereby reports the development, validation and application of a semi-quantitative mass spectrometry-based method for screening of 15 SARM compounds in muscle tissue from nine different classes including arylpropionamide (andarine (S-4), bicalutamide, ostarine (S-22), S-1, S-6, S-9, S-23), diarylhydantoin (GLPG0492), hydantoin (BMS-564929), indole (Ly2452473), isoquinoline (PF-06260414), phenyl-oxadiazole (RAD140), quinolinone (LGD-2226), pyrrolidinyl-benzonitrile (LGD-4033) and tropanol (AC-262536) derivatives.
2. Materials and methods
2.1. Chemicals and reagents
Ultra-pure water (18.2 MOhm) was generated in house using a Millipore water purification system (Millipore, Cork, Ireland). Methanol (MeOH) and acetonitrile (MeCN), both Chromasolv™ LC-MS grade, n-hexane Chromasolv® HPLC grade, as well as acetic acid, eluent additive for LC-MS, were sourced from Honeywell (VWR International, Dublin, Ireland). Ethanol (puriss. p.a., ACS reagent, absolute alcohol, without additive, ≥99.8%), dimethyl sulfoxide (ACS reagent, ≥99.9%) and acetonitrile-D, 99.5% (MeCN-D) were purchased from Sigma-Aldrich (Dublin, Ireland). Bondesil-C18 (40 µm, 100 g, P/N 12213012) came from Agilent Technologies (Cheadle Cheshire, UK). Reinforced tubes (2 mL) for Precellys® homogenisers from Bertin Technologies (VWR International, Dublin, Ireland), stainless steel beads (5 mm) from Qiagen Ltd. (Manchester, UK), a Precellys® 24 homogeniser from Bertin Technologies (Stretton Scientific Ltd, Derbyshire, UK), SafeSeal polypropylene micro tubes (2 mL) from Sarstedt (Nümbrecht, Germany), a DVX-2500 multi-tube vortexer (VWR International, Dublin, Ireland), a Hettich Micro 200R centrifuge from Davidson & Hardy (Belfast, UK) and a Turbovap LV evaporator from Caliper Life Sciences (Mountain View, USA) were used during sample preparation. 750 µL Centrifuge filters PTFE 0.2 µm (P/N F2517-9) were supplied by Thermo Fisher Scientific (Hemel Hempstead, UK). AC-262536 (P/N 96443-25MG), andarine (S-4, P/N 78986-25MG), bicalutamide (P/N PHR-1678-1G), LGD-2226 (P/N 07682-25MG), Ly2452473 (P/N CDS025139-50MG), PF-06260414 (P/N PZ0343-5MG), S-1 (P/N 68114-25MG), S-6 (P/N 79260-25MG) and S-23 (P/N 55939-25MG) were purchased from Sigma-Aldrich (Dublin, Ireland). LGD-4033 (P/N CAY9002046-50 mg), ostarine (S-22, P/N MK-2866) and RAD140 (P/N CAY18773-1 mg) were purchased from Cambridge Bioscience Ltd. (Cambridge, UK). BMS-564929 (10 mM solution in DMSO, P/N HV-12111) and GLPG0492 (10 mM solution in DMSO, P/N HY-18102) were purchased from MedChem Express (Sollentuna, Sweden). S-9 (P/N D289535), bicalutamide-D4 (P/N B382002) and S-1-D4 (P/N D289532) were purchased from Toronto Research Chemicals (TRC; Toronto, Canada). All standards and internal standards stock solutions were prepared at a concentration of 1 mg mL−1 in MeCN, DMSO, EtOH and MeCN-D, respectively. Intermediate mixed standard solutions were prepared in MeCN by serial dilutions. A working quality control standard solution at a concentration of 4/8/16/40 ng mL−1 was prepared in MeCN. Intermediate internal standard mix solutions were prepared using MeCN-D as the diluent. A working internal standard mix solution was prepared at 40 ng mL−1 in MeCN-D.
2.2. Samples
Bovine muscle samples (n = 63) were collected at point of slaughter from abattoirs across Ireland (n = 30) and Scotland (n = 12), whereas retail beef was purchased in local shops (n = 21).
2.3. UHPLC-MS/MS instrumentation and conditions
UHPLC-MS/MS analysis was performed on a Waters (Milford, MA, USA) Acquity I-Class UPLC® system coupled to a Waters Xevo® TQ-MS triple quadrupole mass analyser (Manchester, UK) equipped with an electrospray ionisation (ESI) source operating in positive/negative ionisation switching mode and selected reaction monitoring (SRM). Chromatographic separation (Supplementary data – Fig. S1) was performed on a stainless steel Luna® Omega Polar C18 analytical column (100 × 2.1 mm, 100 Å, 1.6 µm) (Phenomenex, P/N 00D-4748-AN) supplied with KrudKatcher™ Ultra HPLC in-line filter (Phenomenex, P/N AF0-8497) maintained at a temperature of 45 °C and the pump was operated at a flow rate of 0.40 mL min−1. A binary gradient system comprised of mobile phase A, 0.1% (v/v) acetic acid in water and mobile phase B, 0.1% (v/v) acetic acid in MeOH. The gradient profile was as follows: (1) 0.00 min 20% B, (2) 0.50 min 20% B, (3) 4.75 min 60% B, (4) 10.50 min 67.5% B, (5) 11.00 min 99% B, (6) 12.00 min 99% B, (7) 12.10 min 20.0% B, (8) 14.00 min 20% B. The injection volume was 9 µL. After each injection the needle was washed and purged with H2O:MeOH (1:1, v/v) and H2O:MeOH (4:1, v/v) solutions, respectively. A divert valve was used to reduce source contamination (11.00–13.50 min a flow was diverted to waste).
The UHPLC-MS/MS platform was controlled by MassLynx™ software and data was processed using TargetLynx™ software (Waters). The detailed analysis by means of UHPLC-MS/MS is described elsewhere (Ventura et al., 2019), whereas the conditions specific to the current assay are outlined in Supplementary data – Table S1. Stable isotope-labelled analogues of bicalutamide and S-1 (bicalutamide-D4 and S-1-D4) were used as internal standards (IS) for arylpropionamide residues as detailed in Supplementary data – Table S1. The response factor was obtained for arylpropionamides as a ratio between analyte peak area and IS peak area, while in the case of SARM residues assayed without IS, peak area was used as the response.
2.4. Analysis of SARM residues in test samples
Bovine muscle tissue samples were homogenised with kitchen food processor on receipt and stored at −20 °C prior to analysis. A portion of each sample (200 ± 2 mg) was weighed out into a 2 mL reinforced tube. Samples were fortified with 25 µL of a 40 ng mL−1 internal standard mix solution and left to stand for 15 min. Two stainless steel beads (5 mm) were placed into each tube and a 1000 µL volume of MeCN:H2O (4:1, v/v, kept at −20 °C prior to extraction) was added subsequently into each sample. Samples were placed on ice and were kept on ice during homogenisation intervals. The tube contents were homogenised three times for 20 s at 5500 rpm with 30 s intervals using a Precellys® homogeniser and centrifuged at 10,840×g for 10 min at 4 °C. Following the transfer of supernatants into clean empty 2 mL micro tubes, 50 mg end-capped C18 sorbent and 1000 µL of n-hexane pre-saturated with MeCN were added. Samples were vortexed for 30 s, centrifuged at 24,400×g for 10 min at 4 °C and the upper layer was aspirated to waste. A 600 µL portion of the supernatant was transferred into a clean empty 2 mL micro tube and evaporated to dryness under flow of nitrogen (≤5 Bar) at 40 °C on a Turbovap LV system. Samples were reconstituted in H2O:MeCN (4:1, v/v; 150 µL) by vortexing (5 min). Extracts were filtered through 0.22 µm PTFE membranes for 10,840×g for 2 min at 15 °C and 9 µL were injected onto the UHPLC-MS/MS system.
2.5. Preparation of extracted matrix screen positive and recovery control checks
A pool of muscle tissue (n = 5–10) was used for QC purposes. Extracted matrix screen positive controls were prepared by fortifying three negative QC samples (200 mg) prior to extraction with 25 µL of quality control standard solution to give a screening target concentration of 0.5/1/2/5 ng g−1 in muscle (Table 1). Additionally, two blank QC samples were spiked after extraction with quality control standard solution (15 µL) to monitor for loss of analytes during extraction.
Table 1.
Validation results for fortified bovine muscle samples (n = 63).
| Analyte | Transition (m/z) | eLODb (ng g−1) | Cvalc (ng g−1) | CCβ | Relative cut-off factor (RFm)d (%) | Precisione (%) | Sensitivityf (%) |
|---|---|---|---|---|---|---|---|
| AC-262536 | 279.2 > 195.0 | 0.33 | 2 | <Cval | 69 | 19.1 | 97 |
| Andarinea | 440.2 > 150.0 | 0.07 | 2 | <Cval | 80 | 12.1 | 100 |
| Bicalutamidea | 429.2 > 255.0 | 0.09 | 1 | <Cval | 58 | 25.4 | 100 |
| BMS-564929 | 306.1 > 96.0 | 0.26 | 5 | <Cval | 54 | 28.1 | 98 |
| GLPG0492 | 390.2 > 360.2 | 0.18 | 5 | <Cval | 42.6 | 35.0 | 100 |
| LGD-2226 | 393.1 > 241.1 | 0.32 | 2 | <Cval | 24.4 | 46.1 | 100 |
| LGD-4033 | 337.1 > 267.2 | 0.04 | 1 | <Cval | 61 | 23.7 | 98 |
| Ly2452473 | 375.2 > 289.2 | 0.08 | 0.5 | <Cval | 75 | 15.1 | 98 |
| Ostarinea | 388.1 > 118.0 | 0.05 | 1 | <Cval | 66 | 20.8 | 100 |
| PF-06260414 | 303.1 > 168.2 | 0.06 | 2 | <Cval | 54 | 27.8 | 100 |
| RAD140 | 394.1 > 223.1 | 0.19 | 2 | <Cval | 67 | 20.0 | 97 |
| S-1a | 401.1 > 261.1 | 0.07 | 1 | ≤Cval | 93 | 4.5 | 95 |
| S-6a | 435.1 > 145.0 | 0.08 | 2 | ≤Cval | 64 | 22.1 | 95 |
| S-9a | 417.2 > 127.0 | 0.19 | 2 | ≤Cval | 72 | 17.1 | 95 |
| S-23a | 415.2 > 145.0 | 0.05 | 1 | ≤Cval | 82 | 11.0 | 95 |
Values calculated response-based.
Estimated LOD (S/N ≥ 3).
Screening target concentration.
Calculated as percentage based on the ratio of the cut-off factor and the mean response of fortified samples.
Calculated as coefficient of variation (CV) of the response following fortification.
Expressed as percentage based on the ratio of samples detected as positive in true positive samples, following fortification.
2.6. Method validation
The developed assay was ‘in-house’ validated in terms of selectivity, specificity, detection capability (CCβ), sensitivity, precision, limit of detection (LOD), absolute recovery as well as matrix effects and stability, according to the respective EU legislation (CRL, 2010, EC, 2002a). Validation was carried out at the screening target concentration (Cval) of 0.5/1/2/5 ng g−1 as specified in Table 1. The detection capability (CCβ) (EC, 2002a) was calculated in accordance with the EU-RLs 20/1/2010 guidelines, by assessing threshold value (T) and cut-off factor (Fm) as described previously (Ventura et al., 2019). Briefly, the T-value was determined through analysis of blank bovine muscle tissue samples (n = 63) of different origins on three different occasions by two different analysts. Whereas, Fm was determined through analysis of the same bovine muscle tissue samples fortified at Cval on three different occasions by two different analysts. Both T-value and Fm were calculated for at least two transitions for each analyte. CCβ of the screening method is validated when Fm > T (CRL, 2010) and then it can be concluded that CCβ is truly below the validation level. Since a screening assay is required to avoid false-negative (“false-compliant”) results, hence the detection capability of the method was estimated as the concentration level where ≤5% of false-negative results remain.
The sensitivity of the method ≥95% at Cval, expressed as the percentage based on the ratio of samples detected as positive in true positive samples i.e. following the fortification (Fawcett, 2006), means that the number of false-negative samples is truly ≤5%. Precision, which is a required performance characteristic to be determined exclusively for quantitative methods (EC, 2002a), was calculated as the coefficient of variation (CV) of the response following fortification at Cval. Limit of detection (LOD) was estimated at a signal-to-noise ratio (S/N) at least three measured peak-to-peak.
Matrix effects were evaluated through analysis of blank bovine muscle tissue samples (n = 12) from different sources post-extraction spiked at the concentration equal to 2 × Cval, namely 1/2/4/10 ng g−1. Matrix effects for each analyte were calculated as percentage differences between the signals obtained when matrix extracts were injected and when a standard solution of equivalent concentration was injected, divided by the signal of the latter (Trufelli, Palma, Famiglini, & Cappiello, 2011).
2.6.1. Stability studies
Stability of SARMs of interest in solution was reported previously (Ventura et al., 2019). It was found that all SARM standards and internal standards stock solutions were stable for at least one year when stored at −20 °C. Whereas, working quality control standard and working internal standard mix solutions were found to be stable for at least 3 months when stored at −20 °C. Performed stability studies were designed so as to assess the stability of SARM residues in sample matrix as well as in final extracts.
Two studies were executed to assess the stability of residues in sample matrix. The first measured the impact of frozen storage (−20 °C) and the second the effect of freeze thaw cycles. Stability was determined in blank bovine muscle tissue samples fortified at the concentration equal to 5 × Cval, namely 2.5/5/10/25 ng g−1 (n = 5 replicates). In the frozen storage study, fortified muscle tissue samples were analysed following storage in 2 mL reinforced tubes at −20 °C for up to 12 weeks. In the freeze thaw cycle study, the fortified muscle tissue samples were subjected to 0–3 freeze thaw cycles, and at each cycle samples were frozen for at least 24 h before being thawed. A further study investigated the stability of SARM residues in sample extracts – final injection solvent under storage in autosampler vials at −20 °C (0–4 weeks) and 4 °C (0–2 weeks), respectively. The stability was assessed in blank bovine muscle tissue samples spiked post extraction at 5 × Cval (n = 5 replicates). In all stability studies, all relevant samples were tested following randomisation within the same analytical run performed at the end of the stability study period. A criterion of ±20% of the relative response (Gaugain, Chotard, & Verdon, 2013) was applied to assess analyte stability and repeated measures one-way Analysis of Variance (ANOVA) with Tukey’s multiple comparison post test was performed on stability study datasets using GraphPad Prism version 5.00 (GraphPad Software, San Diego, California, USA). A confidence level of 95% was set as an acceptable criterion. Thus, compounds were considered unstable if the relative response varied more than ±20% together with p < 0.05.
2.7. Analysis of incurred muscle tissue samples
Rats (Sprague-Dawley, 9-week-old, 300–350 g bodyweight) were treated daily by oral gavage (1 mL) for 15 days with vehicle, ostarine (3 mg kg−1 bodyweight), LGD-4033 (3 mg kg−1 bodyweight) or RAD140 (3 mg kg−1 bodyweight), respectively. Animals were maintained on a 12 h light, 12 h dark cycle with ad libitum access to food and water. 24 h post-last administration, animals were exsanguinated under terminal general anaesthesia and collected muscle tissue samples were stored at −80 °C prior to analysis. Experimental procedures were performed in adherence to local ethical review procedures and conducted under regulations as outlined within the UK Animals (Scientific Procedures) Act 1986 (UK, 1986). Rodent muscle samples were assayed in triplicate employing the developed screening method as detailed in Section 2.4.
3. Results and discussion
3.1. Method development
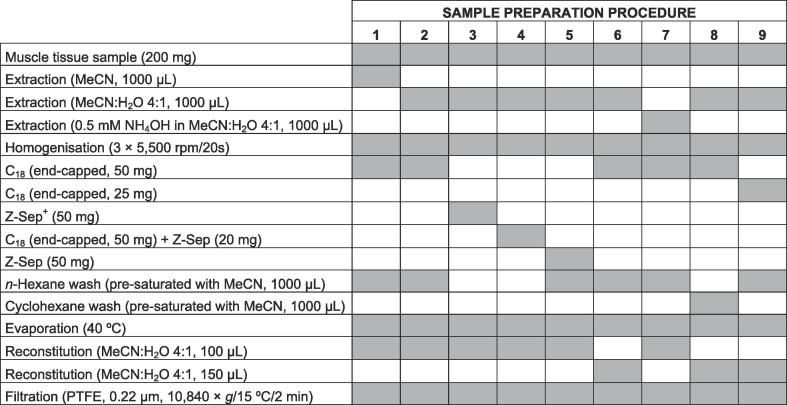
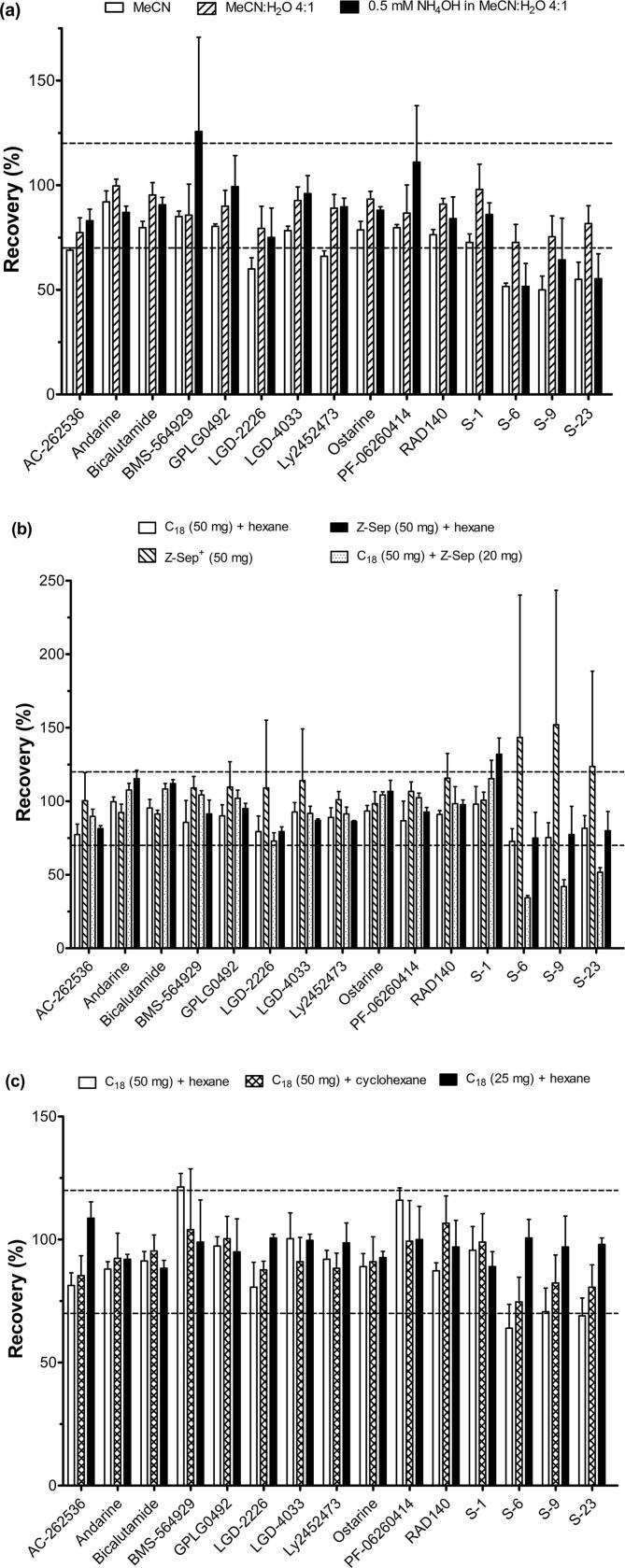
In the current study, SARM residues were analysed by means of a modified UHPLC-MS/MS method previously reported (Ventura et al., 2019). As depicted in Supplementary data – Fig. S1, all SARM compounds were separated within the first 9.55 min of the chromatographic run. The aim of the study was to develop an extraction and clean-up procedure, reducing the amount of matrix co-extractives from bovine muscle tissue, suitable for all SARMs of interest (satisfactory absolute recoveries of 70–120%). Findings from analysis of fortified samples (n = 3) compared to those of blank samples spiked post-extraction (n = 2), were evaluated for various clean-up approaches investigated (Fig. 1). MeCN as well as solvent composed of MeCN:H2O (4:1, v/v) have been shown to be effective extraction solvents for analysis of a broad range of veterinary drug residues in bovine muscle tissue (Geis-Asteggiante et al., 2012, Kinsella et al., 2009). Previous investigations performed in urine and blood have demonstrated the impact of pH on the extraction efficiency of selected SARM compounds (Ventura et al. 2018a, Ventura et al., 2018b, Ventura et al., 2019). Consequently, the abovementioned solvents together with 0.5 mM NH4OH in MeCN:H2O (4:1, v/v) were assessed for their suitability within the proposed assay (Fig. 2a). MeCN provided for low recoveries (≤60%) of LGD-2226, S-6, S-9 and S-23, and whilst 0.5 mM NH4OH in MeCN:H2O (4:1, v/v) gave similar recoveries for S-6, S-9 and S-23, it also led to unacceptable findings in terms of recovery (126%) and precision for BMS-564929. A solvent composed of MeCN:H2O (4:1, v/v) showed the best overall recoveries (73–100%) and was employed in further analyses.
Fig. 1.
Graphic representation highlighting steps involved in various sample preparation procedures (1–9) investigated for extraction of SARM compounds from muscle tissue.
Fig. 2.
Comparison of (a) extraction solvent, (b,c) clean-up efficiency applied for all analytes. Mean recoveries (and standard deviations, shown by error bars) obtained from fortified bovine muscle tissue samples (n = 3). ----- Acceptance limits (70–120%).
To evaluate different sample clean-up parameters, the effects of a range of sorbents for dispersive solid phase extraction (dSPE) as well as solvents for liquid–liquid partitioning were evaluated, namely C18 + hexane, Z-Sep+ only, C18 + Z-Sep, Z-Sep + hexane, and C18 + cyclohexane (Fig. 2b and c) (Geis-Asteggiante et al., 2012). A comprehensive review of sorbent materials has been presented elsewhere (Kinsella et al., 2009). The application of C18 + Z-Sep clean-up resulted in significant loss (recoveries ≤50%) of S-6, S-9 and S-23, with the use of Z-Sep+ sorbent leading to unsatisfactorily high recovery rates (≥120%) and precision for these same analytes. Recoveries of S-6, S-9 and S-23 were improved (78–80%) using Z-Sep + hexane, but the observed recovery for S-1 (132%) fell outside the pre-defined set criteria. Amongst tested clean-up variations (Fig. 2b), a combination of C18 + hexane provided for the best overall recoveries (73–100%), with the most precise results and best signal intensity obtained for the majority of compounds when using C18 (50 mg) + hexane (Fig. 2c). A reconstitution solvent (H2O:MeCN, 4:1, v/v) reported previously as providing for satisfactory sensitivity with acceptable peak shapes for all analytes of interest (Ventura et al., 2018a, Ventura et al., 2018b, Ventura et al., 2019), was employed in the current assay. However, in an attempt to reduce matrix effects and improve method performance, different concentration factors, i.e. 4- and 6-fold of extracts obtained using C18 + hexane were investigated, with a 4-fold concentration found to result in improved analyte signal intensity. The optimised sample preparation procedure detailed above (Section 2.4) gave average absolute recoveries, calculated at Cval, in the range of 81–100% for all SARMs of interest (Table 2).
Table 2.
Recovery and matrix effect data.
| Analyte | Recovery (%)a | RSD (%)a | Ion supression (%) ± SD (%) in matrixb |
|---|---|---|---|
| AC-262536 | 83 | 8.9 | 20.1 ± 12.8 |
| Andarine | 89 | 7.9 | 26.2 ± 9.2 |
| Bicalutamide | 85 | 10.6 | 21.5 ± 12.5 |
| BMS-564929 | 99 | 10.9 | 54 ± 6.5 |
| GLPG0492 | 93 | 7.3 | 36.6 ± 8.9 |
| LGD-2226 | 87 | 11.7 | 25.3 ± 15.5 |
| LGD-4033 | 93 | 7.3 | 28.7 ± 14.4 |
| Ly2452473 | 91 | 7.5 | 21.3 ± 14.3 |
| Ostarine | 87 | 8.2 | 9.5 ± 16.2 |
| PF-06260414 | 100 | 11.0 | 39.8 ± 7.2 |
| RAD140 | 92 | 9.0 | 46.0 ± 10.6 |
| S-1 | 87 | 8.9 | 29.1 ± 18.8 |
| S-6 | 81 | 12.0 | 26.9 ± 24.9 |
| S-9 | 82 | 10.2 | 28.9 ± 22.9 |
| S-23 | 83 | 9.0 | 24.6 ± 22.9 |
Recovery was determined by comparing results from fortified samples to those of negative samples spiked post-extraction at the screening target concentration (Cval). Recovery is based on data collected from six analytical runs.
Ion suppression results for urine matrices are based on the analysis of 12 samples from different sources. Values calculated as described in Section 2.6.
3.2. Method validation
To demonstrate good analytical performance and reliability, method validation required an in-depth study of factors including specificity, selectivity, sensitivity, precision, absolute recovery, matrix effects and stability, as well as confirmation of consistency in reported retention times and MS ion ratios.
3.2.1. Selectivity, specificity, and matrix effect studies
Method specificity has been reported previously (Ventura et al., 2018a, Ventura et al., 2018b), whereas selectivity was established in this study through testing of 63 bovine muscle tissue samples obtained from different (abattoir and food retail) sources without observed matrix interferences. Carry-over was assessed during the validation study by injecting blank solvent (MeOH) following the sample fortified at 5 × Cval. Moreover, carry-over was closely monitored during every analysis by injecting blank solvent (MeOH) following the sample fortified at Cval (screen positive control), with no analyte signal observed in blank solvent. Matrix effect evaluation (Table 2) highlighted suppression in the case of all analytes, with the greatest suppression observed for BMS-564929 (54%) and RAD140 (46.0%). However, for all other analytes with the exception of ostarine (9.5%), the suppression effect was ≥20%. Future availability of affordable isotope-labelled internal standards which could be incorporated into the current method would compensate for signal loss resulting from matrix effects and further improve accuracy and precision.
3.2.2. Detection capability (CCβ)
Since recommended levels for SARMs in food-producing animals have not been established (CRL, 2007), the screening target concentration (Cval) in the current study was based on the ALARA (as low as reasonably achievable) principle (SANCO, 2008), with validation performed at 0.5/1/2/5 ng g−1 as detailed in Table 1. Cut-off factors (Fm) were above T-values for at least two transitions for all SARMs, exceeding the requirements of current legislation (CRL, 2010). Determined CCβ values were below or equal to the validation levels for at least two transitions for all analytes (Table 1 and Supplementary data – Table S2), with sensitivity highlighted as ≥95% for all SARMs. Moreover, ion ratios measured for all transitions of interest were within ±30% permitted tolerance (SANTE, 2017).
The developed screening assay enables detection of 15 emerging SARM compounds in bovine muscle tissue with a risk of a false-negative rate ≤5% as enforced by current EU legislation (CRL, 2010, EC, 2002a). The precision of the method, expressed as CV, was shown to range from 4.5 to 46.1% (Table 1), and absolute recoveries for all 15 SARMs were measured and recorded within each analytical run to verify method performance during routine analysis (Table 2). There is no existing method reporting screening of SARM residues in bovine muscle tissues, and therefore a direct comparison to other methods cannot be made. However, selected SARM deposition in levator ani muscle, liver and kidney has been reported in rodent animals with the levels that accumulate in tissue lower than those found in other matrices (Aikawa et al., 2015, Kim et al., 2013, Perera et al., 2006, Vajda et al., 2009).
As reported previously (Ventura et al., 2019), relative cut-off factor (RFm) was calculated for each analyte (Table 1 and Supplementary data – Table S2) as the percentage based on the ratio of Fm and the mean response of fortified samples. RFm should be applied to screen positive controls (QC samples) during routine application of this screening method and it is suggested that negative controls to be used for routine testing are “average” matrix blanks prepared by pooling n = 5–10 muscle tissue samples.
3.2.3. Stability studies
3.2.3.1. Matrix stability
The stability of SARM residues assessed in fortified bovine muscle tissue samples stored for up to 12 weeks at −20 °C showed significant degradation (relative response difference >±20% with p < 0.05) of the following analytes: AC-262536, BMS-4929, GLPG0492, LGD-2226, Ly2452473, ostarine (S-22), PF-06260414 and RAD140, after one week under frozen storage as depicted in Supplementary data – Fig. S2a. Furthermore, andarine (S-4) and LGD-4033 were found to present a trend towards instability even if set criteria were not fulfilled after one and four weeks of storage under the same conditions, respectively. Consequently, storage conditions are critical and it is recommended that samples are kept ≤ −70 °C, and whenever SARM incurred muscle tissues are available stability under frozen storage conditions should be further examined. Investigation of SARM residue stability under freeze thaw cycle conditions revealed no significant degradation following 0–3 cycles as presented in Supplementary data – Fig. S2b. However, it is recommended that test samples are not subjected to repeated freeze thaw cycles and are aliquoted on day of acquisition for analysis.
3.2.3.2. Processed sample stability
Stability findings of bovine muscle tissue extracts in final injection solvent showed tendency towards instability following storage in the case of LGD-4033 (4 weeks at −20 °C) and S-6 (2 weeks at 4 °C) as illustrated in Supplementary data – Fig. S2c, thus set criteria (relative response difference >±20% with p < 0.05) were not met. Hence, it can be concluded that SARM residues in muscle extracts obtained within the presented method may be stored prior to analysis for up to four weeks at −20 °C and for two weeks at 4 °C.
3.3. Assay applicability
The developed method has been applied to screen bovine muscle samples (n = 63) collected from abattoirs across Ireland and Scotland as well as beef samples obtained from local food store retailers, with none of the analysed samples reported to contain detectable quantities of SARM residues.
3.3.1. Analysis of incurred muscle tissue samples
Due to the lack of either appropriate proficiency test schemes or certified reference materials (CRMs), as an alternative the applicability of the presented method was evidenced through analysis of samples collected from rodent animals exposed to a number of SARM compounds. All tested rodent muscle tissue samples (n = 4) were classified correctly as follows: one sample was screened negative (administration of vehicle) and three samples were screened positive – collected from animals following administration of LGD-4033, ostarine (S-22) and RAD140, respectively (Fig. 3). Thus, this newly presented method is suitable for intended use as demonstrated through analysis of incurred rodent samples.
Fig. 3.
UHPLC-MS/MS traces of (a) blank rodent muscle tissue sample fortified at 1 ng g−1 with ostarine (S-22), rodent muscle tissue sample (b) screened negative (following treatment with vehicle), (c) screened positive following oral administration of ostarine (S-22).
4. Conclusions
Within this study, a fit-for-purpose semi-quantitative UHPLC-MS/MS-based screening assay enabling the simultaneous monitoring of 15 SARM residues in bovine muscle tissue with detection capability (CCβ) determined in the range of 0.5–5 ng g−1 depending on the analyte has been developed and validated. To the best of the authors’ knowledge, this is the first such method reported in the peer reviewed literature enabling screening for a wide range of emerging SARMs in bovine muscle. The method is particularly advantageous due to the high throughput sample preparation procedure generating limited solvent and/or consumable waste during performance. The mass spectrometric-based assay was demonstrated to be suitable for intended use through analysis of survey samples from target species as well as muscle samples from SARM exposed rodent animals, and validation results fulfilling set criteria as stipulated in relevant legislation (CRL, 2010, EC, 2002a). Therefore, the current method is appropriate with regard to routine analysis (e.g. in the frame of residue control programmes), with potential also to be applied as a screening tool for other test matrices (e.g. liver tissue) and/or other species. As SARM compounds have only recently emerged as potential threats in different fields, they have not been significantly investigated with regard to farm livestock (e.g. cattle) and more in vivo studies covering a range of pharmacophores are necessary to better understand their metabolism. Such studies would aid selection of appropriate targets (i.e. intact parent molecules and/or respective metabolites revealing treatment) which could be incorporated into the presented method for routine testing purposes to provide consumers with food of animal origin free from risk of residue contamination from such compounds.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
The research was supported by funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie Grant Agreement No. 642380.
Ethical approval
Ethical consent has been provided by Queen’s University Belfast Animal Welfare and Ethical Review Body (AWERB) (Ref. No. PPL2812).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2019.100056.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Aikawa K., Miyawaki T., Hitaka T., Imai Y.N., Hara T., Miyazaki J.…Yamamoto S. Synthesis and biological evaluation of novel selective androgen receptor modulators (SARMs). Part i. Bioorganic and Medicinal Chemistry. 2015;23:2568–2578. doi: 10.1016/j.bmc.2015.03.032. [DOI] [PubMed] [Google Scholar]
- Beucher L., Dervilly-Pinel G., Cesbron N., Penot M., Gicquiau A., Monteau F., Le Bizec B. Specific characterization of non-steroidal selective androgen peceptor modulators using supercritical fluid chromatography coupled to ion-mobility mass spectrometry: Application to the detection of enobosarm in bovine urine. Drug Testing and Analysis. 2017;9:179–187. doi: 10.1002/dta.1951. [DOI] [PubMed] [Google Scholar]
- Cawley A.T., Smart C., Greer C., Liu Lau M., Keledjian J. Detection of the selective androgen receptor modulator andarine (S-4) in a routine equine blood doping control sample. Drug Testing and Analysis. 2016;8:257–261. doi: 10.1002/dta.1867. [DOI] [PubMed] [Google Scholar]
- Cesbron N., Sydor A., Penot M., Prevost S., Le Bizec B., Dervilly-Pinel G. Analytical strategies to detect enobosarm administration in bovines. Food Additives & Contaminants: Part A. 2017;34:632–640. doi: 10.1080/19440049.2016.1258122. [DOI] [PubMed] [Google Scholar]
- CRL . CRLs View on State of the Art Analytical Methods for National Residue Control Plans; 2007. CRL Guidance paper (7 December 2007) [Google Scholar]
- CRL. (2010). Community reference laboratories residues (CRLs) 20/1/2010. Guidelines for the validation of screening methods for residues of veterinary medicines (initial validation and transfer).
- Dalton, J. T., Miller, D. D., & Kearbey, J. D. (2016). SARMs and method of use thereof, US009278914B2, United States Patent.
- Dalton J.T., Mukherjee A., Zhu Z., Kirkovsky L., Miller D.D. Discovery of nonsteroidal androgens. Biochemical and Biophysical Research Communications. 1998;244:1–4. doi: 10.1006/bbrc.1998.8209. [DOI] [PubMed] [Google Scholar]
- de Rijke E., Essers M.L., Rijk J.C.W., Thevis M., Bovee T.F.H., van Ginkel L.A., Sterk S.S. Selective androgen receptor modulators: In vitro and in vivo metabolism and analysis. Food Additives & Contaminants: Part A. 2013;30:1517–1526. doi: 10.1080/19440049.2013.810346. [DOI] [PubMed] [Google Scholar]
- EC. (1988). Council Directive of 7 March 1988 prohibiting the use in livestock farming of certainsubstances havinga hormonal action (88/146/EEC). Official Journal of the European Communities, L 70, 16–18.
- EC. (2002a). Commission Decision 2002/657/EC of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and interpretation of results. Official Journal of the European Communities, L 221, 8–36.
- EC. (2002b). Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. Official Journal of the European Communities, L 31, 1–24.
- EC. (2017). Regulation (EU) 2017/625 of the European Parliament and of the Council of 15 March 2017 on official controls and other official activities performed to ensure the application of food and feed law, rules on animal health and welfare, plant health and plant protection products, amending Regulations (EC) No 999/2001, (EC) No 396/2005, (EC) No 1069/2009, (EC) No 1107/2009, (EU) No 1151/2012, (EU) No 652/2014, (EU) 2016/429 and (EU) 2016/2031 of the European Parliament and of the Council, Council Regulations (EC) No 1/2005 and (EC) No 1099/2009 and Council Directives 98/58/EC, 1999/74/EC, 2007/43/EC, 2008/119/EC and 2008/120/EC, and repealing Regulations (EC) No 854/2004 and (EC) No 882/2004 of the European Parliament and of the Council, Council Directives 89/608/EEC, 89/662/EEC, 90/425/EEC, 91/496/EEC, 96/23/EC, 96/93/EC and 97/78/ EC and Council Decision 92/438/EEC (Official Controls Regulation). Official Journal of the European Union, L 95, 1–142.
- Fawcett T. An introduction to ROC analysis. Pattern Recognition Letters. 2006;27:861–874. [Google Scholar]
- FEI. (2019). Fédération Equestre Internationale (FEI). 2019 Equine Prohibited Substances List.
- Gaugain M., Chotard M.-P., Verdon E. Stability study for 53 antibiotics in solution and in fortified biological matrixes by LC/MS/MS. Journal of AOAC International. 2013;96:471–480. doi: 10.5740/jaoacint.12-062. [DOI] [PubMed] [Google Scholar]
- Geis-Asteggiante L., Lehotay S.J., Lightfield A.R., Dutko T., Ng C., Bluhm L. Ruggedness testing and validation of a practical analytical method for >100 veterinary drug residues in bovine muscle by ultrahigh performance liquid chromatography–tandem mass spectrometry. Journal of Chromatography A. 2012;1258:43–54. doi: 10.1016/j.chroma.2012.08.020. [DOI] [PubMed] [Google Scholar]
- IABRW. (2019). International Federation of Horseracing Authorities (IFHA). International Agreement on Breeding, Racing and Wagering (IABRW). Article 6A Prohibited substances.
- Kim J., Wang R., Veverka K.A., Dalton J.T. Absorption, distribution, metabolism and excretion of the novel SARM GTx-024 [(S)-N-(4-cyano-3-(trifluoromethyl)phenyl)-3-(4-cyanophenoxy)-2-hydroxy-2-methylpropanamide] in rats. Xenobiotica. 2013;43:993–1009. doi: 10.3109/00498254.2013.788233. [DOI] [PubMed] [Google Scholar]
- Kinsella B., O’Mahony J., Malone E., Moloney M., Cantwell H., Furey A., Danaher M. Current trends in sample preparation for growth promoter and veterinary drug residue analysis. Journal of Chromatography A. 2009;1216:7977–8015. doi: 10.1016/j.chroma.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Narayanan R., Coss C.C., Dalton J.T. Development of selective androgen receptor modulators (SARMs) Molecular and Cellular Endocrinology. 2018;465:134–142. doi: 10.1016/j.mce.2017.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera M.A., Yin D., Wu D., Chan K.K., Miller D.D., Dalton J. In vivo metabolism and final disposition of a novel nonsteroidal androgen in rats and dogs. Drug Metabolism and Disposition. 2006;34:1713–1721. doi: 10.1124/dmd.106.009985. [DOI] [PubMed] [Google Scholar]
- Rojas D., Dervilly-Pinel G., Cesbron N., Penot M., Sydor A., Prévost S., Le Bizec B. Selective androgen receptor modulators: Comparative excretion study of bicalutamide in bovine urine and faeces. Drug Testing and Analysis. 2017;9:1017–1025. doi: 10.1002/dta.2113. [DOI] [PubMed] [Google Scholar]
- SANCO. (2008). SANCO/2004/2726-rev 4-December 2008. Guidelines for the Implementation of Decision 2002/657/EC.
- SANTE. (2017). SANTE/11813/2017. Guidance document on analytical quality control and method validation procedures for pesticide residues and analysis in food and feed.
- Schmidt K.S., Mankertz J. In-house validation of a liquid chromatography–tandem mass spectrometry method for the determination of selective androgen receptor modulators (SARMS) in bovine urine. Food Additives & Contaminants: Part A. 2018;35:1292–1304. doi: 10.1080/19440049.2018.1471222. [DOI] [PubMed] [Google Scholar]
- Thevis M., Kuuranne T., Geyer H. Annual banned-substance review: Analytical approaches in human sports drug testing. Drug Testing and Analysis. 2018;10:9–27. doi: 10.1002/dta.2336. [DOI] [PubMed] [Google Scholar]
- Thevis M., Schänzer W. Detection of SARMs in doping control analysis. Molecular and Cellular Endocrinology. 2018;464:34–45. doi: 10.1016/j.mce.2017.01.040. [DOI] [PubMed] [Google Scholar]
- Thevis M., Volmer D.A. Mass spectrometric studies on selective androgen receptor modulators (SARMs) using electron ionization and electrospray ionization/collision-induced dissociation. European Journal of Mass Spectrometry. 2018;24:145–156. doi: 10.1177/1469066717731228. [DOI] [PubMed] [Google Scholar]
- Trufelli H., Palma P., Famiglini G., Cappiello A. An overview of matrix effects in liquid chromatography-mass spectrometry. Mass Spectrometry Reviews. 2011;30:491–509. doi: 10.1002/mas.20298. [DOI] [PubMed] [Google Scholar]
- UK. (1986). Animals (Scientific Procedures) Act 1986. 1–24.
- Vajda E.G., Lopez F.J., Rix P., Hill R., Chen Y., Lee K.-J.…Lee Y.-H. Pharmacokinetics and Pharmacodynamics of LGD-3303 [9-Chloro-2-ethyl-1-methyl-3-(2,2,2-trifluoroethyl)-3H-pyrrolo-[3,2-f]quinolin-7(6H)-one], an orally available nonsteroidal-selective androgen receptor modulator. Journal of Pharmacology and Experimental Therapeutics. 2009;328:663–670. doi: 10.1124/jpet.108.146811. [DOI] [PubMed] [Google Scholar]
- Ventura E., Gadaj A., Buckley T., Mooney M.H. International mass spectrometry conference (IMSC 2018), August 26th–31st 2018. 2018. A fit-for-purpose UHPLC-MS/MS method for routine screening of SARM residues in equine plasma and bovine serum. [Google Scholar]
- Ventura E., Gadaj A., Buckley T., Mooney M.H. Advances in clinical and forensic analysis 2018, November 27th. 2018. Complementary screening assays to monitor for emerging SARMs in urine and blood by UHPLC-MS/MS. London, UK. [Google Scholar]
- Ventura E., Gadaj A., Monteith G., Ripoche A., Healy J., Botrè F.…Mooney M.H. Development and validation of a semi-quantitative ultra-high performance liquid chromatography-tandem mass spectrometry method for screening of selective androgen receptor modulators in urine. Journal of Chromatography A. 2019;1600:183–196. doi: 10.1016/j.chroma.2019.04.050. [DOI] [PubMed] [Google Scholar]
- WADA. (2019). The World Anti-Doping Agency (WADA). The World Anti-Doping Agency Code. The 2019 Prohibited List International Standard.
- Zhang X., Sui Z. Deciphering the selective androgen receptor modulators paradigm. Expert Opinion on Drug Discovery. 2013;8:191–218. doi: 10.1517/17460441.2013.741582. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.