To the Editor
Recent studies have shown that more than 70% of the mammalian genome is transcribed into RNAs; however, most of these RNAs do not encode proteins and are thus called noncoding RNAs (ncRNAs). In a study published in Nature Immunology, Lin et al. reported the discovery of a new long noncoding RNA (lncRNA), Lnczc3h7a, that promotes the tripartite motif (TRIM25)-mediated K63-linked ubiquitination of retinoic acid-inducible gene-I (RIG-I), in turn augmenting downstream signal transduction.1 The authors showed that Lnczc3h7a binds to the C-terminal domain (CTD) of TRIM25 at steady state. In addition, after RIG-I recognizes pathogenic RNA, activated RIG-I is quickly recruited to form a complex with TRIM25 that serves as a molecular scaffold. This paper presents another component by which innate immunity is controlled and also provides new insight into lncRNA biology.
RIG-I was identified as a cytosolic RNA (Fig. 1) virus sensor in 2004.2 After virus-derived RNA binds to the CTD of RIG-I, a conformational change exposes its caspase recruitment and activation domain (CARD), allowing RIG-I to bind to the C-terminal SPRY domain of TRIM25. RIG-I is subsequently ubiquitinated by TRIM25, which is the key step that activates downstream signaling of RIG-I-mediated antiviral innate immunity.3 TRIM proteins are a family of RING (really interesting new gene) domain-containing proteins comprising more than 70 members in humans, with new members still being described.4 TRIM25, a type I and type II IFN-inducible E3 ligase, was first identified as an “estrogen-responsive finger protein” (EFP) and has been shown to be involved in numerous cellular processes, such as development, cancer, and innate immunity. Emerging data have shown that TRIM25 plays a crucial role in the regulation of the cytoplasmic RNA sensor RIG-I. RIG-I, a DExD/H box RNA helicase, plays a crucial role in the host defense response to eliminate invading RNA viruses. TRIM25 has been shown to have dual roles in RIG-I regulation, while TRIM25-mediated ubiquitination of RIG-I is essential for the transmission of downstream signaling. Furthermore, previous reports showed that the PRY/SPRY domain of TRIM25 is important for RNA binding and that TRIM25 interacts with other RNA-binding proteins using RNA as a scaffold.5 Therefore, the ability of TRIM25 to bind RNA suggests new lines of investigation to uncover additional mechanisms of action and potential targets of this molecule.
Fig. 1.
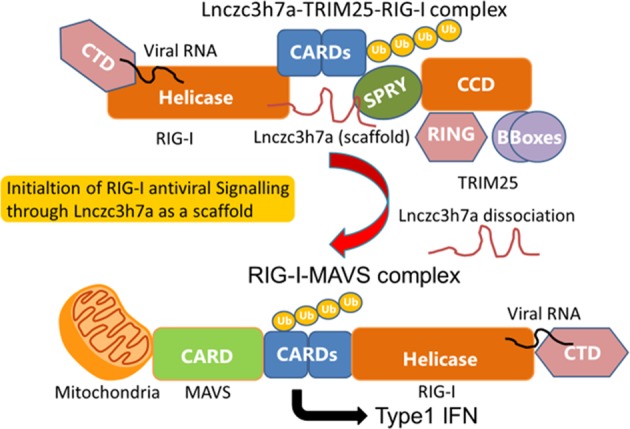
The host lncRNA Lnczc3h7a acts as a molecular scaffold for the Lnczc3h7a–TRIM25–RIG-I complex to initiate RIG-I antiviral signaling
Lin et al. found a novel lncRNA named Lnczc3h7a among lncRNAs that coprecipitated with TRIM25 in murine macrophage RAW264.7 cells before and after RNA virus infection. Lnczc3h7a was shown to bind to the C-terminal SPRY domain of TRIM25, to which the CARD domain of activated RIG-I also binds. While the expression of Lnczc3h7a increased in response to RNA viruses, the lack of Lnczc3h7a abrogated type I IFN signaling and elevated the replication of RNA viruses both in vitro and in vivo. How does Lnczc3h7a promote type I-IFN-mediated antiviral innate immunity? The authors showed that Lnczc3h7a serves as a scaffold to stabilize the RIG-I–TRIM25 complex and facilitates the TRIM25-mediated K63-linked ubiquitination of RIG-I by microscopic colocalization and coimmunoprecipitation assays and an RNA pull-down assay. Furthermore, Lin et al. suggested the binding sites for TRIM25 and RIG-I in Lnczc3h7a.
These findings increase opportunities to develop novel strategies to regulate the innate immune response to reduce viral replication and/or avoid undesired excessive inflammation. LncRNAs, noncoding transcripts longer than 200 nucleotides that bind DNA, RNA, and proteins, can regulate gene expression via diverse mechanisms. Studies have revealed that lncRNAs play important roles in immune responses through different mechanisms.6,7 Notably, individual lncRNAs function through modular domains and often link protein activity to DNA or RNA targets through interacting with both proteins and DNA/RNA. Moreover, these functions are dysregulated in human autoimmune diseases. Thus, lcRNA-mediated regulation of the innate immune response may be common, and further studies should be performed to clarify the role of self RNAs, which were previously unappreciated, in the innate response to eliminate nonself RNAs.
Several TRIM family members are in fact involved in the ubiquitination of RIG-I. TRIM25 and TRIM4 mediate the lysine 63 (K63)-linked ubiquitination of RIG-I and promote signaling. In contrast, TRIM26, TRIM15, TRIM31, TRIM39, and TRIM40 have been reported to mediate the K48-linked ubiquitination of RIG-I. It has also recently emerged that many of these proteins are involved in direct RNA binding and carry out biological roles with lncRNAs.8 Do lncRNAs serve as scaffolds to assist proper TRIMs functioning depending on the situation? To clarify this question, lncRNAs that coprecipitate with other TRIMs, their transcriptional regulation, and their TRIM-binding sites must be explored.
As a component of activation in innate immunity, TRIM25 must dissociate from K63-ubiquitinated RIG-I to facilitate the formation of RIG-I-mitochondrial antiviral signaling protein (MAVS), and RIG-I dissociates from the stabilized complex of Lnczc3h7a–TRIM25–RIG-I. Is there a mechanism by which the RIG-I–MAVS complex is stabilized more than the Lnczc3h7a–TRIM25–RIG-I complex? It would be interesting to explore whether another undiscovered lncRNA facilitates dissociation of the stabilized Lnczc3h7a–TRIM25–RIG-I complex.
Mammalian hosts and pathogens encode lncRNAs that regulate host–pathogen interactions; these lncRNAs play either beneficial or detrimental roles in host survival.9 Viruses are the only group of organisms on the planet that use RNA as their genome, and these RNAs can be replicated through RNA-dependent RNA polymerase, making the spectrum of RNA, including host-derived, virus-derived, and even some chimeric RNAs, more diverse in infected cells. Some viral sensors and downstream immune signaling in the host are regulated directly by host lcRNAs and nonhost viral RNAs through RNA–protein interactions.10 The biological function and importance of this self-recognition in maintaining immune homeostasis need to be clarified.
This study also suggests several other new questions about lncRNA biology. Many previous studies have shown that lncRNAs exert their biological functions in the nucleus.11 In the last decade or so, some cytoplasmic lncRNAs have been discovered, indicating their importance for multiple cellular activities.12 However, the regulatory mechanism of lncRNA localization has not yet been found. How does Lnczc3h7a translocate to the cytoplasm? There should be machinery that either directly or indirectly transports specific lncRNAs into the cytoplasm and perhaps further special subcellular locations or complexes. However, like the mechanism for the precise localization of most other lncRNAs, that of Lnczc3h7a, which preferentially localizes in the cytoplasm, has remained unclear. Furthermore, how does Lnczc3h7a serve as a scaffold to place two specific proteins, RIG-I and TRIM25, in the proper directions? Are there specific sequences or tertiary structures that determine the binding of Lnczc3h7a to specific proteins or the translocation of these proteins? Structural characterization of protein–RNA complexes is a promising approach that should reveal detailed features of this interaction and whether Lnczc3h7a not only serves as a scaffold but also functions via conformational change.
Acknowledgements
We thank Edanz (www.edanzediting.co.jp) for editing the English text of a draft of this paper.
Competing interests
The authors declare no competing interests.
References
- 1.Lin H, et al. The long noncoding RNA Lnczc3h7a promotes a TRIM25-mediated RIG-I antiviral innate immune response. Nat. Immunol. 2019;20:812–823. doi: 10.1038/s41590-019-0379-0. [DOI] [PubMed] [Google Scholar]
- 2.Yoneyama M, et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- 3.Gack MU, et al. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature. 2007;446:916–920. doi: 10.1038/nature05732. [DOI] [PubMed] [Google Scholar]
- 4.Munir M. TRIM proteins: another class of viral victims. Sci. Signal. 2010;3:jc2. doi: 10.1126/scisignal.3118jc2. [DOI] [PubMed] [Google Scholar]
- 5.Choudhury NR, et al. RNA-binding activity of TRIM25 is mediated by its PRY/SPRY domain and is required for ubiquitination. BMC Biol. 2017;15:105. doi: 10.1186/s12915-017-0444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen YG, Satpathy AT, Chang HY. Gene regulation in the immune system by long noncoding RNAs. Nat. Immunol. 2017;18:962–972. doi: 10.1038/ni.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanchez JG, et al. TRIM25 binds RNA to modulate cellular anti-viral defense. J. Mol. Biol. 2018;430:5280–5293. doi: 10.1016/j.jmb.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams F. P., Haubrich K., Perez-Borrajero C., Hennig J. Emerging RNA-binding roles in the TRIM family of ubiquitin ligases. Biol Chem. 10.1515/hsz-2019-0158 (2019). Epub ahead of print. [DOI] [PubMed]
- 9.Wang P. The opening of pandora’s box: an emerging role of long noncoding RNA in viral infections. Front. Immunol. 2018;9:3138. doi: 10.3389/fimmu.2018.03138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang M, et al. Self-recognition of an inducible host lncRNA by RIG-I feedback restricts innate immune response. Cell. 2018;173:906–919 e13. doi: 10.1016/j.cell.2018.03.064. [DOI] [PubMed] [Google Scholar]
- 11.Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol. Cell. 2011;43:904–914. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao RW, Wang Y, Chen LL. Cellular functions of long noncoding RNAs. Nat. Cell Biol. 2019;21:542–551. doi: 10.1038/s41556-019-0311-8. [DOI] [PubMed] [Google Scholar]


