Abstract
Dendritic cell (DC) tumor vaccines exert their antitumor effects through the induction of effector T cells. We recently identified Tc9 cells as a new potent antitumor effector T cell subset. However, approaches to direct DCs to preferably prime antitumor Tc9 cells should be further exploited. Here, we demonstrate that the addition of interleukin (IL)-33 potently promotes the induction of Tc9 cells by DCs in vitro and in vivo. IL-33 treatment also drives the cytotoxic activities of DC-induced Tc9 cells. Notably, IL-33 treatment enhances cell survival and proliferation of DC-primed CD8+ T cells. More importantly, the addition of IL-33 during in vitro priming of tumor-specific Tc9 cells by DCs increases the antitumor capability of Tc9 cells. Mechanistic studies demonstrated that IL-33 treatment inhibits exhaustive CD8+ T cell differentiation by inhibiting PD-1 and 2B4 expression and increasing IL-2 and CD127 (IL-7 receptor-α, IL-7Rα) expression in CD8+ T cells. Finally, the addition of IL-33 further promotes the therapeutic efficacy of DC-based tumor vaccines in the OT-I mouse model. Our study demonstrates the important role of IL-33 in DC-induced Tc9 cell differentiation and antitumor immunity and may have important clinical implications.
Keywords: Interleukin-33, Dendritic cells, Tc9, Cancer immunology
Introduction
Dendritic cells (DCs) are professional antigen-presenting cells (APCs) and play a crucial role in the induction of antitumor immunity.1,2 DCs exert their antitumor effects through the induction of effector T cells.3 CD8+ T cells (cytotoxic T lymphocytes or Tc cells) are superior antitumor effector T cells with immediate cytolytic activity against tumor cells.4,5 CD8+ T cells can be differentiated into different Tc cell subsets with distinct phenotypes, such as Tc1, Tc2, and Tc17 cells.6 In tumor immunology, Tc1 cells produce interferon-γ (IFN-γ) and kill tumor cells by IFN-γ-mediated or perforin-mediated mechanisms.4 Tc1 cells are also the major antitumor Tc cells primed by regular DCs in tumor therapy.7 Tc2 cells secrete type II cytokines, such as IL-4, IL-5, and IL-10.4 Tc17 cells secrete IL-17, which promotes both tumor growth and tumor regression.8–10
We recently described a new Tc cell subset Tc9 characterized by the secretion of IL-9.11 Tc9 cells can be generated in vitro by culturing naive CD8+ T cells under Tc9-polarizing conditions (i.e., anti-CD3/CD28 plus transforming growth factor β (TGF-β)/IL-4).11,12 Tumor-specific Tc9 cells are superior antitumor effector cells.11 Tc9 cells are less cytolytic in vitro than Tc1 cells; however, Tc9 cells elicit greater antitumor responses than Tc1 cells in vivo.11 Tc9 cells have a "younger" phenotype and persist much longer than Tc1 cells in vivo.13 Furthermore, Tc9 cells can be differentiated into Tc1-like effector cells with increased cytolytic activities in vivo.11 These observations suggest that DC tumor vaccines that preferably induce Tc9 cells may potently increase their antitumor effects.
IL-33 is a member of the IL-1 superfamily of cytokines and a ligand for suppression of tumorigenicity 2 (ST2) receptor.14,15 Previous studies reported that IL-33 preferably stimulates antiviral and antitumor CD8+ T cell responses.16,17 IL-33 overexpression in tumors inhibits melanoma tumor growth by activating NK and CD8+ T cell responses18,19 and ILC2-mediated tumor cell apoptosis.20 IL-33 also promotes anti-tumor immunity in mice by inhibiting the differentiation and immunosuppressive activity of granulocytic myeloid-derived suppressor cells and activating DCs and eosinophils.21–23 We recently found that dectin-1-activated DCs drive potent Th9 cell responses.24 T cell differentiation depends mainly on the cytokine microenvironment.25 We further found that dectin-1 activation stimulates DCs to overexpress 42 cytokines and costimulatory molecules, including IL-33, which may contribute to the induction of Th9 cells by dectin-1-activated DCs.24 Given that Th9 and Tc9 cell differentiation may require the same or similar polarizing cytokines, we hypothesized that cytokines overexpressed by dectin-1-activated DCs may also drive the induction of Tc9 cells by DCs.
In this study, we found that the addition of IL-33 promotes the induction of Tc9 cells by DCs. IL-33 treatment increases Tc9 cell survival and proliferation and drives their cytotoxic activities against tumor cells. In addition, IL-33 treatment during in vitro priming of tumor-specific Tc9 cells by DCs increases the antitumor capability of Tc9 cells. Furthermore, the combined use of DC vaccine and IL-33 further promotes the therapeutic efficacy of DC-based tumor vaccines in the OT-I mouse model. Our study demonstrates the important role of IL-33 in DC-induced Tc9 cell differentiation and antitumor immunity and may have important clinical implications.
Materials and methods
Mice and cell lines
C57BL/6 (H-2b) and OT-I (C57BL/6-Tg(TcraTcrb)1100Mjbn/J) mice were purchased from the Jackson Laboratory. All mice were housed and bred under specific pathogen-free conditions at Animal Center of The First Hospital of Jilin University. Mice were used for experiments at age 6–8 weeks. All animal studies were conducted according to the ARRIVE guidelines, the U.K. Animals (Scientific Procedures) Act of 1986, EU Directive 2010/63/EU for animal experiments, and the ethical guidelines of the Animal Ethical Committee of First Hospital of Jilin University.
B16 and B16-OVA melanoma cell lines (ATCC) were cultured in RPMI 1640 medium (CORNING) supplemented with 10% heat-inactivated fetal bovine serum (FBS, HyClone), 100 U/mL penicillin (HyClone) and 100 mg/mL streptomycin (HyClone). Cells were grown in standard (37 °C, 5% CO2) culture incubators.
Reagents and Abs
Recombinant mouse granulocyte-macrophage colony-stimulating factor (GM-CSF), TNF-α, IL-1β, IL-4, and IL-2 were purchased from Peprotech. Recombinant mouse IL-33 and human TGF-β were purchased from R&D Systems. The OVA (257–264, SII NFE KL) peptide used in the OT-I mouse model was purchased from GL Biochem (Shanghai) Ltd. Functional anti-mouse CD3e and CD28 antibodies (mAbs) were purchased from eBioscience.
Generation of DCs
DCs were generated as described previously.24 In brief, bone marrow (BM) cells were cultured in RPMI 1640 complete medium supplemented with GM-CSF (20 ng/mL) and IL-4 (10 ng/mL). At day 4, the culture medium was replaced with fresh GM-CSF (10 ng/mL) and IL-4 (10 ng/mL)-containing medium. At day 7, semi-adherent cells were collected as immature DCs (iDCs) and matured by TNF-α (10 ng/mL) and IL-1β (10 ng/mL) (BMDCs) for 48 h. At day 9, the semi-adherent cells were collected as mature DCs (mDCs) for further experiments.
Flow cytometry analysis
Immunofluorescence surface staining was performed as previously described.24 The fluorescence-labeled mAbs against CD3, CD8, CD25, CD62L, PD-1, 2B4, LAG3, and Annexin V were purchased from BD Biosciences. APC-labeled ST2 Ab was purchased from Biolegend.
Intracellular staining was performed as previously described.24 PE-conjugated or Pacific Blue-conjugated mAbs against IL-9, IFN-γ, granzyme B (GzmB), and Ki67 were purchased from Biolegend. After staining, cell samples were analyzed using a BD LSRFortessaTM cytometer.
In vitro Tc9 cell differentiation
Naive CD8+ T cells (CD8+CD25-CD62Lhi) were purified by fluorescence activated cell sorting (FACS) from mouse spleens and cocultured at 1 × 105 per well with BMDCs (1 × 105/well) in the presence of plate-bound anti-CD3 (2 μg/mL), soluble anti-CD28 (2 μg/mL), Tc9-polarizing cytokines IL-4 (10 ng/mL) and TGF-β (1 ng/mL), and IL-2 (50 ng/mL). Cells from cultures without the addition of TGF-β and IL-4 were used as Tc0 cells. IL-33 (50 ng/mL) was added to some cell cultures. After 2 days of culture, cells were harvested and analyzed by flow cytometry and qPCR.
Quantitative polymerase chain reaction (qPCR)
Total RNA was extracted from cells using an EasyPure RNA Kit (TransGen Biotech), and cDNA was synthesized with an All-in-One First-Strand cDNA Synthesis SuperMix (TransGen). Cd244, Ifng, Il2, Il9, Irf4, Gzmb, Lag3, Pdcd1, Spi1, St2, and Tbx21 mRNA levels in Tc cells were analyzed. Expression was normalized to the expression of the housekeeping gene Gapdh. Primer sets for Ifng, Il9, Irf4, Spi1, and Tbx21 were previously reported.24 Primer sets for Cd244, Il2, Gzmb, Lag3, Pdcd1 and St2 are provided in Supplementary Table 1.
Enzyme-linked immunosorbent assay (ELISA)
IL-9 and IFN-γ concentrations in culture supernatants were detected by ELISA as previously described.24 Capture/detection antibodies for IL-9 and IFN-γ were purchased from BD Biosciences. Recombinant mouse IL-9 and IFN-γ, which were used as ELISA standards, were purchased from R&D Systems and BD Biosciences, respectively. Avidin-HRP was purchased from Biolegend.
In vivo functional tests of IL-33/ST2 in DC-induced T cell differentiation
BMDCs were pulsed with OT-I OVA peptides (5 μg/mL). Mice were administered 2 weekly subcutaneous immunizations with 1 × 106 treated DCs. Mice injected with PBS served as controls. In some experiments, mice were administered IL-33 (250 ng/mouse) every 3 days starting 1 day after the first DC immunization. On day 3 after the second DC immunization, total leukocytes were collected from spleens and lymph nodes. Cells were restimulated with OT-I OVA peptides (5 μg/mL) for 2 days. Cells from PBS control mice were untreated. Culture cells and supernatants were collected and analyzed by flow cytometry and ELISA. CD8+ T cells were isolated by magnetic cell sorting (MACS) and analyzed by qPCR. In some experiments, total leukocytes collected from spleens and lymph nodes were restimulated with OVA peptide-pulsed BMDCs in the presence or absence of IL-33 (50 ng/mL) for 2 days. Culture cells and CD8+ T cells isolated by MACS were analyzed by flow cytometry and/or qPCR.
Cytotoxicity assay
The cytotoxicity assay was performed as previously described.11 To examine the cytotoxicity of in vitro differentiated Tc9 cells, naive CD8+ T cells from OT-I mice were cocultured with BMDCs under Tc9-polarizing conditions in the presence or absence of IL-33 (50 ng/mL) for 2 days. CD8+ T cells were isolated by MACS and used as effector cells. B16-OVA cells labeled with 2.5 μM CFSE were used as target cells, whereas B16 nontarget cells labeled with 0.25 μM CFSE were used as controls. Target cells or nontarget cells were cocultured with effector cells at different ratios for 8 h. Cells from target and nontarget cell cultures were mixed and analyzed by FACS. Percent specific lysis was calculated as (1-target/control) × 100%.
To examine the cytotoxicity of BMDC-primed Tc cells in vivo, OT-I mice (n = 4–5/group) were immunized twice (1 week apart) with 1 × 106 OT-I OVA peptide-pulsed BMDCs. Mice treated with PBS served as controls. In some experimental groups, mice were administered IL-33 (250 ng/mouse) every 3 days starting 1 day after the first DC immunization. On day 2 after the second immunization, spleen cells from the mice were restimulated with OT-I OVA peptides for 48 h. CD8+ T cells were isolated by MACS and used as effector cells. B16-OVA cells labeled with 2.5 μM CFSE were used as target cells, and B16 nontarget cells labeled with 0.25 μM CFSE were used as controls.
Adoptive tumor immunotherapy
Briefly, 5 × 105 B16-OVA cells were injected subcutaneously into C57BL/6 mice. To generate Tc9 cells, naive CD8+ T cells from OT-I mice were cocultured with BMDCs under Tc9-polarizing conditions in the presence or absence of IL-33 for 2 days. Cells from cultures without the addition of Tc9-polarizing cytokines TGF-β and IL-4 were used as Tc0 cells. At day 3 after tumor injection, mice were randomly divided into groups and treated with adoptive transfer of 1 × 106 Tc0 or Tc9 cells. Mice treated with PBS served as controls. Tumor development was monitored over time. The mice were sacrificed when the tumor diameter was between 1.5 and 2 cm. Tumor volume was calculated by the following formula: 3.14 × (mean diameter)3/6.
DC-based tumor therapy
BMDCs were pulsed with OT-I OVA peptides (5 μg/mL) for 2–4 h and then harvested for mouse immunization. Briefly, 5 × 105 B16-OVA were injected subcutaneously into OT-I mice. On day 3 after tumor injection, the mice were randomly divided into groups with five mice per group. One group of mice was treated with IL-33 (250 ng/mouse) via intraperitoneal (i.p.) injection every 3 days. Some mice were administered two weekly subcutaneous immunizations with 1 × 106 treated mDCs with or without i.p. injection of IL-33 (250 ng/mouse) every 3 days. Mice injected with PBS served as controls. Tumor development was monitored over time. The mice were sacrificed when the tumor diameter was between 1.5 and 2 cm.
Statistical analysis
The Student t test (2 groups) and one-way ANOVA (≥3 groups) were used to compare various experimental groups. A P-value less than 0.05 was considered significant.
Results
IL-33 drives DC-induced Tc9 cell differentiation in vitro
We first compared the effects of DCs plus IL-33 and IL-33 alone on Tc9 cell differentiation in vitro. As shown in Supplementary Fig. 1A&B, IL-33 treatment slightly enhanced the development of IL-9-producing Tc9 cells and increased Il9 mRNA expression in Tc9 cells compared with untreated controls. However, the combination of BMDCs and IL-33 further increased Tc9 cell development (Supplementary Fig. 1A) and Il9 mRNA expression (Supplementary Fig. 1B) compared with cells treated with IL-33 alone. Given that we are exploiting new strategies to improve the therapeutic efficacy of DC-based tumor immunotherapy, we therefore focused on the role of BMDCs plus IL-33 in Tc9 cell induction and antitumor immunity in this study.
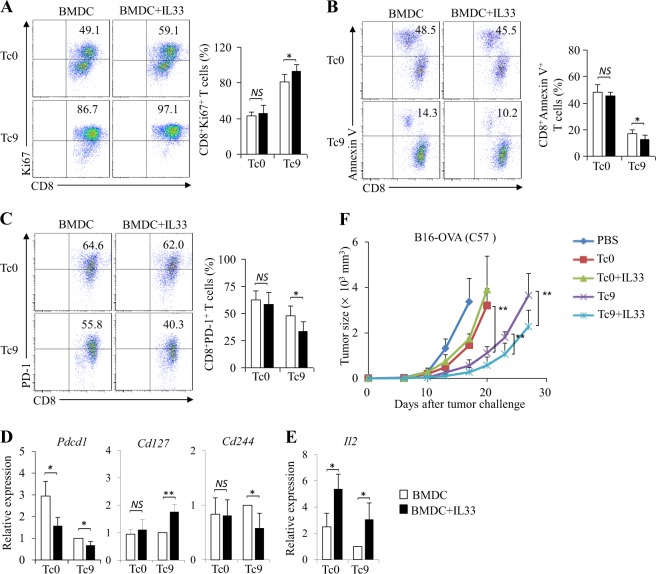
To further exploit the role of IL-33 in DC-induced Tc9 cell differentiation, naive CD8+ T cells were cocultured with BMDCs under Tc9-polarizing conditions with or without the addition of IL-33. Similarly, the addition of IL-33 enhanced BMDC-induced Tc9 cell development (Fig. 1a) and increased IL-9 protein and mRNA expression in BMDC-primed Tc9 cells (Fig. 1c, d). In addition, the addition of IL-33 enhanced Tc9 cell expression of Tc9-related transcription factors Spi1 and Irf4 (Fig. 1e) but not Tc1-related transcription factor Tbx21 (Fig. 1e). Furthermore, IL-33-treated Tc9 cells expressed increased GzmB mRNA and protein compared with regular Tc9 cells (Fig. 1b, d). Interestingly, the addition of IL-33 enhanced the expression of the IL-33 receptor St2 by Tc9 cells (Fig. 1f). Finally, IL-33 treatment increased the cytotoxic activity of BMDC-induced Tc9 cells (Fig. 1g).
Fig. 1.
IL-33 drives Tc9 cell differentiation in vitro. Naive CD8+ T cells were cocultured with BMDCs under Tc9-polarizing conditions with or without the addition of IL-33 for 2 days. Cell cultures without (Tc0) the addition of Tc9-polarizing cytokines TGF-β and IL-4 were used as controls. a, b Flow cytometry analysis of IL-9-expressing CD8+ T (Tc9) cells (a) and GzmB +CD8+ T cells (b). Numbers in the dot plots represent the percentages of CD8+IL-9+ T cells and GzmB +CD8+ T cells. Right, summarized results of three independent experiments obtained as reported on the left. c ELISA assessed IL-9 secretion in the cocultures. d–f qPCR analysis of the indicated cytokines (d), transcription factors (e) and St2 (f) in T cells. Expression was normalized to Gapdh and set at 1 in BMDC-induced Tc9 cells. g Naive CD8+ T cells from OT-I mice were cocultured with BMDCs under Tc9-polarizing conditions in the presence or absence of IL-33 for 2 days. B16-OVA-specific cytotoxicity of the cultured CD8+ T cells was examined. The results presented are the mean ± SD of 3–5 independent experiments. NS nonsignificant; *P < 0.05; **P < 0.01
Next, we analyzed the effects of IL-33 on other Tc1 cell differentiation processes. Naive CD8+ T cells were cocultured with BMDCs under Tc1-polarizing conditions in the presence or absence of IL-33. IL-33 slightly promotes BMDC-induced Tc1 cell differentiation, leading to increased expression of Ifng, GzmB, and Tbx21 (Supplementary Fig. 2A&B). To compare the role of IL-33 in the tumor-specific cytotoxicity of BMDC-primed Tc9 and Tc1 cells, OT-I Tc9, and Tc1 cells were generated in vitro. The addition of IL-33 increased the cytotoxic activity of BMDC-induced Tc9 cells but not Tc1 cells (Supplementary Fig. 2C). Together, these results demonstrated that IL-33 drives BMDC-induced Tc9 cell differentiation in vitro.
IL-33 increases Tc9 cell survival and proliferation in vitro
We next examined the effects of IL-33 treatment on the survival and proliferation of Tc9 cells primed by DCs in vitro. We first examined the effects of IL-33 on Tc9 cell proliferation by flow cytometry analysis of Ki67 (a marker of cell proliferation) expression in cells. As shown in Fig. 2a, IL-33 treatment increased Ki67 expression in Tc9 cells. Furthermore, IL-33 treatment reduced apoptosis in Tc9 cells compared with untreated control cells (Fig. 2b).
Fig. 2.
IL-33 increases the survival and proliferation of Tc9 cells in vitro. Naive CD8+ T cells were cultured as shown in Fig. 1. a–c Flow cytometry of Ki67+CD8+ (a), Annexin V+CD8+ (b) and PD-1+CD8+ (c) T cells. Numbers in the dot plots represent the percentages of double-positive T cells. Right, summarized results of three independent experiments obtained as reported on the left. d, e qPCR analysis of Pdcd1, Cd127, and Cd244 (d) and Il2 (e) expression in CD8+ T cells. f Naive CD8+ T cells from OT-I mice were cocultured with BMDCs under Tc9-polarizing or Tc0-polarizing conditions with or without the addition of IL-33 for 2 days. Cells (1 × 106 per mouse) were adoptively transferred into B16-OVA-bearing C57BL/6 mice. Mice treated with PBS served as controls. Shown are the tumor growth curves. The results presented are the mean ± SD of 3–5 independent experiments. NS nonsignificant; *P < 0.05; **P < 0.01
A younger CD8+ T cell phenotype may favor cell survival and proliferation.26 PD-1 is an immune-checkpoint protein and a marker of exhaustive T cell differentiation.27 We next examined PD-1 expression in Tc9 cells with or without IL-33 treatment. Although Tc9 cells expressed lower levels of PD-1 compared with Tc0 cells (Fig. 2c, d), IL-33 treatment decreased PD-1 expression in both Tc0 and Tc9 cells (Fig. 2c, d). In addition, IL-33 treatment downregulated 2B4 (CD244) mRNA levels in Tc9 cells (Fig. 2d and Supplementary Fig. 3), which is another marker of exhaustive T cell differentiation. Furthermore, IL-33 treatment increased the expression of the IL-7 receptor-α gene Cd127 in Tc9 cells (Fig. 2d) and Il2 in both Tc0 and Tc9 cells (Fig. 2e), which may contribute to the survival and proliferation of CD8+ T lymphocytes. These data demonstrated that IL-33 enhances BMDC-primed Tc9 cell survival and proliferation in vitro.
Tumor-specific Tc9 cells are potent antitumor effector cells.11 We found that IL-33 enhances DC induction of Tc9 cells and promotes their survival and proliferation. Based on these observations, we hypothesized that Tc9 cells primed by DCs plus IL-33 display more potent antitumor activities compared with Tc9 cells primed by DCs alone. To address this issue, OT-I Tc9 cells were primed by BMDCs in the presence or absence of IL-33 in vitro, and cells were used to treat B16-OVA-bearing C57BL/6 mice. Although Tc9 cells mediated increased inhibition of melanoma tumor growth compared with Tc0 cells (Fig. 2f), the addition of IL-33 during Tc9 cell priming further improved their antitumor efficacy (Fig. 2f), demonstrating the role of IL-33 in the improvement of the antitumor capability of BMDC-primed Tc9 cells.
IL-33 promotes the development of BMDC-induced Tc9/1 cells in vivo
We next explored the role of IL-33 in DC-induced Tc9 cell differentiation in vivo. OT-I mice were immunized with OVA peptide-pulsed BMDCs with or without addition of IL-33. Although mice immunized with BMDCs exhibited low levels of IL-9-expressing CD8+ T cells (Tc9) that were comparable to those of PBS control mice (Fig. 3a, b), mice immunized with BMDCs plus IL-33 exhibited increased levels of Tc9 cells compared with mice immunized with BMDCs or PBS control (Fig. 3a, b). ELISA and qPCR further confirmed the increased expression of IL-9 by CD8+ T cells in mice immunized with BMDCs plus IL-33 compared with BMDCs or PBS control (Fig. 3c, d). In addition, CD8+ T cells from mice immunized with BMDCs plus IL-33 expressed higher levels of Spi1 and Irf4 compared with mice treated with BMDCs or PBS control (Fig. 3e). These results indicated that IL-33 enhances the induction of Tc9 cells primed by BMDCs in vivo.
Fig. 3.
IL-33 promotes the development of BMDC-induced Tc9/1 cells in vivo. OT-I mice were administered two weekly subcutaneous immunizations with 1 × 106 OVA-peptide-pulsed BMDCs. Some mice were administered an intraperitoneal injection of IL-33 every 3 days beginning on the day of the first immunization. PBS served as control. On day 2 after the 2nd immunization, mouse spleen cells were restimulated with OT-I OVA peptides for 2 days. Cells from control mice were untreated. a Flow cytometry of IFN-γ-, IL-9-producing or GzmB-producing CD8+ T cells. Numbers in the dot plots represent the percentages of double-positive Tc cells. b Summarized results of three independent experiments obtained in (a). c ELISA assays of IL-9 and IFN-γ in the cultures. d, e CD8+ T cells were isolated by magnetic cell sorting (MACS). qPCR analyses of Il9, Ifng, and Gzmb (d) and Spi1, Irf4, and Tbx21 (e) in CD8+ T cells. f CD8+ T cells were isolated by MACS. B16-OVA-specific cytotoxicity of CD8+ T cells was examined. Data are representative of three (a) independent experiments or presented as the mean ± SD of three (b–f) independent experiments. *P < 0.05; **P < 0.01
We also examined the expression of the antitumor effector molecules IFN-γ and GzmB by CD8+ T cells in mice. Mice immunized with BMDCs plus IL-33 exhibited increased percentages of IFN-γ-producing and GzmB-producing CD8+ T cells (Fig. 3a, b) and increased expression levels of IFN-γ and Gzmb in CD8+ T cells compared with mice treated with BMDCs or PBS control (Fig. 3c, d). Furthermore, CD8+ T cells from mice immunized with BMDCs plus IL-33 expressed higher levels of Tbx21 compared with mice treated with BMDCs or PBS control (Fig. 3e), indicating that IL-33 promoted BMDC induction of Tc1 cells in vivo. Notably, the administration of IL-33 increased the tumor-specific cytotoxicities of CD8+ T cells primed by BMDCs in vivo (Fig. 3f). Together, these results demonstrated that IL-33 stimulates BMDCs to induce Tc9 and Tc1 cells in vivo.
IL-33 increases the proliferation of Tc cells primed by BMDCs in vivo
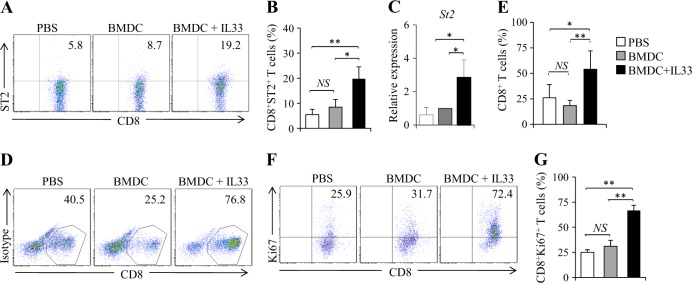
We next examined the role of IL-33 in ST2 expression by CD8+ T cells primed by BMDCs in vivo. OT-I mice were immunized by OVA-peptide-pulsed BMDCs with or without the addition of IL-33. Mice immunized by BMDCs plus IL-33 exhibited increased percentages of ST2-expressing CD8+ (ST2+CD8+) T cells in spleen cells (Fig. 4a, b) and increased St2 mRNA expression in CD8+ T cells (Fig. 4c) compared with mice treated with BMDCs or PBS controls. However, there was no difference in ST2 expression by CD8+ T cells from mice immunized with BMDCs compared with PBS controls (Fig. 4a–c). These results demonstrated that IL-33 stimulates ST2 expression by CD8+ T cells in vivo.
Fig. 4.
IL-33 increases the proliferation capability of Tc cells in vivo. a–c OT-I mice were immunized, and spleen cells were restimulated as presented in Fig. 3. a Flow cytometry of ST2-expressing CD8+ T (ST2+CD8+) cells in mouse spleen cells. b Summarized results of three independent experiments obtained in (a). c CD8+ T cells were isolated by MACS. qPCR analyses of St2 in CD8+ T cells. (d–g) OT-I mice were immunized as presented in Fig. 3. On day 2 after the 2nd immunization, mouse spleen cells were restimulated with BMDCs or BMDCs plus IL-33 for 2 days. Cells from PBS control mice were untreated. d Flow cytometry of CD8+ T cells in mouse spleen cells. e Summarized results of three independent experiments obtained in (d). f Flow cytometry analysis of Ki67+CD8+ T cells. g Summarized results of three independent experiments obtained in f. Data are representative of three (a, d, f) independent experiments or presented as the mean ± SD of three (b, c, e, g) independent experiments. NS nonsignificant; *P < 0.05; **P< 0.01
We next examined the effects of IL-33 on the proliferation of CD8+ T cells primed by BMDCs in vivo. Mice immunized with BMDCs exhibited comparable percentages of CD8+ T cells compared with PBS control mice (Fig. 4d, e). Interestingly, mice immunized by BMDCs plus IL-33 exhibited significantly increased percentages of total CD8+ T cells in mouse spleen cells compared with mice receiving BMDCs or PBS control (Fig. 4d, e). To determine whether IL-33 promoted BMDC-primed CD8+ T cell proliferation, spleen T cells were analyzed using intracellular Ki67 staining. Similarly, IL-33 increased Ki67 expression in Tc cells from mice treated with BMDCs plus IL-33 compared with BMDCs alone or PBS controls (Fig. 4f, g). These results indicated that IL-33 promotes the proliferation of Tc cells primed by DCs in vivo.
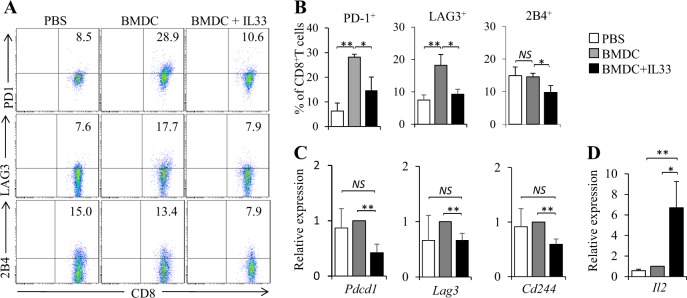
We next examined the phenotype of Tc cells from mice immunized by BMDCs plus IL-33. BMDC immunization increased CD8+ T cell expression of PD-1 and LAG3 compared with PBS controls (Fig. 5a–c). However, immunization with BMDC plus IL-33 potently inhibited PD-1, LAG3, and 2B4 expression by CD8+ T cells compared with BMDC treatment (Fig. 5a–c). Furthermore, mice immunized with BMDCs plus IL-33 exhibited increased Il2 expression in CD8+ T cells compared with mice treated with BMDCs alone or PBS controls (Fig. 5d). These results indicated that IL-33 inhibits exhaustive differentiation of CD8+ cells primed by BMDCs in vivo.
Fig. 5.
IL-33 inhibits the exhaustive differentiation of BMDC-activated CD8+ T cell in vivo. OT-I mice were immunized as shown in Fig. 3, and lymph node cells were collected. a Flow cytometry analysis of PD-1, LAG3, and 2B4 expression in CD8+ T cells. b Summarized results of three independent experiments obtained in (a). c, d qPCR examined the expression of Pdcd1, Lag3, Cd244 (c) and Il2 (d) in CD8+ T cells. Data are representative of three (a) independent experiments or presented as the mean ± SD of three (b–d) independent experiments. NS nonsignificant; *P < 0.05; **P< 0.01
IL-33 increases BMDC-induced antitumor efficacy
To examine the effects of IL-33 on the antitumor efficacy induced by DC vaccines, B16-OVA-bearing OT-I mice were treated with OVA peptide-pulsed BMDCs in the presence or absence of IL-33. As shown in Fig. 6, BMDCs plus IL-33 induced more potent inhibition of melanoma tumor growth than BMDCs or IL-33 alone. Nevertheless, IL-33 alone could not inhibit melanoma growth compared with PBS controls (Fig. 6). These results demonstrated that IL-33 promoted BMDC-induced antitumor immunity in vivo.
Fig. 6.
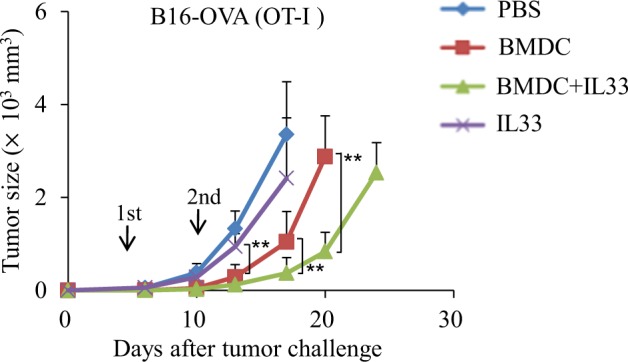
IL-33 increases BMDC-induced antitumor efficacy in vivo. OT-I mice were injected subcutaneously with 1 × 105 B16-OVA cells. On day 3 after tumor challenge, the mice were randomly divided into groups with five mice per group. One group of mice was treated with IL-33 (250 ng/mouse) via intraperitoneal (i.p.) injection every 3 days. Some mice were administered two weekly subcutaneous immunizations with 1 × 106 treated mDCs with or without i.p. injection of IL-33 (250 ng/mouse) every 3 days. Mice that received PBS served as controls. The experiments were performed twice with a total of 10 mice per group (n = 10). Shown are the tumor growth curves. Data are presented as the mean ± SD of the combined experiments. **P < 0.01
Discussion
DC-based immunotherapy is a promising approach for tumor therapy, and tumor-specific Tc9 cells are potent antitumor effector T cells.11 Therefore, strategies to drive the induction of tumor-specific Tc9 cells by DCs may have high clinical significance. In this study, we found that the addition of IL-33 potently promotes the generation of Tc9 cells primed by BMDCs in vitro and in vivo. More importantly, IL-33 treatment further increases the antitumor efficacy induced by BMDC tumor vaccines in OT-I mouse models. Consistently, a recent study demonstrated that IL-33 favors the production of antileukemic IL-9-producing T cells.28 Mechanistically, Tc9 cells primed by DCs plus IL-33 in vitro expressed high levels of IL-9 and GzmB antitumor effector molecules, and such Tc9 cells exhibit increased tumor-specific cytotoxicity and antitumor capabilities. In addition, immunization of BMDCs plus IL-33 stimulates potent Tc9 and Tc1 cell responses in vivo. Tumor-specific Tc9 and Tc1 cells are both potent antitumor effectors.11 Tc9 cells exhibit longer persistence potential and elicit greater antitumor responses compared with Tc1 cells in vivo.11 Thus, our data demonstrate the important role of IL-33 in BMDC-induced Tc9 cell differentiation and antitumor efficacy.
In this study, we found that IL-33 and DCs exert synergistic effects on the induction of Tc9 and Tc1 cells in vivo. The underlying mechanisms must be further defined. However, IL-33 stimulates DCs to overexpress OX40L,29 which is an inducer of Th9 cells.24,30 IL-33 itself may be a driver of Tc9 cell differentiation.28 Regarding Tc1 cells, previous studies demonstrated that IL-33 enhances the production of IFN-γ-expressing CD8+ T cells in vivo.16–18 More importantly, Tc9 cells can also differentiate into IFN-γ- and granzyme-B (GrzB)-producing cytolytic Tc1-like effector cells in vivo.11 These observations suggest that immunization with DC vaccines plus IL-33 may have both direct and indirect effects on the induction of Tc9 and Tc1 cells, which mediate potent antitumor responses.
In this study, we found that IL-33 treatment promotes cell proliferation and inhibits cell apoptosis of BMDC-primed Tc9 cells. Tc9 cells exhibit a “younger” phenotype as demonstrated by the reduced expression of KLRG-1 and higher expression of IL-2 and IL-7Rα in Tc9 cells compared with Tc1 cells, which may contribute to the long-term survival of Tc9 cells in vivo.11,31–33 In this study, we found that IL-33 treatment further increases IL-7Rα and IL-2 expression and inhibits PD-1 and 2B4 expression in BMDC-primed Tc9 cells. PD-1 is an immune-checkpoint protein that has been implicated in tumor immune escape by promoting the apoptosis of tumor-specific T cells.27 In addition, 2B4 is another marker for exhaustive T cell differentiation.34 Thus, our data demonstrated the role of IL-33 in the survival and proliferation of DC-induced Tc9 cells.
Previous studies demonstrated that IL-33 promotes the development and immunosuppressive function of Treg cells.35,36 We also found that IL-33 treatment fails to improve the antitumor efficacy of BMDC vaccines in a B16-OVA OT-II mouse model (unpublished data). However, in this study, we found that IL-33 promotes BMDC-induced Tc9 cell responses and antitumor efficacy in an OT-I mouse model. These observations indicated that IL-33 has different effects on the induction of CD8+ and CD4+ T cell-mediated antitumor immunity primed by DCs. Further studies are necessary to investigate new strategies of blocking the immunosuppressive function of IL-33 on CD4+ T cells but increasing its immunostimulatory activity on CD8+ T cell-mediated antitumor immunity.
In conclusion, our study demonstrates that IL-33 drives the induction of Tc9 cells by BMDCs. IL-33 treatment increases the survival and proliferation of BMDC-induced Tc9 cells and promotes their antitumor activities. The addition of IL-33 further promotes BMDC-induced antitumor efficacy in an OT-I mouse model. Our study demonstrates the important role of IL-33 in DC-induced Tc9 differentiation and antitumor immunity and may have important clinical implications.
Electronic supplementary material
Acknowledgements
This work was supported by funds from National Natural Science Foundation of China (81372536 to S.W., 81502452 to X.C. and 81602485 to Y.Z.).
Author contributions
S.W. and Y.Y. initiated the study. S.W. designed the experiments and wrote the paper. S.W., N.L., Y.J., J.C., H.N, Y.Z., X.C., A.W., D.W. and T.Q. performed the experiments and statistical analyses. A.W. read and edited the manuscript. Q.Y. and S.G. provided critical suggestions to this study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ying Yue, Phone: +0086-13756661254, Email: yying119@126.com.
Siqing Wang, Phone: +0086-15943073518, Email: siw1970@yahoo.com.
Electronic supplementary material
The online version of this article (10.1038/s41423-018-0166-0) contains supplementary material.
References
- 1.Timmerman JM, Levy R. Dendritic cell vaccines for cancer immunotherapy. Annu. Rev. Med. 1999;50:507–529. doi: 10.1146/annurev.med.50.1.507. [DOI] [PubMed] [Google Scholar]
- 2.Randolph GJ, Ochando J, Partida-Sanchez S. Migration of dendritic cell subsets and their precursors. Annu. Rev. Immunol. 2008;26:293–316. doi: 10.1146/annurev.immunol.26.021607.090254. [DOI] [PubMed] [Google Scholar]
- 3.Wang S, et al. Dendritic cell vaccine but not idiotype-KLH protein vaccine primes therapeutic tumor-specific immunity against multiple myeloma. Front. Biosci. 2007;12:3566–3575. doi: 10.2741/2335. [DOI] [PubMed] [Google Scholar]
- 4.Murphy K. A. & Griffith T. S. CD8 T cell-independent antitumor response and its potential for treatment of malignant gliomas. Cancers 8, 71 (2016). [DOI] [PMC free article] [PubMed]
- 5.Reiser J, Banerjee A. Effector, memory, and dysfunctional CD8(+) T cell fates in the antitumor immune response. J. Immunol. Res. 2016;2016:8941260. doi: 10.1155/2016/8941260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shrikant PA, et al. Regulating functional cell fates in CD8 T cells. Immunol. Res. 2010;46:12–22. doi: 10.1007/s12026-009-8130-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye Z, et al. Type 1 CD8+ T cells are superior to type 2 CD8+ T cells in tumor immunotherapy due to their efficient cytotoxicity, prolonged survival and type 1 immune modulation. Cell. Mol. Immunol. 2007;4:277–285. [PubMed] [Google Scholar]
- 8.Yu Y, et al. Adoptive transfer of Tc1 or Tc17 cells elicits antitumor immunity against established melanoma through distinct mechanisms. J. Immunol. 2013;190:1873–1881. doi: 10.4049/jimmunol.1201989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song Y, Yang JM. Role of interleukin (IL)-17 and T-helper (Th)17 cells in cancer. Biochem. Biophys. Res. Commun. 2017;493:1–8. doi: 10.1016/j.bbrc.2017.08.109. [DOI] [PubMed] [Google Scholar]
- 10.Majchrzak K, et al. Exploiting IL-17-producing CD4+ and CD8+ T cells to improve cancer immunotherapy in the clinic. Cancer Immunol., Immunother.: CII. 2016;65:247–259. doi: 10.1007/s00262-016-1797-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu Y, et al. Tumor-specific IL-9-producing CD8+ Tc9 cells are superior effector than type-I cytotoxic Tc1 cells for adoptive immunotherapy of cancers. Proc. Natl Acad. Sci. USA. 2014;111:2265–2270. doi: 10.1073/pnas.1317431111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dardalhon V, et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(-) effector T cells. Nat. Immunol. 2008;9:1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu Y, Wang Q, Yi Q. Anticancer Tc9 cells: long-lived tumor-killing T cells for adoptive therapy. Oncoimmunology. 2014;3:e28542. doi: 10.4161/onci.28542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat. Rev. Immunol. 2010;10:103–110. doi: 10.1038/nri2692. [DOI] [PubMed] [Google Scholar]
- 15.Mehraj V, Ponte R, Routy JP. The dynamic role of the IL-33/ST2 axis in chronic viral-infections: alarming and adjuvanting the immune response. EBioMedicine. 2016;9:37–44. doi: 10.1016/j.ebiom.2016.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonilla WV, et al. The alarmin interleukin-33 drives protective antiviral CD8(+) T cell responses. Science. 2012;335:984–989. doi: 10.1126/science.1215418. [DOI] [PubMed] [Google Scholar]
- 17.Villarreal DO, et al. Alarmin IL-33 acts as an immunoadjuvant to enhance antigen-specific tumor immunity. Cancer Res. 2014;74:1789–1800. doi: 10.1158/0008-5472.CAN-13-2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao K, et al. Transgenic expression of IL-33 activates CD8(+) T cells and NK cells and inhibits tumor growth and metastasis in mice. Cancer Lett. 2013;335:463–471. doi: 10.1016/j.canlet.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Gao X, et al. Tumoral expression of IL-33 inhibits tumor growth and modifies the tumor microenvironment through CD8+ T and NK cells. J. Immunol. 2015;194:438–445. doi: 10.4049/jimmunol.1401344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim J, et al. Intratumorally establishing type 2 innate lymphoid cells blocks tumor growth. J. Immunol. 2016;196:2410–2423. doi: 10.4049/jimmunol.1501730. [DOI] [PubMed] [Google Scholar]
- 21.Lim HX, Choi S, Cho D, Kim TS. IL-33 inhibits the differentiation and immunosuppressive activity of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. Immunol. Cell Biol. 2017;95:99–107. doi: 10.1038/icb.2016.72. [DOI] [PubMed] [Google Scholar]
- 22.Dominguez D, et al. Exogenous IL-33 restores dendritic cell activation and maturation in established cancer. J. Immunol. 2017;198:1365–1375. doi: 10.4049/jimmunol.1501399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lucarini V, et al. IL-33 restricts tumor growth and inhibits pulmonary metastasis in melanoma-bearing mice through eosinophils. Oncoimmunology. 2017;6:e1317420. doi: 10.1080/2162402X.2017.1317420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Y, et al. Dectin-1-activated dendritic cells trigger potent antitumour immunity through the induction of Th9 cells. Nat. Commun. 2016;7:12368. doi: 10.1038/ncomms12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christie D, Zhu J. Transcriptional regulatory networks for CD4 T cell differentiation. Curr. Top. Microbiol. Immunol. 2014;381:125–172. doi: 10.1007/82_2014_372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abu Eid R, et al. Enhanced therapeutic efficacy and memory of tumor-specific CD8 T cells by ex vivo PI3K-delta inhibition. Cancer Res. 2017;77:4135–4145. doi: 10.1158/0008-5472.CAN-16-1925. [DOI] [PubMed] [Google Scholar]
- 27.Topalian SL, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramadan A, et al. Specifically differentiated T cell subset promotes tumor immunity over fatal immunity. J. Exp. Med. 2017;214:3577–3596. doi: 10.1084/jem.20170041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Kleer IM, et al. Perinatal activation of the interleukin-33 pathway promotes type 2 immunity in the developing lung. Immunity. 2016;45:1285–1298. doi: 10.1016/j.immuni.2016.10.031. [DOI] [PubMed] [Google Scholar]
- 30.Xiao X, et al. OX40 signaling favors the induction of T(H)9 cells and airway inflammation. Nat. Immunol. 2012;13:981–990. doi: 10.1038/ni.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duthie KA, Osborne LC, Foster LJ, Abraham N. Proteomics analysis of interleukin (IL)-7-induced signaling effectors shows selective changes in IL-7Ralpha449F knock-in T cell progenitors. Mol. Cell. Proteom. 2007;6:1700–1710. doi: 10.1074/mcp.M600468-MCP200. [DOI] [PubMed] [Google Scholar]
- 32.Liu X, et al. Crucial role of interleukin-7 in T helper type 17 survival and expansion in autoimmune disease. Nat. Med. 2010;16:191–197. doi: 10.1038/nm.2077. [DOI] [PubMed] [Google Scholar]
- 33.Liao W, Lin JX, Leonard WJ. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity. 2013;38:13–25. doi: 10.1016/j.immuni.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bengsch B, et al. Coexpression of PD-1, 2B4, CD160 and KLRG1 on exhausted HCV-specific CD8+ T cells is linked to antigen recognition and T cell differentiation. PLoS Pathog. 2010;6:e1000947. doi: 10.1371/journal.ppat.1000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schiering C, et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature. 2014;513:564–568. doi: 10.1038/nature13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vasanthakumar A, et al. The transcriptional regulators IRF4, BATF and IL-33 orchestrate development and maintenance of adipose tissue-resident regulatory T cells. Nat. Immunol. 2015;16:276–285. doi: 10.1038/ni.3085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.