Abstract
Transglutaminases (TGases), which are widespread cross-linking enzymes in plants, play key roles in photosynthesis and abiotic/biotic stress responses; however, evidence concerning the genetics underlying how TGase improves the capability of photosynthesis and the mechanism of TGase-mediated photosynthesis are not clear in this crop species. In this study, we clarified the function of TGase in the regulation of photosynthesis in tomato by comparing wild-type (WT) plants, tgase mutants generated by the CRISPR/Cas9 system and TGase-overexpressing (TGaseOE) plants. Our results showed that increasing the transcript level of TGase resulted in an enhanced net photosynthetic rate (Pn), whereas the tgase mutants presented significantly inhibited Pns and CO2 assimilation compared with the WT. Although the total RuBisCO activity was not affected by TGase, the initial and activation status of RuBisCO and the activity of RuBisCO activase (RCA) and fructose-1,6-bisphosphatase (FBPase) in TGaseOE plants were significantly higher than that in WT plants. Except for RuBisCO small subunit (RbcS), the transcription levels of Benson–Calvin cycle-related genes were positively related to the endogenous TGase activity. Furthermore, TGaseOE plants had higher protein levels of RuBisCO large subunit (RbcL) and RCA than did WT plants and showed a reduced redox status by enhancing the activity of dehydroascorbate reductase (DHAR) and glutathione reductase (GR), which was compromised in TGase-deficient plants. Overall, TGase positively regulated photosynthesis by maintaining the activation states of the Benson–Calvin cycle and inducing changes in cellular redox homeostasis in tomato.
Subject terms: Photosynthesis, Plant physiology
Introduction
It is well known that photosynthesis is considered the most important metabolic process for biomass accumulation in plants but is limited by carbohydrates and various environmental cues. Therefore, understanding the mechanisms of regulation of photosynthetic processes and improving crop yield potential are critical. Interestingly, increasing evidence supports the hypothesis that polyamines (PAs) regulate photosynthetic capability1,2. It has been reported that PAs are usually found in the photosynthetic apparatus and are closely related to plant growth and stress responses3. Overexpressing the genes involved in PA biosynthesis results in increased photosynthetic rates (Pns) in transgenic plants4,5. In addition, putrescine (Put) plays a positive role in stimulating photophosphorylation, at least to some degree, and regulates adenosine triphosphate synthesis6,7. PAs are an important regulator of redox homeostasis, which increase photosynthesis because of the activation of antioxidant enzymes and the modulation of reactive oxygen species (ROS) homeostasis8,9. However, the functions of PA-regulated photosynthesis are not fully understood.
The regulation of PAs is often mediated by transglutaminases (TGases), which are able to establish ε-(γ-glutamyl) links with PAs for the posttranslational modification of proteins. For instance, PAs cross-linked with tubulin and actin via TGase have been observed during the germination of Malus domestica pollen10,11. TGases are intracellular and extracellular enzymes that are actively regulated by Ca2+ and can be negatively regulated by guanosine triphosphate. It has been demonstrated that TGases exhibit a large number of functions both in eukaryotes and prokaryotes12. In addition, a large amount of TGases are present in the mitochondria, cell walls, and chloroplasts of plants and are continually being identified13–16. In addition, the first TGase gene discovered was AtPng1p, which is most studied gene encoding a TGase protein in Arabidopsis; AtPng1p is expressed under conditions of undisturbed growth and expressed ubiquitously during all stages of plant growth and can be induced by various light conditions14,17. Furthermore, TGases are delivered via a membrane/cytoskeleton-based transport system to the cell wall, where they regulate the apical growth of pollen tubes18. Numerous reports have shown that TGase plays roles not only in nonphotosynthetic tissues but also in photosynthetic organs. Recent proteomic and transcriptomic studies have shown that chloroplast proteins (i.e., light-harvesting complexes of photosystem II (LHCII) and chlorophyll-binding protein of 29, 26, and 24 kDa) are the target proteins of TGase in vivo19. Chloroplast-associated proteins such as the larger subunit of RuBisCO (RbcL) and photosystem II proteins are substrates of TGase in higher plants16,20. In this respect, TGase may have positive roles in photosynthesis or photoprotection reactions. Characterization of transgenic tobacco overexpressing TGase revealed that TGase has a positive role that involves the photosystems21. In addition, photosynthetic complexes are often significantly affected by TGase22. However, the role of TGase in photosynthesis is still unclear. Researchers have been focused on the posttranslational modification of LHCII and the polyamination of thylakoids by TGase, but the function of TGase in redox regulation is often overlooked. PAs may be donors for the biosynthesis of amino acids such as glutamic acid, a key chlorophyll and glutathione precursor; in addition, PAs can regulate plastidial membrane assembly or take part in redox regulation, which has been indicated to be mediated by TGase23. Moreover, some TGases have been identified in rice and maize, but their functions and regulatory mechanisms are largely unknown, especially in horticultural plant species.
Tomato is one of the most economically important horticultural crop species and is distributed worldwide, and this species been widely used to study photosynthesis. The clustered regulatory interspaced short palindromic repeats/CRISPR-associated protein 9 (CRISPR/Cas9) gene editing technology has been established and used to characterize gene functions in plants24. In this study, to analyze the potential role of TGase in tomato, we generated TGase-overexpressing (TGaseOE) plants and tgase mutants by CRISPR/Cas9 technology and compared their photosynthetic capability with that of wild-type (WT) plants. We found a positive relationship between endogenous TGase activity and the capability of photosynthesis. TGase improved photosynthetic capability by altering the cellular redox status and activating the antioxidant capability.
Materials and methods
Plant materials and growth conditions
Tomato (Solanum lycopersicum L. cv Ailsa Craig) was used for expression analysis. The seedlings were grown in a greenhouse at Nanjing Agriculture University in Nanjing city under standard water management and pest control regimens.
Generation and selection of transgenic plants
To generate TGaseOE plants, the CDS of TGase was obtained via PCR amplification using the primers TGaseOE-F (5′-TTGGCGCGCCATGGTTGCTCGGAGACTCGCCGTTA-3′) and TGaseOE-R (5′-CGGGGTACCACTGCTACCTGCAAAGAGGTCAATG-3′) according to the sequence (Sol Genomic Network accession Solyc01g097440). The PCR product was inserted into the binary plasmid vector pFGC1008-HA with a 35S promoter after being digested with AscI and KpnI. The vector was then transformed into Agrobacterium tumefaciens strain EHA105, which was then inserted into Ailsa Craig plants following the method of Fillatti et al25. The overexpression of TGase in plants was identified by real-time quantitative PCR (qPCR) analysis (Fig. S1). Two homozygous F2 lines (OE-#1 and OE-#2) were chosen for the experiment.
To obtain mutants, we used the CRISPR/Cas9 genome-targeting system to modify the TGase according to previous methods26. In the TGase coding region, we chose the guide RNA sequence GGCCCTTCAGTCTCATTACC as the single guide RNA (sgRNA). Double-stranded DNA, generated by annealing the oligo pairs, was inserted into an AtU6-sgRNA-AtUBQ-Cas9 vector, which was then digested by HindIII and KpnI and subsequently introduced into a pCAMBIA1301 vector using T4 DNA ligase (Thermo Fisher Scientific, Waltham, USA, EL0013). The integrated constructs were subsequently transformed into EHA105 bacteria, which were then inserted into Ailsa Craig plants; hygromycin was then used to screen the transgenic plants. The genomic DNA of the mutant seedlings was extracted for PCR using specific primers (Table S1). The PCR products of the mutants were detected by direct sequencing methods. The tgase-1 and tgase-2 mutants, which carried a 10 bp deletion and a 1 bp addition, respectively, were identified and used in this study (Fig. S2).
In the experiments, five genotypes, Ailsa Craig (WT) plants, TGase-deficient mutants (tgase-1 and tgase-2), and overexpression plants (OE-#1 and OE-#2), were used. To analyze photosynthetic capability, germinated seeds were sown in growth media. After full development of the second true leaves, the seedlings were transplanted into 250 cm3 plastic pots that contained media and were watered with 1/2-strength Hoagland’s nutrition solution. Forty-five-day-old plants were used for the experiment. For assay the Calvin cycle enzyme activity, leaf discs were harvested from different genotypes, then frozen immediately in liquid nitrogen, and stored at −80 °C prior to analysis. For detection the activity of TGase and antioxidant enzymes, the leaf samples were collected based on fresh weight. For analysis the gene expression, the whole leaflets were collected.
TGase activity analysis
The hydroxamate method was used to calculate the transglutaminase activity, with some modifications27. Leaf samples (0.3 g) were homogenized with phosphate buffer (50 mM, pH 7.8) at 12,000 g for 15 min at 4 °C for endogenous TGase activity measurement. The colorimetric method (450 nm) was used to measure the activity of TGase in conjunction with N-carbobenzoxy-l-glutaminylglycin (CBZ–Cln–Gly–OH), which is the specific substrate of TGase. The 100 μl of the supernatant was fully mixed with the reaction (50 mM PBS, and 0.5 mM CBZ–Cln–Gly–OH, pH 7.8), then performed at 30 °C for 10 min and performed with 5 mM Ca2+; replaced by 1 mM EDTA in the negative control. l-glutamic acid γ-mono-hydroxamate was used to prepare the standard curve. The enzyme activity was defined as the amount of enzyme that catalyzed the formation of 1μmol of hydroxamate/min.
Leaf gas exchange measurements
Forty-five-day-old plants were used for comparing photosynthetic capabilities. Gas exchange analysis was performed via an LI-6400 portable photosynthesis system (LI-6400; LI-COR, Lincoln, NE, USA). An assimilation vs intercellular CO2 concentration (A/Ci) curve was generated as described by Caemmerer and Farquhar28. The maximum rates of Rubisco (Vc,max) and the maximum rate of electron transport for RuBP regeneration (Jmax) were measured according to the A/Ci curves as described by Ethier and Livingston29.
Determination of Rubisco, Rubisco activase (RCA), and fructose-1, 6-bisphosphatase (FBPase) activity
The Rubisco activity was measured following the method of Ward and Keys30, with slight modifications. Leaf samples were ground with extraction buffer (2% insoluble PVPP, 1 mM EDTA, 50 mM HEPES, 10 mM MgCl2, and 10 mM β-mercaptoethanol; pH 8.0) and then centrifuged at 12,000 g for 15 min at 4 °C. The crude extract was used to assay the total activity. Activation was performed in a 100 μl mixture solution at 28 °C for 15 min. Initial Rubisco activity was determined in a 100 μl reaction medium, and the change in absorbance within 90 s was measured at 340 nm. The RCA activity was measured with a Rubisco Activase Assay Kit (Genmed Scientifics, Wilmington, USA) according to the manufacturer’s instructions.
The activity of FBPase was detected by measuring the increase at A340nm as previously described31. The total activity of FBPase was determined using the crude extract, which was activated in a 100 μl activation mixture reaction (0.1 M Tris-HCl (pH 8.0), 2 mM fructose-1,6-bisphosphate (FBP), 100 mM dithiothreitol (DTT), and 10 mM MgCl2). After homogenization, the initial activity was measured immediately. The reaction was started by the addition of the enzyme extract.
Glutathione content and ascorbate content assays
To determine the glutathione and ascorbate contents, leaf tissue was ground in 2 ml of extraction buffer (6% metaphosphoric acid) and then centrifuged at 12,000 g for 10 min at 4 °C, after which the supernatant was used for further analysis. To assay the GSH and oxidized glutathione (GSSG), the neutralized supernatant with phosphate buffer (0.5 M, pH 7.5) was added to the reaction mixture (100 mM phosphate buffer, 5 mM EDTA, 0.2 mM NADPH, and 0.6 mM 5,5′-dithio-bis (2-nitrobenzoic acid), pH 7.5). After adding the glutathione reductase (GR) (Sigma, Santa Clara, CA, USA) to start the reaction, the changes in absorbance within 1 min at 412 nm were measured. The GSH was masked after adding 40 μl of 2-vinylpyridine to the supernatant for the GSSG assay and the total glutathione assay by adding 40 μl of water. The GSH content was measured by removing the GSSG content from the total content32.
To assay the total levels of AsA, 20 μl of the supernatant was transferred to a new tube containing potassium phosphate buffer (0.5 M, pH 7.4); the same amount of 5 mM DTT was added as was the supernatant, after which the mixture was incubated at 37 °C for 20 min. Afterward, 10 μl of N-ethylmaleimide (NEM, 0.5% w/v) was added to clear the excess DTT via incubation for 1 min at 25 °C. A color reagent (80 μl) (see below) was added to the mixture, which was then incubated at 37 °C for 1 h; the absorbance was measured at 550 nm. To analyze the reduced AsA, 20 μl of potassium phosphate (0.4 M, pH 7.4) was replaced with DTT and NEM, and the procedure was performed according to the total AsA assay. The color reagent was as follows: solution A contained 31% orthophosphoric acid, 0.6% (w/v) iron chloride (FeCl3), and 4.6% (w/v) TCA, whereas solution B contained 4% 2,2-dipyridyl.
Measurements of the activity related to AsA–GSH cycle enzymes
To analyze the enzymatic activities related to the AsA–GSH cycle, leaf samples were ground in 3 ml of extraction buffer (25 mM HEPES, 2% PVP, 2 mM ascorbic acid, and 0.2 mM EDTA, pH 7.8) and centrifuged at 12,000 g for 20 min at 4 °C. The supernatants were then collected and used for the enzymatic activity assay. The activities of ascorbate peroxidase (APX) and dehydroascorbate reductase (DHAR) were evaluated by monitoring the decrease and increase in absorbance at 290 nm and 265 nm, respectively, as described by Nakano and Asada33. To analyze the activity of GR, we monitored the rate of NADPH decrease according to the method reported by Halliwell and Foyer34.
RNA isolation and reverse-transcription qPCR
Tomato leaves were used to extract total RNA following the manufacturer’s instructions of an RNA extraction kit (Tiangen, Beijing, China). Total RNA (1 μg) was used to synthesize cDNA using a HiScript™ Q RT SuperMix for qPCR (+gDNA wiper) kit (Vazyme, Nanjing, China). qPCR analyses were performed using an ABI ViiA7 real-time PCR system (Applied Biosystems, CA, USA). The PCR program consisted of predenaturation at 95 °C for 5 min, followed by 40 cycles of 95 °C for 10 s and 60 °C for 30 s. The Actin gene was used as a reference gene, and the qPCR-specific primers are presented in supplementary Table S1. The relative levels for each expression were calculated according to the methods of Livak and Schmittgen35.
Western blot analysis
Proteins were extracted as described previously35. The proteins were resolved using 12% (w/v) SDS–polyacrylamide gel electrophoresis and then transferred to a nitrocellulose membrane (Millipore, Saint-Quentin, France)36. RuBisCO large subunit (RbcL), RuBisCO small subunit (RbcS), and RCA were detected with commercial antibodies (Agrisera, Vannes, Sweden). At least three independent replicates were used for each determination. The accumulation of proteins was quantified using Quantity One software (Bio-rad, Hercules, California, CA, USA).
Statistics
The data are presented as the means ± SDs and were analyzed by SPSS 20 statistical software. The experimental data were analyzed with Duncan’s multiple range test at P < 0.05.
Results
Expression pattern of TGase in tomato
To detect the expression pattern of TGase, the mRNA transcripts of TGase were measured in different tissues, including the roots, stems, leaves, flowers, and fruits, in tomato (Fig. S1). Transcripts of TGase were highly expressed in the leaves and fruits and accumulated in the flowers (Fig. S1).
To explore the functions of TGase, we first generated TGaseOE tomato lines and obtained several independent transgenic lines. Moreover, the transcripts of TGase in two overexpression lines, OE-#1 and OE-#2, showed 117.5 and 124.3 times greater expression than the WT plants did, respectively (Fig. S2). We also used the CRISPR/Cas9 system to generate mutants, which were named tgase-1 and tgase-2, with a loss of 10 bp and an “A” insertion in an exon of TGase, respectively (Fig. S3). In the mutants, the activity of TGase was almost undetectable; in contrast, the activity of TGase increased in the TGaseOE plants (Fig. S4). These results indicated that TGase deficiency significantly inhibited TGase activity but dramatically increased TGaseOE activity. These homozygous overexpressing lines and mutants were used in subsequent experiments.
TGase enhanced CO2 assimilation in tomato plants
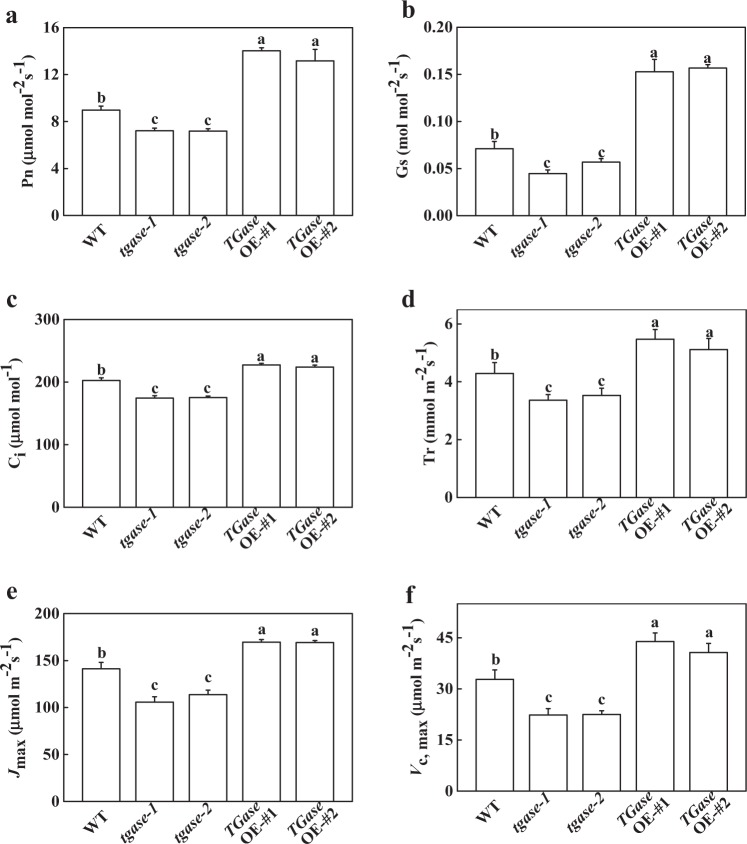
TGases are closely related to photosynthesis in plants. To reveal the role of TGase in photosynthesis capability, we first analyzed the differences in the parameters of gas exchange among the WT, tgase-1, tgase-2, OE-#1, and OE-#2 plants. The results indicated that deficiency of TGase significantly inhibited net Pns compared to WT (Fig. 1a). Moreover, other gas exchange parameters, such as stomatal conductance (Gs), Ci, and transcription rate (Tr), were also reduced in the tgase mutants (Fig. 1b–d). In contrast, the Pn, Gs, Ci, and Tr increased in the OE-#1 and OE-#2 lines compared with WT plants (Fig. 1a–d). To further examine the possible mechanism of TGase regulation of photosynthesis, we used Farquhar’s model to analyze the Vc,max and the Jmax. The results showed that TGase deficiency decreased Vc,max and Jmax; however, compared with those in WT, Vc,max, and Jmax increased in the TGaseOE plants (Fig. 1e, f). Thus, TGase-mediated CO2 assimilation and RuBP carboxylation and regeneration capabilities in tomato plants.
Fig. 1.
Effects of TGase overexpression (OE-#1 and OE-#2) and mutation (tgase-1 and tgase-2) on photosynthesis. Net photosynthetic rate (Pn) (a), stomatal conductance (Gs) (b), intercellular CO2 concentration (Ci) (c), transpiration rate (Tr) (d), maximum ribulose-1,5-bisphosphate (RuBP) regeneration rate (Jmax) (e), and maximum ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) carboxylation rate (Vc,max) (f). Each histogram represents a mean ± SE of three independent experiments (n = 3). Different letters indicate significant differences between treatments (P < 0.05) according to Duncan’s multiple range test
TGase promoted the gene expression and activity of the Benson–Calvin cycle
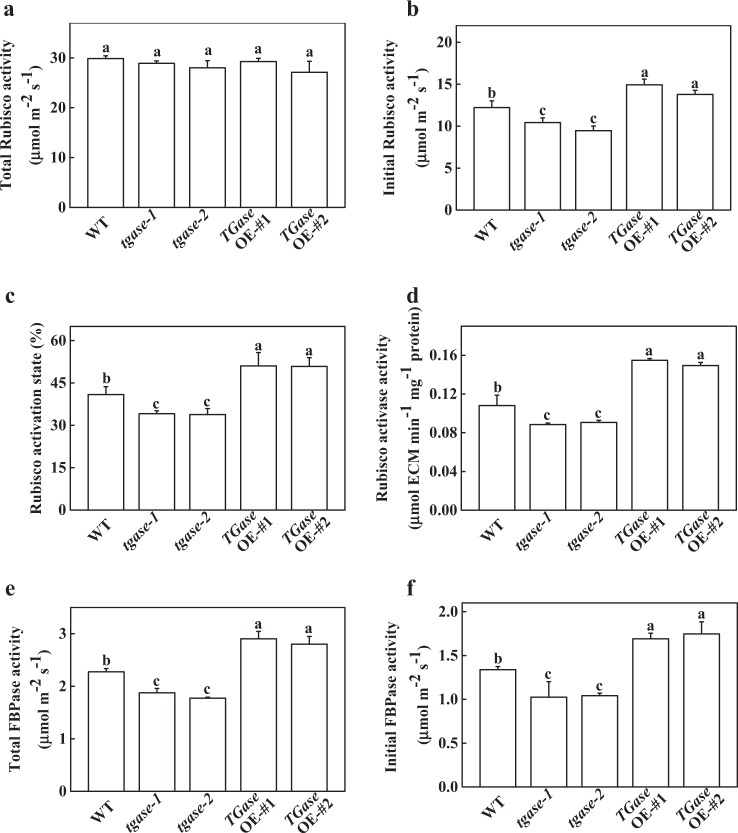
To understand the possible role of TGase in regulating the capability of photosynthesis, we further analyzed the activity of FBPase, which plays key roles in RuBP regeneration. The results showed that changes in the endogenous TGase activity did not induce a significant change in total Rubisco activity (Fig. 2a), but the initial Rubisco activity showed significantly differed among the different genotypes, resulting in a decreased and increased Rubisco activation state in the tgase mutants and TGaseOE plants, respectively (Fig. 2b, c). Similarly, the activity of RCA was reduced in tgase mutants but elevated in TGaseOE plants (Fig. 2d). Moreover, tgase-1 and tgase-2 presented decreased the total FBPase activity, while high FBPase activity was detected in the TGaseOE plants (Fig. 2e). Furthermore, there was a similar change in the initial FBPase activity in the TGaseOE plant (Fig. 2f).
Fig. 2.
Effects of TGase gene overexpression (OE-#1 and OE-#2) and mutation (tgase-1 and tgase-2) on the total and initial carboxylation activity of Rubisco (a, b), the activation status of Rubisco (c), the activity of Rubisco activase (d), and total and initial activity of fructose-1,6-bisphosphatase (FBPase) (e, f). Each histogram represents a mean ± SE of three independent experiments (n = 3). Different letters indicate significant differences between treatments (P < 0.05) according to Duncan’s multiple range test
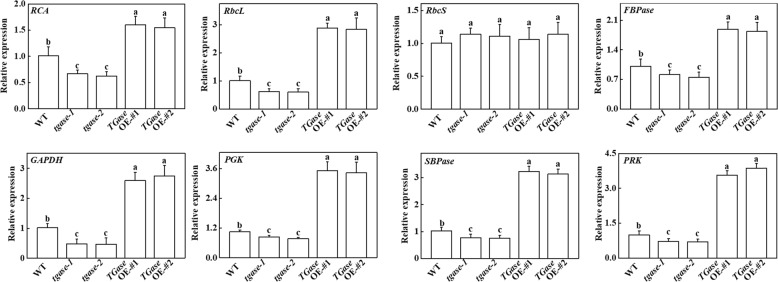
To further analyze the function of TGase in the regulation of photosynthesis, we examined the transcripts of genes related to the Benson–Calvin cycle, including RCA, RbcL, RbcS, glyceraldehyde-3-phosphate dehydrogenase, FBPase, sedoheptulose-1,7-bis-phosphatase, ribulose-5-phosphate kinase, and glycerate-3-phosphate kinase. Except for the expression level of the rbcS gene, the expression levels of the other genes were upregulated in TGaseOE plants (Fig. 3). In contrast, deficiency of the TGase gene led to downregulation of these genes (Fig. 3). Moreover, the protein levels of rbcL, rbcs, and RCA presented similar results as those of their transcription levels. The results showed that TGase deficiency and overexpression induced decreases and increases in rbcL and RCA contents, respectively (Figs. 4 and S5), which are consistent with the total Rubisco activity.
Fig. 3.
Changes in the expression of Benson–Calvin cycle-related genes in overexpression (OE-#1 and OE-#2) and mutant (tgase-1 and tgase-2) plants. Each histogram represents a mean ± SE of three independent experiments (n = 3). Different letters indicate significant differences between treatments (P < 0.05) according to Duncan’s multiple range test
Fig. 4.
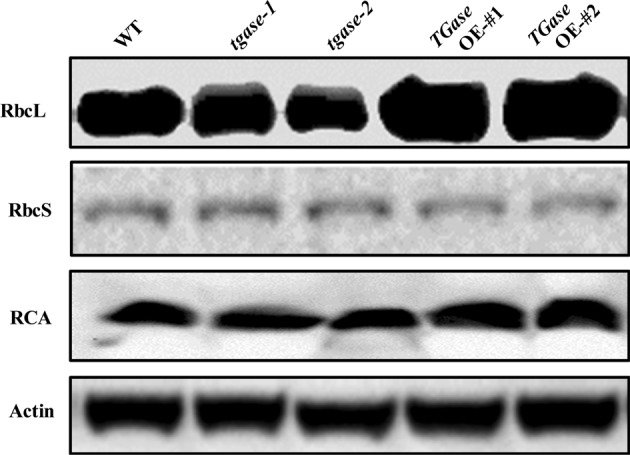
Effects of TGase gene overexpression (OE-#1 and OE-#2) and mutation (tgase-1 and tgase-2) on the protein levels of RuBisCO larger subunit (RbcL), RuBisCO small subunit (RbcS), and RuBisCO activase (RCA)
TGase induced a reduced redox status and activated antioxidant enzymes
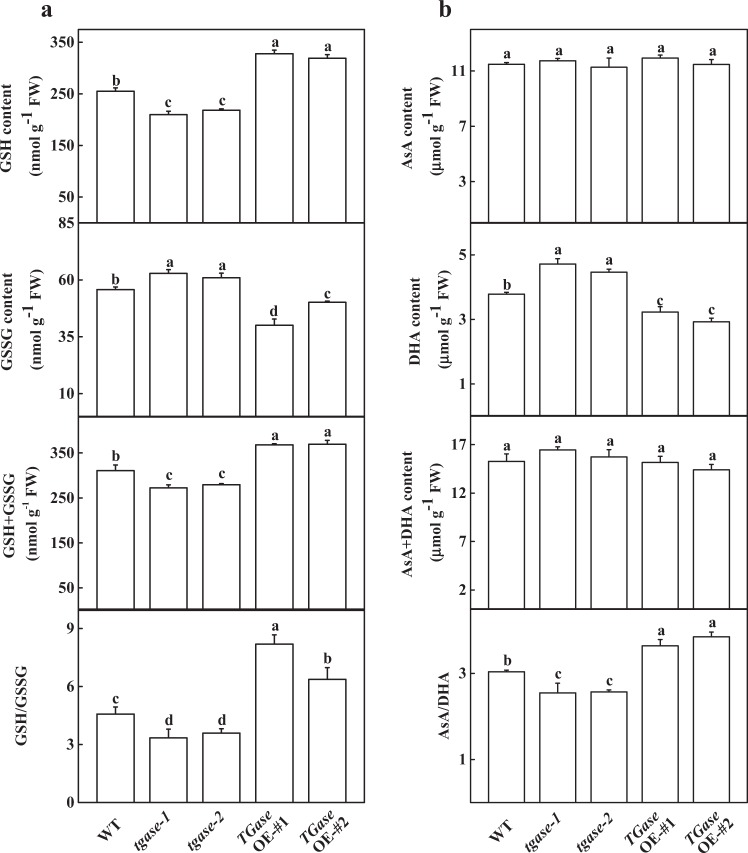
It is well known that redox posttranslational modifications can regulate Calvin cycle enzymes. To reveal the mechanism by which TGase mediates Rubisco and RCA activity, we further examined the different status of glutathione and ascorbate contents. The GSH content was decreased in tgase mutants, whereas high TGase activity in the TGaseOE plants resulted in GSH content accumulation compared to WT (Fig. 5a). However, TGase deficiency led to an increase in GSSG content but a decrease in the TGaseOE plants compared to WT (Fig. 5a). The changes in total glutathione (GSH + GSSG) were similar to those of GSH (Fig. 5a). Importantly, the ratio of GSH/GSSG showed a decline in the tgase mutants, whereas it increased in the TGaseOE plants (Fig. 5a). The content of AsA was not affected by different TGase activity levels in plants (Fig. 5b). TGase deficiency led to an increase in the oxidized ascorbate (DHA) content but a significant decrease in AsA in the TGaseOE lines (Fig. 5b). Although the total ascorbate (AsA + DHA) content was not influenced by the modulation of TGase activity, the AsA/DHA ratio significantly decreased and increased in the tgase mutants and overexpression plants, respectively (Fig. 5b).
Fig. 5.
Effects of TGase overexpression (OE-#1 and OE-#2) and mutation (tgase-1 and tgase-2) on the glutathione content (a) and ascorbate content (b). Each histogram represents a mean ± SE of three independent experiments (n = 3). Different letters indicate significant differences between treatments (P < 0.05) according to Duncan’s multiple range test
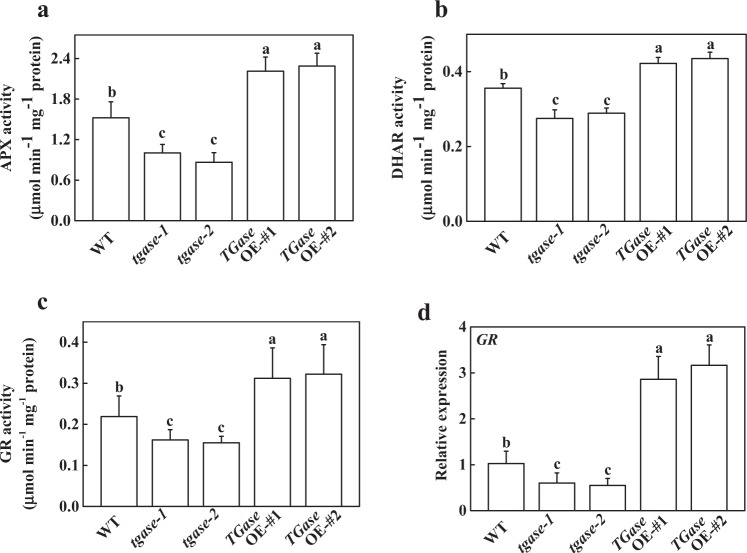
TGase can maintain cellular redox status by mediating the enzymatic activity of the AsA–GSH cycle. In this study, APX, DHAR, and GR, which are involved in the AsA–GSH cycle, were analyzed. The results showed that those activities were inhibited in the tgase mutants but were induced in the TGaseOE plants (Fig. 6a–c). Moreover, the expression of GR was consistent with its activity (Fig. 6c, d).
Fig. 6.
Effects of TGase overexpression (OE-#1 and OE-#2) and mutation (tgase-1 and tgase-2) on the activity of ascorbate peroxidase (APX) (a), dehydroascorbate reductase (DHAR) (b), and glutathione reductase (GR) (c), and on the expression of GR (d). Each histogram represents a mean ± SE of three independent experiments (n = 3). Different letters indicate significant differences between treatments (P < 0.05) according to Duncan’s multiple range test
Discussion
TGases are widely distributed in plants and have been found to be related to all stages of plant growth, programmed cell death, senescence, and stress37. However, until now, there has been no suitable transgenic plant with decreased TGase activity. Moreover, the best tobacco and Arabidopsis experimental lines overexpressed the plastidial transglutaminase from maize, but researchers have not analyzed whether the photosynthetic capability changes in the overexpression plants resulted from the alteration of the Calvin cycle and the promotion of carbohydrate metabolism21,38,39. In this study, we explored the function of TGase using a reverse genetic strategy. We found that high endogenous TGase activity is involved in the enhancement of photosynthesis in TGaseOE plants, but TGase deficiency led to decreased photosynthetic capability (Fig. 1), which suggested that TGase may play crucial roles in the regulation of photosynthesis. The reduction in the rate of photosynthesis in the tgase mutants was associated with a decrease in Gs and Ci (Fig. 1). Our previous study suggested that the application of 8 mM Put enhances stomatal opening and promotes photosynthesis in cucumber40. Here, we also observed increased Ci in the TGaseOE plants but not in the mutants. These results might be due to the positive effect of TGase on the PA level change, which resulted in a significant positive correlation between PAs and the Pn.
To date, several studies have reported that TGase regulates the thylakoid structure and LHCII22,41. For example, overexpression of the maize TGase could remodel tobacco thylakoids38,42. However, little is known about whether TGase can regulate the Calvin cycle and its associated processes. Hence, in this study, we used the A/Ci curve and the Rubisco and FBPase activities to show some evidence for TGase mediation of photosynthetic capability due to the positive regulation of Calvin cycle enzyme activity. We observed that Calvin cycle-related genes were downregulated in the tgase mutant (Fig. 3). Furthermore, TGase deficiency also led to a significant decline in RCA activity and initial Rubisco and FBPase activity, as well as a reduction in Vc,max and activity of RCA, with almost no change in the total activity of Rubisco (Figs. 2 and 4). In contrast, overexpression of TGase increased the expression and activity of these genes (Figs. 2 and 3), indicating that TGase mainly regulated the action of Rubisco activities to activate the state of Rubisco. rbcS and rbcL encode small and large Rubisco subunits, respectively, which compose the Rubisco holoenzyme and are tightly coordinated, but the transcripts of rbcS and rbcL are not correlated43. Indeed, although TGase positively mediates the transcription of rbcL, it did not mediate the expression level of rbcS (Fig. 3b, c). This phenomenon may be due to the synthesis of rbcS being different because a study showed that overexpression of rbcS did not lead to an increase in the content of Rubisco44. Alternatively, pools of cellular glutathione could be a repressor of rbcL proteins when glutathione is in the oxidized state45. Considering that the redox status of glutathione was regulated by TGase, it is possible that TGase affects the levels of rbcL (Fig. 5). The regeneration of RuBP is involved in not only the electron transport chain of photosynthesis but also the Rubisco enzyme in the Calvin cycle46. Both the expression and initial activity of FBPase were reduced in the tgase mutant plants (Fig. 2f). The lack of activation of RCA or FBPase was often related to a decline in Jmax (Fig. 1e), and cellular glutathione homeostasis is related to the activities of these enzyme47.
Some reports have shown that GSH plays a key role in Rubisco activation by enhancing thiol and disulfide exchanges48. The high ratio of GSH to GSSG increased the activation level of RuBisCO and consequently CO2 assimilation49. In addition, the glutathione redox could regulate translation of RbcL through modulating the ROS in the chloroplast50. Overexpression of S-adenosylmethionine synthetase 1 (a PA biosynthesis gene) significantly increased the AsA/DHA and GSH/GSSG ratios in tomato48,51,52. The mechanism of TGase positively regulates the activation state of Rubisco, which may be involved in glutathione redox status changes. Interestingly, in the present study, high ratios of GSH/GSSG and AsA/DHA were observed in TGaseOE lines (Fig. 5), which was caused by an increase in MDAR and GR enzymatic activity (Fig. 6). Activation of the GSH–AsA cycle in TGaseOE plants could maintain a reducing state of the chloroplast, which helps to activate Rubisco. In addition, TGase could bind to the large subunit of ribulose bisphosphate carboxylase–oxygenase, thereby directly activating the activity of Rubisco16. This phenomenon is in agreement with the results by which high TGase activity promoted RCA activity and protein content in TGaseOE plants (Figs. 2d and 4). On the other hand, maintaining the high AsA/DHA and GSH/GSSG ratios may be involved in hormone signaling, cell division, and other physiological processes49.
It is well known that maintaining cellular redox status requires a turnover rate of the ascorbate–glutathione cycle, and APX activity may have an important role in ROS scavenging53. Here, we found that a deficiency in TGase resulted in a decreased activity of antioxidant-related enzymes, while overexpression of TGase increased their activities, as observed in the overexpression of maize plastidial TGase in tobacco42. These results indicated that high endogenous TGase activity induced antioxidant enzymes to regulate the redox system of the plants. This finding further revealed the cross-link between TGase and the glutathione system42.
In conclusion, the current study indicated that manipulation of endogenous TGase activity by overexpression of TGase could promote the CO2 assimilation rate through activating the Calvin cycle enzymes. Moreover, TGase-inducible changes in cellular redox homeostasis may be involved in activation of Calvin cycle enzymes.
Supplementary information
Acknowledgements
We are grateful to Prof. Jie Zhou of Zhejiang University for the tomato seeds and transformation vectors. This work was supported by the National Natural Science Foundation of China (31672199 and 31801902), the China Earmarked Fund for Modern Agro-industry Technology Research System (CARS-23-B12) and the Fundamental Research Funds for the Central Universities (KJQN201928).
Author contributions
S.G. designed the experiment. M.Z. and Y.W. performed the experiments and wrote the manuscript; K.H. collected the plant materials and prepared the figures; and S.S. and J.S. analyzed the data and modified the manuscript. All authors reviewed and approved the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41438-019-0173-z).
References
- 1.Shu S, et al. The role of putrescine in the regulation of proteins and fatty acids of thylakoid membranes under salt stress. Sci. Rep. 2015;5:14390. doi: 10.1038/srep14390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Velikova V, Yordanov I, Edreva A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci. 2000;151:59–66. doi: 10.1016/S0168-9452(99)00197-1. [DOI] [Google Scholar]
- 3.Hamdani S, Yaakoubi H, Carpentier R. Polyamines interaction with thylakoid proteins during stress. J. Photochem. Photobiol. B. 2011;104:314–319. doi: 10.1016/j.jphotobiol.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Wi SJ, Kim WT, Park KY. Overexpression of carnation S-adenosylmethionine decarboxylase gene generates a broad-spectrum tolerance to abiotic stresses in transgenic tobacco plants. Plant Cell Rep. 2006;25:1111–1121. doi: 10.1007/s00299-006-0160-3. [DOI] [PubMed] [Google Scholar]
- 5.Qi YC, Wang FF, Zhang H, Liu WQ. Overexpression of suadea salsa S-adenosylmethionine synthetase gene promotes salt tolerance in transgenic tobacco. Acta Physiol. Plant. 2010;32:263–269. doi: 10.1007/s11738-009-0403-3. [DOI] [Google Scholar]
- 6.Ioannidis NE, Kotzabasis K. Effects of polyamines on the functionality of photosynthetic membrane in vivo and in vitro. Biochim. Biophys. Acta. 2007;1767:1372–1382. doi: 10.1016/j.bbabio.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Ioannidis NE, Sfichi L, Kotzabasis K. Putrescine stimulates chemiosmotic ATP synthesis. Biochim. Biophys. Acta. 2006;1757:821–828. doi: 10.1016/j.bbabio.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 8.Gupta K, Dey A, Gupta B. Plant polyamines in abiotic stress responses. Acta Physiol. Plant. 2013;35:2015–2036. doi: 10.1007/s11738-013-1239-4. [DOI] [Google Scholar]
- 9.Saha J, et al. Polyamines as redox homeostasis regulators during salt stress in plants. Front. Environ. Sci. 2015;3:21. doi: 10.3389/fenvs.2015.00021. [DOI] [Google Scholar]
- 10.Serafini-Fracassini D, Stefano DD. Transglutaminases: widespread cross-linking enzymes in plants. Ann. Bot. 2008;102:145–152. doi: 10.1093/aob/mcn075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai G, Serafini-Fracassini D, Del Duca S. Regulation of pollen tube growth by transglutaminase. Plants. 2013;2:87–106. doi: 10.3390/plants2010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lorand L, Graham RM. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat. Rev. Mol. Cell Boil. 2003;4:140. doi: 10.1038/nrm1014. [DOI] [PubMed] [Google Scholar]
- 13.Lilley GR, Skill J, Griffin M, Bonner PL. Detection of Ca2+-dependent transglutaminase activity in root and leaf tissue of monocotyledonous and dicotyledonous plants. Plant Physiol. 1998;117:1115–1123. doi: 10.1104/pp.117.3.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Della Mea M, Caparrós-Ruiz D, Claparols I, Serafini-Fracassini D, Rigau J. AtPng1p first plant transglutaminase. Plant Physiol. 2004;135:2046–2054. doi: 10.1104/pp.104.042549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Della MM, De FF, Genovesi V, Serafini FD, Del DS. The acropetal wave of developmental cell death of tobacco corolla is preceded by activation of transglutaminase in different cell compartments. Plant Physiol. 2007;144:1211–1222. doi: 10.1104/pp.106.092072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Margosiak SA, et al. Identification of the large subunit of ribulose 1,5-bisphosphate carboxylase/oxygenase as a substrate for transglutaminase in Medicago sativa L. (Alfalfa) Plant Physiol. 1990;92:88–96. doi: 10.1104/pp.92.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andreas D, Guangtao L, Lennarz WJ, Thorsten N, Frédéric B. The ArabidopsisAtPNG1 gene encodes a peptide: N-glycanase. Plant J. 2010;52:94–104. doi: 10.1111/j.1365-313X.2007.03215.x. [DOI] [PubMed] [Google Scholar]
- 18.Del Duca S, et al. Distribution of transglutaminase in pear pollen tubes in relation to cytoskeleton and membrane dynamics. Plant Physiol. 2013;161:1706–1721. doi: 10.1104/pp.112.212225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campos N, et al. Proteomic and transcriptomic analysis of rice tranglutaminase and chloroplast-related proteins. Plant Sci. 2014;229:142–153. doi: 10.1016/j.plantsci.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Dondini L, et al. Suborganellar localisation and effect of light on Helianthus tuberosus chloroplast transglutaminases and their substrates. Planta. 2003;217:84–95. doi: 10.1007/s00425-003-0998-3. [DOI] [PubMed] [Google Scholar]
- 21.Zhong M, et al. Overexpression of transglutaminase from cucumber in tobacco increases salt tolerance through regulation of photosynthesis. Int. J. Mol. Sci. 2019;20:894. doi: 10.3390/ijms20040894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Della Mea, et al. A Zea mays 39-kDa thylakoid transglutaminase catalyses the modification by polyamines of light-harvesting complex II in a light-dependent way. Planta. 2004;219:754–764. doi: 10.1007/s00425-004-1278-6. [DOI] [PubMed] [Google Scholar]
- 23.Sobieszczuk-Nowicka E, Legocka J. Plastid-associated polyamines: their role in differentiation, structure, functioning, stress response and senescence. Plant Biol. 2014;16:297–305. doi: 10.1111/plb.12058. [DOI] [PubMed] [Google Scholar]
- 24.Liu H, et al. CRISPR-P 2.0: an improved CRISPR-Cas9 tool for genome editing in plants. Mol. Plant. 2017;10:530–532. doi: 10.1016/j.molp.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Fillatti JJ, Kiser J, Rose R, Comai L. Efficient transfer of a glyphosate tolerance gene into tomato using a binary Agrobacterium tumefaciens vector. Nat. Biotechnol. 1987;5:726. doi: 10.1038/nbt0787-726. [DOI] [Google Scholar]
- 26.Pan C, et al. CRISPR/Cas9-mediated efficient and heritable targeted mutagenesis in tomato plants in the first and later generations. Sci. Rep. 2016;6:24765. doi: 10.1038/srep24765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grossowicz N, Wainfan E, Borek E, Waelsch H. The enzymatic formation of hydroxamic acids from glutamine and asparagine. J. Bio. Chem. 1950;187:111–125. [PubMed] [Google Scholar]
- 28.von Caemmerer S, Farquhar GD. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta. 1981;153:376–387. doi: 10.1007/BF00384257. [DOI] [PubMed] [Google Scholar]
- 29.Ethier G, Livingston N. On the need to incorporate sensitivity to CO2 transfer conductance into the Farquhar–von Caemmerer–Berry leaf photosynthesis model. Plant Cell. Environ. 2004;27:137–153. doi: 10.1111/j.1365-3040.2004.01140.x. [DOI] [Google Scholar]
- 30.Ward DA, Keys AJ. A comparison between the coupled spectrophotometric and uncoupled radiometric assays for RuBP carboxylase. Photosynth. Res. 1989;22:167–171. doi: 10.1007/BF00035447. [DOI] [PubMed] [Google Scholar]
- 31.Scheibe R, Fickenscher K, Ashton AR. Studies on the mechanism of the reductive activation of NADP-malate dehydrogenase by thioredoxin m and low-molecular-weight thiols. Biochim. Biophys. Acta. 1986;870:191–197. doi: 10.1016/0167-4838(86)90221-9. [DOI] [Google Scholar]
- 32.Rao MV, Ormrod D. Ozone exposure decreases UVB sensitivity in a UVB-sensitive flavonoid mutant of Arabidopsis. Photochem. Photobiol. 1995;61:71–78. doi: 10.1111/j.1751-1097.1995.tb09245.x. [DOI] [PubMed] [Google Scholar]
- 33.Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- 34.Halliwell B, Foyer CH. Ascorbic acid, metal ions and the superoxide radical. Biochem. J. 1976;155:697–700. doi: 10.1042/bj1550697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, et al. Tomato HsfA1a plays a critical role in plant drought tolerance by activating ATG genes and inducing autophagy. Autophagy. 2015;11:2033–2047. doi: 10.1080/15548627.2015.1098798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fracassini DS, Del Duca S. Biochemistry and function of plant transglutaminases. Minerva Biotecnol. 2002;14:135. [Google Scholar]
- 38.Ioannidis NE, et al. Remodeling of tobacco thylakoids by over-expression of maize plastidial transglutaminase. Biochim. Biophys. Acta. 2009;1787:1215–1222. doi: 10.1016/j.bbabio.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 39.Ioannidis NE, Malliarakis D, Torné JM, Santos M, Kotzabasis K. The over-expression of the plastidial transglutaminase from maize in Arabidopsis increases the activation threshold of photoprotection. Front. Plant Sci. 2016;7:635. doi: 10.3389/fpls.2016.00635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuan Y, et al. Effects of exogenous putrescine on chlorophyll fluorescence imaging and heat dissipation capacity in cucumber (Cucumis sativus L.) under salt stress. J. Plant Growth Regul. 2014;33:798–808. doi: 10.1007/s00344-014-9427-z. [DOI] [Google Scholar]
- 41.Del Duca S, Tidu V, Bassi R, Esposito C, Serafmi-Fracassini D. Identification of chlorophyll-a/b proteins as substrates of transglutaminase activity in isolated chloroplasts of Helianthus tuberosus L. Planta. 1994;193:283–289. doi: 10.1007/BF00192542. [DOI] [Google Scholar]
- 42.Ortigosa SM, et al. Oxidative stress induced in tobacco leaves by chloroplast over-expression of maize plastidial transglutaminase. Planta. 2010;232:593–605. doi: 10.1007/s00425-010-1185-y. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki Y, Makino A. Translational downregulation of RBCL is operative in the coordinated expression of Rubisco genes in senescent leaves in rice. J. Exp. Bot. 2013;64:1145–1152. doi: 10.1093/jxb/ers398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suzuki Y, Miyamoto T, Yoshizawa R, Mae T, Makino A. Rubisco content and photosynthesis of leaves at different positions in transgenic rice with an overexpression of RBCS. Plant Cell Environ. 2009;32:417–427. doi: 10.1111/j.1365-3040.2009.01937.x. [DOI] [PubMed] [Google Scholar]
- 45.Cohen I, Sapir Y, Shapira M. A conserved mechanism controls translation of Rubisco large subunit in different photosynthetic organisms. Plant Physiol. 2006;141:1089–1097. doi: 10.1104/pp.106.079046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Long SP, ZHU XG, Naidu SL, Ort DR. Can improvement in photosynthesis increase crop yields? Plant Cell Environ. 2006;29:315–330. doi: 10.1111/j.1365-3040.2005.01493.x. [DOI] [PubMed] [Google Scholar]
- 47.Cheng F, et al. Chloroplastic thioredoxin-f and thioredoxin-m 1/4 play important roles in brassinosteroids-induced changes in CO2 assimilation and cellular redox homeostasis in tomato. J. Exp. Bot. 2014;65:4335–4347. doi: 10.1093/jxb/eru207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moreno J, García-Murria MJ, Marín-Navarro J. Redox modulation of Rubisco conformation and activity through its cysteine residues. J. Exp. Bot. 2008;59:1605–1614. doi: 10.1093/jxb/erm310. [DOI] [PubMed] [Google Scholar]
- 49.Jiang YP, et al. Cellular glutathione redox homeostasis plays an important role in the brassinosteroid-induced increase in CO2 assimilation in Cucumis sativus. New Phytol. 2012;194:932–943. doi: 10.1111/j.1469-8137.2012.04111.x. [DOI] [PubMed] [Google Scholar]
- 50.Irihimovitch V, Shapira M. Glutathione redox potential modulated by reactive oxygen species regulates translation of Rubisco large subunit in the chloroplast. J. Biol. Chem. 2000;275:16289–16295. doi: 10.1074/jbc.275.21.16289. [DOI] [PubMed] [Google Scholar]
- 51.Gong B, et al. Overexpression of S-adenosylmethionine synthetase 1 enhances tomato callus tolerance to alkali stress through polyamine and hydrogen peroxide cross-linked networks. Plant Cell Tissue Organ Cult. 2016;124:377–391. doi: 10.1007/s11240-015-0901-5. [DOI] [Google Scholar]
- 52.Foyer CH, Noctor G. Ascorbate and glutathione: the heart of the redox hub. Plant Physiol. 2011;155:2–18. doi: 10.1104/pp.110.167569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Foyer CH, Lopez-Delgado H, Dat JF, Scott IM. Hydrogen peroxide-and glutathione-associated mechanisms of acclimatory stress tolerance and signalling. Physiol. Plant. 1997;100:241–254. doi: 10.1111/j.1399-3054.1997.tb04780.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.