Splenic marginal zone lymphoma (SMZL) is an incurable indolent small B-cell lymphoma that primarily involves the spleen and arises in elderly patients, who have a median age of 65 years old at diagnosis.1 Splenomegaly is the most common clinical sign and is observed in 75% of patients. Peripheral blood infiltration of tumor B-cells is detected in approximately 60% of cases.2 Dissemination to the bone marrow is almost constantly found.3 The 5-year overall survival ranges from 67.8 to 81% depending on the clinical stage at diagnosis and treatment approach.4 Transformation to aggressive large B-cell lymphoma occurs in ~10% of SMZL cases by 12 years.5
Escape from immune control must be important in the long-term natural course of indolent B-cell lymphomas. The presence of PD-1-positive T-cells, a characteristic of tumors with an immune-inflamed phenotype, has been reported in marginal zone lymphomas.6 The immune-inflamed phenotype is associated with “high” intratumor T-cell content.7 This phenotype suggests the presence of a preexisting antitumor immune response that has been arrested, probably by local immunosuppression in the tumor bed. Other described immune tumor profiles are the immune-excluded phenotype and immune-desert phenotype. The immune-excluded phenotype is also characterized by the presence of abundant T-cells. However, these T-cells are located in the periphery as they are retained in the stroma that surrounds nests of tumor cells. The immune-desert phenotype is characterized by a paucity of T-cells in the tumor (for a review, see Chen et al.7).
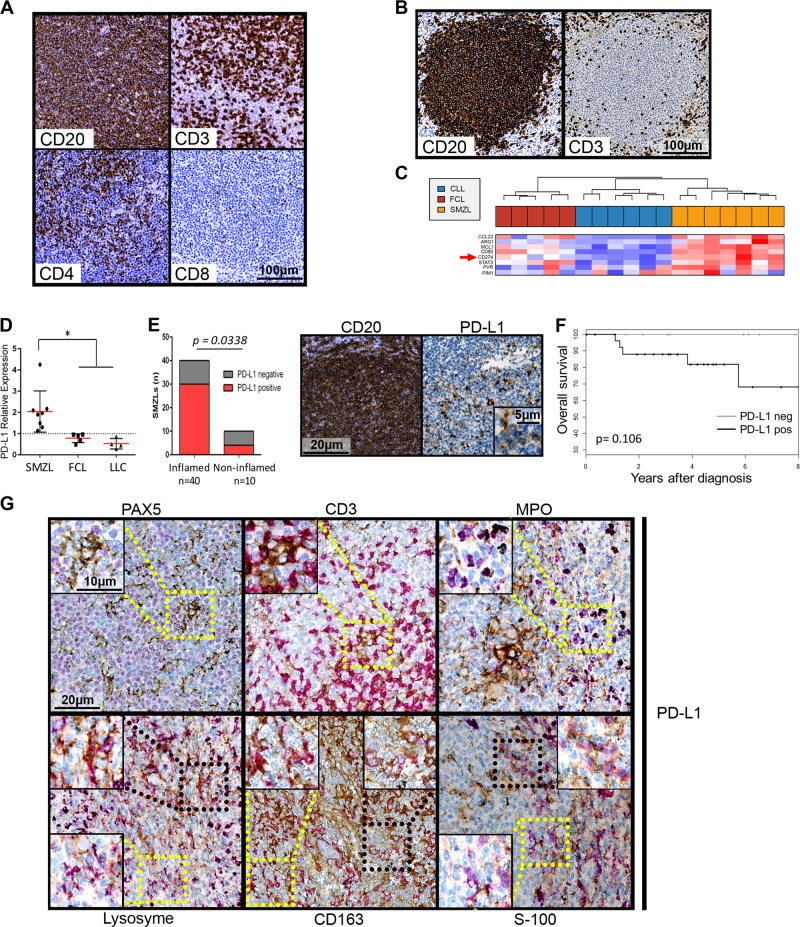
First, we determined the cancer immune phenotype of a set of 54 SMZLs. Patient tumors exhibited a nodular or a nodular and diffuse pattern, as described.3 A “high” intratumor T-cell level was found in 43/54 cases (80%, Fig. 1a). Most intranodular CD3-positive cells were CD4 positive, while some were CD8 positive (Fig. 1a). The remaining 10 tumors exhibited immune-excluded phenotypes, with the majority of the T-cells outside the nodules (Fig. 1b).
Fig. 1.
a and b: example of CD20, CD3, CD4 and CD8 immunohistochemical labeling of spleen sections from two SMZL patients, one with an inflamed phenotype (a) and one with an immune-excluded (b) phenotype. c Clustering of genes from the immune escape gene signature published by Laurent et al.8 for SMZLs, CLLs and FCLs. Detailed legends are in Supplementary Figure 1. d PD-L1 mRNA expression levels shown according to diagnosis (SMZL, CLL and FCL). RT-QPCR was performed using the TaqMan expression assay Hs00204257_m1 from Applied Biosystems. Normalization was performed on HPRT1 expression (Hs02800695_m1) and on a pool of four normal lymph nodes. Normal lymph node expression, which was arbitrarily set equal to 1, is represented by the dashed horizontal line. The mean and standard deviation are shown for each lymphoma type. PD-L1 expression was significantly increased in the SMZLs compared to the CLLs and FCLs, as shown by * p < 0.05. e Left panel: histogram representation of the PD-L1 expression in inflamed or uninflamed SMZL tumors. Right panel: example of immunohistochemical PDL-1 labeling compared to CD20 staining of the same sample. f Overall survival of SMZL patients according to PD-L1 expression. g Example of immunohistochemical double staining for PDL-L1 (in brown) and either Pax5, CD3, myeloperoxidase (MPO), lysozyme, CD163 or S-100 protein. MPO, Lysozyme, CD163 and S-100 protein labeling is shown in purple. Selected microphotographs display the border of a nodule with CD163, lysozyme and S-100 protein labeling to show double-positive cells in the nodule and single-positive cells outside the nodule. Magnified images for PD-L1-negative (yellow square) or PD-L1 double-positive cells (black square) are shown as insets. In this study, 64 SMZL, 5 FCL, and 6 CLL patients were enrolled according to institutional regulations and after approval by the Scientific and Methodological board of the university hospital of Limoges. A tissue microarray (TMA) was performed with formalin-fixed, paraffin-embedded (FFPE) tumor samples from 54 patients. Tissue cores with a 1 mm diameter were arrayed on a recipient paraffin block using a tissue arrayer (Beecher Instruments Tissue Arrayeur, New Jersey, USA). Each tumor sample was punched in triplicate. TMA blocks were sliced at 4 mm. Antibodies against CD20 (clone L26), CD3 (clone 2GV6), Pax5 (clone SP34) and myeloperoxidase (polyclonal immune serum) were purchased from Roche Diagnostics (Meylan, France); those against CD8 (clone C8/144B) and protein S-100 (polyclonal immune serum) were obtained from Dako (Glostrup, Denmark); and those against CD4 (clone 4B12) and CD163 (clone 10D16) were purchased from Novocastra (Leica Biosystems, Wetzlar, Germany). Immunohistochemistry was carried out on a Benchmark®ULTRA (ROCHE -VENTANA) with an UltraView Universal DAB (760–500) detection kit, and the sections were counterstained with hematein eosin. For PD-L1, immunohistochemistry was carried out on Benchmark®ULTRA (ROCHE -VENTANA) with an OptiView DAB IHC (760–700) detection kit for PD-L1 for simple staining (clone SP263; Roche Diagnostics), with a double-staining oDAB-uRed kit (Roche Diagnostics) for double labeling of PD-L1 with CD163, CD3 or Pax5 and with a RUO Discovery Universal DAB-purple kit (Roche Diagnostics) for double labeling of PD-L1 with MPO, lysozyme or protein S-100. The microarray slides were scanned with a Hamamatsu Photonics K. K slide scanner (Hamamatsu Photonics K. K, Hamamatsu City, Japan). Images were visualized with NDP software from Hamamatsu Photonics K.K. Staining for T-cells was scored semiquantitatively as “present (2 + ),” “rare (1 + ),” or “absent (0)”, as previously described.15 For PD-L1, cases were positive when more than 5% of the intratumoral cells were positive
The frequency of the immune-inflamed phenotype raised the question of whether SMZLs could undergo immune escape. We analyzed the immune escape gene signature published by Laurent et al.8 in an Affymetrix transcriptome dataset (HG-U133_Plus_2 array) comprising 8 SMZLs, 5 FCLs (Follicular lymphomas) and 6 CLLs (Chronic lymphocytic leukemias). As shown in Fig. 1c, SMZLs were characterized by the overexpression of PD-L1, MCL1, and CD80 that closely coclustered with STAT3. The expression of these genes in the SMZLs was consistently increased when compared that of the FCLs or CLLs. IDO1, IDO2, LAG3 and CTLA-4 were among the genes with upregulated expression in the FCLs, and BTLA expression was increased in the CLL samples together with its ligand TNFRSF14 (Supplementary Figure 1), which might suggest that these different indolent lymphomas have different mechanisms of immune escape. The overexpression of PD-L1 in the SMZLs was confirmed by RT-PCR (Fig. 1d).
At the protein level, histological characterization of SMZLs revealed an immune-inflamed phenotype with the presence of PD-L1-positive cells in 75% of tumors (Fig. 1e). Even if statistical significance was not reached, Kaplan-Meier overall survival curves suggested that the presence of PD-L1-positive cells in CD20-positive nodules might negatively influence prognosis (Fig. 1f). Intranodular PD-L1 expression seemed to be associated with a morphological pattern evoking the cells of the microenvironment.
Double staining was then performed to identify the PD-L1-positive cells within the SMZL tumor nodules. As shown in Fig. 1g, Pax5-positive tumor B-cells were consistently PD-L1 negative. PD-L1/CD3 double-positive T-cells were extremely rare (Fig. 1g and not shown). Myeloperoxydase-positive granulocytes were scarce within the nodules and were mainly located in the periphery (Fig. 1g). These cells were consistently PD-L1 negative (Fig. 1g). The lysozyme antigen was used as a broad marker of the monocytic/macrophage lineage.9 Most intranodular lysozyme-positive cells were found to express PD-L1, while peripheral lysozyme-positive cells were PD-L1 negative (Fig. 1g). PD-L1 expression also colocalized with CD163, a marker of alternatively activated macrophages (so-called type II tumor-associated macrophages) (Fig. 1g). Extranodular CD163-positive macrophages were PD-L1 negative. Intranodular cells expressing the S-100 protein, a broad marker of immature and mature dendritic cells,10 also expressed PD-L1 (Fig. 1g). Similar to macrophages, S100-positive dendritic cells located in the periphery of nodules did not express PD-L1. Altogether, these results clearly indicated that PD-L1 expression in SMZLs was mainly related to both intratumoral monocytes/macrophages and dendritic cells, and T-lymphocytes concomitantly expressed PD-1.
As reviewed by Chen and Mellman,7 the concept of tumors with an inflamed phenotype, id est with intratumoral infiltration of T-cells that are actively inactivated either by tumor cells or by cells composing the tumor microenvironment such as myeloid-derived suppressor cells or tissue-associated macrophages, has emerged from the characterization of solid cancers. An inflamed tumor phenotype is frequently associated with PD-L1 expression either by tumor cells or cells in the microenvironment. This tumor phenotype is also associated with the response to immunotherapies targeting the PD-L1/PD-1 axis. As reviewed recently, there is presently no standardized approach regarding cutoffs and standards for PD-L1 testing.11 Here, we used a commercial detection kit that is routinely used in lung and colon cancers to predict the response to immunotherapies against the PD-1/PD-L1 axis and is recognized as one of the most consistent in the literature.11,12
Very few studies have examined PD-L1 expression in marginal zone lymphomas. With an antibody different from ours, Andorsky et al. examined PD-L1 expression by flow cytometry in only three cases of marginal zone lymphoma13 and did not find any PD-L1 expression on B-cells. With another antibody, Panjwani et al. looked at PD-L1 expression on a large variety of both T and B-cell lymphomas by immunohistochemistry.14 However, their dataset included only five cases of SMZL and no PD-L1-positive cases. Nothing was said regarding the cells in the microenvironment in these two studies.13,14
With this large dataset of 54 SMZL tumors, we confirmed that Pax5-positive SMZL tumor B-cells were PD-L1 negative. However, PD-L1-positive cells were present in SMZL tumor nodules. The presence of PD-L1 positive cells was associated with an inflamed phenotype with intratumoral infiltration of T-cells, which were predominantly CD4-positive cells. The expression of PD-L1 in SMZL was highly associated with the presence of PD-1-positive cells, which suggests that the PD-L1/PD-1 axis is effective in SMZL. Most in situ lysozyme-positive cells expressed PD-L1. This broad monocytic/macrophage differentiation marker can be found in immature granulocytes.9 However, here, myeloperoxidase and PD-L1 labeling never colocalized. Thus, PD-L1 and lysozyme colocalization in tumor nodules was related to the intratumoral monocytic/macrophage compartment. The presence of either PD-L1/CD163 or PD-L1/S-100 double-positive cells indicated that both type II tumor-associated macrophages and dendritic cells expressed PD-L1. Notably, lysozyme-, CD163- or S-100-positive cells located in the tumor nodule periphery were consistently PD-L1 negative. This suggests that these tumor-associated cells were instructed to express PD-L1.
In conclusion, our results show that SMZL exhibits an inflamed phenotype with an immune escape gene signature involving the expression of PD-L1. The presence of PD-L1-positive cells was associated with numerous PD-1-positive lymphocytes and tended to be associated with shorter overall survival. The expression of PD-L1 in tumor nodules was due to tumor-associated lysozyme-positive monocytes/macrophages and S-100-positive dendritic cells. These cells were very likely to be instructed to express PD-L1 by the tumor since they did not express this marker when located in the periphery. Such an inflamed phenotype is thus very likely to be associated with tumor immune escape, as demonstrated for numerous solid cancers such as melanoma and lung cancers.
Supplementary information
Acknowledgements
We thank Dr J Cook Moreau, UMR CNRS 7276, Limoges, France, for English editing. The group of J Feuillard is supported by grants from the Ligue Nationale Contre le Cancer (Equipe Labellisée Ligue), the Institut National Contre le Cancer (INCa), the Comité Orientation Recherche Cancer (CORC), the Limousin Region and the Haute Vienne and Corrèze committees of the Ligue Nationale Contre le Cancer and the Lyons Club of Corrèze.
Author contributions
C.V.F. and I.S. performed and analyzed the experiments and contributed to the writing. V.V. performed the TMA and immunohistochemistry. I.S. and M.P. contributed to SMZL diagnosis as well as to analyzing the immunohistochemistry results. R.J. and E.L. contributed to the labeling. N.G. performed the Affymetrix* gene expression profiles. J.F. and N.F. designed and directed the study, contributed to the experiments, analyzed the results and wrote the manuscript.
Competing interests
The authors declare no competing interests.
Supplementary information
The online version of this article (10.1038/s41423-019-0228-y) contains supplementary material.
References
- 1.Thieblemont C, et al. Splenic marginal-zone lymphoma: a distinct clinical and pathological entity. Lancet. Oncol. 2003;4:95–103. doi: 10.1016/S1470-2045(03)00981-1. [DOI] [PubMed] [Google Scholar]
- 2.Berger F, et al. Non-MALT marginal zone B-cell lymphomas: a description of clinical presentation and outcome in 124 patients. Blood. 2000;95:1950–1956. [PubMed] [Google Scholar]
- 3.Petit B, et al. Among 157 marginal zone lymphomas, DBA.44(CD76) expression is restricted to tumour cells infiltrating the red pulp of the spleen with a diffuse architectural pattern. Histopathology. 2009;54:626–631. doi: 10.1111/j.1365-2559.2009.03262.x. [DOI] [PubMed] [Google Scholar]
- 4.Perrone S, et al. Splenic marginal zone lymphoma: prognostic factors, role of watch and wait policy, and other therapeutic approaches in the rituximab era. Leuk. Res. 2016;44:53–60. doi: 10.1016/j.leukres.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Casulo C, Friedberg J. Transformation of marginal zone lymphoma (and association with other lymphomas) Best. Pract. Res. Clin. Haematol. 2017;30:131–138. doi: 10.1016/j.beha.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 6.Xu-Monette ZY, Zhou J, Young KH. PD-1 expression and clinical PD-1 blockade in B-cell lymphomas. Blood. 2018;131:68–83. doi: 10.1182/blood-2017-07-740993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321–330. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 8.Laurent C, et al. Several immune escape patterns in non-Hodgkin’s lymphomas. Oncoimmunology. 2015;4:e1026530. doi: 10.1080/2162402X.2015.1026530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nash JR. Macrophages in human tumours: an immunohistochemical study. J. Pathol. 1982;136:72–83. doi: 10.1002/path.1711360202. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi K, Yamaguchi H, Ishizeki J, Nakajima T, Nakazato Y. Immunohistochemical and immunoelectron microscopic localization of S-100 protein in the interdigitating reticulum cells of the human lymph node. Virchows. Arch. B. Cell Pathol. Incl. Mol. Pathol. 1981;37:125–135. doi: 10.1007/BF02892562. [DOI] [PubMed] [Google Scholar]
- 11.Udall M, et al. PD-L1 diagnostic tests: a systematic literature review of scoring algorithms and test-validation metrics. Diagn. Pathol. 2018;13:12. doi: 10.1186/s13000-018-0689-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ionescu DN, Downes MR, Christofides A, Tsao MS. Harmonization of PD-L1 testing in oncology: a Canadian pathology perspective. Curr. Oncol. Tor. Ont. 2018;25:e209–e216. doi: 10.3747/co.25.4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andorsky DJ, et al. Programmed death ligand 1 is expressed by non-hodgkin lymphomas and inhibits the activity of tumor-associated T cells. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011;17:4232–4244. doi: 10.1158/1078-0432.CCR-10-2660. [DOI] [PubMed] [Google Scholar]
- 14.Panjwani PK, et al. Programmed death-1 ligands PD-L1 and PD-L2 show distinctive and restricted patterns of expression in lymphoma subtypes. Hum. Pathol. 2018;71:91–99. doi: 10.1016/j.humpath.2017.10.029. [DOI] [PubMed] [Google Scholar]
- 15.Harlin H, et al. Chemokine expression in melanoma metastases associated with CD8+T-cell recruitment. Cancer Res. 2009;69:3077–3085. doi: 10.1158/0008-5472.CAN-08-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.