Abstract
Traumatic brain injury (TBI) is recognized as a global health problem due to its increasing occurrence, challenging treatment, and persistent impacts on brain pathophysiology. Neural cell death in patients with TBI swiftly causes inflammation in the injured brain areas, which is recognized as focal brain inflammation. Focal brain inflammation causes secondary brain injury by exacerbating brain edema and neuronal death, while also exerting divergent beneficial effects, such as sealing the damaged limitans and removing cellular debris. Recent evidence from patients with TBI and studies on animal models suggest that brain inflammation after TBI is not only restricted to the focal lesion but also disseminates to remote areas of the brain. The dissemination of inflammation has been detected within days after the primary injury and persists chronically. This state of inflammation may be related to remote complications of TBI in patients, such as hyperthermia and hypopituitarism, and may lead to progressive neurodegeneration, such as chronic traumatic encephalopathy. Future studies should focus on understanding the mechanisms that govern the initiation and propagation of brain inflammation after TBI and its impacts on post-trauma brain pathology.
Keywords: disseminated brain inflammation, traumatic brain injury, microglia, post-injury neurodegeneration
Subject terms: Neuroimmunology, Cell death and immune response
Introduction
Traumatic brain injury (TBI) encompasses mechanical brain injuries with diverse causes, severities, pathological changes, and clinical outcomes.1,2 Over the past few decades, the prevalence of TBI has continuously increased, with 27.08 million new cases and over 55 million prevalent cases reported worldwide in 2016.3 This devastating disease causes far-reaching physical, emotional, and economic consequences for patients, their families, and the society-at-large. However, treatment options remain limited to surgical decompression and supportive care. The lack of treatments is partially due to the heterogeneous nature of the injury and the insufficient identification and understanding of the precise mechanisms of secondary brain injury.4–6
The acute primary injury in TBI is characterized by the destruction of the brain tissue by focal intracranial hemorrhage, epidural and subdural hematomas, brain contusions, and direct axonal injury.7,8 These injuries occur at the time of cranial impact and cause immediate, irreversible neuronal damage. This primary brain injury then initiates a series of secondary events, including excitotoxicity, free radical generation, and the inflammatory response.9–11 These events trigger secondary brain injury, which begins within minutes of the primary insult and often persist for months or even years.2 Researchers have increasingly focused on the role of immune system in TBI, both in the acute and long-term stages.
Within minutes after brain injury, damaged neural cells induce a series of immune responses by releasing danger signals, such as damage-associated molecular patterns (DAMPs).12–14 Microglia, which are considered the intrinsic immune cells in the brain, are the first responders to the injury, followed by activated astrocytes and endothelial cells. Peripheral immune cells such as neutrophils, monocytes, and lymphocytes are then recruited to the lesion.15–17 These cells secrete multiple inflammatory mediators (e.g., cytokines and chemokines) that mediate robust and complex interactions among the cells, resulting in post-TBI inflammation. In addition to focal brain inflammation, accumulating evidence obtained from patients and animal models suggests that brain inflammation after TBI is not restricted to the lesions, but instead disseminates to areas remote from the lesions. Furthermore, the disseminated brain inflammation may be persistent and impact brain pathology, causing progressive neurodegeneration. In this review, we summarize the emerging evidence and features of disseminated brain inflammation after TBI and examine the potential mechanisms that govern the process of dissemination and its clinical impacts. We also discuss future efforts that should be undertaken to obtain a better understanding of the biological consequences of the disseminated brain inflammation and develop new treatment strategies targeting inflammation to improve patients’ outcomes.
Focal brain inflammation in TBI
During the acute phase of TBI, the central nervous system (CNS) barrier is immediately damaged by the mechanical impact at the site of the injury, and together with injuries to the meninges, glial limitans and brain parenchyma, injured brain cells release a series of DAMPs, including adenosine triphosphate (ATP), heat shock proteins (HSPs), high-mobility group box 1 (HGMB1), IL-33, and so on.12,18,19 Resident microglia are the first cells to react to these danger signals via their pathogen-associated molecular pattern (PAMP) sensors, including Toll-like receptors (TLRs) and purinergic receptors.20,21 Within minutes of an insult, microglia in the damaged area are activated, extending processes to the glial limitans to maintain the barrier integrity by sealing the gaps left by dead or damaged astrocytes, in addition to helping clear the debris from dead cells through phagocytosis.12 Peripheral immune components, such as the complement system and neutrophils, are also activated and recruited to the injury site during this acute stage.22 These responses are followed by the activation of intracellular signaling pathways that amplify the inflammatory response by increasing the production of chemokines, cytokines, and reactive oxygen species (ROS). Monocytes/macrophages23 and T cells16 are then recruited to the brain, and inflammatory reactions spread from the injury site to the surrounding tissue.
The pathological impact of focal brain inflammation following TBI remains controversial. Several studies suggest a detrimental effect. For example, minocycline, an antibiotic that suppresses microglial activation, exerts beneficial effects when administered to an animal model of TBI.24 Conversely, in another study, the detrimental effect of microglial activation on a cortical brain injury model was discovered in nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-2 (NOX2)−/− mice.25 Additionally, inhibition of C-C chemokine receptor type 2 (CCR2)-mediated myeloid cell migration after TBI in animal studies also suggested a benefit of inhibiting focal brain inflammation.23 However, focal brain inflammation in the acute stages of TBI also actively participates in beneficial functions, such as barrier maintenance, debris clearance, neurotrophin production, and immune regulation.11 Therefore, a detailed analysis of the roles of different immune components and the temporal profiles of their detrimental/beneficial effects after TBI is required, particularly in the context of the failure of systemic inhibition of immune functions (e.g., methylprednisolone and progesterone) to improve the outcomes of patients with TBI.26
Evidence of the dissemination of brain inflammation in TBI
In addition to inflammation localized to the lesion and surrounding area, evidence obtained from patients with TBI and animal models suggests that immune responses disseminate to areas remote from the injury in both the acute and chronic phases. The disseminated inflammation features similar immune components but evolves in a distinct profile compared to focal inflammation (Table 1).
Table 1.
Comparison of the features of focal and disseminated brain inflammation
| Inflammation features | Focal brain inflammation | Disseminated brain inflammation |
|---|---|---|
| Initiating events | Damaged cells release DAMPs | Remote neural cell injury; vascular damage; diffuse axonal injury; CSF inflammation. |
| Dynamic change of components | ||
| Glia cells (microglia and astrocytes) | ||
| Initiation | Minutes after the initial injury | 1 week, after focal inflammation decreasesa |
| Persistence | Approximately 1 week | Increasingly progressive, lifelong |
| Myeloid cells (neutrophils and monocytes) | ||
| Initiation | Hours | Approximately 1 weeka |
| Persistence | Days | Lifelong |
| Lymphocytes | ||
| Initiation | Within 1 day | Within 1 daya |
| Persistence | Days to weeks | Lifelong, with a shift to B cell dominance in the chronic phase |
| Inflammatory mediators | ||
| Components | Many are involved, including TNF, IFNγ, IL‑1β, IL‑6, IL‑10, GM‑CSF, TGFβ, and CCL2. | Many are involved, including CXCL1, IL-13, CCL2, IL-9, IL-10, IL-17, and MIP-1α. |
| Dynamics | Most increase minutes to hours after the initial injury, peak at approximately 1 day, and then decrease over subsequent days. | Appears 1 day after injury, the spectrum changes with the progression of disease stages and persists for at least months. |
| Distribution | Within the lesion and surrounding tissue | Thalamus, hippocampus, pituitary, corpus callosum, occipital cortex, brain stem, and spinal cord. |
| Pathological effects | Detrimental: Edema and cell death in areas surrounding the lesion. Beneficial: Clearance of dead cells; preservation of barrier function. | Disturbance of specific functional regions, such as the thalamus and pituitary; chronic and progressive neurodegeneration. |
| Effects of inhibiting inflammation | Controversial | The impact on chronic brain inflammation may rescue chronic degeneration. |
DAMP damage-associated molecular pattern, CSF cerebrospinal fluid, TNF tumor necrosis factor, IFNγ interferon γ, IL interleukin, GM-CSF granulocyte-macrophage colony-stimulating factor, TGFβ transforming growth factor β, CCL2 C-C chemokine ligand 2, CXCL1 C-X-C chemokine ligand 1, MIP-1α macrophage inflammatory protein 1α
aThe time at which the cells begin to be detected in distal regions
Glial activation
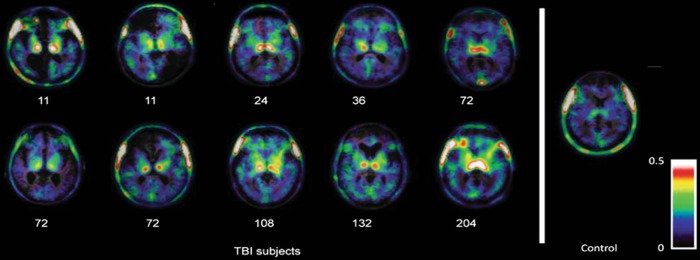
Microglia serve as resident immune cell sentinels of the central nervous system. Microglia react to TBI within minutes by projecting processes toward the sites of injury,12 followed closely by their morphological transformation and proliferation.2 In addition to this focal reaction, activated microglia have also been detected in remote structures, including the thalamus and hippocampus, located both ipsilateral and contralateral to the injury as early as 7 days after TBI in mouse models.27 These contralateral brain structures, which harbor activated microglia, include both cortical and sub-cortical regions that are anatomically located close to the midline. Microglial activation then gradually disperses throughout the brain and persists for more than 1 year after TBI in mice.27 Notably, the activation level and density of microglia in the ipsilateral and contralateral hemispheres during the chronic stage become comparable, suggesting a global response. This phenomenon is mirrored in patients with TBI; autopsy results have confirmed that microglial activation persists for many years after TBI in the corpus callosum, which may be associated with the degeneration of the corpus callosum.28 Additionally, noninvasive PET scans of patients with TBI using PK11195 as the marker (which can bind to TSPO, a specific marker of activated microglia) revealed the presence of activated microglia throughout the brain for years after a single TBI, and the PK11195 signal was primarily detected in the bilateral thalamus, putamen, occipital cortices and internal capsules.29,30 Based on these studies, microglia are progressively activated during the chronic stage of TBI, as a stronger PK11195 signal was observed in those patients with a more remote history of trauma (Fig. 1). Moreover, PET studies using DPA-713, another TSPO-binding marker, of former American National Football League players who had suffered repeated, mild TBI also observed a significant increase in the DPA-713-bound signal throughout the brain31,32 (Table 2).
Fig. 1.
Global microglial activation following TBI as detected using PK11195-PET imaging. The mitochondrial 18 kDa translocator protein (TSPO) is expressed at high levels in activated microglia, macrophages, and, to a lesser extent, astrocytes; PK11195, a ligand for TSPO, is labeled with the 11C isotope and has frequently been used as a contrast agent in positron emission tomography (PET) imaging of neuroinflammation both in the clinic and in animal models. The signals are mainly related to microglial activation. PK11195-PET images are merged with T1 magnetic resonance images (MRI) of 10 patients with traumatic brain injury (TBI) who underwent imaging at different time points after injury, as well as a healthy control subject. Numbers below the images captured from subjects with TBI indicate months elapsed from injury to image scanning. Compared to the control subject, the signal for bound PK11195 apparently increased globally in patients with TBI, indicating a global distribution of activated microglia. Images are reproduced from Ramlackhansingh et al.29 with permission
Table 2.
Evidence for disseminated brain inflammation in patients with traumatic brain injury
| Study design | Patients inclusion (n) | Control group (n) | Age | Approaches | Disease phase during detection | Findings |
|---|---|---|---|---|---|---|
| Cross-sectional29 | Moderate to severe TBIa (10) | Healthy controls (22) | 36-55 | PK11195-PET | 11 months to 17 years after injury | Significantly higher PK11195 binding was observed in the thalamus, putamen, occipital cortices, and posterior limb of the internal capsules of survivors of TBI, without hemispheric differences. |
| Prospective30 | Moderate to severe TBIb (8) | Healthy controls (7) | 18-63 | PK11195-PET | 6 months after injury | PK11195 binding was significantly increased throughout the brains of patients with TBI. |
| Cross-sectional28 | Patients with TBI who survived for different times (52) | Uninjured, no documented history of TBI, Alzheimer’s disease or Down syndrome (44) | 9-89 | Autopsy and immunohisto-chemistry | 10 h to 47 years after injury | Acute phase (< 2 weeks): no obvious change compared with controls; sub-acute phase (2 weeks–1 year): focal clusters of activated microglia; long-term phase (>1 year): activated microglia were distributed throughout the corpus callosum. |
| Cross-sectional31 | Former American football players (11) | Healthy controls (9) | 65 ± 5 | DPA-713-PET | 24–42 years since last playing | DPA-713 binding was significantly increased throughout the brains of retired players. |
| Cross-sectional32 | Active and former American football players (14) | Healthy controls (15) | 31 ± 6 | DPA-713-PET | 7 ± 6.4 years after last self-reported concussion | Players showed higher total distribution volumes. |
(n) indicates the sample size. PK11195 and DPA-713 are 11C-labeled translocator protein (TSPO) ligands used for PET imaging of brain inflammation. TSPO is expressed at high levels in activated microglia, it is also expressed in peripheral monocytes and, to a lesser extent, astrocytes. Therefore, the information about inflammation delivered by this signal may be mediated by multiple cells
PET positron emission tomography, TBI traumatic brain injury
aDefined based on the Mayo classification of patients who meet one or more following criteria: (1) death due to this TBI; (2) loss of consciousness of 30 minutes or more; (3) post-traumatic anterograde amnesia for at least 24 h; (4) worst Glasgow Coma Scale full score recorded in the first 24 h <13; and (5) the presence of one or more of the following symptoms: intracerebral hematoma, subdural hematoma, epidural hematoma, cerebral contusion, hemorrhagic contusion, penetrating TBI (dura penetrated), subarachnoid hemorrhage, or brain stem Injury
bDefined according to the score on the Glasgow Coma Scale, 9–13 points represents a moderate injury and ≤ 8 points represents a severe injury
Astrocytes play an important role in CNS homeostasis as a critical component of the barriers between the blood, CSF and meninges.33 Reactive astrogliosis after an injury restricts the damage and inflammatory responses in the injured area by forming a glial scar to protect the unaffected brain tissue.34 However, astrocytes also participate in the inflammatory response after TBI by secreting cytokines/chemokines, recruiting peripheral immune cells, and interacting with microglia.35,36 Similar to microglia, activated astrocytes have also been detected in remote brain regions in the acute and chronic phases of TBI in animal models. While astrogliosis at the lesion site will gradually subside within 1 week after TBI, astrocytes in the remote area are persistently activated and gradually spread throughout the brain.27 Currently, pathology and imaging data from patients supporting global astrocyte activation are still lacking.
Peripheral inflammatory cells
Regarding peripheral inflammatory cells, neutrophils arrive at the site of brain injury within hours of the primary injury, while macrophages, which are derived from circulating monocytes, begin to migrate into the injured brain 1–2 days after TBI.1,2 According to animal studies using flow cytometry, myeloid cells (CD11b+ CD45high) are observed in the contralateral hemisphere after TBI in the acute and chronic stages,27 suggesting the involvement of neutrophils and macrophages in the dissemination of TBI-induced brain inflammation. However, further studies are needed to characterize the spatial and temporal distributions of these two cell types throughout the brain. The role of adaptive immune cells in the pathophysiology of TBI remains poorly understood. Lymphocytes infiltrate the lesion site hours after brain injury and gradually accumulate over several days. To a lesser extent, lymphocytes are also detected in the contralateral hemisphere of a TBI mouse model during the acute phase and persist for more than 1 year after injury.27
Inflammatory mediators
Gene expression profiles also suggest the dissemination of inflammation following TBI.37–40 Changes in the expression of inflammatory genes are detected as early as 24 h after injury and can persist over several months in tissues located both ipsilateral and contralateral to the brain injury. In addition, differential gene expression has been noted between the site of injury and more remote areas.38,40 Furthermore, the expression profile of inflammatory genes shows time-dependent changes, as suggested by a study reporting that a unilateral brain injury induced by mild TBI cause changes in inflammatory gene expression in both the ipsilateral and contralateral hippocampus. These changes occurred in three distinct phases, suggesting a time-dependent activation of inflammatory pathways following TBI. In the early phase, the chemokine ligands CCL2 and CCL7, lipocalin-2, and tissue inhibitor of metalloproteinase were upregulated within 24 h and returned to baseline levels after 1 week. These changes were followed by an intermediate phase (1 week) characterized by the upregulation of immune cell activators C-C chemokine receptor type 5 and low affinity Fc fragment of IgG IIb (Fcgr2b), complement component C3, major histocompatibility complex II immune response-related genes, CD74 and RT1 class II. Finally, a late phase (1 month) occurs that is categorized by increased expression of Kruppel-like factor 4.37
A protein array also identified cytokines/chemokines that are upregulated throughout the brain, beginning a few hours after TBI and persisting for days. The levels of some proteins, such as CXCL1, IL-13, and CCL2,41,42 are increased in both hemispheres, while others exhibit hemispheric differences.42 Outstanding questions are whether these changes persist in the chronic phase, and what are the relationships and differences between the two phases. Additionally, according to histological studies, cyclooxygenase-2, which catalyzes the transformation of arachidonic acid to prostaglandins, is upregulated in the neurons of the bilateral cortex and hippocampus from 3 h to 3 days after injury in a rat TBI model.43 Thus, neurons in remote areas may contribute to the development of inflammation in situ. However, signals mediating the remote stimulation of neurons have not yet been clearly identified, and further work is necessary in this area.
In summary, neuroinflammation has been detected throughout the brain following TBI and becomes most apparent during the chronic phase. Globally distributed activated microglia are one of the most prominent characteristics of disseminated inflammation following TBI.
Potential mechanisms governing the dissemination of inflammation after TBI
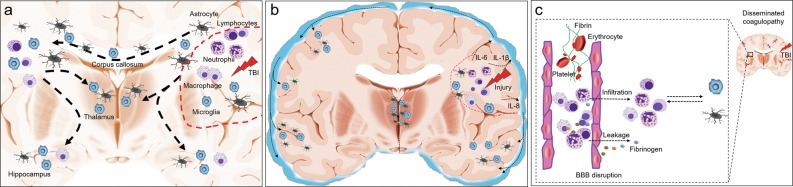
Mechanisms governing the dissemination of TBI-induced brain inflammation remain unclear. However, several possible pathways have been proposed based on recently reported evidence. Remote brain injuries are common in patients who are subjected to repeated impacts, countercoup injury, and diffuse axonal injury, which trigger immune responses in situ; therefore, inflammation can occur in broad areas of the brain. This hypothesis is supported by the finding that activated microglia/astrocytes are often located in large white matter fiber tracts, such as the corpus callosum, which are prone to injury in patients with TBI.28 On the other hand, disseminated brain inflammation may also participate in the damage of remote white matter tracts, causing progressive degeneration of the white matter and brain atrophy28,44,45 (Fig. 2a).
Fig. 2.
Postulated mechanisms that govern the dissemination of brain inflammation after TBI. After the acute primary traumatic brain injury (TBI), immune reactions orchestrated in the injury site are characterized by glial cell activation, the migration of peripheral immune cells, including neutrophils, macrophage, and lymphocytes, as well as the production of many protein products, including cytokines and chemokines. These multiple cellular and protein components induce the formation of focal brain inflammation (a and b: red dashed lines). a TBI commonly induces a remote brain injury, such as diffuse axonal injury, and causes progressive white matter degeneration. Evidence from patients and animal models indicates that globally distributed immune cells, particularly glial cells, are mainly located near or on the white matter, suggesting that brain inflammation is disseminated along the damaged white matter. The disseminated inflammation may further promote white matter degeneration. b Immune mediators are readily released to the cerebrospinal fluid (CSF) through the interstitial fluid, and elevated CSF levels of inflammatory markers, such as IL-6, IL-8, etc., are detected in patients with TBI. Immune cells also enter the CSF, and these cellular and protein components thus disseminate to the central nervous system through the CSF, potentially triggering inflammation in remote areas. c TBI often induces the systemic dysregulation of coagulation characterized by thrombus formation and microbleeding accompanied by BBB disruption. Blood components, including immune cells and proteins such as fibrinogen, are subsequently deposited in the brain parenchyma, orchestrating inflammation. Therefore, inflammation disseminated to the distal areas of the brain after TBI may originate from changes in the blood vessels in the related area
As shown in clinical studies, the levels of several inflammatory cytokines are increased in the cerebrospinal fluid (CSF) from patients with TBI, and the levels of several of these cytokines, including IL-1β, IL-18, and IL-6, are associated with clinical outcomes.46–48 Since the cytokine levels are generally similar between microdialysates (which reflect the extracellular fluid) and CSF,47 these cytokines might be released from the injury site into the CSF and subsequently circulate though CNS to impact remote brain structures and promote the dissemination of brain inflammation (Fig. 2b).
Additionally, CNS inflammation is often preceded by a BBB disruption; inflammation and BBB leakage can exacerbate each other. Furthermore, dysregulated coagulation commonly occurs in patients with TBI and animal models, which can cause micro-thrombosis and microbleeding accompanied by the disruption of microvascular structures.49,50 Microvascular injury has been detected both in acute and chronic stages after TBI,51 and is characterized by microbleeds and the deposition of blood components in the brain parenchyma. These focal microbleeds are related to the magnitude of brain injury.52,53 The areas of these microbleeding sites have been reported to gradually increase and spread to distal anatomical regions, even to the contralateral hemisphere. The sites of microbleeds are accompanied by BBB breakdown, as assessed by serum IgG deposition, microglial/macrophage activation, and astrogliosis.54 White matter demyelination has also been detected in bleeding sites.54 These broadly distributed coagulation and platelet defects may promote the dissemination of inflammation. However, given the close interaction between the immune system and coagulation system,55,56 inflammation may also cause the dysregulation of coagulation (Fig. 2c).
Finally, autoantibodies and autoreactive T cells targeting neurogenic antigens have been detected in the periphery during the acute phase of TBI.71 Autoantibodies can be detected years following initial injury. These autoreactive cells and antibodies may be self-destructive and account for the occurrence of long-term sequelae. According to a recent study, head trauma in adolescence, particularly if it is repeated, is associated with an increased risk of multiple sclerosis.73 We thus speculate that acute brain injury triggers persistent autoimmunity that attacks the brain in a disseminated manner.
The potential clinical relevance of disseminated brain inflammation in TBI
The role of this disseminated immune response to TBI in anatomical regions remote from the focal injury site remains obscure. Some studies suggest that this inflammation may contribute to the dysfunction of certain brain structures during the acute and chronic phases of injury, resulting in neurological complications and long-term degeneration after TBI.
Post-traumatic hyperthermia, a non-infectious elevation in body temperature, is a common complication that occurs in 4–37% of patients with TBI.57,58 Post-traumatic hyperthermia can persist from weeks to months and correlates with negative outcome in several clinical studies of patients with TBI.59–61 Post-traumatic hyperthermia is characterized by the loss of the diurnal temperature variation, is relatively resistant to antipyretic medications, and occurs secondary to hypothalamic thermoregulation center dysfunction.61 In a rat model of severe TBI induced by lateral fluid percussion, the authors reported a significant inflammatory response in the periventricular nucleus of the hypothalamus that was characterized by astrogliosis and the infiltration of microglia/macrophages 1 week after brain injury, suggesting hypothalamic dysfunction.62 However, a detailed timeline of the initiation of hypothalamic inflammation and its causal relationship to post-traumatic hyperthermia remains to be determined.
Hypopituitarism is another common complication occurring in 15–50% of patients with TBI.63 This complication is characterized by decreased serum levels of pituitary hormones, including growth hormone (GH), adrenocorticotropic hormone (ACTH), gonadotropins (FSH and LH), and thyroid-stimulating hormone (TSH), with the growth hormone deficiency being most common finding. An MRI study including 164 patients with TBI revealed a decrease in the apparent diffusion coefficient (ADC) value in diffusion weighted images (DWI) of the pituitary gland as early as 2 weeks after brain injury compared to healthy controls.64 In patients manifesting hypopituitarism, ADC values of the pituitary glands were even lower than in patients without this complication. In a rat model of cortical contusion injury, the authors identified elevated glial fibrillary acidic protein (GFAP) and IL-1β levels in the anterior pituitary 2 months after injury that were associated with reduced serum GH levels, implying that the disseminated and persistent inflammation observed in the pituitary after TBI may contribute to the dysfunction of the HPA axis.65 In a 3-year follow-up study of 29 patients with TBI, serum levels of an anti-pituitary antibody were detected using an indirect immunofluorescence method in 13 (44.8%) patients and were significantly correlated with hypopituitarism,66 further suggesting the involvement of autoimmunity in post-TBI hypopituitarism.
Accumulating evidence suggests a link between TBI and subsequent neurodegenerative diseases, such as Parkinson’s disease and dementia, including Alzheimer’s disease, although the association with Alzheimer’s disease is less certain.5,45,67 In addition, post-mortem studies have revealed a persistent global neurodegeneration in survivors of a single moderate to severe TBI, as well as in patients subjected to repeated mild TBI. This pathology, which has recently spurred significant interest among the scientific community and the lay public, has been termed chronic traumatic encephalopathy (CTE),68–70 which is characterized by perivascular phosphate-tau (pTau) lesions surrounding small vessels.5 Both pathology and imaging studies have shown long-lasting global activation of microglia in patients with TBI,28,29,70 suggesting a role for persistent global inflammation in the development of TBI-induced CTE. In addition, studies of Alzheimer’s disease mouse models have shown that reactive microglia promote the appearance and spread of pTau.71,72 The observation may help explain the link between microglial activation and CTE in patients with TBI. Furthermore, MRI studies of rodent models of TBI have identified an increase in tissue water diffusion and blood–brain barrier permeability in the hippocampus both ipsilateral and contralateral to the injury, with a delayed initiation and attenuated magnitude observed in the ipsilateral hippocampus compared to the contralateral side.73 Moreover, emerging evidence suggests that structural changes in ipsilateral and contralateral hippocampal CA1 neurons, which are manifested as a decrease in dendrite spine density, can persist for 1.5 years after brain injury in mice.27 These data support an ongoing process of cell death and structural changes in the bilateral hippocampus after TBI, which may result from persistent brain inflammation in these regions.
Notably, the mouse spinal cord has been shown to develop neuronal edema accompanied by astrogliosis, microglia/macrophage accumulation, and oxidative damage as early as 24 h after acute brain injury.74 These findings might partially explain the increased risk of developing motor neuron disease in patients with TBI.75–77 Additionally, based on data derived from experimental studies, spinal cord injury (SCI) induces a broad inflammatory response in the thalamus, hippocampus and cerebral cortex that is associated with the development of pain and cognitive decline after SCI.78 Furthermore, the secondary impacts of an injury to the brain or spinal cord disseminate throughout the CNS, and inflammation might play a role in this process.
Potential therapeutic impacts of the modulation of disseminated inflammation following TBI
Researchers have not determined whether strategies targeting the disseminated brain inflammation benefit patients with TBI. Several studies have recently suggested that both pharmacological antagonism and a genetic deficiency in CCR2 reduce macrophage infiltration into the hippocampus and ameliorate the inflammatory environment, leading to improved cognitive function in murine modes of TBI.15,17,79 These data support the hypothesis that distal inflammation contributes to hippocampal dysfunction and cognitive decline following TBI, serving as a therapeutic target to improve patient outcomes. According to another recent study, a single allele deletion of the chemokine receptor CX3CR1 limits the infiltration of peripheral immune cells and substantially prevents chronic degeneration of the injured brain. Interestingly, these changes improve long-term functional recovery in female, but not male, mice after TBI,27 supporting a sex-related difference in this mechanism. The inhibition of peripheral immune cell infiltration might protect the brain from chronic degeneration, which may result from the inhibition of GBI.
Currently, only limited evidence supports a role for lymphocytes in acute brain injury following TBI. One study using recombination-activating gene 1 (RAG-1)-deficient mice (lacking T and B cells) subjected to a closed skull model of head injury reported no differences in injury severity or neurological deficits between wild-type and RAG-1−/− mice.80 In another study, the inhibition of lymphocyte egress with FTY720 failed to reduce the lesion size and improve neurological outcomes in two different mouse models of TBI.81 Thus, lymphocytes may play a minor role in driving the expansion of the acute brain lesion in subjects with TBI. However, further investigations are required to determine whether globally distributed lymphocytes play a role in the functional recovery from TBI, particularly in the chronic phase, and whether suppression of acute lymphocyte migration impacts long-term brain function.
Conclusions and future perspectives
TBI in patients and animals triggers a focal immune response that is initiated within minutes after the primary injury. This focal brain inflammation involves both brain-resident immune cells and peripheral immune cells with complicated interactions. In addition to this focal inflammatory state, the evidence we reviewed here suggests that post-traumatic brain inflammation disseminates globally both in the acute and chronic stages of injury, even throughout life. The disseminated brain inflammation may persistently impact brain pathophysiology and is associated with progressive neurodegeneration. However, a substantial knowledge gap in our understanding of the biological features of the disseminated inflammation still exists.
In future studies, a panoramic picture of the temporal and spatial development of brain inflammation after TBI is essential. In addition, these features should be compared between subjects with different disease severities and primary injury locations, based on the heterogeneity of TBI. The governing mechanisms and many details of this disseminated brain inflammation remain unclear. The events or factors other than acute diffuse brain injury that trigger the initiation and maintenance of disseminated inflammation must be identified to obtain a better understanding of the underlying mechanisms. More importantly, a detailed identification and profile of the immune participants in the disseminated brain inflammation may promote the discovery of new therapeutic targets. In this regard, the use of one or several markers that identify the cell types and functional status is not sufficiently precise. More powerful and precise approaches, including single cell RNA sequencing82 and two-photon live imaging,83,84 should be employed in future studies. For example, the roles of peripheral monocytes and brain-intrinsic myeloid cells, which include microglia, meningeal macrophages, perivascular macrophages, and choroid plexus macrophages, are not clearly defined and have often been mixed with each other in previous studies.85,86 Studies taking advantage of these new approaches will be helpful to clarify these questions. Finally, evidence from patients with neurodegenerative diseases, such as Alzheimer’s disease and Parkinson’s disease, suggests that systemic inflammation might shape the brain pathology and promote degeneration.87,88 Hence, investigations of the roles of systemic inflammation in post-TBI brain inflammation will be interesting and worthwhile to improve monitoring and interventions.
Acknowledgements
Work in the authors’ laboratories was supported in part by grants from the National Science Foundation of China (Grant numbers 81720108015, 91642205, and 81830038), National Key Research and Development Program of China (2018YFC1312200), Tianjin Municipal Science and Technology Commission (15ZXLCSY00060), funds from the Advanced Innovation Center for Human Brain Protection, Capital Medical University, Beijing, China, and Barrow Neurological Foundation. We thank Elaine Shi for providing editorial assistance.
Author contributions
F.-D.S., J.Z. and J.F.D. formulated the concept, K.S. performed the literature search. All authors contributed to drafting the manuscript.
Competing interest
The authors declare that there is no conflict of interest.
References
- 1.Corps KN, Roth TL, McGavern DB. Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol. 2015;72:355–362. doi: 10.1001/jamaneurol.2014.3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jassam YN, Izzy S, Whalen M, McGavern DB, El Khoury J. Neuroimmunology of traumatic brain injury: time for a paradigm shift. Neuron. 2017;95:1246–1265. doi: 10.1016/j.neuron.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD 2016 Traumatic Brain Injury and Spinal Cord Injury Collaborators. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 18, 56–87 (2019). [DOI] [PMC free article] [PubMed]
- 4.Stocchetti N, et al. Severe traumatic brain injury: targeted management in the intensive care unit. Lancet Neurol. 2017;16:452–464. doi: 10.1016/S1474-4422(17)30118-7. [DOI] [PubMed] [Google Scholar]
- 5.Wilson L, et al. The chronic and evolving neurological consequences of traumatic brain injury. Lancet Neurol. 2017;16:813–825. doi: 10.1016/S1474-4422(17)30279-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blennow K, et al. Traumatic brain injuries. Nat. Rev. Dis. Prim. 2016;2:16084. doi: 10.1038/nrdp.2016.84. [DOI] [PubMed] [Google Scholar]
- 7.Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7:728–741. doi: 10.1016/S1474-4422(08)70164-9. [DOI] [PubMed] [Google Scholar]
- 8.Lingsma HF, Roozenbeek B, Steyerberg EW, Murray GD, Maas AI. Early prognosis in traumatic brain injury: from prophecies to predictions. Lancet Neurol. 2010;9:543–554. doi: 10.1016/S1474-4422(10)70065-X. [DOI] [PubMed] [Google Scholar]
- 9.Chandran R, et al. A combination antioxidant therapy to inhibit NOX2 and activate Nrf2 decreases secondary brain damage and improves functional recovery after traumatic brain injury. J. Cereb. Blood. Flow. Metab. 2018;38:1818–1827. doi: 10.1177/0271678X17738701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hinzman JM, Wilson JA, Mazzeo AT, Bullock MR, Hartings JA. Excitotoxicity and metabolic crisis are associated with spreading depolarizations in severe traumatic brain injury patients. J. Neurotrauma. 2016;33:1775–1783. doi: 10.1089/neu.2015.4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russo MV, McGavern DB. Inflammatory neuroprotection following traumatic brain injury. Science. 2016;353:783–785. doi: 10.1126/science.aaf6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roth TL, et al. Transcranial amelioration of inflammation and cell death after brain injury. Nature. 2014;505:223–228. doi: 10.1038/nature12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Y, et al. ST2/IL-33-dependent microglial response limits acute ischemic brain injury. J. Neurosci. 2017;37:4692–4704. doi: 10.1523/JNEUROSCI.3233-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liesz A, et al. DAMP signaling is a key pathway inducing immune modulation after brain injury. J. Neurosci. 2015;35:583–598. doi: 10.1523/JNEUROSCI.2439-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsieh CL, et al. CCR2 deficiency impairs macrophage infiltration and improves cognitive function after traumatic brain injury. J. Neurotrauma. 2014;31:1677–1688. doi: 10.1089/neu.2013.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clausen F, Lorant T, Lewen A, Hillered L. T lymphocyte trafficking: a novel target for neuroprotection in traumatic brain injury. J. Neurotrauma. 2007;24:1295–1307. doi: 10.1089/neu.2006.0258. [DOI] [PubMed] [Google Scholar]
- 17.Morganti JM, et al. CCR2 antagonism alters brain macrophage polarization and ameliorates cognitive dysfunction induced by traumatic brain injury. J. Neurosci. 2015;35:748–760. doi: 10.1523/JNEUROSCI.2405-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen X, et al. Omega-3 polyunsaturated fatty acid supplementation attenuates microglial-induced inflammation by inhibiting the HMGB1/TLR4/NF-kappaB pathway following experimental traumatic brain injury. J. Neuroinflamm. 2017;14:143. doi: 10.1186/s12974-017-0917-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chio CC, et al. Exercise attenuates neurological deficits by stimulating a critical HSP70/NF-κB/IL-6/synapsin I axis in traumatic brain injury rats. J. Neuroinflamm. 2017;14:90. doi: 10.1186/s12974-017-0867-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niemela J, et al. IFN-β regulates CD73 and adenosine expression at the blood-brain barrier. Eur. J. Immunol. 2008;38:2718–2726. doi: 10.1002/eji.200838437. [DOI] [PubMed] [Google Scholar]
- 21.Cekic C, Linden J. Purinergic regulation of the immune system. Nat. Rev. Immunol. 2016;16:177–192. doi: 10.1038/nri.2016.4. [DOI] [PubMed] [Google Scholar]
- 22.Szmydynger-Chodobska J, Strazielle N, Zink BJ, Ghersi-Egea JF, Chodobski A. The role of the choroid plexus in neutrophil invasion after traumatic brain injury. J. Cereb. Blood. Flow. Metab. 2009;29:1503–1516. doi: 10.1038/jcbfm.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Semple BD, Bye N, Rancan M, Ziebell JM, Morganti-Kossmann MC. Role of CCL2 (MCP-1) in traumatic brain injury (TBI): evidence from severe TBI patients and CCL2-/- mice. J. Cereb. Blood. Flow. Metab. 2010;30:769–782. doi: 10.1038/jcbfm.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Homsi S, et al. Blockade of acute microglial activation by minocycline promotes neuroprotection and reduces locomotor hyperactivity after closed head injury in mice: a twelve-week follow-up study. J. Neurotrauma. 2010;27:911–921. doi: 10.1089/neu.2009.1223. [DOI] [PubMed] [Google Scholar]
- 25.Dohi K, et al. Gp91phox (NOX2) in classically activated microglia exacerbates traumatic brain injury. J. Neuroinflamm. 2010;7:41. doi: 10.1186/1742-2094-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edwards P, et al. Final results of MRC CRASH, a randomised placebo-controlled trial of intravenous corticosteroid in adults with head injury-outcomes at 6 months. Lancet. 2005;365:1957–1959. doi: 10.1016/S0140-6736(05)71124-7. [DOI] [PubMed] [Google Scholar]
- 27.Erturk A, et al. Interfering with the chronic immune response rescues chronic degeneration after traumatic brain injury. J. Neurosci. 2016;36:9962–9975. doi: 10.1523/JNEUROSCI.1898-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson VE, et al. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain. 2013;136:28–42. doi: 10.1093/brain/aws322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramlackhansingh AF, et al. Inflammation after trauma: microglial activation and traumatic brain injury. Ann. Neurol. 2011;70:374–383. doi: 10.1002/ana.22455. [DOI] [PubMed] [Google Scholar]
- 30.Folkersma H, et al. Widespread and prolonged increase in (R)-(11)C-PK11195 binding after traumatic brain injury. J. Nucl. Med. 2011;52:1235–1239. doi: 10.2967/jnumed.110.084061. [DOI] [PubMed] [Google Scholar]
- 31.Coughlin JM, et al. Neuroinflammation and brain atrophy in former NFL players: An in vivo multimodal imaging pilot study. Neurobiol. Dis. 2015;74:58–65. doi: 10.1016/j.nbd.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coughlin JM, et al. Imaging of glial cell activation and white matter integrity in brains of active and recently retired national football league players. JAMA Neurol. 2017;74:67–74. doi: 10.1001/jamaneurol.2016.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liddelow SA, Barres BA. Reactive astrocytes: production, function, and therapeutic potential. Immunity. 2017;46:957–967. doi: 10.1016/j.immuni.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 34.Burda JE, Bernstein AM, Sofroniew MV. Astrocyte roles in traumatic brain injury. Exp. Neurol. 2016;275(Pt 3):305–315. doi: 10.1016/j.expneurol.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao TL, et al. Expression of HMGB1 and RAGE in rat and human brains after traumatic brain injury. J. Trauma Acute Care Surg. 2012;72:643–649. doi: 10.1097/TA.0b013e31823c54a6. [DOI] [PubMed] [Google Scholar]
- 36.Pan H, Wang H, Wang X, Zhu L, Mao L. The absence of Nrf2 enhances NF-kappaB-dependent inflammation following scratch injury in mouse primary cultured astrocytes. Mediat. Inflamm. 2012;2012:217580. doi: 10.1155/2012/217580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Almeida-Suhett CP, Li Z, Marini AM, Braga MF, Eiden LE. Temporal course of changes in gene expression suggests a cytokine-related mechanism for long-term hippocampal alteration after controlled cortical impact. J. Neurotrauma. 2014;31:683–690. doi: 10.1089/neu.2013.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.White TE, et al. Gene expression patterns following unilateral traumatic brain injury reveals a local pro-inflammatory and remote anti-inflammatory response. BMC Genom. 2013;14:282. doi: 10.1186/1471-2164-14-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frieler RA, et al. Depletion of macrophages in CD11b diphtheria toxin receptor mice induces brain inflammation and enhances inflammatory signaling during traumatic brain injury. Brain Res. 2015;1624:103–112. doi: 10.1016/j.brainres.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lagraoui M, et al. Controlled cortical impact and craniotomy induce strikingly similar profiles of inflammatory gene expression, but with distinct kinetics. Front. Neurol. 2012;3:155. doi: 10.3389/fneur.2012.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dalgard CL, et al. The cytokine temporal profile in rat cortex after controlled cortical impact. Front. Mol. Neurosci. 2012;5:6. doi: 10.3389/fnmol.2012.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Niesman IR, et al. Traumatic brain injury enhances neuroinflammation and lesion volume in caveolin deficient mice. J. Neuroinflamm. 2014;11:39. doi: 10.1186/1742-2094-11-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dash PK, Mach SA, Moore AN. Regional expression and role of cyclooxygenase-2 following experimental traumatic brain injury. J. Neurotrauma. 2000;17:69–81. doi: 10.1089/neu.2000.17.69. [DOI] [PubMed] [Google Scholar]
- 44.Chen XH, Johnson VE, Uryu K, Trojanowski JQ, Smith DH. A lack of amyloid beta plaques despite persistent accumulation of amyloid beta in axons of long-term survivors of traumatic brain injury. Brain. Pathol. 2009;19:214–223. doi: 10.1111/j.1750-3639.2008.00176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crane PK, et al. Association of traumatic brain injury with late-life neurodegenerative conditions and neuropathologic findings. JAMA Neurol. 2016;73:1062–1069. doi: 10.1001/jamaneurol.2016.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kumar RG, et al. Acute CSF interleukin-6 trajectories after TBI: associations with neuroinflammation, polytrauma, and outcome. Brain Behav. Immun. 2015;45:253–262. doi: 10.1016/j.bbi.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 47.Roberts DJ, et al. Association between the cerebral inflammatory and matrix metalloproteinase responses after severe traumatic brain injury in humans. J. Neurotrauma. 2013;30:1727–1736. doi: 10.1089/neu.2012.2842. [DOI] [PubMed] [Google Scholar]
- 48.Adamczak S, et al. Inflammasome proteins in cerebrospinal fluid of brain-injured patients as biomarkers of functional outcome: clinical article. J. Neurosurg. 2012;117:1119–1125. doi: 10.3171/2012.9.JNS12815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang J, et al. Traumatic brain injury-associated coagulopathy. J. Neurotrauma. 2012;29:2597–2605. doi: 10.1089/neu.2012.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tian Y, et al. Brain-derived microparticles induce systemic coagulation in a murine model of traumatic brain injury. Blood. 2015;125:2151–2159. doi: 10.1182/blood-2014-09-598805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shlosberg D, Benifla M, Kaufer D, Friedman A. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat. Rev. Neurol. 2010;6:393–403. doi: 10.1038/nrneurol.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scheid R, Preul C, Gruber O, Wiggins C, von Cramon DY. Diffuse axonal injury associated with chronic traumatic brain injury: evidence from T2*-weighted gradient-echo imaging at 3 T. AJNR Am. J. Neuroradiol. 2003;24:1049–1056. [PMC free article] [PubMed] [Google Scholar]
- 53.Iwamura A, et al. Diffuse vascular injury: convergent-type hemorrhage in the supratentorial white matter on susceptibility-weighted image in cases of severe traumatic brain damage. Neuroradiology. 2012;54:335–343. doi: 10.1007/s00234-011-0892-9. [DOI] [PubMed] [Google Scholar]
- 54.Glushakova OY, Johnson D, Hayes RL. Delayed increases in microvascular pathology after experimental traumatic brain injury are associated with prolonged inflammation, blood-brain barrier disruption, and progressive white matter damage. J. Neurotrauma. 2014;31:1180–1193. doi: 10.1089/neu.2013.3080. [DOI] [PubMed] [Google Scholar]
- 55.Massberg S, et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat. Med. 2010;16:887–896. doi: 10.1038/nm.2184. [DOI] [PubMed] [Google Scholar]
- 56.Swystun LL, Liaw PC. The role of leukocytes in thrombosis. Blood. 2016;128:753–762. doi: 10.1182/blood-2016-05-718114. [DOI] [PubMed] [Google Scholar]
- 57.Thompson HJ, Pinto-Martin J, Bullock MR. Neurogenic fever after traumatic brain injury: an epidemiological study. J. Neurol. Neurosurg. Psychiatry. 2003;74:614–619. doi: 10.1136/jnnp.74.5.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jiang JY, Gao GY, Li WP, Yu MK, Zhu C. Early indicators of prognosis in 846 cases of severe traumatic brain injury. J. Neurotrauma. 2002;19:869–874. doi: 10.1089/08977150260190456. [DOI] [PubMed] [Google Scholar]
- 59.Behr R, Erlingspiel D, Becker A. Early and longtime modifications of temperature regulation after severe head injury. Prognostic implications. Ann. N. Y. Acad. Sci. 1997;813:722–732. doi: 10.1111/j.1749-6632.1997.tb51774.x. [DOI] [PubMed] [Google Scholar]
- 60.Heindl UT, Laub MC. Outcome of persistent vegetative state following hypoxic or traumatic brain injury in children and adolescents. Neuropediatrics. 1996;27:94–100. doi: 10.1055/s-2007-973756. [DOI] [PubMed] [Google Scholar]
- 61.Sazbon L, Groswasser Z. Outcome in 134 patients with prolonged posttraumatic unawareness. Part 1: Parameters determining late recovery of consciousness. J. Neurosurg. 1990;72:75–80. doi: 10.3171/jns.1990.72.1.0075. [DOI] [PubMed] [Google Scholar]
- 62.Thompson HJ, Hoover RC, Tkacs NC, Saatman KE, McIntosh TK. Development of posttraumatic hyperthermia after traumatic brain injury in rats is associated with increased periventricular inflammation. J. Cereb. Blood. Flow. Metab. 2005;25:163–176. doi: 10.1038/sj.jcbfm.9600008. [DOI] [PubMed] [Google Scholar]
- 63.Tanriverdi F, et al. Pituitary dysfunction after traumatic brain injury: a clinical and pathophysiological approach. Endocr. Rev. 2015;36:305–342. doi: 10.1210/er.2014-1065. [DOI] [PubMed] [Google Scholar]
- 64.Zheng P, He B, Guo Y, Zeng J, Tong W. Decreased apparent diffusion coefficient in the pituitary and correlation with hypopituitarism in patients with traumatic brain injury. J. Neurosurg. 2015;123:75–80. doi: 10.3171/2014.12.JNS132308. [DOI] [PubMed] [Google Scholar]
- 65.Kasturi BS, Stein DG. Traumatic brain injury causes long-term reduction in serum growth hormone and persistent astrocytosis in the cortico-hypothalamo-pituitary axis of adult male rats. J. Neurotrauma. 2009;26:1315–1324. doi: 10.1089/neu.2008.0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tanriverdi F, et al. Antipituitary antibodies after traumatic brain injury: is head trauma-induced pituitary dysfunction associated with autoimmunity? Eur. J. Endocrinol. 2008;159:7–13. doi: 10.1530/EJE-08-0050. [DOI] [PubMed] [Google Scholar]
- 67.Stocchetti N, Zanier ER. Chronic impact of traumatic brain injury on outcome and quality of life: a narrative review. Crit. Care. 2016;20:148. doi: 10.1186/s13054-016-1318-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McKee AC, Daneshvar DH, Alvarez VE, Stein TD. The neuropathology of sport. Acta Neuropathol. 2014;127:29–51. doi: 10.1007/s00401-013-1230-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hayes JP, et al. Mild traumatic brain injury is associated with reduced cortical thickness in those at risk for Alzheimer’s disease. Brain. 2017;140:813–825. doi: 10.1093/brain/aww344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cherry JD, et al. Microglial neuroinflammation contributes to tau accumulation in chronic traumatic encephalopathy. Acta Neuropathol. Commun. 2016;4:112. doi: 10.1186/s40478-016-0382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ghosh S, et al. Sustained interleukin-1β overexpression exacerbates tau pathology despite reduced amyloid burden in an Alzheimer’s mouse model. J. Neurosci. 2013;33:5053–5064. doi: 10.1523/JNEUROSCI.4361-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maphis N, et al. Reactive microglia drive tau pathology and contribute to the spreading of pathological tau in the brain. Brain. 2015;138:1738–1755. doi: 10.1093/brain/awv081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Immonen RJ, et al. Distinct MRI pattern in lesional and perilesional area after traumatic brain injury in rat—11 months follow-up. Exp. Neurol. 2009;215:29–40. doi: 10.1016/j.expneurol.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 74.Evans TM, et al. The effect of mild traumatic brain injury on peripheral nervous system pathology in wild-type mice and the G93A mutant mouse model of motor neuron disease. Neuroscience. 2015;298:410–423. doi: 10.1016/j.neuroscience.2015.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schmidt S, et al. Genes and environmental exposures in veterans with amyotrophic lateral sclerosis: the GENEVA study. Rationale, study design and demographic characteristics. Neuroepidemiology. 2008;30:191–204. doi: 10.1159/000126911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schmidt S, Kwee LC, Allen KD, Oddone EZ. Association of ALS with head injury, cigarette smoking and APOE genotypes. J. Neurol. Sci. 2010;291:22–29. doi: 10.1016/j.jns.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lehman EJ, Hein MJ, Baron SL, Gersic CM. Neurodegenerative causes of death among retired National Football League players. Neurology. 2012;79:1970–1974. doi: 10.1212/WNL.0b013e31826daf50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu J, et al. Spinal cord injury causes brain inflammation associated with cognitive and affective changes: role of cell cycle pathways. J. Neurosci. 2014;34:10989–11006. doi: 10.1523/JNEUROSCI.5110-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gyoneva S, Ransohoff RM. Inflammatory reaction after traumatic brain injury: therapeutic potential of targeting cell-cell communication by chemokines. Trends Pharmacol. Sci. 2015;36:471–480. doi: 10.1016/j.tips.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weckbach S, et al. Challenging the role of adaptive im munity in neurotrauma: Rag1-/- mice lacking mature B and T cells do not show neuroprotection after closed head injury. J. Neurotrauma. 2012;29:1233–1242. doi: 10.1089/neu.2011.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mencl S, et al. FTY720 does not protect from traumatic brain injury in mice despite reducing posttraumatic inflammation. J. Neuroimmunol. 2014;274:125–131. doi: 10.1016/j.jneuroim.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 82.Cuevas-Diaz Duran R, Wei H, Wu JQ. Single-cell RNA-sequencing of the brain. Clin. Transl. Med. 2017;6:20. doi: 10.1186/s40169-017-0150-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Neumann J, et al. Very-late-antigen-4 (VLA-4)-mediated brain invasion by neutrophils leads to interactions with microglia, increased ischemic injury and impaired behavior in experimental stroke. Acta Neuropathol. 2015;129:259–277. doi: 10.1007/s00401-014-1355-2. [DOI] [PubMed] [Google Scholar]
- 84.Odoardi F, et al. T cells become licensed in the lung to enter the central nervous system. Nature. 2012;488:675–679. doi: 10.1038/nature11337. [DOI] [PubMed] [Google Scholar]
- 85.Herz J, Filiano AJ, Smith A, Yogev N, Kipnis J. Myeloid cells in the central nervous system. Immunity. 2017;46:943–956. doi: 10.1016/j.immuni.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Prinz M, Priller J. The role of peripheral immune cells in the CNS in steady state and disease. Nat. Neurosci. 2017;20:136–144. doi: 10.1038/nn.4475. [DOI] [PubMed] [Google Scholar]
- 87.Peter I, et al. Anti-tumor necrosis factor therapy and incidence of Parkinson disease among patients with inflammatory bowel disease. JAMA Neurol. 2018;75:939–946. doi: 10.1001/jamaneurol.2018.0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Heneka MT, Golenbock DT, Latz E. Innate immunity in Alzheimer’s disease. Nat. Immunol. 2015;16:229–236. doi: 10.1038/ni.3102. [DOI] [PubMed] [Google Scholar]