Abstract
OBJECTIVE
African Americans (AA) suffer disproportionately from diabetic nephropathy (DN). C-reactive protein (CRP) has been associated with prevalent DN, but its association with incident DN in AA is unknown. We examined hs-CRP and incident DN in AA.
RESEARCH DESIGN AND METHODS
We conducted a longitudinal analysis of data from exams 1, 2, and 3 in 4,043 eligible Jackson Heart Study (JHS) participants. Participants with DN or without hs-CRP at exam 1 were excluded. Incident DN was defined as urinary albumin-to-creatinine ratio (ACR) >30 mg/g or self-reported dialysis/transplantation and type 2 diabetes mellitus (DM) or HbA1c >6.5% by exam 2 or 3 among participants free of DN at exam 1. Kaplan-Meier curves examined DN event-free survival probability by hs-CRP. With Cox proportional hazards regression we estimated hazard ratios (HRs) and 95% CI for DN by hs-CRP tertiles, adjusting for demographics and clinical and laboratory data.
RESULTS
During 7.8 years of median follow-up time, participants who developed DN had significantly higher baseline hs-CRP, age, fasting glucose, triglycerides, ACR, systolic blood pressure, waist circumference, and duration of DM (P < 0.05). The overall incident rate of DN was 7.9%. The mean time to incident DN was shorter for participants with hs-CRP in the high tertile (>4.24 mg/L) than in the low tertile (<1.46 mg/L); P < 0.001. Participants with high hs-CRP had higher incidence of DN (HR 2.34, 95% CI 1.04–5.24) versus the reference group.
CONCLUSIONS
Inflammation, as measured by hs-CRP levels, may be associated with incident DN in AA. Further studies are warranted to replicate and elucidate the basis for this association.
Introduction
The prevalence of diabetic nephropathy (DN) and end-stage kidney disease (ESKD) among individuals with type 2 diabetes mellitus (DM) has been rising (1). Racial/ethnic minority populations are disproportionately affected by DM and its complications, particularly DN and end-stage kidney disease (2–5). The reasons behind these disparities have not been fully elucidated but may involve a complex interplay of social, cultural, and environmental factors juxtaposed with their impact on gene expression (epigenetics), differing frequencies of select gene polymorphisms, and other biologic pathways (6–10). Among the many biologic mechanisms underlying DN, inflammation in the kidney by mononuclear phagocytic lineage cells (9–11) and the impact of growth factors and proinflammatory cytokines, such as IL-1, IL-6, IL-18, and TNF-α, and other chemokines may have direct or indirect contributions to the pathogenesis of DN (12,13). Increased expression of these cytokines has been reported in both patients with DN (14,15) and experimental animal models of DN (16). In a cross-sectional analysis of National Health and Nutrition Examination Survey (NHANES) data (1999–2008), we recently reported that an important clinical marker of chronic inflammation, C-reactive protein (CRP), and urinary albumin excretion in persons with prevalent DM varied by race/ethnicity (17). The levels of CRP and urinary albumin excretion both were significantly higher in African American (AA) and Hispanic individuals compared with whites, suggesting that the relationship of inflammatory processes and DN may vary by race/ethnicity (17). A cross-sectional analysis of the Jackson Heart Study (JHS) found an association between CRP and both reduced estimated glomerular filtration rate (eGFR) and microalbuminuria, but only the relationship of CRP with reduced eGFR persisted after multiple demographic and clinical variables were controlled for (18).
The JHS is a community-based longitudinal observational study that began in 1998. It was designed to investigate the risk factors for cardiovascular and related diseases (CVD) in AA (19). Thus, taking advantage of the longitudinal JHS design, we posited that higher serum levels of hs-CRP (which detects small elevations in CRP) would predict incident DN and provide more robust evidence for hs-CRP as a risk factor for the development of DN.
Research Design and Methods
Study Population
The JHS recruited 5,306 persons self-reported as AA or black adults aged 21–84 years at baseline, between 2000 and 2004 (exam 1), from the tricounty area (Hinds, Rankin, and Madison Counties) that comprises the city of Jackson, MS. The JHS exam 2 data were collected between 2005 and 2008, and exam 3 was conducted between 2009 and 2013. For the current study, we excluded participants with DN or without information on hs-CRP at exam 1 to assess the association between hs-CRP and incident cases of DN. After these exclusions, 4,043 participants were eligible for the study (Supplementary Fig. 1).
Definition and Ascertainment of DN
Since the data on eGFR were not available at exam 2, we could not use eGFR in ascertaining DN at exam 2. However, the total number of participants with DN did not differ significantly regarding the presence or absence of eGFR in the analysis. The diagnosis of DN was made in 323 patients without using eGFR and 334 patients (an increase of only 3%) when eGFR was used in the diagnosis of DN. We thus captured 97% of new DN cases without the use of eGFR by study end. Therefore, we defined DN by the presence of albuminuria or self-report of dialysis/kidney transplant with a clinical diagnosis of DM and/or HbA1c >6.5%.
To measure incident DN, we excluded those patients who had DN at baseline. Across exams 2 and 3, there were 14 participants who reported receiving dialysis/kidney transplantation but did not have urine albumin-to-creatinine ratio (ACR) >30 mg/g. Albuminuria was defined as an ACR >30 mg/g on a timed 24-h urine collection and a random spot morning urine collection (20). eGFR was assessed using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (21). DM was defined based on the American Diabetes Association guidelines as fasting glucose ≥126 mg/dL and/or use of insulin or antidiabetes medication (actual or self-reported) within 2 weeks prior to the clinic visit and/or HbA1c ≥6.5% (22).
hs-CRP Measurement
hs-CRP was measured at all three exams. Measurement of hs-CRP was performed by the immunoturbidimetric CRP-Latex assay from Kamiya Biomedical Company following the manufacturer’s protocol. All samples were run in duplicate, and any duplicates that were not within a three-assay SD from one another were rerun. The interassay coefficients of variation on control samples were 4.5% and 4.4% at hs-CRP concentrations of 0.45 mg/L and 1.56 mg/L, respectively. Approximately 6% of samples were measured as masked replicates on different dates to assess repeatability of measurements of hs-CRP. The reliability coefficient for masked quality control replicates was 0.95 for the hs-CRP assay. For the current study, tertiles of hs-CRP were used in the analysis, measured at baseline: <1.46 mg/L (reference), 1.46–4.24 mg/L, and >4.24 mg/L.
Covariates
BMI was calculated as the ratio of body weight (kg) and height (m2). Obesity was defined as BMI ≥30 kg/m2. Systolic and diastolic blood pressures (BPs) were taken in the sitting position by trained technicians using a random-zero sphygmomanometer after a 5-min rest. We used an average of the second and third readings. History of CVD was defined as a history of physician-diagnosed stroke, heart attack, acute myocardial infarction, or coronary artery disease. Roche enzymatic methods using a Cobras centrifuge analyzer (Hoffman-La Roche) were used to assess fasting serum total cholesterol, HDL cholesterol, LDL cholesterol, and triglyceride concentrations with the laboratory certified by the Centers for Disease Control and Prevention/National Heart, Lung, and Blood Institute Lipid Standardization Program. Lipid profile was measured under standard laboratory conditions. Smoking status was defined as current smoker, former smoker, and never smoker. Alcohol drinking was defined as regular drinking in the past 12 months (yes/no). Abdominal obesity was defined by waist circumference (WC) >102 cm in men and WC >88 cm in women. Physical activity was categorized as poor, intermediate, and ideal. Ideal physical activity was defined as at least 150 min per week of moderate-intensity aerobic physical activity, 75 min per week of vigorous-intensity aerobic physical activity, or at least 150 min per week of an equivalent combination of moderate- and vigorous-intensity aerobic activity. Intermediate physical activity was defined as <150 min per week of moderate-intensity aerobic physical activity, or <75 min per week of vigorous-intensity aerobic physical activity or <150 min per week of an equivalent combination of moderate- and vigorous-intensity aerobic activity. Poor physical activity was defined as 0 min per week of any physical activity.
Statistical Analysis
Study participants who were free from DN at baseline were divided into three groups according to age- and sex-specific hs-CRP tertiles. We compared the baseline characteristics between those who did not develop DN and those who did develop DN (case subjects) performing t test for continuous variables and χ2 test for categorical variables (education, physical activity, smoking status, and BMI). We constructed Kaplan-Meier curves for event (DN)-free survival time per each hs-CRP category. To assess the association between hs-CRP and the incidence of DN in the multivariable model, we estimated hazard ratios (HRs) using Cox proportional hazards (PH) regression models. We estimated crude and age-adjusted HRs. For further assessment of the independent contribution of serum hs-CRP at baseline to the risk of DN, HRs and their 95% CIs were estimated using the PH model adjusting for potential confounding factors such as age, sex, fasting glucose, total cholesterol, HDL, LDL, triglycerides, systolic and diastolic BP, antihypertensive medication, CVD, years of DM, smoking, alcohol assumption, physical activity, education, BMI, and WC. ACR was not significant in the multiple Cox PH model, and therefore the model was not adjusted for baseline ACR. Subgroup differences in the association (i.e., moderating effect) between hs-CRP and incident DN (yes/no) were determined by testing for statistical interaction, with particular attention to the subgroup free of DM at baseline (Supplementary Table 1). All statistical analyses were performed using SAS statistical software (SAS Institute, Cary, NC).
Results
During a median follow-up of 7.8 years (interquartile range 7.2–8.3) over the three JHS examinations, we identified 705 cases of DM and 323 incident cases of DN, which included 232 (71.8%) subjects with DM at baseline and 91 subjects without DM at baseline. Compared with participants who did not develop DN (no-DN) during follow-up, those who developed DN (i.e., incident cases) had significantly higher hs-CRP, age, fasting glucose, triglycerides, ACR, systolic BP, WC, and duration of DM and lower HDL cholesterol, eGFR, and physical activity at baseline. In addition, participants with incident DN were more likely to have a history of CVD, be less educated, consume alcohol, and be former cigarette smokers and were more likely to be overweight/obese at baseline. There was no difference in sex, total cholesterol, LDL cholesterol, or diastolic BP (Table 1).
Table 1.
Baseline characteristics of study population stratified by subjects who subsequently developed DN and those who did not
| Characteristics | No-DN (N = 3,720) | DN case subjects (N = 323) | P** |
|---|---|---|---|
| hs-CRP (mg/L)* | 2.36 ± 3.40 | 3.36 ± 3.30 | <0.0001 |
| Age (years) | 53.4 ± 12.3 | 59.1 ± 10.4 | <0.0001 |
| Females | 2,392 (64.3) | 208 (64.4) | 0.5124 |
| Fasting glucose (mg/dL) | 94.61 ± 21.7 | 134.1 ± 60.5 | <0.0001 |
| Total cholesterol (mg/dL) | 199.2 ± 38.9 | 198.7 ± 45.0 | 0.849 |
| HDL cholesterol (mg/dL) | 52.0 ± 14.5 | 49.4 ± 13.8 | 0.004 |
| LDL cholesterol (mg/dL) | 127.1 ± 36.0 | 123.7 ± 37.5 | 0.132 |
| Triglycerides (mg/dL) | 100.6 ± 58.8 | 129.2 ± 115.7 | <0.0001 |
| eGFR (mL/min/1.73 m2) | 96.53 ± 19.40 | 88.27 ± 24.94 | <0.0001 |
| ACR (mg/g)* | 6.4 ± 2.6 | 21.3 ± 4.2 | 0.0246 |
| CVD | 272 (7.4) | 61 (19.2) | <0.0001 |
| DM | 473 (12.7) | 232 (71.8)@ | <0.0001 |
| Education | |||
| Less than high school | 529 (14.3) | 84 (26.1) | <0.0001 |
| High school/GED or some college | 1,554 (41.9) | 132 (41.0) | |
| College/associate degree or higher | 1,629 (43.9) | 106 (32.9) | |
| Alcohol consumption | 1,789 (48.3) | 127 (39.7) | 0.003 |
| Physical activity | |||
| Poor | 1,705 (48.9) | 187 (58.1) | <0.0001 |
| Intermediate | 1,237 (33.3) | 98 (30.4) | |
| Ideal | 776 (20.9) | 37 (11.5) | |
| Systolic BP (mmHg) | 124.9 ± 17.1 | 133.2 ± 18.6 | <0.0001 |
| Diastolic BP (mmHg) | 79.0 ± 10.4 | 77.9 ± 10.9 | 0.058 |
| WC (cm) | 99.6 ± 15.9 | 108.6 ± 15.7 | <0.0001 |
| Duration of DM (years) | 8.1 ± 8.6 | 11.9 ± 10.1 | <0.0001 |
| Smoking status | |||
| Nonsmokers | 2,604 (70.6) | 205 (63.9) | 0.040 |
| Former smokers | 664 (18.0) | 74 (23.0) | |
| Current smokers | 423 (11.5) | 42 (13.1) | |
| BMI (kg/m2) | |||
| Normal | 550 (14.8) | 16 (5.0) | <0.0001 |
| Overweight | 1,238 (33.3) | 86 (26.7) | |
| Obese | 1,929 (51.9) | 220 (68.3) |
Data are means ± SD or n (%). GED, General Educational Development degree.
Geometric mean ± SD was used for hs-CRP and ACR due to nonnormality of hs-CRP and ACR.
P values from t test for continuous variables and χ2 test for categorical variables. Baseline study population excludes those with DN or missing hs-CRP values at visit 1.
@A total of 232 participants with DM at the beginning of the study developed DN, and 91 (= 323–232) participants who did not have DM at the beginning developed DN during the study period.
Compared with participants who had hs-CRP levels in the lowest tertile (<1.46 mg/L [reference group]) at baseline, study participants with hs-CRP levels in the highest tertile (>4.24 mg/L) were older and more likely to be female (Table 2). In addition, participants with hs-CRP levels in the highest tertile had significantly higher levels of fasting glucose, total cholesterol, and triglycerides, and a greater proportion had DM, higher systolic BP, and higher WC and were more likely to be overweight/obese but had lower levels of diastolic BP, LDL cholesterol, and physical activity. Also, participants with hs-CRP levels in the highest tertile were more likely to be less educated and to consume alcohol. However, no significant difference was found across hs-CRP tertiles in the level of HDL cholesterol, proportion of preexisting CVD, eGFR, ACR, duration of DM, or smoking status.
Table 2.
Baseline characteristics of study population stratified by hs-CRP tertiles*
| Characteristics | hs-CRP | P** | ||
|---|---|---|---|---|
| <1.46 mg/L (N = 1,342) | 1.46–4.24 mg/L (N = 1,327) | >4.24 mg/L (N = 1,374) | ||
| Age (years) | 52.7 ± 12.6 | 54.9 ± 12.4 | 55.2 ± 11.6 | <0.0001 |
| Females | 650 (48.4) | 846 (63.8) | 1,099 (80.0) | <0.0001 |
| Fasting glucose (mg/dL) | 93.9 ± 20.8 | 97.4 ± 28.3 | 100.9 ± 33.7 | <0.0001 |
| Total cholesterol (mg/dL) | 196.5 ± 39.0 | 202.4 ± 38.4 | 198.6 ± 40.3 | 0.0007 |
| HDL cholesterol (mg/dL) | 52.2 ± 14.8 | 51.5 ± 14.2 | 51.9 ± 14.2 | 0.436 |
| LDL cholesterol (mg/dL) | 125.6 ± 35.3 | 130.2 ± 35.6 | 124.9 ± 37.3 | 0.0004 |
| Triglycerides | 94.3 ± 61.3 | 104.6 ± 59.9 | 109 ± 70.8 | <0.0001 |
| CVD | 110 (8.3) | 108 (8.3) | 115 (8.5) | 0.9771 |
| eGFR (mL/min/1.73 m2) | 96.7 ± 18.8 | 95.4 ± 20.1 | 95.5 ± 21.1 | 0.1824 |
| ACR (mg/g)* | 6.0 ± 2.5 | 7.3 ± 3.0 | 8.1 ± 3.0 | 0.1732 |
| DM | 167 (12.4) | 228 (17.2) | 310 (22.6) | <0.0001 |
| DN | ||||
| Subsequently developed DN | 74 (5.5) | 103 (7.7) | 149 (10.6) | <0.0001 |
| Never developed DN | 1,268 (94.5) | 1,224 (92.2) | 1,228 (89.4) | |
| Education | ||||
| Less than high school | 162 (12.1) | 221 (16.7) | 230 (16.8) | <0.0001 |
| High school/GED or some college | 536 (40.1) | 556 (41.9) | 594 (43.3) | |
| College/associate degree or higher | 638 (47.8) | 549 (41.4) | 548 (39.9) | |
| Alcohol consumption | 648 (48.4) | 710 (53.9) | 752 (54.9) | 0.0015 |
| PA | ||||
| No PA | 555 (41.4) | 638 (48.1) | 699 (51.0) | <0.0001 |
| Light PA | 457 (34.1) | 440 (33.2) | 438 (31.9) | |
| Moderate or vigorous PA | 329 (24.5) | 249 (18.8) | 235 (17.1) | |
| Systolic BP (mmHg) | 124 ± 16.9 | 127 ± 17.7 | 125.8 ± 17.5 | <0.0001 |
| Diastolic BP (mmHg) | 79.5 ± 10.4 | 79.2 ± 10.4 | 78.1 ± 10.5 | 0.0009 |
| WC (cm) | 93.6 ± 13.4 | 100.3 ± 14.6 | 106.9 ± 17.2 | <0.0001 |
| Duration of DM (years) | 9.7 ± 10.1 | 9.4 ± 9.7 | 9.1 ± 8.4 | 0.8591 |
| Smoking status | ||||
| Nonsmokers | 931 (70) | 930 (70.6) | 948 (69.5) | 0.6153 |
| Former smokers | 256 (19.2) | 237 (18.0) | 245 (18.0) | |
| Current smokers | 144 (10.8) | 150 (11.4) | 171 (12.5) | |
| BMI (kg/m2) | ||||
| Normal | 337 (25.1) | 157 (12.0) | 70 (5.1) | <0.0001 |
| Overweight | 598 (44.6) | 434 (32.7) | 292 (21.3) | |
| Obese | 407 (31.3) | 733 (55.3) | 1,009 (73.6) | |
Data are mean ± SD or N (%). GED, General Educational Development degree; PA, physical activity.
Geometric mean ± SD was used for ACR due to nonnormality of ACR.
P values from t test for continuous variables and χ2 test for categorical variables. Baseline study population excludes those with DN or missing hs-CRP values at exam 1.
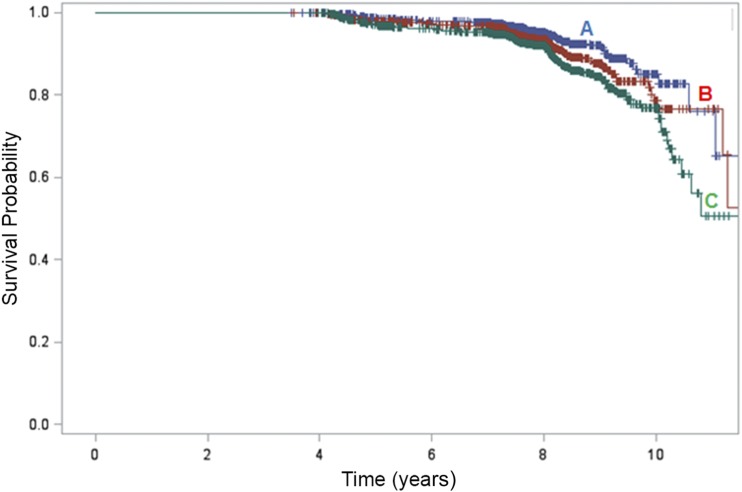
The rate of incident DN increased with higher levels of hs-CRP: 5.5% of those in the lowest hs-CRP tertile group (<1.46 mg/L), 7.7% in the middle tertile (1.46–4.24 mg/L), and 10.6% of in the highest tertile (>4.24 mg/L) developed new DN (Table 2). The probability of survival free of DN is shown in Fig. 1, stratified by hs-CRP tertile. There was a significant difference in survival (free of DN) probabilities (log rank P value < 0.001), with lower probability of being free of DN associated with increasing hs-CRP levels.
Figure 1.
Kaplan-Meier survival plots for time free of DN. Significant difference was observed in estimated mean time free of DN among hs-CRP groups (log rank P value <0.001). The time free of DN was 11.1 years (blue line, A), 10.6 years (red line, B), and 10.1 years (green line, C) in the lowest hs-CRP tertile, <1.46 mg/L; middle, 1.46–4.24 mg/L; and highest, >4.24 mg/L, respectively.
Increasing hs-CRP levels were significantly associated with progressively higher crude incidence rates of DN (HR 1.85, 95% CI 1.40–2.45, P < 0.001) (Table 3) compared with low/reference hs-CRP. While the age-adjusted HR for DN was significantly higher among the highest tertile of hs-CRP than the lowest and middle tertile (HR 1.75, 95% CI 1.33–2.31), there was only a trend for difference between the lowest and middle tertiles. This association persisted after further adjustment in the multivariate analysis for age, sex, fasting glucose, total cholesterol, HDL, LDL, triglycerides, systolic BP, diastolic BP, antihypertensive medication, CVD, years of DM, smoking, alcohol consumption, physical activity, education, BMI, and WC (HR 2.34, 95% CI 1.04–5.24, P < 0.05). There was no significant interaction (P = 0.445) between the overall group and the subgroup of participants free of DM at baseline, suggesting a strong association between hs-CRP and incident DN independent of baseline DM.
Table 3.
HR (95% CI) of DN according to tertile of hs-CRP
| hs-CRP | |||
|---|---|---|---|
| <1.46 mg/L | 1.46–4.24 mg/L | >4.24 mg/L | |
| N | 1,342 | 1,327 | 1,374 |
| Crude HR | Reference | 1.39 (1.03–1.88)* | 1.85 (1.40–2.45)** |
| Age-adjusted HR | Reference | 1.28 (0.95–1.73) | 1.75 (1.33–2.31)** |
| Multivariate-adjusted HR$ | Reference | 1.57 (0.68–3.64) | 2.34 (1.04–5.24)* |
P value <0.05,
P value <0.001, from Cox PH models.
$Adjusted for age, sex, fasting glucose, total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, systolic BP, diastolic BP, antihypertensive medication, CVD, years of DM, smoking, alcohol consumption, physical activity, education, BMI, and WC.
We also examined whether a relationship between incident DN and baseline hs-CRP existed among study subgroups (Supplementary Table 1). A significant increasing trend of HR was found among those who drank alcohol (Ptrend = 0.0001), did not smoke (Ptrend = 0.0206), engaged in an intermediate level of physical activity (Ptrend = 0.0183), and had low systolic (Ptrend = 0.0007) and diastolic (Ptrend = 0.0071) BP. However, the increasing trend of HR with highest hs-CRP was significantly different only between nondrinkers and drinkers (Pinteraction = 0.0114), with the association remaining solely among alcohol drinkers. Therefore, only drinkers with the highest hs-CRP tertile appear to have a trend for increase in incident DN.
Conclusions
In this study of a large cohort of AA followed for a median 7.8 years (interquartile range 7.2–8.3) over the three JHS examinations, we found elevated hs-CRP levels were significantly associated with a higher incidence of DN, irrespective of a diagnosis of DM at baseline. Previous cohort studies have demonstrated a strong association between baseline hs-CRP concentration and prevalence of DN (17,23,24). By contrast, Hayashino et al. (25) reported an independent association between serum baseline hs-CRP and the development but not progression of DN in 2,518 Japanese patients with DM at baseline followed for 1 year. Wang et al. (26) followed 4,213 Japanese civil servants for 6 years (∼15% with DM at baseline) and found an increase in incident cases of DM in the two quartiles with the highest CRP. However, they did not examine these study participants for development of DN. Finally, Overgaard et al. (27) found that CRP levels predicted albuminuria in patients with newly diagnosed type 1 diabetes followed for 30 years. Effoe et al. (28) reported that hs-CRP is also associated with incident DM among AA in the JHS but did not examine participants for incident DN. Thus, our study is unique as the first study to identify an association between hs-CRP and incident DN in persons, regardless of having DM at baseline.
The question now arises, what is the mechanism by which inflammation contributes to the development to DN? The inflammatory response to DM in the kidney has not been well defined. It has been shown that several components of the diabetic state can activate the infiltration of hematopoietic cells, mainly macrophages, to the kidney, which in turn secretes proinflammatory cytokines and reactive oxygen species (29). These cytokines may amplify the inflammatory response and promote cell injury and the development of DN. Therefore, it is logical to propose that hs-CRP is associated with both DM and DN.
Several studies support the mechanistic notion that DM includes an inflammatory component that significantly contributes to the genesis and progression of DN (30). Clinical and experimental studies have shown that a variety of inflammatory molecules, such as CRP, IL-6, and MCP-1, are involved in the setting of DN (31,32). Fujita et al. (33) proposed that IL-18 might have a specific role that contributes more closely to the progression of DN than other DM complications. Using a transgenic Leprdb/db mouse model of DN that expresses human CRP, You et al. (34) have recently shown that CRP is pathogenic in type 2 DN and can activated Smad3 signaling directly through the ERK/p38 MAP kinase cross talk pathway and indirectly via a TGF-β1–dependent mechanism. CRP may also promote renal inflammation via the CD32b–nuclear factor-κB signaling mechanism, whereas CRP may enhance renal fibrosis via the CD32b-Smad3-mTOR signaling pathway (34). CRP was also reported to promote proinflammatory cytokine production (35), leading to mesangial cell proliferation, matrix overproduction, and increased vascular permeability resulting in albuminuria (36). An in vitro study suggests that macrophage-produced CRP, in addition to the effect of circulating and liver-derived CRP, could trigger CRP-mediated proinflammatory effects locally (37). It has been demonstrated that CRP induces macrophage colony–stimulating factor (M-CSF) release via upregulation of nuclear factor-κB, resulting in increased macrophage proliferation (38). Macrophages infiltrate the glomeruli and/or interstitium in the kidney in patients with DN, and the intensity of the interstitial infiltrate is proportional to the rate of subsequent decline in renal function (39). Collectively, these findings support a pivotal role of CRP in the inflammatory process during development of DN.
Our findings need to be considered in light of the limitations and strengths of our study. First, although the JHS participants have detailed phenotypes, JHS participants are not representative of all AA, so our findings may not be generalizable to all AA. Second, while we controlled for numerous potential confounding variables, the potential for residual confounding remains, as we had limited data on disease severity and other unknown factors related to risk of DN. Third, the lack of data on inflammatory markers other than hs-CRP limits our ability to assign a broader generalization of inflammation to the observed association of hs-CRP with DN. Further, eGFR was not considered in ascertaining DN because the data were not available at exam 2. Even so, at exam 3 it was uncommon for incident cases of DN to develop without albuminuria, supporting our approach to DN ascertainment in our cohort. Finally, our study does not address the heterogeneity in diabetic kidney disease phenotypes in which elevated albuminuria (>300 mg/g) and reduced eGFR (<60 mL/min/1.73 m2) are independent risk factors for cardiovascular and renal events among patients with DM and differing mortality risk (40). Balanced against these limitations, strengths of this study include prospectively collected data on the outcomes of interest in a high-risk population with a wealth of high-quality data collected from interviews, physical examination, and laboratory studies including hs-CRP and measures of DM and DN.
In conclusion, we found that elevated serum levels of hs-CRP were associated with, and possibly influenced the development of, DN in AA, even for those without DM at baseline. This makes hs-CRP in its own right a possible risk factor for developing DN in AA. AA have higher hs-CRP than Caucasians (17), perhaps owing to their greater prevalence of risk factors for inflammation. In light of the observed association between hs-CRP and incident DM among AA in the JHS (29), hs-CRP may potentially serve as a biomarker for the development of DN and could theoretically be an alternative indicator to target or follow in “at-risk” AA. Further prospective intervention trials are needed to establish causality. In the interim, advancing our understanding of the mechanism(s) by which inflammatory molecules such as hs-CRP may contribute to the development of DN can help refine new therapeutic interventions and, if successful, reduce the incidence of DN in AA, as well as in the general population.
Supplementary Material
Article Information
Acknowledgments. The authors thank the staff and participants of the JHS.
Funding. Research reported in this publication/press was supported by the National Institute on Minority Health and Health Disparities of the National Institutes of Health (NIH) under award number U54MD008149 (RTRN Small Grants Program to S.K.S.). S.B.N. was supported by NIH grant UL1TR000124. T.B.R. was supported by a Ramalingaswami Fellowship from the Department of Biotechnology, Government of India. K.C.N. was supported by NIH grants UL1TR000124 and P30AG021684. The JHS is supported and conducted in collaboration with Jackson State University (HHSN268201800013I), Tougaloo College (HHSN268201800014I), Mississippi State Department of Health (HHSN268201800015I/HHSN26800001), and University of Mississippi Medical Center (HHSN268201800010I, HHSN268201800011I, and HHSN268201800012I) contracts from the National Heart, Lung, and Blood Institute and the National Institute on Minority Health and Health Disparities.
The views expressed in this article are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. S.K.S. researched data and wrote the manuscript. S.B.N. contributed to data interpretation, discussion, and overall manuscript development. J.H.S. contributed to data extraction and analysis. A.C. and T.B.R. edited the manuscript and contributed to discussion. K.C.N. edited the manuscript and contributed to data interpretation and discussion. J.E.L. designed the program to analyze the data and contributed to the methods and results. S.K.S., J.H.S., and J.E.L. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the American Society of Nephrology Kidney Week, Chicago, IL, 15–20 November 2016.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc18-2563/-/DC1.
References
- 1.Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an ADA Consensus Conference. Diabetes Care 2014;37:2864–2883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brancati FL, Whelton PK, Kuller LH, Klag MJ. Diabetes mellitus, race, and socioeconomic status. A population-based study. Ann Epidemiol 1996;6:67–73 [DOI] [PubMed] [Google Scholar]
- 3.Harris MI, Eastman RC, Cowie CC, Flegal KM, Eberhardt MS. Racial and ethnic differences in glycemic control of adults with type 2 diabetes. Diabetes Care 1999;22:403–408 [DOI] [PubMed] [Google Scholar]
- 4.Nicholas SB, Kalantar-Zadeh K, Norris KC. Racial disparities in kidney disease outcomes. Semin Nephrol 2013;33:409–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tarver-Carr ME, Powe NR, Eberhardt MS, et al. Excess risk of chronic kidney disease among African-American versus white subjects in the United States: a population-based study of potential explanatory factors. J Am Soc Nephrol 2002;13:2363–2370 [DOI] [PubMed] [Google Scholar]
- 6.Harding K, Mersha TB, Webb FA, Vassalotti JA, Nicholas SB. Current state and future trends to optimize the care of African Americans with end-stage renal disease. Am J Nephrol 2017;46:156–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harding K, Mersha TB, Vassalotti JA, Webb FA, Nicholas SB. Current state and future trends to optimize the care of chronic kidney disease in African Americans. Am J Nephrol 2017;46:176–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol 2017;12:2032–2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tuttle KR. Linking metabolism and immunology: diabetic nephropathy is an inflammatory disease. J Am Soc Nephrol 2005;16:1537–1538 [DOI] [PubMed] [Google Scholar]
- 10.Mora C, Navarro JF. Inflammation and diabetic nephropathy. Curr Diab Rep 2006;6:463–468 [DOI] [PubMed] [Google Scholar]
- 11.Galkina E, Ley K. Leukocyte recruitment and vascular injury in diabetic nephropathy. J Am Soc Nephrol 2006;17:368–377 [DOI] [PubMed] [Google Scholar]
- 12.Shikata K, Makino H. Role of macrophages in the pathogenesis of diabetic nephropathy. Contrib Nephrol 2001;1:46–54 [DOI] [PubMed] [Google Scholar]
- 13.Chow F, Ozols E, Nikolic-Paterson DJ, Atkins RC, Tesch GH. Macrophages in mouse type 2 diabetic nephropathy: correlation with diabetic state and progressive renal injury. Kidney Int 2004;65:116–128 [DOI] [PubMed] [Google Scholar]
- 14.Navarro-González JF, Mora-Fernández C. The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol 2008;19:433–442 [DOI] [PubMed] [Google Scholar]
- 15.Ihm CG. Monocyte chemotactic peptide-1 in diabetic nephropathy. Kidney Int Suppl 1997;60:S20–S22 [PubMed] [Google Scholar]
- 16.Chow FY, Nikolic-Paterson DJ, Ozols E, Atkins RC, Rollin BJ, Tesch GH. Monocyte chemoattractant protein-1 promotes the development of diabetic renal injury in streptozotocin-treated mice. Kidney Int 2006;69:73–80 [DOI] [PubMed] [Google Scholar]
- 17.Sinha SK, Shaheen M, Rajavashisth TB, Pan D, Norris KC, Nicholas SB. Association of race/ethnicity, inflammation, and albuminuria in patients with diabetes and early chronic kidney disease. Diabetes Care 2014;37:1060–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fox ER, Benjamin EJ, Sarpong DF, et al. The relation of C–reactive protein to chronic kidney disease in African Americans: the Jackson Heart Study. BMC Nephrol 2010;11:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor HA Jr, Wilson JG, Jones DW, et al. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethn Dis 2005;15:S6-4-17. [PubMed] [Google Scholar]
- 20.Carpenter MA, Crow R, Steffes M, et al. Laboratory, reading center, and coordinating center data management methods in the Jackson Heart Study. Am J Med Sci 2004;328:131–144 [DOI] [PubMed] [Google Scholar]
- 21.Levey AS, Stevens LA. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis 2010;55:622–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33(Suppl. 1):S62–S69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Q, Jiang CY, Chen BX, Zhao W, Meng D. The association between high-sensitivity C-reactive protein concentration and diabetic nephropathy: a meta-analysis. Eur Rev Med Pharmacol Sci 2015;19:4558–4568 [PubMed] [Google Scholar]
- 24.Phosat C, Panprathip P, Chumpathat N, et al. Elevated C-reactive protein, interleukin 6, tumor necrosis factor alpha and glycemic load associated with type 2 diabetes mellitus in rural Thais: a cross-sectional study. BMC Endocr Disord 2017;17:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayashino Y, Mashitani T, Tsujii S, Ishii H; Diabetes Distress and Care Registry at Tenri Study Group . Serum high-sensitivity C-reactive protein levels are associated with high risk of development, not progression, of diabetic nephropathy among Japanese type 2 diabetic patients: a prospective cohort study (Diabetes Distress and Care Registry at Tenri [DDCRT7]). Diabetes Care 2014;37:2947–2952 [DOI] [PubMed] [Google Scholar]
- 26.Wang C, Yatsuya H, Tamakoshi K, et al. Positive association between high-sensitivity C-reactive protein and incidence of type 2 diabetes mellitus in Japanese workers: 6-year follow-up. Diabetes Metab Res Rev 2013;29:398–405 [DOI] [PubMed] [Google Scholar]
- 27.Overgaard AJ, McGuire JN, Hovind P, Parving HH, Rossing P, Pociot F. Serum amyloid A and C-reactive protein levels may predict microalbuminuria and macroalbuminuria in newly diagnosed type 1 diabetic patients. J Diabetes Complications 2013;27:59–63 [DOI] [PubMed] [Google Scholar]
- 28.Effoe VS, Correa A, Chen H, Lacy ME, Bertoni AG. High-sensitivity C-reactive protein is associated with incident type 2 diabetes among African Americans: the Jackson Heart Study. Diabetes Care 2015;38:1694–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim AKH, Tesch GH. Inflammation in diabetic nephropathy. Mediators Inflamm 2012;2012:146154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Navarro-González JF, Mora-Fernández C. The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol 2008;19:433–442 [DOI] [PubMed] [Google Scholar]
- 31.Dalla Vestra M, Mussap M, Gallina P, et al. Acute-phase markers of inflammation and glomerular structure in patients with type 2 diabetes. J Am Soc Nephrol 2005;16(Suppl. 1):S78–S82 [DOI] [PubMed] [Google Scholar]
- 32.Banba N, Nakamura T, Matsumura M, Kuroda H, Hattori Y, Kasai K. Possible relationship of monocyte chemoattractant protein-1 with diabetic nephropathy. Kidney Int 2000;58:684–690 [DOI] [PubMed] [Google Scholar]
- 33.Fujita T, Ogihara N, Kamura Y, et al. Interleukin-18 contributes more closely to the progression of diabetic nephropathy than other diabetic complications. Acta Diabetol 2012;49:111–117 [DOI] [PubMed] [Google Scholar]
- 34.You YK, Huang XR, Chen HY, Lyu XF, Liu HF, Lan HY. C-reactive protein promotes diabetic kidney disease in db/db mice via the CD32b-Smad3-mTOR signaling pathway. Sci Rep 2016;6:26740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verma S, Li SH, Badiwala MV, et al. Endothelin antagonism and interleukin-6 inhibition attenuate the proatherogenic effects of C-reactive protein. Circulation 2002;105:1890–1896 [DOI] [PubMed] [Google Scholar]
- 36.Horii Y, Iwano M, Hirata E, et al. Role of interleukin-6 in the progression of mesangial proliferative glomerulonephritis. Kidney Int Suppl 1993;39:S71–S75 [PubMed] [Google Scholar]
- 37.Kaplan M, Tendler Y, Mahamid R, Shiner M, Aviram M, Hayek T. High glucose upregulates C-reactive protein synthesis in macrophages. Clin Chem 2010;56:1036–1038 [DOI] [PubMed] [Google Scholar]
- 38.Devaraj S, Yun J-M, Duncan-Staley C, Jialal I. C-reactive protein induces M-CSF release and macrophage proliferation. J Leukoc Biol 2009;85:262–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nguyen D, Ping F, Mu W, Hill P, Atkins RC, Chadban SJ. Macrophage accumulation in human progressive diabetic nephropathy. Nephrology (Carlton) 2006;11:226–231 [DOI] [PubMed] [Google Scholar]
- 40.Ninomiya T, Perkovic V, de Galan BE, et al.; ADVANCE Collaborative Group . Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol 2009;20:1813–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.