Abstract
OBJECTIVE
A recent study raises concerns that dipeptidyl peptidase 4 inhibitors (DPP4i) are associated with increased risk of inflammatory bowel disease (IBD). We evaluated the association between new use of DPP4i and IBD risk compared with other second-line antihyperglycemics.
RESEARCH DESIGN AND METHODS
We implemented an active-comparator, new-user cohort design using two U.S. administrative claims databases for commercially insured (MarketScan) and older adult (Medicare fee-for-service, 20% random sample) patients from January 2007 to December 2016. We identified patients, aged ≥18 years, who initiated DPP4i versus sulfonylureas (SUs) or initiated DPP4i versus thiazolidinediones (TZDs) and were without prior diagnosis, treatment, or procedure for IBD. The primary outcome was incident IBD, defined by IBD diagnosis preceded by colonoscopy and biopsy and followed by IBD treatment. We performed propensity score weighting to control for measured baseline confounding, estimated adjusted hazard ratios (aHRs [95% CI]) using weighted Cox proportional hazards models, and used random-effects meta-analysis models to pool aHRs across cohorts.
RESULTS
We identified 895,747 eligible patients initiating DPP4i, SU, or TZD; IBD incidence rates ranged from 11.6 to 32.3/100,000 person-years. Over a median treatment duration of 1.09–1.69 years, DPP4i were not associated with increased IBD risk across comparisons. The pooled aHRs for IBD were 0.82 (95% CI 0.41–1.61) when comparing DPP4i (n = 161,612) to SU (n = 310,550) and 0.76 (0.46–1.26) when comparing DPP4i (n = 205,570) to TZD (n = 87,543).
CONCLUSIONS
Our population-based cohort study of U.S. adults with diabetes suggests that short-term DPP4i treatment does not increase IBD risk.
Introduction
Dipeptidyl peptidase 4 inhibitors (DPP4i), a commonly prescribed second-line glucose-lowering drug (GLD) class, reduce hyperglycemia by increasing the levels of incretin hormones, which in turn increase insulin and decrease glucagon in a glucose-dependent fashion (1). DPP4, also known as CD26, is expressed on leukocytes and can inactivate various cytokines as well as act as a costimulator of T lymphocytes (2), thus raising concerns about potential immunologic effects of DPP4i (3). A recent retrospective cohort study by Abrahami et al. (4) involving 141,170 patients in the British Clinical Practice Research Datalink (CPRD) database found that, over a median use period of 1.6 years and follow-up of 3.6 years, new use of DPP4i was significantly associated with an increased incidence of inflammatory bowel disease (IBD) compared with other GLDs (hazard ratio [HR] 1.75 [95% CI 1.22–2.49]), with HR 2.23 (1.32–3.76) for ulcerative colitis (UC) and HR 0.87 (0.37–2.09) for Crohn disease (CD), respectively.
To date, the study by Abrahami et al. (4) is the only cohort study available assessing DPP4i’s IBD risk. Other studies have yielded uncertainty regarding the effect of DPP4i on IBD risk, which necessitates replication using multiple sources and study designs. While a meta-analysis of randomized trials suggests no association between DPP4i and IBD risk (5), a disproportionality analysis of the U.S. Food and Drug Administration Adverse Event Reporting System database suggested a weak to moderate signal of CD; the reporting odds ratio (≥2 defined as a positive signal) was 2.63 (95% CI 1.74–3.99) compared with a comparator group consisting of both thiazolidinediones (TZDs) and sodium–glucose cotransporter 2 inhibitors (6). A cross-sectional analysis of health care databases in Israel also suggests a signal for CD (odds ratio 3.56 [1.04–12.21]) (7). Given these results, we conducted a retrospective cohort study, using the active-comparator, new-user (ACNU) cohort design (8), to address the methodological limitations of prior studies and to further investigate whether new use of DPP4i is associated with an increased risk of IBD.
Research Design and Methods
Data Source
We used two large U.S.-based administrative claims databases: the IBM MarketScan Commercial Claims and Encounters (MarketScan) and a 20% random sample (University of North Carolina at Chapel Hill has access for only 20% random sample) of Medicare Fee-for-Service (Medicare) from January 2007 to December 2016. The MarketScan database contains data primarily on adults aged <65 years from ∼350 insurance payers across the U.S. Medicare provides medical coverage primarily for the U.S. population aged ≥65 years, including Parts A (in- patient), B (outpatient physician services), and D (dispensed prescription drugs) coverage. Both databases contain longitudinal, individual-level data, including demographics, inpatient and outpatient diagnosis and procedures, and pharmacy claims. The study protocol was registered in the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance electronic register of studies (http://www.encepp.eu/encepp/viewResource.htm?id=26246) and approved by the University of North Carolina at Chapel Hill Institutional Review Board.
Study Population
The eligible population consisted of MarketScan enrollees, aged 18–64 years, as well as Medicare enrollees, aged ≥65 years, both with at least 12 months of continuous enrollment (for Medicare, continuous enrollment in Parts A, B, and D and without HMO coverage) before initiation. We first independently identified all new use periods from 1 January 2008 to 30 September 2015 of each of the three second-line therapies of interest (9)—DPP4i, sulfonylureas (SUs), and thiazolidinediones (TZDs)—simultaneously (i.e., the three groups of new users are not mutually exclusive) based on the first dispensing of a prescription in a given drug class after a 12-month washout period. A patient could have multiple new-user periods of the same drug class and could enter the study in both drug classes (after at least 12 months with no prescription fill after the end of the days’ supply of a previous prescription fill or after the start of enrollment). Then, we constructed two comparison cohorts from the three groups of new users—DPP4i versus SU and DPP4i versus TZD, additionally requiring no evidence of use of the comparator drug in the 12 months prior to initiation (therefore, the number of participants and events for DPP4i differed in the two comparisons). We used two comparators, as both are oral treatment alternatives, thereby increasing the clinical relevance by addressing the question, “which second-line treatment is safer?” Additionally, we required patients to have a second prescription-dispensing claim within the sum of the first prescription’s days’ supply and a 90-day grace period to increase the probability that patients actually took the medication (10). The use of active comparators helps to reduce bias by only selecting individuals at similar disease severity and with an indication for initiating a second-line GLD, and the new-user design ensures appropriate temporal ordering of baseline confounders, treatment, and outcome (8). We did not restrict cohorts to those with a diagnosis code for diabetes, as these GLDs are primarily indicated for diabetes treatment (9).
We excluded patients with the following diagnoses, treatments, or procedures in the 12 months before the first prescription (index date): 1) diagnosis of IBD, including CD and UC; 2) prior exposure to IBD treatments, including aminosalicylates, anti–tumor necrosis factor, enteral budesonide, and immunosuppressive/immunoregulatory agents (11,12); 3) common diseases that could be confused with IBD, including diverticulitis, ischemic colitis, pseudomembranous colitis, or unspecific colitis; 4) prior colectomy, colostomy, ileostomy, or receipt of ostomy supplies; and 5) prior colonoscopy or sigmoidoscopy before 50 years of age (because current U.S. Preventative Services Task Force guidelines recommend colonoscopy for colorectal cancer only for individuals aged 50–75 years [13]). We additionally excluded patients with a diagnosis of congestive heart failure when comparing DPP4i to TZDs (TZDs are contraindicated in patients with heart failure [14]).
IBD Outcome
The primary outcome was incident IBD, defined by the first IBD diagnosis (ICD-9 and ICD-10, Clinical Modification codes) that was preceded by a colonoscopy/sigmoidoscopy and biopsy within 30 days before diagnosis and followed by a prescription claim for IBD medication treatment within 30 days after diagnosis (Supplementary Tables 1 and 2A). The date of IBD diagnosis was considered as the event date. Secondary analyses assessed the risk of incident CD and UC, respectively. The majority of the first three diagnoses were used to distinguish between CD and UC.
Follow-up
Because short duration of exposure is unlikely to be associated with incident IBD, and IBD diagnosis is unlikely to be made immediately after symptom onset (15), we started follow-up for the outcome 180 days (induction period) after the second prescription (cohort entry date) and excluded patients with the outcome or discontinued enrollment within 180 days after their second prescription. Similarly, follow-up for IBD events continued 180 days (latency period) after treatment was changed or stopped. Follow-up ended at the earliest of the following events: 1) 180 days after the date of treatment change, including discontinuation (defined as no refill within a period equal to the prescribed days’ supply of the last filled prescription plus a 90-day grace period) or initiating the comparator drug (initiating noncomparator GLDs would not result in censoring); 2) the end of enrollment (the end of enrollment for Parts A, B, or D or enrollment for HMO for Medicare beneficiaries); 3) death (for Medicare only); 4) administrative study end (31 December 2016); or 5) observation of an incident IBD event, per the definition above. We used the first incident IBD event date during follow-up to define the outcome date.
Statistical Analyses
We controlled for a variety of covariates defined based on claims during the 12 months prior to the index prescription (Supplementary Table 3), including demographics, markers for diabetes severity (retinopathy, nephropathy, and neuropathy), gastroenterological disease, autoimmune disease, comorbidities, comedications, health care utilization (e.g., gastroenterologist encounters), appendectomy, and low-income subsidy status (for Medicare only) (11,12,16). To control for factors that may influence the decision to prescribe a given treatment, we used these covariates to estimate propensity scores (PSs) for each patient in each comparison. The PS, which represents the predicted probability of receiving the index treatment conditional on baseline covariates, was estimated separately for each comparison. We then applied asymmetric PS trimming in order to exclude patients who were treated most contrary to prediction (i.e., in the tails of the PS distribution), using a cut point corresponding to the 0.5th and 99.5th percentiles of the PS distribution in the treated and untreated patients, respectively (17).
To directly compare the estimates across two comparator groups for each of the DPP4i comparisons, we standardized the covariate distribution of comparator initiators to the covariate distribution of DPP4i initiators using standardized mortality/morbidity ratio (SMR) weights (PS/[1 − PS]) (18). SMR weighting aims to create comparator cohorts with the same covariate distribution as the DPP4i cohort, allowing for the estimation of the average treatment effect in the treated. This allowed us to estimate what would have happened to the actual DPP4i initiators if they had, contrary to fact, initiated the comparator drug class instead. We measured covariate balance using SDs between cohorts, with SDs <0.1 (10%) after weighting indicating successful control for measured confounding.
We calculated crude incidence rates by dividing the number of patients with observed outcome by the total amount of observed person-time for each exposure cohort, with 95% CIs estimated using Poisson regression. We constructed SMR-weighted (adjusted) Kaplan-Meier curves to compare the cumulative incidence of IBD (19) and fit Cox proportional hazards models in the PS-weighted populations to estimate adjusted HRs (aHRs) and 95% CIs for IBD associated with initiating DPP4i versus comparators. Finally, we performed a meta-analysis of both estimates from MarketScan and Medicare data using random-effects models with inverse variance weighting (20) to report pooled HRs (pHRs); a fixed-effects model was used for sensitivity analysis. Between-database heterogeneity was assessed using I2 statistics (21), which represents the proportion of the total variance in the meta-analysis that is attributed to between-database heterogeneity.
Secondary Analyses
We stratified analyses by age at cohort entry (aged <50 and ≥50 years in MarketScan and <75 and ≥75 years in Medicare), sex, duration of treatment (≤12 and >12 months), and preexisting gastroenterological disease (as patients with preexisting conditions tend to have more physician visits and thus a higher chance for IBD diagnosis). We also restricted to patients without autoimmune disease at baseline (only ∼5% had preexisting autoimmune disease). Finally, we estimated IBD risk for each individual DPP4i agent (sitagliptin, saxagliptin, and linagliptin).
Sensitivity Analyses
To assess the robustness of estimated IBD risk, we performed the following sensitivity analyses based on our primary analysis (as-treated, second prescription as cohort entry date, using a 180-day lag for both induction and latency periods) unless stated otherwise. First, we repeated the analyses of IBD and the IBD subtypes CD and UC changing both induction and latency periods from 180 days to 0 days, 90 days, and 365 days. Second, we performed initial-treatment analysis ignoring treatment changes during follow-up, which mimics the intention-to-treat analysis in a randomized trial. The follow-up ended at the earliest of the following events: death (Medicare only), the end of insurance enrollment, end of study, or an incident IBD event. We also stratified by time since the first prescription (≤2, >2–4, and >4 years) in these initial-treatment analyses. Third, we required only one study drug prescription in the exposure definition and used the first prescription as cohort entry date (i.e., follow-up started 180 days after the first prescription and ended 180 days after treatment change). Fourth, we constructed cohorts using only the first new-user period (i.e., patients could only enter the cohort once) and reconstructed Kaplan-Meier curves. Fifth, we applied the abovementioned exclusion criteria (IBD diagnoses, treatments, or procedures) in the all-available look-back history before the first prescription. Sixth, we modified our primary outcome (Supplementary Fig. 2) to: 1) use the date of IBD treatment instead of the date of IBD diagnosis as event date, to quantify the potential for time-related bias; 2) remove the biopsy requirement; 3) remove both colonoscopy/sigmoidoscopy and biopsy requirements; and 4) define patients with IBD as those with at least three diagnoses on different days within 90 days (22), in which the third diagnosis date was considered as the event date. Seventh, we relaxed our exclusion criteria to include additional patients with: 1) prior use of the abovementioned IBD treatments except mesalamine and enteral budesonide; 2) prior partial colectomy, colostomy, ileostomy, and ostomy supplies; and 3) MarketScan patients who received colonoscopy/sigmoidoscopy prior to 50 years of age. Eighth, we additionally censored patients when they received medications that could potentially induce or progress IBD (23) (Supplementary Table 2B). Ninth, we conducted conventional multivariable-adjusted Cox regression instead of PS-weighted Cox regression to control for potential confounders. Lastly, we excluded patients who originally qualified for Medicare due to end-stage renal disease and disability. All database-specific analyses described above were performed using SAS version 9.4. Meta-analyses were conducted using STATA version 14.0.
Results
Study Population
Across the two databases, we identified 896,084 eligible patients who filled at least two prescriptions for each cohort drug, including 382,475 (MarketScan) and 170,326 (Medicare) initiators for the DPP4i versus SU comparison and 249,750 (MarketScan) and 93,533 (Medicare) initiators for the DPP4i versus TZD comparison (Supplementary Fig. 3). The key baseline covariates are shown in Table 1. The mean age ranged between 51.0 and 74.8 years; 40.8–58.2% were men. Patients were more likely to have been treated with DPP4i compared with TZD since 2011. Overall, the prevalence of comorbidities was similar in the comparison cohorts, except that DPP4i initiators were more likely to have dyslipidemia and take metformin as background therapy. In the Medicare population, DPP4i initiators had more physician visits and more health care utilization (e.g., more likely to take flu shot). In the third column of each comparison (Table 1), we present PS-weighted covariate distributions for the comparator drug initiators. The well-balanced distribution of the covariates in DPP4i initiators and the PS-weighted comparator initiators (all weighted standardized absolute mean differences <0.1; the proportion of standardized absolute mean difference <0.01 was 63% for DPP4i vs. SU and 76% for DPP4i vs. TZD in MarketScan and 98% for DPP4i vs. SU and 79% for DPP4i vs. TZD in Medicare) shows that we were able to balance all measured covariates and, thus, remove measured confounding by these covariates.
Table 1.
Key patient characteristics of DPP4i and comparator initiators*
| Characteristic | MarketScan | Medicare | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DPP4i vs. SU | DPP4i vs. TZD | DPP4i vs. SU | DPP4i vs. TZD | |||||||||
| DPP4i (N = 137,341)† | SU (N = 234,727)† | Weighted SU (N = 154,551)‡ | DPP4i (N = 171,576)† | TZD (N = 69,222)† | Weighted TZD (N = 181,586)‡ | DPP4i (N = 46,518)† | SU (N = 117,820)† | Weighted SU (N = 46,375)‡ | DPP4i (N = 61,283)† | TZD (N = 28,532)† | Weighted TZD (N = 61,554)‡ | |
| Age, years, mean (SD) | 52.4 (8.32) | 51.0 (9.47) | 51.8 (8.86) | 52.4 (8.32) | 52.1 (8.52) | 52.4 (8.46) | 74.8 (7.29) | 74.4 (7.51) | 74.7 (7.29) | 74.1 (6.91) | 73.3 (6.76) | 74.2 (6.85) |
| Male | 75,265 (54.8) | 124,936 (53.2) | 82,043 (53.1) | 94,798 (55.3) | 40,286 (58.2) | 98,755 (54.4) | 19,033 (40.9) | 51,610 (43.8) | 18,930 (40.8) | 25,798 (42.1) | 12,611 (44.2) | 26,323 (42.8) |
| Race | ||||||||||||
| White | NA | NA | NA | NA | NA | NA | 34,729 (74.7) | 91,280 (77.5) | 34,714 (74.9) | 45,818 (74.8) | 20,887 (73.2) | 46,934 (76.2) |
| Black | NA | NA | NA | NA | NA | NA | 5,436 (11.7) | 14,633 (12.4) | 5,398 (11.6) | 6,865 (11.2) | 3,323 (11.6) | 6,315 (10.3) |
| Other | NA | NA | NA | NA | NA | NA | 6,353 (13.7) | 11,907 (10.1) | 6,262 (13.5) | 8,600 (14.0) | 4,322 (15.1) | 8,305 (13.5) |
| Diabetes complications | ||||||||||||
| Retinopathy | 7,399 (5.4) | 10,267 (4.4) | 7,905 (5.1) | 10,212 (6.0) | 4,089 (5.9) | 10,974 (6.0) | 6,388 (13.7) | 12,068 (10.2) | 6,384 (13.8) | 8,878 (14.5) | 3,989 (14.0) | 8,787 (14.3) |
| Nephropathy | 3,607 (2.6) | 5,980 (2.5) | 4,055 (2.6) | 5,190 (3.0) | 2,114 (3.1) | 5,620 (3.1) | 4,133 (8.9) | 7,763 (6.6) | 4,137 (8.9) | 5,021 (8.2) | 1,938 (6.8) | 5,284 (8.6) |
| Neuropathy | 6,759 (4.9) | 10,098 (4.3) | 7,272 (4.7) | 9,863 (5.7) | 3,578 (5.2) | 10,606 (5.8) | 8,928 (19.2) | 16,961 (14.4) | 8,925 (19.2) | 11,226 (18.3) | 4,412 (15.5) | 11,470 (18.6) |
| Autoimumune diseases | ||||||||||||
| Psoriasis | 1,153 (0.8) | 1,758 (0.7) | 1,299 (0.8) | 1,397 (0.8) | 491 (0.7) | 1,497 (0.8) | 505 (1.1) | 1,266 (1.1) | 512 (1.1) | 692 (1.1) | 284 (1.0) | 681 (1.1) |
| Rheumatoid arthritis | 747 (0.5) | 1,109 (0.5) | 800 (0.5) | 807 (0.5) | 258 (0.4) | 866 (0.5) | 1,281 (2.8) | 2,588 (2.2) | 1,232 (2.7) | 1,308 (2.1) | 578 (2.0) | 1,281 (2.1) |
| Systemic lupus erythematosus | 285 (0.2) | 439 (0.2) | 348 (0.2) | 291 (0.2) | 103 (0.1) | 377 (0.2) | 155 (0.3) | 293 (0.2) | 153 (0.3) | 136 (0.2) | 54 (0.2) | 152 (0.2) |
| Comedications | ||||||||||||
| Metformin | 114,686 (83.5) | 170,299 (72.6) | 124,346 (80.5) | 145,477 (84.8) | 55,188 (79.7) | 152,163 (83.8) | 29,720 (63.9) | 67,079 (56.9) | 29,875 (64.4) | 43,172 (70.4) | 18,855 (66.1) | 43,513 (70.7) |
| SU§ | NA | 234,727 (100.0) | NA | 62,101 (36.2) | 28,397 (41.0) | 66,876 (36.8) | NA | 117,820 (100.0) | NA | 30,897 (50.4) | 14,955 (52.4) | 31,373 (51.0) |
| TZD§ | 23,222 (16.9) | 24,889 (10.6) | 24,320 (15.7) | NA | 69,222 (100.0) | NA | 8,203 (17.6) | 13,577 (11.5) | 8,184 (17.6) | NA | 28,532 (100.0) | NA |
| DPP4i§ | 137,341 (100.0) | NA | NA | 171,576 (100.0) | NA | NA | 46,518 (100.0) | NA | NA | 61,283 (100.0) | NA | NA |
| GLP1RA | 6,740 (4.9) | 9,964 (4.2) | 7,028 (4.5) | 8,857 (5.2) | 5,025 (7.3) | 9,157 (5.0) | 927 (2.0) | 1,662 (1.4) | 944 (2.0) | 1,277 (2.1) | 723 (2.5) | 1,290 (2.1) |
| SGLT2i | 1,751 (1.3) | 1,024 (0.4) | 1,589 (1.0) | 3,780 (2.2) | 722 (1.0) | 3,573 (2.0) | 199 (0.4) | 235 (0.2) | 207 (0.4) | 413 (0.7) | 108 (0.4) | 431 (0.7) |
| LAI | 15,343 (11.2) | 18,587 (7.9) | 16,412 (10.6) | 19,506 (11.4) | 8,089 (11.7) | 20,127 (11.1) | 8,862 (19.1) | 14,793 (12.6) | 8,894 (19.2) | 8,969 (14.6) | 3,916 (13.7) | 9,099 (14.8) |
| α-Glucosidase inhibitor | 307 (0.2) | 365 (0.2) | 363 (0.2) | 562 (0.3) | 230 (0.3) | 797 (0.4) | 243 (0.5) | 394 (0.3) | 250 (0.5) | 457 (0.7) | 205 (0.7) | 514 (0.8) |
| Meglitinide | 1,911 (1.4) | 1,615 (0.7) | 1,903 (1.2) | 1,999 (1.2) | 844 (1.2) | 2,250 (1.2) | 1,642 (3.5) | 2,472 (2.1) | 1,633 (3.5) | 1,436 (2.3) | 613 (2.1) | 1,581 (2.6) |
| ACE inhibitors | 57,376 (41.8) | 99,885 (42.6) | 62,382 (40.4) | 76,517 (44.6) | 32,038 (46.3) | 80,689 (44.4) | 20,193 (43.4) | 54,905 (46.6) | 20,098 (43.3) | 28,632 (46.7) | 14,134 (49.5) | 28,468 (46.2) |
| ARBs | 33,912 (24.7) | 41,161 (17.5) | 36,134 (23.4) | 40,818 (23.8) | 14,579 (21.1) | 42,558 (23.4) | 15,033 (32.3) | 27,843 (23.6) | 15,017 (32.4) | 18,837 (30.7) | 7,404 (25.9) | 19,138 (31.1) |
| β-Blockers | 34,159 (24.9) | 62,317 (26.5) | 38,862 (25.1) | 42,071 (24.5) | 15,872 (22.9) | 44,602 (24.6) | 24,134 (51.9) | 59,727 (50.7) | 24,056 (51.9) | 29,406 (48.0) | 12,213 (42.8) | 29,781 (48.4) |
| CCBs | 25,956 (18.9) | 42,379 (18.1) | 28,561 (18.5) | 33,049 (19.3) | 12,562 (18.1) | 35,212 (19.4) | 16,703 (35.9) | 40,483 (34.4) | 16,580 (35.8) | 21,871 (35.7) | 9,497 (33.3) | 21,815 (35.4) |
| Statins | 78,516 (57.2) | 114,397 (48.7) | 84,516 (54.7) | 98,732 (57.5) | 39,178 (56.6) | 104,233 (57.4) | 32,624 (70.1) | 74,008 (62.8) | 32,543 (70.2) | 43,072 (70.3) | 18,974 (66.5) | 43,403 (70.5) |
| Loop diuretics | 8,721 (6.3) | 14,409 (6.1) | 9,476 (6.1) | 8,488 (4.9) | 3,177 (4.6) | 9,490 (5.2) | 11,994 (25.8) | 30,305 (25.7) | 11,977 (25.8) | 9,531 (15.6) | 3,949 (13.8) | 9,625 (15.6) |
| Other diuretics | 44,413 (32.3) | 71,214 (30.3) | 48,294 (31.2) | 55,311 (32.2) | 21,951 (31.7) | 58,944 (32.5) | 17,979 (38.6) | 43,787 (37.2) | 17,888 (38.6) | 23,744 (38.7) | 10,883 (38.1) | 23,932 (38.9) |
| Drugs that may induce IBD | ||||||||||||
| Oral contraceptives‖ | 2,220 (3.6) | 5,387 (4.9) | 3,178 (4.4) | 2,654 (3.5) | 1,086 (3.8) | 3,035 (3.7) | NTSR | NTSR | NA | NTSR | NTSR | NA |
| Hormonal therapy‖ | 6,915 (11.1) | 11,630 (10.6) | 8,713 (12.0) | 8,083 (10.5) | 3,294 (11.4) | 9,226 (11.1) | 1,178 (4.3) | 2,667 (4.0) | 1,173 (4.3) | 1,488 (4.2) | 725 (4.6) | 1,462 (4.1) |
| Aspirin | 4,770 (3.5) | 6,849 (2.9) | 5,120 (3.3) | 5,869 (3.4) | 2,256 (3.3) | 6,117 (3.4) | 2,150 (4.6) | 4,107 (3.5) | 2,130 (4.6) | 2,093 (3.4) | 935 (3.3) | 2,124 (3.5) |
| NSAIDs | 34,549 (25.2) | 55,152 (23.5) | 38,233 (24.7) | 43,134 (25.1) | 16,973 (24.5) | 45,903 (25.3) | 12,192 (26.2) | 25,560 (21.7) | 12,109 (26.1) | 15,674 (25.6) | 7,082 (24.8) | 15,562 (25.3) |
| Other drugs that may induce IBD | 577 (0.4) | 1,245 (0.5) | 704 (0.5) | 675 (0.4) | 249 (0.4) | 711 (0.4) | 80 (0.2) | 169 (0.1) | 78 (0.2) | 84 (0.1) | 41 (0.1) | 82 (0.1) |
| Health care utilization | ||||||||||||
| Physician encounters | ||||||||||||
| 0 | 3,368 (2.5) | 9,405 (4.0) | 3,884 (2.5) | 4,335 (2.5) | 2,272 (3.3) | 4,836 (2.7) | 3,641 (7.8) | 14,055 (11.9) | 3,593 (7.7) | 4,576 (7.5) | 2,945 (10.3) | 4,328 (7.0) |
| 1–3 | 39,527 (28.8) | 82,469 (35.1) | 46,368 (30.0) | 48,858 (28.5) | 21,062 (30.4) | 51,712 (28.5) | 6,564 (14.1) | 22,470 (19.1) | 6,524 (14.1) | 8,913 (14.5) | 5,140 (18.0) | 8,738 (14.2) |
| 4–6 | 44,901 (32.7) | 71,131 (30.3) | 49,455 (32.0) | 57,290 (33.4) | 22,838 (33.0) | 59,148 (32.6) | 8,724 (18.8) | 23,460 (19.9) | 8,727 (18.8) | 12,891 (21.0) | 6,357 (22.3) | 12,881 (20.9) |
| ≥7 | 49,545 (36.1) | 71,722 (30.6) | 54,844 (35.5) | 61,093 (35.6) | 23,050 (33.3) | 65,889 (36.3) | 27,589 (59.3) | 57,835 (49.1) | 27,531 (59.4) | 34,903 (57.0) | 14,090 (49.4) | 35,607 (57.8) |
| Gastroenterologist encounters | ||||||||||||
| 0 | 129,218 (94.1) | 224,254 (95.5) | 146,194 (94.6) | 161,662 (94.2) | 65,710 (94.9) | 171,161 (94.3) | 42,331 (91.0) | 109,632 (93.1) | 42,264 (91.1) | 56,264 (91.8) | 26,636 (93.4) | 56,682 (92.1) |
| 1–3 | 5,632 (4.1) | 7,144 (3.0) | 5,515 (3.6) | 6,872 (4.0) | 2,363 (3.4) | 6,948 (3.8) | 2,473 (5.3) | 5,123 (4.3) | 2,446 (5.3) | 3,042 (5.0) | 1,147 (4.0) | 2,854 (4.6) |
| 4–6 | 1,694 (1.2) | 2,191 (0.9) | 1,867 (1.2) | 2,028 (1.2) | 754 (1.1) | 2,314 (1.3) | 1,044 (2.2) | 1,826 (1.5) | 953 (2.1) | 1,209 (2.0) | 449 (1.6) | 1,250 (2.0) |
| ≥7 | 797 (0.6) | 1,138 (0.5) | 974 (0.6) | 1,014 (0.6) | 395 (0.6) | 1,164 (0.6) | 670 (1.4) | 1,239 (1.1) | 712 (1.5) | 768 (1.3) | 300 (1.1) | 768 (1.2) |
| ED visits | ||||||||||||
| 0 | 113,067 (82.3) | 187,321 (79.8) | 126,447 (81.8) | 141,079 (82.2) | 57,687 (83.3) | 149,653 (82.4) | 31,207 (67.1) | 78,650 (66.8) | 31,080 (67.0) | 45,561 (74.3) | 21,919 (76.8) | 45,931 (74.6) |
| 1 | 17,434 (12.7) | 33,398 (14.2) | 20,081 (13.0) | 21,857 (12.7) | 8,311 (12.0) | 22,556 (12.4) | 7,758 (16.7) | 19,801 (16.8) | 7,733 (16.7) | 9,329 (15.2) | 4,016 (14.1) | 9,325 (15.1) |
| ≥2 | 6,840 (5.0) | 14,008 (6.0) | 8,023 (5.2) | 8,640 (5.0) | 3,224 (4.7) | 9,376 (5.2) | 7,553 (16.2) | 19,369 (16.4) | 7,561 (16.3) | 6,393 (10.4) | 2,597 (9.1) | 6,298 (10.2) |
| Flu vaccine | 38,131 (27.8) | 59,913 (25.5) | 42,749 (27.7) | 47,518 (27.7) | 17,499 (25.3) | 50,477 (27.8) | 24,498 (52.7) | 56,327 (47.8) | 24,501 (52.8) | 32,500 (53.0) | 13,647 (47.8) | 33,453 (54.3) |
| Smoking¶ | 7,047 (5.1) | 13,504 (5.8) | 8,024 (5.2) | 8,844 (5.2) | 3,079 (4.4) | 9,073 (5.0) | 5,535 (11.9) | 13,635 (11.6) | 5,542 (12.0) | 6,076 (9.9) | 2,075 (7.3) | 6,052 (9.8) |
| Appendectomy | 120 (0.1) | 252 (0.1) | 134 (0.1) | 153 (0.1) | 65 (0.1) | 202 (0.1) | 15 (0.0) | 46 (0.0) | 15 (0.0) | 26 (0.0) | 14 (0.0) | 22 (0.0) |
Data are n (%) unless otherwise indicated. ARB, angiotensin receptor blockers; CCB, calcium-channel blocker; ED, emergency department; GLP1RA, glucagon-like peptide 1 receptor agonist; LAI, long-acting insulin; NA, not applicable; NSAID, nonsteroidal anti-inflammatory drug; NTSR, numbers too small (<11) to report based on Center for Medicare and Medicaid Services rules and data use agreement; SGLT2i, sodium–glucose cotransporter 2 inhibitor.
The comparisons were defined by use of DPP4i and PS-weighted comparator. Covariates were measured in 12 months before the first prescription including the index date. (New users appear to 100% have the treatment at baseline.) Initiation defined as having no prescriptions of either drug class during the 12 months prior to initiation.
The size of the population for a specific drug differed across cohorts because of the requirement not to have been treated prior to index date with the comparator drug class. As shown in Supplementary Fig. 3, before PS trimming, the sample size for MarketScan population is 140,641 vs. 241,834 in DPP4i vs. SU comparison and 178,036 vs. 71,714 in DPP4i vs. TZD comparison, respectively, and the sample size for Medicare population is 47,927 vs. 122,399 in DPP4i vs. SU comparison and 63,474 vs. 30,059 in DPP4i vs. TZD comparison, respectively.
Weighted by standardizing to their distribution in incretin-based therapy initiators by using weights of 1 for DPP4i initiators and the odds of the estimated PS for comparator initiators.
Patients with congestive baseline heart failure were excluded for DPP4i vs. TZD comparison, and patients are required not to have been treated prior to index date with the comparator drug class.
The denominator of percentage is number of female patients.
Smoking was defined using a previously validated algorithm that was a composite of tobacco use diagnosis codes or consultation Current Procedural Terminology codes or prescription filled for smoking cessation. Although it has perfect specificity and positive predictive value, this measure has poor sensitivity (27.9% [95% CI 16.6–39.1%]) (25).
DPP4i and IBD
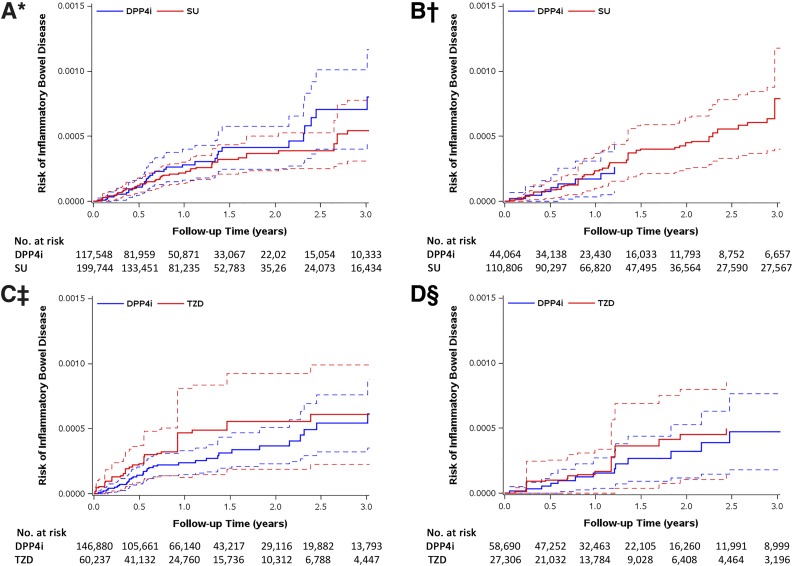
In Table 2, we present results of the primary as-treated analysis for IBD risk. Among the eight cohorts, the observed number of events ranged from <11 to 53, and the median treatment duration ranged from 1.09 to 1.69 years with an average duration of 1.71 years. Crude incidence of IBD ranged from 11.6 to 32.3 events/100,000 person-years, and average incidence was 22.0/100,000 person-years. After adjustment for confounding, DPP4i was not associated with increased risk of IBD. The estimated pHR was 0.82 (95% CI 0.41–1.61) when comparing DPP4i (n = 161,612) to SU (n = 310,550) and 0.76 (0.46–1.26) when comparing DPP4i (n = 205,570) to TZD (n = 87,543) (Supplementary Fig. 5). The median time between first and second prescriptions ranged from 32 to 35 days, the median number of prescriptions during follow-up ranged from 8 to 12, and the majority of patients were censored due to end of enrollment and discontinuation of treatment (Supplementary Tables 4A–C). Weighted Kaplan-Meier curves for each database demonstrated similar patterns; CI bands are wide, especially for TZD (Fig. 1).
Table 2.
Crude HRs and aHRs for IBD associated with use of DPP4i compared with therapeutic alternatives*
| Comparison | Database | Cohort | Number of patients | Duration (years) of treatment, median (IQR) | Person-years | Number of IBD events | IBD rate per 100,000 patient-years | Crude HR (95% CI) | PS-weighting HR (95% CI)† |
|---|---|---|---|---|---|---|---|---|---|
| DPP4i vs. SU | MarketScan | DPP4i | 117,548‡ | 1.21 (0.73–2.04) | 146,171 | 35 | 23.9 (17.2–33.3) | 1.08 (0.70–1.65) | 1.08 (0.70–1.68) |
| SU | 199,744‡ | 1.12 (0.66–1.96) | 238,558 | 53 | 22.2 (17.0–29.1) | ||||
| Medicare | DPP4i | 44,064§ | 1.42 (0.75–2.49) | NA | NTSR | 11.6 (5.8–23.1) | 0.52 (0.25–1.10) | 0.53 (0.24–1.15) | |
| SU | 110,806§ | 1.69 (0.90–2.91) | 202,385 | 44 | 21.7 (16.2–29.2) | ||||
| DPP4i vs. TZD | MarketScan | DPP4i | 146,880‖ | 1.27 (0.75–2.13) | 189,987 | 40 | 21.1 (15.4–28.7) | 0.67 (0.40–1.11) | 0.68 (0.37–1.26) |
| TZD | 60,237‖ | 1.09 (0.67–1.88) | 71,268 | 23 | 32.3 (21.4–48.6) | ||||
| Medicare | DPP4i | 58,690¶ | 1.50 (0.81–2.57) | 95,351 | 17 | 17.8 (11.1–28.7) | 0.58 (0.28–1.21) | 0.97 (0.40–2.37) | |
| TZD | 27,306¶ | 1.27 (0.74–2.18) | 39,834 | 12 | 30.1 (17.1–53.0) |
IQR, interquartile range; NTSR, numbers too small (<11) to report based on Center for Medicare and Medicaid Services rules and data use agreement (person-years is not shown in this case to block the number of events).
Analysis was based on as-treated exposure definition, follow-up started from 180 days (induction period) after the second prescription and ended at the earliest of the following events: 1) 180 days after the date of prescription change including discontinuation or initiating the other drug in the drug pair that is being compared; 2) the end of enrollment (the end of enrollment for Parts A, B, or D or enrollment for HMO for Medicare beneficiaries); 3) death (for Medicare beneficiaries only); 4) administrative study end (31 December 2016); or 5) observation of an incident IBD event.
PS-weighted HRs were standardized to the distribution of baseline covariates in DPP4i initiators.
Before the start of follow-up, the following patients were excluded from the PS-trimmed cohort (sample size shown in Table 1): 19,793 DPP4i initiators, including 21 with IBD events and 19,772 with discontinuation of enrollment or who reached end of study date; and 34,983 SU initiators, including 29 with IBD events and 34,957 with discontinuation of enrollment or who reached end of study date.
Before the start of follow-up, the following patients were excluded from the PS-trimmed cohort: 2,454 DPP4i initiators, including a few (NTSR) with IBD events and 2,449 who died, discontinued enrollment, or reached end of study date; and 7,014 SU initiators, including 14 with IBD events and 7,000 who died, discontinued enrollment, or reached end of study date.
Before the start of follow-up, the following patients were excluded from PS-trimmed cohort: 24,696 DPP4i initiators, including 20 with IBD events and 24,676 with discontinuation of enrollment or who reached end of study date; and 8,985 TZD initiators, including 6 with IBD events and 8,979 with discontinuation of enrollment or who reached end of study date.
Before the start of follow-up, the following patients were excluded from the PS-trimmed cohort: 2,593 DPP4i initiators, including a few (NTSR) with IBD events and 2,588 who died, discontinued enrollment, or reached end of study date; and 1,226 TZD initiators, including a few (NTSR) with IBD events and 1,223 who died, discontinued enrollment, or reached end of study date.
Figure 1.
SMR-weighted Kaplan-Meier plots of IBD: DPP4i vs. SU cohort in MarketScan (A), DPP4i vs. SU cohort in Medicare (B), DPP4i vs. TZD cohort in MarketScan (C), and DPP4i vs. TZD cohort in Medicare (D). Follow-up started for the outcome 180 days (induction period) after the second prescription (cohort entry date). SMR weights create a pseudopopulation of the untreated (comparators: SU or TZD), which has the same covariate distribution as the treated (DPP4i). Every patient receiving DPP4i has a weight of 1, while every patient in the comparator group is weighted by (PS/[1 − PS]). The risks on the y-axis were obtained by SMR-weighted Cox model (weighting comparator drug initiators by the PS odds [PS/(1 − PS)]). HR treating comparators as reference; aHR <1 indicates a lower risk for DPP4i. Dotted lines around the survival curve point estimates represent 95% CI bands. *HR 1.08 (95% CI 0.70–1.68); †HR 0.53 (0.24–1.15); ‡HR 0.68 (0.37–1.26); §HR 0.97 (0.40–2.37).
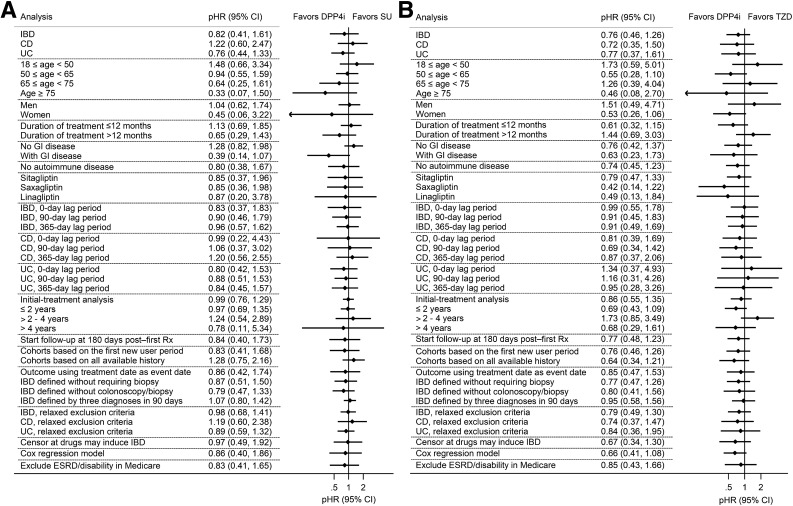
Results for secondary analyses are shown in Fig. 2. Compared with SU, DPP4i showed a trend for increased risk for CD (pHR 1.22 [95% CI 0.60–2.47]). With stratification by age, we observed a higher HR in younger patients in the MarketScan population. DPP4i showed a lower risk versus SU in patients with preexisting gastrointestinal disease (pHR 0.39 [0.14–1.07]), and a lower risk versus TZD in women (pHR 0.53 [0.26–1.06]). Overall, the pHRs did not differ meaningfully in stratified analysis and CIs widely overlapped.
Figure 2.
Forest plot summarizing primary, secondary, and sensitivity analyses showing the association between initiating DPP4i and the risk of IBD compared with SU (A) and TZD (B), respectively. The effect estimate for each analysis is the pooled aHR from the two databases. ESRD, end-stage renal disease; GI, gastrointestinal; Rx, prescription.
Sensitivity Analyses
Overall, sensitivity analysis results for IBD were consistent with our primary analysis (Fig. 2); detailed results for each analysis were shown in the Supplementary Data. Weighed Kaplan-Meier curves for the first new-user period–based cohort (Supplementary Fig. 19B) showed a pattern similar to that of the primary analysis. In initial-treatment analysis, the median follow-up time ranged from 1.78 to 3.86 years, with an average follow-up time of 2.58 years. When stratified by time since initiation, pHR reached a peak between 2 and 4 years after initiation (pHR 1.24 [0.54–2.89] vs. SU and 1.73 [0.85–3.49] vs. TZD, respectively) and then decreased after 4 years.
Conclusions
Summary
Our study examined data from two large U.S. databases and observed no evidence of increased risk of IBD for DPP4i compared with therapeutic alternatives, over an average treatment duration of ∼1.5–2 years. Instead, we observed effect estimates that suggested a possibility of decreased IBD risk in new use of DPP4i compared to TZD. The results were robust across secondary and sensitivity analyses.
DPP4i and IBD Risk
Our results are in line with the recent meta-analysis of randomized trials (5), which reported similar IBD risk between DPP4i users and comparators (relative risk 1.01 [0.31–3.41] over a duration of 2.4 years). Notably, we observed slightly decreased pHRs of DPP4i compared with therapeutic alternatives, especially versus TZD, suggesting the possibility of a protective effect. This is consistent with previous studies on mouse models suggesting that DPP4i can lead to decreased IBD activity (24–28). A proposed role for DPP4i as a novel pharmacological agent for IBD is based in its anti-inflammatory and immunomodulatory effects. An interaction between the DPP4i and DPP4 is essential for a reduced inflammatory response, and DPP4 expressed on the cell surface is critical for DPP4i’s immunomodulatory effects (25).
However, some clinical data also suggest that patients with IBD have lower serum DPP4 concentration, which is inversely associated with increased IBD activity, and it is undetermined whether the lower serum DPP4 is the consequence or cause of IBD (29–31). We also detected a weak signal for increased CD risk of initiating DPP4i versus SU and a trend for increased risk between 2 and 4 years after initiation in the initial-treatment analysis. Thus, a more complex mechanism may exist, and future studies are needed to investigate effects of DPP4i on IBD.
Comparison With Previous Studies
Our results are consistent with the recent meta-analysis of 13 trials (5). Both cross-sectional analyses suggested weak to moderate signal (6,7); however, pharmacovigilance databases are prone to reporting bias and cannot be used to assess incident IBD.
The CPRD study by Abrahami et al. (4) suggested an increased risk of both IBD (HR 1.75 [95% CI 1.22–2.49]) and UC (HR 2.23 [1.32–3.76]) associated with DPP4i use. The risk appeared to increase between 2 and 4 years after treatment initiation, and there was no significant association in the first 2 years or after 4 years of use. No signal for CD (HR 0.87 [0.37–2.09]) was detected. In contrast, our study did not suggest an association between DPP4i initiation and IBD risk, and we observed a weak signal only for CD. We also observed a trend for an increased risk between 2 and 4 years after initiation in our initial-treatment analysis.
Our study differed from the CPRD study in several ways, including exclusion criteria, outcome definition, study design, covariates, study population, and follow-up time. We found no meaningful difference between treatments in sensitivity analyses in which we relaxed exclusion criteria and used less specific outcome definitions that were similar to the CPRD study. Notably, the follow-up time for our initial-treatment analysis (pertinent information to allow for a comparison) was shorter than the CPRD study. Additionally, the inconsistent results may be due to differences in study design and analysis choices.
In our ACNU cohort, we compared DPP4i to other second-line treatments, SU or TZD, which may be a more reasonable choice, as it leads to less clinical heterogeneity between index and comparator groups and therefore reduces the potential for unmeasured confounding. Our ACNU design also requires a washout period before initiating therapy for DPP4i and comparator drug classes, which minimizes bias from treatment choice affected by previous treatment (other GLDs that may influence subsequent prescribing are balanced by PSs). Both DPP4i and comparator initiators were analyzed in the same fashion (i.e., in either as-treated [censoring patients who initiated the other drug in the drug pair that is being compared] or initial-treatment [ignoring treatment changes] analysis).
By comparison, the CPRD study (4) compared DPP4i with non-DPP4i GLDs, including first-line metformin therapy. Moreover, patients were allowed to switch from comparator to DPP4i after cohort entry but not the other way around (thereby allowing the same patient to contribute unexposed and then exposed person-time, but not vice versa). Although DPP4i users and nonswitchers were analyzed by initial-treatment analysis, those switchers were handled in an as-treated fashion (censored when switching) and then were included in the DPP4i group and analyzed in initial-treatment fashion (Supplementary Fig. 26). Therefore, DPP4i and comparator groups were not actually analyzed in the same way. A proper initial-treatment analysis handles both treatment cohorts in the same manner (i.e., ignoring treatment changes in both groups), which in turn avoids selection bias. Considering the high switching rate among second-line GLD users in CPRD data (∼45% at 18 months [32]), a large proportion of patients likely switched to DPP4i in the CPRD study. Such switchers may be affected by previous therapy—for example, physicians may switch patients on metformin or glucagon-like peptide 1 receptor agonists to DPP4i due to development of gastrointestinal symptoms (which may be either drug adverse events [33] or early symptoms of IBD). Notably, in a head-to-head comparison of the CPRD study, a higher risk was also observed comparing DPP4i versus insulin (HR 2.28 [1.07–4.85]), which might be due to a speculative potential protective effect of insulin on UC (34).
Limitations
Our findings should be viewed in light of limitations. First, we had no information on diabetes duration, and data on measures of glycemic control were available for only a small proportion of the population. The presence of risk factors, such as smoking, in claims data has high specificity but low sensitivity (35). However, Wang et al. (36) have demonstrated that clinical measures such as hemoglobin A1c are well balanced between DPP4i versus SU and DPP4i versus TZD comparisons in their ACNU study.
Second, our algorithm for detection of the outcome has not been validated. However, it is unlikely that cases that met our disease definition (with colonoscopy, biopsy, and treatments) are not incident IBD. A high-specificity outcome definition minimizes bias in the HR, even in the presence of nonperfect sensitivity (37).
Third, we only assessed short-term DPP4i use (average duration 1.71 years) because real-world adherence and persistence are low (10), which may impair the potential for detecting differences with long-term use of these drugs, and our conclusion is only generalizable to short-term use. Censoring treatment change in the primary analysis is a potential source of selection bias. As information that drives treatment decisions (e.g., laboratory data or subtle side effects) is often missing in secondary databases, we did not predict adherence or use inverse probability of censoring weights to address censoring analytically. Our initial-treatment analysis ignoring treatment change avoids selection bias and yielded similar results, but we cannot necessarily rule out the possibility that informative censoring has affected results of our primary analysis.
Lastly, our ACNU cohorts are a selection of DPP4i initiators with no recent history of comparator drug use and therefore do not cover all DPP4i initiators in the real world. Using a 12-month baseline period and including multiple new-user periods that meet the washout period cannot completely rule out prevalent IBD. However, our analyses for only the first new-user period and Kaplan-Meier plots are consistent with primary analysis, suggesting no immortal-time bias. In addition, all available look-back history–based cohorts also yielded similar results, indicating that the inclusion of patients with prevalent IBD was not a major limitation. We therefore do not believe that such biases played a major role in our results.
In conclusion, our population-based, ACNU cohort study of older U.S. adults with diabetes suggests that the short-term real-world use of DPP4i does not increase the risk of IBD compared with second-line alternatives. This finding should be reassuring to physicians and patients who are considering the potential benefits and risks of DPP4i.
Supplementary Material
Article Information
Acknowledgments. The authors thank Dr. Blànaid Hicks from Queen’s University Belfast, U.K., for providing Supplementary Fig. 26 and expert advice.
Funding and Duality of Interest. J.B.B. has received contracted consulting fees paid to the University of North Carolina (UNC) at Chapel Hill by Adocia, AstraZeneca, Dance Biopharm Inc., Eli Lilly and Company, MannKind Corporation, NovaTarg Therapeutics, Novo Nordisk, Senseonics, vTv Therapeutics, and Zafgen and grant support from Novo Nordisk, Sanofi, and vTv Therapeutics; is a consultant to Cirius Therapeutics, CSL Behring, Neurimmune AG, and Whole Biome; holds stock options in Mellitus Health, PhaseBio, Stability Health, and Whole Biome; and is supported by a grant from the National Institutes of Health (UL1-TR-002489, U01-DK-098246, UC4-DK-108612, and U54-DK-118612), the Patient-Centered Outcomes Research Institute, and the American Diabetes Association. J.Y.Y. receives funding support as a trainee from the National Institutes of Health (T32-DK-007634) and through the UNC Royster Society of Fellows. V.P. receives salary support from National Institutes of Health grants R01-AG-056479 and R01-HL-118255 and the National Center for Advancing Translational Sciences (UL1-TR-002489). T.S. receives investigator-initiated research funding and support as principal investigator from the National Institute on Aging (R01-AG-056479) and as co-investigator from the National Institutes of Health (R01-CA-174453, R01-HL-118255, and R21-HD-080214); he also receives salary support as Director of Comparative Effectiveness Research (CER), North Carolina Translational and Clinical Sciences Institute, UNC Clinical and Translational Science Award (UL1-TR-002489), the Center for Pharmacoepidemiology (current members: GlaxoSmithKline, UCB Biosciences Inc, Merck, and Takeda), from pharmaceutical companies (GlaxoSmithKline, Amgen, AstraZeneca, and Novo Nordisk), and from a generous contribution from Dr. Nancy A. Dreyer to the Department of Epidemiology, UNC at Chapel Hill. T.S. does not accept personal compensation of any kind from any pharmaceutical company but owns stock in Novartis, Roche, BASF, AstraZeneca, and Novo Nordisk. Medicare: the database infrastructure used for this project was funded by the Pharmacoepidemiology Gillings Innovation Lab for the Population-Based Evaluation of Drug Benefits and Harms in Older US Adults (GIL200811.0010), the Center for Pharmacoepidemiology, Department of Epidemiology, UNC Gillings School of Global Public Health, the CER Strategic Initiative of UNC’s Clinical Translational Science Award (UL1-TR-002489), the Cecil G. Sheps Center for Health Services Research, UNC, and the UNC School of Medicine. MarketScan (Truven Health Analytics Inc.): the database infrastructure used for this project was funded by the Department of Epidemiology, UNC Gillings School of Global Public Health, the Cecil G. Sheps Center for Health Services Research, UNC, the CER Strategic Initiative of UNC’s Clinical Translational Science Award (UL1-TR-002489), and the UNC School of Medicine. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. T.W. wrote the first draft of the manuscript and did the statistical analysis. V.P. oversaw and supported programing. T.W., J.Y.Y., J.B.B., H.T., E.L.B., R.S.S., and T.S. were involved in data review and interpretation. T.W., J.Y.Y., J.B.B., E.L.B., R.S.S., and T.S. contributed to critical revision of the manuscript for important intellectual content. T.W., J.Y.Y., and T.S. developed the protocol. T.W., J.B.B., and T.S. conceived and designed the study. T.W., J.Y.Y., J.B.B., V.P., H.T., E.L.B., R.S.S., and T.S. approved the final version of the manuscript. J.Y.Y., E.L.B., and R.S.S. participated in the design of the study. H.T. performed meta-analyses. T.S. supervised the study. T.W. and T.S. are the guarantors of this work and, as such, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc19-0162/-/DC1.
References
- 1.Drucker DJ, Sherman SI, Gorelick FS, Bergenstal RM, Sherwin RS, Buse JB. Incretin-based therapies for the treatment of type 2 diabetes: evaluation of the risks and benefits. Diabetes Care 2010;33:428–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Price JD, Linder G, Li WP, et al. Effects of short-term sitagliptin treatment on immune parameters in healthy individuals, a randomized placebo-controlled study. Clin Exp Immunol 2013;174:120–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim SC, Schneeweiss S, Glynn RJ, Doherty M, Goldfine AB, Solomon DH. Dipeptidyl peptidase-4 inhibitors in type 2 diabetes may reduce the risk of autoimmune diseases: a population-based cohort study. Ann Rheum Dis 2015;74:1968–1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abrahami D, Douros A, Yin H, et al. Dipeptidyl peptidase-4 inhibitors and incidence of inflammatory bowel disease among patients with type 2 diabetes: population based cohort study. BMJ 2018;360:k872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li G, Crowley MJ, Tang H, Yang JY, Sandler RS, Wang T. Dipeptidyl peptidase 4 inhibitors and risk of inflammatory bowel disease among patients with type 2 diabetes: a meta-analysis of randomized controlled trials. Diabetes Care 2019;42:e119–e121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang T, Lu W, Li D, et al. Assessing the association between dipeptidyl peptidase 4 inhibitor use and inflammatory bowel disease through drug adverse event reporting. Diabetes Care 2019;42:e89–e91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kridin K, Amber K, Khamaisi M, Comaneshter D, Batat E, Cohen AD. Is there an association between dipeptidyl peptidase-4 inhibitors and autoimmune disease? A population-based study. Immunol Res 2018;66:425–430 [DOI] [PubMed] [Google Scholar]
- 8.Lund JL, Richardson DB, Stürmer T. The active comparator, new user study design in pharmacoepidemiology: historical foundations and contemporary application. Curr Epidemiol Rep 2015;2:221–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Diabetes Association 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019;42(Suppl. 1):S90–S102 [DOI] [PubMed] [Google Scholar]
- 10.Carls GS, Tuttle E, Tan RD, et al. Understanding the gap between efficacy in randomized controlled trials and effectiveness in real-world use of GLP-1 RA and DPP-4 therapies in patients with type 2 diabetes. Diabetes Care 2017;40:1469–1478 [DOI] [PubMed] [Google Scholar]
- 11.Podolsky DK. Inflammatory bowel disease. N Engl J Med 2002;347:417–429 [DOI] [PubMed] [Google Scholar]
- 12.Feuerstein JD, Nguyen GC, Kupfer SS, Falck-Ytter Y, Singh S; American Gastroenterological Association Institute Clinical Guidelines Committee . American Gastroenterological Association institute guideline on therapeutic drug monitoring in inflammatory bowel disease. Gastroenterology 2017;153:827–834 [DOI] [PubMed] [Google Scholar]
- 13.Bibbins-Domingo K, Grossman DC, Curry SJ, et al.; US Preventive Services Task Force . Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA 2016;315:2564–2575 [DOI] [PubMed] [Google Scholar]
- 14.Gilbert RE, Krum H. Heart failure in diabetes: effects of anti-hyperglycaemic drug therapy. Lancet 2015;385:2107–2117 [DOI] [PubMed] [Google Scholar]
- 15.Nguyen VQ, Jiang D, Hoffman SN, et al. Impact of diagnostic delay and associated factors on clinical outcomes in a U.S. inflammatory bowel disease cohort. Inflamm Bowel Dis 2017;23:1825–1831 [DOI] [PubMed] [Google Scholar]
- 16.Loftus EV., Jr Clinical epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology 2004;126:1504–1517 [DOI] [PubMed] [Google Scholar]
- 17.Stürmer T, Rothman KJ, Avorn J, Glynn RJ. Treatment effects in the presence of unmeasured confounding: dealing with observations in the tails of the propensity score distribution--a simulation study. Am J Epidemiol 2010;172:843–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sato T, Matsuyama Y. Marginal structural models as a tool for standardization. Epidemiology 2003;14:680–686 [DOI] [PubMed] [Google Scholar]
- 19.Cole SR, Hernán MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed 2004;75:45–49 [DOI] [PubMed] [Google Scholar]
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–188 [DOI] [PubMed] [Google Scholar]
- 21.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–1558 [DOI] [PubMed] [Google Scholar]
- 22.Bernstein CN, Blanchard JF, Rawsthorne P, Wajda A. Epidemiology of Crohn’s disease and ulcerative colitis in a central Canadian province: a population-based study. Am J Epidemiol 1999;149:916–924 [DOI] [PubMed] [Google Scholar]
- 23.Dubeau M-F, Iacucci M, Beck PL, et al. Drug-induced inflammatory bowel disease and IBD-like conditions. Inflamm Bowel Dis 2013;19:445–456 [DOI] [PubMed] [Google Scholar]
- 24.Klemann C, Wagner L, Stephan M, von Hörsten S. Cut to the chase: a review of CD26/dipeptidyl peptidase-4's (DPP4) entanglement in the immune system. Clin Exp Immunol 2016;185:1–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yazbeck R, Howarth GS, Abbott CA. Dipeptidyl peptidase inhibitors, an emerging drug class for inflammatory disease? Trends Pharmacol Sci 2009;30:600–607 [DOI] [PubMed] [Google Scholar]
- 26.Yazbeck R, Howarth GS, Geier MS, Demuth HU, Abbott CA. Inhibiting dipeptidyl peptidase activity partially ameliorates colitis in mice. Front Biosci 2008;13:6850–6858 [DOI] [PubMed] [Google Scholar]
- 27.Mimura S, Ando T, Ishiguro K, et al. Dipeptidyl peptidase-4 inhibitor anagliptin facilitates restoration of dextran sulfate sodium-induced colitis. Scand J Gastroenterol 2013;48:1152–1159 [DOI] [PubMed] [Google Scholar]
- 28.Bank U, Heimburg A, Helmuth M, et al. Triggering endogenous immunosuppressive mechanisms by combined targeting of dipeptidyl peptidase IV (DPIV/CD26) and aminopeptidase N (APN/ CD13)--a novel approach for the treatment of inflammatory bowel disease. Int Immunopharmacol 2006;6:1925–1934 [DOI] [PubMed] [Google Scholar]
- 29.Ohnuma K, Hosono O, Dang NH, Morimoto C. Dipeptidyl peptidase in autoimmune pathophysiology. Adv Clin Chem 2011;53:51–84 [DOI] [PubMed] [Google Scholar]
- 30.Magro DO, Kotze PG, Martinez CAR, et al. Changes in serum levels of lipopolysaccharides and CD26 in patients with Crohn’s disease. Intest Res 2017;15:352–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moran GW, O’Neill C, Padfield P, McLaughlin JT. Dipeptidyl peptidase-4 expression is reduced in Crohn’s disease. Regul Pept 2012;177:40–45 [DOI] [PubMed] [Google Scholar]
- 32.Wilding J, Godec T, Khunti K, et al. Changes in HbA1c and weight, and treatment persistence, over the 18 months following initiation of second-line therapy in patients with type 2 diabetes: results from the United Kingdom Clinical Practice Research Datalink. BMC Med 2018;16:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Du YT, Rayner CK, Jones KL, Talley NJ, Horowitz M. Gastrointestinal symptoms in diabetes: prevalence, assessment, pathogenesis, and management. Diabetes Care 2018;41:627–637 [DOI] [PubMed] [Google Scholar]
- 34.Yassin M, Sadowska Z, Tritsaris K, et al. Rectal insulin instillation inhibits inflammation and tumor development in chemically induced colitis. J Crohn’s Colitis 2018;12:1459–1474 [DOI] [PubMed] [Google Scholar]
- 35.Desai RJ, Solomon DH, Shadick N, Iannaccone C, Kim SC. Identification of smoking using Medicare data—a validation study of claims-based algorithms. Pharmacoepidemiol Drug Saf 2016;25:472–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang T, Hong J-L, Gower EW, et al. Incretin-based therapies and diabetic retinopathy: real-world evidence in older U.S. adults. Diabetes Care 2018;41:1998–2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chubak J, Pocobelli G, Weiss NS. Tradeoffs between accuracy measures for electronic health care data algorithms. J Clin Epidemiol 2012;65:343–349.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.