Abstract
OBJECTIVE
GRADE (Glycemia Reduction Approaches in Diabetes: A Comparative Effectiveness Study) is a 36-center unmasked, parallel treatment group, randomized controlled trial evaluating four diabetes medications added to metformin in people with type 2 diabetes (T2DM). We report baseline characteristics and compare GRADE participants to a National Health and Nutrition Examination Survey (NHANES) cohort.
RESEARCH DESIGN AND METHODS
Participants were age ≥30 years at the time of diagnosis, with duration of T2DM <10 years, HbA1c 6.8–8.5% (51–69 mmol/mol), prescribed metformin monotherapy, and randomized to glimepiride, sitagliptin, liraglutide, or insulin glargine.
RESULTS
At baseline, GRADE’s 5,047 randomized participants were 57.2 ± 10.0 years of age, 63.6% male, with racial/ethnic breakdown of 65.7% white, 19.8% African American, 3.6% Asian, 2.7% Native American, 7.6% other or unknown, and 18.4% Hispanic/Latino. Duration of diabetes was 4.2 ± 2.8 years, with mean HbA1c of 7.5 ± 0.5% (58 ± 5.3 mmol/mol), BMI of 34.3 ± 6.8 kg/m2, and metformin dose of 1,944 ± 204 mg/day. Among the cohort, 67% reported a history of hypertension, 72% a history of hyperlipidemia, and 6.5% a history of heart attack or stroke. Applying GRADE inclusion criteria to NHANES indicates enrollment of a representative cohort with T2DM on metformin monotherapy (NHANES cohort average age, 57.9 years; mean HbA1c, 7.4% [57 mmol/mol]; BMI, 33.2 kg/m2; duration, 4.2 ± 2.5 years; and 7.2% with a history of cardiovascular disease).
CONCLUSIONS
The GRADE cohort represents patients with T2DM treated with metformin requiring a second diabetes medication. GRADE will inform decisions about the clinical effectiveness of the addition of four classes of diabetes medications to metformin.
Introduction
The optimal medication management of hyperglycemia in type 2 diabetes (T2DM) is not established. In addition to lifestyle intervention, metformin is the recommended initial medication in T2DM due to its glycemic effectiveness, lack of associated hypoglycemia or weight gain, low cost, and evidence of long-term benefit and safety (1,2). Over time, most patients are unable to maintain glycemic control with metformin alone, with an estimated 20–50% incidence of metformin monotherapy failure within 5 years (3–6). The UK Prospective Diabetes Study (UKPDS) demonstrated that only 50% of patients with newly diagnosed diabetes could maintain glycemic goals with monotherapy after 3 years, declining to ∼25% by 9 years (5). Hence, most patients with T2DM will require a second medication in addition to metformin for glycemic management.
Clinicians may choose among many medication classes and multiple options within each class, in addition to metformin, for the treatment of T2DM (7). In the absence of cardiovascular disease (CVD), current guidelines propose choosing from among individual medications or medication classes based on patient characteristics and treatment goals (8,9). Although patients take diabetes medications for many years, there has been a paucity of long-term head-to-head comparison trials, and, for the most part, only limited comparisons, usually involving two medications or classes, have been performed (1,10,11). The Glycemia Reduction Approaches in Diabetes: A Comparative Effectiveness Study (GRADE) aims to fulfill a primary goal of comparative effectiveness research: testing commonly used medication combinations in randomly assigned treatment groups over time to aid in real-world clinical decision making (12). GRADE will compare four medications in combination with metformin over ∼5 years.
This report describes the baseline characteristics of the 5,047 participants enrolled in GRADE, providing a novel description of a large randomized cohort with T2DM of <10 years’ duration prescribed metformin monotherapy. In addition, we compare the GRADE cohort to a National Health and Nutrition Examination Survey (NHANES) cohort meeting GRADE inclusion criteria to assess the broader generalizability of GRADE.
Research Design and Methods
General
GRADE is being conducted at 36 centers across the U.S. (Fig. 1). The full protocol is available at https://portal.bsc.gwu.edu/web/grade and in the Supplementary Data. The Institutional Review Board at each clinical center approved the protocol, and all participants gave written informed consent before any study procedures. The first patient was enrolled in July 2013, and enrollment concluded in August 2017. The trial is registered on ClinicalTrials.gov, identifier NCT01794143.
Figure 1.
Map of GRADE clinical centers.
Participants
Eligibility requirements for GRADE at screening and randomization have been previously reported (1) and are updated in the Supplementary Data (Protocol 1.6.1, pages 13–15). Eligibility in the final protocol included patients with T2DM, with a diagnosis of diabetes <10 years prior (initially 5 years; Protocol v.1.3, released 15 January 2014, extended eligibility to 10 years), diagnosed at age ≥30 years in non-American Indian (AI)/Alaska Native (AN) patients or age ≥20 for AI/AN, taking metformin monotherapy (at least 1,000 mg/day), HbA1c 6.8–8.5% (51–69 mmol/mol) at randomization, and willingness to take a second diabetes medication, including daily injections of insulin if required. Key exclusion criteria included evidence of type 1 or secondary forms of diabetes, use of other diabetes medications within the last 6 months, history of intolerance or allergy to any of the proposed study medications or sulfa drugs, estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2, major cardiovascular event within the previous year, history of pancreatitis, congestive heart failure (New York Heart Association Functional Classification ≥III), new diagnosis or treatment for any cancer (other than nonmelanoma skin cancer) within the previous 5 years, planned major surgery, or planned pregnancy for women of childbearing potential.
Study Design
GRADE is a parallel treatment group, unmasked clinical trial. Eligible participants were randomly assigned to one of four diabetes medications (1:1:1:1) in combination with metformin, representing the four main treatment classes of diabetes medications that were approved by the U.S. Food and Drug Administration (FDA) in combination with metformin and in common use at the time the trial was designed: glimepiride (sulfonylurea), sitagliptin (dipeptidyl peptidase 4 [DPP-4] inhibitor), liraglutide (glucagon-like peptide 1 [GLP-1] receptor agonist), and glargine (basal insulin) (see Supplementary Data: Protocol Fig. 1). Medications were selected based on data regarding efficacy, safety profile, daily (rather than twice-daily) dosing, and availability of a donated supply by a subset of investigators without conflicts of interest and used in accordance with their labeling (1).
GRADE is an intention-to-treat study in which all participants are requested to continue quarterly follow-up for all study outcomes until the close of the study in April 2021. The planned follow-up period for participants ranges from 3.25 to 7.5 years, with an estimated mean duration of follow-up of 5.2 years, not accounting for losses to follow-up.
Clinical Centers
Clinical centers were chosen by peer review of applications received in response to a request for support announcement. Clinical centers were selected in part to ensure broad national representation, including representation of the overall racial and ethnic diversity of people with T2DM. As shown in Fig. 1, GRADE has 36 clinical centers varying in size, region, and practice environment (e.g., academic, community, closed-model HMOs, and Veterans Administration health care systems).
Recruitment
Participants were identified through Institutional Review Board-approved electronic health record queries and other local outreach methods. After an initial contact, participants attended a screening visit at which eligibility was assessed. Eligible participants then initiated a run-in period of 4–8 weeks during which the dose of metformin was escalated. Participants who were still eligible after the run-in attended the randomization visit.
Variables and Assessments
Assessments were completed during screening and run-in and at the baseline randomization visits. Participant race and ethnicity, medical history, current medications, alcohol intake, smoking status, and educational attainment were self-reported and obtained through interviews conducted by research staff. All assessments in this report were attempted for all participants, with the exception of querying use of medications for depression or anxiety; this question was added after study initiation and was collected on 2,502 participants only. All physical and metabolic measurements were obtained by certified staff. Height, weight, and blood pressure were taken in duplicate by trained clinical research staff. Height was recorded to the nearest 0.1 cm and weight to the nearest 0.1 kg. Seated blood pressure was taken after resting for 5 min and repeated after 1 min; measurements were averaged.
History of hypertension, hyperlipidemia, heart attack or stroke, and retinopathy were obtained by self-report. Diabetic peripheral neuropathy was measured by combining the 15-item symptom questionnaire and the 4 physical examination components of the Michigan Neuropathy Screening Instrument (MNSI). A value of 3.2883 on the combined questionnaire and examination index correctly classifies 80% of diabetic peripheral neuropathy with a sensitivity of 48% and specificity of 93% (13).
All laboratory tests were performed by the Central Biochemistry Laboratory (Advanced Research and Diagnostic Laboratory, Department of Laboratory Medicine and Pathology, at the University of Minnesota) using standardized laboratory procedures. HbA1c in GRADE, as for NHANES, is standardized per NGSP protocol. Baseline physical assessment and laboratory values are reported, with laboratory values obtained at the final run-in visit or at randomization.
Outcomes
Details of outcome ascertainment have been previously described (1). The primary outcome for GRADE is the time to primary failure of the randomly assigned treatment, defined as the time to an initial HbA1c ≥7% (≥53 mmol/mol), subsequently confirmed at the next visit, while being treated at maximum tolerable doses of both metformin and the second randomly assigned medication. Participants will be analyzed as part of their randomly assigned medication group according to intention-to-treat principles (14) regardless of adherence to the assigned medication.
Additional outcomes have been previously described (1), including metabolic outcomes, cardiovascular outcomes, microvascular outcomes, adverse effects, side-effect profiles, adherence, safety and tolerability, quality of life, and health-economic evaluation.
Statistical Analysis
For this baseline report, descriptive statistics are provided for all baseline characteristics presented. Data are presented as mean ± SD or median (interquartile range) for continuous variables and n (%) for categorical variables.
Comparisons to NHANES Cohort
We report baseline characteristics of GRADE participants compared with an NHANES subsample meeting GRADE eligibility criteria. NHANES is a set of stratified, multistage probability surveys conducted by the National Center for Health Statistics that are designed to represent the U.S. civilian noninstitutionalized population. NHANES uses standardized questionnaires and measurements, as previously described (15,16). We report characteristics of NHANES respondents in 2011–2014, age ≥30 years with diabetes for <10 years, HbA1c of 6.8–8.5% (51–69 mmol/mol), and taking metformin alone. We used the NHANES 2011–2014 cycle because it overlapped in time with the onset of GRADE recruitment and contained all relevant variables, including metformin use. NHANES analyses use weights provided by NHANES so that estimates are representative of the U.S. civilian noninstitutionalized population and to account for the complex survey design and survey nonresponse.
Results
Enrollment
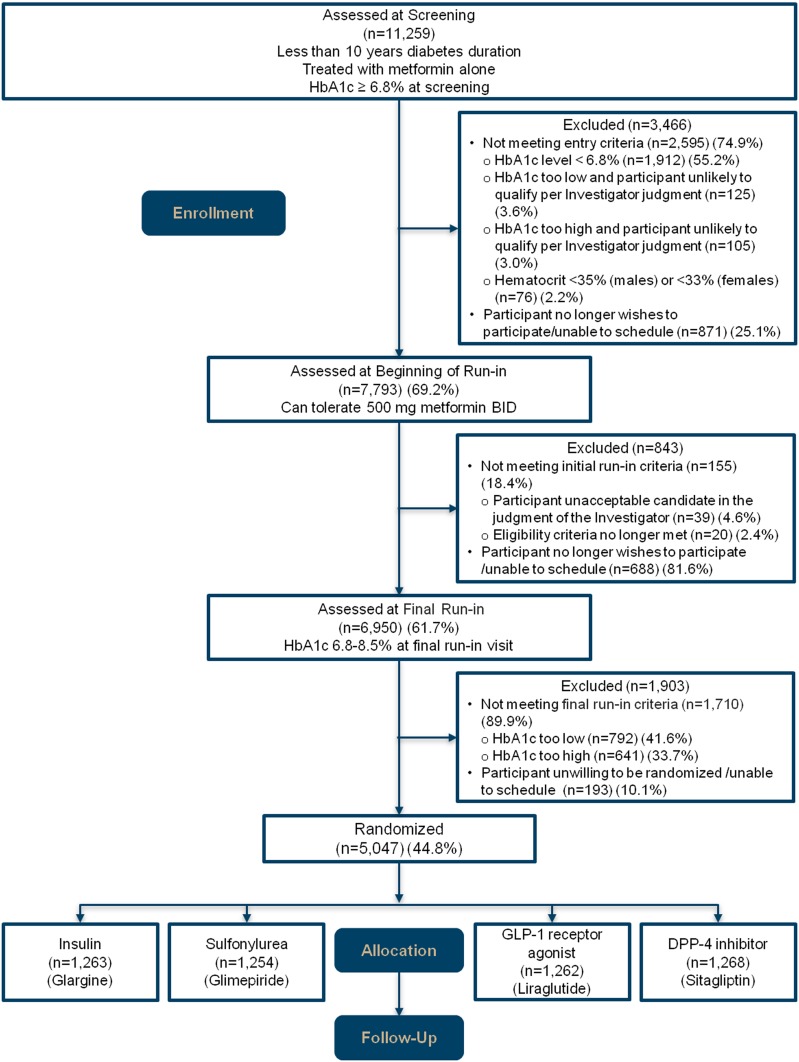
GRADE screened 11,259 patients in person (Fig. 2). Of these, 3,466 were immediately excluded, 58.8% because HbA1c was too low at the time of screening or was deemed likely to fall below the inclusion criterion of 6.8% (51 mmol/mol) by the end of run-in. The final run-in visit was attended by 61.7% of screened participants, after which 1,903 were excluded, 41.6% because the HbA1c was <6.8% (51 mmol/mol) and 33.7% because the HbA1c was >8.5% (69 mmol/mol). Of those screened, 5,047 (44.8%) were randomly assigned to one of the four study treatment groups.
Figure 2.
Consolidated Standards of Reporting Trials diagram.
Demographic Characteristics
Baseline characteristics of participants are summarized in Table 1. Mean age is 57.2 ± 10.0 years, and 64% of the study participants are male. The racial composition of the cohort is 65.7% white, 19.8% African American, 3.6% Asian, 2.7% AI/AN, 0.6% Native Hawaiian or other Pacific Islander, 6.3% other or more than one race, and 1.3% unknown or not reported. Hispanic/Latino ethnicity was reported by 18.4% of participants.
Table 1.
Baseline characteristics of participants in GRADE
| Overall (n = 5,047)* | Normal range for laboratory tests | |
|---|---|---|
| Age at baseline visit (years) | 57.2 ± 10.0 | |
| Age group (years) | ||
| <45 | 619 (12.3) | |
| 45–59 | 2,327 (46.1) | |
| ≥60 | 2,101 (41.6) | |
| Male sex | 3,210 (63.6) | |
| Race | ||
| White | 3,314 (65.7) | |
| African American or black | 1,000 (19.8) | |
| Asian | 182 (3.6) | |
| AI/AN | 137 (2.7) | |
| Native Hawaiian or other Pacific Islander | 28 (0.6) | |
| Other or more than one race | 319 (6.3) | |
| Unknown or not reported | 67 (1.3) | |
| Ethnicity | ||
| Hispanic/Latino | 929 (18.4) | |
| Not Hispanic/Latino | 4,077 (80.8) | |
| Unknown/not reported | 41 (0.8) | |
| Education completed | ||
| <High school | 364 (7.2) | |
| High school graduate | 1,039 (20.6) | |
| Some college | 1,463 (29.0) | |
| ≥College degree | 2,180 (43.2) | |
| Duration of diabetes (years) | 4.2 ± 2.8 | |
| Duration of diabetes (years), median (IQR) | 3.8 (1.9, 6.4) | |
| Screening metformin dose (mg/day) | 1,575.5 ± 525.2 | |
| Baseline metformin dose (mg/day) | 1,944.2 ± 204.5 | |
| Family history of any first-degree relatives with diabetes | 3,522 (69.8) | |
| Medical history | ||
| Heart attack/stroke | 330 (6.5) | |
| Retinopathy | 49 (1.0) | |
| Neuropathy | 1,083 (21.5) | |
| Hypertension | 3,360 (66.6) | |
| Elevated blood lipids | 3,646 (72.2) | |
| Current medications | ||
| Blood pressure medications | 3,495 (69.2) | |
| Lipid-lowering medications | 3,317 (65.7) | |
| Statin | 3,209 (63.6) | |
| Aspirin | 2,288 (45.3) | |
| Depression/anxiety medication(s)† | 472/2,502 (18.9)† | |
| Smoking status | ||
| Current smoker | 695 (13.8) | |
| Former smoker | 1,617 (32.0) | |
| Never smoked | 2,735 (54.2) | |
| Physical measurements | ||
| Weight (kg) | 100.0 ± 22.3 | |
| BMI (kg/m2) | 34.3 ± 6.8 | |
| Blood pressure | ||
| Systolic (mmHg) | 128.3 ± 14.7 | |
| Diastolic (mmHg) | 77.3 ± 9.9 | |
| Blood pressure <140/90 mmHg | 3,802 (75.3) | |
| Blood pressure <130/80 mmHg | 2,172 (43.0) | |
| Laboratory tests* | ||
| HbA1c (%) | 7.5 ± 0.5 | ≥6.5% or 48 mmol/mol may indicate diabetes |
| HbA1c (mmol/L) | 58 ± 5.3 | |
| HbA1c <7% | 725 (14.4) | |
| Cholesterol (mg/dL) | 163.8 ± 37.8 | <200 mg/dL |
| Cholesterol (mmol/L) | 4.2 ± 0.98 | 5.172 mmol/L |
| Triglycerides (mg/dL) | 154.0 ± 121.6 | 0–100 mg/dL |
| Triglycerides (mmol/L) | 1.7 ± 1.4 | 0–1.7 mmol/L |
| HDL (mg/dL) | 43.4 ± 10.6 | Female >50 mg/dL; male >40 mg/dL |
| HDL (mmol/L) | 1.1 ± 0.3 | Female >1.3 mmol/L; male >1.0 mmol/L |
| LDL (mg/dL) | 90.5 ± 31.7 | <129 mg/dL |
| LDL (mmol/L) | 2.3 ± 0.8 | <3.4 mmol/L |
| LDL <100 mg/dL | 3,348 (66.3) | |
| UACR (mg/g) | 6.4 (3.1, 16.9) | <30 mg albumin/g creatinine |
| UACR <30 mg/g creatinine | 4,241 (84.1) | |
| Fasting glucose (mg/dL) | 151.5 ± 30.9 | 60–99 mg/dL |
| Fasting glucose (mmol/L) | 8.4 ± 1.7 | 3.3–5.5 mmol/L |
| eGFR (mL/min/1.73 m2) | 95.3 ± 16.9 | ≥60 mL/min/1.73 m2 |
| eGFR <60 mL/min/1.73 m2 | 121 (2.4) | |
| Serum creatinine (mg/dL) | 0.83 ± 0.2 | Female 0.4–1.1 mg/dL; male 0.5–1.2 mg/dL |
| Fasting C-peptide (nmol/L) | 1.34 ± 0.6 | 0.37–1.47 nmol/L |
| Fasting insulin (pmol/L) | 129.4 ± 95.4 | 12–150 pmol/L |
| Fasting insulin (mU/L) | 21.6 ± 15.9 | 2–25 mU/L |
Continuous data are presented as the mean ± SD or as the median (interquartile range), and categorical data are presented as n (%). UACR, urinary albumin-to-creatinine ratio.
*N was 5,047 except for depression/anxiety medication question (see next note).
†This question was added after the study started and was answered by 2,498 participants at baseline. Of these, 472 participants answered “yes” and 2,032 participants answered “no”: 472/2,502 = 0.19.
Clinical Characteristics
At baseline, HbA1c was 7.5 ± 0.5% (58 ± 5.3 mmol/mol), fasting glucose was 151 ± 31 mg/dL (8.4 ± 1.7 mmol/L), and duration of diabetes was 4.2 ± 2.8 years. BMI was 34.3 ± 6.8 kg/m2. The prevalence of hypertension and dyslipidemia was 66.6% and 72.2%, respectively. Self-reported history of heart attack or stroke was 6.5%. History of self-reported eye disease due to diabetes was 1.0%. Baseline neuropathy prevalence was 21.5% by combined MNSI index. Nondiabetes medication use and metabolic parameters are listed in Table 1. Among the cohort, 69% were treated with antihypertensive medications, with mean blood pressure for the entire cohort of 128 ± 15/77 ± 10 mmHg. Mean total cholesterol was 164 ± 38 mg/dL (4.24 ± 0.98 mmol/L) and mean LDL was 91 ± 32 mg/dL (2.3 ± 0.8 mmol/L), with 64% of all participants reporting statin use. Approximately one-fifth reported taking antidepressant or anxiolytic medications (see Table 1, second footnote).
Comparison of GRADE to NHANES Respondents Meeting GRADE Inclusion Criteria
For this report, we applied GRADE inclusion but not exclusion criteria to unpublished data available from NHANES respondents ≥18 years with diabetes in the 2011–2014 surveys (Table 2). The number of respondents with diabetes was 1,432. After applying GRADE inclusion criteria, 201 NHANES respondents with diabetes met criteria of age ≥30 years, diabetes duration of <10 years, and HbA1c of 6.8–8.5% (51–69 mmol/mol). Of these, 120 were taking metformin alone, representing 2,000,987 of the 21,686,032 Americans with diabetes, a weighted percentage of 9.1% (95% CI 7.4–11.2) of American adults with diabetes. The NHANES cohort had a mean age of 57.9 ± 12.0 years, with a mean HbA1c of 7.4 ± 0.56% (57 ± 6.6 mmol/mol) and BMI of 33.2 ± 8.2 kg/m2, and 7.2% had a history of CVD (Table 2).
Table 2.
Comparison of GRADE study to UKPDS, ADOPT, and GRADE-eligible NHANES cohort
| GRADE (1) | UKPDS (20) | ADOPT (3,21) | NHANES (16) | |
|---|---|---|---|---|
| Primary study aim | Glycemic durability of second diabetes medication after metformin | Diabetes outcomes of intensive vs. conventional control after initial diagnosis of T2DM | Glycemic durability of initial diabetes medication | Subsample of NHANES participants meeting similar criteria (below) as GRADE (n = 120 [unweighted]) |
| Study characteristics | ||||
| Key eligibility criteria | • Age ≥30 years | • Age 25–65 years | • Age 30–75 years | • Age ≥30 years |
| • T2DM <10 years | • Newly diagnosed with T2DM | • T2DM ≤3 years | • T2DM <10 years | |
| • HbA1c 6.8–8.5% (51–69 mmol/mol) taking metformin monotherapy | • Mean FPG 110–270 mg/dL (6.1–15.0 mmol/L) after 3 months’ diet treatment | • FPG 126–180 mg/dL (7–10 mmol/L) with lifestyle management alone | • HbA1c 6.8–8.5% (51–69 mmol/mol) taking metformin monotherapy | |
| Randomized intervention | Medications representing four classes: Sulfonylurea (glimepiride), DPP-4 inhibitor (sitagliptin), GLP-1 analog (liraglutide), or insulin (glargine) | Intensive glycemic control with sulfonylurea or insulin or metformin (aim FPG <108 mg/dL (6 mmol/L), or conventional control with diet | Rosiglitazone, metformin, or glyburide | NA |
| Primary outcome | Time to primary failure, defined as HbA1c ≥7% (53 mmol/mol), confirmed | Any diabetes-related end point,* diabetes-related death, all-cause mortality | Time to monotherapy failure (FPG >180 mg/dL [10 mmol/L], confirmed) for rosiglitazone, compared with metformin or glyburide | NA |
| Years of study conduct | 2013–2021 (planned) | 1977–1997 | 2000–2006 | 2011–2014 |
| Follow-up (years) | 5.2 (planned) | 10.0 (median) | 4.0 (median) | NA |
| Baseline characteristics of randomized cohort | ||||
| Demographic | ||||
| N | 5,047 | 3,867 | 4,360 | 120 (representing population n = 2,000,987) |
| Age (years) | 57.2 ± 10.0 | 53.2 ± 8.6 | 57 ± 10 | 57.9 ± 12.0 |
| Sex (% male) | 63.6 | 61.0 | 57.7 | 55.9 |
| Race/ethnicity | ||||
| Caucasian | 65.7 | 81 | 88.4 | 62.1† |
| African Ancestry | 19.8 | 8 | 4.0 | 15.1† |
| Hispanic | 18.4 | — | 4.4 | 12.1 |
| Asian | 3.6 | 10 (Indian Asian) | 2.4 | 8.5† |
| AI | 2.7 (AI/AN) | — | — | — |
| Clinical | ||||
| Duration of diabetes (years) | 4.2 ± 2.8 | New-onset | 96% <2 years | 4.2 ± 2.5 |
| Weight (kg) | 100.0 ± 22.3 | 77.5 ± 15.5 | 91.7 ± 19.5 | 95.8 ± 27.2 |
| BMI (kg/m2) | 34.3 ± 6.8 | 27.5 ± 5.2 | 32.2 ± 6.4 | 33.2 ± 8.2 |
| Systolic BP (mmHg) | 128.3 ± 14.7 | 135 ± 20 | 133 ± 15.3 | 132.2 ± 18.2 |
| Diastolic BP (mmHg) | 77.3 ± 9.9 | 82 ± 10 | 79.7 ± 9.0 | 74.1 ± 11.4 |
| Current smoker | 13.8 | 31 | 15 | 14.2 |
| History of CVD | 6.5 | NA | NA | 7.2 |
| Education | ||||
| <High school | 7.2 | 16.8 | ||
| High school graduate | 20.6 | 24.9 | ||
| Some college | 29.0 | 30.8 | ||
| ≥College degree | 43.2 | 27.5 | ||
| Biochemical | ||||
| Glycemia | ||||
| Fasting plasma glucose | ||||
| mg/dL | 151.5 ± 30.9 | 144 (128, 175)**‡ | 151.7 ± 26.2 | 161.7 ± 35.0 |
| mmol/L | 8.41 ± 1.72 | 8.0 (7.1, 9.7)**‡ | 8.42 ± 1.45 | 9.0 ± 1.9 |
| HbA1c | ||||
| % | 7.5 ± 0.5 | 7.1 ± 1.51 | 7.4 ± 0.93 | 7.4 ± 0.6 |
| mmol/mol | 58 ± 5.3 | 54 ± 16.5 | 57 ± 10.2 | 57 ± 6.6 |
| Fasting insulin | ||||
| pmol/L | 129.4 ± 95.4 | 92 (52, 160)§ | 150.7 ± 111 | 122.17 ± 96.36 |
| mU/L | 21.57 ± 15.9 | 15 (8.7, 27)§ | 25.12 ± 18.5 | 20.362 ± 16.06 |
| Lipids | ||||
| Total cholesterol | ||||
| mmol/L | 4.236 ± 0.976 | 5.4 ± 1.1 | 5.276 (4.58, 5.98)‡ | 4.74 ± 1.51 |
| mg/dL | 163.8 ± 37.8 | 209 ± 43 | 203.7 (177, 231)‡ | 183.19 ± 58.46 |
| LDL cholesterol | ||||
| mmol/L | 2.3 ± 0.8 | 3.5 ± 1.0 | 3.1 (2.5, 3.73)‡ | NA |
| mg/dL | 90.5 ± 31.7 | 135 ± 39 | 120 (97, 144)‡ | |
| HDL cholesterol | ||||
| mmol/L | 1.12 ± 0.27 | 1.07 ± 0.24 | 1.21 (1.02, 1.42)‡ | 1.12 ± 0.2 |
| mg/dL | 43.4 ± 10.6 | 41.4 ± 9.3 | 46.9 (39.2, 55.0)‡ | 43.3 ± 10.9 |
| Triglycerides | ||||
| mmol/L | 1.740 ± 1.374 | 2.35 (0.84–6.55)§ | 1.823 (1.28, 2.58)‡ | 2.8 ± 5.9 |
| mg/dL | 154.0 ± 121.6 | 208 (74–580)§ | 161.3 (113, 228)‡ | 246.5 ± 518.7 |
Continuous data are reported as the mean ± SD or as indicated and categorical data as the percentage. FPG, fasting plasma glucose; NA, not available.
*Defined as sudden death, hyper- or hypoglycemia-related death, myocardial infarction, angina, heart failure, stroke, renal failure, amputation, vitreous hemorrhage, retinopathy requiring photocoagulation, and blindness.
**Fasting serum glucose reported (not plasma).
†Non-Hispanic.
‡Median (interquartile range) reported.
§Geometric mean, 1 SD reported.
Conclusions
GRADE has met its first goal of enrolling a national cohort of people with T2DM treated with metformin alone who require a second diabetes medication. Although GRADE is not a populationbased study, it is informative to compare the GRADE cohort to the general population of Americans with diabetes. The Centers for Disease Control and Prevention reported in 2017 that 84.4% of adults with diabetes had HbA1c of ≤9% (75 mmol/mol) (17). In a published report of the 2005–2010 NHANES cohort, 57.8% of Americans with diagnosed diabetes were on oral antihyperglycemics only, and 13.4% took no diabetes medication. Among those taking medication, 77.6% had an HbA1c of <8% (64 mmol/mol) (18). In GRADE, all participants were treated with metformin and had a mean HbA1c of 7.5 ± 0.5% (58 ± 5.3 mmol/mol).
Focusing on the analysis performed for this report of NHANES respondents meeting GRADE inclusion criteria, it is apparent that GRADE participants are similar with respect to mean age, BMI (with 4-kg difference in body weight with broad CIs), HbA1c, current smoking, and self-reported history of CVD (Table 2) despite the small actual number of NHANES respondents from which these data are derived.
There are, however, notable differences. GRADE enrolled a higher proportion of men, and GRADE participants had higher educational attainment than NHANES respondents. In addition, GRADE selected centers specifically to ensure enrollment of populations disproportionately affected by diabetes and as such was more racially and ethnically diverse than the NHANES population: GRADE participants are 19.8% African American and 18.4% Hispanic compared with 15.1% African American and 12.1% Hispanic in the NHANES subset meeting GRADE inclusion criteria (Table 2). It is important to note that inclusion criteria for a clinical trial narrow the eligible population substantially: applying GRADE inclusion criteria to the NHANES yielded 9.1% of the original sample. Expanding the eligibility for duration of diabetes early on during recruitment likely yielded a slightly longer duration of diabetes than would otherwise have been seen but likely did not affect other characteristics because the overall sample is similar to the NHANES-eligible cohort.
GRADE in the Context of Other Major Diabetes Studies
GRADE fits into a spectrum of large trials of patients with T2DM evaluating durability of glycemic therapy (Table 2). The UKPDS enrolled patients with newly diagnosed diabetes starting in 1977 (19,20). This study, which reported initial results in 1998, nonetheless provides the basis of our knowledge of T2DM treatment over a prolonged period. GRADE participants have a longer duration of diabetes and higher baseline HbA1c than those who participated in UKPDS. A Diabetes Outcome Progression Trial (ADOPT), conducted in the U.S. between 2000 and 2006, was a trial of initial glucose-lowering therapy in which 96% of participants had been diagnosed with diabetes for ≤3 years (21). Participants in GRADE are similar in age but more racially and ethnically diverse than those in ADOPT. These major diabetes trials disproportionately enrolled men (61% in UKPDS and 58% in ADOPT), and GRADE is similar in this respect.
GRADE is different from the major diabetes clinical trials of the last decade that tested the hypothesis that intensive glycemic control would reduce CVD outcomes in T2DM. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) (22), Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) (23), and Veterans Affairs Diabetes Trial (VADT) (24) trials enrolled patients with established CVD or at high cardiovascular risk. The intervention treatment groups of ACCORD and ADVANCE aimed for and achieved lower glycemic targets than were then or are currently recommended. All three trials used complex diabetes medication regimens to achieve glycemic targets. Moreover, the choice of diabetes medication was not protocolized. By contrast, GRADE aims to achieve a uniform glycemic target (<7%) using medications representative of four major diabetes medication classes while allowing investigator discretion to adjust individual participant glycemic targets over time for changes in clinical status.
GRADE is also unlike the major cardiovascular outcomes trials (CVOT) reported over the last half-decade. CVOT trials, mandated by the FDA starting in 2008 to demonstrate the cardiovascular safety of new diabetes drugs, have enrolled participants with T2DM who have established CVD or are at very high cardiovascular risk to accrue a sufficient number of outcomes to evaluate cardiovascular safety during relatively brief follow-up periods. The prevalence of established CVD in ACCORD, ADVANCE, and VADT was 30–40% and was even higher in CVOT trials of sodium–glucose cotransporter 2 (SGLT2) inhibitors and GLP-1 receptor agonists (usually ≥70%) (25). The prevalence of CVD in GRADE at baseline is much lower, with only 6.5% reporting a history of myocardial infarction or stroke at study entry. Determining the overall prevalence of CVD in patients with T2DM can be difficult, but one large U.S. electronic medical record database reported CVD prevalence of 21% among 1,389,016 patients with T2DM (26). This is consistent with an NHANES report showing 18.3% prevalence of CVD among adults with diabetes in 2012 (27). Although the prevalence of CVD in GRADE is lower than in the general U.S. population with diabetes, it is representative of the age-similar NHANES population meeting GRADE eligibility criteria (7.2%) (Table 2).
Compared with UKPDS, which enrolled patients with newly diagnosed T2DM, and with ACCORD, ADVANCE, and VADT and the more recent CVOTs that focused on participants with T2DM of longer duration and established CVD, GRADE represents an intermediate stage of treatment of T2DM. Immediately after diabetes diagnosis, as was seen in UKPDS and ADOPT, lifestyle change and initial single-agent pharmacotherapy are effective for a period of time, usually followed by deterioration in glycemic control. At the other end of the spectrum, long-standing diabetes may require insulin treatment if β-cell deficiency is advanced.
Although there are data from CVOTs that certain medications reduce the risk of heart failure and renal outcomes, or, in the case of one long-term GLP-1 receptor agonist trial, major adverse cardiac events, even in those without established CVD (28–30), the subgroups without established atherosclerotic CVD in these studies had two or more cardiovascular risk factors and longer duration of diabetes than participants in GRADE. The evidence remains inconclusive regarding which medication to choose for individuals with diabetes who have had deterioration in glycemic control despite initial management with metformin and lifestyle intervention but who are younger, have fewer cardiovascular risk factors, and do not yet have significant complications. This has been highlighted in the American Diabetes Association’s and numerous other position statements (9,31). As a large-scale, longitudinal trial of patients with T2DM conducted in the current treatment era, characterized by more aggressive blood pressure and statin treatment, GRADE will allow comparative assessment of different diabetes medication classes with regard to efficacy and durability of achieving a target HbA1c of <7% (53 mmol/mol) and patient-centered outcomes, including the safety of treatment.
The major limitation of GRADE is the lack of an SGLT2 inhibitor treatment group. SGLT2 inhibitors were not approved at the time the study was designed in 2012 and were in limited use at the time of study launch (32). As a comparative effectiveness study, GRADE selected commonly used, FDA-approved medication combinations (1). An inherent pitfall of long-term trials is that evidence and practice patterns may change within the time frame of the study. It is notable that despite the emergence of new evidence supporting use of GLP-1 receptor agonists and SGLT2 inhibitors in patients with T2DM and established CVD or high CVD risk, the best medication choice in the population enrolled in GRADE remains unclear. Similarly, pioglitazone was considered as a fifth treatment group of the study but was not included based on budgetary concerns, safety concerns, and declining use at the time the study was designed. Another limitation is that each medication is but one representative of a class and may have different properties than others in that class. Nonetheless, the four medications studied in this trial have long safety records, with each representing classes with distinct pathophysiologic approaches to the treatment of T2DM. Finally, the primary focus of GRADE is glycemic outcomes, and although some microvascular outcomes are included, the trial is not adequately powered to determine the myriad effects of individual treatment assignments on other outcomes of interest in patients with diabetes.
These limitations are balanced by other strengths. GRADE is a prospective randomized trial with a large number of participants recruited from 36 U.S. clinical centers. Participants were recruited not only from academic centers but also from community practices, Veterans Affairs medical centers, and closed-model HMOs. Finally, it is notable that GRADE’s racial and ethnic composition, although similar to other large, National Institutes of Health–funded trials, such as the Diabetes Prevention Program (33) and ACCORD, is more diverse than often seen in diabetes clinical development programs (34,35). Also, GRADE is a comparative effectiveness trial in which each medication is used according to its product label to maximal effect over a sustained period of time. The current state of knowledge of the comparative effectiveness of diabetes medications stems largely from observational trials, which are limited by allocation and time-related biases (36). As a randomized controlled trial that will monitor participants for a planned mean follow-up of >5 years, GRADE will provide valid comparisons unhindered by allocation and time-related bias. Results from GRADE, expected in late 2021, will inform the choice of the most durable diabetes medication added to metformin.
In conclusion, GRADE’s 5,047 participants are broadly representative of U.S. patients with T2DM who require a second diabetes medication after metformin to achieve and maintain HbA1c ≤7% (53 mmol/mol). Results of the GRADE study will inform decisions about clinical effectiveness of the addition of four commonly used classes of diabetes medications to metformin.
Supplementary Material
Article Information
Acknowledgment. The authors thank Catherine Cowie, National Institute of Diabetes and Digestive and Kidney Diseases, and Danita Byrd-Clark, Social & Scientific Systems, for their guidance and support for the NHANES analysis.
Funding. The GRADE Study is supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases (U34-DK-088043 and U01-DK-098246). The American Diabetes Association supported the initial planning meeting for the U34 proposal. The National Heart, Lung, and Blood Institute and the Centers for Disease Control and Prevention are also providing funding support. Educational materials have been provided by the National Diabetes Education Program. Material support in the form of donated medications and supplies has been provided by BD, Bristol-Myers Squibb, Merck, Novo Nordisk, Roche Diagnostics, and Sanofi.
Duality of Interest. V.R.A. reports grants and personal fees from AstraZeneca/Bristol-Myers Squibb, Novo Nordisk, and Sanofi, personal fees from BD and Zafgen, grants from Calibra, Eisai, Janssen, and Theracos, other from Adocia, and other from Merck, outside the submitted work. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. D.J.W. contributed to the design, interpretation of data, supervision and management of the research, writing, and critical review of this manuscript. H.K.-S. contributed to the design, acquisition of data, statistical analysis, interpretation of data, supervision and management of research, writing, and critical review of this manuscript. J.P.C. contributed to the design, acquisition and interpretation of data, and critical review of this manuscript. H.J.F. contributed to the design, interpretation of data, and critical review of this manuscript. S.H.H. contributed to the acquisition of data and critical review of this manuscript. A.K. contributed to the acquisition of data, writing, and critical review of this manuscript. A.S. contributed to the acquisition and interpretation of data, supervision and management of research, and critical review of this manuscript. C.U. contributed to the acquisition of data and critical review of the manuscript. V.R.A. contributed to the design, acquisition, and interpretation of data, supervision and management of research, writing, and critical review of this manuscript. All authors affirm that authorship is merited based on the International Committee of Medical Journal Editors authorship criteria. D.J.W. and H.K.-S. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 78th Scientific Sessions of the American Diabetes Association, Orlando, FL, 22–28 June 2018.
Footnotes
Clinical trial reg. no. NCT01794143, clinicaltrials.gov
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc19-0901/-/DC1.
A complete list of the members of the GRADE Research Group can be found in the Supplementary Data online.
This article is featured in a podcast available at http://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
Contributor Information
Collaborators: GRADE Research Group, Jill P. Crandall, Melissa Diane McKee, Janet Brown-Friday, Entila Xhori, Keisha Ballentine-Cargill, Sally Duran, Jennifer Lukin, Stephanie Beringher, Susana Gonzalez de la Torre, Lawrence Phillips, Elizabeth Burgess, Darin Olson, Mary Rhee, Peter Wilson, Tasha Stephanie Raines, Julie Costello, Chona Gullett, Maxine Maher-Albertelli, Folayan Morehead, Radhika Mungara, Saranjit Person, Louise Savoye, Mabil Sibymon, Sridhar Tanukonda, Carol Ann White, Leah Holloway, Cynthia Adams, April Ross, Ashok Balasubramanyam, Erica Gonzalez, Charlyne Wright, Priscilla Hollander, Erin Roe, Analyn Uy, Polly Burt, Lorie Estrada, Kris Chionh, Faramarz Ismail-Beigi, Corinna Falck-Ytter, Laure Sayyed Kassem, Ajay Sood, Margaret Tiktin, Bethany Cramer, Jacalyn Iacoboni, Maria V. Kononets, Tanya Kulow, Cynthia Newman, Katherine A. Stancil, Cristina Sanders, Lisa Tucker, Amanda Werner, Adrienne Krol, Gloria McPhee, Christine Patel, Linda Colosimo, Robin Goland, James Pring, Patricia Kringas, Jessica Tejada, Camille Hausheer, Harvey Schneier, Kelly Gumpel, Amanda Kirpitch, Jennifer B. Green, Hiba AbouAssi, Ranee Chatterjee, Mark N. Feinglos, Jennifer English, Shubi A. Jones, Jeanne B. Khan, Ronna P. Kimpel, Mary Zimmer, Barbara M. Furst, Connie R. Satterwhite, Kathryn Thacker, Kieren J. Evans Kreider, Amale Mather, Tonya Lteif, Nick Hamilton, Gabriela Patel, Marcia Riera, Vivian Jackson, Devin Pirics, Danielle Howard, Sloan Aguillar, Richard Hurt, Anders Bergenstal, Thomas Carlson, Mary Martens, Renae Johnson, Jamie Hill, Connie Hyatt, Marcia Jensen, Dianna Madden, Holly Martin, Wanda Willis, Rebecca Konerza, Kathleen Passi, Stephen Kleeberger, Michael Fortmann, Karen Herson, Harry Mularski, James Glauber, Britt Prihoda, Christina Ash, Phyllis Anne Carlson, Emily Ramey, Britta Schield, Kathy Torgrimson-Ojerio, Bryan Arnold, Elease Kauffman, Samantha Panos, Kristi Sahnow, Jennifer Bays, Jennifer Cook, Debra Gluth, Katrina Sasaki, Jennifer Schell, Camille Criscola, Suzi Friason, Sergey Jones, Joshua Nazarov, Negah Barzilay, Rachel Rassouli, Michelle Puttnam, Kia Curtis, Bonita Stokes, Cynthia Hollis, Roslin Sanders-Jones, Zakiah Nelson, Abby El-Haqq, Tu Kolli, Deborah Tran, James Wexler, Amy Meigs, Gianna Dushkin, Brittany Rocchio, Mike Chambers, Barbara Yepes, Hilary Steiner, Melody Dulin, Andrea Cayford, Lindsey DeManbey, Mallory Gurry, Kimberly Hillard, Christine Martin, Nopporn Stevens, Raquel Thangthaeng, Elyse Kochis, Valerie Raymond, Jean Ripley, Vanita Park, Adline Aroda, Amy Ghazi, Maria Loveland, Alexander Hurtado, Florence Kuhn, Hermes J. Mofor, Willy Marcos Florez, Jennifer Valencia, Lisset Marks, Ana K. Oropesa-Gonzalez, Riccio, Ramfis Veliz, Miriam Nieto-Martinez, Andrew Gutt, Diana Ahmann, Farahnaz Aby-Daniel, Victoria Joarder, Carol Morimoto, Daisuke Sprague, Nancy Yamashita, Patricia Cady, Nadia Kirchhoff, Joseph Rivera-Eschright, Brianna Adducci, Alina Morales Gomez, Sophia H. Goncharova, Helen Hox, Michael Petrovitch, Victoria Matwichyna, Nina O. Jenkins, Renée R. Bermudez, Daniel S. Ishii, William T. Hsia, Frank L. Cefalu, Celeste Greenway, Erin Waguespack, Natalie King, Amy Haynes, Brandi Thomassie, Claire Bourgeois, Robert Hazlett, Sunder Henry, Schafer Mudaliar, Jeremy Boeder, Elsa Pettus, Catherine Diaz, Erick DeLue, Sylvia Castro, Jonathan Hernandez, Jeffrey M. Krakoff, Tina Curtis, Erica Killean, Enrique Joshevama, Denelle Diaz, Tracey Martin, Jeanine Karshner, F. Xavier Albu, Sylvaine Pi-Sunyer, Carol Frances, Emily Maggio, Joseph Ellis, Xiuqun Bastawrose, Mary Ann Gong, Phyllis Banerji, Daniel August, Necole M. Lorber, Debra H. Brown, Lorraine L. Josephson, Mari Thomas, Ajini Tsovian, Marlo H. Cherian, Motria M. Jacobson, M. Sue Mishko, Katherine Kirkman, John B. Bergamo, Jean Buse, Laura Dostou, April Young, Jeffrey Goley, Joseph F. Kerr, Sonia Largay, Juanita Guarda, Dawn Cuffee, Rachael Culmer, Hope Fraser, Samantha Almeida, Elizabeth Coffer, Lauren Debnam, Sarah Kiker, Kim Morton, Gail Josey, W. Timothy Fuller, Andrea Garvey, Dana Cherrington, Olivia Golson, Mary Catherine Griffith, April Robertson, Steve Agne, Robert M. McCullars, Jacqueline Cohen, Kimberly Craig, M. Colleen Kersey, Carla Rogge, Kathryn Wilson, Sonia Burton, Mary Beth Lipp, Neda Vonder Meulen, Emily Rasouli, Stephanie Schroeder, Chelsea Steiner, Chantal Baker, Sara Underkofler, William Douglass, Erin Sivitz, Laura Cline, Jennifer Knosp, Tamara McConnell, William H. Lowe, Rodica Herman, Meng H. Pop-Busui, Catherine Tan, Andrea Martin, Lynn Waltje, Rebecca Goodhall, Shihchen Eggleston, Stephanie Kuo, Nancy Bule, Elizabeth Kessler, Elizabeth R. LaSalle, Anne Seaquist, Anjali Bantle, Bruce Kumar, John Redmon, Tasma Bantle, Mary Harindhanavudhi, Michael Coe, Abdisa Mech, Lesia Taddese, Shannon Lesne, Cyrus Smith, Lisa Desouza, Vijay Kuechenmeister, Ana Laura Shivaswamy, Maria Grace Morales, Kris Rodriguez, Alissa Seipel, Jenna Alfred, Grace Eggert, William Lord, Renee Taylor, David S. Tillson, Allen Schade, Mark Adolphe, Elizabeth Burge, Janae Duran-Valdez, Doris Martinez, Hernandez, Benjamin McGinnis, Elizabeth Pucchetti, Ralph A. Scripsick, Eugenio DeFronzo, Muhammad Cersosimo, Curtis Abdul-Ghani, Hector Triplitt, Rosa Irene Verastiqui, Kathryn Garza, Curtiss Wright, Philip Puckett, Chanhaeng Raskin, Soma Rhee, Lin Fan Abraham, Serey Jordan, Luisa Sao, Oralenda Morton, Laura Smith, Laura Osornio Walker, Rosa Schnurr-Breen, Robert Brian Ayala, Daytheon Kraymer, Kristina M. Sturgess, Steven E. Utzschneider, Lorena Kahn, Edward J. Alarcon-Casas Wright, Elaine C. Boyko, Dace L. Tsai, Basma N. Trence, Brenda K. Fattaleh, Karen M. Montgomery, Tessa Atkinson, Alexandra Concepcion, Cameron Kozedub, Samantha Moak, Tom A. Rhothisen, Stephanie Elasy, Laura Martin, Rita Shackelford, Nina Goidel, Janie Hinkle, Cynthia Lipps Hogan, Janet Lovell, Janet B. Myers, Maamoun McGill, Sarah Salam, Toni Kissel, Carol Schweiger, William Recklein, Patricia Tamborlane, Anne Gatcomb, Barbara Camp, Silvio Gulanski, Kim Inzucchi, Michele Pham, Katarzyna Alguard, Magalys Lessard, Elizabeth Perez, Abmaridel Magenheimer, David M. Montoza, John Nathan, Heidi Lachin, Mary Krause-Steinrauf, Henry B. Larkin, Barbara Burch, Andrew Linder, Naji Bremer, Michael Younes, Ionut Backman, C.J. Bebu, Anna Buys, Yuping Fagan Murphy, Michaela Gao, Stephanie Gramzinski, Elizabeth Hall, Alyssa Legowski, Joel Arey, Claire Bethepu, Pam Lund, Mangat, Paula Dhaliwal, Emily McGee, Lisa Mesimer, Michael Ngo, Jesse Steffes, Amy Seegmiller, Valerie Saenger, Deanna Arends, Todd Gabrielson, Stuart Conner, Jolene Warren, Alexandra Day, Elsayed Z. Scrymgeour, Zhu-Ming Soliman, Charles Zhang, Julie Campbell, Lisa Hu, Susan Keasler, Yabing Hensley, Rada Li, Veronica Mihalcea, Lisa Perez-Rosas, Kenneth Prosser, Wen Resnicow, Hui Ye, Ping Shao, Jose Zhang, Danurys Luchsinger, Judith Sanchez, Erik Fradkin, Helen Groessl, Naomi Chong, Ivan Hillery, Paula Abdouch, Frances E. Brantley, Gay Broyles, Paul Canaris, Jeri J. Copeland, Warren L. Craine, Melissa S. Fein, Rebecca Lee, Vaughn Meiners, Hollis Meiners, James E. O’Neal, Edward Park, Jeanne Sledge, Jr, Alexander Steppel-Resnick, Barbara Turchin, Christiane S. Brooks-Worrell, Christopher B. Hampe, Jerry P. Newgard, Ali Palmer, John Shojaie, Lawrence Higgins, Sherita Fischer, Jeffery Golden, Aanand Gonzalez, Elizabeth Naik, Lynne Walker, Joanne M. Doner Lotenberg, Joanne Gallivan, Diane M. Lim, Tuncer, and Stephanie Behringer-Massera
References
- 1.Nathan DM, Buse JB, Kahn SE, et al.; GRADE Study Research Group . Rationale and design of the glycemia reduction approaches in diabetes: a comparative effectiveness study (GRADE). Diabetes Care 2013;36:2254–2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nathan DM, Buse JB, Davidson MB, et al.; American Diabetes Association; European Association for Study of Diabetes . Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009;32:193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kahn SE, Haffner SM, Heise MA, et al.; ADOPT Study Group . Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006;355:2427–2443 [DOI] [PubMed] [Google Scholar]
- 4.Ekström N, Svensson AM, Miftaraj M, et al. . Durability of oral hypoglycemic agents in drug naïve patients with type 2 diabetes: report from the Swedish National Diabetes Register (NDR). BMJ Open Diabetes Res Care 2015;3:e000059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turner RC, Cull CA, Frighi V, Holman RR; UK Prospective Diabetes Study (UKPDS) Group . Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). JAMA 1999;281:2005–2012 [DOI] [PubMed] [Google Scholar]
- 6.Brown JB, Conner C, Nichols GA. Secondary failure of metformin monotherapy in clinical practice. Diabetes Care 2010;33:501–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuhn A, Park J, Ghazi A, Aroda VR. Intensifying treatment beyond monotherapy in type 2 diabetes mellitus: where do newer therapies fit? Curr Cardiol Rep 2017;19:25. [DOI] [PubMed] [Google Scholar]
- 8.American Diabetes Association 8. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018;41(Suppl. 1):S73–S85 [DOI] [PubMed] [Google Scholar]
- 9.Davies MJ, D’Alessio DA, Fradkin J, et al. . Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018;41:2669–2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inzucchi SE, Bergenstal RM, Buse JB, et al. . Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015;38:140–149 [DOI] [PubMed] [Google Scholar]
- 11.Inzucchi SE, Bergenstal RM, Buse JB, et al.; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD) . Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012;35:1364–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sox HC, Greenfield S. Comparative effectiveness research: a report from the Institute of Medicine. Ann Intern Med 2009;151:203–205 [DOI] [PubMed] [Google Scholar]
- 13.Herman WH, Pop-Busui R, Braffett BH, et al.; DCCT/EDIC Research Group . Use of the Michigan Neuropathy Screening Instrument as a measure of distal symmetrical peripheral neuropathy in Type 1 diabetes: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications. Diabet Med 2012;29:937–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lachin JM. Statistical considerations in the intent-to-treat principle. Control Clin Trials 2000;21:167–189 [DOI] [PubMed] [Google Scholar]
- 15.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA 2015;314:1021–1029 [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention (CDC) , National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data. Hyattsville, MD, U.S. Department of Health and Human Services and Centers for Disease Control and Prevention, 2018 [Google Scholar]
- 17.Centers for Disease Control and Prevention. National Diabetes Statistics Report [Internet], 2017. Available from https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed 13 November 2017
- 18.Selvin E, Parrinello CM, Sacks DB, Coresh J. Trends in prevalence and control of diabetes in the United States, 1988-1994 and 1999-2010. Ann Intern Med 2014;160:517–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.UK Prospective Diabetes Study (UKPDS) Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) [published correction appears in Lancet 1998;352:1558]. Lancet 1998;352:854–865 [PubMed] [Google Scholar]
- 20.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) [published correction appears in Lancet 1999;354:602]. Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 21.Viberti G, Lachin J, Holman R, et al.; ADOPT Study Group . A Diabetes Outcome Progression Trial (ADOPT): baseline characteristics of Type 2 diabetic patients in North America and Europe. Diabet Med 2006;23:1289–1294 [DOI] [PubMed] [Google Scholar]
- 22.Cushman WC, Evans GW, Byington RP, et al.; ACCORD Study Group . Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010;362:1575–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerstein HC, Miller ME, Byington RP, et al.; Action to Control Cardiovascular Risk in Diabetes Study Group . Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duckworth W, Abraira C, Moritz T, et al.; VADT Investigators . Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–139 [DOI] [PubMed] [Google Scholar]
- 25.Cefalu WT, Kaul S, Gerstein HC, et al. . Cardiovascular outcomes trials in type 2 diabetes: where do we go from here? Reflections from a Diabetes Care editors’ Expert Forum. Diabetes Care 2018;41:14–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iglay K, Hannachi H, Joseph Howie P, et al. . Prevalence and co-prevalence of comorbidities among patients with type 2 diabetes mellitus. Curr Med Res Opin 2016;32:1243–1252 [DOI] [PubMed] [Google Scholar]
- 27.Yoon SS, Dillon CF, Illoh K, Carroll M. Trends in the prevalence of coronary heart disease in the U.S.: National Health and Nutrition Examination Survey, 2001-2012. Am J Prev Med 2016;51:437–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perkovic V, Jardine MJ, Neal B, et al. . Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380:2295–2306 [DOI] [PubMed] [Google Scholar]
- 29.Zelniker TA, Wiviott SD, Raz I, et al. . Comparison of the effects of glucagon-like peptide receptor agonists and sodium-glucose cotransporter 2 inhibitors for prevention of major adverse cardiovascular and renal outcomes in type 2 diabetes mellitus. Circulation 2019;139:2022–2031 [DOI] [PubMed] [Google Scholar]
- 30.Gerstein HC, Colhoun HM, Dagenais GR, et al.; REWIND Investigators . Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 2019;394:121–130 [DOI] [PubMed] [Google Scholar]
- 31.Qaseem A, Humphrey LL, Sweet DE, Starkey M, Shekelle P; Clinical Guidelines Committee of the American College of Physicians . Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2012;156:218–231 [DOI] [PubMed] [Google Scholar]
- 32.Toscano D. Market Brief – The Market for Type 2 Diabetes Therapeutics – Key Findings From a Recent Analysis of Global Drug Development Efforts. Drug Development and Delivery, November/December 2013 [Internet]. Available from http://www.specialtypharma.com/Main/Back-Issues/MARKET-BRIEF-The-Market-for-Type-2-Diabetes-Therap-641.aspx. Accessed 4 June 2018
- 33.Knowler WC, Barrett-Connor E, Fowler SE, et al.; Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Food and Drug Administration. Dialogues on diversifying clinical trials: Successful strategies for engaging women and minorities in clinical trials [Internet], 2011. Available from https://www.fda.gov/downloads/ScienceResearch/SpecialTopics/WomensHealthResearch/UCM334959.pdf. Accessed 13 November 2017 [DOI] [PMC free article] [PubMed]
- 35.Food and Drug Administration. Collection of race and ethnicity data in clinical trials: Guidance for industry and Food and Drug Administration staff [Internet], 2016. Available from https://www.fda.gov/downloads/RegulatoryInformation/Guidances/ucm126396.pdf. Accessed 13 November 2017
- 36.Suissa S. Lower risk of death with SGLT2 inhibitors in observational studies: real or bias? Diabetes Care 2018;41:6–10 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.