Abstract
Introduction
Trials in Alzheimer's disease (AD) now include participants at the earliest stages to prevent further decline. However, the lack of tools sensitive to subtle functional changes in early-stage AD hinders the development of new therapies as it is difficult to prove their clinical relevance.
Methods
We assessed functional changes over three years in 289 elderly memory complainers from the Investigation of Alzheimer's Predictors in subjective memory complainers cohort using the Amsterdam Instrumental-Activities-of-Daily-Living questionnaire (A-IADL-Q).
Results
No overall functional decline related to AD imaging markers was evidenced. However, five distinct classes of A-IADL-Q trajectories were identified. The largest class (212 [73.4%]) had stable A-IADL-Q scores over 3 years. A second group (23 [8.0%]) showed a persistent functional decline, higher amyloid load (P = .0005), and lower education (P = .0392).
Discussion
The A-IADL-Q identified a subtle functional decline in asymptomatic at-risk AD individuals. This could have important implications in the field of early intervention in AD.
Keywords: Alzheimer's disease, Autonomy, Amsterdam-IADL, Linear mixed model, Latent class analysis
1. Introduction
Therapeutic trials in Alzheimer's disease (AD) start including participants at the earliest clinical stages to prevent the future onset of dementia [1]. Cognitively normal individuals who later progress to prodromal AD [2] or AD dementia [3], [4], [5], [6] may already show subtle functional changes, that is, changes in the person's ability to manage tasks and activities that are usually required in daily life such as managing finances and driving.
Activities of daily living (ADLs) are divided into basic (BADLs) and instrumental (IADLs) [7]. BADLs include fundamental skills that are needed to manage basic physical needs (personal hygiene, eating, dressing, toileting, continence). IADLs include activities related to living in community, mostly done with forethought, and for which multiple cognitive processes are needed (e.g., cleaning, washing clothes, managing medications and finances, driving) [8]. Because IADLs are by definition more complex that BADLs, they are more vulnerable to early brain pathological events [9].
There is currently a lack of tools that are sensitive enough to detect such subtle functional changes in patients with early-stage AD. Performance in IADLs is often measured using questionnaires, which are sometimes outdated, not nuanced enough (answers to questions being “impaired or not”), or self-report measures, being possibly biased by anosognosia (i.e. loss of insight), which may already be present in the early stages [6]. To overcome these limitations, Sikkes et al. [10] developed an informant-based computerized questionnaire in 2012, the Amsterdam IADL Questionnaire (A-IADL-Q). The initial version of the A-IADL-Q was an adaptive 47- to 70-item questionnaire, covering a broad range of cognitive IADLs, aimed to detect early dementia and early-onset dementia. The A-IADL-Q short version has been recently developed and has maintained the psychometric quality of the original version [11]. It consists of 30 items (derived from the original version) that can be used to detect functional decline from normal aging to dementia. We used this innovative questionnaire to identify subtle functional decline in our cohort of individuals with subjective cognitive decline (SCD) followed up for three years.
In this study, we hypothesized a subtle functional decline in preclinical AD. In the population of elderly subjective memory complainers, we expect heterogeneity in the functional trajectories. Using the A-IADL-Q in the Investigation of Alzheimer's Predictors in subjective memory complainers (INSIGHT-preAD) cohort, we wanted (1) to investigate whether we can identify subgroups of functional evolution and, if these subgroups exist, (2) to explore whether functional decline can be characterized by a distinct pattern of demographic and biological factors.
2. Method
2.1. Study design and participants
Participants eligible for this study were included in a longitudinal observational study, the INSIGHT-preAD study, which has been described previously [12]. This is an ongoing single-center study at the Institute of Memory and Alzheimer's disease of the Pitié-Salpêtrière University Hospital located in Paris, France. The INSIGHT-preAD study was designed to identify risk factors and markers of progression to AD in asymptomatic at-risk (due to their age and memory complaints) individuals [12].
Three hundred and eighteen participants were recruited by the neurologists of the memory clinic and through announcement of the study in the media, and they were enrolled between May 25, 2013, and January 20, 2015. The INSIGHT-preAD study has been approved by the ethics committee of the Pitié-Salpêtrière Hospital, and all participants provided written informed consent. Participants are between 70 and 85 years of age with SCD but unimpaired cognition (Mini–Mental State Examination ≥27 and Clinical Dementia Rating Scale = 0) and without episodic memory deficit (Free and Cued Selective Reminding Test [13], [14] total recall score ≥41). To this day, participants have now been followed up for 3 years out of the 5-year-long protocol.
A comprehensive cognitive and functional assessment was performed every 12 months, including Mini–Mental State Examination, Free and Cued Selective Reminding Test, Trail Making Test, Frontal Assessment Battery, Verbal fluency, and A-IADL-Q (this latter filled by the patient's study partner).
The brain glucose metabolism was measured with 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) every 24 months. The means of standardized uptake value ratio (SUVr) in four bilateral regions, specifically affected by AD [15], were used to calculate cortical metabolic indices: posterior cingulate cortex, inferior parietal lobule, precuneus, and inferior temporal gyrus (the reference region is the pons) [12].
Brain amyloid load was also measured every 24 months, by 18F-florbetapir (18F-AV45) PET. In particular, the 18F-AV45 SUVr was calculated by averaging the mean activity of the following regions of interests (ROIs): left and right precuneus, cingulum posterior, cingulum anterior and parietal, temporal, and orbitofrontal cortices (the reference region is a combination of the whole cerebellum and pons regions). Then, the threshold set for normal versus abnormal uptake is 0.7918.
In the present study, relevant data were collected from the INSIGHT-preAD cohort, including age, gender, level of education, neuropsychological scores, functional autonomy, level of amyloid deposition, and brain glucose metabolism. Global amyloid-PET SUVr and FDG-PET mean SUVr in AD-related ROIs were used as markers of preclinical AD for amyloid deposition and hypometabolism, respectively.
2.2. Amsterdam IADL Questionnaire
The A-IADL-Q is an informant-based questionnaire covering a broad range of complex IADLs such as household, administration, work, computer use, leisure time, appliances, and transport activities. For the present study, the short version was used, consisting of 30 items [11]. For each item, difficulty in performance is rated on a 5-point Likert scale (ranging from “no difficulty in performing this task” to “no longer able to perform this task”). Scoring is based on item response theory, a paradigm linking item responses to an underlying latent trait. This results in a latent trait score, which is converted to a T-score, with a mean of 50 and SD of 10. This score reflects one's level of IADL functioning, with higher scores indicating better IADL functioning. The A-IADL-Q was previously found to have a high test-retest reliability, good content and construct validity, and able to detect changes over time [16], [17].
We collected answers from each A-IADL-Q as reported by the study partner of every participant. An independent blinded person entered the answers on an online version of the questionnaire. We used the short version of A-IADL-Q because it has the same psychometric quality than the original version and it is shorter thus more user friendly/less burdensome to the informant [11]. Results were compiled and extracted for each patient and each visit of the 3-year follow-up (M0, M12, M24, and M36).
2.3. Statistical analysis
Subjects with at least two time points of A-IADL-Q score and with no missing data for amyloid load, glucose metabolism, age, gender, and education were included in the analysis. Baseline characteristics were compared between subjects included and excluded in the analysis using the χ2 test for categorical variables and the Student's t-test for continuous variables.
To evaluate the impact of amyloid load and glucose metabolism on A-IADL-Q scores evolution in 3 years of follow-up, we first conducted linear mixed-effects model (LMM). Amyloid load at baseline, glucose metabolism at baseline, age at baseline, gender, educational level, and visit were included as fixed effects in the model and participant as a random effect. We also included interaction between both PET biomarkers to study their concomitant impact on global measures of IADL; interaction of each PET biomarker with visit to evaluate if baseline PET biomarkers impact on IADL scores was different across time; and interaction between both PET biomarkers and visit to investigate the different effects of PET biomarker concomitant impact on IADL scores across time. Type II likelihood ratio tests were used to test each fixed effect and interaction. Cohen's f2 were calculated, using the marginal R2 [18], for each effect to estimate their size. Normality of residuals and random effects as well as heteroskedasticity were checked visually. Influencers and outliers were checked computing hat values and Cook's distance.
As LMMs are restricted to homogeneous population, we subsequently decided to perform latent class linear mixed model (LCLMM) [19] to investigate heterogeneous trajectories. Therefore, G latent classes of subjects characterized by G mean profiles of trajectories were computed. We compared models from one up to seven classes using Bayesian information criterion, and we selected the one which minimize Bayesian information criterion. Mean of posterior probabilities and percentage of posterior probabilities higher than 0.7 were computed. IADL scores evolution was modeled by the interaction between classes and visits. Using a multinomial logistic model, characteristics of classes were compared to the class with the largest number of subjects on amyloid load at baseline, glucose metabolism at baseline, age at baseline, gender, and educational level. Normality of residuals and random effects as well as heteroskedasticity were checked visually.
Statistical analyses were performed using R 3.5.0. Packages lme4 (version 1.1-17) and LCMM (version 1.7.9) were used to perform LMM and LCLMM, respectively.
3. Results
As shown in Supplementary Table 1, 289 subjects were included in the analysis. The 29 excluded subjects were older, with lower educational level and had lower scores for Trail Making Test B–A time. However, no statistical differences in the A-IADL-Q score at baseline were found. Participants were mostly women (61.94%), with a mean age of 75.9 ± 3.44 years at baseline and a diploma equivalent of a high school diploma or higher (69.55%). At baseline, they had an average Mini–Mental State Examination score of 28.68 ± 0.95 and an A-IADL-Q score of 67.01 ± 3.66.
LMM results are presented in Table 1. Lower overall score of A-IADL-Q score, indicating worse IADL functioning, was associated with older age at baseline (−0.16 ± 0.06, P = .0028) and with higher 18F-AV45 SUVr (−26.40 ± 11.53, P = .0372). Male gender was associated with a higher overall A-IADL-Q score (0.97 ± 0.39, P = .0128). All three effects had similar impact size (f2: 0.018, 0.015, and 0.011 for age, gender, and amyloid-PET, respectively). No overall functional decline was found (P = .4904), and impact of amyloid-PET and FDG-PET did not change over time (P = .6128 and P = .5827, respectively). We also investigated the interaction between visit and age, gender, and educational level, but they were not significant (data not shown).
Table 1.
Linear mixed effects model (LMM) results on Amsterdam IADL score
| Variables | Coefficient ± SE† | Cohen's f2 | P value |
|---|---|---|---|
| Age at baseline | −0.16 ± 0.06 | .018 | .0028∗ |
| Gender (M) | 0.97 ± 0.39 | .015 | .0128∗ |
| Educational level (low) | 0.16 ± 0.41 | <.001 | .7008 |
| Amyloid-PET | −26.40 ± 11.53 | .011 | .0372∗ |
| FDG-PET | −7.16 ± 3.83 | .002 | .5421 |
| Visit | .002 | .4904 | |
| M12 | −8.00 ± 9.44 | ||
| M24 | −0.22 ± 9.55 | ||
| M36 | −15.00 ± 9.90 | ||
| Visit:PET-amyloid | .001 | .6128 | |
| M12 | 10.62 ± 11.73 | ||
| M24 | 3.41 ± 11.91 | ||
| M36 | 19.34 ± 12.45 | ||
| Visit:PET-FDG | .001 | .5827 | |
| M12 | 3.28 ± 3.91 | ||
| M24 | 0.45 ± 3.94 | ||
| M36 | 6.14 ± 4.09 | ||
| PET-amyloid:PET-FDG | 10.22 ± 4.80 | .008 | .0711 |
| Visit:PET-amyloid:PET-FDG | <.001 | .4439 | |
| M12 | −4.36 ± 4.90 | ||
| M24 | −2.02 ± 4.95 | ||
| M36 | −8.02 ± 5.19 |
NOTE. Coefficients and standard error (SE) were extracted from complete GLMs with all interactions.
Abbreviations: OR, odd ratio; SE, standard errors.
P < .05.
For the following effect, the reference categories were: gender: women; educational level: high; visit: M0.
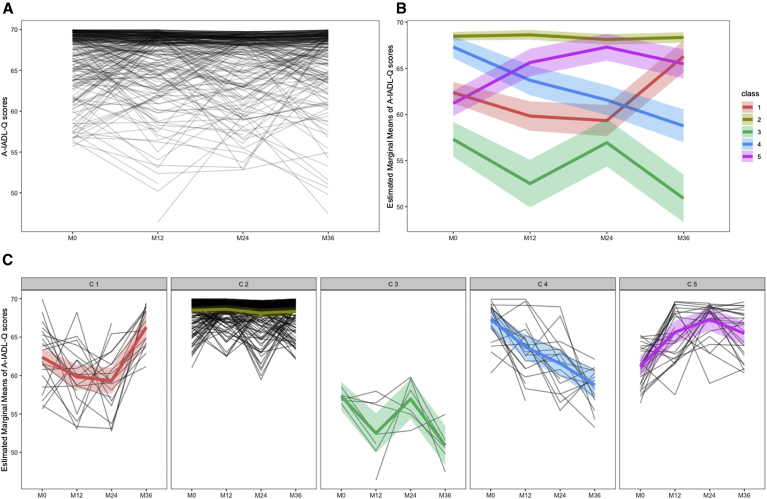
Five groups of A-IADL-Q evolution were identified by the LCLMM in the INSIGHT-preAD cohort (Fig. 1B and C). Group 1 (n = 20; 7% of our cohort) included subjects with a slight but significant improvement in A-IADL-Q scores (mainly between M24 and M36). In group 2 (n = 212, 74%), A-IADL-Q scores were high and stable over time. A-IADL-Q scores in group 3 (n = 7; 2%) were around the pathological threshold for clinical diagnosis of dementia (51.4; [20]). Their overall average trajectory had slightly diminished over time, with a fluctuating trend. Group 4 (n = 23; 8%) showed a persistent decline over time in A-IADL-Q scores. In group 5 (n = 27; 9%), A-IADL-Q scores were initially lower than in group 2 and then statistically improved on average over time, with a peak at M24 and a slight decrease at M36.
Fig. 1.
(A) Individual evolution of the A-IADL-Q scores in the 289 INSIGHT-preAD cohort participants over three years. (B) The five mean A-IADL-Q trajectories identified by the LCLMM. (C) Individuals' A-IADL-Q trajectory according to the group of evolution identified by the LCLMM [Group 1: n = 20 (6.9%); Group 2: n = 212 (73.4%); Group 3: n = 7 (2.4%); Group 4: n = 23 (8.0%); Group 5: n = 27 (9.3%)]. Abbreviations: A-IADL-Q, Amsterdam Instrumental Activities of Daily Living Questionnaire; INSIGHT-preAD, Investigation of Alzheimer's Predictors in subjective memory complainers; LCLMM, latent class linear mixed model.
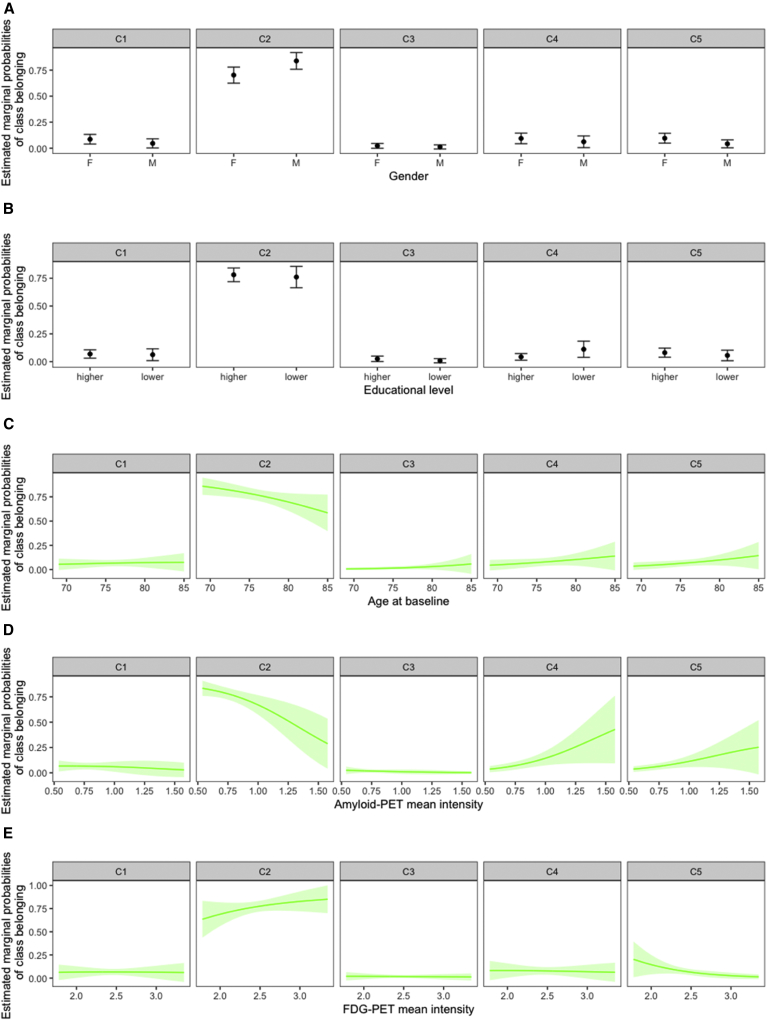
We compared characteristics of groups 1, 3, 4, and 5 with that of group 2. This latter group was chosen as a reference because of its highest and stable values of A-IADL-Q (Table 2, Fig. 2). Individuals included in group 4 had a higher amyloid load (OR = 33.86 ± 34.26, P = .0005) and a lower level of education (OR = 2.66 ± 1.26, P = .0392), compared with the reference group (Fig. 2). In group 5, subjects were less likely to be men (OR = 0.36 ± 0.18, P = .0428), had higher amyloid load (OR = 18.91 ± 18.25, P = .0023), and lower brain metabolism on FDG-PET (OR = 0.14 ± 0.13, P = .0383).
Table 2.
Comparison between each group and group 2 (reference group)
| Classes | Age at baseline |
Gender (reference:women) |
Educational level (reference:high) |
PET-amyloid at baseline |
PET-FDG at baseline |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR ± SE | P value | OR ± SE | P value | OR ± SE | P value | OR ± SE | P value | OR ± SE | P value | |
| Class 1 | 1.05 ± 0.07 | .5035 | 0.45 ± 0.25 | .1464 | 0.93 ± 0.48 | .8899 | 1.34 ± 1.99 | .8440 | 0.79 ± 0.76 | .8106 |
| Class 3 | 1.18 ± 0.13 | .1314 | 0.45 ± 0.41 | .3777 | 0.35 ± 0.38 | .3397 | 0.29 ± 0.89 | .6874 | 0.60 ± 0.98 | .7542 |
| Class 4 | 1.10 ± 0.08 | .1722 | 0.55 ± 0.29 | .2532 | 2.66 ± 1.26 | .0392∗ | 33.86 ± 34.26 | .0005∗ | 0.69 ± 0.65 | .6986 |
| Class 5 | 1.12 ± 0.07 | .0723 | 0.36 ± 0.18 | .0428∗ | 0.71 ± 0.35 | .4809 | 18.91 ± 18.25 | .0023∗ | 0.14 ± 0.13 | .0383∗ |
Abbreviations: OR, odd ratio; SE, standard errors.
P < .05.
Fig. 2.
Estimated marginal probabilities of class belonging using a multinomial logistic model. Group 2 is the reference class with stable A-IADL-Q scores over 3 years according to (A) gender status, (B) educational level, (C) age at baseline, (D) amyloid-PET SUVr, and (E) FDG-PET SUVr in AD-related ROIs. Abbreviations: A-IADL-Q, Amsterdam Instrumental Activities of Daily Living Questionnaire; FDG, 18F-fluorodeoxyglucose; PET, positron emission tomography; SUVr, standardized uptake value ratio.
Among the participants of the INSIGHT-preAD cohort (n = 6) who cognitively declined and were clinically diagnosed with prodromal AD within the 3 years of follow-up, 4 (1.9% of group) were in group 2 and 2 (8.7% of group) in group 4.
4. Discussion
In this longitudinal observational study, we aimed at exploring functional changes in individuals at risk of developing AD, due to their age, cognitive complaints, and in a part of the subjects, brain amyloid positivity. We chose to use the A-IADL-Q to assess functional status because it is shown to be valid and reliable and to correlate longitudinally with cognitive decline, which underlines its ability to detect changes over time [16]. It has been used to identify AD-related functional decline and have been validated in different countries [10], [21]. This explains its growing use in research (e.g., ClinicalTrials.gov Identifier: NCT03980730 and NCT03174886). To investigate functional decline in our cohort of subjective memory complainers, we initially performed an LMM. First, we found a negative association between A-IADL-Q and age, which was expected since functional decline and progressive reduction of autonomy is a condition known to be associated with growing age. Elderly people appear to be more vulnerable because of the effects of biological or psychological aging: a slight function loss is commonly present in normal aging, whereas function loss patterns that imply a progressively greater disability may be driven by causes other than age, such as a neurodegenerative disease [22], [23]. Second, participants with poorer performances in IADLs also had higher brain amyloid burden. This result is particularly interesting if we consider that our sample included cognitively healthy individuals with an SCD, who were potentially in the preclinical phase of AD (“asymptomatic at-risk individuals” according to [24]). The literature about the relationship between amyloid deposition and loss of function in predementia stages of AD is still rather scarce. As an example, Lilamand et al. [25] found that the presence of amyloid plaques was associated with lower functional ability, in both individuals with prodromal AD and cognitively normal, consistently with the present study. This suggests that functional impairment could be one of the first consequences of AD neuropathology, therefore representing a potential marker for early-stage AD. Third, male gender was associated with higher ability in IADLs, consistently with previous studies showing that women generally encounter a greater functional impairment than men [26], [27]. This gender difference could be linked to the use of different strategies to perform IADLs between men and women [28]. For instance, gender-specific navigational strategies have been evidenced in AD [29], which can in turn have an impact on IADLs such as driving. Moreover, sociocultural issues may also have a role, due to the historically gendered division of household labor, especially for individuals who are now in old age. Indeed, in a recent model, the (simply) greater exposure to IADLs (due to sociocultural expectations, physical health, and socioeconomic factors, among others) is seen to be responsible for cognitive and physical improvements (called functional reserve), which in turn delay the onset of functional disability [30]. However, we must also consider that A-IADL-Q was developed to specifically address the need of a measurement tool free from gender-based issues, and other studies found no relationship between this score and gender [17]. Also, an additional explanation is that women in our cohort may be suffering from more pathological conditions, such as arthritis, osteoporosis, high blood pressure, and cataracts, which generally affect women more than men and which are known to reduce the ability to perform IADLs [31]. Finally, the LMM showed no overall change in A-IADL-Q scores over the 3 years. Our 3-year-long follow-up may have been too short to observe a clear decline in IADLs, considering that our cohort includes cognitively normal subjects. Excluding that the A-IADL-Q may not be sensitive enough to detect subtle changes in IADLs (which has been previously demonstrated), an additional and plausible explanation may be that our sample is not homogeneous enough to detect clear longitudinal changes on average. In fact, asymptomatic at-risk individuals with SCD represent a highly heterogeneous population (as shown in Fig. 1A), and for this reason, we have chosen to also perform an LCLMM, which seems to be a more appropriate approach for our purpose. LCLMM identifies groups of subjects on the basis of a given criterion (here, performance on IADLs over the 3 years) and then compares them to a reference group. In this way, it allows to study subpopulations or latent classes. In the present study, the LCLMM identified five latent classes and tended to model the largest one (group 2) over the others. Thanks to the partitioning in subpopulations, we have been able to explore which factors were associated with the longitudinal trajectory of IADL abilities. Interestingly, the group with consistently high IADL skills over the follow-up (group 2, 74% of the sample) included 4 of the 6 converters of our cohorts. These were participants with SCD and normal cognition at baseline, who progressed to prodromal AD during the follow-up. This finding confirmed that a functional deterioration is not a mandatory criterion for the diagnosis of prodromal AD [24] because the performance on IADL of patients at the prodromal stage was comparable with that of cognitively normal elderly. About 10% of our cohort experienced an either fluctuating (group 3) or persistent (group 4) worsening in IADLs. Those with fluctuating difficulties were entirely comparable with our reference group with stable IADL performance, whereas those with progressive functional decline had a lower level of education, a greater amyloid load, and 2 of the 6 converters in our cohort were part of this group. Thus, this group could represent a subpopulation of elderly individuals with a relatively lower educational level (and therefore less cognitive reserve and compensation capacity), where a higher amyloid deposition affects IADLs before cognitive functioning (except for two of 20 subjects belonging to this group who also presented overt cognitive deficits at follow-up testing). An interesting and a somewhat unexpected finding of this study is that subjects with amyloid deposition and a persistent functional decline did not show lower brain glucose metabolism. We did expect functional decline to be more directly associated with hypometabolism than with amyloid deposition [32]. Probably, the progressive decline in performing IADLs in this group was associated with a lower metabolism in ROIs that are not AD-related (for example in the frontal lobe) and therefore not explored here. In addition, a part of our cohort (about 16%; groups 1 and 5) showed a statistically significant improvement in IADL performance during the follow-up, with two different trajectories (see Fig. 1C). A similar trend has been previously demonstrated in studies in which elderly with more or less marked functional deterioration regained some degree of independence. The reason behind this recovery is not yet known, but it has been proposed to lie in a higher level of resilience [33]. In particular, the subjects who experienced a slight improvement in performance mainly between M24 and M36 (group 1) were entirely comparable to our reference group with stable IADLs. On the contrary, those who improved in IADLs, eventually encountering a decline from M24 to M36, were proportionately more men and had a greater amyloid load and less brain glucose metabolism. We propose that this group includes subjects with an underlying AD pathology who are in a compensatory phase, in which they show no cognitive or functional impairment, thanks to a high level of cognitive reserve and resilience. The slight decline observed between M24 and M36 could represent the beginning of a process of decompensation. This hypothesis would be consistent with a previous study on the INSIGHT-preAD cohort that analyzed data from the first 2 years of follow-up [12]. Our group failed to detect overall cognitive changes and discussed this finding as the result of a compensation mechanism mediated by the frontal lobe, where at-rest α oscillations on EEG were increased.
5. Conclusion
To conclude, the present study showed that slight changes in IADLs may be already present in very early, mostly “preclinical,” AD which might be useful to inform about potential future onset of AD dementia, contrary to current leading models of disease progression. The A-IADL-Q appears to be a good tool to use in the predementia phases to detect this subtle functional decline.
However, we should better understand if a functional measure has sufficient sensitivity and specificity to constitute an endpoint of studies on the progression from preclinical to clinical. Our study is data-driven and explorative and needs replication in larger samples. Thus, the implementation of the A-IADL-Q in large cohorts of individuals at risk for AD such as the European Prevention on Alzheimer's Dementia [34], [35] cohort could help in disentangling this interrogation.
Research in Context.
-
1.
Systematic review: We did not find any publication pertaining specifically to functional decline in preclinical Alzheimer's disease. There are, however, many studies describing a subtle cognitive decline in the preclinical stage of the disease, which would support such functional changes.
-
2.
Interpretation: In a longitudinal cohort study of 318 elderly memory complainers, we identified a group of participants with persistent functional decline on the Amsterdam Instrumental-Activities-of-Daily-Living questionnaire. This group displayed higher amyloid load on positron emission tomography and had a lower education level compared with a control group with stable functional scores.
-
3.
Future directions: The Amsterdam Instrumental-Activities-of-Daily-Living questionnaire could be used in the fields of early detection and intervention in Alzheimer's disease.
Acknowledgments
The authors are grateful to Hovagim Bakardjian and Marine Sole who contributed to collect the A-IADL-Q of the INSIGHT-preAD participants. The authors also wish to thank the 318 INSIGHT-preAD volunteers and their informants for their altruism and dedication to research against Alzheimer's disease.
Funding: The study was promoted by INSERM in collaboration with ICM, Instituts Hospitalo-Universitaires à ICM, and Pfizer and has received support within the “Investissement d'Avenir” (ANR-10-AIHU-06) program. The study was promoted in collaboration with the “CHU de Bordeaux” (coordination CIC EC7), the promoter of Memento cohort, and funded by the Foundation Plan-Alzheimer. The study was further supported by AVID/Lilly. This project/research has received funding from the European Union's Horizon 2020 Framework Programme for Research and Innovation under the Specific Grant Agreement No. 785907 (Human Brain Project SGA2). The funding sources had no role in the study design, data collection, data analysis, or data interpretation.
Footnotes
S.E. has received honoraria as a speaker or consultant for Eli Lilly, Biogen, Astellas Pharma, Roche, and GE Healthcare.
Supplementary data related to this article can be found at 10.1016/j.trci.2019.08.009.
Contributor Information
Stéphane Epelbaum, Email: stephane.epelbaum@aphp.fr.
MEMENTO study group and the INSIGHT-preAD study group:
Hovagim Bakardjian, Habib Benali, Hugo Bertin, Joel Bonheur LaurieBoukadida, Nadia Boukerrou, Enrica Cavedo, Patrizia Chiesa, Olivier Colliot, Bruno Dubois, Marion Dubois, Stéphane Epelbaum, Geoffroy Gagliardi, Remy Genthon, Marie-Odile Habert, Harald Hampel, Marion Houot, Aurélie Kas, Foudil Lamari, Marcel Levy, Simone Lista, Christiane Metzinger, Fanny Mochel, Francis Nyasse, Catherine Poisson, Marie-Claude Potier, Marie Revillon, Antonio Santos, Katia Santos Andrade, Marine Sole, Mohmed Surtee, Michel Thiebaud de Schotten, Andrea Vergallo, and Nadjia Younsi
Appendix
INSIGHT-preAD study group: Hovagim Bakardjian, Habib Benali, Hugo Bertin, Joel Bonheur, LaurieBoukadida, Nadia Boukerrou, Enrica Cavedo, Patrizia Chiesa, Olivier Colliot, Bruno Dubois, Marion Dubois, Stéphane Epelbaum, Geoffroy Gagliardi, Remy Genthon, Marie-Odile Habert, Harald Hampel, Marion Houot, Aurélie Kas, Foudil Lamari, Marcel Levy, Simone Lista, Christiane Metzinger, Fanny Mochel, Francis Nyasse, Catherine Poisson, Marie-Claude Potier, Marie Revillon, Antonio Santos, Katia Santos Andrade, Marine Sole, Mohmed Surtee, Michel Thiebaud de Schotten, Andrea Vergallo, and Nadjia Younsi.
Supplementary data
1
References
- 1.Cummings J., Ritter A., Zhong K. Clinical trials for disease-modifying therapies in Alzheimer's disease: a primer, lessons learned, and a blueprint for the future. J Alzheimers Dis. 2018;64:S3–S22. doi: 10.3233/JAD-179901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jekel K., Damian M., Wattmo C., Hausner L., Bullock R., Connelly P.J. Mild cognitive impairment and deficits in instrumental activities of daily living: a systematic review. Alzheimer's Res Ther. 2015;7:17. doi: 10.1186/s13195-015-0099-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marshall G.A., Amariglio R.E., Sperling R.A., Rentz D.M. Activities of daily living: where do they fit in the diagnosis of Alzheimer's disease? Neurodegener Dis Manag. 2012;2:483–491. doi: 10.2217/nmt.12.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sikkes S.A., Visser P.J., Knol D.L., de Lange-de Klerk E.S., Tsolaki M., Frisoni G.B. Do instrumental activities of daily living predict dementia at 1-and 2-year follow-up? Findings from the development of screening guidelines and diagnostic criteria for predementia Alzheimer's disease study. J Am Geriatr Soc. 2011;59:2273–2281. doi: 10.1111/j.1532-5415.2011.03732.x. [DOI] [PubMed] [Google Scholar]
- 5.Farias S.T., Chou E., Harvey D.J., Mungas D., Reed B., DeCarli C. Longitudinal trajectories of everyday function by diagnostic status. Psychol Aging. 2013;28:1070. doi: 10.1037/a0034069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sunderaraman P., Cosentino S. Integrating the constructs of anosognosia and metacognition: a review of recent findings in dementia. Curr Neurol Neurosci Rep. 2017;17:27. doi: 10.1007/s11910-017-0734-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albert S.M., Bear-Lehman J., Burkhardt A. Lifestyle-adjusted function: variation beyond BADL and IADL competencies. Gerontologist. 2009;49:767–777. doi: 10.1093/geront/gnp064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lawton M.P., Brody E.M. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 9.Peres K., Helmer C., Amieva H., Orgogozo J.M., Rouch I., Dartigues J.F. Natural history of decline in instrumental activities of daily living performance over the 10 years preceding the clinical diagnosis of dementia: a prospective population-based study. J Am Geriatr Soc. 2008;56:37–44. doi: 10.1111/j.1532-5415.2007.01499.x. [DOI] [PubMed] [Google Scholar]
- 10.Sikkes S.A., de Lange-de Klerk E.S., Pijnenburg Y.A., Gillissen F., Romkes R., Knol D.L. A new informant-based questionnaire for instrumental activities of daily living in dementia. Alzheimers Dement. 2012;8:536–543. doi: 10.1016/j.jalz.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Jutten R.J., Peeters C.F., Leijdesdorff S.M., Visser P.J., Maier A.B., Terwee C.B. Detecting functional decline from normal aging to dementia: development and validation of a short version of the Amsterdam IADL Questionnaire. Alzheimers Dement (Amst) 2017;8:26–35. doi: 10.1016/j.dadm.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubois B., Epelbaum S., Nyasse F., Bakardjian H., Gagliardi G., Uspenskaya O. Cognitive and neuroimaging features and brain β-amyloidosis in individuals at risk of Alzheimer's disease (INSIGHT-preAD): a longitudinal observational study. Lancet Neurol. 2018;17:335–346. doi: 10.1016/S1474-4422(18)30029-2. [DOI] [PubMed] [Google Scholar]
- 13.Grober E., Buschke H., Crystal H., Bang S., Dresner R. Screening for dementia by memory testing. Neurology. 1988;38:900–903. doi: 10.1212/wnl.38.6.900. [DOI] [PubMed] [Google Scholar]
- 14.Van der Linden M., Coyette F., Poitrenaud J., Kalafat M., Calicis F., Wyns C. L'épreuve de rappel libre/rappel indicé à 16 items (RL/RI-16) In: Solal, editor. L'évaluation des troubles de la mémoire: présentation de quatre tests de mémoire épisodique avec leur étalonnage. De Boeck Supérieur; Marseille, France: 2004. pp. 25–47. [Google Scholar]
- 15.Jack C.R., Jr., Knopman D.S., Weigand S.D., Wiste H.J., Vemuri P., Lowe V. An operational approach to National Institute on Aging-Alzheimer's Association criteria for preclinical Alzheimer disease. Ann Neurol. 2012;71:765–775. doi: 10.1002/ana.22628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koster N., Knol D.L., Uitdehaag B.M., Scheltens P., Sikkes S.A. The sensitivity to change over time of the Amsterdam IADL Questionnaire©. Alzheimers Dement. 2015;11:1231–1240. doi: 10.1016/j.jalz.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Sikkes S.A., Knol D.L., Pijnenburg Y.A., De Lange-de Klerk E.S., Uitdehaag B.M., Scheltens P. Validation of the Amsterdam IADL Questionnaire©, a new tool to measure instrumental activities of daily living in dementia. Neuroepidemiology. 2013;41:35–41. doi: 10.1159/000346277. [DOI] [PubMed] [Google Scholar]
- 18.Nakagawa S., Schielzeth H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol. 2013;4:133–142. [Google Scholar]
- 19.Proust C., Jacqmin-Gadda H. Estimation of linear mixed models with a mixture of distribution for the random effects. Comput Methods Programs Biomed. 2005;78:165–173. doi: 10.1016/j.cmpb.2004.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sikkes S.A., Pijnenburg Y.A., Knol D.L., de Lange-de Klerk E.S., Scheltens P., Uitdehaag B.M. Assessment of instrumental activities of daily living in dementia: diagnostic value of the Amsterdam Instrumental Activities of Daily Living Questionnaire. J Geriatr Psychiatry Neurol. 2013;26:244–250. doi: 10.1177/0891988713509139. [DOI] [PubMed] [Google Scholar]
- 21.Facal D., Carabias M.A., Pereiro A.X., Lojo-Seoane C., Jutten R.J., Sikkes S.A. Assessing everyday activities across the dementia spectrum with the Amsterdam IADL Questionnaire. Curr Alzheimer Res. 2018;15:1261–1266. doi: 10.2174/1567205015666180925113411. [DOI] [PubMed] [Google Scholar]
- 22.Gore P.G., Kingston A., Johnson G.R., Kirkwood T.B.L., Jagger C. New horizons in the compression of functional decline. Age Ageing. 2018;47:764–768. doi: 10.1093/ageing/afy145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hebert R. Functional decline in old age. CMAJ. 1997;157:1037–1045. [PMC free article] [PubMed] [Google Scholar]
- 24.Dubois B., Feldman H.H., Jacova C., Cummings J.L., Dekosky S.T., Barberger-Gateau P. Revising the definition of Alzheimer's disease: a new lexicon. Lancet Neurol. 2010;9:1118–1127. doi: 10.1016/S1474-4422(10)70223-4. [DOI] [PubMed] [Google Scholar]
- 25.Lilamand M., Cesari M., del Campo N., Cantet C., Soto M., Ousset P.J. Brain amyloid deposition is associated with lower instrumental activities of daily living abilities in older adults. Results from the MAPT study. J Gerontol A Biol Sci Med Sci. 2016;71:391–397. doi: 10.1093/gerona/glv155. [DOI] [PubMed] [Google Scholar]
- 26.Sinforiani E., Citterio A., Zucchella C., Bono G., Corbetta S., Merlo P. Impact of gender differences on the outcome of Alzheimer's disease. Dement Geriatr Cogn Disord. 2010;30:147–154. doi: 10.1159/000318842. [DOI] [PubMed] [Google Scholar]
- 27.Lin K.A., Choudhury K.R., Rathakrishnan B.G., Marks D.M., Petrella J.R., Doraiswamy P.M. Marked gender differences in progression of mild cognitive impairment over 8 years. Alzheimers Dement (N Y) 2015;1:103–110. doi: 10.1016/j.trci.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hall J.R., Vo H.T., Johnson L.A., Barber R.C., O'Bryant S.E. The link between cognitive measures and ADLs and IADL functioning in mild Alzheimer's: what has gender got to do with it? Int J Alzheimers Dis. 2011;2011:276734. doi: 10.4061/2011/276734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cushman L.A., Duffy C.J. The sex specificity of navigational strategies in Alzheimer disease. Alzheimer Dis Assoc Disord. 2007;21:122–129. doi: 10.1097/WAD.0b013e318047df2f. [DOI] [PubMed] [Google Scholar]
- 30.Berezuk C., Zakzanis K.K., Ramirez J., Ruocco A.C., Edwards J.D., Callahan B.L. Functional reserve: experience participating in instrumental activities of daily living is associated with gender and functional independence in mild cognitive impairment. J Alzheimers Dis. 2017;58:425–434. doi: 10.3233/JAD-161227. [DOI] [PubMed] [Google Scholar]
- 31.Leveille S.G., Fried L., Guralnik J.M. Disabling symptoms. J Gen Intern Med. 2002;17:766–773. doi: 10.1046/j.1525-1497.2002.20229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fukai M., Hirosawa T., Kikuchi M., Hino S., Kitamura T., Ouchi Y. Different patterns of glucose hypometabolism underlie functional decline in frontotemporal dementia and Alzheimer's disease: FDG-PET study. Neuropsychiatry. 2018;8:441–447. [Google Scholar]
- 33.Arenaza-Urquijo E.M., Vemuri P. Resistance vs resilience to Alzheimer disease: clarifying terminology for preclinical studies. Neurology. 2018;90:695–703. doi: 10.1212/WNL.0000000000005303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solomon A., Kivipelto M., Molinuevo J.L., Tom B., Ritchie C.W. European prevention of Alzheimer's dementia longitudinal cohort study (EPAD LCS): study protocol. BMJ Open. 2019;8:e021017. doi: 10.1136/bmjopen-2017-021017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vermunt L., Veal C.D., Ter Meulen L., Chrysostomou C., van der Flier W., Frisoni G.B. European prevention of Alzheimer's dementia registry: recruitment and prescreening approach for a longitudinal cohort and prevention trials. Alzheimers Dement. 2018;14:837–842. doi: 10.1016/j.jalz.2018.02.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1