Abstract
We examined the relationship between insulin clearance, insulin sensitivity, and β-cell function and the longitudinal effect of insulin clearance on β-cell function in lean and obese insulin-sensitive and insulin-resistant adolescents. A hyperinsulinemic-euglycemic and a hyperglycemic clamp were performed in 110 youths to quantify hepatic and peripheral clearance, insulin sensitivity, and β-cell function (disposition index, DIh-clamp). Participants underwent an oral glucose tolerance test at baseline and after 2 years to assess glucose tolerance and oral β-cell function (oDIcpep) and were sorted into four groups (lean and obese normal glucose tolerance, insulin sensitive, insulin resistant, and impaired glucose tolerance). Insulin sensitivity was defined based on the median of insulin stimulated glucose disposal (M) measured during the hyperinsulinemic-euglycemic clamp. Lean and obese insulin-sensitive participants did not differ with respect to hepatic and peripheral clearance or for insulin sensitivity. Insulin sensitivity was linearly correlated with whole-body insulin clearance. Hepatic insulin extraction at baseline acted as an independent determinant of β-cell function at follow-up. The decline in insulin sensitivity, even in the absence of an impairment of glucose tolerance, is associated with lowering of hepatic insulin clearance in obese youth, which in turn may contribute to the decline in β-cell function over time.
Introduction
Hyperinsulinemia is a hallmark of prediabetes in youth (1–3), resulting from the hyperresponsivness of the β-cell to insulin resistance along with a reduction in insulin clearance (4–6), and has a pathogenic role in type 2 diabetes progression (7–9).
A reduced insulin clearance has been described in obese adults (10) and youths (3,11,12), even in the absence of dysglycemia, and associated with a higher risk of incident diabetes in Hispanic and African American adults from the Insulin Resistance Atherosclerosis Study (IRAS) (10). Recently, the Restoring Insulin Secretion (RISE) study, using a surrogate measure of whole-body insulin clearance (fasting C-peptide over insulin ratio), reported an almost 25% lower clearance in youths with prediabetes or recent-onset type 2 diabetes compared with the adults (13). This age-specific feature is highly suggestive for a low insulin clearance to play a pivotal role in the rapid progression of diabetes described in obese youths (14,15).
Liver and peripheral insulin-sensitive tissues, such as kidney and skeletal muscle, clear plasma insulin, with the liver removing a high and variable fraction of insulin after first pass (16,17). In animal models, a reduced hepatic insulin clearance has been proposed as an early homeostatic mechanism to preserve β-cell function in the context of increased insulin resistance by reducing the hepatic insulin extraction (18). Early changes in insulin sensitivity and clearance are expected to precede the rise in 2-h glucose toward abnormal levels as measured by the oral glucose tolerance test (OGTT).
To the best of our knowledge, no studies have examined the potential differences in insulin clearance across groups of youths with different levels of insulin sensitivity and glucose tolerance using accurate measures of β-cell function and insulin sensitivity. The simultaneous measurement of both insulin and C-peptide concentrations during two dynamic metabolic tests (the hyperinsulinemic-euglycemic clamp and hyperglycemic clamp) and the use of a model-based method for insulin clearance allowed us to estimate multiple insulin clearance parameters and determine their impact on β-cell function over time in youth.
We hypothesized that the decline in insulin sensitivity, observed in obese youths even before an overt impairment in glucose tolerance, is paralleled by a lowering of insulin clearance and that this latter acts as an independent determinant of β-cell function over time. This hypothesis was tested in a multiethnic cohort of lean and obese youths with normal glucose tolerance (NGT) and impaired glucose tolerance (IGT) using hyperglycemic and hyperinsulinemic-euglycemic clamps and the OGTT to assess β-cell function and insulin sensitivity and estimate hepatic and extrahepatic insulin clearance. This cohort was then reevaluated longitudinally with a second OGTT after a 2-year period to assess the effect of baseline hepatic and peripheral clearance on β-cell function and glucose tolerance.
Research Design and Methods
We conducted a longitudinal study in 110 lean and obese youths recruited from a multiethnic cohort participating in the Yale Pathogenesis of Youth Onset Type 2 Diabetes (PYOD) study (ClinicalTrials.gov identifier NCT01967849), a long-term project aimed at studying early alterations in β-cell function and insulin sensitivity in obese youths (19).
Inclusion criteria for the lean group were BMI <85th percentile for age and sex and age 14–21 years at the screening visit. The overweight/obese cohorts (Ob) included subjects with BMI in the ≥85th percentile for age and sex. We excluded individuals using medications affecting glucose metabolism, diagnosed with syndromic obesity, or participating in other clinical trials. Also excluded were participants who tested positive for at least one of the autoantibodies associated with type 1 diabetes (anti-islet cell antibody, anti-insulin antibody, anti-GAD, anti-protein tyrosine phosphatase [IA2], and anti-zinc transporter 8 [ZnT8]) in case of negativity of the previous ones. The Yale School of Medicine Human Investigations Committee approved the study protocol.
All of the eligible individuals who consented to the study underwent an OGTT at baseline, along with a euglycemic-hyperinsulinemic clamp and a hyperglycemic clamp and were reassessed after ∼2 years with a second OGTT to test the effect of baseline hepatic insulin clearance on changes of β-cell function. The three tests at baseline were performed within a 3-month interval. During the clinical evaluation, Tanner stage was assessed, as previously described (20,21), by rating genital development and pubic hair growth for boys and breast development and pubic hair growth for girls.
Study Procedures
OGTT
All eligible participants who consented to the study underwent two OGTTs, the first at baseline and the second after the 2-year follow-up evaluation. Before the test, they were asked to follow a weight-maintenance diet consisting of at least 250 g of carbohydrates daily for 7 days and to avoid intense physical activity. After an ∼10-h overnight fast, all subjects arrived at 8:00 a.m. at the Yale Center for Clinical Investigation. One intravenous catheter was inserted and two baseline samples were obtained for measurements of plasma glucose, insulin, and C-peptide. Thereafter, flavored dextrose (Orangedex; Custom Laboratories, Baltimore, MD) in a dose of 1.75 g/kg of body wt (up to a maximum of 75 g) was given orally, and blood samples were obtained every 30 min for 180 min for the measurement of plasma glucose, insulin, and C-peptide. We defined IGT as a plasma glucose concentration 2 h after the OGTT (2-h glucose) of 140–199 mg/dL and type 2 diabetes as fasting plasma glucose of ≥126 mg/dL or 2-h glucose of ≥200 mg/dL (22). Participants from the Ob cohort who resulted in having type 2 diabetes at a confirmatory OGTT were excluded from the study as well as those from the lean cohort with IGT or type 2 diabetes. After the baseline OGTT, eligible subjects underwent an abdominal MRI to assess the body fat partitioning, a hyperinsulinemic-euglycemic clamp, and a hyperglycemic clamp procedure.
Abdominal Fat Partition
Abdominal MRI studies were performed to assess the abdominal fat partitioning on a GE or Siemens Sonata 1.5-Tesla system, as previously reported (23). A single slice, obtained at the level of the L4/L5 disc space, was analyzed for each subject. Body fat distribution was expressed as the ratio between visceral adipose tissue (VAT, cm2) over the sum of VAT and subcutaneous adipose tissue (VAT + SAT, cm2) (23,24).
Hyperinsulinemic-Euglycemic Clamp
Participants were admitted to the Yale Center for Clinical Investigation Research Unit at 7:30 a.m. A catheter was inserted in the antecubital retrograde arm vein for blood sampling, and the hand was warmed throughout the study for blood arterialization. An antecubital catheter was inserted in the contralateral arm for glucose and insulin infusion. Insulin sensitivity and insulin clearance were measured by a two-step euglycemic clamp, first infusing insulin at a low insulin dose as a continuous primed infusion at 4 mU ⋅ m−2 ⋅ min−1 for the first 2 h, followed by high insulin dose of 80 mU ⋅ m−2 ⋅ min−1 from 120 to 240 min (14,25,26). The glucose infusion was adjusted to maintain a target glucose value of 92 mg/dL during the test. Baseline glucose, insulin, and C-peptide levels were measured at −20 and 0 min before the glucose and insulin infusion. During the last 30 min of each step, plasma insulin and C-peptide levels were collected at 10-min intervals to calculate insulin and C-peptide steady state.
Thus, the baseline glucose and C-peptide at −20 and 0 min were used to estimate the endogenous insulin secretion rate (ISR), while the low- and high-dose insulin phases were used to calculate insulin clearance and its components, as detailed in the calculations.
Arterialized blood samples were collected every 5 min during each step of the clamp and immediately analyzed at the bedside using the glucose oxidase method on a YSI 2300 STAT Plus Glucose Analyzer (YSI Life Sciences, Yellow Springs, IL).
Hyperglycemic Clamp
Hyperglycemic clamp was performed to assess β-cell function in the context of whole-body insulin sensitivity. As described for the two-step hyperinsulinemic-euglycemic clamp, one catheter was inserted for blood sampling, and the hand was warmed for blood arterialization. A second catheter was inserted in the contralateral arm for glucose infusion. Baseline glucose, insulin, and C-peptide were measured at −20 and 0 min before the glucose infusion, and the average value was used to calculate baseline values (t = 0). Blood for plasma glucose was drawn every 2 min during the first 10 min and then every 5 min and immediately centrifuged and analyzed using the glucose oxidase method (YSI 2300 STAT Plus Glucose Analyzer).
A standardized priming 20% dextrose (Hospira, Lake Forest, IL) infusion was administered during the first 10 min (200 mg/kg body wt), and then infusion rates were adjusted every 5 min to maintain plasma glucose at 11.1 mmol/L (200 mg/dL) for 120 min (26). Blood samples for subsequent assays were drawn at 2, 4, 6, 8, 10, 20, 30, 40, 60, 80, 100, 110, and 120 min.
Data on 30 subjects from both the hyperglycemic and hyperinsulinemic-euglycemic clamp have been previously reported (27,28).
Assays
Insulin and C-peptide were measured by a double-antibody radioimmunoassay kit (EMD Millipore, Billerica, MA).
Calculations
Insulin Clearance
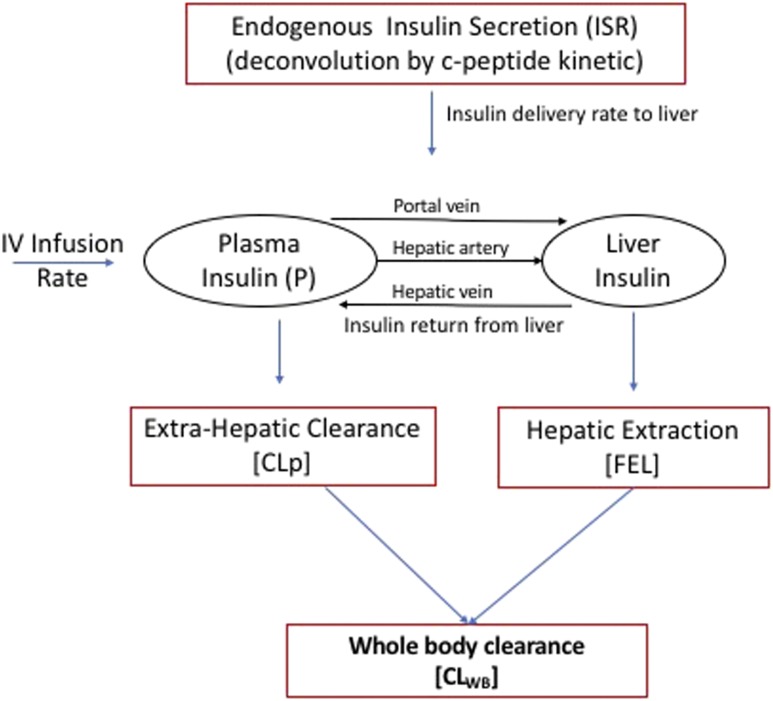
The model we adapted to estimate insulin clearance has been previously described (29) and modified according to the current experimental design based on euglycemic clamp measurements only. The outline of the model is described in Fig. 1. The equation used for describing extrahepatic and hepatic contributions to insulin clearance (17) was used in the basal (preinfusion, −20 and 0 time) period and during the high insulin infusion (80 mU ⋅ m−2 ⋅ min−1) to estimate extrahepatic insulin clearance (CLp) and hepatic fractional extraction (FEL) by assuming the subjects were at steady state in both the basal period and during the last 30 min of the insulin infusion. Denoting the average values of plasma insulin and ISR during the basal and clamp periods as Pbasal and Pclamp and ISRbasal and ISRclamp (where ISR values are calculated using measured C-peptide values and deconvolution), and assuming steady-state conditions in each of these periods, gives the following two equations:
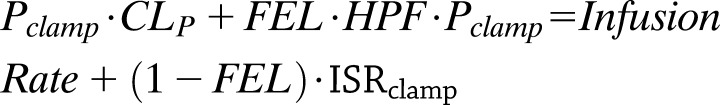 |
where HPF is the hepatic plasma flow (estimated to be 0.576 L/m2 [17]). Inserting the measured/calculated values for P, ISR, and HPF in these equations leaves two equations for the two unknown values of FEL and CLP, and the equations were solved using standard linear algebra methods in Matlab. Subjects where FEL was estimated to be <0 were excluded from the analysis. Whole-body clearance (CLWB) was defined as the whole-body clearance for secreted insulin and was calculated from these parameters as previously described (17).
Figure 1.
Outline of the insulin clearance model (hepatic plasma flow 0.576 L/m2). CLWB is calculated for constant ISR (with infusion = 0) as the ratio of insulin delivery to plasma insulin concentration (CLWB = ISR/P). IV, intravenous.
Insulin Sensitivity
During the two-step hyperinsulinemic-euglycemic clamp, steady state and insulin sensitivity (M value) were measured during the last 30 min of the second step of the study (210–240 min) and expressed as milligrams of glucose infused per kilogram of lean body mass (LBM) per minute (M = mg/kgLBM/min). Insulin sensitivity was estimated as the insulin-stimulated glucose disposal during the steady state of the hyperinsulinemic clamp (210–240 min) and used to sort the Ob-NGT group into two subgroups, insulin-sensitive (Ob-NGT-IS) and insulin-resistant (Ob-NGT-IR), based on the median of insulin sensitivity of the group (M = 4.5 mg/kgLBM/min). Insulin-stimulated glucose disposal will be referred to the M value as a surrogate measure of insulin sensitivity from now on. The coefficient of correlation between the M value derived from the euglycemic-hyperinsulinemic clamp and the M/I value derived from the hyperglycemic clamp (see below) was r = 0.45 (P < 0.001).
Insulin Secretion (Hyperglycemic Clamp and OGTT)
During the hyperglycemic clamp, insulin sensitivity was estimated as the ratio (M to I) of insulin-stimulated glucose disposal (Mh-clamp)—equal to the mean glucose infusion rate at 100, 110, and 120 min—over insulin at steady state (I), computed as the mean steady-state plasma insulin concentration (I) for the same time points, as previously described (13,30). Acute C-peptide response to glucose (ACPRg) was calculated as the mean incremental response over the baseline value for the first 10 min after the glucose bolus (13). Steady-state C-peptide response was defined as the mean C-peptide concentration at 100, 110, and 120 min above baseline (13). The baseline disposition index (DIh-clamp) was estimated as the product of ACPRg and M/Ih-clamp (13,31). The follow-up assessment of insulin secretion relied on the DI estimated from the OGTT (oDIcpep) calculated as previously described (32). Briefly, the oDIcpep was calculated as the product of the insulinogenic index (IGIcpep) and the whole-body insulin sensitivity index computed on the 3-h OGTT performed at follow-up: the IGIcpep: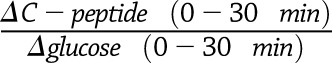 is a surrogate measure of the acute insulin response (33,34), whereas whole-body insulin sensitivity index
is a surrogate measure of the acute insulin response (33,34), whereas whole-body insulin sensitivity index
is a measure of insulin sensitivity (35). A lower oDIcpep indicates a reduced β-cell function in the context of ambient insulin sensitivity (35). The adoption of the C-peptide–based index is meant to minimize the effect of the differential insulin clearance described in some of the ethnic groups enrolled in our study (3), as recently described by the Restoring Insulin Secretion (RISE) study (13) and by our group (32). The correlation between oDIcpep and DIh-clamp has been previously demonstrated (30,36).
Statistical Analysis
Participants were stratified into four groups based on BMI, glucose tolerance, and estimated insulin sensitivity (M) during the hyperinsulinemic clamp for the analysis. Specifically, obese participants were grouped as Ob-NGT-IS, Ob-NGT-IR, and Ob-IGT. The Ob-NGT-IS group was defined as those with an M value during the hyperinsulinemic-euglycemic clamp >4.5 mg/kgLBM/min, whereas the Ob-NGT-IR included those with an M value of ≤4.5 mg/kgLBM/min. The Ob-NGT-IS group was used as comparison term for the analysis.
The primary outcome of the study was the difference in the CLWB across the four groups. Its two components, namely, extrahepatic (CLp) and hepatic (FEL) insulin clearance, were similarly compared.
Distribution of continuous variables was examined for skewness and kurtosis. Nonnormally distributed variables were compared by the Kruskal-Wallis test, followed by the post hoc pairwise Dunn test. Normally distributed variables were analyzed by ANOVA, followed by the post hoc pairwise Dunnett test. Statistical significance was established with α = 0.025, with Bonferroni adjustment for multiple comparisons. Categorical variables were compared using the χ2 test. Data were summarized in tables using median (25th, 75th percentile) for continuous variables and count (%) for categorical variables. Multivariable regression analysis was performed to estimate the effect of age, BMI, sex, Tanner stage, ethnicity (non-Hispanic white, non-Hispanic black, and Hispanic), baseline 2-h glucose and oDIcpep, and FEL and CLp insulin clearance rate on the continuous variable oDIcpep at follow-up (37–39). The variables were selected a priori based on the published literature (37–39). Before including the covariates into the model, we examined them for multicollinearity using the Spearman correlation coefficient (all estimates were <0.40) and variance inflation factor (all estimates were <2.0). Statistical significance for the parameter estimates of the individual predictors was established using the Wald test (α = 0.05).
Linear regression analysis was adopted to test the relationship between CLWB and insulin sensitivity (M ) as well as ISR, adjusted for BMI, age, sex, and Tanner stage.
Analyses were performed using Stata 13 software (StataCorp, College Station, TX) and Prism 8.0 software (GraphPad Software, San Diego, CA).
Data and Resource Availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request. No applicable resources were generated or analyzed during the current study.
Results
Participants
We enrolled 110 participants who had an OGTT, along with a hyperglycemic clamp and a hyperinsulinemic-euglycemic clamp at baseline and a second OGTT at 2.1 (1.5, 2.6) years of follow-up. Baseline characteristics are described in Table 1. Participants were sorted into four groups: 43 Ob-NGT-IS, 43 obese Ob-NGT-IR, 14 Ob-IGT, and 10 lean NGT (lean). The three ethnicities (non-Hispanic white, non-Hispanic black, and Hispanic) of our cohort were equally represented in the four groups, and the BMI was similar across the three obese subgroups. Fasting glucose and HbA1c did not differ across the three obese groups or between lean and Ob-NGT-IS. As expected, 2-h glucose was increasingly higher from the Ob-NGT-IS to the Ob-IGT. Despite similar level of obesity (BMI), baseline insulin and C-peptide were progressively higher across the three obese groups, whereas the lean participants had a lower baseline C-peptide and insulin than their Ob-NGT-IS peers. The VAT-to-SAT ratios were similar among the three obese groups.
Table 1.
Participants’ characteristics
| Ob-NGT-IS (n = 43) | Ob-NGT-IR (n = 43) | Ob-IGT (n = 14) | P | Lean-NGT (n = 10) | P* (vs. Ob-NGT-IS) | |
|---|---|---|---|---|---|---|
| Age (years) | 15 (12, 16) | 14 (13, 15) | 13.5 (11, 16) | 0.523 | 16.5 (15, 19) | 0.029 |
| Female sex, n (%) | 18 (38) | 28 (60) | 14 (39) | 0.006 | 13 (56) | 0.456 |
| BMI (kg/m2) | 31.8 (29.8, 40) | 37 (32.7, 40.1) | 33.5 (28, 38.7) | 0.064 | 22.6 (21.6, 23.3) | <0.001 |
| Ethnicity, n (%) | 0.267 | 0.829 | ||||
| Non-Hispanic white | 17 (40) | 15 (35) | 6 (42) | 5 (50) | ||
| Non-Hispanic black | 15 (35) | 12 (28) | 1 (7) | 3 (30) | ||
| Hispanic | 11 (25) | 16 (37) | 7 (51) | 2 (20) | ||
| Tanner stage, n (%) | 0.511 | 0.236 | ||||
| II–III | 12 (72) | 9 (55) | 5 (58) | 1 (4) | ||
| IV–V | 31 (28) | 34 (45) | 9 (42) | 9 (96) | ||
| Fasting glucose (mg/dL) | 93 (87, 97) | 94 (89, 102) | 88 (85, 94) | 0.285 | 93.5 (90, 101) | 0.320 |
| 2-h glucose (mg/dL) | 114 (101, 124) | 121 (110, 134) | 156.5 (147, 165) | <0.001 | 97 (92, 114) | 0.478 |
| Fasting insulin (μU/mL) | 26.5 (22, 34.5) | 35 (25, 46) | 35.5 (26.5, 63.5) | 0.014 | 12 (12, 13) | 0.048 |
| Fasting C-peptide (pmol/L) | 995 (880, 1,130) | 1,170 (880, 1,570) | 1,192.5 (1,092.5, 1,927.5) | <0.001 | 610 (585, 765) | 0.006 |
| HbA1c (%) | 5.4 (5, 5.6) | 5.4 (5, 5.6) | 5.2 (5.1, 5.4) | 0.929 | 5.3 (5.2, 5.7) | 0.710 |
| HbA1c % (mmol/mol) | 36 (31,38) | 36 (31, 38) | 33 (32,36) | 34 (33,39) | ||
| VAT (cm2) | 57.4 (43.6, 92.2) | 55.4 (40.3, 68.9) | 52.1 (41.1, 87.3) | 0.015 | 26.3 (17.3, 29.9) | 0.022 |
| SAT (cm2) | 515.2 (455, 655.7) | 508 0.9 (423.3, 722.2) | 518.3 (403.8, 609) | 0.040 | 326.5 (245.1, 370.7) | <0.001 |
| VAT/(VAT + SAT) | 0.098 (0.079, 0.143) | 0.123 (0.088, 0.158) | 0.075 (0.062, 0.087) | 0.477 | 0.109 (0.084, 0.134) | 0.481 |
| DIh-clamp (pmol ⋅ m−2 ⋅ min−1) | 7.3 (4.4, 10.3) | 5.1 (4.0, 7.5) | 3.0 (2.1, 3.9) | <0.001 | 6.06 (5.70, 23.03) | 0.474 |
| M value (mg/kgLBM/min) | 7.3 (5.6, 8.9) | 3.5 (2.8, 4.1) | 3.8 (3.3, 4.8) | <0.001 | 10.8 (10.2, 13.1) | <0.001 |
Data are median (interquartile range) unless otherwise indicated. Continuous variables have been compared with ANOVA; categorical variables compared with χ2 test. The M value has been computed at the steady state of the hyperinsulinemic-euglycemic clamp. Bold indicates P < 0.05.
Ob-NGT-IS has been adopted as comparison group for the lean group.
Insulin Clearance (Hyperinsulinemic-Euglycemic Clamp)
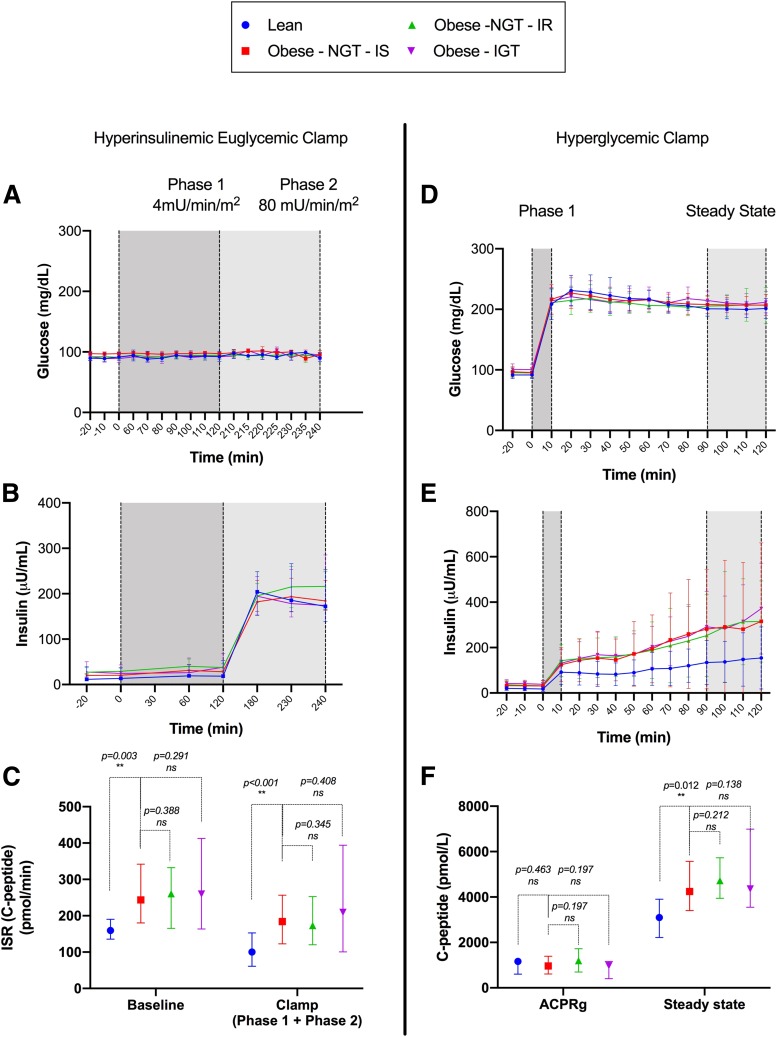
Plasma glucose levels during the clamp did not differ across the four groups, with an average glucose of 95 ± 7 mg/dL (Fig. 2A). Endogenous ISRbasal (Fig. 2B and C) was lower in the lean group compared with the Ob-NGT-IS group (P < 0.003) but did not differ between the Ob-NGT-IS and the Ob-NGT-IR and Ob-IGT groups (P = 0.388 and P = 0.291, respectively). During the test, the ISRclamp (Fig. 2C) mirrored the baseline difference in ISR, with the lean group exhibiting a lower ISRclamp than the Ob-NGT-IS group (P < 0.001). ISRclamp for the lean group resulted ∼30% lower than the baseline value (159.5 [190.3, 135.6] vs. 100 [61.1, 152.6], P < 0.001), with a significant drop of ISRclamp in all of the groups compared with the ISRbasal (P < 0.001), except for the IGT participants (P = 0.064).
Figure 2.
Glucose and insulin profiles during hyperinsulinemic-euglycemic clamp (A and B), ISR at baseline and during the test (C), glucose and insulin during the hyperglycemic clamp (D and E), and ACPRg and steady-state C-peptide (F). Data are reported as the median and interquartile range (25th, 75th). **Indicates statistical significance.
Conversely, the peripheral insulin sensitivity, as described by the M value, did not differ significantly between lean and Ob-NGT-IS (P = 0.067), whereas Ob-NGT-IR and Ob-IGT had a lower peripheral insulin sensitivity (P < 0.001 for both the groups) than the Ob-NGT-IS group (Table 1).
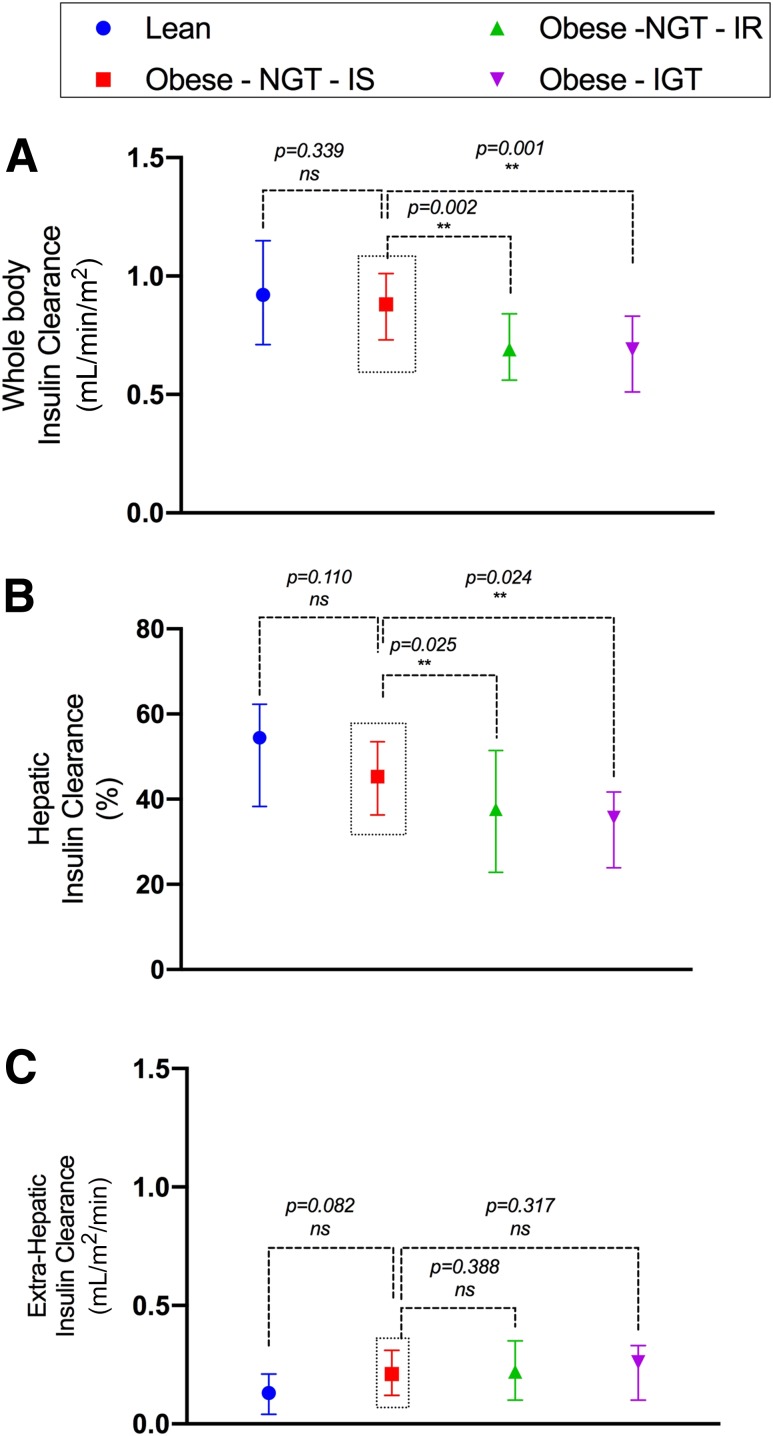
Mirroring the trend observed for insulin sensitivity, CLWB did not differ between lean and Ob-NGT-IS participants (P = 0.339), whereas it was ∼20% lower in Ob-NGT- IR (P < 0.001) and Ob-IGT (P < 0.001) compared with the Ob-NGT-IS participants (Fig. 3A). This difference was supported by a reduced FEL in Ob-NGT-IR and Ob-IGT compared with the Ob-NGT-IS group (P = 0.025) (Fig. 3B), with similar FEL in the lean and Ob-NGT-IS groups (P = 0.110). The four groups did not differ with respect to extrahepatic clearance (Fig. 3C).
Figure 3.
CLWB (A) and hepatic (B) and extrahepatic (C) insulin clearance. Data are reported as the median and interquartile range (25th, 75th). **Indicates statistical significance.
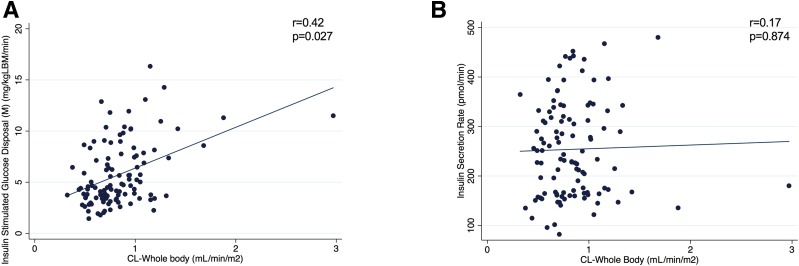
The relationship between insulin clearance and endogenous ISR and sensitivity is depicted in Fig. 4. Herein, we described a linear correlation between CLWB and insulin sensitivity (M) during the hyperinsulinemic-euglycemic clamp (r = 0.42, adjusted P = 0.027) (Fig. 4A), in the absence of a similar relationship between CLWB and ISRbasal (r = 0.17, adjusted P = 0.874) (Fig. 4B), with ISRbasal estimating endogenous ISR independently of the whole-body insulin sensitivity.
Figure 4.
Correlation analysis between CLWB and M (A) and ISR (B). Data are reported as median and interquartile range (25th, 75th).
β-Cell Function (Hyperglycemic Clamp)
We estimated the β-cell function, in the context of whole-body insulin sensitivity, performing a hyperglycemic clamp (DIh-clamp) (Fig. 2). Baseline and steady-state plasma glucose were similar across the four groups, supporting the robustness of the test, with an average glucose of 207 ± 17 mg/dL at the steady state (Fig. 2D). Notably, despite the similar hyperglycemic levels, pronounced differences were observed in the stimulated insulin levels among the four groups. Indeed, the β-cell function, as described by DIh-clamp, was significantly lower in the Ob-IGT group (P = 0.013) than in the Ob-NGT-IS group, whereas lean subjects had a more than double DIh-clamp than the Ob-NGT-IS subjects (P = 0.007) (Fig. 3C).
This finding does not conflict with the observation of a higher ISR in the obese groups during the euglycemic clamp (Fig. 2C), because DIh-clamp estimates endogenous secretion in the context of insulin sensitivity, whereas ISRbasal does not account for individual sensitivity.
Acute insulin response to glucose and ACPRg did not differ among the Ob-NGT-IS and the other three groups (Fig. 2E and F). As expected, steady-state insulin and C-peptide were both lower in lean compared with Ob-NGT-IS (P = 0.022 and P = 0.012, respectively), without a difference among the three obese groups (Fig. 2E and F).
Longitudinal Effect of Insulin Clearance on Insulin Secretion
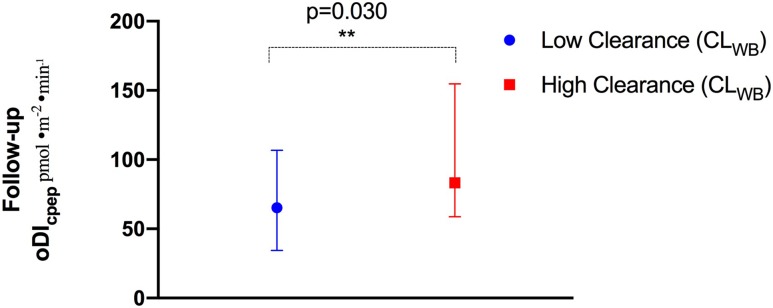
We evaluated the effect of insulin clearance at baseline on β-cell function, as measured by the oDIcpep at follow-up. Using a multivariate regression model, we estimated the FEL was the sole significant determinant of follow-up oDIcpep (P = 0.018) when the other clinical and metabolic variables were held constant (age, sex, baseline BMI and 2-h glucose, Tanner stage, baseline oDIcpep), with a 1% drop in hepatic insulin extraction associated with a 8.0 3.2 pmol/m2/min decrease of oDIcpep at the follow-up OGTT (Supplementary Table 1). Confirming findings from the model, we divided the original cohort into two groups according to the median CLWB and observed that those from the high clearance group exhibited an ∼30% higher oDIcpep at follow-up compared with their low clearance peers (P = 0.030) (Fig. 5).
3.2 pmol/m2/min decrease of oDIcpep at the follow-up OGTT (Supplementary Table 1). Confirming findings from the model, we divided the original cohort into two groups according to the median CLWB and observed that those from the high clearance group exhibited an ∼30% higher oDIcpep at follow-up compared with their low clearance peers (P = 0.030) (Fig. 5).
Figure 5.
oDIcpep at follow-up in the low and high CLWB groups. Low and high insulin clearance are defined as those with a CLWB at or below the median CLWB (low clearance) or above the median (high clearance). Data are reported as the median and interquartile range (25th, 75th). **Indicates statistical significance.
Discussion
In the current study, we found that in obese youths, the decrease in insulin sensitivity, more than the obesity per se, is paralleled by a progressive lowering of hepatic and extrahepatic insulin clearance. Notably, hepatic, but not extrahepatic, insulin clearance acts as an independent risk determinant for a lower β-cell function over time.
Herein, we adopted two robust dynamic metabolic tests in a multiethnic cohort of lean and obese youths to quantify the three main components of glucose homeostasis—insulin sensitivity, secretion, and clearance—and the OGTT to evaluate the longitudinal effect of baseline hepatic and extrahepatic clearance on the β-cell function (DI).
Previous studies adopted surrogate measures of insulin clearance (13), were limited to specific ethnic groups (3,10,40), or lacked the simultaneous quantification of insulin and C-peptide during dynamic hyperglycemic tests (30) as well as a longitudinal assessment of the impact of insulin clearance on insulin secretion.
Our study has several points of strengths. First, we identified a subgroup of Ob-NGT youth who share key metabolic features with their lean peers. Indeed, by the use of peripheral glucose disposal (M), measured during the hyperinsulinemic-euglycemic clamp, we separated the Ob-NGT participants into an Ob-NGT-IS and an Ob-NGT-IR group (41). The whole-body insulin clearance and the hepatic clearance were two key features that characterized the metabolic phenotype of the Ob-NGT-IS group. Indeed, the latter shared a comparable insulin clearance with their lean peers that was, in turn, significantly higher than the Ob-NGT-IR and Ob-IGT groups. This observation suggests that changes in insulin clearance could antedate the overt impairment in glucose tolerance and can be described even in the presence of NGT. Hepatic and extrahepatic clearance are differently regulated (17), with only the former being directly influenced by dietary changes, as shown in dog models using a high-fat diet (42), and diminished in African American and Hispanic groups at high risk for diabetes (10,43).
Second, we described a linear relation between insulin clearance and sensitivity that supports the existence of a homeostatic interplay of these two variables. This is supported by a number of previous reports in adults (9,44,45).
Third, we obtained two distinct measures of β-cell function by the use of hyperglycemic clamp (DIh-clamp) and the deconvolution method adopted during the preinfusion hyperinsulinemic-euglycemic clamp to estimate endogenous ISR. This latter mirrored insulin secretion independently from the whole-body insulin sensitivity and described an “hypersecretive” phenotype for the three obese groups, as confirmed by the C-peptide and insulin levels during the steady state of the hyperglycemic clamp. In contrast, DIh-clamp described the insulin secretion in the context of whole-body sensitivity, because it is computed by both measurements. DIh-clamp demonstrated two downfalls across the phenotypes of our cohort: Ob-NGT-IS had a lower DI than their lean peers but did not differ from the Ob-NGT-IR, whereas the Ob-IGT group had a significantly lower DI than their Ob-NGT-IS peers. This finding demonstrates how a decline in DI, that is supported by a contextual decrease in both insulin sensitivity and secretion, is accompanied by the disruption of glucose tolerance and the IGT phenotype.
The method used here for estimating whole-body insulin clearance and the contributions of hepatic and extrahepatic clearance used the same model as previously described (17,29), but the analysis method was modified in two important ways based on this study design. First, we used data from only the hyperinsulinemic clamp rather than combined data from both a hyperinsulinemic clamp + OGTT, as previously reported (29), or from a frequently sampled intravenous GTT (17), as in previous studies. The primary reason for using only the hyperinsulinemic clamp data in this study is that in a pediatric population, we expected insulin clearance parameters, as necessary for modeling purpose, could have shown a high intraindividual variability even over a short period of time affecting the robustness of the entire analysis. In addition, the contribution of other individual determinants of insulin secretion that occur during the OGTT, such as the incretin response (46), could have increased the variability of the response and acted as a major confounder to dissect the role of insulin clearance in the observed phenotypes. Therefore, the analyses were done only under conditions of relatively low ISR (compared with ISR during OGTT or hyperglycemic clamps), and thus hepatic insulin extraction was assumed to be linear (i.e., nonsaturated) over this range, whereas in previous studies with OGTT or intravenous GTT, it was possible to estimate saturable models of hepatic insulin clearance (17,29).
Fourth, we observed the longitudinal effect of baseline clearance on DI. Although our study could not rely on a second hyperglycemic clamp–derived measure, we obtained a second OGTT from the initial cohort. The follow-up oDIcpep was significantly influenced by the baseline hepatic but not extrahepatic clearance, with a lower hepatic clearance associated with a reduction of the DI independently of the other clinical and metabolic variables. Supporting this finding, we described how participants belonging to the lower half of whole-body insulin clearance resulted in having a reduced oDIcpep at follow-up. This finding needs to be supported by a larger study powered to detect longitudinal changes in glucose tolerance accompanying the decline in the DI.
A limitation of our study design stands in the age difference between the lean and obese group. Indeed, the lean group was ∼5 years older than the obese group. However, the metabolic phenotypical overlap between obese insulin-sensitive and lean subjects may somewhat diminish this problem as well as the correction, across the comparisons, for Tanner stage.
The major strength of this study is the contextual performance of two robust dynamic metabolic tests in the same subject as well as of a robust estimate of insulin clearance that has allowed us to assess the relationship between insulin clearance and insulin secretion, in vivo, for the first time, using both serial insulin and C-peptide measures to describe their phenotypes.
The longitudinal nature of the study is unique because it has allowed us to critically assess the role of the low hepatic insulin clearance in the pathogenesis of glucose intolerance in youth, although it was not powered to evaluate changes in glucose tolerance over time.
Hepatic insulin clearance appears as a major player in the decline of β-cell function in obese youths (10,18,42), because it acts as a gatekeeper that might prevent peripheral tissues and β-cells from being exposed to the insulin overload that features obesity and insulin-resistant stages. Failure to regulate the liver-insulin gate may represent a major step toward the progression to diabetes in youths that needs to be addressed in future studies. Dietary and pharmacological interventions targeting insulin clearance to prevent progression toward overt diabetes should consider obese “insulin-sensitive” adolescents as an ideal window of opportunity to maximize their long-term efficacy.
Supplementary Material
Article Information
Acknowledgments. The authors thank all volunteers for their participation in the study and Rachel Goldberg, Cindy Guandalini, and Mary Savoye for their help in the Yale Pediatric Clinic.
Funding. This study was supported by the International Society for Pediatric and Adolescent Diabetes Research Fellowship and the Robert Leet Patterson and Clara Guthrie Patterson Trust Mentored Research Award (to A.G.), the National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health and Human Development (grants R01-HD-40787, R01-HD-28016, and K24-HD-01464, to S.C.), the National Center for Research Resources (Clinical and Translational Science Award UL1-RR-0249139, to S.C.), the American Diabetes Association (Distinguished Clinical Scientist Award, to S.C.), and the National Institute of Diabetes and Digestive and Kidney Diseases (grant R01-DK-111038, to S.C.).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. A.G., D.P., R.W., C.G., B.P., D.T., and S.C. contributed to reviewing the data and analyses and to writing, editing, and reviewing the manuscript. A.G., R.W., and S.C. designed the clinical study and collected patient data. D.P. developed the modeling approach. C.G. and D.T. contributed to data analysis. B.P. contributed to patients’ enrollment and data collection. S.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 79th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 7–11 June 2019.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db19-0120/-/DC1.
References
- 1.Amiel SA, Sherwin RS, Simonson DC, Lauritano AA, Tamborlane WV. Impaired insulin action in puberty. A contributing factor to poor glycemic control in adolescents with diabetes. N Engl J Med 1986;315:215–219 [DOI] [PubMed] [Google Scholar]
- 2.Hannon TS, Bacha F, Lin Y, Arslanian SA. Hyperinsulinemia in African-American adolescents compared with their American white peers despite similar insulin sensitivity: a reflection of upregulated β-cell function? Diabetes Care 2008;31:1445–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arslanian SA, Saad R, Lewy V, Danadian K, Janosky J. Hyperinsulinemia in african-american children: decreased insulin clearance and increased insulin secretion and its relationship to insulin sensitivity. Diabetes 2002;51:3014–3019 [DOI] [PubMed] [Google Scholar]
- 4.Polonsky KS, Given BD, Hirsch L, et al. . Quantitative study of insulin secretion and clearance in normal and obese subjects. J Clin Invest 1988;81:435–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polonsky KS, Pugh W, Jaspan JB, et al. . C-peptide and insulin secretion. Relationship between peripheral concentrations of C-peptide and insulin and their secretion rates in the dog. J Clin Invest 1984;74:1821–1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergman RN, Phillips LS, Cobelli C. Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest 1981;68:1456–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corkey BE. Banting lecture 2011: hyperinsulinemia: cause or consequence? Diabetes 2012;61:4–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corkey BE. Diabetes: have we got it all wrong? Insulin hypersecretion and food additives: cause of obesity and diabetes? Diabetes Care 2012;35:2432–2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tricò D, Natali A, Arslanian S, Mari A, Ferrannini E. Identification, pathophysiology, and clinical implications of primary insulin hypersecretion in nondiabetic adults and adolescents. JCI Insight 2018;3:e124912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee CC, Haffner SM, Wagenknecht LE, et al. . Insulin clearance and the incidence of type 2 diabetes in Hispanics and African Americans: the IRAS Family Study. Diabetes Care 2013;36:901–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiss R, Dziura JD, Burgert TS, Taksali SE, Tamborlane WV, Caprio S. Ethnic differences in beta cell adaptation to insulin resistance in obese children and adolescents. Diabetologia 2006;49:571–579 [DOI] [PubMed] [Google Scholar]
- 12.Gower BA, Granger WM, Franklin F, Shewchuk RM, Goran MI. Contribution of insulin secretion and clearance to glucose-induced insulin concentration in african-american and caucasian children. J Clin Endocrinol Metab 2002;87:2218–2224 [DOI] [PubMed] [Google Scholar]
- 13.RISE Consortium Metabolic contrasts between youth and adults with impaired glucose tolerance or recently diagnosed type 2 diabetes: I. Observations using the hyperglycemic clamp. Diabetes Care 2018;41:1696–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiss R, Dufour S, Taksali SE, et al. . Prediabetes in obese youth: a syndrome of impaired glucose tolerance, severe insulin resistance, and altered myocellular and abdominal fat partitioning. Lancet 2003;362:951–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiss R, Santoro N, Giannini C, Galderisi A, Umano GR, Caprio S. Prediabetes in youth - mechanisms and biomarkers. Lancet Child Adolesc Health 2017;1:240–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hovorka R, Powrie JK, Smith GD, Sönksen PH, Carson ER, Jones RH. Five-compartment model of insulin kinetics and its use to investigate action of chloroquine in NIDDM. Am J Physiol 1993;265:E162–E175 [DOI] [PubMed] [Google Scholar]
- 17.Polidori DC, Bergman RN, Chung ST, Sumner AE. Hepatic and extrahepatic insulin clearance are differentially regulated: results from a novel model-based analysis of intravenous glucose tolerance data. Diabetes 2016;65:1556–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim SP, Ellmerer M, Kirkman EL, Bergman RN. Beta-cell “rest” accompanies reduced first-pass hepatic insulin extraction in the insulin-resistant, fat-fed canine model. Am J Physiol Endocrinol Metab 2007;292:E1581–E1589 [DOI] [PubMed] [Google Scholar]
- 19.Cropano C, Santoro N, Groop L, et al. . The rs7903146 variant in the TCF7L2 gene increases the risk of prediabetes/type 2 diabetes in obese adolescents by impairing β-cell function and hepatic insulin sensitivity. Diabetes Care 2017;40:1082–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child 1969;44:291–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child 1970;45:13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American Diabetes Association 2. Classification and diagnosis of diabetes. Diabetes Care 2017;40(Suppl. 1):S11–S24 [DOI] [PubMed] [Google Scholar]
- 23.Umano GR, Shabanova V, Pierpont B, et al. . A low visceral fat proportion, independent of total body fat mass, protects obese adolescent girls against fatty liver and glucose dysregulation: a longitudinal study. Int J Obes 2019;43:673–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kursawe R, Eszlinger M, Narayan D, et al. . Cellularity and adipogenic profile of the abdominal subcutaneous adipose tissue from obese adolescents: association with insulin resistance and hepatic steatosis. Diabetes 2010;59:2288–2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D’Adamo E, Cali AM, Weiss R, et al. . Central role of fatty liver in the pathogenesis of insulin resistance in obese adolescents. Diabetes Care 2010,33:1817–1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214–E223 [DOI] [PubMed] [Google Scholar]
- 27.Weiss R, Caprio S, Trombetta M, Taksali SE, Tamborlane WV, Bonadonna R. β-cell function across the spectrum of glucose tolerance in obese youth. Diabetes 2005;54:1735–1743 [DOI] [PubMed] [Google Scholar]
- 28.Cali’ AM, Bonadonna RC, Trombetta M, Weiss R, Caprio S. Metabolic abnormalities underlying the different prediabetic phenotypes in obese adolescents. J Clin Endocrinol Metab 2008;93:1767–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Utzschneider KM, Kahn SE, Polidori DC. Hepatic insulin extraction in NAFLD is related to insulin resistance rather than liver fat content. J Clin Endocrinol Metab 2019;104:1855–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sjaarda L, Lee S, Tfayli H, Bacha F, Bertolet M, Arslanian S. Measuring β-cell function relative to insulin sensitivity in youth: does the hyperglycemic clamp suffice? Diabetes Care 2013;36:1607–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bergman RN, Ader M, Huecking K, Van Citters G. Accurate assessment of β-cell function: the hyperbolic correction. Diabetes 2002;51(Suppl. 1):S212–S220 [DOI] [PubMed] [Google Scholar]
- 32.Galderisi A, Giannini C, Van Name M, Caprio S. Fructose consumption contributes to hyperinsulinemia in adolescents with obesity through a GLP-1-mediated mechanism. J Clin Endocrinol Metab 2019;104:3481–3490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yeckel CW, Weiss R, Dziura J, et al. . Validation of insulin sensitivity indices from oral glucose tolerance test parameters in obese children and adolescents. J Clin Endocrinol Metab 2004;89:1096–1101 [DOI] [PubMed] [Google Scholar]
- 34.Yeckel CW, Taksali SE, Dziura J, et al. . The normal glucose tolerance continuum in obese youth: evidence for impairment in beta-cell function independent of insulin resistance. J Clin Endocrinol Metab 2005;90:747–754 [DOI] [PubMed] [Google Scholar]
- 35.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–1470 [DOI] [PubMed] [Google Scholar]
- 36.Sjaarda LG, Bacha F, Lee S, Tfayli H, Andreatta E, Arslanian S. Oral disposition index in obese youth from normal to prediabetes to diabetes: relationship to clamp disposition index. J Pediatr 2012;161:51–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiss R, Taksali SE, Tamborlane WV, Burgert TS, Savoye M, Caprio S. Predictors of changes in glucose tolerance status in obese youth. Diabetes Care 2005;28:902–909 [DOI] [PubMed] [Google Scholar]
- 38.Lyssenko V, Almgren P, Anevski D, et al.; Botnia study group . Predictors of and longitudinal changes in insulin sensitivity and secretion preceding onset of type 2 diabetes. Diabetes 2005;54:166–174 [DOI] [PubMed] [Google Scholar]
- 39.Perreault L, Kahn SE, Christophi CA, Knowler WC, Hamman RF; Diabetes Prevention Program Research Group . Regression from pre-diabetes to normal glucose regulation in the diabetes prevention program. Diabetes Care 2009;32:1583–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lorenzo C, Wagenknecht LE, Rewers MJ, et al. . Disposition index, glucose effectiveness, and conversion to type 2 diabetes: the Insulin Resistance Atherosclerosis Study (IRAS). Diabetes Care 2010;33:2098–2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weiss R, Taksali SE, Dufour S, et al. . The “obese insulin-sensitive” adolescent: importance of adiponectin and lipid partitioning. J Clin Endocrinol Metab 2005;90:3731–3737 [DOI] [PubMed] [Google Scholar]
- 42.Ader M, Stefanovski D, Kim SP, et al. . Hepatic insulin clearance is the primary determinant of insulin sensitivity in the normal dog. Obesity (Silver Spring) 2014;22:1238–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Piccinini F, Polidori DC, Gower BA, Bergman RN. Hepatic but not extrahepatic insulin clearance is lower in African American than in European American women. Diabetes 2017;66:2564–2570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haffner SM, Stern MP, Watanabe RM, Bergman RN. Relationship of insulin clearance and secretion to insulin sensitivity in non-diabetic Mexican Americans. Eur J Clin Invest 1992;22:147–153 [DOI] [PubMed] [Google Scholar]
- 45.Ahrén B, Thorsson O. Increased insulin sensitivity is associated with reduced insulin and glucagon secretion and increased insulin clearance in man. J Clin Endocrinol Metab 2003;88:1264–1270 [DOI] [PubMed] [Google Scholar]
- 46.Michaliszyn SF, Mari A, Lee S, et al. . β-cell function, incretin effect, and incretin hormones in obese youth along the span of glucose tolerance from normal to prediabetes to type 2 diabetes. Diabetes 2014;63: 3846–3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.