Abstract
Inflammation of the nervous system (neuroinflammation) is now recognized as a hallmark of virtually all neurological disorders. In neuroinflammatory conditions such as multiple sclerosis, there is prominent infiltration and a long-lasting representation of various leukocyte subsets in the central nervous system (CNS) parenchyma. Even in classic neurodegenerative disorders, where such immense inflammatory infiltrates are absent, there is still evidence of activated CNS-intrinsic microglia. The consequences of excessive and uncontrolled neuroinflammation are injury and death to neural elements, which manifest as a heterogeneous set of neurological symptoms. However, it is now readily acknowledged, due to instructive studies from the peripheral nervous system and a large body of CNS literature, that aspects of the neuroinflammatory response can be beneficial for CNS outcomes. The recognized benefits of inflammation to the CNS include the preservation of CNS constituents (neuroprotection), the proliferation and maturation of various neural precursor populations, axonal regeneration, and the reformation of myelin on denuded axons. Herein, we highlight the benefits of neuroinflammation in fostering CNS recovery after neural injury using examples from multiple sclerosis, traumatic spinal cord injury, stroke, and Alzheimer’s disease. We focus on CNS regenerative responses, such as neurogenesis, axonal regeneration, and remyelination, and discuss the mechanisms by which neuroinflammation is pro-regenerative for the CNS. Finally, we highlight treatment strategies that harness the benefits of neuroinflammation for CNS regenerative responses.
Subject terms: Neuroimmunology, Mechanisms of disease
General introduction
Following injury to the central nervous system (CNS), there is an influx of leukocytes to the site of injury and an activation of CNS-intrinsic microglia; these phenomena are collectively referred to as neuroinflammation. There is a well-defined body of evidence showing that, in conditions such as multiple sclerosis (MS), an excessive uncontrolled inflammatory response in the normally immune-homeostatic CNS is destructive through an increase in the levels of toxic cytokines, proteases, glutamate, and free radicals.1–3 The literature is replete with evidence of the detrimental effects of extensive neuroinflammation on CNS constituents, such as injury to and the destruction of axons and myelin, the loss of oligodendrocytes and neurons, and the death of regenerating elements, including neural progenitor cells.4,5 In this light, strong immunomodulators that ablate or suppress the activity of immune cells have been successfully used to reduce clinical relapses in MS, which are associated with the prominent influx of leukocytes across the blood–brain barrier.6
Neuroinflammation, however, is not synonymous with poor CNS outcomes, and lessons from the peripheral nervous system indicate that, for the successful regeneration of axons after their transection, an important dialog between infiltrating macrophages and Schwann cells must occur.7 In correspondence, there are now multiple examples of the significant benefits of inflammatory responses to the injured CNS for protection against further deterioration (neuroprotection) and for regenerative responses.8,9
The findings that neuroinflammation can be beneficial should not be surprising given that the inflammatory response in other tissues is often a natural healing process in the recovery from an insult. Moreover, a vast amount of data now affirms that the microglia intrinsic to the CNS are important for supporting brain development, effectively pruning synapses during learning throughout life, and alerting the CNS to a threat, among other functions.10,11
In this review, we highlight the beneficial impact of neuroinflammation in fostering recovery after neural injury, focusing on the CNS regenerative responses of neurogenesis and axonal regeneration and culminating with remyelination. We further highlight the mechanisms by which neuroinflammation can be pro-regenerative within the CNS and discuss medicinal strategies to harness such benefits. We integrate the results of studies on various neurological conditions (including MS, traumatic spinal cord injury, stroke, and Alzheimer’s disease) to draw generalities on the mechanisms of the benefits of neuroinflammation for CNS repair. Finally, we discuss medications that harness these benefits for therapeutics.
Neuroinflammation promotes neurogenesis
The uninjured adult hippocampus is a region involved in neurogenesis, the formation of new neurons, throughout life. Learning-induced hippocampal neurogenesis is influenced by T lymphocytes. This is evident in an enriched environment, in which wild-type mice form elevated numbers of new neurons while SCID mice and nude mice (lacking T and B cells) exhibit impaired neurogenesis.12 Further, in uninjured adult mice, cognitive performance is dependent on the presence of IL-4-producing T cells in the meninges; these T cells prevent their myeloid counterparts from skewing towards a proinflammatory phenotype.13 The formation of adult hippocampal neurons in vitro and in vivo from neural progenitor cells has beem reported to occur through toll-like receptor (TLR)-2 signaling, while signaling through TLR-4 may retard neurogenesis;14 TLRs are important receptors in innate immune cells such as macrophages/microglia and regulate their activity. In culture, microglia have been shown to influence neurogenesis by providing instructive signals; microglia or their secretory products rescue the progressive decline of neural stem-like cells in culture to produce committed neuroblasts.15 It is thought that the exposure of cultured neural stem/progenitor cells to M2-like microglia activates the PPARgamma signaling pathway, enabling the formation of new neurons and oligodendrocytes.16
While inflammatory cells are important for neurogenesis in uninjured adults, there is also good data that neuroinflammation regulates the repopulation of neurons in the CNS after an insult. In experimental autoimmune encephalomyelitis (EAE), an animal model of MS, ependymal cells proliferate, and their progenies migrate to areas of neuroinflammation and mature into neuroblasts, despite a pronounced inflammatory response within the CNS.17 This repopulation of neurons in an inflammatory setting is likely regulated by a chemokine gradient, with the elevation of chemotactic molecules at the site of injury guiding the progenitors from their niche to areas of injury.18 Antagonizing TLR-4 in a model of intracerebral hemorrhage in rats further emphasizes the importance of inflammatory cells in neurogenesis; here, neurogenesis and angiogenesis are reduced and the recovery of neurological functions is inhibited.19
Specific cytokines have also been implicated in promoting neurogenesis. Interferon (IFN)-γ enhances neurogenesis in a mouse model of Alzheimer’s disease, and IFN-γ transgenic mice have elevated numbers of neurons in the neurogenic dentate gyrus compared to those in wild-type controls.20 Many other inflammatory molecules, including tumor necrosis factor-α (TNF-α), interleukin (IL)-4 and -6, and several chemokines, have also been recognized to have proliferative effects on neuronal progenitors and other precursor populations in the CNS.21
In a fascinating study of irradiated mice, the proliferation of neural progenitors in neurogenic zones and cognition were promoted by granulocyte-colony-stimulating factor (G-CSF), a cytokine important for the proliferation and maturation of myeloid progenitor cells and the activation of mature granulocytes; moreover, by using G-CSF receptor knockout mice along with bone marrow transplantation, the authors concluded that bone marrow-derived G-CSF-responsive cells are required for brain repair.22 Overall, there is ample evidence that aspects of neuroinflammation are required for neurogenesis after CNS insults.
Neuroinflammation facilitates axonal regeneration
That immune cell subsets alter the CNS microenvironment to promote axonal elongation was suggested by early experiments from David et al.23 using cocultured adult rat optic nerve sections with dorsal root ganglia; the exposure of the optic nerve sections to macrophages enabled neurites to invade the previously nonpermissive optic nerve. Extending this line of research in vivo, Prewitt et al.24 reported that the injection of activated macrophages or microglia into the lesioned spinal cord of rodents induces axonal regeneration.
After spinal cord injury, it appears that phagocytic macrophages/microglia are required to clear dead cells and debris that would otherwise impair axonal elongation;25,26 their secretion of complement C1q may also help shape newly formed synapses.27 In support of these mechanisms, one study reported that the injection of the TLR-2 agonist zymosan into the eyes of rats induces the regeneration of axons from retinal ganglion neurons into the lesioned optic nerve; the authors identified oncomodulin as a potent macrophage-derived factor that promotes axonal growth in neurons.28,29 Conversely, inhibiting microglial activity using nonspecific pharmacological inhibitors (e.g., cyclosporine A) after monocular enucleation inhibits plasticity related to the reorganization of the retinotectal pathway in the remaining eye.30 Using this knowledge, Chen et al.31 enabled axonal plasticity in the chronic period following spinal cord injury by treatment with lipopolysaccharide (LPS), a TLR-4 ligand with potent effects on macrophages/microglia. More recently, another group found that mild LPS stimulation plus rehabilitative training in chronic spinal cord injury in rats enhances functional recovery and axonal regeneration of the corticospinal tract.32
Macrophages and microglia are now recognized to have a tremendous range of functions and can exist as members of one of two subtypes: as cells with proinflammatory and potentially toxic functions (“M1”-like) or as cells with anti-inflammatory/regulatory and potentially reparative functions (“M2”-like).33 In culture, M2-like cells promote neurite outgrowth in conditions where M1-like cells do not.34 The adoptive transfer of M2-like macrophages35 or the intraspinal injection of IL-4 to elevate the levels of “M2”-like cells36 after spinal cord injury in rats promotes locomotor recovery. IL-33, expressed by many neural cell types, including oligodendrocytes, serves as a CNS-derived alarmin that recruits M2-like macrophages to the contused spinal cord; IL-33 null mice have reduced recovery of motor scores following traumatic spinal cord injury, which is associated with lower representation of M2-like cells in the site of injury.37
T lymphocytes are another inflammatory subset of cells that have been found to facilitate axonal regeneration.38 The adoptive transfer of CD4+ T helper (Th) 1, but not Th2 or Th17 cells, 4 days after traumatic spinal cord injury promotes locomotor and tactile recovery associated with the regrowth of corticospinal tract and serotonergic fibers.39 The many interactions between T lymphocytes and neural cells for CNS recovery have been well summarized elsewhere.40,41
Neuroinflammation is critical for remyelination
Extensive literature exists on the benefits of immune cells for CNS remyelination (Fig. 1).42,43 This was first highlighted by Triarhou and Herndon44, who showed that depleting macrophages with silica quartz dust impairs the clearance of myelin debris and delays remyelination. Kotter et al.45 described that the depletion of monocytes soon after injury using clodronate liposomes significantly reduces subsequent remyelination. In mice with genetic deficiencies in interleukin (IL)-1β or tumor necrosis factor (TNF)-α, remyelination following injury is delayed.46,47 Complementing these depletion experiments, the stimulation of immune cells with TLR ligands enhances the recruitment of OPCs into lesions, thus improving remyelination.48,49 Further, the introduction of IL-4-treated regulatory microglia into the cerebrospinal fluid of rodents with EAE increases oligodendrogenesis in the spinal cord.50 The treatment of cuprizone-demyelinated mice with macrophage colony-stimulating factor (M-CSF), a cytokine that stimulates survival, proliferation, and the differentiation of myeloid cells, stimulates myelin debris clearance and enhanced remyelination.51
Fig. 1.
Immune ablation inhibits remyelination, while an appropriate increase in the immune response and select medications promote myelin formation. The left panel shows OPCs in culture, while the right panel is a single oligodendrocyte (arrow points to the cell body) and its myelin sheaths in vivo (images captured in the Yong laboratory). Please refer to the text for the references
Both the M1- and M2-like macrophage/microglia phenotypes have roles in remyelination.52 In a postdemyelination state in animals, proinflammatory (M1) macrophages/microglia predominate at early stages, and the depletion of these cells impairs OPC proliferation; at later stages of postdemyelination, regulatory (M2) macrophages/microglia predominate, and the depletion of these cells inhibits OPC differentiation.53 This study emphasizes the difficulty of simply subclassifying macrophages/microglia into subtypes that are either beneficial or detrimental. The types of molecules affected by the predominant subset in a particular injury microenvironment is likely an important determinant of whether overall harm or benefit results.
An evaluation of human MS brain samples supports the contention that macrophages/microglia are beneficial for remyelination. An examination of these samples documented that the density of O4-positive oligodendrocyte precursor cells correlates with the density of macrophages/microglia.54 Furthermore, the elevated density of HLA-DR-positive macrophages/microglia at the lesion border of MS plaques correlates with more prominent remyelination.55
T lymphocytes are also implicated in myelin repair, although their effect on remyelination is not well understood. In a model of demyelination in mice, the ablation of CD4+ and CD8+ T cells reduces subsequent remyelination.56 In another study, the infiltration of myelin-specific T cells was shown to enhance oligodendrogenesis in the mouse dentate gyrus,57 although the transfer of myelin-reactive Th17 cells was found to attenuate remyelination after cuprizone demyelination.58 In culture, Th2, but not Th1, cells promote OPC maturation.59 More recently, in mice genetically deficient in regulatory T cells, spontaneous remyelination after injury was shown to be attenuated. Spontaneous remyelination was shown to be subsequently rescued by the adoptive transfer of T regulatory cells, which deliver an important oligodendrocyte trophic factor, CCN3.60
Overall, the process of oligodendrocyte repopulation and remyelination is critically dependent on support from the inflammatory response within the CNS.
Mechanisms of the benefits of neuroinflammation for CNS regeneration
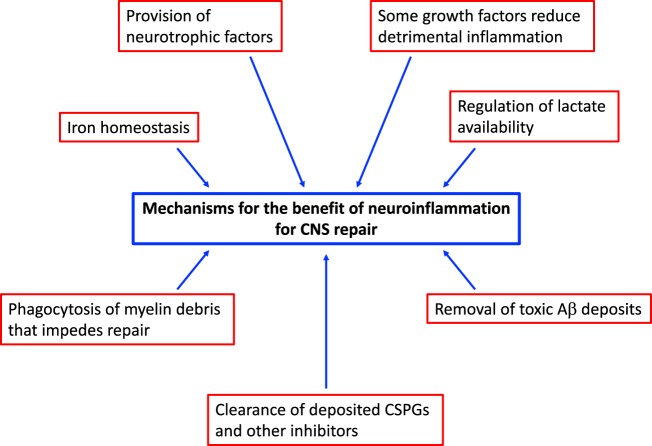
Several mechanisms have been identified to account for the benefits of neuroinflammation in promoting CNS repair (Fig. 2), a prominent one of which is the production of trophic factors that favor recovery. In general, leukocytes and microglia are prominent producers of neurotrophic factors, including oncomodulin, osteopontin, platelet-derived growth factor, epidermal growth factor (EGF), fibroblast growth factor-2 (FGF-2), ciliary neurotrophic factor (CNTF), activin-A, glial-derived growth factor (GDNF), endothelin-2, insulin-like growth factor-1 (IGF-1), and the neurotrophin nerve growth factors brain-derived neurotrophic factor (BDNF) and neurotrophin-3.61,62 Many of these neurotrophic factors have been shown to be beneficial for the proliferation and/or differentiation of OPCs and for neurogenesis.53,63–70 Recently, the sequential use of osteopontin, IGF-1, and CNTF before spinal cord injury and FGF-2, EGF, and GDNF after injury was shown to stimulate robust axonal regrowth of propriospinal axons across astrocyte scars.71 We theorize that leukocytes that aggregate at the site of CNS insult synthesize and release these growth factors with various beneficial effects. Recently, microglia were also shown to produce transglutaminase-2, which interacts with laminin to signal OPC proliferation (through the ADGRG1 receptor).72 Moreover, neurotrophic factors such as NGF can be immunomodulatory; one study observed that NGF can decrease detrimental neuroinflammation by modulating the surface molecules on microglia (specifically by lowering the levels of MHC class II).4
Fig. 2.
Mechanisms of the benefits of neuroinflammation for myelin repair. Contributory mechanisms for remyelination include the production of several neurotrophic factors by all types of immune cells, the reduction of immune overactivity by growth factors produced by immune cells, the phagocytic clearance of inhibitory myelin debris and toxic substances such as Aβ, the removal of CSPGs that impede remyelination and axonal regeneration, the maintenance of iron homeostasis, and the regulation of lactate bioavailability
Perhaps more importantly, macrophages/microglia are needed for the phagocytic clearance of myelin debris45,65,73–75 that otherwise hinders the recruitment of reparative cells.76 Remyelination in spontaneously regenerative models is impaired upon the depletion of monocytes/macrophages by the infusion of clodronate liposomes, which is associated with an accumulation of myelin debris; by studying the time period of depletion, the authors concluded that early activity of these cells is important for effective remyelination.73
Other inhibitors, including members of the extracellular matrix, such as chondroitin sulfate proteoglycans (CSPGs), are also deposited in lesions. CSPGs have long been recognized as inhibitors of axonal regeneration and are also inhibitors of remyelination, particularly OPC adherence and maturation.77–80 The clearance of CSPG deposition from areas of injury is a therapeutic target.81,82 The regulatory M2-like subset of macrophages helps to degrade anchored CSPGs and promote axon regrowth, at least in culture,34 and the breakdown of CSPGs by matrix metalloproteinases (MMPs) aids the subsequent maturation of oligodendrocytes, allowing remyelination to occur.77 Moreover, the phagocytic removal of lesion-deposited CSPGs by macrophages/microglia favors the differentiation of OPCs for subsequent remyelination and mitigates the proinflammatory properties of CSPGs.79,80,83 Phagocytic myeloid cells are also important in the clearance of toxic accumulations of substances such as amyloid-beta (Aβ) in addition to that of CSPGs and myelin debris. Rivest et al.84 reported that macrophages recruited from the bone marrow clear intracerebral Aβ deposits to reduce the latter’s neurotoxic potential in a model of Alzheimer’s disease. In CCR2-deficient mice, where monocytes/macrophages are not recruited to lesions, Alzheimer’s-like disease progression is accelerated, with an increase in cognitive impairments and amyloid deposition.85,86 As a further demonstration of the benefits of microglia, their stimulation through TLR-4 improves Alzheimer’s-like pathology.86 The roles of macrophages/microglia in Alzheimer’s disease are heavily debated, but there is an increasingly strong view that these myeloid phagocytes are likely beneficial early on in the disease process for the clearance Aβ deposits and less helpful in the later phases, when their prolonged activation and the amplification of many toxic molecules exacerbate injury.87,88 In support of this hypothesis, Hamelin et al.89 used positron emission tomography of 18F-DPA-714, a ligand that binds to macrophages/microglia in the brain, in patients with Alzheimer’s disease and found that those with high levels of 18F-DPA-714 in the initial stages of disease correlated with a favorable clinical evolution, while patients with low initial levels of 18F-DPA-714 had a poor clinical outcome.
Iron is a co-factor for many enzymes with important functions in CNS cells;90 for example, oligodendrocytes normally use iron for growth and differentiation. However, its unbuffered ferrous form can be a source of highly damaging free radicals. The role of macrophages in iron homeostasis has been studied, and the results are complex.91,92 Activated macrophages transfer ferritin, the iron storage protein, to OPCs, enhancing their differentiation into oligodendrocytes.93 Macrophage polarization differentially influences the activity of ferritin and ferroportin, which affect the capacity of macrophages to store and release iron, respectively. Proinflammatory macrophages tend to sequester iron, whereas regulatory macrophages express genes that promote iron release.92 In EAE, macrophages/microglia appear to have a defect in iron efflux, disrupting their ability to handle iron and resulting in the gradual accumulation of iron as the disease progresses.94 Another study conducted by the same group showed that an increased loading of iron in macrophages induces a proinflammatory phenotype that may be detrimental to repair.95 More work is necessary to determine how best to harness the pro-regenerative capacity of macrophages in the context of iron handling.
Another mechanism underlying the benefits of neuroinflammation is the generation of lactate. LPS-activated macrophages engage in glycolysis rather than oxidative phosphorylation for energy production, producing lactate as a byproduct.96 Lactate is important for oligodendrogenesis,97 so it is conceivable that proinflammatory macrophages responding to demyelination may provide lactate as a metabolic factor to support remyelination.
In summary, there are multiple mechanisms by which neuroinflammation is beneficial to the CNS (Fig. 2). In addition to those discussed above, there are likely other mechanisms that remain to be elucidated. Although it is speculated that leukocytes that infiltrate the injured CNS may transdifferentiate into neural progenitors to repopulate the CNS, there is no good evidence to support this possibility.
Learning to harness the benefits of neuroinflammation for CNS recovery
The pioneer of the field of beneficial neuroinflammation, Dr. Michal Schwartz (Weizmann Institute, Israel) stated it best: the question is “no longer if but how” to harness immune cells for CNS recovery.98 How can one tap into the obvious necessity for a properly directed immune response for enabling CNS reparative goals without running into the dangers of overzealous immunity? One strategy is to employ existing medications (Fig. 1) that polarize immune subsets into those that are regulatory/anti-inflammatory. A medication used in MS, glatiramer acetate (GA), generates GA-reactive T cells that are Th2-polarized and macrophages that are M2-like.99,100 These GA-reactive cells are not toxic to cells in culture, and there is evidence that they enter the CNS to release various neurotrophic factors locally.101 Accordingly, GA treatment has resulted in beneficial CNS outcomes, including neuroprotection, neurogenesis, and oligodendrogenesis, in animal models of MS, Parkinson’s disease, Alzheimer’s disease, and Huntington’s disease.101–103 We described that the treatment of mice with lysolecithin-induced demyelination of the spinal cord with GA fosters the repopulation of oligodendrocytes and remyelination.104
We note that there are other agents that have been shown to modulate macrophage polarization towards an M2-like regulatory phenotype, including interferon-β and the peroxisome proliferator-activated receptor α/γ agonist, DSP-8658.105,106 Additionally, the antibiotic azithromycin and a purinergic receptor P2X4 agonist were recently reported to induce a regulatory phenotype in models of traumatic spinal cord injury and MS, respectively.107–109 While promising, the timing of the administration of a cellular polarization approach is important, as remyelination may require an initial early proinflammatory macrophage/microglia response and a subsequent regulatory response as previously mentioned.53 Perhaps then, medications that amplify a regulatory phenotype in combination with a mild proinflammatory nonneurotoxic signature may be useful. We described that the combination of TLR-stimulating amphotericin B and the myeloid cell mobilizer/activator M-CSF enhances OPC recruitment and remyelination by activating both proinflammatory and regulatory macrophage/microglia signatures.110 While we did not encounter neurotoxicity with this approach, the known systemic toxicity of amphotericin B precludes its use as a remyelination agent.
As the accumulation of cellular and myelin debris and CSPGs in lesions are impediments to axonal regeneration and remyelination, therapies that enhance their phagocytic removal are promising approaches to elicit CNS regeneration. The retinoid X receptor agonist bexarotene stimulates phagocytosis in monocytes derived from MS patients and promotes remyelination in animals.111 Other agents that may aid in the removal of myelin debris and Aβ are Protollin and monophosphoryl lipid A.86,112 Both medications have been studied in the context of Aβ removal, and it is conceivable that they would be effective in the clearance of myelin debris. The phagocytic clearance of deposited CSPGs by locally injected chondroitinase-ABC is an area of strong interest in focal injuries,113,114 while inhibitors of CSPG production such as “fluorosamine” (peracetylated-4-F-N-acetylglucosamine) offer a more systemic approach to reduce CSPG production,80,83 which would seem more reasonable for diffuse multifocal lesions, such as those in MS.
A strong initiative to clear the Aβ deposited in Alzheimer’s disease is ongoing. Antibody-induced Aβ uptake by myeloid phagocytes has been the subject of many clinical trials,115 and small molecules such as cromolyn have been shown to increase Aβ phagocytosis in mouse models of Alzheimer’s disease.116 Research has also been undertaken to ascertain whether particular properties of activated immune cells, such as the production of proinflammatory molecules versus the phagocytosis of inhibitors, can be separately targeted. The timing of treatment with these medications must also be considered, as specific properties of neuroinflammation for particular repair purposes occur at different stages of specific neurological conditions. The age of the recipient would also have to be considered given the changes to the immune system that occur with aging.117 It would also be pertinent to distinguish the functions of CNS-resident microglia from peripherally derived cells to inform whether a treatment needs to be able to penetrate the blood–brain barrier. As noted by others, the “devil is in the details” for neuroinflammation.118
In summary, there still remain significant barriers to harnessing the benefits of neuroinflammation without encountering its detriments. However, given the emerging knowledge of the details of “when and why” an inflammatory response is helpful and appropriate, there is a strong likelihood that safe strategies to promote beneficial neuroinflammation for CNS recovery will become an increasing reality in the future.
Conclusions
Immune cells of all types can direct a beneficial CNS outcome by producing neurotrophic factors, phagocytosing cellular debris and potentially toxic products, and producing proteases to remove inhibitory molecules such as CSPGs for repair. Increasing knowledge of the details of neuroinflammation’s role in eliciting injury or repair and an accompanied arsenal of medications to tap into the benefits of neuroinflammation will enable the immune system to be utilized in its original intent: to facilitate recovery from an insult, including one to the CNS.
Acknowledgements
The authors acknowledge operating grant support from the Canadian Institutes of Health Sciences and the Multiple Sclerosis Society of Canada (to V.W.Y.) and from the National Natural Science Foundation of China (grants no: 81870942, 81471174, and 81520108011) and Innovation Scientists and Technicians Troop Constructions Projects of Henan Province of China (for M.X.).
Author contributions
All coauthors provided sections of the first draft, with the majority being contributed by H.Y.F.Y. and K.S.R. All authors edited the manuscript, and V.W.Y. approved the final version.
Competing interests
The authors declare no competing interests.
References
- 1.Takeuchi H. Neurotoxicity by microglia: mechanisms and potential therapeutic strategy. Clin. Exp. Neuroimmunol. 2010;1:12–21. doi: 10.1111/j.1759-1961.2009.00001.x. [DOI] [Google Scholar]
- 2.Yong VW. Inflammation in neurological disorders: a help or a hindrance? Neuroscientist. 2010;16:408–420. doi: 10.1177/1073858410371379. [DOI] [PubMed] [Google Scholar]
- 3.Czeh M, Gressens P, Kaindl AM. The yin and yang of microglia. Dev. Neurosci. 2011;33:199–209. doi: 10.1159/000328989. [DOI] [PubMed] [Google Scholar]
- 4.Kerschensteiner M, Meinl E, Hohlfeld R. Neuro-immune crosstalk in CNS diseases. Neuroscience. 2009;158:1122–1132. doi: 10.1016/j.neuroscience.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson AJ, Baranzini SE, Geurts J, Hemmer B, Ciccarelli O. Multiple sclerosis. Lancet. 2018;391:1622–1636. doi: 10.1016/S0140-6736(18)30481-1. [DOI] [PubMed] [Google Scholar]
- 7.Faroni A, Mobasseri SA, Kingham PJ, Reid AJ. Peripheral nerve regeneration: experimental strategies and future perspectives. Adv. Drug Deliv. Rev. 2015;82-83:160–167. doi: 10.1016/j.addr.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz M, Moalem G, Leibowitz-Amit R, Cohen IR. Innate and adaptive immune responses can be beneficial for CNS repair. Trends Neurosci. 1999;22:295–299. doi: 10.1016/S0166-2236(99)01405-8. [DOI] [PubMed] [Google Scholar]
- 9.Bollaerts I, Van Houcke J, Andries L, De Groef L, Moons L. Neuroinflammation as fuel for axonal regeneration in the injured vertebrate central nervous system. Mediat. Inflamm. 2017;2017:9478542. doi: 10.1155/2017/9478542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Labzin LI, Heneka MT, Latz E. Innate immunity and neurodegeneration. Annu. Rev. Med. 2018;69:437–449. doi: 10.1146/annurev-med-050715-104343. [DOI] [PubMed] [Google Scholar]
- 11.Norris GT, Kipnis J. Immune cells and CNS physiology: microglia and beyond. J. Exp. Med. 2019;216:60–70. doi: 10.1084/jem.20180199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ziv Y, et al. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat. Neurosci. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]
- 13.Derecki NC, et al. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J. Exp. Med. 2010;207:1067–1080. doi: 10.1084/jem.20091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rolls A, et al. Toll-like receptors modulate adult hippocampal neurogenesis. Nat. Cell Biol. 2007;9:1081–1088. doi: 10.1038/ncb1629. [DOI] [PubMed] [Google Scholar]
- 15.Walton NM, et al. Microglia instruct subventricular zone neurogenesis. Glia. 2006;54:815–825. doi: 10.1002/glia.20419. [DOI] [PubMed] [Google Scholar]
- 16.Yuan J, et al. M2 microglia promotes neurogenesis and oligodendrogenesis from neural stem/progenitor cells via the PPARgamma signaling pathway. Oncotarget. 2017;8:19855–19865. doi: 10.18632/oncotarget.15774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Danilov AI, et al. Neurogenesis in the adult spinal cord in an experimental model of multiple sclerosis. Eur. J. Neurosci. 2006;23:394–400. doi: 10.1111/j.1460-9568.2005.04563.x. [DOI] [PubMed] [Google Scholar]
- 18.Imitola J, et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc. Natl. Acad. Sci. USA. 2004;101:18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lei C, Wu B, Cao T, Liu M, Hao Z. Brain recovery mediated by toll-like receptor 4 in rats after intracerebral hemorrhage. Brain Res. 2016;1632:1–8. doi: 10.1016/j.brainres.2015.11.045. [DOI] [PubMed] [Google Scholar]
- 20.Baron R, et al. IFN-gamma enhances neurogenesis in wild-type mice and in a mouse model of Alzheimer’s disease. FASEB J. 2008;22:2843–2852. doi: 10.1096/fj.08-105866. [DOI] [PubMed] [Google Scholar]
- 21.Bosak V, Murata K, Bludau O, Brand M. Role of the immune response in initiating central nervous system regeneration in vertebrates: learning from the fish. Int. J. Dev. Biol. 2018;62:403–417. doi: 10.1387/ijdb.180033vb. [DOI] [PubMed] [Google Scholar]
- 22.Dietrich J, et al. Bone marrow drives central nervous system regeneration after radiation injury. J. Clin. Invest. 2018;128:2651. doi: 10.1172/JCI121592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.David S, Bouchard C, Tsatas O, Giftochristos N. Macrophages can modify the nonpermissive nature of the adult mammalian central nervous system. Neuron. 1990;5:463–469. doi: 10.1016/0896-6273(90)90085-T. [DOI] [PubMed] [Google Scholar]
- 24.Prewitt CM, Niesman IR, Kane CJ, Houle JD. Activated macrophage/microglial cells can promote the regeneration of sensory axons into the injured spinal cord. Exp. Neurol. 1997;148:433–443. doi: 10.1006/exnr.1997.6694. [DOI] [PubMed] [Google Scholar]
- 25.Popovich PG, et al. Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp. Neurol. 1999;158:351–365. doi: 10.1006/exnr.1999.7118. [DOI] [PubMed] [Google Scholar]
- 26.Barrette B, et al. Requirement of myeloid cells for axon regeneration. J. Neurosci. 2008;28:9363–9376. doi: 10.1523/JNEUROSCI.1447-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Shea TM, Burda JE, Sofroniew MV. Cell biology of spinal cord injury and repair. J. Clin. Invest. 2017;127:3259–3270. doi: 10.1172/JCI90608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yin Y, et al. Oncomodulin is a macrophage-derived signal for axon regeneration in retinal ganglion cells. Nat. Neurosci. 2006;9:843–852. doi: 10.1038/nn1701. [DOI] [PubMed] [Google Scholar]
- 29.Yin Y, et al. Oncomodulin links inflammation to optic nerve regeneration. Proc. Natl. Acad. Sci. USA. 2009;106:19587–19592. doi: 10.1073/pnas.0907085106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chagas LDS, et al. Rapid plasticity of intact axons following a lesion to the visual pathways during early brain development is triggered by microglial activation. Exp. Neurol. 2019;311:148–161. doi: 10.1016/j.expneurol.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 31.Chen Q, Smith GM, Shine HD. Immune activation is required for NT-3-induced axonal plasticity in chronic spinal cord injury. Exp. Neurol. 2008;209:497–509. doi: 10.1016/j.expneurol.2007.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torres-Espin A, et al. Eliciting inflammation enables successful rehabilitative training in chronic spinal cord injury. Brain. 2018;141:1946–1962. doi: 10.1093/brain/awy128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mishra MK, Yong VW. Myeloid cells—targets of medication in multiple sclerosis. Nat. Rev. Neurol. 2016;12:539–551. doi: 10.1038/nrneurol.2016.110. [DOI] [PubMed] [Google Scholar]
- 34.Kigerl KA, et al. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J. Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma SF, et al. Adoptive transfer of M2 macrophages promotes locomotor recovery in adult rats after spinal cord injury. Brain Behav. Immun. 2015;45:157–170. doi: 10.1016/j.bbi.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 36.Francos-Quijorna I, Amo-Aparicio J, Martinez-Muriana A, Lopez-Vales R. IL-4 drives microglia and macrophages toward a phenotype conducive for tissue repair and functional recovery after spinal cord injury. Glia. 2016;64:2079–2092. doi: 10.1002/glia.23041. [DOI] [PubMed] [Google Scholar]
- 37.Gadani SP, Walsh JT, Smirnov I, Zheng J, Kipnis J. The glia-derived alarmin IL-33 orchestrates the immune response and promotes recovery following CNS injury. Neuron. 2015;85:703–709. doi: 10.1016/j.neuron.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hauben E, et al. Passive or active immunization with myelin basic protein promotes recovery from spinal cord contusion. J. Neurosci. 2000;20:6421–6430. doi: 10.1523/JNEUROSCI.20-17-06421.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ishii H, et al. Adoptive transfer of Th1-conditioned lymphocytes promotes axonal remodeling and functional recovery after spinal cord injury. Cell Death Dis. 2012;3:e363. doi: 10.1038/cddis.2012.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwartz M, Raposo C. Protective autoimmunity: a unifying model for the immune network involved in CNS repair. Neuroscientist. 2014;20:343–358. doi: 10.1177/1073858413516799. [DOI] [PubMed] [Google Scholar]
- 41.Kipnis J. Multifaceted interactions between adaptive immunity and the central nervous system. Science. 2016;353:766–771. doi: 10.1126/science.aag2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldstein EZ, Church JS, Hesp ZC, Popovich PG, McTigue DM. A silver lining of neuroinflammation: beneficial effects on myelination. Exp. Neurol. 2016;283:550–559. doi: 10.1016/j.expneurol.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 43.Rawji KS, Mishra MK, Yong VW. Regenerative capacity of macrophages for remyelination. Front. Cell Dev. Biol. 2016;4:47. doi: 10.3389/fcell.2016.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Triarhou LC, Herndon RM. Effect of macrophage inactivation on the neuropathology of lysolecithin-induced demyelination. Br. J. Exp. Pathol. 1985;66:293–301. [PMC free article] [PubMed] [Google Scholar]
- 45.Kotter MR, Setzu A, Sim FJ, Van Rooijen N, Franklin RJ. Macrophage depletion impairs oligodendrocyte remyelination following lysolecithin-induced demyelination. Glia. 2001;35:204–212. doi: 10.1002/glia.1085. [DOI] [PubMed] [Google Scholar]
- 46.Mason JL, Suzuki K, Chaplin DD, Matsushima GK. Interleukin-1beta promotes repair of the CNS. J. Neurosci. 2001;21:7046–7052. doi: 10.1523/JNEUROSCI.21-18-07046.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arnett HA, et al. TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat. Neurosci. 2001;4:1116–1122. doi: 10.1038/nn738. [DOI] [PubMed] [Google Scholar]
- 48.Setzu A, et al. Inflammation stimulates myelination by transplanted oligodendrocyte precursor cells. Glia. 2006;54:297–303. doi: 10.1002/glia.20371. [DOI] [PubMed] [Google Scholar]
- 49.Glezer I, Lapointe A, Rivest S. Innate immunity triggers oligodendrocyte progenitor reactivity and confines damages to brain injuries. FASEB J. 2006;20:750–752. doi: 10.1096/fj.05-5234fje. [DOI] [PubMed] [Google Scholar]
- 50.Butovsky O, et al. Induction and blockage of oligodendrogenesis by differently activated microglia in an animal model of multiple sclerosis. J. Clin. Invest. 2006;116:905–915. doi: 10.1172/JCI26836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Laflamme N, et al. mCSF-induced microglial activation prevents myelin loss and promotes its repair in a mouse model of multiple sclerosis. Front. Cell Neurosci. 2018;12:178. doi: 10.3389/fncel.2018.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miron VE, Franklin RJ. Macrophages and CNS remyelination. J. Neurochem. 2014;130:165–171. doi: 10.1111/jnc.12705. [DOI] [PubMed] [Google Scholar]
- 53.Miron VE, et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat. Neurosci. 2013;16:1211–1218. doi: 10.1038/nn.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wolswijk G. Oligodendrocyte precursor cells in the demyelinated multiple sclerosis spinal cord. Brain. 2002;125:338–349. doi: 10.1093/brain/awf031. [DOI] [PubMed] [Google Scholar]
- 55.Patani R, Balaratnam M, Vora A, Reynolds R. Remyelination can be extensive in multiple sclerosis despite a long disease course. Neuropathol. Appl. Neurobiol. 2007;33:277–287. doi: 10.1111/j.1365-2990.2007.00805.x. [DOI] [PubMed] [Google Scholar]
- 56.Bieber AJ, Kerr S, Rodriguez M. Efficient central nervous system remyelination requires T cells. Ann. Neurol. 2003;53:680–684. doi: 10.1002/ana.10578. [DOI] [PubMed] [Google Scholar]
- 57.Hvilsted Nielsen H, Toft-Hansen H, Lambertsen KL, Owens T, Finsen B. Stimulation of adult oligodendrogenesis by myelin-specific T cells. Am. J. Pathol. 2011;179:2028–2041. doi: 10.1016/j.ajpath.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baxi, E. G., et al. Transfer of myelin-reactive th17 cells impairs endogenous remyelination in the central nervous system of cuprizone-fed mice. J. Neurosci. 35, 8626–8639 (2015). [DOI] [PMC free article] [PubMed]
- 59.Zhang Y, et al. Glatiramer acetate-reactive T lymphocytes regulate oligodendrocyte progenitor cell number in vitro: role of IGF-2. J. Neuroimmunol. 2010;227:71–79. doi: 10.1016/j.jneuroim.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dombrowski Y, et al. Regulatory T cells promote myelin regeneration in the central nervous system. Nat. Neurosci. 2017;20:674–680. doi: 10.1038/nn.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yong VW, Rivest S. Taking advantage of the systemic immune system to cure brain diseases. Neuron. 2009;64:55–60. doi: 10.1016/j.neuron.2009.09.035. [DOI] [PubMed] [Google Scholar]
- 62.Sousa-Victor P, Jasper H, Neves J. Trophic factors in inflammation and regeneration: the role of MANF and CDNF. Front. Physiol. 2018;9:1629. doi: 10.3389/fphys.2018.01629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O’Donnell SL, Frederick TJ, Krady JK, Vannucci SJ, Wood TL. IGF-I and microglia/macrophage proliferation in the ischemic mouse brain. Glia. 2002;39:85–97. doi: 10.1002/glia.10081. [DOI] [PubMed] [Google Scholar]
- 64.Higashiyama S, Abraham JA, Miller J, Fiddes JC, Klagsbrun M. A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science. 1991;251:936–939. doi: 10.1126/science.1840698. [DOI] [PubMed] [Google Scholar]
- 65.Ruckh JM, et al. Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell. 2012;10:96–103. doi: 10.1016/j.stem.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McMorris FA, Smith TM, DeSalvo S, Furlanetto RW. Insulin-like growth factor I/somatomedin C: a potent inducer of oligodendrocyte development. Proc. Natl. Acad. Sci. USA. 1986;83:822–826. doi: 10.1073/pnas.83.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Scafidi J, et al. Intranasal epidermal growth factor treatment rescues neonatal brain injury. Nature. 2014;506:230–234. doi: 10.1038/nature12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Woodruff RH, Fruttiger M, Richardson WD, Franklin RJ. Platelet-derived growth factor regulates oligodendrocyte progenitor numbers in adult CNS and their response following CNS demyelination. Mol. Cell. Neurosci. 2004;25:252–262. doi: 10.1016/j.mcn.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 69.Armstrong RC, Le TQ, Frost EE, Borke RC, Vana AC. Absence of fibroblast growth factor 2 promotes oligodendroglial repopulation of demyelinated white matter. J. Neurosci. 2002;22:8574–8585. doi: 10.1523/JNEUROSCI.22-19-08574.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yuen TJ, et al. Identification of endothelin 2 as an inflammatory factor that promotes central nervous system remyelination. Brain. 2013;136:1035–1047. doi: 10.1093/brain/awt024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Anderson MA, et al. Required growth facilitators propel axon regeneration across complete spinal cord injury. Nature. 2018;561:396–400. doi: 10.1038/s41586-018-0467-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Giera, S., et al. Microglial transglutaminase-2 drives myelination and myelin repair via GPR56/ADGRG1 in oligodendrocyte precursor cells. Elife7, e33385 (2018). [DOI] [PMC free article] [PubMed]
- 73.Kotter MR, Zhao C, van Rooijen N, Franklin RJ. Macrophage-depletion induced impairment of experimental CNS remyelination is associated with a reduced oligodendrocyte progenitor cell response and altered growth factor expression. Neurobiol. Dis. 2005;18:166–175. doi: 10.1016/j.nbd.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 74.Neumann H, Kotter MR, Franklin RJ. Debris clearance by microglia: an essential link between degeneration and regeneration. Brain. 2009;132:288–295. doi: 10.1093/brain/awn109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rawji KS, et al. Deficient surveillance and phagocytic activity of myeloid cells within demyelinated lesions in aging mice visualized by ex vivo live multiphoton imaging. J. Neurosci. 2018;38:1973–1988. doi: 10.1523/JNEUROSCI.2341-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kotter MR, Li WW, Zhao C, Franklin RJ. Myelin impairs CNS remyelination by inhibiting oligodendrocyte precursor cell differentiation. J. Neurosci. 2006;26:328–332. doi: 10.1523/JNEUROSCI.2615-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Larsen PH, Wells JE, Stallcup WB, Opdenakker G, Yong VW. Matrix metalloproteinase-9 facilitates remyelination in part by processing the inhibitory NG2 proteoglycan. J. Neurosci. 2003;23:11127–11135. doi: 10.1523/JNEUROSCI.23-35-11127.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Siebert JR, Osterhout DJ. The inhibitory effects of chondroitin sulfate proteoglycans on oligodendrocytes. J. Neurochem. 2011;119:176–188. doi: 10.1111/j.1471-4159.2011.07370.x. [DOI] [PubMed] [Google Scholar]
- 79.Lau LW, et al. Chondroitin sulfate proteoglycans in demyelinated lesions impair remyelination. Ann. Neurol. 2012;72:419–432. doi: 10.1002/ana.23599. [DOI] [PubMed] [Google Scholar]
- 80.Keough MB, et al. An inhibitor of chondroitin sulfate proteoglycan synthesis promotes central nervous system remyelination. Nat. Commun. 2016;7:11312. doi: 10.1038/ncomms11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Morgenstern DA, Asher RA, Fawcett JW. Chondroitin sulphate proteoglycans in the CNS injury response. Prog. Brain. Res. 2002;137:313–332. doi: 10.1016/S0079-6123(02)37024-9. [DOI] [PubMed] [Google Scholar]
- 82.Tran AP, Warren PM, Silver J. The biology of regeneration failure and success after spinal cord injury. Physiol. Rev. 2018;98:881–917. doi: 10.1152/physrev.00017.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stephenson EL, Yong VW. Proinflammatory roles of chondroitin sulfate proteoglycans in disorders of the central nervous system. Matrix Biol. 2018;71-72:432–442. doi: 10.1016/j.matbio.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 84.Simard AR, Soulet D, Gowing G, Julien JP, Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron. 2006;49:489–502. doi: 10.1016/j.neuron.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 85.Naert G, Rivest S. CC chemokine receptor 2 deficiency aggravates cognitive impairments and amyloid pathology in a transgenic mouse model of Alzheimer’s disease. J. Neurosci. 2011;31:6208–6220. doi: 10.1523/JNEUROSCI.0299-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Michaud JP, et al. Toll-like receptor 4 stimulation with the detoxified ligand monophosphoryl lipid A improves Alzheimer’s disease-related pathology. Proc. Natl. Acad. Sci. USA. 2013;110:1941–1946. doi: 10.1073/pnas.1215165110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.ElAli A, Rivest S. Microglia in Alzheimer’s disease: a multifaceted relationship. Brain Behav. Immun. 2016;55:138–150. doi: 10.1016/j.bbi.2015.07.021. [DOI] [PubMed] [Google Scholar]
- 88.Sarlus H, Heneka MT. Microglia in Alzheimer’s disease. J. Clin. Invest. 2017;127:3240–3249. doi: 10.1172/JCI90606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hamelin L, et al. Distinct dynamic profiles of microglial activation are associated with progression of Alzheimer’s disease. Brain. 2018;141:1855–1870. doi: 10.1093/brain/awy079. [DOI] [PubMed] [Google Scholar]
- 90.Stephenson E, Nathoo N, Mahjoub Y, Dunn JF, Yong VW. Iron in multiple sclerosis: roles in neurodegeneration and repair. Nat. Rev. Neurol. 2014;10:459–468. doi: 10.1038/nrneurol.2014.118. [DOI] [PubMed] [Google Scholar]
- 91.Corna G, et al. Polarization dictates iron handling by inflammatory and alternatively activated macrophages. Haematologica. 2010;95:1814–1822. doi: 10.3324/haematol.2010.023879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Recalcati S, et al. Differential regulation of iron homeostasis during human macrophage polarized activation. Eur. J. Immunol. 2010;40:824–835. doi: 10.1002/eji.200939889. [DOI] [PubMed] [Google Scholar]
- 93.Schonberg DL, et al. Ferritin stimulates oligodendrocyte genesis in the adult spinal cord and can be transferred from macrophages to NG2 cells in vivo. J. Neurosci. 2012;32:5374–5384. doi: 10.1523/JNEUROSCI.3517-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zarruk JG, et al. Expression of iron homeostasis proteins in the spinal cord in experimental autoimmune encephalomyelitis and their implications for iron accumulation. Neurobiol. Dis. 2015;81:93–107. doi: 10.1016/j.nbd.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 95.Kroner A, et al. TNF and increased intracellular iron alter macrophage polarization to a detrimental M1 phenotype in the injured spinal cord. Neuron. 2014;83:1098–1116. doi: 10.1016/j.neuron.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 96.Jha AK, et al. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity. 2015;42:419–430. doi: 10.1016/j.immuni.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 97.Rinholm JE, et al. Regulation of oligodendrocyte development and myelination by glucose and lactate. J. Neurosci. 2011;31:538–548. doi: 10.1523/JNEUROSCI.3516-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shechter R, Schwartz M. Harnessing monocyte-derived macrophages to control central nervous system pathologies: no longer ‘if’ but ‘how’. J. Pathol. 2013;229:332–346. doi: 10.1002/path.4106. [DOI] [PubMed] [Google Scholar]
- 99.Yong VW. Differential mechanisms of action of interferon-beta and glatiramer aetate in MS. Neurology. 2002;59:802–808. doi: 10.1212/WNL.59.6.802. [DOI] [PubMed] [Google Scholar]
- 100.Lalive PH, et al. Glatiramer acetate in the treatment of multiple sclerosis: emerging concepts regarding its mechanism of action. CNS Drugs. 2011;25:401–414. doi: 10.2165/11588120-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Aharoni R. Immunomodulation neuroprotection and remyelination—the fundamental therapeutic effects of glatiramer acetate: a critical review. J. Autoimmun. 2014;54:81–92. doi: 10.1016/j.jaut.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 102.Aharoni R, et al. The immunomodulator glatiramer acetate augments the expression of neurotrophic factors in brains of experimental autoimmune encephalomyelitis mice. Proc. Natl. Acad. Sci. USA. 2005;102:19045–19050. doi: 10.1073/pnas.0509438102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.From R, et al. Oligodendrogenesis and myelinogenesis during postnatal development effect of glatiramer acetate. Glia. 2014;62:649–665. doi: 10.1002/glia.22632. [DOI] [PubMed] [Google Scholar]
- 104.Skihar V, et al. Promoting oligodendrogenesis and myelin repair using the multiple sclerosis medication glatiramer acetate. Proc. Natl. Acad. Sci. USA. 2009;106:17992–17997. doi: 10.1073/pnas.0909607106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cohen M, et al. Chronic exposure to TGFbeta1 regulates myeloid cell inflammatory response in an IRF7-dependent manner. EMBO J. 2014;33:2906–2921. doi: 10.15252/embj.201489293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yamanaka M, et al. PPARgamma/RXRalpha-induced and CD36-mediated microglial amyloid-beta phagocytosis results in cognitive improvement in amyloid precursor protein/presenilin 1 mice. J. Neurosci. 2012;32:17321–17331. doi: 10.1523/JNEUROSCI.1569-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang B, et al. Azithromycin drives alternative macrophage activation and improves recovery and tissue sparing in contusion spinal cord injury. J. Neuroinflamm. 2015;12:218. doi: 10.1186/s12974-015-0440-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gensel JC, Kopper TJ, Zhang B, Orr MB, Bailey WM. Predictive screening of M1 and M2 macrophages reveals the immunomodulatory effectiveness of post spinal cord injury azithromycin treatment. Sci. Rep. 2017;7:40144. doi: 10.1038/srep40144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zabala, A., et al. P2X4 receptor controls microglia activation and favors remyelination in autoimmune encephalitis. EMBO Mol. Med. 10, e8743 (2018). [DOI] [PMC free article] [PubMed]
- 110.Doring A, et al. Stimulation of monocytes, macrophages, and microglia by amphotericin B and macrophage colony-stimulating factor promotes remyelination. J. Neurosci. 2015;35:1136–1148. doi: 10.1523/JNEUROSCI.1797-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Natrajan, M. S., et al. Retinoid X receptor activation reverses age-related deficiencies in myelin debris phagocytosis and remyelination. Brain2, 1071–1084 (2015). [DOI] [PMC free article] [PubMed]
- 112.Frenkel D, et al. Scara1 deficiency impairs clearance of soluble amyloid-beta by mononuclear phagocytes and accelerates Alzheimer’s-like disease progression. Nat. Commun. 2013;4:2030. doi: 10.1038/ncomms3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fawcett JW. The extracellular matrix in plasticity and regeneration after CNS injury and neurodegenerative disease. Prog. Brain. Res. 2015;218:213–226. doi: 10.1016/bs.pbr.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 114.Orr MB, Gensel JC. Spinal cord injury scarring and inflammation: therapies targeting glial and inflammatory responses. Neurotherapeutics. 2018;15:541–553. doi: 10.1007/s13311-018-0631-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Biber K, Moller T, Boddeke E, Prinz M. Central nervous system myeloid cells as drug targets: current status and translational challenges. Nat. Rev. Drug Discov. 2016;15:110–124. doi: 10.1038/nrd.2015.14. [DOI] [PubMed] [Google Scholar]
- 116.Zhang C, et al. Cromolyn reduces levels of the Alzheimer’s disease-associated amyloid beta-protein by promoting microglial phagocytosis. Sci. Rep. 2018;8:1144. doi: 10.1038/s41598-018-19641-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rawji KS, et al. Immunosenescence of microglia and macrophages: impact on the ageing central nervous system. Brain. 2016;139:653–661. doi: 10.1093/brain/awv395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.DiSabato DJ, Quan N, Godbout JP. Neuroinflammation: the devil is in the details. J. Neurochem. 2016;139(Suppl 2):136–153. doi: 10.1111/jnc.13607. [DOI] [PMC free article] [PubMed] [Google Scholar]