Highlights
-
•
ECG abnormalities after chemo-RT in LA-NSCLC are common (35%–67%).
-
•
Non-specific ECG abnormalities are associated with a high superior vena cava dose.
-
•
Reducing cardiopulmonary dose is likely to lead to less radiation-induced cardiac toxicity.
Keywords: Radiotherapy, Lung cancer, Cardiac, Dose, Toxicity, Survival
Abstract
Introduction
High dose radiotherapy (RT) has been associated with unexpectedly short survival times for locally advanced Non-Small Cell Lung Cancer (LA-NSCLC) patients. Here we tested the hypothesis that cardiac substructure dose is associated with electrocardiography (ECG) assessed abnormalities after RT for LA-NSCLC.
Materials and methods
Pre- and post-RT ECGs were analyzed for 155 LA-NSCLC patients treated to a median of 64 Gy in 1.8–2.0 Gy fractions using intensity-modulated RT plus chemotherapy (concurrent/sequential: 64%/36%) between 2004 and 2014. ECG abnormalities were classified as new Arrhythmic, Ischemic/Pericardial, or Non-specific (AΔECG, I/PΔECG, or NSΔECG) events. Abnormalities were modeled as time to ECG events considering death a competing risk, and the variables considered for analysis were fractionation-corrected dose-volume metrics (α/β = 3 Gy) of ten cardio-pulmonary structures (aorta, heart, heart chambers, inferior and superior vena cava, lung, pulmonary artery) and 15 disease, patient and treatment characteristics. Each abnormality was modelled using bootstrapping and a candidate predictor was suggested by a median multiple testing-adjusted p-value ≤0.05 across the 1000 generated samples. Forward-stepwise multivariate analysis was conducted in case of more than one candidate.
Results
At a median of eight months post-RT, the rate of AΔECG, I/PΔECG, and NSΔECG was 66%, 35%, and 67%. Both AΔECG and I/PΔECG were associated with worse performance status (p = 0.007, 0.03), while a higher superior vena cava minimum dose was associated with NSΔECG (p = 0.002).
Conclusion
This study suggests that higher radiation doses to the cardio-pulmonary system lead to non-specific ECG abnormalities. Reducing dose to this system, along with effective tumor control, is likely to decrease radiation-induced cardiac toxicity.
1. Introduction
Lung cancer has been the leading cause of cancer death in the US for the last 25 years [1], and is among the cancer entities with the lowest 5-year relative survival rates for all stages (18%) [2]. The recently updated standard-of-care for locally advanced inoperable non-small cell lung cancer (LA-NSCLC) is concurrent chemotherapy and radiation therapy (RT) followed by adjuvant durvalumab [3]. However, most toxicity data have been based on the preceding standard of care, i.e., concurrent chemo-RT [4], [5]. The most commonly observed acute effects involve the lungs and esophagus, while cardiac toxicity has typically been overlooked given that the overall survival (OS) times have in general been expected to be shorter than the manifestation of cardiac toxicity [6].
In view of earlier, encouraging data suggesting improved OS for LA-NSCLC with RT dose-escalation [7], [8], [9], [10], [11], the phase III RTOG 0617 trial compared high- to standard dose (74 Gy vs. 60 Gy) chemo-RT with or without Cetuximab [12]. Unexpectedly, the OS times in the high-dose arm were shorter, and increased heart dose was suggested an independent predictor to this finding. Based on these results, Faivre-Finn et al. hypothesized that under-reported cardiac toxicity might have contributed to the inferiority of the high-dose arm [13]. In general, radiation-induced cardiac toxicity has been considered a late effect as observed from breast cancer data [14], [15] and Hodgkin’s lymphoma data [16], [17], but there are a few lung cancer studies suggesting that cardiac toxicity manifests already six months after completed RT [18], [19]. Even though this has recently spurred increased recognition on cardiac toxicity in lung cancer RT [20], [21], [22], [23], [24], [25], [26], there is limited associated dose tolerance data [27], [28], [29], [30], [31] particularly for early cardiac toxicity assessed using electrocardiography (ECG) [32], [33], [34], [35].
In this study, we explored the hypothesis that RT dose to the cardio-pulmonary system (including cardiac substructures) is associated with arrhythmic, ischemic/pericardial, or non-specific ECG abnormalities in stage III inoperable LA-NSCLC treated with intensity-modulated RT (IMRT).
2. Materials and methods
2.1. Cohort data and inclusion criteria
From a total of 241 inoperable stage III LA-NSCLC patients treated with IMRT in combination with concurrent/sequential chemotherapy between 2004 and 2014 at the Memorial Sloan Kettering Cancer Center [36], [37], this Institutional Review Board approved retrospective study included the 155 patients that had ECG acquired before and after RT. Among these patients, 65% received concurrent chemotherapy, while 35% received sequential chemotherapy. The population median prescribed dose was 64 Gy (range: 50–80 Gy) in 1.8–2.0 Gy fractions. Dose was prescribed to the primary tumor, and positron emission tomography positive lymph nodes. All dose distributions were re-calculated with our currently used dose-calculation algorithm (Eclipse AAA v.13, Varian Medical Systems, Palo Alto, CA, US). Patient characteristics and ECG reports were gathered from electronic medical records (EMRs), and records from all specialties were utilized to retrieve this information. A summary of the cohort data is depicted in Table 1.
Table 1.
Distribution of all disease, patient, and treatment characteristics investigated. *Pre-RT angina, arrhythmia, coronary artery disease, congestive heart failure, endocarditis, myocardial infarct, pericardial effusion, tamponade, and valve disease; **Pre-RT abdominal aortic aneurysm, cerebrovascular accident, deep vein thrombosis, middle cerebral artery syndrome, peripheral vascular disease, pulmonary embolism, and transient ischemic attack. ***Any ECG abnormality pre-RT. Abbreviations: COPD: Chronic obstructive pulmonary disease; KPS: Karnofysky performance status; LLL: Left lower lobe; LUL: Left upper lobe; RLL: Right lower lobe; RML: Right middle lobe; RUL: Right upper lobe.
| Variable | N = 155 ( % (n) or median (range)) |
|---|---|
| Age at RT [y] | 66 (25–86) |
| Cardiac disease* | 29 (45) |
| Cardiovascular disease** | 13 (20) |
| Chemotherapy timing | |
| Concurrent | 65 (101) |
| Sequential | 35 (54) |
| COPD | 28 (43) |
| Diabetes | 17 (27) |
| ECG abnormality*** | 61 (95) |
| Histology | |
| Adenocarcinoma | 67 (104) |
| Squamous cell carcinoma | 28 (44) |
| Other | 5 (7) |
| Hyperlipidemia | 35 (54) |
| Hypertension | 48 (75) |
| KPS | 80 (60–100) |
| Sex | |
| Female | 49 (76) |
| Male | 51 (79) |
| Smoking status | |
| Current | 29 (45) |
| Former | 61 (95) |
| Never | 10 (15) |
| Stage | |
| IIIA | 46 (71) |
| IIIB | 54 (84) |
| Tumor location | |
| LLL | 12 (19) |
| LUL | 25 (39) |
| RLL | 14 (22) |
| RML | 9 (14) |
| RUL | 55 (35) |
| N/A | 4 (6) |
2.2. Electrocardiogram abnormalities
Electrocardiograms were retrospectively reviewed by A.H, and ECG abnormalities were defined as any new event after compared to immediately before RT. For the post-RT ECG readings, if a patient experienced a new ECG event at time point two and not at time point one, time point two was recorded. Any new ECG event was categorized into being primarily Arrhythmic (atrial fibrillation/flutter, atrioventricular block, left or right bundle branch block, prolonged/widened QRS, sinus bradycardia, and sinus tachycardia), Ischemic/Pericardial (anterior infarct, septal infarct etc., and Q/ST/T change), or Non-specific (left/right axis deviation, low voltage QRS, poor R wave progression, premature ventricular complex, atrial enlargement, and prolonged/short QT interval) [30], [35].
2.3. Cardio-pulmonary structures and dose representation
Ten structures were considered for analysis: the aorta, heart, chambers (left and right atrium and ventricles, LA, RA, LV, RV), inferior and superior vena cava (IVC, SVC), lung, and the pulmonary artery (PA). Anatomical definitions followed those from Feng et al. [38]; further segmentation details are given in Supplementary material S1 and S2.
The dose-volume histograms (DVHs) for each patient and structure were converted into equivalent doses in 2 Gy fractions (α/β = 3 Gy). From these DVHs, the maximum, minimum, and the mean dose (Max[Gy], Min[Gy], and Mean[Gy]), and the minimum dose to the hottest x% volume, Dx%[Gy] (x = 5–95 in 5% steps) were extracted (dose-volume nomenclature according to TG 263 [39]). It was assumed that the DVH for each structure could be explained by Max[Gy], Min[Gy], and Mean[Gy], and that all remaining 19 Dx%[Gy] metrics were strongly correlated with any of these three metrics. This was, however, tested using an unsupervised approach prior to modeling: the dimensionality of each structure’s DVH was assessed using principal component analysis (PCA). For each structure, the number of PCs accounting for ≥95% of the explained variance in the DVH was assessed. In parallel, all Dx%[Gy] metrics were investigated for correlation with Max[Gy], Min[Gy], and Mean[Gy]. If the Spearman’s rank correlation coefficient (Rs) exceeded 0.70 then a particular Dx%[Gy] metric was judged redundant. If, on the other hand, the Rs was below 0.70, the number of PCs was larger than three for a 95% explained variance, and Max[Gy], Min[Gy], or Mean[Gy] of that particular structure was a candidate predictor, that particular Dx%[Gy] was considered for modeling as well. Both the PCA and the correlation assessment were conducted with resampling (1000 bootstrap samples; median value over all samples is reported).
2.4. Modeling
The three ECG abnormalities were modelled as time to event accounting for death as the competing risk using a Fine and Gray sub distribution [40]. Variables considered for analysis were, unless otherwise specified, Max[Gy], Min[Gy], and Mean[Gy] for each of the ten structures, and 15 disease, patient and treatment characteristics (age, cardiac disease (baseline), cardiovascular disease (baseline), chemotherapy timing, COPD, diabetes, ECG abnormality (baseline), histology, hyperlipidemia, hypertension, karnofsky performance status (KPS), sex, smoking status, stage, and tumor location; Table 1).
Each variable was first subject to univariate analysis using a same resampling approach as previously outlined. If a variable presented with a median p-value ≤0.05 across these samples (p ≤ 0.05 also after adjusted for false discovery rate), that variable was considered a candidate predictor. If multiple DVH metrics of the same structure were candidate predictors only the metric with the lowest p-value was considered a candidate predictor. If there was more than one candidate predictor per ECG abnormality, these candidates were included in a bootstrapped multivariate analysis. However, preceding multivariate analysis, the correlation between candidate predictors was investigated and a candidate predictor presenting with Rs ≥ 0.70 (over 1000 bootstrap samples) with any other candidate predictor with a lower p-value was considered redundant, and was not included in the multivariate analysis. The multivariate analysis was conducted using a forward-stepwise procedure minimizing the Bayesian Information Criterion (BIC) [41]. In each bootstrap sample, all candidate predictors with a BIC ≤ 0–2 of the minimum BIC were considered final predictors [42], and the most frequently selected multivariate models across all samples were considered final.
The association between candidate predictors and predictors included in the final models of each ECG abnormality was plotted as cumulative incidence functions (CIFs). All statistical analyses were conducted in R version 3.5.1 using packages ‘cmprskg’ [40] and ‘crrstep’ [41].
3. Results
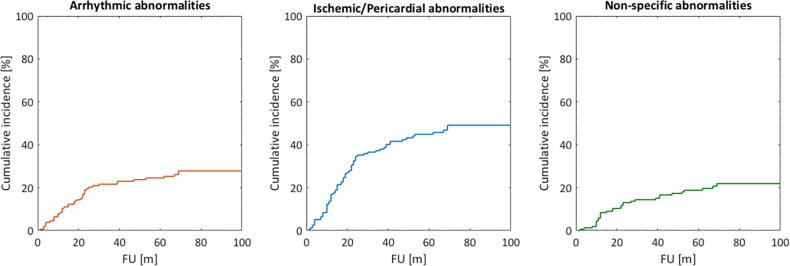
The raw rate of Arrhythmic, Ischemic/Pericardial, and Non-specific abnormalities were 66%, 35%, and 67%, respectively. The corresponding median times to each abnormality (including all 155 patients) were seven, nine, and eight months (range: 0–96 months) after completed RT. The CIFs taking into account the competing risk of death for each abnormality is given in Fig. 1.
Fig 1.
Cumulative incidence functions for the three studied ECG abnormalities.
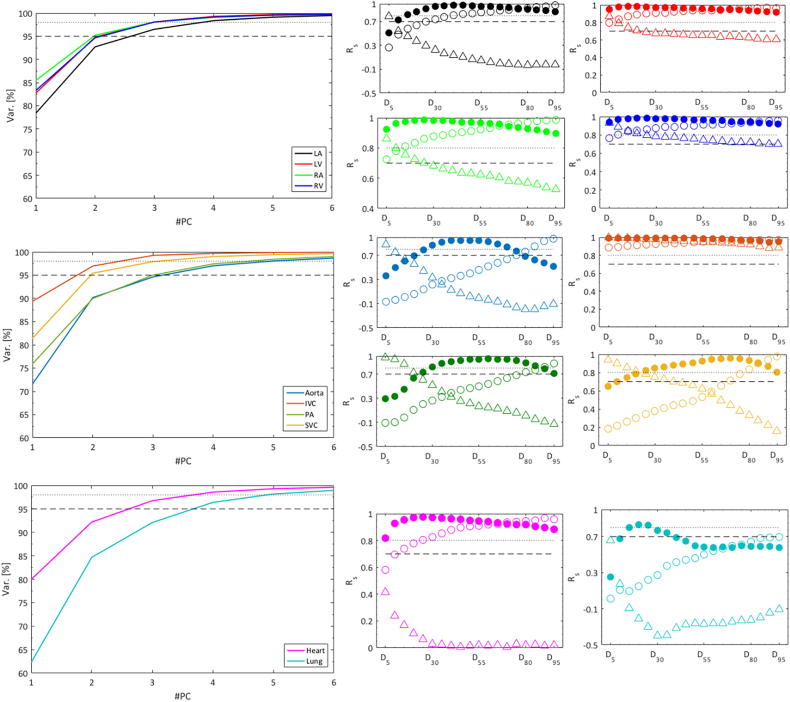
Three PCs explained a median of 97% (range: 92–99%) of the DVH variance, and only for the lung was the explained variance with three PCs < 95% (i.e., 92%), but four PCs accounted for more than 96% of the lung DVH variance (Fig. 2). Also, the correlation between the 19 Dx%[Gy] metrics and Min[Gy], Mean[Gy], or Max[Gy] was strong with Rs exceeding 0.70 for all metrics across all structures except for aorta D15%[Gy] and D20%[Gy], and lung D5%[Gy], D10%[Gy], and D40-90%[Gy] (Fig. 2). These 15 metrics were, therefore, also investigated univariately.
Fig 2.
Left: Explained variance as a function of the number of principal components (#PC) based on the substructure DVHs for all patients. Dashed and dotted lines denote a 95% and 98% explained variance, respectively. Right: Spearman’s correlation coefficients (Rs) between the min/mean/max dose (open circles/filled circles/open triangles) and Dx metrics ranging between D5 and D95 (in 5 Gy intervals) with the same substructure color coding as on the left. Dashed and dotted lines denote Rs = 0.70, and Rs = 0.80. Abbreviations: IVC: Inferior vena cava; LA: Left atrium; LV: Left ventricle; PA: Pulmonary artery; RA: Right atrium; RV: Right ventricle; SVC: Superior vena cava.
For Arrhythmic abnormalities, KPS, aorta Min[Gy] and lung Mean[Gy] were candidate predictors (p = 0.007, 0.008, 0.02; Table 2). None of the additional 15 aorta or lung Dx%[Gy] metrics predicted this abnormality better than aorta Min[Gy] and lung Mean[Gy] (p = 0.02–0.47). The correlation between KPS, aorta Min[Gy] and lung Mean[Gy] was at most modest with Rs reaching 0.56 between aorta Min[Gy] and lung Mean[Gy]. Thus, all three variables were considered candidate predictors and were passed on to multivariate analysis. The final multivariate model included KPS only (model frequency: 39%; Fig. 3). With a considerably lower frequency, two other single-variable models were selected that included either aorta Min[Gy] or lung Mean[Gy] (model frequency: 20%, 15%; Fig. 3), whereas non-single variable models were selected less frequently (model frequency: 5–8%).
Table 2.
Univariate analysis results for the three investigated ECG abnormalities based on all investigated disease, patient and treatment characteristics, as well as the DVH metrics. Note: P-value refers to the median p-value across the 1000 Bootstrap samples; Final candidate predictors for each ECG abnormality are denoted by *. Abbreviations: AR: Arrhythmic; I/P: Ischemic/Pericardial; NS: Non-specific; SCC: Squamous cell carcinoma.
| Variable | AR p-value |
I/P p-value |
NS p-value |
|---|---|---|---|
| Age at RT [y] | 0.47 | 0.47 | 0.40 |
| Cardiac disease | 0.37 | 0.06 | 0.38 |
| Cardiovascular disease | 0.09 | 0.07 | 0.13 |
| Chemotherapy timing | |||
| (Ref: Sequential) | 0.47 | 0.25 | 0.40 |
| COPD | 0.43 | 0.42 | 0.25 |
| Diabetes | 0.21 | 0.23 | 0.29 |
| ECG abnormality | 0.49 | 0.33 | 0.41 |
| Histology | |||
| (Ref: Adenocarcinoma) | 0.22 | 0.20 | 0.45 |
| (Ref: SCC) | 0.05 | 0.37 | 0.41 |
| Hyperlipidemia | 0.17 | 0.45 | 0.35 |
| Hypertension | 0.47 | 0.37 | 0.36 |
| KPS | 0.007* | 0.03* | 0.24 |
| Sex | |||
| (Ref: Female) | 0.26 | 0.46 | 0.03 |
| Smoking status | |||
| (Ref: Current) | 0.40 | 0.43 | 0.47 |
| (Ref: Former) | 0.39 | 0.47 | 0.40 |
| Stage | |||
| (Ref: IIIA) | 0.10 | 0.39 | 0.50 |
| Tumor laterality | |||
| (Ref: Left) | 0.41 | 0.52 | 0.19 |
| (Ref: Right) | 0.44 | 0.52 | 0.07 |
| Tumor inferiority | |||
| (Ref: Inferior) | 0.29 | 0.24 | 0.47 |
| (Ref: Superior) | 0.52 | 0.10 | 0.16 |
| Aorta | |||
| Min[Gy] | 0.008* | 0.48 | 0.10 |
| Mean[Gy] | 0.40 | 0.40 | 0.03 |
| Max[Gy] | 0.47 | 0.48 | 0.45 |
| Heart | |||
| Min[Gy] | 0.43 | 0.46 | 0.10 |
| Mean[Gy] | 0.22 | 0.46 | 0.05 |
| Max[Gy] | 0.45 | 0.29 | 0.15 |
| IVC | |||
| Min[Gy] | 0.20 | 0.31 | 0.0004* |
| Mean[Gy] | 0.08 | 0.30 | 0.23 |
| Max[Gy] | 0.22 | 0.35 | 0.22 |
| Lung | |||
| Min[Gy] | 0.47 | 0.40 | 0.34 |
| Mean[Gy] | 0.02* | 0.09 | 0.005* |
| Max[Gy] | 0.29 | 0.30 | 0.35 |
| LA | |||
| Min[Gy] | 0.48 | 0.32 | 0.35 |
| Mean[Gy] | 0.43 | 0.51 | 0.02 |
| Max[Gy] | 0.49 | 0.28 | 0.03 |
| LV | |||
| Min[Gy] | 0.33 | 0.25 | 0.44 |
| Mean[Gy] | 0.49 | 0.43 | 0.46 |
| Max[Gy] | 0.46 | 0.34 | 0.01 |
| PA | |||
| Min[Gy] | 0.41 | 0.50 | 0.02 |
| Mean[Gy] | 0.42 | 0.25 | 0.005* |
| Max[Gy] | 0.48 | 0.29 | 0.44 |
| RA | |||
| Min[Gy] | 0.35 | 0.24 | 0.11 |
| Mean[Gy] | 0.04 | 0.50 | 0.07 |
| Max[Gy] | 0.04 | 0.49 | 0.04 |
| RV | |||
| Min[Gy] | 0.45 | 0.16 | 0.45 |
| Mean[Gy] | 0.35 | 0.35 | 0.44 |
| Max[Gy] | 0.33 | 0.40 | 0.14 |
| SVC | |||
| Min[Gy] | 0.11 | 0.27 | 0.002* |
| Mean[Gy] | 0.15 | 0.46 | 0.11 |
| Max[Gy] | 0.32 | 0.50 | 0.47 |
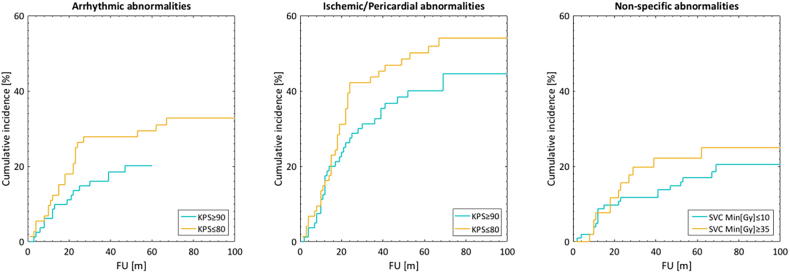
Fig 3.
Cumulative incidence functions stratified by the tertiles of the three predictors included in the final model for each ECG abnormality.
Only KPS was a candidate and, thus, final predictor for Ischemic/Pericardial abnormalities (p = 0.03; Table 2; Fig. 3). Among the DVH metrics, lung Mean[Gy] showed a predictive tendency (p = 0.09). In summary, a worse performance status, i.e. lower KPS, was associated with a higher probability of both Arrhythmic and Ischemic/Pericardial abnormalities.
The largest number of candidate predictors was observed for Non-specific abnormalities: four in total; all DVH metrics, i.e., IVC Min[Gy], lung Mean[Gy], PA Mean[Gy], and SVC Min[Gy] (p = 0.0004–0.005; Table 2). Given that the additional 13 lung Dx%[Gy] metrics were not better predictors than lung Mean[Gy] (p = 0.01–0.52 vs. p = 0.005), and that Rs was at most 0.42 between the four candidate predictors (IVC Min[Gy] and lung Mean[Gy]), these four metrics were passed on to multivariate analysis. The final multivariate model included SVC Min[Gy] (model frequency: 23%; Fig. 3) such that a higher minimum dose (i.e., less sparing) is associated with Non-specific abnormalities. The subsequent three most frequently selected models (model frequency: 11–14%) were single variable models and included any of the remaining three DVH metrics. The most frequently selected non-single variable model was that including lung Mean[Gy], and SVC Min[Gy], but this model presented with a frequency of only 8%.
4. Discussion
The underlying hypothesis of RT dose to the cardio-pulmonary system being associated with ECG abnormalities after RT for LA-NSCLC was confirmed between non-specific ECG abnormalities and high superior vena cava (SVC) dose. Also, the examined ECG abnormalities manifested at a median of eight months post-RT, which further adds to the growing evidence of early onset radiation-induced cardiac toxicity in NSCLC patients [12], [18], [19].
Pre-treatment cardiac toxicity is common in this patient population. Among our 155 patients, 61% experienced pre-treatment ECG abnormalities, which is considerably higher than reported for the general American population (adults; similar age distribution), which has been estimated to be at most 37% (accumulation of all possible ECG abnormalities) for Afro-American men older than 65 years [43]. However, in that national population study only major ECG abnormalities were addressed and as such some of the ECG abnormalities included in the current study (e.g., sinus tachycardia/bradycardia, axis deviation, wide QRS, and poor R wave progression) were not. Further in [43], the prevalence of ECG abnormalities was associated with the presence of hypertension, diabetes, high blood pressure, and advanced age. A similar pattern was also found in the current cohort with 42% presenting with pre-existing cardiovascular disease, 57% with hypertension, and 19% with diabetes among the 95 patients with pre-existing ECG abnormalities. The corresponding rates for the remaining 60 patients with no ECG abnormalities prior to RT were 27%, 35%, and 15% for pre-existing cardiovascular disease, hypertension, and diabetes, respectively. Also, patients with pre-RT ECG abnormalities were slightly older (population averages: 67 vs. 62 years). Thus, our data indicate a higher prevalence of ECG abnormalities among LA-NSCLC patients compared to the general population, motivating the need for closer monitoring and follow-up of cardio-pulmonary status in this patient population.
Following RT for breast cancer and Hodgkin’s lymphoma, primarily long-term severe ECG abnormalities, such as arrhythmias and conduction blocks, have been reported [44]. However, Strender et al. described earlier ECG abnormalities and found that 45% of their 197 breast cancer patients had conduction abnormalities six months after treatment; among the left-sided patients, the major ECG event was T-wave abnormalities [45]. Furthermore, in a limited-sized prospective study including 25 patients treated to a median of 50 Gy in 2 Gy fractions for primary thoracic malignancies including lung cancer, Gomez et al. observed that approximately every other patient presented with ECG abnormalities during or near the end of treatment (i.e., end of RT or 1–2 months post-RT) with most abnormalities being non-specific; however, no ECG assessments were made later than 1–2 months post-RT [35]. In another small cohort including only LA-NSCLC patients, Vivekanandan et al. instead observed that ischemic/pericarditis like ECG abnormalities were most common (N = 53; rate: 14%) at the six months post-RT assessment [30]. Unlike in [30], the rate of ECG abnormalities in the current study was the lowest for ischemic/pericardial abnormalities (35%) compared to 53% for arrhythmic, and 89% for non-specific abnormalities six months post-RT. In our cohort, non-specific abnormalities were more prevalent than both arrhythmic and ischemic/pericardial abnormalities within the studied follow-up period, which spanned from the end of RT up to nine years after completed RT. The pattern of non-specific ECG abnormalities being most prevalent was also observed within the much shorter 1–2 months time period studied in [35]. Thus, the high rate of non-specific ECG abnormalities observed in the current study, which includes to the best of our knowledge by far the largest number of LA-NSCLC with ECG data published so far, suggest that these ECG abnormalities are more related to radiation-induced cardiac toxicity than are arrhythmic and ischemic/pericardial abnormalities. The role as the primary radiation-induced cardiac toxicity is also supported by the identified cardio-pulmonary dose-response relationship, which was only established for non-specific ECG abnormalities.
The dose-response relationship identified for non-specific ECG abnormalities suggests that higher minimum SVC doses are likely to generate higher incidences of non-specific ECG abnormalities. More specifically, reducing the minimum SVC dose from 35 Gy to 10 Gy would lead to a 4% reduction (from 16% to 12%) in the cumulative incidence of non-specific ECG abnormalities two years post-RT according to this model (Fig. 3). The three residual candidate predictors for non-specific ECG abnormalities involved IVC, lung and pulmonary artery dose, but our multivariate analysis demonstrated that the minimum SVC dose was the final predictor. Similarly, Stam et al. found that near minimum (D90%[Gy]) SVC doses were significantly correlated with non-cancer death (i.e., cause of death not indicated to be related to the disease) in patients treated with stereotactic body RT for early stage NSCLC patients [28]. Their multivariate model also included left atrial maximum dose, which we did not observe. In a subsequent analysis we established a significant association between the non-specific ECG abnormalities and OS (Fig S3; Authors’ note: OS was chosen as endpoint over non-cancer death given that patients were lost to follow-up and scoring of cause-specific mortality is, therefore, questionable): we demonstrated that higher SVC doses lead to non-specific ECG abnormalities and shorter survival times. Thus, conforming the IMRT dose distributions to maximally avoid irradiation of the SVC should be warranted.
While the pathophysiologic mechanism on how RT affects SVC is not clear, radiation-induced toxicity on vessels in the short- and long-term have been described: initially, irradiation causes endothelial dysfunction, which ultimately leads to intimal hyperplasia [46]. In parallel, RT activates nuclear factor-kappa B, which can cause inflammation due to oxidative stress [47]. In the later phase, vasa vasorum damage (i.e., microvasculature that nourishes the walls of large blood vessels) produces focal necrosis of the media, which results in fibrosis [48]. Additionally, case reports of SVC obstruction caused by vascular fibrosis due to RT years earlier have been described [49]. Rather than slowly progressing fibrosis explaining our SVC results, other potential mechanisms are microvascular damage or disturbances of the conduction system given that the sinoatrial node is located at the junction between the right atrium and the SVC. Also, accounting for organ motion uncertainty (including that from the heart) nearby located structures such as the RA, origin of the coronary artery origin and/or the sinoatrial node may also play a role to our SVC finding. Monitoring SVC pathological conditions may be further improved using e.g. MRI venography imaging [49].
Even though no dose-response relationship was established for arrhythmic or ischemic/pericardial ECG abnormalities, the identified association between these and KPS could be used as an indicator to precautious planning involving more stringent dose/volume constraints to structures of the cardio-pulmonary system for patients presenting with a KPS ≤ 80 (Fig. 3), also given that ischemic/pericardial ECG abnormalities were associated with worse OS (Fig S3). In [30], an association was found between ‘any’ ECG abnormality six months post-RT and OS, but this relationship did not persist as the ECG abnormalities were studied separately as ischemic/pericardial or conduction changes, yet again, this inconclusiveness may be hampered by the size of their cohort (N = 53). As for arrhythmic abnormalities, the superior heart region (including the SVC) is where arrhythmias are more likely to originate and high dose can ablate cardiac conductive tissue [50]. Thus, further studies are needed to better understand how RT affects the cardiac conduction system and more specifically, arrhythmias.
This study is associated with a couple of limitations: ECGs were identified retrospectively from the EMRs and patient selection bias is likely, i.e., patients presenting with symptoms are more likely to have an ECG acquired. Therefore, the true ECG rate in this population may have been misjudged. Detailed data on all such symptoms was not available, however, comparing the 86 patients from the original cohort (N = 241) that were not included in this study with the 155 included patients, did not indicate any significant difference in either mortality rate or follow-up time (mortality rate: 71% vs. 77% (p = 0.32); median follow-up time: 20 months vs. 20 months (p = 0.13)). Additionally, to assess ECG abnormalities we relied on reports, which are mainly used as a screening tool in clinical practice. Also, reversible and irreversible ECG abnormalities post-RT were not differentiated. Lastly, given that the 155 patients were treated at only one institution, we advise others to externally validate our results and assess the treatment planning feasibility of the SVC minimum dose before routinely implementing such a dose-volume constraint.
In conclusion, this study indicates that non-specific ECG abnormalities are more common after RT for LA-NSCLC than ECG abnormalities related to arrhythmic or ischemic/pericardial abnormalities, and that these are caused by dose to the superior vena cava (SVC). Prior to adopting a minimum SVC dose-volume constraint (except for As Low As Reasonably Achievable), this finding should be explored externally. In addition, patients with KPS ≤ 80 may also benefit from a lowered SVC dose, and may constitute a subgroup that could also profit from additional and close cardiac monitoring. Lastly and due to heterogeneity in cardiac toxicity ranging from myocardial to vascular, thrombotic, or metabolic, using ECG in combination with biomarkers (e.g., troponins, BNP) and advanced imaging, such as cardiac MRI may provide more insight into radiation-induced cardiac toxicity.
Declaration of Competing Interest
None. AR reports relevant grants outside the submitted work from Varian Medical Systems, Boehringer Ingelheim, Pfizer, and AztraZeneca, personal fees from AztraZeneca, Merck, Research to Practice, and Cybrexa, as well as non-financial support from Philips/Elekta.
Acknowledgements
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748 (AH, AR, JOD, MT), as well as through NIH/NCI R01 CA198121 (JOD, MT).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2019.09.003.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Global Burden of Disease Cancer Collaboration, Fitzmaurice C., Allen C. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3:524–548. doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 3.Antonia S.J., Villegas A., Daniel D. Durvalumab after chemoradiotherapy in stage III non-small cell lung cancer. N Eng J Med. 2017;377:1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 4.Chang J.Y., Kestin K.K., Barriger R.B. ACR appropriateness criteria: nonsurgical treatment for non-small-cell lung cancer: good performance status/definitive intent. Oncology. 2014;28:706–710. [PubMed] [Google Scholar]
- 5.Bezjak A., Temin S., Franklin G. Definitive and adjuvant radiotherapy in locally advanced non-small-cell lung cancer: American Society of Clinical Oncology practice guideline endorsement of the American Society for Radiation Oncology evidence-based clinical practice guideline. J Clin Oncol. 2015;33:2100–2105. doi: 10.1200/JCO.2014.59.2360. [DOI] [PubMed] [Google Scholar]
- 6.Werner-Wasik M., Paulus R., Curran W.J. Acute esophagitis and late lung toxicity in concurrent chemoradiotherapy trials in patients with locally advanced non-small-cell lung cancer: analysis of the Radiation Therapy Oncology Group (RTOG) database. Clin Lung Cancer. 2011;12:245–251. doi: 10.1016/j.cllc.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 7.Bradley J.D., Bae K., Graham M.V. Primary analysis of the phase II component of a phase I/II dose intensification study using three-dimensional conformal radiation therapy and concurrent chemotherapy for patients with inoperable non-small-cell lung cancer: RTOG 0117. J Clin Oncol. 2010;28:2475–2480. doi: 10.1200/JCO.2009.27.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schild S.E., McGinnis W.L., Graham D. Results of a phase I trial of concurrent chemotherapy and escalating doses of radiation for unresectable non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2006;65:1106–1111. doi: 10.1016/j.ijrobp.2006.02.046. [DOI] [PubMed] [Google Scholar]
- 9.Socinski M.A., Blackstock A.W., Bogart J.A. Randomized phase II trial of induction chemotherapy followed by concurrent chemotherapy and dose-escalated thoracic conformal radiotherapy (74 Gy) in stage III non-small-cell lung cancer: CALGB 30105. J Clin Oncol. 2008;26:2457–2463. doi: 10.1200/JCO.2007.14.7371. [DOI] [PubMed] [Google Scholar]
- 10.Stinchcombe T.E., Lee C.B., Moore D.T. Long-term follow-up of a phase I/II trial of dose escalating three-dimensional conformal thoracic radiation therapy with induction and concurrent carboplatin and paclitaxel in unresectable stage IIIA/B non-small cell lung cancer. J Thorac Oncol. 2008;3:1279–1285. doi: 10.1097/JTO.0b013e31818b1971. [DOI] [PubMed] [Google Scholar]
- 11.Curran W.J., Jr, Paulus R., Langer C.J. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011;103:1452–1460. doi: 10.1093/jnci/djr325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bradley J.D., Paulus R., Komaki R. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non- small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16:187–199. doi: 10.1016/S1470-2045(14)71207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faivre-Finn C. Dose escalation in lung cancer: Have we gone full circle? Lancet Oncol. 2015;16:125–127. doi: 10.1016/S1470-2045(15)70001-X. [DOI] [PubMed] [Google Scholar]
- 14.Darby S.C., McGale P., Taylor C.W., Peto R. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300 000 women in US SEER cancer registries. Lancet Oncol. 2005;6:557–565. doi: 10.1016/S1470-2045(05)70251-5. [DOI] [PubMed] [Google Scholar]
- 15.Darby S.C., Ewertz M., McGale P. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 16.Aleman B.M., van den Belt-Dusebout A.W., De Bruin M.L., van‘t Veer M.B., Baaijens M.H., de Boer J.P. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood. 2007;109:1878–1886. doi: 10.1182/blood-2006-07-034405. [DOI] [PubMed] [Google Scholar]
- 17.Hancock S.L., Donaldson S.S., Hoppe R.T. Cardiac disease following treatment of Hodgkin’s disease in children and adolescents. J Clin Oncol. 1993;11:1208–1215. doi: 10.1200/JCO.1993.11.7.1208. [DOI] [PubMed] [Google Scholar]
- 18.Phlips P., Rocmans P., Vanderhoeft P. Postoperative radiotherapy after pneumonectomy: impact of modern treatment facilities. Int J Radiat Oncol Biol Phys. 1993;27:525–529. doi: 10.1016/0360-3016(93)90375-6. [DOI] [PubMed] [Google Scholar]
- 19.Dautzenberg B., Arriagada R., Chammard A.B. A controlled study of postoperative radiotherapy for patients with completely resected nonsmall cell lung carcinoma. Cancer. 1999;86:265–273. doi: 10.1002/(sici)1097-0142(19990715)86:2<265::aid-cncr10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 20.Lally B.E., Detterbeck F.C., Geiger A.M. The risk of death from heart disease in patients with nonsmall cell lung cancer who receive postoperative radiotherapy: analysis of the Surveillance, Epidemiology, and End Results database. Cancer. 2007;110:911–917. doi: 10.1002/cncr.22845. [DOI] [PubMed] [Google Scholar]
- 21.Douillard J.Y., Rosell R., De Lena M. Impact of postoperative radiation therapy on survival in patients with complete resection and stage I, II, or IIIA non-small-cell lung cancer treated with adjuvant chemotherapy: the adjuvant Navelbine International Trialist Association (ANITA) Randomized Trial. Int J Radiat Oncol Biol Phys. 2008;72:695–701. doi: 10.1016/j.ijrobp.2008.01.044. [DOI] [PubMed] [Google Scholar]
- 22.Dess R.T., Sun Y., Matuszak M.M. Cardiac events after radiation therapy: combined analysis of prospective multicenter trials for locally advanced non- small-cell lung cancer. J Clin Oncol. 2017;35:1395–1402. doi: 10.1200/JCO.2016.71.6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tucker S.L., Liu A., Gomez D. Impact of heart and lung dose on early survival in patients with non-small cell lung cancer treated with chemoradiation. Radiother Oncol. 2016;119:495–500. doi: 10.1016/j.radonc.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 24.Wang K., Eblan M.J., Deal A.M. Cardiac toxicity after radiotherapy for stage III non-small-cell lung cancer: pooled analysis of dose-escalation trials delivering 70 to 90 Gy. J Clin Oncol. 2017;35:1387–1394. doi: 10.1200/JCO.2016.70.0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ning M.S., Tang L., Gomez D.R. Incidence and predictors of pericardial effusion after chemoradiation therapy for locally advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2017;99:70–79. doi: 10.1016/j.ijrobp.2017.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee C.C., Zheng H., Soon Y.Y., Foo L.L., Koh W.Y., Leong C.N. Association between radiation heart dosimetric parameters, myocardial infarct and overall survival in stage 3 non-small cell lung cancer treated with definitive thoracic radiotherapy. Lung Cancer. 2018;120:54–59. doi: 10.1016/j.lungcan.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 27.McWilliam A., Kennedy J., Hodgson C. Radiation dose to heart base linked with poorer survival in lung cancer patients. Eur J Cancer. 2017;85:106–113. doi: 10.1016/j.ejca.2017.07.053. [DOI] [PubMed] [Google Scholar]
- 28.Stam B., Peulen H., Guckenberger M. Dose to heart substructures is associated with non-cancer death after SBRT in stage I-II NSCLC patients. Radiother Oncol. 2017;123:370–375. doi: 10.1016/j.radonc.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 29.Wang K., Pearlstein K.A., Patchett N.D. Heart dosimetric analysis of three types of cardiac toxicity in patients treated on dose-escalation trials for Stage III non-small-cell lung cancer. Radiother Oncol. 2017;125:293–300. doi: 10.1016/j.radonc.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vivekanandan S., Landau D.B., Counsell N., Warren D.R., Khwanda A., Rosen S.D. The impact of cardiac radiation dosimetry on survival after radiation therapy for non-small cell lung cancer. Int J Radiat Oncol. 2017;99:51–60. doi: 10.1016/j.ijrobp.2017.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yegya-Raman N., Wang K., Kim S. Dosimetric predictors of symptomatic cardiac events after conventional-dose chemoradiation therapy for inoperable non-small cell lung cancer. J Thorac Oncol. 2018 doi: 10.1016/j.jtho.2018.05.028. June 5 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Byhardi R., Brace K., Ruckdeschel J., Chang P., Martin R. Dose and treatment factors in radiation-related pericardial effusion associated with the mantle technique for hodgkin's disease. Cancer. 1975;35:795–802. doi: 10.1002/1097-0142(197503)35:3<795::aid-cncr2820350335>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 33.McRaynoids R.A., Gold G.L., Roberts W.C. Coronary heart disease after mediastinal irradiation for hodgkin's disease. Am J Med. 1976;60:39–45. doi: 10.1016/0002-9343(76)90531-3. [DOI] [PubMed] [Google Scholar]
- 34.Dollinger M.R., Lavine D.M., Foye L.V. Myocardial infarction due to postiradiation fibrosis of the coronary arteries. JAMA. 1996;195:316–319. [PubMed] [Google Scholar]
- 35.Gomez D.R., Yusuf S.W., Munsell M.F. Prospective exploratory analysis of cardiac biomarkers and electrocardiogram abnormalities in patients receiving thoracic radiation therapy with high-dose heart exposure. J Thorac Oncol. 2014;9:1554–1560. doi: 10.1097/JTO.0000000000000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oberije C., De Ruysscher D., Houben R. A validated prediction model for overall survival from stage III non-small cell lung cancer: Toward survival prediction for individual patients. Int J Radiat Oncol Biol Phys. 2015;92:935–944. doi: 10.1016/j.ijrobp.2015.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sonnick M.A., Oro F., Yan B. Identifying the optimal radiation dose in locally advanced non-small-cell lung cancer treated with definitive radiotherapy without concurrent chemotherapy. Clin Lung Cancer. 2018;19:131–140. doi: 10.1016/j.cllc.2017.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feng M., Moran J.M., Koelling T. Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int J Radiat Oncol Biol Phys. 2011;79:10–18. doi: 10.1016/j.ijrobp.2009.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mayo C.S., Moran J.M., Bosch W. American Association of Physicists in Medicine Task Group 263: Standardizing Nomenclatures in Radiation Oncology. Int J Radiat Oncol Biol Phys. 2018;100:1057–1066. doi: 10.1016/j.ijrobp.2017.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fine J.P., Gray R.J. A proportional hazards model for the sub distribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 41.Kuk D., Varadhan R. Model selection in competing risk regression. Stat Med. 2013;32:3077–3088. doi: 10.1002/sim.5762. [DOI] [PubMed] [Google Scholar]
- 42.Kass R.E., Raftery A.E. Bayes factors. J Am Stat Assoc. 1995;90:773–795. [Google Scholar]
- 43.Prineas R.J., Le A., Soliman E.Z. United States national prevalence of electrocardiographic abnormalities in black and white middle-age (45- to 64-year) and older (≥65-year) adults (from the Reasons for Geographic and Racial Differences in Stroke Study) Am J Cardiol. 2012;109:1223–1228. doi: 10.1016/j.amjcard.2011.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heidenreich P.A., Kapoor J.R. Radiation induced heart disease: systemic disorders in heart disease. Heart. 2009;95:252–258. doi: 10.1136/hrt.2008.149088. [DOI] [PubMed] [Google Scholar]
- 45.Strender L.E., Lindahl J., Larsson L.E. Incidence of heart disease and functional significance of changes in the electrocardiogram 10 years after radiotherapy for breast cancer. Cancer. 1986;57(5):929–934. doi: 10.1002/1097-0142(19860301)57:5<929::aid-cncr2820570509>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 46.Murros K.E., Toole J.F. The effect of radiation on carotid arteries. Arch Neurol. 1989;46:449–455. doi: 10.1001/archneur.1989.00520400109029. [DOI] [PubMed] [Google Scholar]
- 47.Zhao W., Robbins M.E. Inflammation and chronic oxidative stress in radiation-induced late normal tissue injury: therapeutic implications. Curr MedChem. 2009;16:130–143. doi: 10.2174/092986709787002790. [DOI] [PubMed] [Google Scholar]
- 48.Zidar N., Ferluga D., Hvala A., Popovic M., Soba E. Contribution to the pathogenesis of radiation-induced injury to large arteries. J Laryngol Otol. 1997;111:988–990. doi: 10.1017/s0022215100139167. [DOI] [PubMed] [Google Scholar]
- 49.Van Putten J.W., Schlosser N.J., Vujaskovic Z., Leest A.H., Groen H.J. Superior vena cava obstruction caused by radiation induced venous fibrosis. Thorax. 2000;55:245–246. doi: 10.1136/thorax.55.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cuculich P.S., Schill M.R., Kashani R. Noninvasive cardiac radiation for ablation of ventricular tachycardia. N Engl J Medi. 2017;377(24):2325–2336. doi: 10.1056/NEJMoa1613773. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.