Highlights
-
•
Benefits of proton beam therapy for treatment of prostate cancer are unknown.
-
•
Data comparing pencil beam vs. passive scatter/uniform scanning protons are limited.
-
•
Significant differences in acute toxicity between proton modalities were observed.
-
•
Future studies evaluating differences between the two proton modalities are needed.
Abbreviations: CTCAE, Common Terminology Criteria for Adverse Events; GI, gastrointestinal; GU, genitourinary; LET, linear energy transfer; MVA, multivariable analysis; PBT, proton beam therapy; PBS, pencil beam scanning; PC, prostate cancer; PCG, Proton Collaborative Group; PS/US, passive scattering/uniform scanning; RBE, relative biological effectiveness; RT, radiation therapy; PARTIQoL, Prostate Advanced Radiation Technologies Investigating Quality of Life
Keywords: Prostate cancer; Proton therapy; Pencil beam scanning; Passive scattering, uniform scanning; Comparative effectiveness, Toxicity
Abstract
Background and purpose
Patient-level benefits of proton beam therapy (PBT) relative to photon therapy for prostate cancer (PC) continue to be the focus of debate. Although trials comparing the two modalities are underway, most are being conducted using “conventional” PBT (passive scattering/uniform scanning [PS/US]) rather than pencil beam scanning (PBS). The dosimetric benefits of PBS are well-known, but comparative data are limited. This analysis compares PBS toxicity rates with those of PS/US in a prospective multicenter registry.
Methods
We evaluated acute/late gastrointestinal (GI) and genitourinary (GU) toxicity rates for men with low-to-intermediate risk PC enrolled in PCG 001-09. Acute toxicities with the two techniques were compared using χ2 tests, and the cumulative incidence methods for late toxicity. Multivariable analyses (MVAs) for acute toxicity were performed using logistic regression, and cox proportional hazards models for late toxicity.
Results
Patients were treated using PS/US (n = 1105) or PBS (n = 238). Acute grade ≥2 GI toxicity in PBS did not significantly differ from that with PS/US (2.9% and 2.1%, respectively; P = 0.47). Acute grade ≥2 GU toxicity was significantly higher with PBS (21.9% and 15.1%; P < 0.01). In MVA, PBS was significantly associated with increased acute grade ≥2 GU toxicity (RR = 1.57, p < 0.001). Late grade ≥2 GI and GU toxicities did not differ significantly between groups.
Conclusions
This is the first multi-institutional comparative effectiveness evaluation of PBT techniques in PC. Differences in acute GU toxicity warrant further evaluation, and highlight the urgent need for prospective data using PBT.
1. Introduction
Patient-level benefits of proton beam therapy (PBT) relative to photon therapy for prostate remain controversial. The American Society of Radiation Oncology (ASTRO) and Choosing Wisely© campaign specifically recommend against routine use of PBT to treat PC outside of a clinical trial or prospective registry study [1].
A pivotal randomized controlled trial of protons vs. photons for treatment of PC is currently underway, but the results of this study are not expected to be available for several years [2]. Since this study was first activated, PBT technology has evolved significantly, and two alternative modes of PBT delivery are now in routine clinical use: passive scattering/uniform scanning (PS/US) and pencil beam scanning (PBS). PBS is able to generate a spot-weighted dose delivery that allows for highly conformal dose distribution [3].
It has been hypothesized that PBS will have a more favorable toxicity profile than that with PS/US – similar to the transition of photon therapy dose delivery from three-dimensional conformal radiation to intensity-modulated radiation therapy (IMRT) [4], [5]. The relevance of randomized comparison of protons delivered with PS/US with IMRT has been questioned, given that PBS technology is now clinically available and is expected to replace PS/US in the coming years [6].
The clinical relevance of differences between the two PBT approaches is not well documented and this could have an impact on the results of ongoing randomized proton vs. photon studies. The purpose of this study was to compare toxicity profiles following PBS and PS/US in a large cohort of PC patients enrolled on a prospective, multicenter registry study.
2. Methods
2.1. Study design and setting
Proton Collaborative Group (PCG) 001-09 (NCT01255748) is an ongoing multi-institutional prospective registry study for patients treated with PBT at 10 participating proton centers across the United States. After obtaining written informed consent, patients are enrolled on PCG 01-009 and are followed to collect data on treatment-related toxicities, tumor control, and overall survival. Details of PBT delivery are also collected, including delivery type (PBS or PS/US), treatment dose, number of fractions, and neoadjuvant/concurrent/adjuvant systemic therapies. Data quality and toxicities are monitored through the PCG Data Safety Monitoring Board.
2.2. Patient population
This analysis was limited to the subset of men enrolled on PCG 01-009 with low- to intermediate-risk PC treated with PBT to the prostate ± seminal vesicles with conventionally-fractionated radiation to a dose of >75 Gy using passive scatter or PBS techniques. Patients on androgen deprivation therapy were excluded from the analysis, as were patients with a history of prior pelvic radiation. GI and GU toxicities were prospectively scored using the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE) v4.0 scoring system. Toxicities occurring within 90 days of treatment were categorized as ‘acute’, whereas toxicities after this time point were categorized as ‘late’ toxicities.
2.3. Statistical analysis
Baseline characteristics for patients receiving PBS and PS/US were reported and compared using χ2 tests. Univariate comparisons of acute GI and GU toxicities between the two treatment modalities were compared using χ2 tests. Logistic regression was utilized to perform multivariable comparisons for acute toxicity. Cumulative incidence of late GU and GI toxicities were compared using the Gray’s test. Cox proportional hazards models were utilized for univariate and multivariable comparisons of rates of GI and GU toxicities in the two treatment modalities. To account for the effect of confounders, propensity score adjustments were utilized on the study sample for patient characteristics such as age, race, baseline PSA levels, Gleason score, cT stage, and baseline IPSS/AUA. Statistical analyses were performed using SAS version 9.4 (SAS Institute; Cary, NC). All statistical tests were performed at a significance level of 0.05.
3. Results
3.1. Patient characteristics
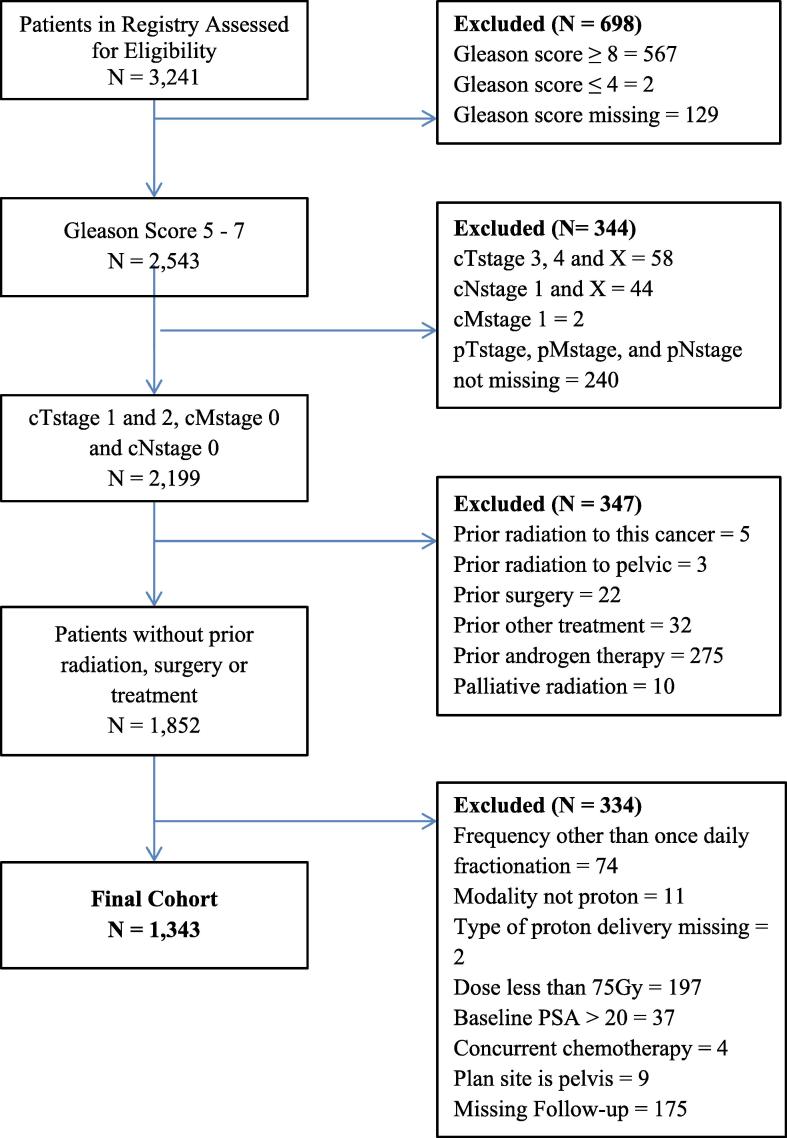
A total of 3241 patients with PC enrolled in PCG 001-09 from 2009 to 2017 were assessed for eligibility in the study, 1343 of whom were included in this analysis (Fig. 1). PBS was received by 238 patients (17.8%), and the remaining 1105 (82.2%) received PS/US. Patient characteristics are summarized in Table 1. Median follow-up was significantly shorter for the PBS group than the passive scatter group (16.5 months and 27.2 months, respectively; P < 0.001). The mean ages of patients receiving PBS and PS/US were 66.6 years and 65.2 years, respectively (P < 0.001). Of the nine centers at which patients underwent treatment, one offered only PS/US, four offered only PBS, and the remaining four offered both. A significantly lower proportion of patients receiving PBS were non-Hispanic white than in the passive scattering group (62.6% and 79.1%, respectively; P < 0.001).
Fig. 1.
CONSORT diagram.
Table 1.
Baseline characteristics of the study cohort.
| Patient Characteristics | Pencil Beam n = 238 | Passive Scatter N = 1.105 | Unadjusted P value* | Adjusted P value* |
|---|---|---|---|---|
| Age at baseline (Years) | <0.001 | 0.22 | ||
| Mean | 66.6 ± 6.4 | 65.2 ± 7.6 | ||
| Baseline PSA | 0.01 | 0.42 | ||
| Mean | 6.9 ± 3.5 | 6.3 ± 3.2 | ||
| Radiation Dose | 0.72 | 0.81 | ||
| Mean | 79.3 ± 0.7 | 79.4 ± 0.5 | ||
| IPSS/AUA prior to Radiation | 0.79 | 0.91 | ||
| 0–7 | 161 (67.7) | 730 (66.0) | ||
| 8–19 | 68 (28.6) | 323 (29.2) | ||
| 20–35 | 9 (3.8) | 52 (4.7) | ||
| Race | <0.001 | 0.91 | ||
| Non-Hispanic White | 149 (62.6) | 874 (79.1) | ||
| Non-Hispanic Black | 19 (8.0) | 72 (6.5) | ||
| Other | 70 (29.4) | 159 (14.4) | ||
| Treatment Location | <0.001 | – | ||
| ProCure Proton Therapy Center – Oklahoma | 0 (0.0) | 592 (53.6) | ||
| Northwestern Medicine – Chicago Proton Center | 74 (31.1) | 351 (31.8) | ||
| ProCure Proton Therapy Center – New Jersey | 46 (19.3) | 131 (11.9) | ||
| Seattle Cancer Care Alliance Proton Therapy Center | 35 (14.7) | 31 (2.8) | ||
| Scripps Proton Therapy Center | 22 (9.2) | 0 (0.0) | ||
| Willis-Knighton Proton Therapy Center | 56 (23.5) | 0 (0.0) | ||
| Maryland Proton Treatment Center | 4 (1.7) | 0 (0.0) | ||
| Mayo Clinic – Arizona | 1 (0.4) | 0 (0.0) | ||
| Gleason Score | <0.001 | 0.97 | ||
| 6 | 81 (34.0) | 580 (52.5) | ||
| 7 | 157 (66.0) | 525 (47.5) | ||
| cT Stage | 0.18 | 0.89 | ||
| cT1 | 171 (71.9) | 792 (76.0) | ||
| cT2a | 45 (18.9) | 193 (18.5) | ||
| cT2b | 11 (4.6) | 28 (2.7) | ||
| cT2c | 11 (4.6) | 29 (2.8) | ||
| Patient Follow-up Status | <0.001 | – | ||
| In Follow-up | 234 (98.3) | 928 (83.9) | ||
| Lost to Follow-up | 4 (1.7) | 177 (16.0) | ||
Propensity score adjustment performed using inverse probability of treatment weighting, adjusting for age, race, baseline PSA level, Gleason score, clinical stage, and baseline IPSS/AUA.
3.2. Acute GI/GU toxicity
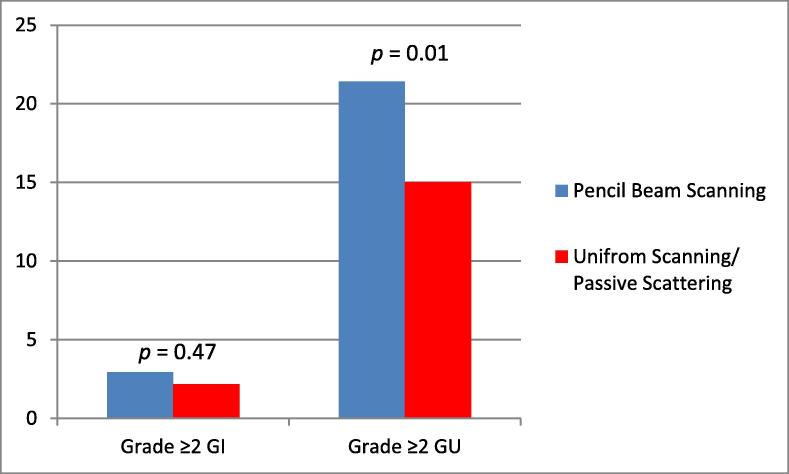
Table 2 summarizes the incidence of grade ≥2 acute GI and GU toxicities in the PBS and passive scatter groups. Fig. 2 shows the difference in acute GI and GU toxicities observed. A significantly higher proportion of patients experienced grade ≥2 acute GU toxicity in the PBS group than in the PS/US group (21.9% and 15.1%, respectively; P = 0.01). However, the incidence of grade ≥2 acute GI toxicity did not differ significantly between the PBS and PS/US groups but the incidence of this toxicity was low in both groups (2.9% and 2.1%, respectively; P = 0.47). Multivariate analysis adjusted for age, race, baseline PSA level, Gleason score, clinical stage, and baseline International Prostate Symptom Score/American Urological Association (AUA) Symptom Score (Table 3; full model: Supplementary Table 1). PBS was associated with a higher rate grade ≥2 acute GU toxicity (RR) = 1.57, 95% CI = 1.28–1.94, p < 0.001). No significant difference was observed in rates of grade ≥2 acute GI toxicity between the two groups (RR = 1.32; 95% CI, 0.79–2.19).
Table 2.
Acute and late toxicities by radiation modality.
| Toxicity | Pencil Beam n = 238 |
Passive Scatter n = 1,105 |
||||
|---|---|---|---|---|---|---|
| Grade |
Grade |
|||||
| 1 | 2 | 3 | 1 | 2 | 3 | |
| Acute Toxicities | ||||||
| GI | 40 | 7 | 0 | 223 | 24 | 0 |
| GU | 160 | 51 | 1 | 783 | 165 | 2 |
| SF | 23 | 11 | 0 | 149 | 45 | 0 |
| Late Toxicities | ||||||
| GI | 38 | 10 | 1 | 212 | 71 | 0 |
| GU | 52 | 15 | 0 | 490 | 129 | 0 |
| SF | 30 | 40 | 0 | 260 | 251 | 9 |
Fig. 2.
Incidence of acute ≥grade 2 GI and GU toxicities by proton modality.
Table 3.
Multivariable analysis of acute late grade ≥2 GI and GU.
| Model | Categories | Relative risk | 95%CI |
P value | |
|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||
| Grade 2 + acute GI toxicity | Passive scattering/uniform scanning | Ref. | |||
| Pencil beam | 1.32 | 0.79 | 2.19 | 0.29 | |
| Grade 2 + acute GU toxicity | Passive scattering/uniform scanning | Ref. | |||
| Pencil beam | 1.57 | 1.28 | 1.94 | <0.001 | |
Note: Adjusted for age, race, baseline PSA level, Gleason score, clinical stage, and baseline IPSS/AUA.
3.3. Late GI/GU toxicity
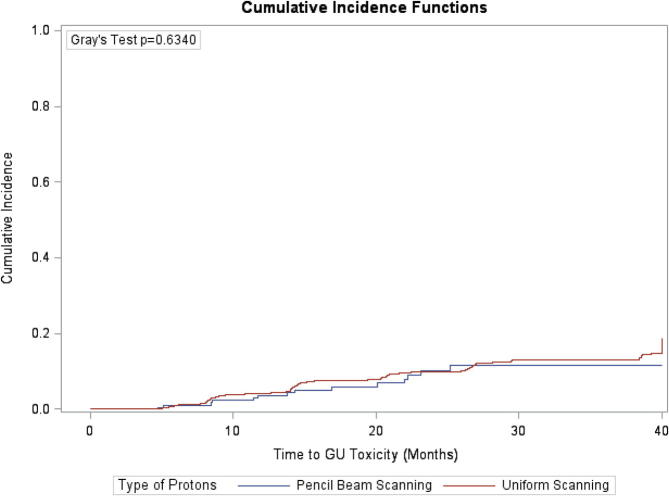
Table 2 summarizes the incidence of grade ≥2 late GI and GU toxicities in the PBS and PS/US groups. Fig. 3, Fig. 4 compare the time to late GI and GU toxicities by treatment group. No significant differences were observed in the cumulative incidence of late GI and GU toxicities at 3 years across the treatment groups. Specifically, patients treated with PBS had a 7.34% cumulative incidence of grade ≥2 GI toxicity compared to 6.3% for patients treated with PS/US at 2 years (P = 0.89). Patients treated with PBS also had a 10.20% cumulative incidence of grade ≥2 GU toxicity compared to 9.9% for patients treated with PS/US at 2 years (P = 0.63). Multivariate analysis that adjusted for age, race, baseline PSA level, Gleason score, clinical stage, and baseline IPSS/AUA found no significant differences in times to grade ≥2 late GI toxicity (hazard ratio [HR] = 0.94; 95%CI: 0.47–1.90, p = 0.87) or GU toxicity (HR = 0.78; 95%CI: 0.44–1.39, p = 0.40) between the treatment groups (Table 4; full model: Supplementary Table 2).
Fig. 3.
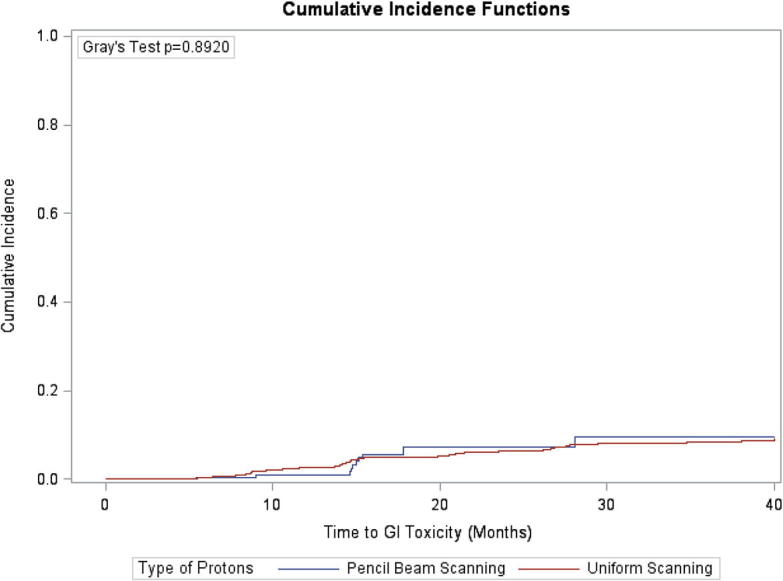
Actuarial time to development of late grade ≥2 GI toxicities by proton modality.
Fig. 4.
Actuarial time to development of late grade ≥2 GU toxicities by proton modality.
Table 4.
Multivariable analysis of late grade ≥2 GI and GU toxicities.
| Model | Categories | Hazard ratio | 95%CI |
P value | |
|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||
| Grade 2 + late GI toxicity | Passive scattering/uniform scanning | Ref. | |||
| Pencil beam scanning | 0.94 | 0.47 | 1.90 | 0.87 | |
| Grade 2 + late GU toxicity | Passive scattering/uniform | Ref. | |||
| Pencil beam scanning | 0.78 | 0.44 | 1.39 | 0.47 | |
Note: Adjusted for age, race, baseline PSA level, Gleason score, clinical stage, and baseline IPSS/AUA.
4. Discussion
This study represents real-world outcomes of one of the largest cohorts of men treated with modern PBT at over a third of all operational proton centers in the United States. We found an increase in acute grade ≥2 GU toxicity with PBS compared to PS/US. No difference in late GU toxicity was noted, but longer-term follow-up will be required given that acute GU toxicity is an independent predictor of late GU toxicity [7], which may not manifest until >5 years after treatment completion [7]. No significant difference in acute or late GI toxicities was observed.
A randomized study of comparing PBS and PS/US would be challenging to complete and is unlikely to ever occur. Therefore, comparisons of PBS and US/PS will be limited to non-randomized cohort studies, such as the present one. We did apply propensity score adjustments to match baseline characteristics of patients treated with PBS and PS/US. Even after adjusting for baseline IPSS scores between the two cohorts, differences in acute GU toxicity remained significant. A limitation of this study includes lack of data on dosimetric correlates with toxicity that could have had an impact on the toxicity profiles observed, as well as the limited follow-up for both cohorts (median time of 16.5 month for PBS and 26.5 months for PS/US). Nevertheless, the data represents real-world outcomes following treatment with PBT treatment plans that were deemed appropriate by physicians at a number of academic- and community-based proton centers across the US.
The exact cause of differences identified in this study is unclear, but there are a number of variables that could have had an impact on the observed results. First, although toxicities in this study were prospectively scored according to CTCAE criteria, scoring of grade 2 toxicity can be strongly physician dependent, which is important to note given that nearly 50% of patients treated with PS/US were treated at a single center. However, this is an inherent limitation to any study using clinician-scored toxicities as a study end point, and the same endpoint (grade ≥2 toxicity) was used to compare toxicity profiles following IMRT vs. 3D-CRT for prostate cancer patients treated in RTOG 0126 [8]. Next, it is possible that centers with both PS/US and PBS technologies were preferentially treating patients with more complex anatomy (e.g., large prostate size or large median lobe) with PBS. These would be patients who would be expected to have a higher burden of pre-treatment urinary symptoms. However, there was no difference in pre-treatment urinary symptoms between the two cohorts, as measured by IPSS. Finally, a longer experience with PBT has been associated with reduced toxicity [9]. PBS is a relatively recent development in PBT technology and is more likely to be in clinical use in centers that are newer.
There are also a number of treatment- and planning-specific variables that could have an impact on PBT treatment-related toxicity. Proton dose distribution is very sensitive to range and setup uncertainties [10]. Small changes in anatomy and/or inter- and intra-fraction motion can potentially lead to differences between the dose distribution that what was planned vs. what is actually delivered to the patient. These changes can be even more significant with PBS than with PS/US. Fortunately, treatment-planning techniques have been developed to optimize a plan that can be ‘robust’ to such changes [10]. However, use of such robust optimization methods is a relatively recent development, and its’ use is not required for participation most clinical trials, nor is it a specific variable that is collected in many ongoing proton clinical trials.
Other treatment-specific variables that would be of significance when studying outcomes following PBT include: image-guidance used during treatment, pencil beam scanning spot size, treatment planning margins, number of beam angles used, and treatment with alternating fields vs. treatment with all treatment fields daily. Such variables were not collected in the PCG 001-09 trial, but would be of interest given the findings of our analysis. We therefore recommend that data about specific treatment and planning techniques be collected in future PBT clinical trials, including registry studies.
This study also raises the question of whether inherent differences between PBS and PS/US exist. The two PBT delivery techniques are currently assumed to be radiobiologically equivalent, with a constant relative biological effectiveness (RBE) of 1.1 [11]. However, a number of preclinical studies have indicated subtle differences between linear energy transfer (LET) and, therefore, RBE, with the two proton delivery techniques, and the clinical relevance of these findings has not been explored [12], [13], [14].
RBE is known to increase with increasing LET and decreasing dose/fraction, with this effect most pronounced in low α/β tissues (e.g., most normal tissues) [15]. LET values are known to increase at the distal range for both types of PBT, but studies have indicated this may be slightly higher with PBS than with PS (1.57 keV/μM and 1.43 keV/μM, respectively) [16]. Moreover, spot-weighted delivery with PBS allows for a homogeneous dose distribution but with highly inhomogeneous LET distributions within the treatment target [17]. In PBS, the individual spot weights are inversely-optimized (driven by physical dose distribution), and the ratio of the highest to the lowest spot weights can be as high as 100:1. Furthermore the most highly weighted spots are often generated near normal tissue, consequently increasing the RBE dose in these regions. In contrast, the variations within a PS/US beam are typically 12:1 [18]. PBS also has a higher instantaneous dose-rate than PS/US and is known to be associated with distinct (and increased) changes in gene expression and chromosomal changes compared to passive scatter [13].
The ongoing PARTIQoL (Prostate Advanced Radiation Technologies Investigating Quality of Life) randomized study of PBT and photons, will stratify patients by PBT treatment type with pencil beam scanning and PS/US. Moreover, the COMPPARE study, “A comparative study of outcomes with proton and photon radiation in Prostate cancer,” is comparative (non-randomized) study of protons and photons that has been recently activated, and also allows for patients to be treated with pencil beam scanning or PS/US techniques [19]. Secondary analyses comparing outcomes following PS/US and pencil beam scanning from both of these important studies will be possible and may lead to further insights into differences between the two PBT delivery technologies.
Taken together, these results highlight the continued need for long-term data following modern PBT techniques that are currently being used to treat patients with prostate cancer. The results of this study raise the possibility of clinically significant differences between the two most commonly used forms of PBT, and warrant further evaluation though well-designed prospective clinical trials.
Authors’ disclosure of potential conflicts of interest
Dr. Mishra reports grants from ASTRO Comparative Effectiveness Research, during the conduct of the study; personal fees from Varian. No other authors reported potential conflicts of interest.
Acknowledgments
This research was funded through an American Society of Radiation Oncology Comparative Effectiveness Grant and was presented at the 2018 American Society of Radiation Oncology Annual Meeting.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2019.08.006.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.American Society of Radiation Oncology. http://www.choosingwisely.org/clinician-lists/american-society-radiation-oncology-proton-beam-therapy/ [accessed October 5 2018].
- 2.Mishra M.V., Aggarwal S., Bentzen S.M., Knight N., Mehta M.P., Regine W.F. Establishing evidence-based indications for proton therapy: an overview of current clinical trials. Int J Radiat Oncol Biol Phys. 2017;97:228–235. doi: 10.1016/j.ijrobp.2016.10.045. [DOI] [PubMed] [Google Scholar]
- 3.Langen K., Zhu M. Concepts of PTV and robustness in passively scattered and pencil beam scanning proton therapy. Semin Radiat Oncol. 2018;28:248–255. doi: 10.1016/j.semradonc.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Mouw K.W., Trofimov A., Zietman A.L., Efstathiou J.A. Clinical controversies: proton therapy for prostate cancer. Semin Radiat Oncol. 2013;23:109–114. doi: 10.1016/j.semradonc.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trofimov A., Nguyen P.L., Coen J.J., Doppke K.P., Schneider R.J., Adams J.A. Radiotherapy treatment of early-stage prostate cancer with IMRT and protons: a treatment planning comparison. Int J Radiat Oncol Biol Phys. 2007;69:444–453. doi: 10.1016/j.ijrobp.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yegya-Raman N., Zou W., Nie K., Malhotra J., Jabbour S.K. Advanced radiation techniques for locally advanced non-small cell lung cancer: intensity-modulated radiation therapy and proton therapy. J Thorac Dis. 2018;10:S2474–S2491. doi: 10.21037/jtd.2018.07.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zelefsky M.J., Levin E.J., Hunt M., Yamada Y., Shippy A.M., Jackson A. Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70:1124–1129. doi: 10.1016/j.ijrobp.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 8.Michalski J.M., Yan Y., Watkins-Bruner D., Bosch W.R., Winter K., Galvin J.M. Preliminary toxicity analysis of 3-dimensional conformal radiation therapy versus intensity modulated radiation therapy on the high-dose arm of the Radiation Therapy Oncology Group 0126 prostate cancer trial. Int J Radiat Oncol Biol Phys. 2013;87:932–938. doi: 10.1016/j.ijrobp.2013.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim S., Shen S., Moore D.F., Shih W., Lin Y., Li H. Late gastrointestinal toxicities following radiation therapy for prostate cancer. Eur Urol. 2011;60:908–916. doi: 10.1016/j.eururo.2011.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu W., Zhang X., Li Y., Mohan R. Robust optimization of intensity modulated proton therapy. Med Phys. 2012;39:1079–1091. doi: 10.1118/1.3679340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giantsoudi D., Adams J., MacDonald S., Paganetti H. Can differences in linear energy transfer and thus relative biological effectiveness compromise the dosimetric advantage of intensity-modulated proton therapy as compared to passively scattered proton therapy? Acta Oncol. 2018;1–6 doi: 10.1080/0284186X.2018.1468090. [DOI] [PubMed] [Google Scholar]
- 12.Michaelidesova A., Vachelova J., Puchalska M., Brabcova K.P., Vondracek V., Sihver L. Relative biological effectiveness in a proton spread-out Bragg peak formed by pencil beam scanning mode. Australas Phys Eng Sci Med. 2017;40:359–368. doi: 10.1007/s13246-017-0540-8. [DOI] [PubMed] [Google Scholar]
- 13.Gridley D.S., Pecaut M.J., Mao X.W., Wroe A.J., Luo-Owen X. Biological effects of passive versus active scanning proton beams on human lung epithelial cells. Technol Cancer Res Treat. 2015;14:81–98. doi: 10.7785/tcrt.2012.500392. [DOI] [PubMed] [Google Scholar]
- 14.Grassberger C., Paganetti H. Varying relative biological effectiveness in proton therapy: knowledge gaps versus clinical significance. Acta Oncol. 2017;56:761–762. doi: 10.1080/0284186X.2017.1316516. [DOI] [PubMed] [Google Scholar]
- 15.Wedenberg M., Lind B.K., Hardemark B. A model for the relative biological effectiveness of protons: the tissue specific parameter alpha/beta of photons is a predictor for the sensitivity to LET changes. Acta Oncol. 2013;52:580–588. doi: 10.3109/0284186X.2012.705892. [DOI] [PubMed] [Google Scholar]
- 16.Tran L.T., Chartier L., Bolst D., Pogossov A., Guatelli S., Petasecca M. Characterization of proton pencil beam scanning and passive beam using a high spatial resolution solid-state microdosimeter. Med Phys. 2017;44:6085–6095. doi: 10.1002/mp.12563. [DOI] [PubMed] [Google Scholar]
- 17.Unkelbach J., Botas P., Giantsoudi D., Gorissen B.L., Paganetti H. Reoptimization of intensity modulated proton therapy plans based on linear energy transfer. Int J Radiat Oncol Biol Phys. 2016;96:1097–1106. doi: 10.1016/j.ijrobp.2016.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paganetti H. 2nd ed. CRC Press, Taylor & Francis Group; Boca Raton, FL: 2019. Proton therapy physics. [Google Scholar]
- 19.Comparing Radiation Treatments for Localized Prostate Cancer. https://www.pcori.org/research-results/2017/comparing-radiation-treatments-localized-prostate-cancer [accessed March 11, 2019].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.