Abstract
CD70 is the unique ligand of CD27 and is expressed on immune cells only upon activation. Therefore, engagement of the costimulatory CD27/CD70 pathway is solely dependent on upregulation of CD70. However, the T cell-intrinsic effect and function of human CD70 remain underexplored. Herein, we describe that CD70 expression distinguishes proinflammatory CD4+ T lymphocytes that display an increased potential to migrate into the central nervous system (CNS). Upregulation of CD70 on CD4+ T lymphocytes is induced by TGF-β1 and TGF-β3, which promote a pathogenic phenotype. In addition, CD70 is associated with a TH1 and TH17 profile of lymphocytes and is important for T-bet and IFN-γ expression by both T helper subtypes. Moreover, adoptive transfer of CD70−/−CD4+ T lymphocytes induced less severe experimental autoimmune encephalomyelitis (EAE) disease than transfer of WT CD4+ T lymphocytes. CD70+CD4+ T lymphocytes are found in the CNS during acute autoimmune inflammation in humans and mice, highlighting CD70 as both an immune marker and an important costimulator of highly pathogenic proinflammatory TH1/TH17 lymphocytes infiltrating the CNS.
Keywords: CD70+CD4+ T lymphocytes, multiple sclerosis, CD27/CD70 pathway, TGF-β1, TGF-β3, soluble CD70, blood-brain barrier, endothelial cells, experimental autoimmune encephalitis, TCR1640 transgene mouse model
Subject terms: Autoimmunity, Mechanisms of disease
Introduction
CD70, a member of the tumor necrosis factor (TNF) receptor superfamily, is the unique ligand of CD27.1 CD27 is constitutively expressed by conventional T lymphocytes, while CD70 is only transiently expressed after T cell activation.2 Therefore, costimulation through CD70-CD27 interactions is primarily regulated by the expression of CD70, and this pathway can directly regulate T cell–T cell interactions and influence effector T cell development.3 A large body of evidence has confirmed that the interaction of CD27 on T lymphocytes with CD70 on antigen-presenting cells drives TH1 polarization in vitro and in vivo4,5 and TH17 polarization in vivo in the gut.6 Given that expression of CD70 is limited to activated as opposed to naive lymphocytes, these data may indicate that CD70 could be a reliable marker of pathogenic T lymphocytes during inflammatory processes. Accordingly, blocking the CD27/CD70 pathway by administering CD70-specific neutralizing antibody led to substantial inhibition of disease in experimental autoimmune encephalomyelitis (EAE).7 Likewise, overexpression of CD70 on B cells worsened disease outcome.8 Furthermore, CD70 expression on CD4+ T lymphocytes was shown to be significantly upregulated in patients affected by rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE).9,10 Despite this evidence supporting a role for CD27/CD70 in autoimmune disorders, another study demonstrated that CD70−deficient animals suffered from exacerbated EAE,11 highlighting the need to further explore the complex role of the CD27/CD70 pathway in autoimmune disorders, as suggested by Libergts and Wang.12,13
Multiple sclerosis (MS) is the most common chronic autoimmune disorder of the central nervous system (CNS), affecting more than 2 million people worldwide. Because the exact cause of MS has not yet been defined, treatment is largely symptomatic, and the disease is currently incurable. TH1 and TH17 lymphocytes are generally considered to be the major drivers of pathogenesis in both MS and EAE.14–18 These encephalitogenic proinflammatory lymphocytes develop pathogenic features enabling them to migrate across the blood–brain barrier (BBB) and infiltrate into the CNS.19 Both TH1 and TH17 lymphocytes express a number of cellular adhesion molecules (CAMs) involved in the process of transendothelial migration, such as intercellular cell adhesion molecule (ICAM), vascular cell adhesion molecule (VCAM), activated leukocyte cell adhesion molecule (ALCAM) and melanoma cell adhesion molecule (MCAM).20–22 These molecules can be seen as activation markers, with specific functions related to diapedesis and have been shown to be most important for the development of EAE.20 However, a costimulatory marker that defines both proinflammatory TH1 and TH17 lymphocytes invading the CNS in MS patients is still lacking. In addition, whether the CAM signature on proinflammatory TH lymphocytes can define molecular pathways critical for autoimmunity remains to be established.
Herein, we performed a proteomic screen comparing MCAM+ TH17 polarized human lymphocytes with their MCAMneg counterparts and found important differences in a number of immune pathways, including costimulatory molecules. Human MCAM+ TH17 polarized lymphocytes express significantly more costimulatory molecules, including CD70, than MCAMneg lymphocytes. Furthermore, we found that CD70 was upregulated on TH1 and TH17 lymphocytes and was coexpressed with specific adhesion molecules needed for CNS infiltration. In MS patients, increased expression of CD70 was found on peripheral ex vivo CD4+ T lymphocytes and on TH1 and TH17 polarized lymphocytes in vitro compared to healthy controls. CD70+CD4+ T lymphocytes were found both in the cerebrospinal fluid (CSF) and in perivascular infiltrates in the brains of MS patients, as well as in the CNS of acute and chronic TCR-transgenic EAE animals. Finally, adoptive transfer of CD70−/−CD4+ T lymphocytes in RAG2CGN−/− mice induced less severe EAE disease course than transfer of WT CD4+ T lymphocytes, highlighting the CNS-pathogenic effects of these cells in vivo.
Results
CD70 expression on ex vivo CD4+ T lymphocytes defines a pathogenic and proinflammatory subpopulation
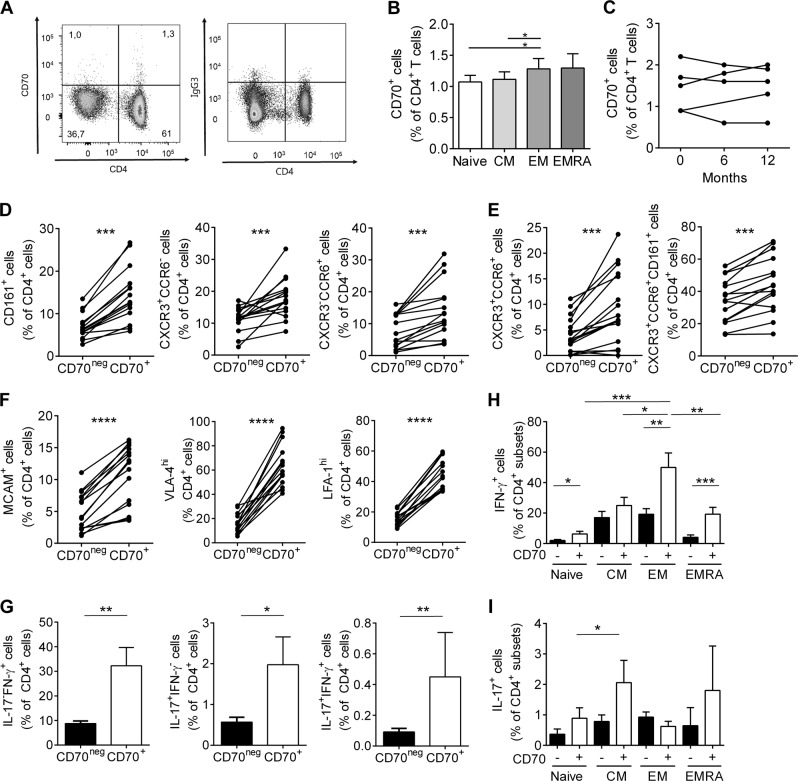
In humans, CD70 is mainly induced after activation and predominantly expressed on antigen-presenting cells. However, resting CD4+ T lymphocytes can also express this unique ligand for CD27.10 We confirmed that CD70 is expressed on ex vivo human CD4+ T lymphocytes, albeit very few (Fig. 1a), and that CD70 is mostly expressed on effector memory CD4+ T lymphocytes (Fig. 1b). Moreover, longitudinal ex vivo analysis of CD70 expression on CD4+ T lymphocytes from healthy donors illustrated stable expression over a period of 1 year (Fig. 1c). To further characterize CD70+CD4+ T lymphocytes, we used specific surface markers to define the ex vivo phenotype of these lymphocytes in human peripheral blood (Fig. 1d–f). CD70+CD4+ T lymphocytes coexpressed significantly increased amounts of CD161, CCR6, and CXCR3, which are all established markers of the human proinflammatory TH1/TH17 phenotype (Fig. 1d).23,24 In addition, CD70+CD4+ T lymphocytes were also CXCR3+CCR6+ and CXCR3+CCR6+CD161+, suggesting that CD70 expression by CD4+ T lymphocytes is associated with pathogenic human TH17 lymphocytes (Fig. 1e).24 In MS, specific CAMs and integrins, such as MCAM, very late activation antigen 4 (VLA-4) and lymphocyte function associated antigen 1 (LFA-1) are essential to endow pathogenic lymphocytes to infiltrate into the CNS.20,21,25 We therefore analyzed the expression of adhesion molecules on CD70+ and CD70negCD4+ T lymphocytes and found that CD70 expression correlated with the expression of MCAM, LFA-1 and even more so with VLA-4 (Fig. 1f), giving CD70+CD4+ T lymphocytes the hypothetical advantage to infiltrate the CNS. Furthermore, a short in vitro stimulation demonstrated that a significantly higher proportion of CD70+CD4+ T lymphocytes expressed IL-17 and IFN-γ compared to their CD70neg counterparts (Fig. 1g). We further demonstrated that CD70+ central memory and effector memory CD4+ T lymphocytes expressed significantly more IL-17 and IFN-γ (Fig. 1h, i) compared to naive and CD70neg populations.
Fig. 1.
CD70 expression on human ex vivo CD4+ T lymphocytes defines a pathogenic and proinflammatory subpopulation. a Representative dot plot of expression of CD70 and isotype on freshly isolated CD4+ peripheral T lymphocytes from a healthy control, gated within live CD3+ T lymphocytes. b Percentage peripheral CD4+ T lymphocyte subtypes (naive (CCR7+CD45RA+), central memory (CM, CCR7+CD45RA−), effector memory (EM, CCR7−CD45RA−), and terminally differentiated effector (EMRA, CCR7-CD45RA+)) coexpressing CD70 in healthy controls (n = 22). c Expression of CD70 on ex vivo CD4+ T lymphocytes of individual healthy controls collected over 12 months (n = 5). d–f Percentage of CD70+ or CD70neg cells that coexpress CD161+, CXCR3+CCR6−, CXCR3−CCR6+, e CXCR3+CCR6+, CXCR3+CCR6+CD161+ and f MCAM, VLA-4hi and LFA-1hi within ex vivo CD4+ T lymphocytes from healthy controls (n = 15). g PercentageS of IL-17+IFN-γ-, IL-17-IFN-γ+, and IL-17+IFN-γ+ cells within ex vivo CD4+ T lymphocytes that are CD70+ or CD70neg from healthy controls (n = 12). h, i Expression of IFN-γ (h) and IL-17 (i) in different ex vivo CD4+ T lymphocyte subsets separated into CD70+ and CD70neg from healthy controls (n = 11). Statistical differences were assessed by a paired t-test. Data are presented as paired data or as the mean ± SEM, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001
These data demonstrate that in humans, CD70 expression defines a population of proinflammatory effector CD4+ T lymphocytes with an enhanced potential to infiltrate the CNS.
Stimulation of CD4+ T lymphocytes with TGF-β1 and TGF-β3 upregulates CD70 and CD70 engagement promotes proinflammatory cytokine expression
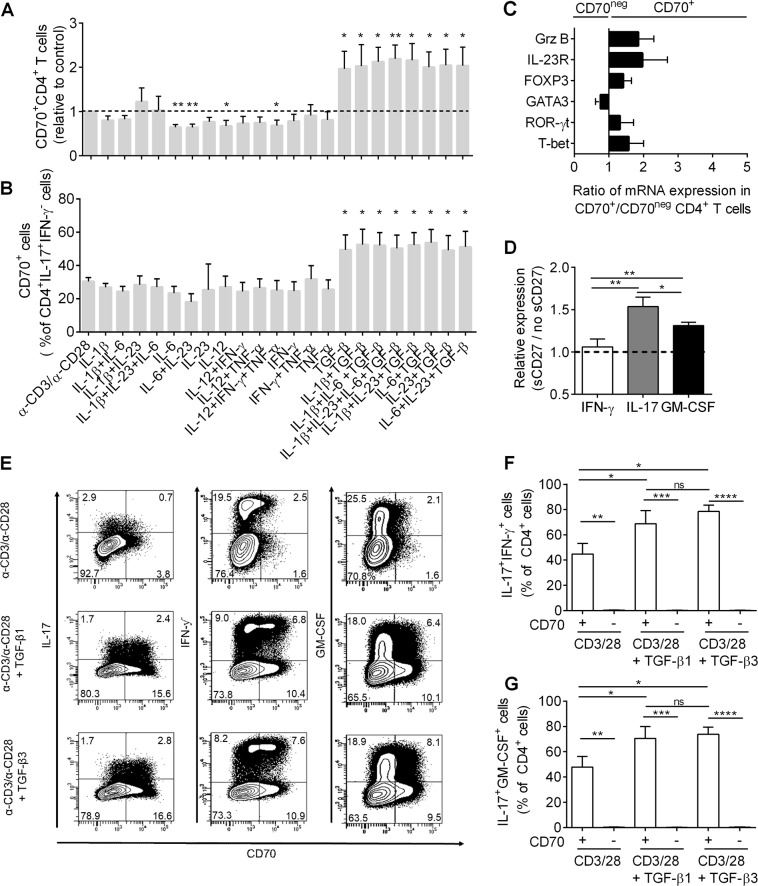
To identify signals regulating CD70 expression on the surface of CD4+ T lymphocytes, we performed a cytokine stimulation assay in which total CD4+ T lymphocytes were stimulated with anti-CD3 and anti-CD28 in combination with various cytokines. In line with a previous report,26 the addition of TGF-β1 led to a significant upregulation of CD70 on CD4+ T lymphocytes (Supplemental Fig. 1a) and a significant increase in the number of CD4+ T lymphocytes expressing CD70 (Fig. 2a). TGF-β1-induced CD70 expression was also associated with IL-17 (Fig. 2b) and IFN-γ (Supplemental Fig. 1b) expression by CD4+ T lymphocytes. Furthermore, the effect of adding various cytokines to CD8+ T lymphocytes was also measured. TGF-β1 alone does not have an effect on CD70 expression by CD8+ T lymphocytes, while the addition of IL-1β leads to a significant upregulation of CD70 (Supplemental Fig. 1c-d). To further characterize the effect of TGF-β stimulation, we selected CD70+ and CD70neg CD4+ T lymphocytes after stimulation with TGF-β and measured the expression of a panel of relevant genes. A relative increase in IL-23 receptor (IL-23R), granzyme B (GrzB), FOXP3, ROR-γt, and T-bet mRNAs was detected in CD70+CD4+ T lymphocytes compared to CD70neg lymphocytes. GATA3 mRNA seemed to be upregulated in CD70neg lymphocytes (Fig. 2c). Next, to evaluate the downstream effect of CD70 activation on CD4+ T lymphocytes, we added soluble CD27 (sCD27) to in vitro TGF-β1-stimulated CD4+ T lymphocytes. We found that the proportion of IL-17- and GM-CSF-expressing lymphocytes was significantly increased in sCD27-treated cells (Fig. 2d). These results demonstrate that activation of CD70, by binding CD27, induced a proinflammatory phenotype in human CD4+ T lymphocytes.
Fig. 2.
Stimulation of CD4+ T lymphocytes with TGF-β1 and TGF-β3 upregulates CD70, and CD70 engagement promotes proinflammatory cytokine expression. a Proportion of CD70+CD4+ T lymphocytes after in vitro stimulation with α-CD3/α-CD28 alone or together with various cytokines (α-CD3/α-CD28 used as baseline) in healthy controls (n = 7). b Percentage of CD70+ lymphocytes within IFN-γ−IL-17+ producing CD4+ T lymphocytes after in vitro stimulation with α-CD3/α-CD28 alone or together with various cytokines in healthy controls (n = 7). c Quantitative PCR analysis of granzyme B (Grz B), IL-23 receptor (IL23-R), FOXP3, ROR-γt, GATA3, and T-bet mRNA in CD70+CD4+ T lymphocytes and CD70negCD4+ T lymphocytes after in vitro stimulation with TGF-β1. The results are shown as ratios (CD70+/CD70neg) of mRNA transcripts relative to 18S ribosomal RNA, in healthy controls (n = 5). d Relative expression of IL-17, IFN-γ, and GM-CSF within CD4+ T lymphocytes (stimulated with TGF-β1) incubated with or without soluble CD27 (sCD27, 8 ng/ml) for 3 days in healthy controls (n = 6). The results are shown as the ratio (addition of soluble CD27/no addition of soluble CD27). e Representative dot plot of CD70 expression on IL-17, IFN-γ, and GM-CSF-expressing CD4+ T lymphocytes after in vitro stimulation with α-CD3/α-CD28 alone or together with TGF-β1 or TGF-β3 in a healthy control (representative of n = 6). f, g Percentage of IFN-γ+IL-17+ and IL-17+GM-CSF+ in CD70+ and CD70neg CD4+ T lymphocytes after in vitro stimulation with α-CD3/α-CD28 alone or together with TGF-β1 or TGF-β3 in healthy controls (n = 6). A one-way ANOVA was used to compare various stimulation conditions and a paired t-test to compare CD70+ with CD70neg and TGF-β1 with TGF-β3. Data can be expressed relative to control (α-CD3/α-CD28), with or without the addition of soluble CD27, and relative to CD70+. Statistical analysis was performed on raw data. Data are represented as the mean ± SEM *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001
The influence of TGF-β1 and TGF-β3 on the regulation of encephalitogenic T lymphocytes and regulatory T cells (Tregs) in EAE remains debated.27,28 To investigate whether TGF-β1 and TGF-β3 act differently on human CD4+ T lymphocytes, we compared CD70 expression and cytokine production in CD4+ T lymphocytes stimulated with TGF-β1 or TGF-β3. We found that both TGF-β1 and TGF-β3 induced the expression of CD70 (Fig. 2e) and upregulated IL-17, IFN-γ, and GM-CSF, preferentially in CD70+CD4+ T lymphocytes (Fig. 2e–g). However, we did not detect a difference between the effect of TGF-β1 and TGF-β3 (Fig. 2e–g), even at higher concentrations (Supplemental Fig. 1e).
In conclusion, our data demonstrate that upregulation of CD70 by TGF-β1 or TGF-β3 and stimulation of CD70 by soluble CD27 induces a proinflammatory phenotype in CD4+ T lymphocytes, skewing them towards a mixed TH1/TH17 phenotype in humans.
CD70 expression is associated with TH1 and TH17 polarization in humans
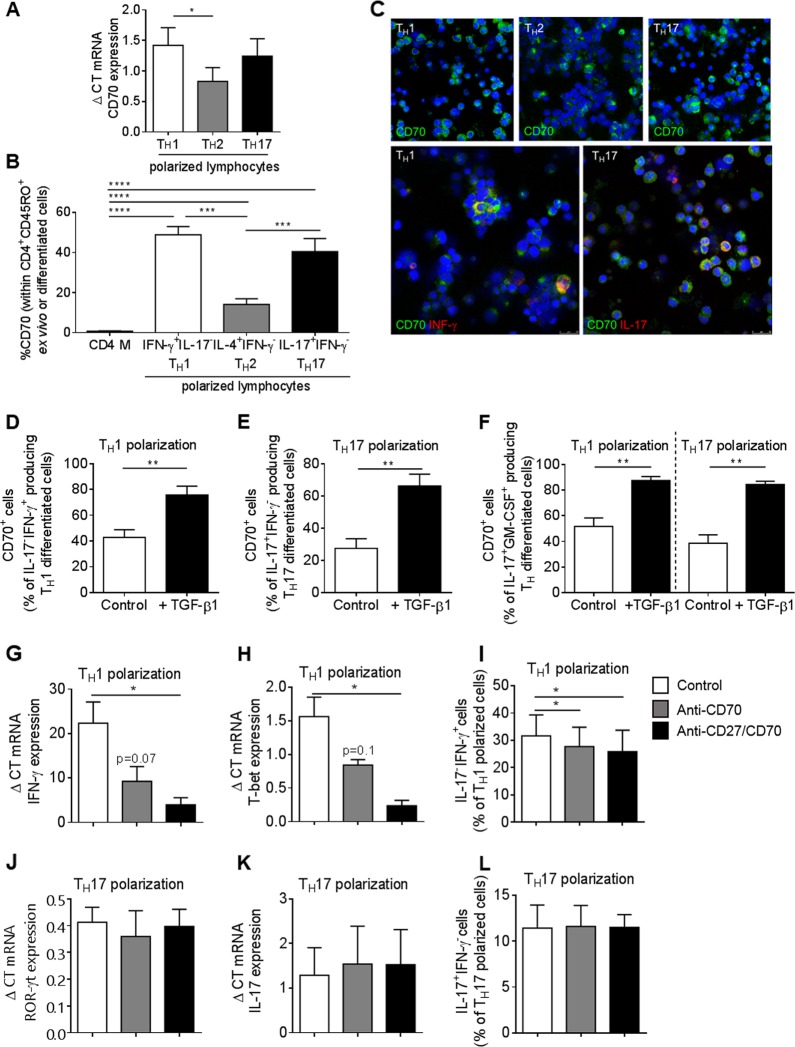
To investigate whether CD70 is expressed after in vitro TH1, TH17, and TH2 polarization, human peripheral blood CD4+ memory T lymphocytes were stimulated with IL-23, IL-12, and IL-4 using our previously established differentiation protocol.20,29 We found that CD70 mRNA expression was significantly increased upon IL-23-driven TH17 polarization and IL12-driven TH1 polarization (Fig. 3a). At the protein level, CD70 was significantly upregulated on IL-23-driven IL-17+IFN-γ− TH17 and IL-12-driven IL-17−IFN-γ+ TH1 polarization compared to IL-4-driven IL-4+IFN-γ− TH2 and ex vivo CD4+ memory T lymphocytes (Fig. 3b). These results were confirmed using immunocytofluorescence and confocal microscopy, where we found that CD70 is upregulated on TH1 and TH17 lymphocytes compared to TH2 lymphocytes (Fig. 3c, upper panel). CD70 expression was restricted to IL-17- and IFN-γ-expressing cells (Fig. 3c, lower panel). Furthermore, CD70 was gradually upregulated during 6 days of in vitro TH polarization and reached a plateau at days 4-5, depending on the polarization (Supplemental Fig. 2a).
Fig. 3.
CD70 expression is associated with TH17 and TH1 polarization in humans. a Quantitative PCR analysis of CD70 mRNA expression relative to 18S ribosomal RNA in TH1, TH2, and TH17 differentiated lymphocytes in healthy controls (n = 9). b Expression of CD70 on CD4+CD45RO+ memory T lymphocytes and IFN-γ+IL-17- TH1, IL-4+IFN-γ- TH2 and IL-17+IFN-γ- TH17 lymphocytes in healthy controls (n≥14). c Representative immunocytofluorescent confocal images of expression of CD70 (green), IFN-γ (red), and IL-17 (red) on TH1, TH2, and TH17 differentiated lymphocytes in a healthy control (images representative of n = 5). d–f Percentage of CD70+ lymphocytes within IFN-γ+IL-17-, IFN-γ-IL-17+, IL-17+GM-CSF+ TH1 and TH17 differentiated lymphocytes with or without the addition of TGF-β1 (control or +TGF-β1) in healthy controls (n = 8). g–l Gene and protein expression levels of specific markers under normal TH1 and TH17 polarizing conditions (control) or when adding neutralizing antibody against CD70 alone or against both CD27 and CD70 every day for 5 days (5 µg/ml). Analysis of the expression levels of the lymphocytes was performed on day 6. g, h Gene expression of IFN-γ and T-bet mRNA relative to 18S ribosomal RNA expression in TH1 differentiated lymphocytes (control) or TH1 lymphocytes treated with anti-CD70 neutralizing antibody alone or with both anti-CD27 and anti-CD70 in healthy controls (n = 6). i Percentage of IFN-γ+IL-17− production in TH1 differentiated lymphocytes (control) or treated with anti-CD70 neutralizing antibody alone or with both anti-CD27 and anti-CD70 in healthy controls (n = 7). j, k Gene expression of IL-17 and ROR-γt mRNA, relative to 18S ribosomal RNA expression, in TH17 differentiated lymphocytes (control) or TH17 lymphocytes treated with anti-CD70 neutralizing antibody alone or with both anti-CD27 and anti-CD70, in healthy controls (n = 3). i Percentage of INF-γ-IL-17+ production in TH17 differentiated lymphocytes (control) or treated with anti-CD70 neutralizing antibody alone or with both anti-CD27 and anti-CD70 in healthy controls (n = 3). Statistical testing was performed with unpaired or paired t-tests when appropriate. Data are represented as the mean ± SEM. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001
TGF-β1 was proposed to induce a pathogenic TH17 phenotype in both mouse and human.30–32 Therefore, we evaluated whether TGF-β1 alters cytokine production by CD70+ cells during polarization of human CD4+ T lymphocytes into TH1 and TH17 cells. We found that the addition of TGF-β1 to TH1 and TH17 polarizing conditions significantly increased the proportion of CD70-expressing IL-17−IFN-γ+ (Fig. 3d), IL-17+IFNγ− (Fig. 3e), and IL-17+GM-CSF+ CD4+ polarized T lymphocytes (Fig. 3f).
Because CD70 is an activation marker, and while we found CD70 to be highly upregulated upon TH1 and TH17 polarization, we hypothesized that blocking CD70 during polarization could possibly affect the differentiation of lymphocytes. To test this hypothesis, anti-CD70 or anti-CD70 together with anti-CD27 neutralizing antibodies were added every day during TH1 and TH17 polarization of human CD4+ T lymphocytes. During TH1 polarization, neutralization of the CD27/CD70 pathway led to a reduction in IFN-γ and T-bet mRNA expression (Fig. 3g, h) and to a significant reduction in IFN-γ production (Fig. 3i). The CD27/CD70 pathway did not affect ROR-γt or IL-17 when CD4+ T lymphocytes were polarized into TH17 lymphocytes (Fig. 3j–l). However, and most interestingly, neutralization of the CD27/CD70 pathway during TH17 polarization resulted in a significant reduction of IFN-γ and T-bet mRNA (Supplemental Fig. 2b-c).
Collectively, these results demonstrate that CD70 is highly expressed on human proinflammatory TH1 and TH17 lymphocytes, especially in the presence of TGF-β and that CD70 expression on T lymphocytes specifically controls IFN-γ and T-bet expression, both under TH1 and TH17 polarization programming.
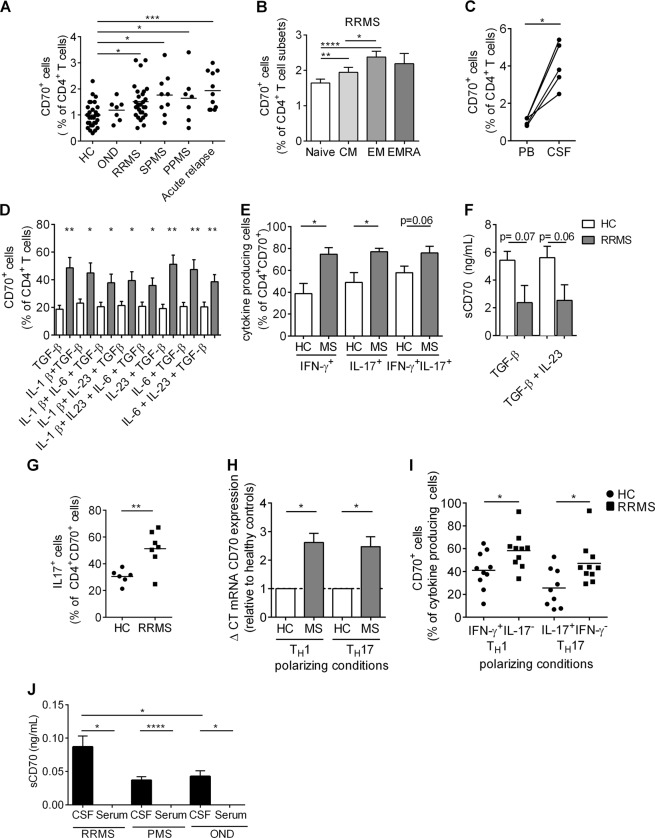
CD70 is upregulated on CD4+ T lymphocytes of MS patients
Pathogenic proinflammatory CD4+ T lymphocytes, both TH1 and TH17, are important contributors to disease activity in patients affected by MS.29,33,34 Therefore, we next sought to investigate whether CD70 is involved in these pathogenic responses found in MS. We first compared the ex vivo expression of CD70 on peripheral blood CD4+ T lymphocytes from healthy controls (HC), untreated MS patients (relapsing-remitting MS (RRMS) patients, secondary progressive MS (SPMS) patients, primary progressive MS (PPMS) patients), MS patients undergoing acute relapse and patients with other neurological diseases (OND). A significantly higher proportion of peripheral CD70+CD4+ T lymphocytes was observed in all types of MS patients compared to HC and OND (Fig. 4a), and even more so during an acute relapse. Interestingly, central memory and effector memory CD4+ T lymphocytes, two subtypes highly implicated in MS pathogenesis, expressed more frequently CD70 in RRMS patients compared to naive CD4+ T lymphocytes (Fig. 4b). Furthermore, CD70+CD4+ T lymphocytes from untreated RRMS patients coexpressed significantly more CXCR3, MCAM, and CCR6 compared to HC (Supplemental Fig. 3a). Additionally, a paired comparison of CD70+CD4+ T lymphocytes from the peripheral blood and the CSF of the same MS patients demonstrated a significant enrichment of these cells in the CSF (Fig. 4c). To investigate whether the increased frequency of CD70+CD4+ T lymphocytes in MS patients was specific to T lymphocytes, we compared the proportion of CD70+ lymphocytes between MS and HC within other peripheral immune cell populations (CD19+ B lymphocytes, CD8+ T lymphocytes, CD14+ monocytes, CD11c+ conventional dendritic cells, and CD11c−CD14−CD304+CD303+ plasmacytoid dendritic cells). The frequency of CD70+ CD19+ B lymphocytes, CD14+ monocytes, CD11c+ conventional dendritic cells, and CD11c−CD14−CD304+CD303+ plasmacytoid dendritic cells remained unchanged in MS and HC. However, in addition to CD4+ T lymphocytes, we observed that the frequency of CD70+CD8+ T lymphocytes was increased in MS patients when compared to HC, highlighting the importance of T cell-specific CD70 pathogenicity in MS (Supplemental Fig. 3b-f).
Fig. 4.
CD70 is upregulated on CD4+ T lymphocytes of MS patients. a CD70 expression on ex vivo CD4+ T lymphocytes in the peripheral blood of healthy controls (HC, n = 30), patients with other neurological diseases (OND, n = 7), untreated relapsing-remitting MS in remission (RRMS, n = 53), secondary progressive MS (SPMS, n = 9), primary progressive MS (PPMS, n = 7) and patients with an acute MS relapse (n = 11). b Expression of CD70 expression on ex vivo CD4+ T lymphocyte subtypes (naive (CCR7+CD45RA+), central memory (CM, CCR7+CD45RA-), effector memory (EM, CCR7−CD45RA−) and terminally differentiated effector re-expressing CD45RA (EMRA, CCR7−CD45RA+)) in peripheral blood of untreated RRMS patients (n = 53). c Paired expression of CD70 on CD4+ T lymphocytes isolated from peripheral blood (PB) and cerebrospinal fluid (CSF) of untreated RRMS patients (n = 5). d CD70 expression on CD4+ T lymphocytes after in vitro stimulation with α-CD3/α-CD28 together with various cytokines in HC (n = 7) and untreated RRMS patients (n = 7). e Expression of cytokines (IFN-γ, IL-17) by CD70+CD4+ T lymphocytes after in vitro stimulation with TGF-β1 and IL-23 in HC (n = 6) and untreated RRMS patients (n = 5). f Concentration of soluble CD70 (sCD70) secreted after stimulation of CD4+ T lymphocytes with TGF-β1 alone and TGF-β1 with IL-23 in HC (n = 6) and untreated RRMS patients (n = 5). g Expression of IL-17 in CD70+CD4+ T lymphocytes after in vitro stimulation with α-CD3/α-CD28 in healthy controls (HC, n = 6) and untreated RRMS patients (RRMS, n = 7). h Expression levels of CD70 mRNA, relative to 18S ribosomal RNA, in TH1 and TH17 differentiated lymphocytes in healthy controls (n = 8) and untreated MS patients (n = 8) (results shown as relative to HC). i Expression of CD70 on IFN-γ+IL-17− TH1 and IL-17+IFN-γ− TH17 polarized lymphocytes in HC (n = 10) and untreated RRMS patients (n = 10). j Concentration of soluble CD70 in the serum and cerebrospinal fluid (CSF) of patients with relapsing-remitting MS (RRMS, n = 27, untreated), progressive MS (PMS, n = 4, untreated), and other neurological diseases (OND, n = 12). Flow cytometry was used for a–e, g, and i, and ELISA was used for f and j. An unpaired t-test was used to compare patients with healthy controls, and a paired t-test was used to compare CSF and PB of patients. Data are represented as the mean ± SEM. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001
Next, we compared the effect of in vitro stimulation of CD4+ T lymphocytes on CD70 expression between untreated RRMS patients and HC (Fig. 4d–i). The percentage of CD70+CD4+ T lymphocytes (Fig. 4d) and the expression of CD70 on CD4+ T lymphocytes (Supplemental Fig. 3g) after TGF-β1 stimulation alone, or in the presence of other cytokines, was significantly higher in RRMS patients compared to HC. In addition, the proportion of IFN-γ- and IL-17-producing CD4+ T lymphocytes that coexpress CD70 after stimulation with TGF-β1 and IL-23 was significantly higher in MS patients compared to HC (Fig. 4e). Interestingly, the extent to which soluble CD70 (sCD70) was secreted after stimulation with TGF-β1 alone or TGF-β1 with IL-23 was lower in RRMS compared to HC, indicating that HC shed their CD70 more after stimulation than MS patients (Fig. 4f). Furthermore, to assess a difference in nonspecific stimulation (α-CD3/ α-CD28) between RRMS and HC, we measured the frequency of CD70+IL-17-producing CD4+ T lymphocytes generated with RRMS PBMCs and found it to be higher than the frequency generated with PBMCs from HC (Fig. 4g). We also measured CD70 mRNA expression in TH1- and TH17-polarized lymphocytes of HC and RRMS patients and found that CD70 was significantly upregulated in both TH1- and TH17-polarized lymphocytes of patients (Fig. 4h). These results were confirmed when comparing CD70 expression of untreated RRMS and HC on IL23-driven IL-17+IFNγ− TH17- and IL-12-driven IL-17−IFNγ+ TH1 polarized lymphocytes (Fig. 4i).
Finally, we measured sCD70 in the serum and CSF of patients with RRMS, progressive MS (PMS) and OND. Soluble CD70 was found to be significantly higher in the CSF of RRMS patients compared to CSF of patients with OND. Soluble CD70 was undetected or very low in all the sera samples (Fig. 4j). Endothelial cells can express members of the TNF superfamily.3 We therefore hypothesized that the striking increase in sCD70 in the CSF of MS patients could be partly explained by the secretion of CD70 by blood–brain barrier endothelial cells (BBB-EC). To investigate this, we first analyzed the gene expression of CD70 mRNA on human BBB-EC, meningeal-blood barrier endothelial cells (MBB-EC) and astrocytes and found that CD70 mRNA expression was unique to human BBB-EC (Supplemental Fig. 4a). Next, we measured the expression of CD70 mRNA (Supplemental Fig. 4b) and CD70 protein (Supplemental Fig. 4c and Supplemental Fig. 4d) on resting and inflamed (IFN-γ/TNF-α) human BBB-EC and found an upregulation of both CD70 mRNA and protein by inflammatory culture conditions. Moreover, soluble CD70 was found to be secreted by BBB-EC in these various conditions. Intriguingly, human primary cultures of BBB-EC grown from the brain of an MS patient showed a remarkable secretion of sCD70 (Supplemental Fig. 4e).
Overall, these data demonstrate that CD70 is significantly upregulated on both ex vivo and stimulated peripheral proinflammatory CD4+ T lymphocytes of MS patients and that both soluble CD70 and CD70+CD4+ T lymphocytes can be found in the CSF of RRMS patients, suggesting an association between CD70 and disease activity.
CD70+CD4+ T lymphocytes are present in the MS brain and retain their proinflammatory features
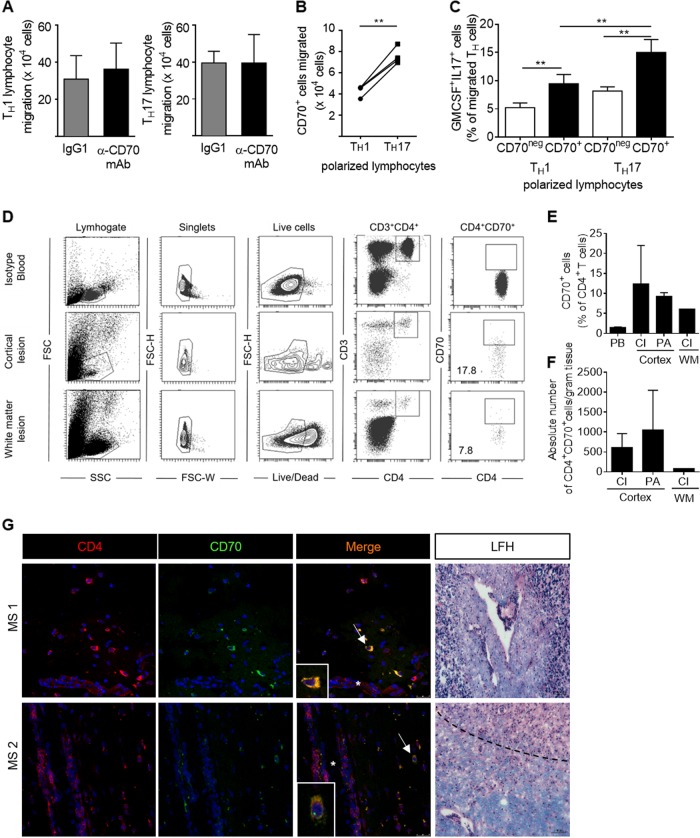
TH17 polarized lymphocytes migrate faster across the BBB compared to TH1 lymphocytes, suggesting that lesion formation in MS is driven by CNS-infiltrating pathogenic TH17.29,33 To investigate whether CD70 is involved in pathogenic CD4+ T lymphocyte migration across the BBB, we pretreated TH1 and TH17 cells with a CD70-neutralizing antibody and found that CD70 does not influence the migratory potential of CD4+ T lymphocytes (Fig. 5a). Interestingly, when comparing the number of CD70+ lymphocytes within the TH1 and TH17 lymphocytes that had migrated across human BBB-ECs, we found a significant increase in the number of CD70+ TH17 lymphocytes compared to CD70+ TH1 lymphocytes (Fig. 5b). This increase was not due to an overall increase in TH17 migration, as it was not observed for CD70neg lymphocytes (data not shown). In addition, after migration across human BBB-ECs, CD70+ TH1 and TH17 lymphocytes produce significantly more GM-CSF and IL-17 than their CD70neg counterparts (Fig. 5c). These results demonstrate that human CD70+CD4+ T lymphocytes represent a stable proinflammatory population that retains their phenotype after migration across the BBB.
Fig. 5.
CD70+CD4+ T lymphocytes are present in the MS brain and retain their proinflammatory features. a Absolute number of TH1 and TH17 polarized lymphocytes that have migrated across resting human blood–brain barrier endothelial cells (BBB-ECs) after treatment with anti-CD70 neutralizing antibody or with the appropriate isotype in healthy controls (n = 5). b Absolute number of CD70+ TH1 and TH17 polarized cells that have migrated across resting human BBB-ECs in healthy controls (n = 4). c Percentage of GM-CSF+IL-17+ CD70+ and CD70neg TH1 and TH17 lymphocytes that have migrated across resting BBB-EC in healthy controls (n = 5). d Representative dot plots showing the gating strategy used for flow cytometry performed on immune lymphocytes that were isolated from cortical lesions and white matter lesions from the brain of an MS patient (at autopsy). Blood from a healthy control was used to set the gates and the isotype control for CD70. e Expression of CD70 on CD4+ T lymphocytes present in chronic inactive lesions (CI) of the cortex (n = 2) and white mater (WM) (n = 1), and preactive lesions of the cortex (n = 2) isolated from the brain of an MS patient at autopsy (n = 1) and compared to CD70 expression in peripheral blood (PB) CD4+ T lymphocytes from untreated RRMS patients (n = 53). f Absolute number of CD70+CD4+ T lymphocytes per gram tissue present in chronic inactive lesions (CI) of the cortex (n = 2) and white mater (WM) (n = 1) and preactive lesions of the cortex (n = 2) isolated the brain of an MS patient (at autopsy). g Representative immunofluorescent images of frozen sections of MS brains. Staining for CD4 (red), CD70 (green), and overlay (orange) in active (MS1) and preactive (MS2) white matter MS lesions (classified using Luxol Fast Blue and hematoxylin staining (LFH). Arrowhead shows colocalization of CD4 and CD70 and asterisks blood vessels. Representative of n = 3, a total of 18 sections. Nuclei in blue. The dotted line shows the border of the active MS lesion. Statistical significance was assessed with unpaired or paired t-tests when appropriate. Data are represented as paired data or as the mean ± SEM. *p<0.05, **p<0.01
Next, to examine whether CD70+CD4+ T lymphocytes are present in lesions in MS-affected brains, we performed both flow cytometry on immune cells isolated from MS cortical and white matter lesions obtained after rapid autopsy (Fig. 5d–f) and immunofluorescent staining of frozen sections of MS brains (Fig. 5g). Each MS lesion was used for both classification of lesion type (preactive (PA), active, chronic inactive (CI)35 and for immune cell isolation followed by flow cytometry. A representative dot plot illustrates the gating strategy that was used to identify and quantify the CD70+CD4+ T lymphocytes within the live CD3+ T lymphocytes of a cortical and a white matter lesion (Fig. 5d). In general, the percentage of CD70+CD4+ T lymphocytes was higher in the lesions than in the peripheral blood of RRMS patients. Moreover, the expression seemed to be highest in preactive lesions (Fig. 5e, f). To localize CD70+CD4+ lymphocytes within MS lesions, we performed immunofluorescent staining/confocal microscopy on our collection of frozen MS brains. Using 3 different patients, we found numerous CD70+CD4+ lymphocytes in the perivascular infiltrates of active and preactive lesions (Fig. 5g), more so than in chronic inactive plaques. In addition, CD70-expressing dendritic cells were also found in MS lesions (Supplemental Fig. 5).
Overall, these data confirm the encephalitogenic potential of CD70+CD4+ T lymphocytes and suggest an implication of these lymphocytes in the formation of (pre)active lesions in MS.
CD70+CD4+ T lymphocytes contribute to increased disease severity in experimental autoimmune encephalomyelitis
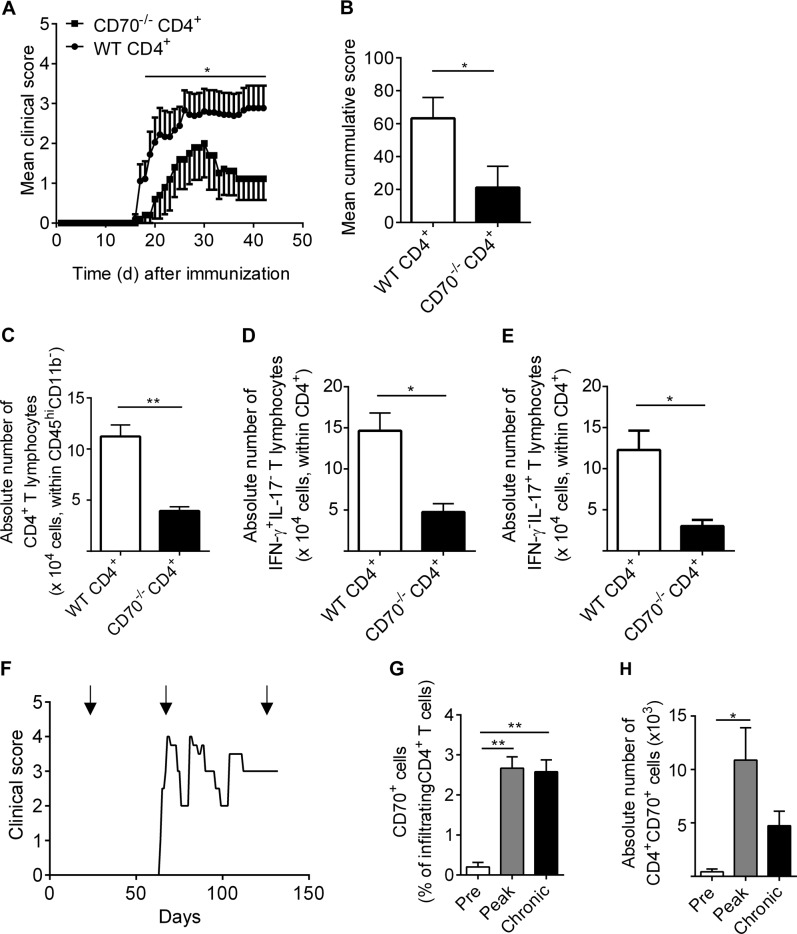
Previous reports have shown an involvement of the CD70/CD27 pathway in the development and severity of experimental autoimmune encephalomyelitis (EAE).7,8,11 However, it remains unclear whether these effects are intrinsic to T cells. Herein, we performed an adoptive T cell transfer EAE using CD4+ T lymphocytes isolated from either CD70−/− mice or from wild-type C57BL/6 mice into RAG2CGN−/− mice that were subsequently immunized with MOG35–55. We observed slightly later disease onset and a less severe clinical course in animals that received CD70−/−CD4+ T lymphocytes than in animals that were injected with WT CD4+ T lymphocytes (Fig. 6a, b). Furthermore, a significant reduction in the number of CNS-infiltrating CD4+ T lymphocytes was observed in RAG2CGN−/− mice that received CD70−/− CD4+ T lymphocytes (Fig. 6c). Moreover, the absolute numbers of both TH1 and TH17 lymphocytes infiltrating the CNS were significantly lower in animals injected with CD70−/−CD4+ T lymphocytes (Fig. 6d, e).
Fig. 6.
CD70+CD4+ T lymphocytes contribute to increased disease severity in experimental autoimmune encephalomyelitis. a Clinical course of EAE following CD4+ T cell adoptive transfer isolated from either CD70−/− mice or from bC57BL/6 WT mice into RAG2CGN−/− mice, immunized with MOG35–55 with n = 9 animals injected with WT CD4+ T cells and n = 5 animals injected with CD70−/−CD4+ T cells. b Cumulative mean clinical EAE score of RAG2CGN−/− mice injected with WT CD4+ T cells or CD70−/−CD4+ T cells. c–e Absolute number of CNS-infiltrating CD4+ T cells (c), IFN-γ+IL-17− TH1 (d), and IL-17+IFN-γ− TH17 (e) in RAG2CGN−/− mice injected with CD4+ T cells isolated from CD70−/− mice or from B57BL/6 WT mice. f Female TCR1640 transgenic mice, which developed spontaneous RR-EAE, were killed at presymptomatic (pre), acute (peak), and chronic disease phases, and immune cells were isolated from the CNS. g, h The percentage and absolute number of CD4+CD70+ lymphocytes were assessed at each time point (n≥3 animals per time point). Statistical significance was assessed with unpaired or paired t-tests when appropriate. Data are represented as paired data or as the mean ± SEM. *p<0.05, **p<0.01
Finally, to assess the relevance of CNS-infiltrating CD70+CD4+ T lymphocytes in presymptomatic, acute, and chronic neuroinflammatory disease, a TCR-transgenic mouse model for MS was used.36 The TCR1640 mouse, an animal that carries a TCR specific to MOG92–106, develops a TH1-driven spontaneous RR-EAE disease followed by a stable chronic decline (Fig. 6f, representative example). Similar to what we observed in the brains of MS patients, a significant increase in the proportion and number of CD70+CD4+ T lymphocytes was primarily found in the CNS of animals undergoing active disease (Fig. 6g, h).
Altogether, our data demonstrate that human CD70 regulates the production of IFN-γ by CD4+ T lymphocytes and identifies encephalitogenic CD4+ T lymphocytes.
Discussion
The expression of CD70 is mostly induced on professional antigen-presenting cells (APCs), such as B cells, macrophages, and dendritic cells, although CD4+ and CD8+ T lymphocytes can also express this CD27 ligand. CD27/CD70 pathway engagement is therefore fully dependent on the presence of CD70, indicating that overexpression of CD70 is an excellent marker for both acute and chronic immune activation.3 In contrast to previous studies, in which the interaction of CD70+ APC with CD27+ T lymphocytes was investigated,4,37 we focused on CD70 expression by CD4+ T lymphocytes specifically and found that CD70 expression on ex vivo CD4+ T lymphocytes is associated with a proinflammatory phenotype. We also found a notable increase in CD70+CD4+ T lymphocytes during chronic and even more so during acute autoimmune activation. Our results also point to a general dysregulation of this pathway in MS because activation of CD4+ T lymphocytes led to an overexpression of CD70 on the one hand and a decrease in effectiveness of CD70 shedding on the other hand, possibly inhibiting their ability to dampen the immune response.
An association between the CD27/CD70 pathway and TH1 differentiation has been reported in humans5,38 and mice4,37 or using mouse cell lines.39 Furthermore, Atarashi et al. found that CD70hi cells are essential in driving TH17 differentiation in the gut.6 In these reports, the focus was again on CD70 on APC aiding CD27+ T lymphocytes to differentiate. Intriguingly, our results show that in humans, the involvement of CD70+APC is not essential for the polarization of TH1 lymphocytes because (i) CD70 is highly upregulated on TH1 and TH17 polarized lymphocytes and because (ii) inhibiting CD70 on these lymphocytes leads to a downregulation of T-bet and a decrease in IFN-γ production. This confirms a central role for the CD27/CD70 pathway in IFN-γ production in humans, even in the absence of CD70+APC.
Overexpression of CD70 on human CD4+ T lymphocytes has been reported in patients affected by autoimmune diseases, such as SLE and RA.9,10 In addition, studies using the EAE model demonstrated that either anti-CD70 treatment7 or constitutive CD70 expression on B cells8 prevented or enhanced, respectively, the susceptibility to EAE, pointing to a proinflammatory role of CD70 in autoimmune sterile neuroinflammation. Our results support these findings because we demonstrated an increase in CD70+CD4+ T lymphocytes during active disease both in MS patients and in TCR1640 transgenic mice. However, another study using CD70−/− mice demonstrated an exacerbation of EAE, suggesting an anti-inflammatory role of CD70.11 This is supported by a recent report in which T cell-derived adoptive transfer was used to induce experimental colitis, where disease was more severe when transferring CD4+CD25−CD70−/− T cells.40 Because we show that CD70−/−CD4+ T lymphocytes induce a less severe disease course in adoptive transfer EAE, we propose that, as already proposed,13 downstream effects of CD27/CD70 axis activation are intrinsic to immune cells and dependent on both the experimental model and the site of inflammation, highlighting once again the complex role of the CD27/CD70 pathway in mice and humans. In addition, a potential role for CD70 on regulatory T cells in adoptive transfer EAE needs to be examined further.
TGF-β is a cytokine essential in both the differentiation and function of effector CD4+ T lymphocytes and Tregs.41 The combined results of a previous study26 and the present one demonstrate that the expression of CD70 on CD4+ T lymphocytes is directly related to the presence of TGF-β1. Yang et al. observed that TGF-β1 upregulated CD70, particularly on human effector memory CD4+ T lymphocytes, while simultaneously inducing the expression of Foxp3 on naive CD4+ T lymphocytes. In EAE, it has also been reported that TGF-β can have opposite effects on naive and effector CD4+ T lymphocytes.41,42 These results are in line with ours, demonstrating that CD70 upregulation by TGF-β1 was associated with a proinflammatory effector phenotype. Furthermore, the increase in the frequency of CD70+CD4+ T lymphocytes upon TGF-β stimulation was significantly higher in MS patients than in healthy controls. In the context of MS, effector memory and central memory CD4+ T lymphocytes were also the cells expressing CD70, suggesting that TGF-β1-mediated CD70 expression is enhanced during neuroinflammation. We postulate that this is in relation to the increased expression of various TGF-β-receptors on the surface of CD4+ T lymphocytes of MS patients, as already demonstrated for monocytes.43 In our hands, both TGF-β1 and TGF-β3 upregulate CD70, and a similar CD70-specific proinflammatory phenotype was observed, indicating that, as previously suggested, these two cytokines signal through the same receptor. However, the efficacy of TGF-β1+IL-6-stimulated CD4+ T lymphocytes to induce EAE and the relative contribution of TGF-β3, compared to TGF-β1, to induce a stable pathogenic TH17 phenotype remains debated.27,28,41 Notably, TNF-α is able to drive human CD14+ monocytes to differentiate into CD70+ dendritic cells, leading to TH1 and TH17 responses, but this was not observed for T lymphocytes.44 It would be interesting to analyze this induction in MS patients. Lastly, as TGF-β is present in the CNS45 and as we detected CD70+CD4+ T lymphocytes in the CNS of MS patients, investigating whether TGF-β can change the function and phenotype of effector CD4+ T lymphocytes infiltrating the CNS remains to be established.
Several ligands of the TNF family can be shed, either by proteolysis of the stalk region or by alternative splicing.46 The presence of native soluble CD70 and its function have been a subject of debate, although recombinant soluble variants have been found to interact with CD27.47,48 We observed naturally occurring soluble CD70 in the CSF of humans, especially during neuroinflammation, but not in the serum. Of interest, soluble CD27 was also found in the CSF of MS patients and was suggested as a biomarker for intrathecal T cell activation.49 Collectively, these data demonstrate that both soluble CD70 and soluble CD27 are present in the CSF of MS patients, although their functional role remains to be investigated.
In conclusion, we have demonstrated that CD70 is specific to and involved in the differentiation of proinflammatory pathogenic lymphocytes characterized by a TH1/TH17 phenotype with the ability to infiltrate the CNS. Because CD70 is a reliable marker of active disease, targeting CD70 may be particularly suited for the treatment of IFN-γ-dependent autoimmune disorders, such as MS. Furthermore, as other immune cells that undergo activation may also express this marker, depletion of these cells could lead to a general downscaling of an overactivated immune system.
Materials and methods
TCR1640 transgenic mouse model
The TCR1640 transgenic mice, kindly donated by the laboratory of Dr. Harmut Werkele,36 express a T cell receptor (TCR) that is specific to the MOG peptide 92-106 on an SJL/j background. Female mice were scored daily to assess spontaneous EAE development based on paralysis and/or ataxia as previously described.21 Mice were killed at presymptomatic phase (d35), at acute phase (score = 4) and at chronic phase (score ≥3 for more than 20 days). The animal protocol was approved by the Comité Institutionnel de Protection des Animaux du CHUM (N15035APs).
Adoptive T cell-transfer EAE
CD70−/− mice were kindly provided by the professional group of Dr. Jannie Borst (The Netherlands Cancer Institute).11 C57BL/6 mice and C57BL/6 RAG2CGN−/− mice were purchased from The Jackson Laboratory. All mice were maintained in specific pathogen-free conditions. The animal protocol was approved by the Comité Institutionnel de Protection des Animaux du CHUM (N15035APs). Adoptive CD4+ T cell transfer using either CD70−/− mice or C57BL/6 mice was performed as previously described.50,51 Briefly, CD4+ T cells were isolated from the spleen and lymph nodes of CD70−/− mice or WT C57BL/6 mice using MACS beads (Miltenyi,130-104-454) according to the manufacturer’s guidelines. A total of 4 × 106 CD4+ T cells were injected i.v. into RAG2CGN−/− mice, and EAE was induced 28 days after engraftment.
Healthy donors, MS patients, and patients with OND
Informed consent was signed by every donor prior to sample collection. Venous puncture and CSF samples were taken in accordance with institutional guidelines (Centre Hospitalier de l’Université de Montréal [CHUM] research ethic committee approval number SL05.22 and 023 and BH07.001). The patients included in these studies were never treated, or the sample was taken after a wash-out period of 2 months. Two certified neurologists classified patients with MS according to McDonald’s 2010 revised criteria52 as previously described.20 Relapse was defined as a new neurological deficit at acute/subacute onset lasting longer than 48 h within 3 weeks before sample collection. Other noninflammatory neurological diseases (OND) include focal refractory epilepsy, frontal epilepsy, and focal epilepsy. Brain samples from MS patients were obtained in accordance with institutional guidelines (CRCHUM research ethics committee approval number SL05.022, SL05.023 and BH07.001). The MS brain autopsy used for flow cytometry analysis of MS lesions was received 3 h after the time of death. The male RRMS patient was 26 years old, had an EDSS of 9 and underwent assisted euthanasia after failure of efficacy of various treatments (cyclophosphamide, Rebif, Copaxone, Tysabri, Gilenya, and a trial for cyclophosphamide).
Human primary brain-derived endothelial cell cultures
Informed consent and ethical approval (ethical approval number HD04.046) was acquired, and human temporal lobe material was obtained from patients undergoing surgical treatment for intractable temporal lobe epilepsy.
Human immune lymphocyte isolation, expansion, and culture
CSF was taken from subjects undergoing lumbar puncture for clinical indication. The type of disease was confirmed after, and only patients classified as RRMS were included. Immune lymphocytes were obtained by centrifugation and immediately used for flow cytometry analysis as previously published.20 Peripheral blood mononuclear cells were isolated from the peripheral blood using density gradient centrifugation on Ficoll-Paque (GE Healthcare) as previously described.15 Human CD4+ T lymphocytes were isolated using a positive selection kit or an enrichment kit according to the manufacturer’s protocol (Stemcell Technologies). When indicated, CD70+ (positive selection) and CD70− lymphocytes (negative selection) were selected using anti-PE microbeads and MACS isolation columns according to the manufacturer’s protocol (Miltenyi) and then analyzed by quantitative PCR. The purity of isolated immune cells was assessed by flow cytometry and the cells were used only if the purity was >90%. Nonspecific stimulation of CD4+ T lymphocytes was performed in vitro by culturing 0.5 million cells/ml with plate-bound anti-CD3 (2.5 µg/ml; eBioscience, San Diego, CA; clone OKT3) and soluble anti-CD28 (BD Biosciences, San Diego, CA; 2 µg/ml) in X-vivo 15 medium (Lonza, Walkersville, MD) without serum and supplemented with 2 mM L-glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin (Sigma, St. Louis, MO). For the cytokine stimulation assay, CD4+ T lymphocytes were cultured as described above with anti-CD3 and anti-CD28 in combination with specific cytokines for 5 days at 37 °C. The cytokines that were used are IL-1β (R&D Systems, 10 ng/ml), IL-6 (20 ng/ml), IL-23 (25 ng/ml), TGF-β (3 ng/ml), IL12 (20 ng/ml), all from R&D Systems, as well as TNF-α (Thermo Fisher, 10 U/ml) and IFN-γ (Thermo Fisher, 10 U/ml). To investigate the downstream effects of activating CD70 by binding with soluble CD27, CD4+ T lymphocytes were stimulated with anti-human CD3, anti-human CD28 and TGF-β1 (3 ng/ml) as previously described. Soluble CD27 (8 ng/ml, Abcam (ab114342)) or nothing was added to the wells. Lymphocytes were harvested for quantitative PCR analysis and for flow cytometry. For polarization of CD4+CD45RO+ T lymphocytes, lymphocytes were selected using MACS isolation columns according to the manufacturer’s protocol (negative selection, Miltenyi) and cultured as previously described.20 For TH1 differentiation, IL-12 (10 ng/ml) and anti-IL-4 (5 µg/ml) were added, while for TH17 differentiation, IL-23 (25 ng/ml), anti-IL-4 antibody (5 µg/ml), and IFN-γ antibody (5 µg/ml) were added. For TH2 differentiation, IL-4 (200 ng/ml), and anti-IFN-γ antibody and anti-IL-12 antibody (10 ng/ml) were added, and lymphocytes were incubated at 37 °C for 6 days. All antibodies used are from BioXcell and all cytokines from R&D systems. When indicated, TGF-β1 (R&D systems) or TGF-β3 (Miltenyi) was added to the lymphocytes (3 ng/ml) at the start of polarization. To assess the effect of CD70 and CD27 blocking during polarization, human anti-CD70 antibody (Abcam, ab77868, 5 µg/ml) or human anti-CD27 antibody (Abcam ab131254, 5 µg/ml) was added every day. MS lesions were cut out of the tissue, and ex vivo immune lymphocytes were isolated after homogenization, passage through filters and Percoll density centrifugation.
Human primary brain-derived endothelial cell cultures
Primary human brain-derived endothelial cells were isolated from nonepileptic CNS material as previously described.53 The purity and characterization of these blood–brain barrier endothelial cells was performed as previously published.15
Extracellular and intracellular flow cytometry staining
Flow cytometric analysis of ex vivo human lymphocytes
Immune cells were analyzed by flow cytometry as previously described.15 The antibodies used were CD3 BV605, CCR7 APC, CD138 AF700, CD49d BV605 (all from Biolegend), CD4 Percp-Cy5.5, CD8 APC-Cy7, CD27 PE, CD70 BV786, CD45RA FITC, CD19 v450, IgD PE-Cy7, CD3 AF700, CD4 PE-Cy7, CD8 BV711, CD27 APC-H7, CCR6 PE, CXCR3 BV421, CD161 FITC, CD11a FITC (all from BD Biosciences), and MCAM APC (from Miltenyi). For each staining, Live/Dead stain (from Thermo Fisher) was added, and the appropriate isotypes were used.
Flow cytometric analysis of stimulated human T lymphocytes
For intracellular staining of cytokines, purified T lymphocytes were restimulated for 4 h at 37 °C with 1 µg/ml ionomycin, 25 ng/ml PMA (both from Sigma) and GolgiPlug (1:1000 from BD Biosciences). Lymphocytes were stained for surface markers and then fixed and permeabilized for 20 min at 4 °C with a BD Cytofix/Cytoperm kit (BD Biosciences). Next, intracellular staining was performed as previously described.29 The antibodies used were CD3 BV605, CD70 PE (all from Biolegend), CD70 BV786, CD27 FITC, CD4 Pacific Blue, CD45RA FITC, CCR7 PE-Cy7, IFN-γ AF700, IL-4 PE, IL-4 PE-Cy7, IL-4 PE, GM-CSF PE, GM-CSF AF647 (all from BD Biosciences), IL-17 APC, and IL-17 eFluor 660 (all from Thermo Fisher). The results were acquired on a BD LSRII and analyzed using BD FACSDiva Software and FlowJo.
Flow cytometric analysis of blood–brain barrier endothelial cells
To investigate whether CD70 is expressed on human brain-derived endothelial cells (BBB-ECs), human brain-derived endothelial cells were grown to full confluency, either left in new culture media or culture media supplemented with IFN-γ and TNF-α (100 U/ml) for 24 h and isolated and characterized as previously described to.54 BBB-EC were stained with anti-human CD70 BV786 (BD Biosciences) or the appropriate isotype control and Live/Dead staining (Thermo Fisher).
Flow cytometric analysis of mouse lymphocytes
Perfused brains, spinal cords, spleen, and lymph nodes were harvested and processed as previously described.33 Immune cells were further processed for flow cytometry as previously described.33 The antibodies that were used for flow cytometry for TCR1640 mice were CD45 percp-cy5.5, CD11b BV785, CD3 BV605, CD4 PB, and CD8 AF488. The antibodies that were used for flow cytometry for RAG−/− mice were CD45 percp-cy5.5, CD11b BV785, CD3 FITC, CD4 PE-Cy7, CD8 AF488, IFN-γ APC, and IL-17 BV605. The antibodies were purchased from BD Biosciences or Biolegend, and Live/Dead staining (Thermo Fisher) was used each time.
Enzyme-linked immunosorbent assay (ELISA)
To quantify the levels of soluble CD70 in serum, in CSF, in supernatants of stimulated CD4+ T lymphocytes and in conditioned supernatants from human BBB-EC, samples were analyzed by ELISA. For this purpose, a human ELISA detection kit from LSBio (LS-F6520) was purchased and used following the manufacturer’s guidelines.
Western blot analysis
Confluent human brain-derived endothelial cells were treated for 24 h with a mix of TNF-α and IFN-γ (each at 100 U/ml Thermo Fisher) and compared to untreated human brain-derived endothelial lymphocytes (control). Human ex vivo CD4+ T lymphocytes and human TH1 and TH17 polarized lymphocytes were acquired as described above. All cells were frozen as pellets at −80 °C until use. For protein isolation, cells were lysed with RIPA buffer with 1X proteinase inhibitor (both from Thermo Fisher) and kept on ice for 30 min. After centrifugation, the supernatant containing the protein was quantified using the standard BCA detection kit (Pierce). SDS-PAGE (Bio-Rad) was performed by loading 10 µg of denatured protein per lane and blotted onto a PVDF membrane (Bio-Rad). After blocking with 5% milk in Tris-buffered saline–0.1% Tween (TBST), the anti-human CD70 antibody (Abcam, ab96323 1/1000) was applied overnight at 4 °C, followed by washes with TBST and by incubation with secondary goat anti-rabbit horseradish peroxidase HRP (Jackson Immuno Research Labs 111-035-144, 1/10000). Protein bands were developed with the ECL substrate (Amersham, ECLPlus detection kit). Protein bands were then measured by densitometry using Bio-Rad Gel Doc System and Quantity One software.
RNA isolation and real-time quantitative polymerase chain reaction
In vitro stimulated and polarized T lymphocytes were harvested at days 5 and 6, respectively. When indicated, selection for CD70 into a CD70+ and CD70− population was performed as described above (purity >90%). Total T lymphocytes or CD70 selected T lymphocytes were frozen as a dry pellet and kept at -80 °C until use. Human brain-derived endothelial lymphocytes were cultured to confluency and then treated for 24 hours in fresh endothelial cell medium, supplemented without any treatment or with TNF-α and IFN-γ (both at 100 U/ml), trypsinized, washed, and then lysed with TRIzol (Thermo Fisher). Quantitative PCR was performed as previously described.20 Briefly, total RNA was extracted using the RNeasy Mini kit (Qiagen) and transcribed into cDNA using a reverse transcription kit (Qiagen). The following primers were used to assess relative gene expression levels: CD70, T-bet, ROR-γt, GATA-3, Foxp3, IFN-γ, IL-17, granzyme B, IL-23 receptor, and GM-CSF (all TaqMan® FAMTM labeled; Life Technologies) and 18S ribosomal RNA (VIC®-labeled probe; Life Technologies) according to the manufacturer’s instructions. The CT method (CT = cycle threshold = cycle at which gene of interest is detected in a linear range; CT = difference between CT of the gene of interest and the CT of the internal gene control) was used to compare levels of mRNA. When indicated, the results are shown as a relative ratio (CD70+/CD70− and sCD27/no sCD27). An overview of primers that were used can be found in Supplementary Table 1.
Immunocytofluorescent staining
Human TH1, TH2, and TH17 polarized lymphocytes were used for immunocytofluorescent staining as previously described.11,53 The following primary antibodies were used: CD70 mouse anti-human (Abcam 1/100), IFN-γ goat anti-human (R&D systems, 1/100), IL-17 mouse anti-human (R&D systems, 1/30), and CD70 mouse anti-human FITC (Abcam, 1/100). Qualitative microscopic evaluation was performed using a LEICA SP5 confocal microscope.
Immunostaining of human CNS
Tissue
Frozen brain tissue from 3 MS patients (3 blocks per patient) was studied and sectioned into 8-μm-thick slices with a Leica CM3050S cryostat (Leica Microsystems; Wetzlar, Germany). Lesion identification was performed by Luxol Fast Blue and hematoxylin–eosin (LHE) staining.
Luxol fast blue and hematoxylin and eosin staining
To define the type of lesions (preactive, active, and chronic inactive) and perivascular infiltration combined with demyelination, Luxol Fast Blue-hematoxylin–eosin (LHE) staining was performed as previously described.55 Briefly, the sections were fixed, washed, incubated with Luxol Fast Blue at 60 °C and differentiated with hydroquinone and sodium sulfite solution in water. Next, slides were incubated with Harris hematoxylin for 10 min and with eosin Y for 1 min. Leica DM6000 microscope and Improvision OpenLab 4.0.4 Software (Weltham, MA) was used for imaging.
Immunofluorescent staining
Human sections were immunostained as previously described.20,33 Primary antibodies against CD4 rabbit anti-human (Abcam, 1/30) and CD70 mouse anti-human (Abcam, 1/40) in 3% donkey serum) were incubated overnight at 4 °C, and secondary antibodies (donkey anti-rabbit Alexa Fluor 488 and donkey anti-mouse Cy3 in 1x PBS, both 1/400 and from Jackson laboratories) were incubated at room temperature for 30–40 min. Finally, the sections were washed and mounted in Gelvatol containing TOPRO-3 (1:300; Thermo Fisher; Carlsbad, CA). Sections were imaged using a Leica SP5 confocal microscope and the Leica LAS AF software.
Blood–brain barrier transmigration assay
Transmigration assays using a modified Boyden chamber were performed as previously described.20,29 To investigate the migration of TH1 and TH17 lymphocytes, CD4+ T memory lymphocytes were polarized for 6 days as described above before migration for 18 hours. When indicated, an antibody specific for CD70 (Abcam, ab77868 40 µg/ml, or an appropriate isotype control (mouse IgG1, 40 µg/ml) was incubated with lymphocytes 1 hour before immune cell migration. After migration, T lymphocytes were collected from the upper and lower chambers, counted to assess the absolute number of migrations and further analyzed using intracellular flow cytometry. All conditions were performed in triplicate for each donor.
Statistical analysis
Data are presented as the mean ± SEM unless otherwise indicated in the figure legends. Sample number (n) indicates the number of independent biological samples in each experiment. Sample numbers are indicated in the figures and figure legends or in the methods section above. Data are considered to be statistically significant when p<0.05 by an unpaired Student’s t-test, a paired Student’s t-test or a one-way ANOVA as indicated in the figure legend. In the figures, asterisks denote statistical significance (*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001) or ns, nonsignificant. Statistical analysis was performed with the software GraphPad Prism 6.0.
Supplementary information
Acknowledgements
T.D. holds a fellowship from the Fonds de Recherche du Québec-Santé (FRQS). L.T. holds scholarships from Université de Montréal and CRCHUM. E.P. holds a fellowship from the Multiple Sclerosis Society of Canada (MSSC) and the FRQS. S.Z. is supported by a fellowship from Biogen Canada. C.L. is supported by FRQS. A.P. holds the T1 (senior) Canada Research Chair in Multiple Sclerosis. This work was funded by operating grants from the Canadian Institutes of Health Research (MOP 89885, PJI-153195) and from the MSSC (EGID 2382). We thank Jannie Borst for providing us with the CD70−/− mice. We thank Hartmut Wekerle for providing us with the TCR1640 mice. Special thanks to Magdalena Paterka and Volker Siffrin for providing the protocol for CD4+ adoptive T cell transfer in RAG null mice. We would also like to thank the imaging platform, the pathology platform, and the flow cytometry platform from the CRCHUM for the excellent technical support and Alice M Roy and Elvia Gonzalez for their excellent technical animal support.
Author contributions
T.D. and A.P. conceived, designed, and supervised the research, analyzed the data and wrote the paper. T.D., L.T., L.B., S.Z., E.P., C.L. and C.G. performed the experiments. X.A. and R.M.R. provided clinical information. J.P., B.L., P.D., M.G., R.M., A.P., C.L., and A.B. secured human blood, CSF, and brain samples. C.L., E.P., and S.Z. provided key scientific input.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
The online version of this article (10.1038/s41423-018-0198-5) contains supplementary material.
References
- 1.Hintzen RQ, et al. CD70 represents the human ligand for CD27. Int. Immunol. 1994;6:477–480. doi: 10.1093/intimm/6.3.477. [DOI] [PubMed] [Google Scholar]
- 2.Hintzen RQ, et al. Engagement of CD27 with its ligand CD70 provides a second signal for T cell activation. J. Immunol. 1995;154:2612–2623. [PubMed] [Google Scholar]
- 3.Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat. Rev. Immunol. 2009;9:271–285. doi: 10.1038/nri2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soares H, et al. A subset of dendritic cells induces CD4+ T cells to produce IFN-gamma by an IL-12-independent but CD70-dependent mechanism in vivo. J. Exp. Med. 2007;204:1095–1106. doi: 10.1084/jem.20070176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Oosterwijk MF, et al. CD27-CD70 interactions sensitise naive CD4+ T cells for IL-12-induced Th1 cell development. Int. Immunol. 2007;19:713–718. doi: 10.1093/intimm/dxm033. [DOI] [PubMed] [Google Scholar]
- 6.Atarashi K, et al. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–812. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- 7.Nakajima A, et al. Involvement of CD70-CD27 interactions in the induction of experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2000;109:188–196. doi: 10.1016/S0165-5728(00)00324-6. [DOI] [PubMed] [Google Scholar]
- 8.Francosalinas G, et al. Enhanced costimulation by CD70+ B cells aggravates experimental autoimmune encephalomyelitis in autoimmune mice. J. Neuroimmunol. 2013;255:8–17. doi: 10.1016/j.jneuroim.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 9.Han BK, et al. Increased prevalence of activated CD70+CD4+ T cells in the periphery of patients with systemic lupus erythematosus. Lupus. 2005;14:598–606. doi: 10.1191/0961203305lu2171oa. [DOI] [PubMed] [Google Scholar]
- 10.Park JK, et al. CD70-expressing CD4 T cells produce IFN-gamma and IL-17 in rheumatoid arthritis. Rheumatology. 2014;53:1896–1900. doi: 10.1093/rheumatology/keu171. [DOI] [PubMed] [Google Scholar]
- 11.Coquet JM, et al. The CD27 and CD70 costimulatory pathway inhibits effector function of T helper 17 cells and attenuates associated autoimmunity. Immunity. 2013;38:53–65. doi: 10.1016/j.immuni.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Libregts S, van Olffen RW, van der Sluijs KF, van Lier RA, Nolte MA. Function of CD27 in helper T cell differentiation. Immunol. Lett. 2011;136:177–186. doi: 10.1016/j.imlet.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Dong C. The CD70-CD27 axis, a new brake in the T helper 17 cell response. Immunity. 2013;38:1–3. doi: 10.1016/j.immuni.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Codarri L, et al. RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat. Immunol. 2011;12:560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- 15.Ifergan I, et al. The blood-brain barrier induces differentiation of migrating monocytes into Th17-polarizing dendritic cells. Brain. 2008;131:785–799. doi: 10.1093/brain/awm295. [DOI] [PubMed] [Google Scholar]
- 16.Cao Y, et al. Functional inflammatory profiles distinguish myelin-reactive T cells from patients with multiple sclerosis. Sci. Transl. Med. 2015;7:287ra74. doi: 10.1126/scitranslmed.aaa8038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peters A, et al. Th17 cells induce ectopic lymphoid follicles in central nervous system tissue inflammation. Immunity. 2011;35:986–996. doi: 10.1016/j.immuni.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lai WCY, Zhou J, Chen S, Qin C, Yang C. Deficiency of the G protein Gαq ameliorates experimental autoimmune encephalomyelitis with impaired DC-derived IL-6 production and Th17 differentiation. Cell. Mol. Immunol. 2017;14:557–567. doi: 10.1038/cmi.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alvarez JI, Cayrol R, Prat A. Disruption of central nervous system barriers in multiple sclerosis. Biochim. Biophys. Acta. 2011;1812:252–264. doi: 10.1016/j.bbadis.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 20.Larochelle C, et al. Melanoma cell adhesion molecule identifies encephalitogenic T lymphocytes and promotes their recruitment to the central nervous system. Brain. 2012;135:2906–2924. doi: 10.1093/brain/aws212. [DOI] [PubMed] [Google Scholar]
- 21.Larochelle C, et al. Melanoma cell adhesion molecule-positive CD8 T lymphocytes mediate central nervous system inflammation. Ann. Neurol. 2015;78:39–53. doi: 10.1002/ana.24415. [DOI] [PubMed] [Google Scholar]
- 22.Schlager C, et al. Effector T-cell trafficking between the leptomeninges and the cerebrospinal fluid. Nature. 2016;530:349–353. doi: 10.1038/nature16939. [DOI] [PubMed] [Google Scholar]
- 23.Acosta-Rodriguez EV, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat. Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 24.Wacleche VS, et al. New insights into the heterogeneity of Th17 subsets contributing to HIV-1 persistence during antiretroviral therapy. Retrovirology. 2016;13:59. doi: 10.1186/s12977-016-0293-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ifergan I, et al. Central nervous system recruitment of effector memory CD8+ T lymphocytes during neuroinflammation is dependent on alpha4 integrin. Brain. 2011;134:3560–3577. doi: 10.1093/brain/awr268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang ZZ, et al. TGF-beta upregulates CD70 expression and induces exhaustion of effector memory T cells in B-cell non-Hodgkin's lymphoma. Leukemia. 2014;28:1872–1884. doi: 10.1038/leu.2014.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee PW, Yang Y, Racke MK, Lovett-Racke AE. Analysis of TGF-beta1 and TGF-beta3 as regulators of encephalitogenic Th17 cells: implications for multiple sclerosis. Brain Behav. Immun. 2015;46:44–49. doi: 10.1016/j.bbi.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee Y, et al. Induction and molecular signature of pathogenic TH17 cells. Nat. Immunol. 2012;13:991–999. doi: 10.1038/ni.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kebir H, et al. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat. Med. 2007;13:1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang L, et al. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454:350–352. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korn T, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kebir H, et al. Preferential recruitment of interferon-gamma-expressing TH17 cells in multiple sclerosis. Ann. Neurol. 2009;66:390–402. doi: 10.1002/ana.21748. [DOI] [PubMed] [Google Scholar]
- 34.Tzartos JS, et al. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am. J. Pathol. 2008;172:146–155. doi: 10.2353/ajpath.2008.070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuhlmann T, et al. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol. 2017;133:13–24. doi: 10.1007/s00401-016-1653-y. [DOI] [PubMed] [Google Scholar]
- 36.Pollinger B, et al. Spontaneous relapsing-remitting EAE in the SJL/J mouse: MOG-reactive transgenic T cells recruit endogenous MOG-specific B cells. J. Exp. Med. 2009;206:1303–1316. doi: 10.1084/jem.20090299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dhainaut M, et al. Thymus-derived regulatory T cells restrain pro-inflammatory Th1 responses by downregulating CD70 on dendritic cells. EMBO J. 2015;34:1336–1348. doi: 10.15252/embj.201490312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pen JJ, et al. Modulation of regulatory T cell function by monocyte-derived dendritic cells matured through electroporation with mRNA encoding CD40 ligand, constitutively active TLR4, and CD70. J. Immunol. 2013;191:1976–1983. doi: 10.4049/jimmunol.1201008. [DOI] [PubMed] [Google Scholar]
- 39.Kawamura T, et al. CD70 is selectively expressed on Th1 but not on Th2 cells and is required for Th1-type immune responses. J. Invest. Dermatol. 2011;131:1252–1261. doi: 10.1038/jid.2011.36. [DOI] [PubMed] [Google Scholar]
- 40.O'Neill RE, et al. T cell-derived CD70 delivers an immune checkpoint function in inflammatory T cell responses. J. Immunol. 2017;199:3700–3710. doi: 10.4049/jimmunol.1700380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee PW, Severin ME, Lovett-Racke AE. TGF-beta regulation of encephalitogenic and regulatory T cells in multiple sclerosis. Eur. J. Immunol. 2017;47:446–453. doi: 10.1002/eji.201646716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huss DJ, et al. TGF-beta enhances effector Th1 cell activation but promotes self-regulation via IL-10. J. Immunol. 2010;184:5628–5636. doi: 10.4049/jimmunol.1000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Healy LM, et al. MerTK-mediated regulation of myelin phagocytosis by macrophages generated from patients with MS. Neurol. Neuroimmunol. Neuroinflamm. 2017;4:e402. doi: 10.1212/NXI.0000000000000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iwamoto S, et al. TNF-alpha drives human CD14+ monocytes to differentiate into CD70+ dendritic cells evoking Th1 and Th17 responses. J. Immunol. 2007;179:1449–1457. doi: 10.4049/jimmunol.179.3.1449. [DOI] [PubMed] [Google Scholar]
- 45.Pratt BM, McPherson JM. TGF-beta in the central nervous system: potential roles in ischemic injury and neurodegenerative diseases. Cytokine Growth Factor Rev. 1997;8:267–292. doi: 10.1016/S1359-6101(97)00018-X. [DOI] [PubMed] [Google Scholar]
- 46.Wajant H. Therapeutic targeting of CD70 and CD27. Expert Opin. Ther. Targets. 2016;20:959–973. doi: 10.1517/14728222.2016.1158812. [DOI] [PubMed] [Google Scholar]
- 47.Miller J, et al. Soluble CD70: a novel immunotherapeutic agent for experimental glioblastoma. J. Neurosurg. 2010;113:280–285. doi: 10.3171/2009.11.JNS09901. [DOI] [PubMed] [Google Scholar]
- 48.Wyzgol A, et al. Trimer stabilization, oligomerization, and antibody-mediated cell surface immobilization improve the activity of soluble trimers of CD27L, CD40L, 41BBL, and glucocorticoid-induced TNF receptor ligand. J. Immunol. 2009;183:1851–1861. doi: 10.4049/jimmunol.0802597. [DOI] [PubMed] [Google Scholar]
- 49.Komori M, et al. Cerebrospinal fluid markers reveal intrathecal inflammation in progressive multiple sclerosis. Ann. Neurol. 2015;78:3–20. doi: 10.1002/ana.24408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schulze-Topphoff U, et al. Activation of kinin receptor B1 limits encephalitogenic T lymphocyte recruitment to the central nervous system. Nat. Med. 2009;15:788–793. doi: 10.1038/nm.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Herz J, et al. In vivo imaging of lymphocytes in the CNS reveals different behaviour of naive T cells in health and autoimmunity. J. Neuroinflamm. 2011;8:131. doi: 10.1186/1742-2094-8-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Polman CH, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alvarez JI, et al. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science. 2011;334:1727–1731. doi: 10.1126/science.1206936. [DOI] [PubMed] [Google Scholar]
- 54.Cayrol R, et al. Activated leukocyte cell adhesion molecule promotes leukocyte trafficking into the central nervous system. Nat. Immunol. 2008;9:137–145. doi: 10.1038/ni1551. [DOI] [PubMed] [Google Scholar]
- 55.Alvarez JI, et al. Focal disturbances in the blood-brain barrier are associated with formation of neuroinflammatory lesions. Neurobiol. Dis. 2015;74:14–24. doi: 10.1016/j.nbd.2014.09.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.