Abstract
Background
Hydrogen therapy has been reported to convert exhausted programmed cell death receptor (PD-1)+CD8+ T cells to PD-1-CD8+ T cells, in advanced colorectal cancer patients, which is associated with significantly prolonged survival.
Case presentation
A 72-year-old female patient presented with metastatic gallbladder cancer and underwent symptomatic treatment combined with hydrogen therapy. The tumors were initially enlarged and displayed increased tumor marker expression following hydrogen inhalation therapy, after which they continued to remit, similar to the pseudo-progression that occurs after anti-PD-1 treatment. During one month of hydrogen therapy, the patient’s gallbladder and liver tumors continued to progress, and intestinal obstruction occurred. The intestinal obstruction was gradually relieved after symptomatic treatment, and the metastases in the abdominal cavity gradually decreased in size, anemia and hypoalbuminemia were corrected, and both the lymphocyte and tumor marker levels returned to normal. The patient was able to resume normal life two and a half months after hydrogen inhalation and survived over 10 months.
Conclusion
This is the first report of pseudo-progression followed by sustained remission after hydrogen inhalation. This phenomenon is similar to the pseudo-progression-remission pattern that occurs following PD-1 antibody treatment. These findings suggest that hydrogen may have an inhibitory effect on PD-1 expression.
Keywords: hydrogen gas, metastatic gallbladder cancer, pseudo-progression, programmed cell death receptor-1
Introduction
Hydrogen gas is a type of “physiological gas” that is continuously produced by human intestinal bacteria,1,2 and has been confirmed to have selective antioxidant and anti-inflammatory effects.3 Several studies have shown that hydrogen can inhibit the formation, migration, and invasion of cancer cells through its anti-oxidative effects.4–6 Due to technical challenges, the ability of hydrogen to directly kill cancer cells in vivo or lead to tumor shrinkage has been difficult to confirm. In 2019, Akagi et al first reported a long-term follow-up study involving 55 participants and found that continued hydrogen inhalation reversed the exhausted status of PD-1+CD8+ T cells to a CD8+PD-1- phenotype, and could extend the survival time from 18 to 46 months.7 To study the effect of hydrogen therapy on ovarian cancer, we report a case for whom the disease first worsened and then continued to improve along with changes in CD8+ T cell immune functionality.
Case Presentation
A 72-year-old female patient developed a gallbladder tumor in December 2017 due to pain in the right upper quadrant. The biopsy showed poorly differentiated adenocarcinoma. She was diagnosed as stage IIIA gallbladder carcinoma (T3N1M0) bacause of lymph node metastases at hepatic hilar and pancreatic head by positron emission tomography (PET) scan. Since the patient had been previously diagnosed with rheumatic heart disease and diabetes, irreversible electroporation ablation was performed. The patient’s abdominal pain was relieved after ablation and she then received oral tegafur (a fluoropyrimidine derivative) chemotherapy. In September 2018, the patient developed severe pain in her upper right abdomen, with vomiting associated with the inability to eat, and a subsequent rapid reduction in body weight. After being admitted to our hospital, the patient was diagnosed with heart failure (class IV heart function), severe anemia, and hypoproteinemia. A computed tomography (CT) examination revealed gallbladder tumors (6.3 × 4.9 cm), multiple spotted high-density lesions in the liver parenchyma, tumor invasion of the duodenum, accompanied by gallbladder-duodenal descending fistula, multiple enlarged lymph nodes around the pancreatic head (maximum 2.7 × 2.1 cm), and compression of the inferior vena cava. Moreover, the gallbladder cancer had progressed to stage IV (T4N1aM0). On the second day after admission, the patient entered the Intensive Care Unit and received both symptomatic and systemic supportive treatments, including parenteral nutrition, an infusion of albumin, red blood cells, insulin, cardiotonic diuretics, and antibiotics, as well as gastrointestinal catheter drainage.
This patient was enrolled in a hydrogen therapy clinical trial (NCT03818347) on October 24, 2018. The inhalation of hydrogen gas via a hydrogen oxygen atomizer (AMS-H-03, Asclepius Meditec, Shanghai, China) was initiated simultaneously with symptomatic treatments. Initially, the patient underwent hydrogen inhalation for 2 h/day (3 L/min), and was gradually increased to 6 h/day. In addition to continuing to administer the above symptomatic and systemic supportive treatments, no routine anti-cancer therapy was provided. Post-treatment evaluations included: 1) adverse reactions: referred to in the Common Terminology Standard Version 5.0 (CTCAE 5.0). Adverse events were classified and scored weekly after treatment initiation. Laboratory indicators included peripheral blood cell subsets, serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TB), and gamma-glutamyl transpeptidase (GGT), and expression of tumor markers; 2) clinical symptoms and PS scores; 3) imaging changes: the tumor response was measured using computed tomography (CT) according to the RECIST 1.1 guidelines, and the tumor response was classified as complete remission (CR), partial remission (PR), stable disease (SD), or progressive disease (PD).
Following hydrogen inhalation, the patient’s daily agitation gradually decreased and disappeared completely after two weeks. In addition, the patient’s sleep time was extended from 4–5 h/day to 8–10 h/day within two weeks. No adverse reactions related to hydrogen inhalation were observed during the first three months. The patient’s upper abdominal pain persisted, requiring the daily use of analgesics prior to hydrogen treatment; however, after two weeks of hydrogen inhalation, the pain gradually decreased and painkillers were no longer required. The PS score gradually decreased from 4 points prior to hydrogen treatment to 1 point after two and a half months. By the end of April 2019, the patient had a PS score of 0 and had resumed normal diet and activity. The disease has been continuously relieved for 10 months and has exhibited increased improvement.
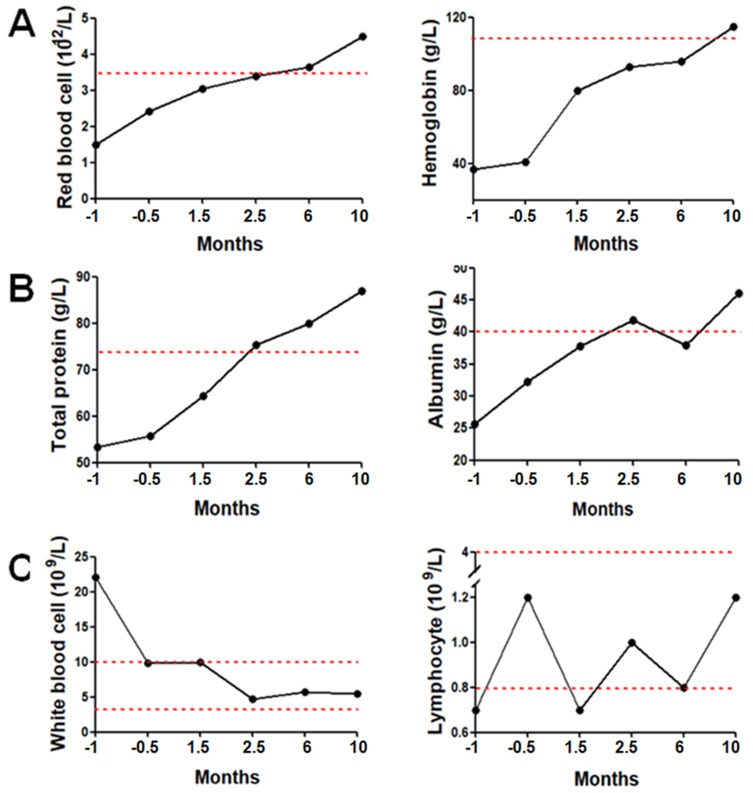
During the entire period of hydrogen treatment, serum levels of ALT, AST, TB, and GGT exhibited no abnormalities; the red blood cell count and hemoglobin levels stopped decreasing; and the interval between blood transfusions was extended from one week before hydrogen inhalation to two weeks. Moreover, these two indexes began to increase continuously one month later (Figure 1A), and the blood transfusion was completely stopped after two months. The level of serum total protein and albumin began to rise after supportive treatments and continued to increase after hydrogen inhalation, reaching a normal range after two and a half months, at which time, supplements were no longer required (Figure 1B). In the two weeks before hydrogen treatment, the peripheral blood leukocyte count was significantly increased due to the intestinal fistula. The leukocyte count decreased to the upper limit of the reference range after antibiotic treatment and continued to decrease after hydrogen inhalation, falling to normal levels after 1.5 months (Figure 1C left). The total number of lymphocytes was low at the time of admission, and the number began to rebound after one and a half months of hydrogen inhalation, returning to normal after two and a half months (Figure 1C right).
Figure 1.
Blood parameters before and after hydrogen treatment. (A) Red blood cell counts and hemoglobin levels; the red line in the represents the lower limit of the reference range. (B) Total protein and albumin concentrations; the red lines in the figures represent the lower limit of the reference range. (C) White blood cell and lymphocyte counts; the red lines in the figures represent the reference range.
Serum CA19-9, AFP, and CEA were all elevated at the time of admission (2, 1.8, and 8 times the upper limit of the reference values, respectively). Following hydrogen inhalation, the three markers did not decline and the AFP even exceeded the level at the time of admission; however, these levels rapidly declined afterwards, and all fell to within the normal reference range after two and a half months (Figure 2).
Figure 2.
Changes in the tumor markers before and after hydrogen treatment. The red lines in the figures represent the upper limit of the reference ranges.
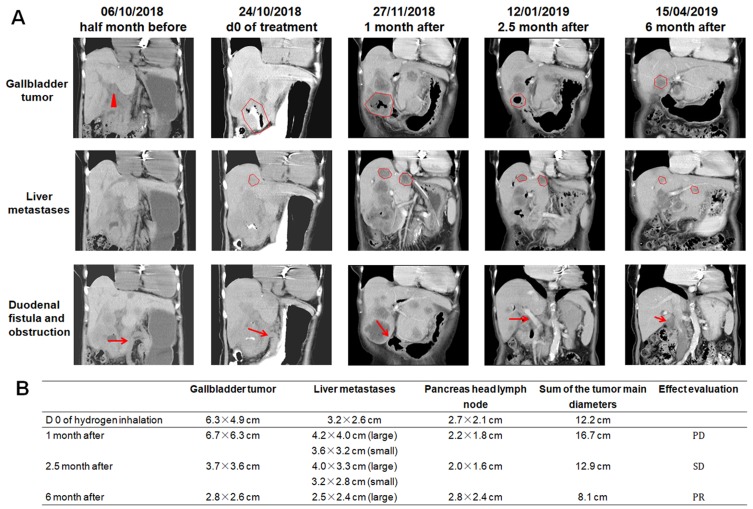
Abdominal CT images were analyzed at different time points before and after hydrogen treatment (Figure 3A). Before hydrogen treatment, the sum of the diameter of multiple tumors was 12.2 cm. After hydrogen treatment for: 1) one month, the sum was 16.7 cm (37% increase compared with pretreatment, PD); 2) two and a half months, the sum was 12.9 cm (6% increase compared with pretreatment, SD); and 3) six months, the total was 8.1 cm (33% decrease compared with pretreatment, PR) (Figure 3B).
Figure 3.
(A) CT imaging findings at different time-points before and after hydrogen treatment. In the first line of figures, the triangle and the contour lines represent the location and range of the gallbladder. In the second line of figures, the contour lines represent the location and range of the liver metastases. In the third line of figures, the arrows represent the location of the duodenal fistula or obstruction. (B) Evaluation of the effect of hydrogen treatment at different time-points according to CT imaging and RECIST 1.1.
Abbreviations: PD, progressed disease; SD, stable disease; PR, partial remission; CT, computed tomography.
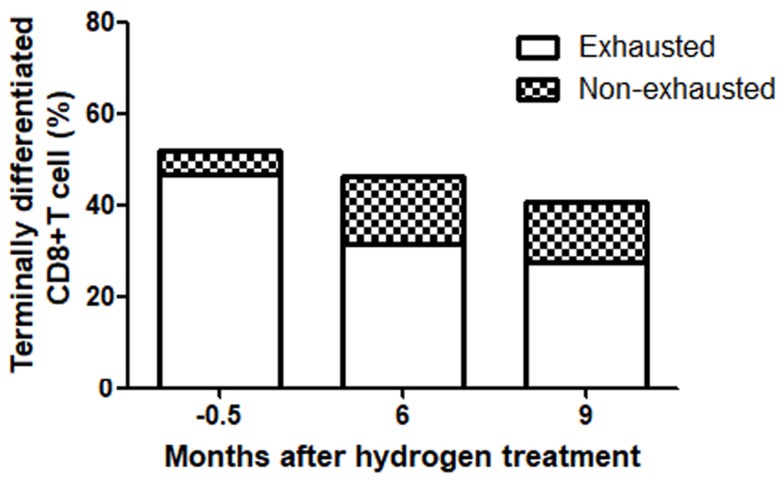
The patient was tested for immunological function before hydrogen treatment, as well as at six and nine months after treatment. In the terminally differentiated CD8+ T cells, the proportion of exhausted cells was 46.8% at two weeks before treatment, 31.3% at six months after treatment, and 27.6% after nine months (Figure 4).
Figure 4.
In terminally differentiated CD8+ T cells, the proportion of exhausted cells varied with the treatment time. The results were tested by flow cytometry, in which the terminally differentiated CD8+ T cells were labeled CD3+CD8+CD27-, in which PD-1+, was considered a marker of exhaustion and non-exhausted cells were PD-1.
Discussion
The treatment for gallbladder cancer is difficult, primarily due to the development of early intrahepatic and hepatic metastases.8 There is no evidence that minimally invasive ablation and chemotherapy can be used to obtain survival gains.9 In this patient, intrahepatic and hepatic portal metastases were present at the time of diagnosis. Although irreversible electroporation ablation and chemotherapy were performed, the lesion progressed further after nine months, invading the duodenum and appearing around the head of the pancreas. Multiple lymph node metastases involving the inferior vena cava was indicative of metastatic cancer (T4N1aM0). In addition to the patient’s original rheumatic heart disease with mitral valve replacement, the patient was 72 years old with diabetes (fasting blood glucose up to 22 mmol/L) and severe anemia (hemoglobin only 37 g/L), making it impossible for her to receive routine anti-tumor treatments.
The patient’s disease status changed following hydrogen inhalation. One month after hydrogen therapy, the patient’s general condition and blood parameters (including total protein, albumin, red blood cell count, and hemoglobin levels) gradually improved. After two and a half months of therapy, her duodenal obstruction was relieved and subsequently disappeared, the gastric tube was removed, the level of tumor marker expression began to decline, the CT examination showed that the tumors in the gallbladder and liver had gradually reduced, and the PS score was significantly improved. The patient has undergone hydrogen treatment for 10 months to date, and her condition has further improved. However, in this case, although the symptoms and systemic conditions improved within one month of hydrogen inhalation, the tumor size and tumor marker expression increased and subsequently decreased following continued treatment. Clearly, the initial changes observed regarding the tumors and associated markers are false indicators of progression. Moreover, these findings are highly similar to the pseudo-remission-response patterns that occur during immunotherapy with PD-1 inhibitors.10,11
Pseudo-progression refers to an increase in the original lesions at the beginning of treatment or the emergence of new lesions, which is reduced following continued treatment. This response appears to be “exclusive” to anti-PD-1/PD-L1 immunotherapy, first observed in melanoma patients,12,13 and has subsequently been reported in other cancers.14,15 The mechanism for this phenomenon may be that during immunotherapy, immunocytes (i.e., cytotoxic T cells) infiltrate the tumor, causing edema and necrosis.16
PD-1 is a typical marker of senescent apoptotic CD8+ T cells.17,18 Recent studies have shown that hydrogen can inhibit the expression of PD-1 on T cells in vivo. Moreover, Akagi et al.7 reported that after the hydrogen treatment of 55 patients with stage IV colorectal cancer, PD-1+CD8+ T cells decreased and PD-1−CD8+ T cells increased in the peripheral blood. As the hydrogen inhalation time increases, the ratio of the two cells is further reduced. Additionally, the greater the reduction in PD-1+CD8+ T cells, the longer the progression-free survival and overall survival of the patients. Indeed, this study showed that hydrogen therapy restored exhausted CD8+ T cell activity by inhibiting PD-1 expression. We observed a similar phenomenon in this case, which supports the evidence that hydrogen therapy may exhibit an effect similar to the pseudo-progression phenomenon observed following anti-PD-1 treatment.
It has been found that an increased number of CD8+ T cells expressing PD-1 in the peripheral blood of cancer patients represents a serious prognostic marker.19 In addition, PD-1+CD8+ T cells are functionally exhausted and have lost their anti-tumor functionality.20,21 Further studies have found that mitochondrial dysfunction results in an insufficient energy supply to such T cells, which leads to functional impairment.22 Hydrogen can stimulate peroxisome proliferator-activated receptor gamma cofactor 1 alpha (PGC1α),23,24 which positively regulates several metabolic processes, including mitochondrial biosynthesis, respiration, adaptive caloric production, and gluconeogenesis,25 thereby enhancing and improving mitochondrial activity and exerting immunomodulatory effects.
Conclusion
Here, we reported a case of progressive gallbladder cancer that received only hydrogen inhalation therapy in addition to symptomatic treatment. Pseudo-progression and subsequent remission after hydrogen inhalation exhibited a similar response pattern following anti-PD-1 immunotherapy. We found a significant decrease in the proportion of exhausted CD8+PD-1+ T cells by immunological function tests before and after hydrogen inhalation, which suggests that hydrogen can revitalize senescent apoptotic CD8+ T cells through mitochondrial rescue. However, the process of tumor killing and shrinking may induce pseudo-progression. The potential clinical use of this therapy still needs validation with additional large-scale trials.
Acknowledgments
We would like to thank the doctors and nurses in the three wards of Fuda Cancer Hospital for their careful observation and care of this patient. Thanks to Shanghai Elixigen Bioengineering Co., Ltd. for the retouching and modification of this article.
Ethics Approval And Consent For Publication
This research was approved by the research ethics committee of Fuda Cancer Hospital of Jinan University, and written informed consent for publication of the clinical details and images was obtained from the patient.
Author Contributions
All listed authors took part in the design of the study, performed the experiment and acquired the data, performed the data analysis and interpretation, drafted and revised the article, approved the version to be published, and agreed to be accountable for all aspects of this work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Levitt MD. Production and excretion of hydrogen gas in man. N Engl J Med. 1969;281(3):122–127. doi: 10.1056/NEJM196907172810303 [DOI] [PubMed] [Google Scholar]
- 2.Levitt MD. Intestinal gas production–recent advances in flatology. N Engl J Med. 1980;302(26):1474–1475. doi: 10.1056/NEJM198006263022610 [DOI] [PubMed] [Google Scholar]
- 3.Ohsawa I, Ishikawa M, Takahashi K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13(6):688–694. doi: 10.1038/nm1577 [DOI] [PubMed] [Google Scholar]
- 4.Yang Y, Zhu Y, Xi X. Anti-inflammatory and antitumor action of hydrogen via reactive oxygen species. Oncol Lett. 2018;16(3):2771–2776. doi: 10.3892/ol.2018.9023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D, Wang L, Zhang Y, Zhao Y, Chen G. Hydrogen gas inhibits lung cancer progression through targeting SMC3. Biomed Pharmacother. 2018;104:788–797. doi: 10.1016/j.biopha.2018.05.055 [DOI] [PubMed] [Google Scholar]
- 6.Liu MY, Xie F, Zhang Y, et al. Molecular hydrogen suppresses glioblastoma growth via inducing the glioma stem-like cell differentiation. Stem Cell Res Ther. 2019;10(1):145. doi: 10.1186/s13287-019-1241-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akagi J, Baba H. Hydrogen gas restores exhausted CD8+ T cells in patients with advanced colorectal cancer to improve prognosis. Oncol Rep. 2019;41(1):301–311. doi: 10.3892/or.2018.6841 [DOI] [PubMed] [Google Scholar]
- 8.Rakic M, Patrlj L, Kopljar M, et al. Gallbladder cancer. Hepatobiliary Surg Nutr. 2014;3(5):221–226. doi: 10.3978/j.issn.2304-3881.2014.09.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shukla SK, Singh G, Shahi KS, Pant P. Staging, treatment, and future approaches of gallbladder carcinoma. J Gastrointest Cancer. 2018;49(1):9–15. doi: 10.1007/s12029-017-0036-5 [DOI] [PubMed] [Google Scholar]
- 10.Kao C, McNamara M, Alley C, et al. A complete response after pseudo-progression: pembrolizumab for metastatic Squamous Cell Carcinoma (SCC) of the bladder. Clin Genitourin Cancer. 2019;17(3):e672–e677. doi: 10.1016/j.clgc.2019.03.019 [DOI] [PubMed] [Google Scholar]
- 11.Furubayashi N, Negishi T, Uozumi T, et al. Isolated adrenocorticotropic hormone deficiency potentially induced by nivolumab following pseudo-progression in clear cell renal cell carcinoma: a case report. Mol Clin Oncol. 2019;10(2):304–308. doi: 10.3892/mco.2018.1781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atrash S, Makhoul I, Mizell JS, Hutchins L, Mahmoud F. Response of metastatic mucosal melanoma to immunotherapy: it can get worse before it gets better. J Oncol Pharm Pract. 2017;23(3):215–219. doi: 10.1177/1078155215627503 [DOI] [PubMed] [Google Scholar]
- 13.Markovic SN, Galli F, Suman VJ, et al. Non-invasive visualization of tumor infiltrating lymphocytes in patients with metastatic melanoma undergoing immune checkpoint inhibitor therapy: a pilot study. Oncotarget. 2018;9(54):30268–30278. doi: 10.18632/oncotarget.25666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saada-Bouzid E, Defaucheux C, Karabajakian A, et al. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol. 2017;28(7):1605–1611. doi: 10.1093/annonc/mdx178 [DOI] [PubMed] [Google Scholar]
- 15.Kazandjian D, Keegan P, Suzman DL, Pazdur R, Blumenthal GM. Characterization of outcomes in patients with metastatic non-small cell lung cancer treated with programmed cell death protein 1 inhibitors past RECIST version 1.1-defined disease progression in clinical trials. Semin Oncol. 2017;44(1):3–7. doi: 10.1053/j.seminoncol.2017.01.001 [DOI] [PubMed] [Google Scholar]
- 16.Cohen JV, Alomari AK, Vortmeyer AO, et al. Melanoma brain metastasis pseudoprogression after pembrolizumab treatment. Cancer Immunol Res. 2016;4(3):179–182. doi: 10.1158/2326-6066.CIR-15-0160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knaus HA, Berglund S, Hackl H, et al. Signatures of CD8+ T cell dysfunction in AML patients and their reversibility with response to chemotherapy. JCI Insight. 2018;3(21). doi: 10.1172/jci.insight.120974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Narayanan S, Kawaguchi T, Yan L, Peng X, Qi Q, Takabe K. Cytolytic activity score to assess anticancer immunity in colorectal cancer. Ann Surg Oncol. 2018;25(8):2323–2331. doi: 10.1245/s10434-018-6506-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmadzadeh M, Johnson LA, Heemskerk B, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114(8):1537–1544. doi: 10.1182/blood-2008-12-195792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun S, Fei X, Mao Y, et al. PD-1(+) immune cell infiltration inversely correlates with survival of operable breast cancer patients. Cancer Immunol Immunother. 2014;63(4):395–406. doi: 10.1007/s00262-014-1519-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zarour HM. Reversing T-cell dysfunction and exhaustion in cancer. Clin Cancer Res. 2016;22(8):1856–1864. doi: 10.1158/1078-0432.CCR-15-1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogando J, Saez ME, Santos J, et al. PD-1 signaling affects cristae morphology and leads to mitochondrial dysfunction in human CD8(+) T lymphocytes. J Immunother Cancer. 2019;7(1):151. doi: 10.1186/s40425-019-0628-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sobue S, Inoue C, Hori F, Qiao S, Murate T, Ichihara M. Molecular hydrogen modulates gene expression via histone modification and induces the mitochondrial unfolded protein response. Biochem Biophys Res Commun. 2017;493(1):318–324. doi: 10.1016/j.bbrc.2017.09.024 [DOI] [PubMed] [Google Scholar]
- 24.Kamimura N, Ichimiya H, Iuchi K, Ohta S. Molecular hydrogen stimulates the gene expression of transcriptional coactivator PGC-1alpha to enhance fatty acid metabolism. NPJ Aging Mech Dis. 2016;2:16008. doi: 10.1038/npjamd.2016.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev. 2006;27(7):728–735. doi: 10.1210/er.2006-0037 [DOI] [PubMed] [Google Scholar]