The Toll-like receptor (TLR) signaling pathway, which is composed of a group of highly conserved molecules, plays a critical role in the recognition of pathogen-associated molecular patterns (PAMPs). Innate immunity activated through the TLR signaling pathway serves as a first defense against infectious diseases.1 However, the exact function of TLR signaling in viral infections remains to be elucidated. Previously, a number of clinical reports indicated that TLR expression and function are impaired in chronic hepatitis B virus (HBV) infection.2 A specific question was raised regarding whether deficient TLR function may contribute to chronic HBV infection, characterized by low or absent T cell responses against HBV.3 In our previous study published in Cellular and Molecular Immunology, Ma et al. demonstrated that deficiency in TLRs or adaptor molecules (MyD88/Trif or IRAK4) resulted in not only elevated expression of HBsAg and HBV DNA but also delayed HBV clearance in the hydrodynamic injection HBV mouse model.4 HBV-specific T cell responses, which play a key role in HBV control and clearance,5 were detectable but functionally impaired in IL-1R/TLR deficient mice. This study highlighted the essential role of the IL-1R/TLR signaling pathway in adaptive immunity and HBV clearance in vivo. This finding suggested that TLR deficiency may be involved in the low HBV-specific T cell responses observed during chronic HBV infection in humans.
The obvious question is whether TLR signaling provides direct help for hepatic T cells to enhance their immune functions. The liver is an immune-privileged organ with potent mechanisms for tolerance induction.6 Hepatic tolerance, though crucial for maintaining liver homeostasis, limits antiviral immune responses. Typically, liver nonparenchymal cells, including liver sinusoidal endothelial cells (LSECs) and Kupffer cells (KCs), facilitate intrahepatic T cell priming usually followed by T cell tolerance and functional impairment. Our previous studies showed that TLR stimulation may render LSECs but not KCs immune-activating instead of tolerogenic, indicating that T cell tolerance could be overcome by TLRs targeting the proper responding cell types.7,8
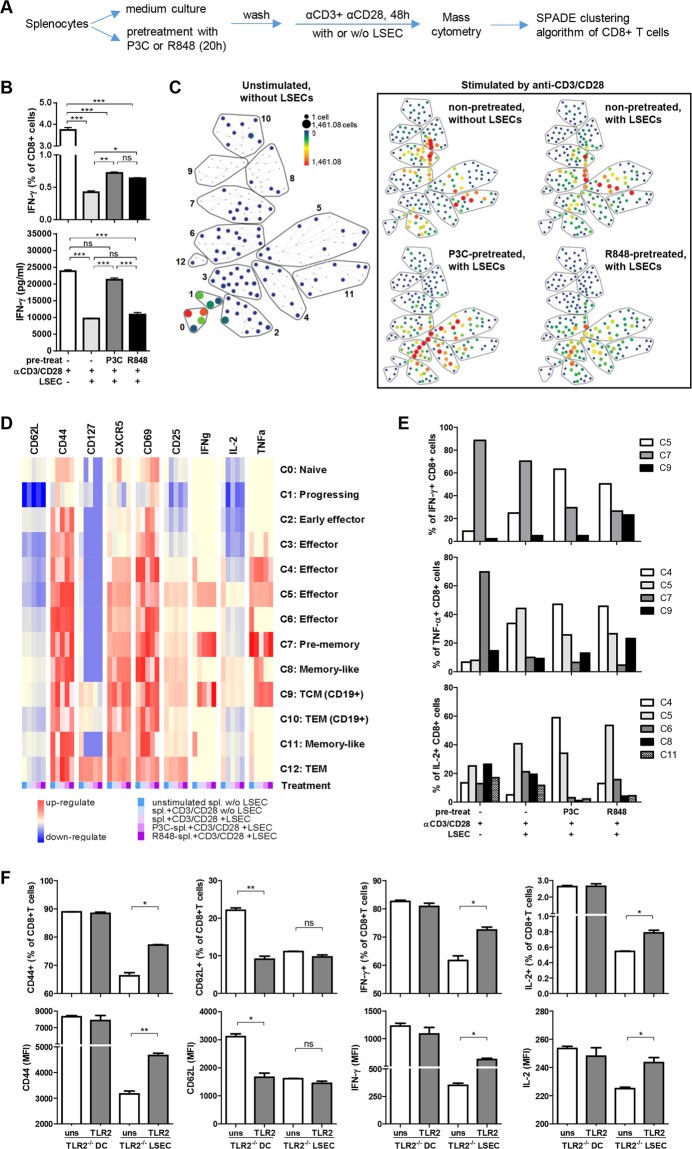
Recent studies point out that some TLRs are expressed by activated CD8+ T cells and serve as costimulatory molecules.9 For example, TLR2 signaling in CD8+ T cells results in a reduced antigen threshold, activation of the mTOR pathway and IFN-γ production. Therefore, we presume that the TLR activation of CD8+ T cells may promote their ability to maintain functionality in the liver. To address this hypothesis, murine splenocytes were prestimulated with TLR2 or TLR7 ligands (P3C or R848, respectively) and activated by anti-CD3 antibodies in the presence of naïve LSECs after washing out free TLR ligands. The CD8+ T cell subpopulations were analyzed by mass cytometry (CyTOF)10 (Fig. 1a). While IFN-γ production of anti-CD3 antibody-activated T cells was suppressed by LSECs, P3C-pretreated CD8+ T cells displayed reduced sensitivity to LSEC-induced suppression (Fig. 1b). Further simultaneous analysis of CD8+ T cell differentiation and function in the coculture system by using CyTOF revealed an immune landscape of the CD8+ T cell response based on 17 surface and functional markers. Thirteen major CD8+ T cell populations could be annotated based on the SPADE algorithm (Fig. 1c). Different expression patterns of the major activation and functional markers for each population are shown by heatmap (Fig. 1d).
Fig. 1.
Activation of TLRs in CD8+ T cells improves resistance to LSEC-induced tolerance. a Experimental design of T cell pretreatment, coculture and analysis. Splenocytes derived from naïve C57BL/6 mice were prestimulated with P3C (2.5 µg/ml) or R848 (10 µg/ml) for 24 h, washed with 50 ml of PBS 3 times, and then cocultured with LSECs from naïve C57BL/6 mice for an additional 48 h in the presence of anti-CD3 and anti-CD28 antibodies (1 µg/ml). b The frequency of IFN-γ+ CD8+ T cells was analyzed by flow cytometry. Secreted IFN-γ in the supernatants was detected by ELISA (in triplicate, n = 3). c Cells from each group were harvested and pooled for mass cytometry staining and measured with a Helios instrument. The SPADE algorithm was performed on gated CD45+ CD11b− CD3+ NK1.1− CD8+ live single cells to generate the immune landscape for each sample using the surface antigens CD3, CD4, CD8, CD19, CD62L, CD44, CD25, CD127, and CD69 and functional markers IL-2, IL-4, IL-5, IL-6, IL-17a, TNF-α, IFN-γ, and CXCR5. The color gradient and node size indicate cell counts. CD8+ T cells were annotated into 13 major cell populations. d The expression of CD62L, CD44, CD127, CD69, CD25, CXCR5, TNF-α, IFN-γ, and IL-2 is indicated by the mean value in the heatmap of each population. e The number of IFN-γ+, TNF-α+, or IL-2+ cells in each population was counted, and the composition of cytokine-producing cells in each treatment was calculated by count(cytokine+ cells in each population)/count(total cytokine+ cells) × 100%. f To analyze antigen-specific T cell activation, FV-TCR CD8+ T cells were activated by peptide-loaded DCs or LSECs from TLR2−/− mice with or without P3C costimulation (in triplicate, n = 3). The frequency of CD44+, CD62L+, IFN-γ+, or IL-2+ CD8 T cells and MFI of CD44, CD62L, IFN-γ, or IL-2 in CD8+ T cells were analyzed by flow cytometry. The experiments were repeated at least 3 times (b, f). Significant differences are indicated by *(p < 0.05), **(p < 0.01), and ***(p < 0.001)
Clearly, unstimulated CD8+ T cells did not express T cell activation or functional markers (Fig. 1c, unstimulated, without LSECs, clusters 1 and 2), whereas the anti-CD3 antibody activated CD8+ T cells (Fig. 1c, non-pretreated, without LSECs) developed into mainly prememory and memory-like CD62hiCD44hiCD127lo subsets (clusters 7, 8, and 11), with functional IFN-γ+ and TNF-α+ CD8+ T cells located in cluster 7. In the presence of LSECs, these activated CD8+ T cells differentiated into mainly effector cell populations with diminished cytokine production (clusters 5 and 6; Fig. 1c, non-pretreated, with LSECs), indicating that the presence of LSECs changed the differentiation and function of activated CD8+ T cells. Interestingly, P3C-pretreated CD8+ T cells (Fig. 1c, P3C-pretreated, with LSECs) polarized the effector populations, which expressed increased CD44, CD69, and CXCR5 and reduced CD62L markers (clusters 3 and 4, Fig. 1d). However, P3C pretreatment did not restore the number or function of prememory cells (cluster 7) but did improve effector populations with enhanced IFN-γ and TNF-α/IL-2 production (Fig. 1e, clusters 5 and 4, respectively). Similar results were obtained with R848-pretreated splenocytes. These results reveal the complexity of T cell activation and differentiation in response to LSECs and TLR stimulation. While TLR stimulation apparently compensates for the inhibitory effect of LSECs on CD8+ T cells, refined analysis demonstrated that CD8+ T cells develop additional phenotypes in the course of this complex interaction.
To investigate whether the direct TLR-activation of CD8+ T cells regulates hepatic T cell priming, TCR transgenic CD8+ T cells specific for the DbGagL Friend retrovirus (FV) epitope (FV-TCR CD8+ T cells) were activated with or without P3C costimulation during priming with peptide-loaded TLR2-deficient DCs or LSECs, which were P3C-nonresponding APCs. Antigen presentation by TLR2−/− DCs led to the expression of activation markers and cytokine production by CD8+ T cells, which was not further enhanced by P3C, except for a slight reduction in CD62L expression (Fig. 1f). Priming with TLR2−/− LSECs induced only partial activation of CD8+ T cells with a phenotype of CD62LloCD44dim and reduced the abundance of cytokine-producing cells. The presence of P3C significantly increased the expression of CD44, IFN-γ, and IL-2 in FV-TCR CD8+ T cells, suggesting that TLR2 activation in CD8+ T cells is beneficial for efficient activation during LSEC priming. These findings support the conclusion that TLR signaling is important for T cell activation and function in the tolerogenic microenvironment of the liver.
Our present studies imply that activation of the TLR signaling pathway in CD8+ T cells directly regulates their activation, function and sensitivity to an immune tolerance environment, which provides an alternative view for understanding how TLRs may regulate intrahepatic CD8+ T cell responses to viral infections. In general, TLRs likely contribute to viral control at multiple levels, including the induction of an antiviral status in host cells, production of antiviral and proinflammatory cytokines, and activation of various immune cells.11 TLRs also link innate and adaptive immunity via liver-specific immune regulatory cells, resulting in the recruitment and activation of specific T cells to the liver.12,13 Accordingly, our present findings provide evidence that costimulation of CD8+ T cells with TLRs render them more resistant to intrahepatic tolerance induced by LSECs. A relevant perspective is how to translate these findings into treatments that restore T cell functions in patients with chronic HBV infection. It is anticipated that manipulating T cells by TLR stimulation may overcome the negative immune regulation and achieve effective immune control over HBV in the liver.
Acknowledgments
This work was supported by grants from Deutsche Forschungsgemeinschaft (TRR60 and GK1949) and the National Science Foundation of China (No. 81771688 and 81461130019). We thank Juan Min and Ding Gao in the core facility for technical support of the Wuhan Institute of Virology for their kind help in flow cytometry and CyTOF analysis, respectively.
Competing interests
The authors declare no competing interests.
References
- 1.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Chen Z, et al. Expression profiles and function of Toll-like receptors 2 and 4 in peripheral blood mononuclear cells of chronic hepatitis B patients. Clin. Immunol. 2008;128:400–408. doi: 10.1016/j.clim.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Bertoletti A, Kennedy PT. The immune tolerant phase of chronic HBV infection: new perspectives on an old concept. Cell. Mol. Immunol. 2015;12:258–263. doi: 10.1038/cmi.2014.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma Z, et al. The IL-1R/TLR signaling pathway is essential for efficient CD8(+) T-cell responses against hepatitis B virus in the hydrodynamic injection mouse model. Cell. Mol. Immunol. 2017;14:997–1008. doi: 10.1038/cmi.2017.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertoletti A, Ferrari C. Innate and adaptive immune responses in chronic hepatitis B virus infections: towards restoration of immune control of viral infection. Gut. 2012;61:1754–1764. doi: 10.1136/gutjnl-2011-301073. [DOI] [PubMed] [Google Scholar]
- 6.Kubes P, Jenne C. Immune responses in the liver. Annu. Rev. Immunol. 2018;36:247–277. doi: 10.1146/annurev-immunol-051116-052415. [DOI] [PubMed] [Google Scholar]
- 7.Liu J, et al. TLR2 stimulation strengthens intrahepatic myeloid-derived cell-mediated t cell tolerance through inducing Kupffer cell expansion and IL-10 production. J. Immunol. 2018;200:2341–2351. doi: 10.4049/jimmunol.1700540. [DOI] [PubMed] [Google Scholar]
- 8.Liu J, et al. TLR1/2 ligand-stimulated mouse liver endothelial cells secrete IL-12 and trigger CD8+ T cell immunity in vitro. J. Immunol. 2013;191:6178–6190. doi: 10.4049/jimmunol.1301262. [DOI] [PubMed] [Google Scholar]
- 9.Salerno F, Freen-van Heeren JJ, Guislain A, Nicolet BP, Wolkers MC. Costimulation through TLR2 drives polyfunctional CD8(+) T cell responses. J. Immunol. 2019;202:714–723. doi: 10.4049/jimmunol.1801026. [DOI] [PubMed] [Google Scholar]
- 10.Simoni Y, Chng MHY, Li S, Fehlings M, Newell EW. Mass cytometry: a powerful tool for dissecting the immune landscape. Curr. Opin. Immunol. 2018;51:187–196. doi: 10.1016/j.coi.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 11.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 12.Wu J, et al. Poly(I:C) treatment leads to interferon-dependent clearance of hepatitis B virus in a hydrodynamic injection mouse model. J. Virol. 2014;88:10421–10431. doi: 10.1128/JVI.00996-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang LR, et al. Intrahepatic myeloid-cell aggregates enable local proliferation of CD8(+) T cells and successful immunotherapy against chronic viral liver infection. Nat. Immunol. 2013;14:574–583. doi: 10.1038/ni.2573. [DOI] [PubMed] [Google Scholar]