Abstract
Fatigue is often one of the most commonly reported symptoms in prostate cancer survivors, but it is also one of the least understood cancer-related symptoms. Fatigue is associated with psychological distress, disruptions in sleep quality, and impairments in health-related quality of life. Moreover, inflammatory processes and changes related to the hypothalamic-pituitary-adrenal (HPA) axis and/or autonomic nervous system may also play a role in cancer-related fatigue. Thus, effective treatments for fatigue in prostate cancer survivors represent a current unmet need. Prior research has shown that Tai Chi Qigong, a mind-body exercise intervention, can improve physical and emotional health. Herein, we describe the protocol of the ongoing 3-arm randomized controlled Health Empowerment & Recovery Outcomes (HERO) clincal trial. One hundred sixty-six prostate cancer survivors with fatigue are randomized to a modified Tai Chi Qigong intervention (TCQ), intensity-matched body training intervention (BT), or usual care (UC) condition. Guided by biopsychosocial and psychoneuroimmunology models, we propose that TCQ, as compared to BT or UC will: i) reduce fatigue (primary outcome) in prostate cancer survivors; ii) reduce inflammation; and iii) regulate the expression of genes from two major functional clusters: a) inflammation, vasodilation and metabolite sensing and b) energy and adrenergic activation. Assessments are conducted at baseline, the 6-week midpoint of the intervention, and 1 week, 3 months, and 12 months post-intervention. If our findings show that TCQ promotes recovery from prostate cancer and its treatment, this type of intervention can be integrated into survivorship care plans as the standard of care. The study's findings will also provide novel information about underlying biobehavioral mechanisms of cancer-related fatigue.
Trial registration number
Keywords: Prostate cancer, Clinical trial, Tai chi, Qigong, Cancer survivors
1. Introduction
Prostate cancer is the most common malignant tumor among men in the United States. Survival rates are high, with over 3.6 million men in the U.S alive with this diagnosis. Over 90% of men are diagnosed with local or regional prostate cancer, and the 5-year survival rate is 99%. Prostate cancer develops mainly in older and in African American men [1]. The median age at diagnosis is 66 years [2]. Both short- and long-term treatment-related side effects are often compounded with age-related comorbidities and declines in physical, mental, and social functioning [3,4]. Moreover, many survivors have unique challenges because they are coping with late and long-term effects of having had a cancer diagnosis and treatment [[3], [4], [5], [6]]. Together, these factors may influence their ability to engage in physical activity because of slower post-treatment recovery, increased functional limitations, and other quality of life (QOL) impairments.
Fatigue is one of the most commonly reported symptoms by cancer patients at large, including men with prostate cancer [[6], [7], [8], [9], [10], [11]]. Furthermore, it is one of the least understood cancer-related symptoms by patients and healthcare providers [12]. Fatigue is associated with psychological distress, disruptions in sleep quality, and impairments in health-related QOL. Inflammatory processes and changes in the hypothalamic-pituitary-adrenal (HPA) axis and autonomic nervous system may also play a role in cancer-related fatigue [13]. Many prostate cancer patients report fatigue lasting 6 months or more after completion of treatment [14,15]. Fatigue is common, under-recognized, undertreated, and leads patients to become more isolated, dependent on others, and less physically active, and contributes to deconditioning, which exacerbates fatigue [11,[16], [17], [18], [19]].
Research on exercise interventions has shown benefits in treating fatigue and improving QOL in cancer patients [[20], [21], [22], [23], [24], [25]]. However, a systematic review and meta-analysis of the effects of exercise in modulating cancer-related fatigue reported that only 11% of studies (n = 4) enrolled prostate cancer survivors exclusively [26]. Recommendations from the American Society of Clinical Oncology (ASCO) and the National Comprehensive Cancer Network (NCCN) for the management of cancer-related fatigue advise tailoring exercise interventions to the tolerance of the individuals [11,27]. Limited published evidence exists to inform health promotion survivorship guidelines for reducing fatigue in men who have been diagnosed with and treated for prostate cancer [28,29]. Indeed, most randomized controlled trials that have targeted fatigue focused on breast cancer survivors [30,31]. Thus, there is a relative absence of randomized efficacy trials that have focused on fatigue symptoms as a primary outcome in men with prostate cancer [32].
According to biopsychosocial models [[33], [34], [35]] and the field of psychoneuroimmunology [[36], [37], [38]], mind-body interventions promote exercise, relaxation and psychological well-being and may lead to substantial improvements in fatigue, distress, and QOL, along with reductions of inflammation. Mind-body interventions have been developed for cancer patients during and following their medical treatment. Data from these studies indicate benefits for physical and mental aspects of health-related QOL [[39], [40], [41], [42], [43], [44], [45], [46], [47]] and are hypothesized to be associated with reductions in markers of inflammation [44]. Tai Chi Qigong (TCQ) is one such mind-body intervention. TCQ differs from traditional exercise interventions by including a focus on deep breathing techniques, synchronized and rhythmic movements, specific postures, and meditation to induce relaxation [[48], [49]]. It has been effectively used in the elderly and medically-compromised populations, including those with mobility limitations and cancer patients [[48], [49], [50], [51], [52], [53], [54]]. For prostate cancer survivors suffering from fatigue, mind-body interventions such as TCQ may be more appealing because they are not overly physically exertive and they are safe for elderly populations. In a small-scale safety and feasibility randomized controlled trial, we found that TCQ reduced fatigue and distress in inactive, elderly, prostate cancer survivors [39]. However, these findings require replication in a larger sample with evaluation of the durability of these effects.
The rationale for this study is that it will establish the efficacy of a 12-week manualized TCQ intervention for reductions in fatigue, the primary outcome. Secondary outcomes include changes in the biomarkers of inflammation, including genome wide transcriptional factors, and expression of fatigue-related genes. To date, no definitive randomized controlled trial has studied the biological effects of Tai Chi and/or Qigong compared to an exercise intensity-matched intervention in fatigued prostate cancer survivors. Prior work has shown TCQ improves physical and emotional health but research has not provided a mechanistic understanding of the efficacy of TCQ on fatigue. Inflammation is associated with fatigue, and mind-body interventions influence cellular and molecular markers of inflammation, especially in cancer survivors [55]. Furthermore, distinct functional clusters of genes are associated with fatigue in men with prostate cancer. One cluster includes genes related to inflammation and vasodilation/metabolite-sensing and the other cluster is related to energy and adrenergic activation [56], which may represent different pathways by which TCQ could reduce fatigue. The absence of definitive data on these outcomes underscores the innovation, significance, and overall impact of the present study.
2. Scientific rationale and conceptual framework
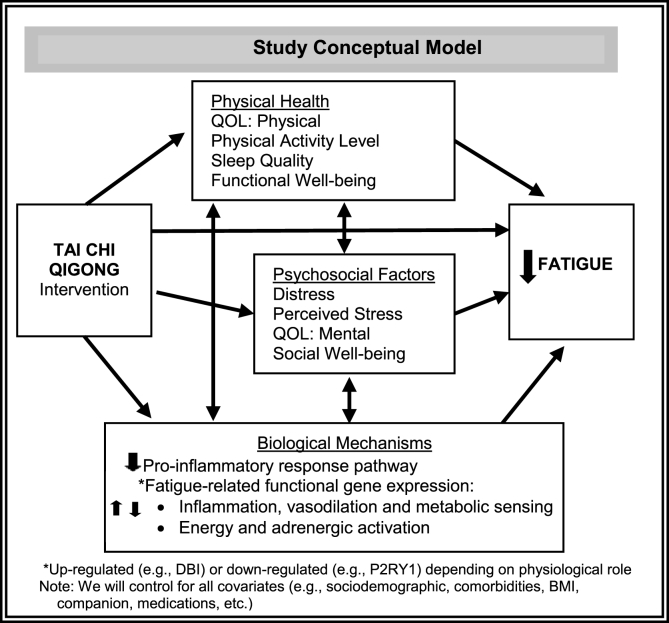
Biopsychosocial and psychoneuroimmunology models consider both psychosocial and biological mechanisms. Psychological and social factors may interact and affect health and illness through neuroendocrine (hypothalamic-pituitary-adrenal and sympathetic-adrenal-medullary axes) and biologic (e.g., immune) pathways [33,36,37,[57], [58], [59]]. Mindfulness-based and light exercise interventions such as TCQ target stress effector mechanisms. Such interventions can improve fatigue directly and through effects on psychological distress and stress, social well-being, QOL, physical well-being, and biological processes in cancer survivors as delineated in Fig. 1 [42,48,52,55,[59], [60], [61], [62], [63]].
Fig. 1.
Study conceptual model.
Basic research on neural-immune signaling indicates that pro-inflammatory cytokines can signal the central nervous system to generate symptoms of fatigue and other behavioral and physical changes [64], and there is growing evidence for a role of pro-inflammatory cytokines in cancer-related fatigue [65]. Elevations in systemic, cellular, and genomic markers of inflammation have been found in women with breast cancer who experience persistent post-treatment fatigue [[66], [67], [68]]. Prior randomized controlled trials have found that mind-body interventions are associated with robust and sustained (i.e., one-year follow-up) decreases in markers of systemic inflammation such as C-reactive protein (CRP) and interleukin-6 (IL-6), decreases of TLR-4 stimulated production of pro-inflammatory cytokines, and decreases in the expression of genes regulated by the transcription factor NFκB [59,[69], [70], [71]]. Further, Tai Chi and Qigong are also associated with decreased blood pressure and cortisol [44,48,[50], [51], [52],72,73]. While some studies did not find effects of mind-body interventions on pro-inflammatory cytokines [46,73], a recent meta-analysis [55] showed that mind-body interventions reduce markers of inflammation such as CRP and possibly IL-6, and reverse inflammatory gene expression profiles [69,70]. Seemingly inconsistent findings that may be due to variation in selection of the control condition [59,70,71], sample population, intervention protocol and fidelity, control for confounding factors, and length of treatment and follow-up, are addressed in this study design [19,59,70,71]. Overall, available literature provides compelling evidence that pro-inflammatory cytokines and the signaling pathways associated with inflammatory activation contribute to fatigue during and particularly after cancer treatment [59,[69], [70], [71]]. However, the effects of TCQ on these mechanisms in aging fatigued prostate cancer survivors are unknown, further underscoring the significance of our study.
Although a growing body of work supports the role of inflammation pathways as a biological mechanism associated with fatigue in cancer patients and survivors, other work has suggested that multiple neural and immune pathways may be involved [19,74]. It has been hypothesized that the etiology of cancer-related fatigue likely involves the dysregulation of several physiological and biochemical systems including 5-HT neurotransmitter dysregulation, vagal afferent activation, alterations in muscle and ATP metabolism, HPA axis dysfunction, circadian rhythm disruption, and cytokine dysregulation [75]. The specific fatigue-related neurological, energy metabolism and immune pathways functionally altered by cancer, its treatment, and mind-body therapies such as TCQ have not as of yet been established. Examining the complex mechanisms of fatigue through leukocyte gene expression (mRNA) allows multiple pathways to be examined efficiently from a single peripheral blood sample. Testing of mRNA outcomes could confirm these suggested genes as biomarkers for fatigue in prostate cancer survivors and as genes that predict successful response to our treatment (i.e., TCQ), including reductions in fatigue [39,74]. Genes that track improvement could also be clues as to the mechanisms of fatigue and the mechanisms of improvement caused by TCQ.
Applying a biopsychosocial and psychoneuroimmunology model to TCQ in inactive, fatigued prostate cancer survivors, we hypothesize that TCQ, as compared to an exercise intensity matched body training (BT) or usual care (UC), will: (i) reduce fatigue (primary outcome); (ii) reduce inflammation as indexed by a vertically integrated approach: systemic levels of pro- and anti-inflammatory cytokines, Toll-like receptor (TLR)-4 stimulated monocyte production of inflammatory cytokines, inflammatory signaling (e.g., nuclear factor, NF-κB) and promoter-based bioinformatics inflammatory transcriptional profiling; and (iii) alter expression of two major fatigue-associated functional clusters of genes: a) inflammation, vasodilation and metabolite sensing and b) energy and adrenergic activation. We are also examining potential underlying theoretical mediating factors (changes in distress, perceived stress, sleep quality, functional and social well-being, physical activity, inflammation pathway markers, and gene expression) and moderating mechanisms (sociodemographic and clinical factors such as age and receiving androgen deprivation/hormone manipulation therapy, body mass index (BMI), and participation of a companion in intervention classes) that are related to improvements in fatigue, which will further specify and elucidate intervention effects.
If TCQ is proven effective, results of this trial can contribute to a needed evidence base for prostate cancer survivorship care and be broadly disseminated to a population who suffer extensively from fatigue.
3. Study design and eligibility criteria
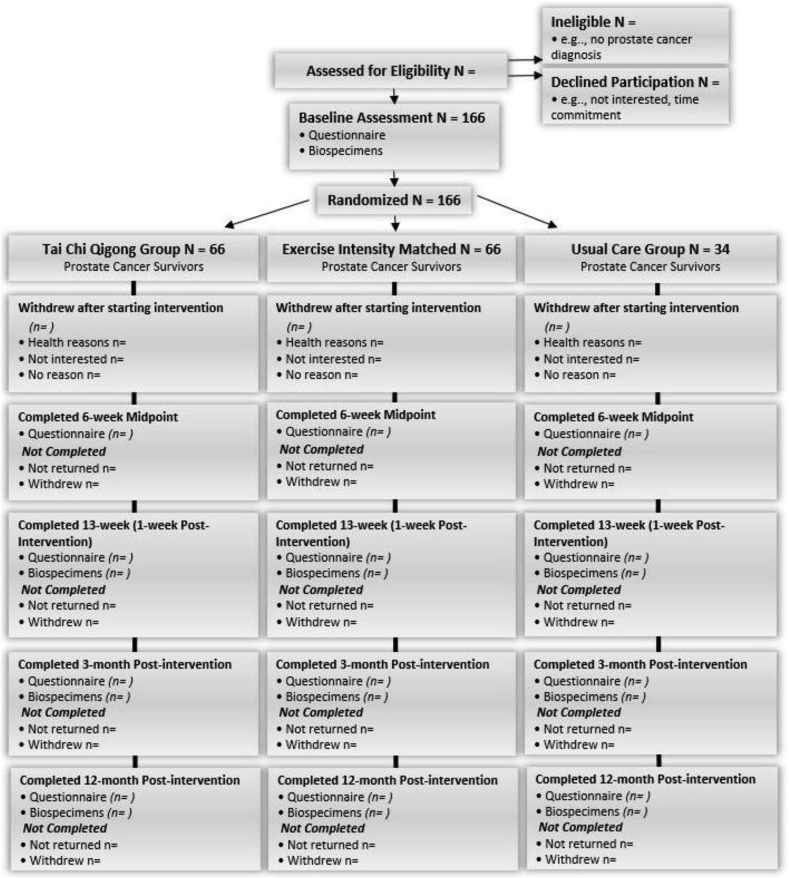
This 3-arm, parallel arm, randomized superiority clinical trial adheres to the CONSORT guidelines for conducting and reporting clinical trial results [76]. The study design and CONSORT diagram are depicted in Fig. 2 and the inclusion and exclusion criteria are delineated in Table 1. After obtaining written informed consent and baseline assessments, 166 fatigued prostate cancer survivors age 55 years and older are randomized to the TCQ arm, BT arm, or UC arm. Patient-reported assessments (surveys) occur at baseline (within 1-week pre-intervention), mid-intervention (6 weeks), 1-week post-intervention (13 weeks), and at 3 months and 12 months post-intervention. Phlebotomy for biomarker assessment occurs at baseline, 13 weeks, 3 months, and 12 months post-intervention.
Fig. 2.
Proposed Study Design and CONSORT Diagram showing the flow of participants and anticipated numbers at each stage.
Table 1.
Eligibility criteria.
| Inclusion Criteria |
|---|
| Age ≥55 years |
| Previous or current diagnosis of local, regional, or metastatic prostate CA |
| Chemotherapy, radiation therapy and/or surgery completed 3 ≥ months ago and/or on ADT/Hormone manipulation ≥4 months |
| Fatigue: SF-36 Vitality Scale score of ≤13 [78,79] or PROMIS Fatigue Scale score of ≥9 [80] |
| Live ≤ 75 miles of New Brunswick |
| Inactive (<150 min of moderate intensity exercise/week within past 3 months) |
| Has transportation and is willing to attend on-site classes and assessments |
| Exclusion Criteria |
| Prostate CA metastasis to liver, brain or lung OR diagnosis of other cancer (except non-melanoma skin CA/CA free ≥ 5 years) |
| Patient Health Questionnaire PHQ-9 score > 12 indicating moderate to severe depression and/or indication of suicidality from response to PHQ-9 [81] |
| Karnofsky performance status score ≤ 50 [82] |
| Non-ability to consent/Non-English proficient |
| Mind-body intervention is a current regular practice within 12 months (2–3 times a week of uninterrupted practice over 2 months) |
| Severe respiratory, cardiovascular, or neurological problems |
| Myalgic Encephalomyelitis/Chronic fatigue syndrome, current major depression, alcohol use disorder, shift work |
| Currently receiving some chemotherapy agents (VePesid, Cytoxan) or radionucleotides (Strontium-89, Samarium (Quadramet®), Radium-223 (Xofigo ®)) & some other therapies |
3.1. Recruitment and retention
Prostate cancer survivors are identified and recruited through a variety of sources in New Jersey (NJ) and New Mexico (NM): 1) invitation letters to prostate cancer patients and clinician referrals from health care providers; 2) face-to-face clinic-based recruitment; 3) community-based recruitment strategies (e.g., radio, newspaper, and flyers) and 4) cancer registries.
As a recruitment and retention strategy we permit participants to have a social network member (e.g., family member/caretaker/significant other) accompany them to classes as was done in our feasibility trial [39]. We will evaluate this possible design effect in the analysis (although we did not observe appreciable subgroup differences in our feasibility trial). Several additional strategies are used to promote retention and adherence to the interventions: 1) Project staff meet and greet each participant before classes and collect/distribute study-related surveys and logs/diaries; 2) weekly phone calls to non-adherent participants to promote adherence with motivational strategies and barriers counseling; 3) reminder postcards, email, text messages, and/or letters are sent to participants about appointments or class schedules based on participants’ preferences; 4) monetary incentives (in the form of pre-paid merchandise cards: $50 per completion of each assessment at baseline, mid-point, and 1-week, 3-month and 12-month post intervention classes and an additional $100 for completion of all of the assessments for up to $350 in total per participant; 5) continued emphasis on the importance of the study at the beginning or end of classes; and 6) class instructors emphasize the importance of attendance for personal benefit and the scientific integrity of the study at the end of each class.
3.2. Randomization and blinding
For participant recruitment and research materials such as the participant consent forms, recruitment letters, advertisements, assessments, and intervention implementation, we avoid the use of the terms Tai Chi and Qigong specifically. Instead, we refer to the intervention groups as follows: TCQ intervention is referred to as body-mind training (BMT) and the intensity-matched condition is referred to as body training (BT). This is done to help to reduce bias by minimizing participants’ expectations or perceived differences between the groups.
After obtaining informed consent and baseline assessments, 166 eligible men are randomized by using computer-generated random numbers with an allocation ratio of 2:2:1 to the TCQ, BT, or UC arms, respectively. Participants are instructed not to discuss their arm assignment with study personnel during their assessments; questionnaires are self-administered. The study investigators, biostatisticians, and the biometric testing and lab technicians are blinded to intervention allocation. The technicians who analyze biospecimens are blind to all other data. Recruitment documents, screening materials, and informed consent indicate that the purpose of this study is to evaluate the effects of two types of light exercise on well-being. Thus, participants are not blinded to the condition to which they are assigned, but they are not informed of the specific study hypotheses. Potential therapeutic benefit in general terms (improvements in well-being) of the interventions is discussed equally and with equal confidence by study staff and instructors to support retention of participants and control expectancy effects [77].
3.3. Study arms
3.3.1. Tai Chi Qigong
TCQ provides a unifying, short-hand label for the many types of Qigong and Tai Chi (including Tai Chi Chih) that have been used, as these practices have similar theoretical foundations, proposed mechanisms of action, and expected benefits [52]. The intervention includes Tai Chi practices with a primary Qigong focus, and most Qigong has a Tai Chi component. The different types of TCQ vary in the amount of Qigong (i.e., relatively static moves making them generally simple to perform) vs. Tai Chi (i.e., dynamic moves that can take months to learn, years to master, and are more cognitively demanding). Hence, we have manualized a TCQ intervention that incorporates Qigong and simplified forms of Tai Chi (e.g., Tai Chi Ruler, Cloud Hands and Wild Goose). These manualized TCQ movements and postures can be learned relatively quickly because they involve repeating a single movement or small number of movements. They can be done seated or standing. Further, TCQ is very adaptive to different levels of physical functioning, making it well suited for inactive senior men with prostate cancer and fatigue [48].
The TCQ sessions are led by instructors who have prior training in TCQ, and training in the project's manualized TCQ intervention. During the first session, the instructor explains the underlying TCQ theory and orients participants to the procedures of TCQ. In all subsequent group sessions, participants practice TCQ under the instruction of a TCQ instructor. The rationale that is communicated to participants randomized to this arm is that TCQ is a health promotion intervention that incorporates meditation, body training, and repetitive movements to promote health and well-being. Each session includes: 1) warm up and a review of TCQ principles; 2) meditation with TCQ movement; 3) breathing techniques; and 4) relaxation procedures. Eccentrically-biased movements that can be completed while seated or standing (depending on participants ability) were chosen for the intervention (e.g., rocking chair, Tai Chi ruler, etc.) to increase the intensity of the intervention while maintaining a level of safety [83]. The classes last 60 min, take place twice per week, and are supplemented with home-based practice by using an instructional DVD and handouts delineating the specific poses to help with their independent practice. The TCQ manual and handouts for home practice are included in the supplemental materials. Participants are instructed to practice at least 30 min per day, at least 3 days per week throughout the intervention and post-intervention follow-up period.
3.3.2. Body training
The rationale for the BT condition is that it will serve as a movement-based, intensity/volume-matched and attention-matched control arm. It matches the TCQ classes in non-specific factors such as contact time, getting out of the home at least twice per week, experiencing a therapeutic environment, interactions with trained instructors and other research participants, attention, and expectation of therapeutic benefit. In addition, this intervention controls for flowing, stretching (e.g., extending arms upward and outward) and eccentric (e.g., bending and lowering) movements, and home practice with a DVD. The therapeutic benefit of participation in this arm is emphasized by both study staff and the instructor to support retention of participants and control expectancy effects [77]. The rationale communicated to participants is that this exercise program incorporates repetitive movements to promote health and well-being. Like the TCQ classes, the 60-min exercise classes will meet twice per week for 12 weeks, and participants will be provided with an instructional DVD and handouts delineating the specific movements to help with their independent practice, and given the same expectations for home practice (minimum of 3 days per week). As with the TCQ classes, BT classes begin within two weeks after the baseline assessment.
The BT program was developed by two exercise specialists (F.A. and E.H.) with previous experience designing exercise-training programs. Initially, the TCQ program was presented to the two professionals and they designed the exercise program simulating the TCQ movement pattern in muscle action (eccentric, concentric and isometric), posture (seated or standing), number of sets and repetitions. After the developing the BT program, a preliminary study was conducted with healthy individuals performing one session of TCQ and BT. The oxygen consumption through indirect calorimetry (Parvomedics, Trueone 2400, Sandy, Utah) heart rate Polar M400 (Polar Electro Oy, Kempele, Finland), and rate of perceived exertion using the Borg Rating of Perceived Exertion Scale [84] were measured. No differences were found between the TCQ and BT sessions.
3.3.3. Usual care
Participants in the UC arm receive care as normal and do not attend classes. However, they are asked to complete the same assessments as participants in the TCQ and the BT classes.
3.4. Standardization and intervention fidelity
For all classes, instructors and study staff who observe each class complete intervention fidelity checklists to ensure that the intervention is being administered as intended according to the intervention manuals and study protocol. To assess the perceived intensity of the two exercise interventions, self-reported Borg Rating of Perceived Exertion (RPE) scale measures [84] are collected following every class session for 24 time points. The completed scales are reviewed by the study team bi-weekly to ensure that both interventions are similar with regard to being intensity-matched.
3.5. Study assessments and measures
The timing of assessments is delineated in Table 2.
Table 2.
Timing of participant assessments.
| Assessment | Baseline | 6 weeks | 13 weeks | 3 months | 12 months |
|---|---|---|---|---|---|
| Informed Consent | X | ||||
| List of Medications | X | X | X | X | X |
| Survey | X | X | X | X | X |
| Sleep Diary (7 days) | X | X | X | X | X |
| Food Diary (3 days) | X | X | X | X | |
| Food Frequency Questionnaire | X | ||||
| Stool | X | X | X | X | |
| Clinical Measures: Height, weight, blood pressure, hip:waist ratio | X | X | X | X | |
| Heart Rate Variability | X | X | X | X | |
| Biospecimen Collection | X | X | X | X | |
| Timed Up & Go (TUG) Test | X | X | X | X | |
| 6-min Walk Test | X | X | X | X | |
| Chair Stand Test | X | X | X | X | |
| Credibility/Expectancy Questions | X | X | X | ||
| Satisfaction Questions | X | ||||
| Adherence Questions | X | X |
3.6. Outcome measures
Fatigue: Fatigue, the primary outcome of the study, is assessed with the Functional Assessment of Chronic Illness (FACIT)-Fatigue Scale [85], is often used with cancer survivor populations. This 13-item scale assesses level of fatigue during usual activities over the past 7 days, with higher scores indicating less fatigue (score range = 0–52; Cronbach's alpha = 0.86).
Psychological Distress, Sleep, Perceived Stress, Well-Being and QOL: We will also examine whether intervention effects might extend to behavioral symptoms and health-related measures associated with fatigue and serve as mediators of these effects. We will assess: 1) psychological distress (depression and anxiety) using the Brief Symptom Inventory-18 (BSI-18; α = 0.79–0.91) [86]; 2) stress perceptions using the Perceived Stress Scale (α = 0.78) [87]; 3) sleep quality and amount by using the Pittsburgh Sleep Quality Index (α = .83) [88] and the Berlin Sleep Questionnaire [89] and Consensus Sleep Diary [90]; 4) social well-being with validated PROMIS 2 Version 2 measures: SF4a Satisfaction with Participation in Social Roles and activities, SF4a Emotional Support, SF4a Ability to Participate in Social Roles, and SF4a Social Isolation [80]; 5) functional well-being with the Functional Well-being Scale [91]; 6) health-related quality of life (mental and physical) using the SF-36v2™ Health Survey [78]; and 7) spirituality using the FACIT-Sp-12 Spiritual Well-Being Scale [92,93]. Follow-up questions will be asked of all participants at 1-week post intervention. These questions are to briefly assess their experience during the intervention period.
Clinic Physical Assessments: Participants are asked to attend appointments at the two study sites (in New Jersey or New Mexico) at baseline, and at one week, three months, and 12 months post-intervention. During these appointments, participants receive a brief physical assessment that includes blood pressure, height, weight, waist/hip circumference measurements, heart rate variability, a 6-min walk test, the Timed Up and Go (TUG) test [94], and a chair stand test. These appointments coincide with the collection of biospecimens and collection of surveys by study staff.
Inflammation Biology Measures and Fatigue-Related Functional Cluster Gene Expression: Blood specimens for inflammation biology and gene expression measures are collected at the study enrollment sites, prepared and shipped to the Cousins Psychoneuroimmunology Center at the University of California Los Angeles (UCLA) for laboratory analysis by using methods found to be highly reliable [59,[69], [70], [71]]. Our pilot work identified differentially expressed genes associated with fatigue in prostate cancer patients compared to healthy controls [74]. These genes are grouped into two major functional gene clusters by mRNA level intercorrelations: 1) Inflammation, Vasodilation, and Metabolite Sensing and 2) Energy and Adrenergic Activation. To test the hypothesis that genes within these defined functional clusters are affected by TCQ and associated changes in fatigue, we will target our analysis of gene expression for these biomarkers. Following these a priori tests, we will examine expression of additional genes/pathways from the full genomic data yielded by the gene expression assays. Stool is collected and processed for gut microbes (microbiome analysis), including DNA and RNA sequencing. The stool samples are stored for future research. A 3-day food diary and a full food frequency questionnaire are collected for future analyses.
3.7. Covariates, moderators, and mediators
Participants complete demographic questions, including self-reported age, race, ethnicity, income, education level, stage of diagnosis, types of cancer treatment, date of diagnosis, and date of primary cancer treatment. Comorbidity, BMI, and other medication information is collected at each survey time point, including current prescription and non-prescription medications and over-the-counter supplements. Body-mass index (BMI) and heart rate variability is assessed during the phlebotomy visits. Physical activity outside the intervention is assessed at all survey time points with the Godin-Shephard Leisure-Time Physical Activity Questionnaire [95] and the Measurement of Older Adults Sedentary Time (MOST) Questionnaire [96]. In addition, participant activity levels throughout the intervention period are recorded with weekly activity logs of home performance of the interventions and other forms of physical activity. The log includes a measurement of perceived exertion using the Borg scale for each activity performed and the number of times a specific activity was performed [84].
Participants are also asked to wear a pedometer and are trained on how to use it to record the number of steps they take each day [97]. Activity and home practice logs are collected once a week at class check-in and at the 3-month and 12-month follow-ups. At the end of the 12-week intervention, participants are encouraged to continue practicing what they learned during their classes (TCQ and BT), ideally 5 times a week, by using the DVDs provided to them. UC participants are asked to continue with their normal daily activities. All participants are also asked to turn in weekly logs to capture activity and capture frequency and duration of engagement (DVD, handouts, and non-aerobic exercise and Borg scale) for their respective assigned study arm. UC participants are called weekly by study staff to collect their activity log and pedometer readings because they will not attend classes. Logs are collected once a week at class check-in, or by telephone and at the 3-month and 12-month follow-up. After the 13-week follow-up, TCQ and BT arm participants receive monthly mailed reminders, phone calls, text messages, or other social media contact (as preferred by each individual participant) to continue their home practice. They are asked to report the average frequency of their home practice and exercise frequency at the 3- and 12-month follow-up assessments.
Treatment credibility and expectations are assessed during the first week of class, mid-intervention and post-intervention by using the 6-item Credibility/Expectancy Scale modified for a class-based intervention with health and well-being as outcomes [98]. This scale assesses both expectancy and credibility factors and includes items about thoughts and feelings of each individual participant about the likelihood that the intervention will have the desired effect (i.e., on health and well-being), and expectations about the size of the effects. Across several treatment populations the scale demonstrated high internal consistency (α = 0.79–0.81) and high test-retest reliability (0.75–0.82) [98].
3.8. Data accuracy and protocol compliance
Several strategies are used to assure data accuracy and protocol compliance. The database uses logic and range checks to minimize data entry errors. Double data entry strategies are used to detect data entry errors. The project coordinator and database manager regularly assess for errors and generate reports to discuss data entry accuracy and quality improvement measures. Completion of surveys and blood draws is monitored by study staff. Intervention fidelity checklists and participant intervention logs are used to assess whether the interventions were implemented as intended and the level that participants are engaging in home practice.
3.9. Sample size and power calculations
The primary goal of the statistical analyses is to assess the efficacy of the TCQ intervention in reducing fatigue (primary outcome), compared to the BT and UC arms. To assess both short-term and long-term intervention effects, fatigue is measured at baseline, followed by four additional measurements: 6 weeks as an interim, 13 weeks, and 3- and 12-months post-intervention. We will use a 2:2:1 allocation ratio (TCQ, BT, and UC, respectively) and a repeated measures design (1 between factor for intervention group and 1 within factor for time) for a total of 123 subjects (49, 49, 25 subjects for TCQ, BT, and UC groups, respectively). In our TCQ feasibility trial [39], we found an average fatigue score for the target population of 32.4, a standard deviation of 8.5, and an autocorrelation between adjacent measurements on the same subject of 0.7. We assume that first-order autocorrelation (AR1) adequately represents the autocorrelation pattern. We assume the group average fatigue score means of 39.9, 34.4, and 32.4 after the immediate post-intervention (3 months post-intervention), reflecting a 23%, 6%, and 0% increase (improvement) in fatigue score for TCQ, BT, and UC, respectively. We will have at least 80% power to detect a minimum of 15% difference in fatigue score among the study arms to test group by time interaction factors by using a Hotelling-Lawley Test [99] with a 2.5% significance level and an actual effect size of 0.3, 0.45, and 0.46 for group, time, and interaction, respectively. The Hotelling-Lawley test includes adjustment for Type I error at 2.5%. Based on t-test evaluation, group sample sizes of 40 and 20 achieve 81% power to reject the null hypothesis of equal means when the population mean difference is 7.5 (39.9 vs. 32.4 post-intervention) with a standard deviation for both groups of 8.5 and with a significance level of 0.025 using a two-sided two-sample equal-variance t-test [[100], [101], [102]]. The group sample sizes are inflated to account for 25% attrition. Thus, 166 participants will be randomized to the three arms, with 66 receiving TCQ, 66 receiving BT, and 34 receiving usual care.
Noncompliance and missing data will be handled by intention-to-treat (ITT) analysis for the primary and secondary outcomes. To manage missing data, multiple imputation under the Missing at Random assumption will be applied using a Markov Chain Monte Carlo method [103,104] via PROC MI in SAS, given the expected pattern of non-monotonic missing data. A post hoc approach [103] will address the influence of Missing Not at Random (MNAR) on fatigue. Sensitivity analyses will be performed to assess alternative multiple imputation techniques upon the extent of MNAR influences.
3.10. Data analysis
The analytical goal of the primary outcome analysis is to test if a TCQ intervention will reduce fatigue in inactive, older prostate cancer survivors, compared to exercise or UC. The primary outcome for this study is fatigue as measured by the FACIT [91]. Generalized linear mixed effects model (GLMM) with an appropriate link function (e.g., identity for continuous and logit for binary outcomes) will be fitted to examine the effects of the intervention assignment on the longitudinally measured primary outcome (at baseline, 6 weeks, 13 weeks, and 3- and 12-months post intervention) with the main covariates of intervention condition, time (in weeks), and their interaction between condition and time. The interaction term will be the main test of interest as it tests for the average departure from the slope due to the intervention. The potential correlations within the same subjects due to the repeated measurements will be accounted for in the GLMM by using unstructured, AR(1) or compound symmetric correlation structures. If baseline values of potential confounders (e.g., medications, BMI, age, stage, prior treatment, co-morbidities, psychosocial factors, presence of a companion and companion dose) are not balanced across the 3-study arms, their influence on outcomes of interest will be assessed by adjustment in multivariable models. We will explore intervention effects on patient-reported outcomes 12 months after completion of the group classes. Analysis of secondary outcomes, include the hypotheses that TCQ will decrease biomarkers associated with inflammatory mechanisms such as serum levels of CRP, IL1, IL6, IL8, IL10, and TNF, decrease TLR-4 stimulated production of inflammatory cytokines (all baseline, 13-week, and 3-month specimens), and decrease expression of several genes regulated by NF-κB pathways (all baseline and 13-week specimens). GLMM with L1-Penalized Estimation (LASSO method) [105,106] for variable selection will handle each of the biomarkers with the main covariates of intervention conditions, time, and their interaction between condition and time, as described above. We will also test the hypothesis that TCQ versus exercise and UC, will regulate the expression of genes from two major functional clusters: 1) inflammation, vasodilation and metabolite sensing and 2) energy and adrenergic activation. Descriptive statistics will be summarized for each gene at baseline and 1-week post intervention (Week 13) and for the change over time by intervention arms. Then, general linear model/ANOVA or the corresponding non-parametric methods will be used to determine the difference in each gene expression across arms. For the separate functional clusters, GLMM will be utilized, while accounting for the correlation among the genes within the cluster by including a random effect of gene and specifying a correlation structure. Multiple comparisons adjustment will be done by using the false discovery rate (FDR) method.
To explore the impact of potential mediators and moderators of TCQ's effect on fatigue, we will use multiple generalized linear regression models. Specifically, based on the pattern of observed results and the study's conceptual model depicted in Fig. 1, mediation analysis [107] will be conducted to examine whether psychosocial (changes in distress, perceived stress, sleep, mental QOL, physical QOL, social well-being, functional well-being, presence of a companion) and/or biological mechanisms (inflammation biology pathways, treatment expectations, fatigue-related gene expression) mediated or explained intervention effects on the fatigue outcome. Adjusted analysis for all aims will control for these factors and will also control for dose (physical activity level, class attendance level, dose of home practice, and dose of companion involvement) as well as sociodemographics and clinical factors. Path coefficients for the mediator model and 95% bootstrap confidence intervals for effects will be estimated to determine the statistical significance of a potential mediator [107]. Further investigation with possible exposure-mediator interactions and causal interpretation will be investigated by using VanderWeele methods [108]. Subgroup analyses (e.g., age <75 vs. ≥75, companion participation, co-morbidity index, ADT/hormone manipulation treatment status, and intervention dose) will be conducted as sensitivity analyses for the primary and secondary outcomes. All subgroup and interaction analyses will be interpreted cautiously as the Type I error rates are unknown.
4. Ethical considerations
All participants are given information about the study and an opportunity to ask questions. Before obtaining written informed consent, all participants are provided with an explanation of 1) the purpose of the study; 2) randomization process; 3) the use of data and procedures for ensuring confidentiality; 4) voluntary participation and the right of the participant to withdraw from the study at any time; and 5) potential harm that could occur as a result of participation. Informed consent is obtained before any study measurements. The study has been approved by the institutional review boards at Rutgers University (The State University of New Jersey) and the University of New Mexico. Trial registration number: ClinicalTrials.gov Identifier: NCT03345563; Date registered: November 17, 2017.
5. Discussion
This 3-arm randomized clinical trial will determine the efficacy of TCQ for reducing fatigue in prostate cancer survivors and examine inflammatory biology and selected gene-expression pathways hypothesized to contribute to the intervention's effect. Rigorous clinical trials with activity-matched and usual care control groups are needed to assess whether TCQ is a specific and efficacious intervention for fatigue in this cancer survivor population. Our study will fill this knowledge gap. Additionally, examining inflammatory biology and gene expression outcomes relevant to fatigue and other key symptoms that cluster with fatigue is a clinically meaningful and novel direction, as this trial will provide an unprecedented opportunity to explore TCQ's effect at the cellular and molecular level.
The impact of this trial is underscored in several important ways. First, despite the increasing and large numbers of prostate cancer survivors living with increasing disease burden and/or treatment-related impairments to their quality of life, there is limited evidence regarding interventions targeting fatigue in this population. Research on mind-body interventions to improve patient-reported symptoms and quality of life is much less frequent for prostate and senior cancer survivors than breast cancer survivors [109,110] and to our knowledge, no definitive randomized controlled trials have studied the effects of a TCQ intervention in fatigued prostate cancer survivors. Our study addresses this knowledge gap and the findings will provide decision-makers and practitioners with information needed to make evidence-based decisions and recommendations regarding the management of cancer-related fatigue.
Second, we utilize a 3-arm randomized design with both a UC and an intensity-matched, exercise condition. Outcome differences between the TCQ and UC conditions will provide an estimate of the efficacy of the TCQ intervention for improving fatigue. In addition, we will compare TCQ to the BT condition, consisting of intensity-matched non-TCQ and eccentric movements, but no meditation or focused deep breathing, to determine the benefits of these relaxation components above the effects of exercise, and social support and other salient non-specific factors (same dose of class attendance, attention by study staff and instructors, social setting) that could influence outcomes.
Third, a growing body of literature supports the use of mind-body interventions for treatment of fatigue in individuals with cancer and reduction of biological markers of inflammation related to fatigue particularly for breast cancer patients. It is unknown whether the fatigue, psychological distress, sleep impairments, and quality of life/well-being prevalent in aging prostate cancer survivors [16,21,[111], [112], [113]] will be as responsive to TCQ interventions. We have shown that the TCQ intervention is well-tolerated and acceptable by elderly, fatigued prostate cancer survivors [39], but a larger efficacy clinical trial such as ours is required before this intervention can be recommended as part of survivorship care plans.
The importance of this study is further underscored by using both an unprecedented discovery and targeted approach to identify the biological pathways involved in inflammation biology associated with fatigue in aging, fatigued prostate cancer survivors practicing TCQ. We will examine the inflammatory signaling changes that result from TCQ practice, and genome-wide transcriptional factors to determine if they are plausible candidates for molecular mediators of inflammation and glucocorticoid receptor-related signaling pathways that may underlie increased inflammatory signaling related to fatigue. Last, we are just beginning to understand how gene expression relates to the fatigue experienced by cancer survivors. Recent work has identified two functional gene clusters (i.e., 2 distinct pathways) that are correlated with the level and severity of fatigue experienced by prostate cancer patients [74]. Coupling a TCQ intervention with patient-reported fatigue and gene expression measurement pre- and post-intervention in fatigued men with prostate cancer will allow a better understanding of the causal role of gene expression in fatigue in this patient population.
In summary, a TCQ intervention may be of great benefit for fatigued and inactive prostate cancer survivors because it is of relatively low intensity, acceptable, convenient, and it can be practiced from a seated posture or standing. Its safety, preliminary efficacy, clinical implications, and low cost make this an attractive intervention for this survivor population. The study's findings will provide novel information about underlying biobehavioral mechanisms as well as novel and needed data to inform evidence-based recommendations for survivorship care.
Acknowledgements
We would like to acknowledge David Medrano for his assistance with project implementation and Dr. Ji-Hyun Lee for her input into the study design, including the statistical analysis plan and calculating statistical power and the sample size. We would also like to thank James McIntire, Sifu Dug Corpolongo, Curtis Hardison, Jerry LaSarre Gardner, and John Henry Moore for their contributions to the TCQ and BT intervention implementation and treatment fidelity. This work is supported by the National Cancer Institute of the National Institutes of Health [grant number R01CA203939 to A.Y.K and M.I.]; Biometrics Shared Resource and Biospecimen Repository and Histopathology Service of Rutgers Cancer Institute of New Jersey [NIH/NCI P30CA072720], the New Jersey Alliance for Clinical Translational Science; NJ ACTS, and the Behavioral and Population Science and Biostatistics shared resources of the UNM Comprehensive Cancer Center core grant [NIH/NCI P30 CA118100], and the University of New Mexico Clinical and Translational Science Center [UL1TR001449-04].
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.conctc.2019.100431.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Miller K.D., Nogueira L., Mariotto A.B., Rowland J.H., Yabroff K.R., Alfano C.M., Jemal A., Kramer J.L., Siegel R.L. Cancer treatment and survivorship statistics. CA A Cancer J. Clin. 2019 doi: 10.3322/caac.21565. 2019. [DOI] [PubMed] [Google Scholar]
- 2.Noone A., Howlader N., Krapcho M., Miller D., Brest A., Yu M., Ruhl J., Tatalovich Z., Mariotto A., Lewis D., Chen H., Feuer E., Cronin K., editors. SEER Cancer Statistics Review, 1975-2015. National Cancer Institute; Bethesda, MD: 2018. [Google Scholar]
- 3.Yancik R. Population aging and cancer: a cross-national concern. Cancer J. 2005;11(6):437–441. doi: 10.1097/00130404-200511000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Rowland J.H., Yancik R. Cancer survivorship: the interface of aging, comorbidity, and quality care. J. Natl. Cancer Inst. 2006;98(8):504–505. doi: 10.1093/jnci/djj154. [DOI] [PubMed] [Google Scholar]
- 5.Rowland J.H., Bellizzi K.M. Cancer survivors and survivorship research: a reflection on today's successes and tomorrow's challenges. Hematol. Oncol. Clin. N. Am. 2008;22(2):181–200. doi: 10.1016/j.hoc.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Rao A.V., Demark-Wahnefried W. The older cancer survivor. Crit. Rev. Oncol. Hematol. 2006;60(2):131–143. doi: 10.1016/j.critrevonc.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Rao A., Cohen H.J. Symptom management in the elderly cancer patient: fatigue, pain, and depression. J. Natl. Cancer Inst. 2004;(32):150–157. doi: 10.1093/jncimonographs/lgh031. Monographs. [DOI] [PubMed] [Google Scholar]
- 8.Borneman T., Piper B.F., Sun V.C., Koczywas M., Uman G., Ferrell B. Implementing the fatigue guidelines at one NCCN member institution: process and outcomes. J. Natl. Compr. Cancer Netw. 2007;5(10):1092–1101. doi: 10.6004/jnccn.2007.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langston B., Armes J., Levy A., Tidey E., Ream E. The prevalence and severity of fatigue in men with prostate cancer: a systematic review of the literature. Support. Care Cancer. 2013;21(6):1761–1771. doi: 10.1007/s00520-013-1751-5. [DOI] [PubMed] [Google Scholar]
- 10.Wang X.S., Zhao F., Fisch M.J., O'Mara A.M., Cella D., Mendoza T.R., Cleeland C.S. Prevalence and characteristics of moderate to severe fatigue: a multicenter study in cancer patients and survivors. Cancer. 2014;120(3):425–432. doi: 10.1002/cncr.28434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berger A.M., Mooney K., Alvarez-Perez A., Breitbart W.S., Carpenter K.M., Cella D., Cleeland C., Dotan E., Eisenberger M.A., Escalante C.P., Jacobsen P.B., Jankowski C., LeBlanc T., Ligibel J.A., Loggers E.T., Mandrell B., Murphy B.A., Palesh O., Pirl W.F., Plaxe S.C., Riba M.B., Rugo H.S., Salvador C., Wagner L.I., Wagner-Johnston N.D., Zachariah F.J., Bergman M.A., Smith C. Cancer-related fatigue, version 2.2015. J. Natl. Compr. Cancer Netw. 2015;13(8):1012–1039. doi: 10.6004/jnccn.2015.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barsevick A.M., Irwin M.R., Hinds P., Miller A., Berger A., Jacobsen P., Ancoli-Israel S., Reeve B.B., Mustian K., O'Mara A., Lai J.S., Fisch M., Cella D. Recommendations for high-priority research on cancer-related fatigue in children and adults. J. Natl. Cancer Inst. 2013;105(19):1432–1440. doi: 10.1093/jnci/djt242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barsevick A., Frost M., Zwinderman A., Hall P., Halyard M., GENEQOL Consortium I’m so tired: biological and genetic mechanisms of cancer-related fatigue. Qual Life Res. 2010;19(10):1419–1427. doi: 10.1007/s11136-010-9757-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forlenza M.J., Hall P., Lichtenstein P., Evengard B., Sullivan P.F. Epidemiology of cancer-related fatigue in the Swedish twin registry. Cancer. 2005;104(9):2022–2031. doi: 10.1002/cncr.21373. [DOI] [PubMed] [Google Scholar]
- 15.Storey D.J., McLaren D.B., Atkinson M.A., Butcher I., Liggatt S., O'Dea R., Smyth J.F., Sharpe M. Clinically relevant fatigue in recurrence-free prostate cancer survivors. Ann. Oncol. 2012;23(1):65–72. doi: 10.1093/annonc/mdr034. [DOI] [PubMed] [Google Scholar]
- 16.Maliski S.L., Kwan L., Elashoff D., Litwin M.S. Symptom clusters related to treatment for prostate cancer. Oncol. Nurs. Forum. 2008;35(5):786–793. doi: 10.1188/08.ONF.786-793. [DOI] [PubMed] [Google Scholar]
- 17.Lawrence D.P., Kupelnick B., Miller K., Devine D., Lau J. Evidence report on the occurrence, assessment, and treatment of fatigue in cancer patients. J. Natl. Cancer Inst. Monogr. 2004;(32):40–50. doi: 10.1093/jncimonographs/lgh027. [DOI] [PubMed] [Google Scholar]
- 18.Curt G.A., Breitbart W., Cella D., Groopman J.E., Horning S.J., Itri L.M., Johnson D.H., Miaskowski C., Scherr S.L., Portenoy R.K., Vogelzang N.J. Impact of cancer-related fatigue on the lives of patients: new findings from the Fatigue Coalition. The Oncologist. 2000;5:353–360. doi: 10.1634/theoncologist.5-5-353. [DOI] [PubMed] [Google Scholar]
- 19.Bower J.E. Cancer-related fatigue-mechanisms, risk factors, and treatments. Nat. Rev. Clin. Oncol. 2014 doi: 10.1038/nrclinonc.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Segal R.J., Reid R.D., Courneya K.S., Malone S.C., Parliament M.B., Scott C.G., Venner P.M., Quinney H.A., Jones L.W., D'Angelo M.E., Wells G.A. Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. J. Clin. Oncol. : Off. J. Am. Soc. Clin. Oncol. 2003;21(9):1653–1659. doi: 10.1200/JCO.2003.09.534. [DOI] [PubMed] [Google Scholar]
- 21.Thorsen L., Courneya K.S., Stevinson C., Fossa S.D. A systematic review of physical activity in prostate cancer survivors: outcomes, prevalence, and determinants. Support. Care Cancer. 2008;16(9):987–997. doi: 10.1007/s00520-008-0411-7. [DOI] [PubMed] [Google Scholar]
- 22.Culos-Reed S.N., Robinson J.W., Lau H., Stephenson L., Keats M., Norris S., Kline G., Faris P. Physical activity for men receiving androgen deprivation therapy for prostate cancer: benefits from a 16-week intervention. Support. Care Cancer. 2010;18(5):591–599. doi: 10.1007/s00520-009-0694-3. [DOI] [PubMed] [Google Scholar]
- 23.Galvao D.A., Taaffe D.R., Spry N., Joseph D., Newton R.U. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trial. J. Clin. Oncol. : Off. J. Am. Soc. Clin. Oncol. 2010;28(2):340–347. doi: 10.1200/JCO.2009.23.2488. [DOI] [PubMed] [Google Scholar]
- 24.Culos-Reed S.N., Robinson J.L., Lau H., O'Connor K., Keats M.R. Benefits of a physical activity intervention for men with prostate cancer. J. Sport Exerc. Psychol. 2007;29(1):118–127. doi: 10.1123/jsep.29.1.118. [DOI] [PubMed] [Google Scholar]
- 25.Gardner J.R., Livingston P.M., Fraser S.F. Effects of exercise on treatment-related adverse effects for patients with prostate cancer receiving androgen-deprivation therapy: a systematic review. J. Clin. Oncol. : Off. J. Am. Soc. Clin. Oncol. 2014;32(4):335–346. doi: 10.1200/JCO.2013.49.5523. [DOI] [PubMed] [Google Scholar]
- 26.Brown J.C., Huedo-Medina T.B., Pescatello L.S., Pescatello S.M., Ferrer R.A., Johnson B.T. Efficacy of exercise interventions in modulating cancer-related fatigue among adult cancer survivors: a meta-analysis. Cancer Epidemiol. Biomark. Prev.: Publ. Am. Assoc. Cancer Res., Am. Soc. Prev. Oncol. 2011;20(1):123–133. doi: 10.1158/1055-9965.EPI-10-0988. [DOI] [PubMed] [Google Scholar]
- 27.Bower J.E., Bak K., Berger A., Breitbart W., Escalante C.P., Ganz P.A., Schnipper H.H., Lacchetti C., Ligibel J.A., Lyman G.H., Ogaily M.S., Pirl W.F., Jacobsen P.B. Screening, assessment, and management of fatigue in adult survivors of cancer: an American Society of Clinical oncology clinical practice guideline adaptation. J. Clin. Oncol. : Off. J. Am. Soc. Clin. Oncol. 2014;32(17):1840–1850. doi: 10.1200/JCO.2013.53.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Resnick M.J. Prostate cancer: optimizing prostate cancer survivorship care. Nat. Rev. Urol. 2015;12(7):366–367. doi: 10.1038/nrurol.2015.123. [DOI] [PubMed] [Google Scholar]
- 29.Skolarus T.A., Wolf A.M., Erb N.L., Brooks D.D., Rivers B.M., Underwood W., 3rd, Salner A.L., Zelefsky M.J., Aragon-Ching J.B., Slovin S.F., Wittmann D.A., Hoyt M.A., Sinibaldi V.J., Chodak G., Pratt-Chapman M.L., Cowens-Alvarado R.L. American Cancer Society prostate cancer survivorship care guidelines. CA Cancer J. Clin. 2014 doi: 10.3322/caac.21234. [DOI] [PubMed] [Google Scholar]
- 30.Kessels E., Husson O., van der Feltz-Cornelis C.M. The effect of exercise on cancer-related fatigue in cancer survivors: a systematic review and meta-analysis. Neuropsychiatric Dis. Treat. 2018;14:479–494. doi: 10.2147/NDT.S150464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cramp F., Byron-Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst. Rev. 2012;11:CD006145. doi: 10.1002/14651858.CD006145.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mishra S.I., Scherer R.W., Geigle P.M., Berlanstein D.R., Topaloglu O., Gotay C.C., Snyder C. Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database Syst. Rev. 2012;8 doi: 10.1002/14651858.CD007566.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Engel G.L. From biomedical to biopsychosocial. 1. Being scientific in the human domain. Psychother. Psychosom. 1997;66(2):57–62. doi: 10.1159/000289109. [DOI] [PubMed] [Google Scholar]
- 34.Borrell-Carrio F., Suchman A.L., Epstein R.M. The biopsychosocial model 25 years later: principles, practice, and scientific inquiry. Ann. Fam. Med. 2004;2(6):576–582. doi: 10.1370/afm.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hatala A.R. The status of the “biopsychosocial” model in health psychology: towards an integrated approach and a critique of cultural conceptions. OJMP. 2012;1:51–62. [Google Scholar]
- 36.Kiecolt-Glaser J.K., Glaser R. Psychoneuroimmunology and cancer: fact or fiction? Eur. J. Cancer. 1999;35(11):1603–1607. doi: 10.1016/s0959-8049(99)00197-5. [DOI] [PubMed] [Google Scholar]
- 37.Lutgendorf S.K., Costanzo E.S. Psychoneuroimmunology and health psychology: an integrative model. Brain Behav. Immun. 2003;17(4):225–232. doi: 10.1016/s0889-1591(03)00033-3. [DOI] [PubMed] [Google Scholar]
- 38.Green McDonald P., O'Connell M., Lutgendorf S.K. Psychoneuroimmunology and cancer: a decade of discovery, paradigm shifts, and methodological innovations. Brain Behav. Immun. 2013;30(Suppl):S1–S9. doi: 10.1016/j.bbi.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campo R.A., Agarwal N., LaStayo P.C., O'Connor K., Pappas L., Boucher K.M., Gardner J., Smith S., Light K.C., Kinney A.Y. Levels of fatigue and distress in senior prostate cancer survivors enrolled in a 12-week randomized controlled trial of Qigong. J. Cancer Surviv.: Res. Pract. 2014;8(1):60–69. doi: 10.1007/s11764-013-0315-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stan D.L., Collins N.M., Olsen M.M., Croghan I., Pruthi S. The evolution of mindfulness-based physical interventions in breast cancer survivors. Evid. Based Complement Altern. Med. 2012 doi: 10.1155/2012/758641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mansky P., Sannes T., Wallerstedt D., Ge A., Ryan M., Johnson L.L., Chesney M., Gerber L. Tai chi chuan: mind-body practice or exercise intervention? Studying the benefit for cancer survivors. Integr. Cancer Ther. 2006;5(3):192–201. doi: 10.1177/1534735406291590. [DOI] [PubMed] [Google Scholar]
- 42.Carlson L.E., Speca M., Patel K.D., Goodey E. Mindfulness-based stress reduction in relation to quality of life, mood, symptoms of stress, and immune parameters in breast and prostate cancer outpatients. Psychosom. Med. 2003;65(4):571–581. doi: 10.1097/01.psy.0000074003.35911.41. [DOI] [PubMed] [Google Scholar]
- 43.Carlson L.E., Speca M., Faris P., Patel K.D. One year pre-post intervention follow-up of psychological, immune, endocrine and blood pressure outcomes of mindfulness-based stress reduction (MBSR) in breast and prostate cancer outpatients. Brain Behav. Immun. 2007;21(8):1038–1049. doi: 10.1016/j.bbi.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 44.Oh B., Butow P., Mullan B., Clarke S., Beale P., Pavlakis N., Kothe E., Lam L., Rosenthal D. Impact of medical Qigong on quality of life, fatigue, mood and inflammation in cancer patients: a randomized controlled trial. Ann. Oncol. 2010;21(3):608–614. doi: 10.1093/annonc/mdp479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bower J.E., Garet D., Sternlieb B., Ganz P.A., Irwin M.R., Olmstead R., Greendale G. Yoga for persistent fatigue in breast cancer survivors: a randomized controlled trial. Cancer. 2012;118(15):3766–3775. doi: 10.1002/cncr.26702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kiecolt-Glaser J.K., Bennett J.M., Andridge R., Peng J., Shapiro C.L., Malarkey W.B., Emery C.F., Layman R., Mrozek E.E., Glaser R. Yoga's impact on inflammation, mood, and fatigue in breast cancer survivors: a randomized controlled trial. J. Clin. Oncol. : Off. J. Am. Soc. Clin. Oncol. 2014;32(10):1040–1049. doi: 10.1200/JCO.2013.51.8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Larkey L.K., Roe D.J., Weihs K.L., Jahnke R., Lopez A.M., Rogers C.E., Oh B., Guillen-Rodriguez J. Randomized controlled trial of Qigong/Tai Chi Easy on cancer-related fatigue in breast cancer survivors. Ann. Behav. Med.: Publ. Soc. Behav. Med. 2014 doi: 10.1007/s12160-014-9645-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Larkey L., Jahnke R., Etnier J., Gonzalez J. Meditative movement as a category of exercise: implications for research. J. Phys. Act. Health. 2009;6(2):230–238. doi: 10.1123/jpah.6.2.230. [DOI] [PubMed] [Google Scholar]
- 49.Lan C., Chou S.W., Chen S.Y., Lai J.S., Wong M.K. The aerobic capacity and ventilatory efficiency during exercise in Qigong and Tai Chi Chuan practitioners. Am. J. Chin. Med. 2004;32(1):141–150. doi: 10.1142/S0192415X04001734. [DOI] [PubMed] [Google Scholar]
- 50.Rogers C.E., Larkey L.K., Keller C. A review of clinical trials of tai chi and qigong in older adults. West. J. Nurs. Res. 2009;31(2):245–279. doi: 10.1177/0193945908327529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ng B.H., Tsang H.W. Psychophysiological outcomes of health qigong for chronic conditions: a systematic review. Psychophysiology. 2009;46(2):257–269. doi: 10.1111/j.1469-8986.2008.00763.x. [DOI] [PubMed] [Google Scholar]
- 52.Jahnke R., Larkey L., Rogers C., Etnier J., Lin F. A comprehensive review of health benefits of qigong and tai chi. Am. J. Health Promot. : AJHP. 2010;24(6):e1–e25. doi: 10.4278/ajhp.081013-LIT-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang C.W., Chan C.L., Ho R.T., Tsang H.W., Chan C.H., Ng S.M. The effect of qigong on depressive and anxiety symptoms: a systematic review and meta-analysis of randomized controlled trials. Evid. Based Complement Altern. Med.: eCAM. 2013;2013:716094. doi: 10.1155/2013/716094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang F., Man J.K., Lee E.K., Wu T., Benson H., Fricchione G.L., Wang W., Yeung A. The effects of qigong on anxiety, depression, and psychological well-being: a systematic review and meta-analysis. Evid. Based Complement Altern. Med.: eCAM. 2013;2013:152738. doi: 10.1155/2013/152738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morgan N., Irwin M.R., Chung M., Wang C. The effects of mind-body therapies on the immune system: meta-analysis. PLoS One. 2014;9(7) doi: 10.1371/journal.pone.0100903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Light A.R., Bateman L., Jo D., Hughen R.W., VanHaitsma T.A., White A.T., Light K.C. Gene expression alterations at baseline and following moderate exercise in patients with chronic fatigue syndrome and fibromyalgia syndrome. J. Intern. Med. 2012;271(1):64–81. doi: 10.1111/j.1365-2796.2011.02405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fava G.A., Sonino N. The biopsychosocial model thirty years later. Psychother. Psychosom. 2008;77(1):1–2. doi: 10.1159/000110052. [DOI] [PubMed] [Google Scholar]
- 58.Bower J.E. Cancer-related fatigue: links with inflammation in cancer patients and survivors. Brain Behav. Immun. 2007;21(7):863–871. doi: 10.1016/j.bbi.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bower J.E., Irwin M.R. Mind-body therapies and control of inflammatory biology: a descriptive review. Brain Behav. Immun. 2016;51:1–11. doi: 10.1016/j.bbi.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wayne P.M., Walsh J.N., Taylor-Piliae R.E., Wells R.E., Papp K.V., Donovan N.J., Yeh G.Y. Effect of Tai Chi on cognitive performance in older adults: systematic review and meta-analysis. J. Am. Geriatr. Soc. 2014 doi: 10.1111/jgs.12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sprod L.K., Fernandez I.D., Janelsins M.C., Peppone L.J., Atkins J.N., Giguere J., Block R., Mustian K.M. Effects of yoga on cancer-related fatigue and global side-effect burden in older cancer survivors. J. Geriatr. Oncol. 2015;6(1):8–14. doi: 10.1016/j.jgo.2014.09.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McCain N.L., Gray D.P., Elswick R.K., Robins J.W., Tuck I., Walter J.M., Rausch S.M., Ketchum J.M. A randomized clinical trial of alternative stress management interventions in persons with HIV infection. J. Consult. Clin. Psychol. 2008;76(3):431–441. doi: 10.1037/0022-006X.76.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Robins J.L., McCain N.L., Elswick R.K., Jr., Walter J.M., Gray D.P., Tuck I. Psychoneuroimmunology-based stress management during adjuvant chemotherapy for early breast cancer. Evid. Based Complement Altern. Med. 2013;2013:372908. doi: 10.1155/2013/372908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dantzer R., Capuron L., Irwin M.R., Miller A.H., Ollat H., Perry V.H., Rousey S., Yirmiya R. Identification and treatment of symptoms associated with inflammation in medically ill patients. Psychoneuroendocrinology. 2008;33(1):18–29. doi: 10.1016/j.psyneuen.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schubert C., Hong S., Natarajan L., Mills P.J., Dimsdale J.E. The association between fatigue and inflammatory marker levels in cancer patients: a quantitative review. Brain Behav. Immun. 2007;21(4):413–427. doi: 10.1016/j.bbi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 66.Bower J.E., Ganz P.A., Aziz N., Fahey J.L. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom. Med. 2002;64(4):604–611. doi: 10.1097/00006842-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 67.Bower J.E., Ganz P.A., Aziz N., Fahey J.L., Cole S.W. T-cell homeostasis in breast cancer survivors with persistent fatigue. J. Natl. Cancer Inst. 2003;95(15):1165–1168. doi: 10.1093/jnci/djg0019. [DOI] [PubMed] [Google Scholar]
- 68.Collado-Hidalgo A., Bower J.E., Ganz P.A., Cole S.W., Irwin M.R. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin. Cancer Res. : Off. J. Am. Assoc. Cancer Res. 2006;12(9):2759–2766. doi: 10.1158/1078-0432.CCR-05-2398. [DOI] [PubMed] [Google Scholar]
- 69.Bower J.E., Greendale G., Crosswell A.D., Garet D., Sternlieb B., Ganz P.A., Irwin M.R., Olmstead R., Arevalo J., Cole S.W. Yoga reduces inflammatory signaling in fatigued breast cancer survivors: a randomized controlled trial. Psychoneuroendocrinology. 2014;43:20–29. doi: 10.1016/j.psyneuen.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Irwin M.R., Olmstead R., Breen E.C., Witarama T., Carrillo C., Sadeghi N., Arevalo J.M.G., Ma J., Nicassio P., Ganz P.A., Bower J.E., Cole S. Tai Chi, cellular inflammation, and transcriptome dynamics in breast cancer survivors with insomnia: a randomized controlled trial. JNCI Monogr. 2014;(50):295–301. doi: 10.1093/jncimonographs/lgu028. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Black D.S., Irwin M.R., Olmstead R., Ji E., Crabb Breen E., Motivala S.J. Tai Chi meditation effects on nuclear factor-kappaB signaling in lonely older adults: a randomized controlled trial. Psychother. Psychosom. 2014;83(5):315–317. doi: 10.1159/000359956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jones B.M. Changes in cytokine production in healthy subjects practicing Guolin Qigong : a pilot study. BMC Complement Altern. Med. 2001;1:8. doi: 10.1186/1472-6882-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oh B., Butow P.N., Mullan B.A., Clarke S.J., Beale P.J., Pavlakis N., Lee M.S., Rosenthal D.S., Larkey L., Vardy J. Effect of medical Qigong on cognitive function, quality of life, and a biomarker of inflammation in cancer patients: a randomized controlled trial. Support. Care Cancer. 2012;20(6):1235–1242. doi: 10.1007/s00520-011-1209-6. [DOI] [PubMed] [Google Scholar]
- 74.Light K.C., Agarwal N., Iacob E., White A.T., Kinney A.Y., Vanhaitsma T.A., Aizad H., Hughen R.W., Bateman L., Light A.R. Differing leukocyte gene expression profiles associated with fatigue in patients with prostate cancer versus chronic fatigue syndrome. Psychoneuroendocrinology. 2013;38(12):2983–2995. doi: 10.1016/j.psyneuen.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ryan J.L., Carroll J.K., Ryan E.P., Mustian K.M., Fiscella K., Morrow G.R. Mechanisms of cancer-related fatigue. The Oncologist. 2007;12(Suppl 1):22–34. doi: 10.1634/theoncologist.12-S1-22. [DOI] [PubMed] [Google Scholar]
- 76.Schulz K.F., Altman D.G., Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann. Intern. Med. 2010;152(11):726–732. doi: 10.7326/0003-4819-152-11-201006010-00232. [DOI] [PubMed] [Google Scholar]
- 77.Kinser P.A., Robins J.L. Control group design: enhancing rigor in research of mind-body therapies for depression. Evid. Based Complement Altern. Med. 2013;2013:140467. doi: 10.1155/2013/140467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ware J.E. second ed. QualityMetric Health Outcomes Solutions; Lincoln, RI: 1993. User's Manual for the SF-36v2™ Health Survey –. [Google Scholar]
- 79.Brown L.F., Kroenke K., Theobald D.E., Wu J. Comparison of SF-36 vitality scale and Fatigue Symptom Inventory in assessing cancer-related fatigue. Support. Care Cancer. 2011;19(8):1255–1259. doi: 10.1007/s00520-011-1148-2. [DOI] [PubMed] [Google Scholar]
- 80.PROMIS dynamic tools to measure health outcomes from a patient perspective. http://www.nihpromis.org
- 81.Kroenke K., Spitzer R.L., Williams J.B. The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern. Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yates J.W., Chalmer B., McKegney F.P. Evaluation of patients with advanced cancer using the Karnofsky performance status. Cancer. 1980;45(8):2220–2224. doi: 10.1002/1097-0142(19800415)45:8<2220::aid-cncr2820450835>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 83.Lastayo P., Marcus R.L., Dibble L., Frajacomo F., Lindstedt S.L. Eccentric exercise in rehabilitation: safety, feasibility and application. J. Appl. Physiol. 2013 doi: 10.1152/japplphysiol.00008.2013. Bethesda, Md. : 1985. [DOI] [PubMed] [Google Scholar]
- 84.Borg G.A. Psychophysical bases of perceived exertion. Med. Sci. Sport. Exerc. 1982;14(5):377–381. [PubMed] [Google Scholar]
- 85.Cella D., Lai J.S., Chang C.H., Peterman A., Slavin M. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer. 2002;94(2):528–538. doi: 10.1002/cncr.10245. [DOI] [PubMed] [Google Scholar]
- 86.Derogatis L.R. NCS Pearson, Inc.; Minneapolis, MN: 2000. BSI 18 Brief Symptom Inventory 18: Administration, Scoring, and Procedures Manual. [Google Scholar]
- 87.Cohen S., Kamarck T., Mermelstein R. A global measure of perceived stress. J. Health Soc. Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 88.Buysse D., Reynolds C., III, Monk T., Berman S., Kupfer D. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. J. Psychiatr. Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 89.Netzer N.C., Stoohs R.A., Netzer C.M., Clark K., Strohl K.P. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann. Intern. Med. 1999;131(7):485–491. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 90.Carney C.E., Buysse D.J., Ancoli-Israel S., Edinger J.D., Krystal A.D., Lichstein K.L., Morin C.M. The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep. 2012;35(2):287–302. doi: 10.5665/sleep.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.FACIT.Org questionniares. http://www.facit.org/FACITOrg/Questionnaires
- 92.Canada A.L., Murphy P.E., Fitchett G., Peterman A.H., Schover L.R. A 3-factor model for the FACIT-Sp. Psycho Oncol. 2008;17(9):908–916. doi: 10.1002/pon.1307. [DOI] [PubMed] [Google Scholar]
- 93.Haugan G. The FACIT-Sp spiritual well-being scale: an investigation of the dimensionality, reliability and construct validity in a cognitively intact nursing home population. Scand. J. Caring Sci. 2015;29(1):152–164. doi: 10.1111/scs.12123. [DOI] [PubMed] [Google Scholar]
- 94.Podsiadlo D., Richardson S. The Timed "Up & Go": a test of basic functional mobility for frail elderly persons. JAGS. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 95.Amireault S., Godin G. The Godin-Shephard leisure-time physical activity questionnaire: validity evidence supporting its use for classifying healthy adults into active and insufficiently active categories. Percept. Mot. Skills. 2015;120(2):604–622. doi: 10.2466/03.27.PMS.120v19x7. [DOI] [PubMed] [Google Scholar]
- 96.Gardiner P.A., Clark B.K., Healy G.N., Eakin E.G., Winkler E.A., Owen N. Measuring older adults' sedentary time: reliability, validity, and responsiveness. Med. Sci. Sport. Exerc. 2011;43(11):2127–2133. doi: 10.1249/MSS.0b013e31821b94f7. [DOI] [PubMed] [Google Scholar]
- 97.Lee J.M., Kim Y., Welk G.J. Validity of consumer-based physical activity monitors. Med. Sci. Sport. Exerc. 2014;46(9):1840–1848. doi: 10.1249/MSS.0000000000000287. [DOI] [PubMed] [Google Scholar]
- 98.Devilly G.J., Borkovec T.D. Psychometric properties of the credibility/expectancy questionnaire. J. Behav. Ther. Exp. Psychiatry. 2000;31(2):73–86. doi: 10.1016/s0005-7916(00)00012-4. [DOI] [PubMed] [Google Scholar]
- 99.Edwards L.K. Marcel Dekker; New York: 1993. Applied Analysis of Variance in the Behavior Sciences. [Google Scholar]
- 100.Muller K.E., LaVange L.E., Ramey S.L., Ramey C.T. Power calculations for general linear multivariate models including repeated measures applications. J. Am. Stat. Assoc. 1992;87(420):1209–1226. doi: 10.1080/01621459.1992.10476281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Muller K.E., Peterson B.L. Practical methods for computing power in testing the multivariate general linear hypothesis. Comput. Stat. Data Anal. 1984;2:143–158. [Google Scholar]
- 102.Muller K.E., Edwards L.J., Simpson S.L., Taylor D.J. Statistical tests with accurate size and power for balanced linear mixed models. Stat. Med. 2007;26:3639–3660. doi: 10.1002/sim.2827. [DOI] [PubMed] [Google Scholar]
- 103.Rubin D.B. John Wiley & Sons; New York: 1987. Multiple Imputation for Nonresponse in Surveys. [Google Scholar]
- 104.Schafer J.L. Chapman & Hall; London: 1997. Analysis of Incomplete Multivariate Data. [Google Scholar]
- 105.Groll A., Tutz G. Variable selection for generalized linear mixed models by L1- penalized estimation. Stat. Comput. 2014;24(2):137–154. [Google Scholar]
- 106.Goeman J.J. L1 penalized estimation in the cox proportional hazards model. Biom. J. 2010;52:70–84. doi: 10.1002/bimj.200900028. [DOI] [PubMed] [Google Scholar]
- 107.Preacher K.J., Hayes A.F. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav. Res. Methods Instrum. 2004;36:717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- 108.VanderWeele T.J. A unification of mediation and interaction: a four-way decomposition. Epidemiology. 2014;25:749–761. doi: 10.1097/EDE.0000000000000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Minton O., Berger A., Barsevick A., Cramp F., Goedendorp M., Mitchell S.A., Stone P.C. Cancer-related fatigue and its impact on functioning. Cancer. 2013;119(Suppl 11):2124–2130. doi: 10.1002/cncr.28058. [DOI] [PubMed] [Google Scholar]
- 110.Hutton B., Yazdi F., Bordeleau L., Morgan S., Cameron C., Kanji S., Fergusson D., Tricco A., Straus S., Skidmore B., Hersi M., Pratt M., Mazzarello S., Brouwers M., Moher D., Clemons M. Comparison of physical interventions, behavioral interventions, natural health products, and pharmacologics to manage hot flashes in patients with breast or prostate cancer: protocol for a systematic review incorporating network meta-analyses. Syst. Rev. 2015;4:114. doi: 10.1186/s13643-015-0099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Joly F., Alibhai S.M., Galica J., Park A., Yi Q.L., Wagner L., Tannock I.F. Impact of androgen deprivation therapy on physical and cognitive function, as well as quality of life of patients with nonmetastatic prostate cancer. J. Urol. 2006;176(6 Pt 1):2443–2447. doi: 10.1016/j.juro.2006.07.151. [DOI] [PubMed] [Google Scholar]
- 112.Miaskowski C., Paul S.M., Cooper B.A., Lee K., Dodd M., West C., Aouizerat B.E., Swift P.S., Wara W. Trajectories of fatigue in men with prostate cancer before, during, and after radiation therapy. J. Pain Symptom Manag. 2008;35(6):632–643. doi: 10.1016/j.jpainsymman.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Potosky A.L., Reeve B.B., Clegg L.X., Hoffman R.M., Stephenson R.A., Albertsen P.C., Gilliland F.D., Stanford J.L. Quality of life following localized prostate cancer treated initially with androgen deprivation therapy or no therapy. J. Natl. Cancer Inst. 2002;94(6):430–437. doi: 10.1093/jnci/94.6.430. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.