Abstract
Purpose
To report a case of bilateral non-leaking cystoid macular degeneration induced by docetaxel, possibly potentiated by hydroxychloroquine.
Observations
A 63-year-old female patient with a long-term history of rheumatoid arthritis controlled on hydroxychloroquine for 33 years with no evidence of retinopathy developed bilateral loss of vision after having been on docetaxel chemotherapy for breast cancer. Optical coherence tomography showed bilateral cystic maculopathy with no angiographic evidence of leakage on fluorescein angiography. The patient was treated conservatively with no further interventions. Marked improvement of the macular degeneration occurred over the subsequent 9 months, but without visual improvement, although a cataract likely confounded final visual acuity measurement.
Conclusions and importance
Docetaxel-induced maculopathy has been previously reported, but with only four case reports in literature, and most often in conjunction with concurrent therapies or conditions also known to cause macular edema. This is the first case report of docetaxel-induced maculopathy in a setting of hydroxychloroquine therapy which may possibly has potentiated the effect of docetaxel to induce maculopathy. Impaired transcellular retinal pigment epithelial transport might be the cause of non-leaking cystic maculopathy.
Keywords: Docetaxel, Hydroxychloroquine, Non-leaking cystoid macular degeneration, Retinal pigment epithelium pump
1. Introduction
Cystoid macular edema (CME) is characteristically recognized as a vision-threatening condition due to vascular leakage usually due to some degree of inflammation. It has classically been diagnosed, quantified, and monitored by the appearance of fluorescein extravasation and is exquisitely apparent even in mild degrees by optical coherence tomography (OCT). More recently, several drugs have been identified as causing non-leaking CME-cystoid spaces demonstrable by OCT, but not associated with fluorescein leakage which has come to be referred to as cystoid macular degeneration (CMD).1
We describe a case of bilateral, non-leaking cystic maculopathy likely induced by docetaxel chemotherapy, possibly potentiated by long-term hydroxychloroquine use, in patient treated for invasive breast cancer.
2. Case report
A 62-year-old female presented with a 2-month history of bilateral loss of vision. She had a past medical history rheumatoid arthritis for which she was receiving hydroxychloroquine (HCQ) 200mg/day for 33 years. During this long period of follow-up, no signs of HCQ-retinopathy were detected by annual macular Humphrey visual field testing and her best corrected visual acuity was 20/20 OU. Her medical history was also notable for a diagnosis of right invasive breast cancer treated by right partial mastectomy followed by a combination of both radiotherapy and chemotherapy including 4 cycles of cyclophosphamide (Endoxan®) docetaxel (Taxotere®) and corticosteroids. Shortly after completion of the chemotherapy regimen she noticed bilateral blurry vision.
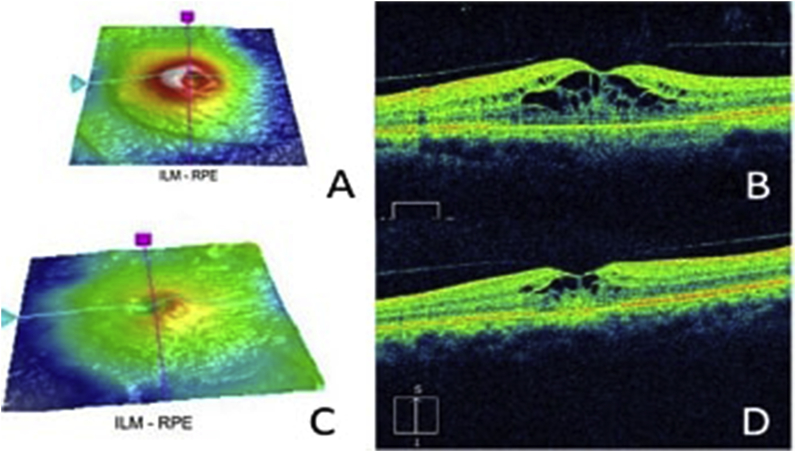
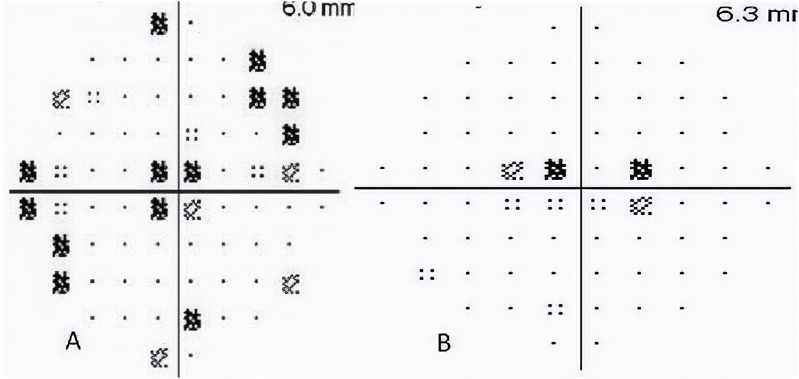
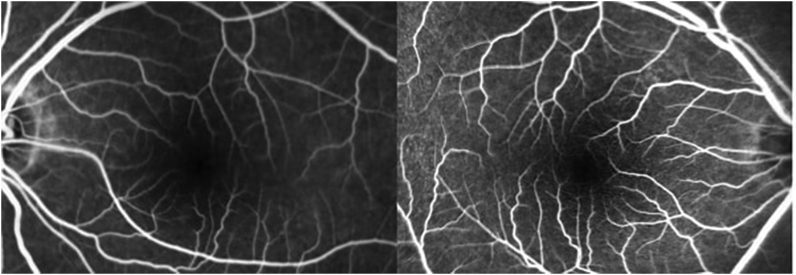
The BCVA was 20/70 OD and 20/60 OS. The anterior segment, free extraocular motility, pupil, and intraocular pressure examinations were normal. The dilated fundus examination disclosed bilateral macular spaces without evidence of inflammation. The optical coherence tomography (OCT) showed intraretinal fluid spaces with multiple parafoveal cystic spaces (Fig. 1). Visual field showed bilateral central scotomas whereas the study from the previous year had been normal (Fig. 2). Fundus fluorescein angiography (FA) showed normal filling of choroidal and retinal vasculature without angiographic evidence of leakage (Fig. 3).
Fig. 1.
Optical coherence tomography (OCT) of (A–B) the left, (C–D) the right eye showing intraretinal fluid spaces with multiple parafoveal cystic spaces.
Fig. 2.
Visual field of the A) left, and B) right eye showing bilateral central scotomas.
Fig. 3.
Fluorescein angiography (FA) of (A) the left, (B) the right eye showing no evidence of vascular leakage with normal filling of retinal vasculature.
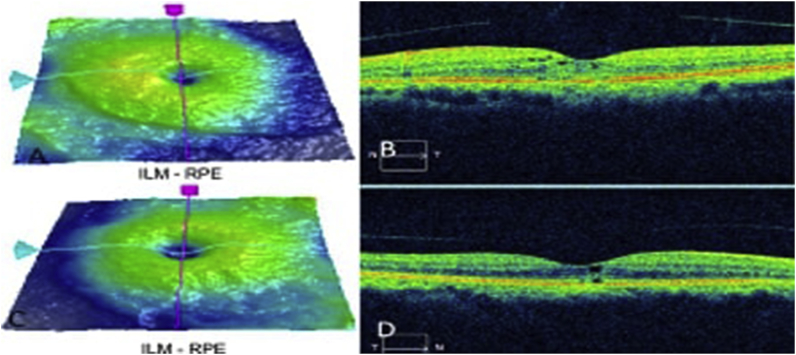
Docetaxel induced maculopathy was diagnosed, possibly potentiated by long term HCQ therapy. Since the patient had already completed her last cycle of therapy, no therapeutic intervention was recommended. Eight weeks later, the macular edema had improved markedly with reduction in the retinal thickness (Fig. 4). At the most recent follow-up examination, 9 months after onset, the BCVA was 20/80 OD and 20/60, but using the potential acuity meter the vision improved to 20/40 OU, consistent he presence of bilateral nuclear sclerotic cataracts.
Fig. 4.
OCT of (A–B) the left, (C–D) the right eye showing marked improvement of the macular spaces with reduction of the retinal thickness at 2 months follow-up.
3. Discussion
Taxane-induced CME has been reported with 4 cases involving docetaxel,2, 3, 4, 5 but previous case reports have been clouded by the concurrent use of other agents (such as tamoxiphen) or conditions (retinitis pigmentosa or the fluid retention syndrome) also known to cause CME. This has led some to hypothesize that taxanes might potentiate the tendency towards CME in such settings.4,5 Generally, the CME resolved with cessation of the drug, although in some cases topical or oral acetazolamide was also used.2,6 We present this case as the first with docetaxel-induced CME possibly potentiated by long-term use of HCQ, and also one that was peculiar in that its onset seemed to occur after cessation of the docetaxel. Moreover, we hypothesize that RPE transcellular transport disruption is the site of this action.
Taxanes comprise a group of chemotherapeutic agents commonly used in the management of various types of solid malignant tumors including breast carcinoma.7 These include paclitaxel (Taxol®, Bristol-Myers Squibb), docetaxel (Taxotere®, Rhone-Poulenc Rhorer) and cabazitaxel (Jevatana®, Sanofi-Aventis). Their mechanism of action is to disrupt the function of the microtubules by binding to beta-tubulin subunit stabilizing microtubules, inhibiting cell replication, and inducing apoptosis.8 Reported ocular adverse effects of docetaxel are rare but include canalicular stenosis, toxic optic neuropathy, conjunctivitis, and non-leaking cystoid macular degeneration.2, 3, 4, 5,9, 10, 11 One member of the taxane class of drugs, paclitaxel, is reported to be more toxic than docetaxel, possibly related to its more quickly achieving a cumulative dose needed to induce CME.6,12,13
The pathogenesis of taxane-induced CME is unclear, but several mechanisms have been suggested. One study suggested paclitaxel-induced dysfunction of Muller cells with subsequent fluid accumulation.13 This theory is consistent with Jampol's suggestion that the non-leaking nature of niacin-induced CME represented subclinical leakage or niacin-induced toxic effects on Muller cells leading to intracellular deregulation and fluid accumulation.14 Based on OCT-findings, Kuznetcova et al. proposed that paclitaxel's toxic effect on microtubules leads to arrest of microtubule-dependent fluid absorption across the RPE, intimating that nonleaking CME might be a consequence of dysfunctional RPE rather than vascular leakage.15 such a role has been postulated for microtubules in Sertoli cells where the seminiferous tubule secretion requires an intact microtubule transport system.16 The reported occurrence of taxane-induced CME in patients already treated with systemic bevacizumab, corroborates the theory that excessive permeability does not underlie the pathogenesis of non-leaking CME.17 Another pathogenic theory for non-leaking CME (by FA) proposes that the blood retinal barrier is only partially compromised (as Jampol hypothesized for niacin's effect) such that diffusion of small molecules but not larger ones such as fluorescein.18
Hydroxychloroquine (HCQ) is used to manage arthropathies associated with some autoimmune disorders, most commonly rheumatoid arthritis or systemic lupus erythematosis. Its mechanism of action is not completely established but may involve immune function suppression by disrupting antigen processing and presentation; its lysosomotrophic characteristics cause pH elevation which seems to affect this result. It is associated with various forms of retinopathy including photoreceptor damage, bull's eye maculopathy via RPE damage, and even diffuse retinal atrophy; these effects are related to dosage and duration of treatment.19,20 HCQ has also been reported to be associated with leaking21 as well as non-leaking22 CME with 12 cases reported. Although the mechanism of HCQ-induced CME is not well understood, it may be by inducing Increased RPE permeability subsequently causing partial loss of blood retinal barrier (BRB) but without affecting tight junction molecules expression.23 Accumulation of HCQ in the RPE may disrupt its pump function, resulting in intraretinal fluid accumulation. . In fact, the rationale for using carbonic anhydrase inhibitors is to enhance RPE pumping function.21,24,25 Whatever the exact mechanism, taxanes could potentiate the (non-leaking) CME effect of other agents (like tamoxifen5,12 or HCQ) at the level of the RPE pump as was suggested by Buffet et al.5 The occurrence of CME in a retinitis pigmentosa patient also supports the possibility of RPE dysfunction as a possible mechanism triggered or compounded by the taxane effect.4
4. Conclusion
We can propose that the pathogenic site of the non-leaking CME caused by taxanes, hydroxychloroquine,and possibly tamoxifen is malfunctioning RPE rather than retinal vasculature, resulting in inhibition of transcellular fluid transport across the RPE to the choriocapillaris, and that such agents are cumulative or synergistic. This explains the absence of leakage pattern by FA in our case - and perhaps in other drug-induced,non-leaking CME. Following discontinuation of the drug(s) there is usually spontaneous resolution. This mechanism is consistent with reports that oral or topical carbonic anhydrase inhibitors may improve refractory edema since they enhance RPE cell transport.3 Taxane-induced maculopathy has been rarely reported in literature, but oncologists and ophthalmologists should be aware of its existence and response to non-interventional management.
Patient consent
No consent was needed as we did not publish any identifying information.
Funding
“This study was supported by the NIH Center Core Grant P30EY014801 and an unrestricted grant to the University of Miami from the Research to Prevent Blindness."
Authorship
All authors attest that they meet the current ICMJE criteria for authorship.
Declaration of competing interest
The following authors have no financial disclosures:
Acknowledgments
None.
References
- 1.Makri O.E., Georgalas I., Georgakopoulos C.D. Drug-induced macular edema. Drugs. 2013;73:789–802. doi: 10.1007/s40265-013-0055-x. [DOI] [PubMed] [Google Scholar]
- 2.Teitelbaum B., Tresley A. Cystic maculopathy with normal capillary permeability secondary to docetaxel. Optom Vis Sci. 2003;80(4):277–279. doi: 10.1097/00006324-200304000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Telander D., Sarraf D. Cystoid macular edema with docetaxel chemotherapy and the fluid retention syndrome. Semin Ophthalmol. 2007;22(3):151–153. doi: 10.1080/08820530701457373. [DOI] [PubMed] [Google Scholar]
- 4.Enzsoly A., Kammerer K., Nemeth J., Schneider M. Bilateral cystoid macular edema following docetaxel chemotherapy in a patient with retinitis pigmentosa: a case report. BMC Ophthalmol. 2015;15(1) doi: 10.1186/s12886-015-0020-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nghiem-Buffet S., Cohen S., Giocanti-Auregan A. Docetaxel retinopathy: a case report. Case Rep Ophthalmol. 2017;8(1):21–25. doi: 10.1159/000455088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bassi E., Loizzi V., Furino C. Cystoid macular edema secondary to paclitaxel therapy for ovarian cancer: a case report. Mol Clin Oncol. 2017;7(2):285–287. doi: 10.3892/mco.2017.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nabholtz J., Gligorov J. The role of taxanes in the treatment of breast cancer. Expert Opin Pharmacother. 2005;6(7):1073–1094. doi: 10.1517/14656566.6.7.1073. [DOI] [PubMed] [Google Scholar]
- 8.Rowinsky E. The development and clinical utility of the taxane class of antimicrotubule chemotherapy agents. Annu Rev Med. 1997;48(1):353–374. doi: 10.1146/annurev.med.48.1.353. [DOI] [PubMed] [Google Scholar]
- 9.Moloney T, Xu W, Rallah-Baker K, Oliveira N, Woodward N, Farrah J. Toxic optic neuropathy in the setting of docetaxel chemotherapy: a case report. BMC Ophthalmol. 201 ;Feb 24;14(1):18. [DOI] [PMC free article] [PubMed]
- 10.Esmaeli B., Valero V., Ahmadi M.A., Booser D. Canalicular stenosis secondary to docetaxel (taxotere): a newly recognized side effect. Ophthalmology. 2001;108:994–995. doi: 10.1016/s0161-6420(00)00640-0. [DOI] [PubMed] [Google Scholar]
- 11.Skolnick C., Doughman D. Erosive conjunctivitis and punctal stenosis secondary to docetaxel (taxotere) Eye Contact Lens. 2003;29(2):134–135. doi: 10.1097/01.ICL.0000062464.79558.5A. [DOI] [PubMed] [Google Scholar]
- 12.Chung H., Kim D., Ahn S. Early detection of tamoxifen-induced maculopathy in patients with low cumulative doses of tamoxifen. Ophthalmic Surg Lasers Imaging. 2010:1–5. doi: 10.3928/15428877-20100215-06. Mar 9. [DOI] [PubMed] [Google Scholar]
- 13.Joshi M.M., Garretson B.R. Paclitaxel maculopathy. Arch Ophthalmol. 2007;125:709–710. doi: 10.1001/archopht.125.5.709. [DOI] [PubMed] [Google Scholar]
- 14.Jampol L.M. Niacin maculopathy. Ophthalmology. 1988;95:1704–1705. doi: 10.1016/s0161-6420(88)32955-6. [DOI] [PubMed] [Google Scholar]
- 15.Kuznetcova T., Cech P., Herbort C. The mystery of angiographically silent macular oedema due to taxanes. Int Ophthalmol. 2012;32(3):299–304. doi: 10.1007/s10792-012-9558-9. [DOI] [PubMed] [Google Scholar]
- 16.Richburg J., Redenbach D., Boekelheide K. Seminiferous tubule fluid secretion is a sertoli cell microtubule-dependent process inhibited by 2,5-hexanedione exposure. Toxicol Appl Pharmacol. 1994;128(2):302–309. doi: 10.1006/taap.1994.1210. [DOI] [PubMed] [Google Scholar]
- 17.Yokoe T., Fukada I., Kobayashi K. Cystoid macular edema during treatment with paclitaxel and bevacizumab in a patient with metastatic breast cancer: a case report and literature review. Case Rep Oncol. 2017;10(2):605–612. doi: 10.1159/000477897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith S. Cystoid macular edema secondary to albumin-bound paclitaxel therapy. Arch Ophthalmol. 2008;126(11):1605. doi: 10.1001/archopht.126.11.1605. [DOI] [PubMed] [Google Scholar]
- 19.Geamanu Panca A., Popa-Cherecheanu A., Marinescu B., Geamanu C.D., Voinea L.M. Retinal toxicity associated with chronic exposure to hydroxychloroquine and its ocular screening. review. J Med Life. 2014;7:322–326. [PMC free article] [PubMed] [Google Scholar]
- 20.Mackenzie A.H. Dose refinements in long-term therapy of rheumatoid arthritis with antimalarials. Am J Med. 1983;75(1A):40–45. doi: 10.1016/0002-9343(83)91269-x. [DOI] [PubMed] [Google Scholar]
- 21.Hong E., Ahn S., Lim H., Lee B. The effect of oral acetazolamide on cystoid macular edema in hydroxychloroquine retinopathy: a case report. BMC Ophthalmol. 2017;17(1) doi: 10.1186/s12886-017-0517-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parikh V., Modi Y., Au A. Nonleaking cystoid macular edema as a presentation of hydroxychloroquine retinal toxicity. Ophthalmology. 2016;123(3):664–666. doi: 10.1016/j.ophtha.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 23.Korthagen N., Bastiaans J., van Meurs J., van Bilsen K., van Hagen P., Dik W. Chloroquine and hydroxychloroquine increase retinal pigment epithelial layer permeability. J Biochem Mol Toxicol. 2015;29(7):299–304. doi: 10.1002/jbt.21696. [DOI] [PubMed] [Google Scholar]
- 24.Kim D.G., Yoon C.K., Kim H.W., Lee S.J. Effect of topical dorzolamide therapy on cystoid macular edema in hydroxychloroquine retinopathy. Can J Ophthalmol. 2018;53:e103–e107. doi: 10.1016/j.jcjo.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 25.Ahn S., Joung J., Lee S., Lee B. Intravitreal dexamethasone implant therapy for the treatment of cystoid macular oedema due to hydroxychloroquine retinopathy: a case report and literature review. BMC Ophthalmol. 2018;18(1) doi: 10.1186/s12886-018-0985-x. [DOI] [PMC free article] [PubMed] [Google Scholar]