ABSTRACT
Wnt signalling pathway is widely studied in many processes of biological development, like embryogenesis, tissue homeostasis and wound repair. It is universally known that Wnt signalling pathway plays an important role in tooth development. Here, we summarized the function of Wnt signalling pathway during tooth initiation, crown morphogenesis, root formation, and discussed the therapeutic potential of Wnt modulators.
KEYWORDS: crown morphogenesis, Dentin regeneration, root formation, Wnt signalling
INTRODUCTION
The Wnt signalling pathway is widely studied in many processes of biological development, including embryogenesis, tissue homeostasis and wound repair. It controls several developmental processes via regulating cell proliferation, differentiation, polarization, and apoptosis. The Wnt signalling pathway is a complex system that consist of 19 ligands (Wnts), 10 Frizzled receptors (Frz) and 2 co-receptors (Lrp5/6), as well as antagonists (WISE, Dkks, Notum, sclerostin, WIFs, sFRPs) and co-activators (Norrin, R-Spondins).1–3 For the canonical Wnt signalling pathway, soluble Wnt protein ligands bind to Frz receptors to mediate their biological effects, leading to the accumulation of β-catenin and its translocation into nuclei to activate downstream transcriptions.4 The non-canonical Wnt signalling pathway includes Wnt-PCP and Wnt-Ca2+ pathway, which extensively participate in biological processes; nevertheless, the core mechanisms remain unclear.
It is universally known that Wnt signalling pathway plays an important role in tooth development. Our recent research using RNA sequencing analysis of rat tooth germs demonstrated that the Wnt/β-catenin signalling is upstream to most of other signalling pathways at the initiation stage of tooth development.5 Researches about Wnt signalling have made new progress in other tissue, which may have suggestive effects on tooth-related development. Therefore, we summarized the function of Wnt signalling pathway during tooth initiation, crown morphogenesis, root formation, and discussed the therapeutic potential of Wnt modulators.
OVERVIEW OF THE WNT SIGNALLING PATHWAY
The Wnt sigalling pathway can be classified into two distinct categories: the canonical or β-catenin dependent pathway and non-canonical pathway (Fig. 1), some of which have been verified in tooth development, while others remain to be verified. As a class of secretory glycoproteins, at least 19 Wnt proteins are identified to activate Wnt signalling pathway in mammals. Wnt1, Wnt3, Wnt3a, Wnt7a, Wnt7b, and Wnt8a activate the canonical pathway, while Wnt4, Wnt5a and Wnt11 activate the non-canonical pathway.6 The canonical Wnt pathway controls the cell behavior by regulating transcription of the DNA binding proteins of T cell factor/lymphoid enhancer factor (TCF/LEF) family. In the absence of Wnt proteins, the complex consisting of GSK3, APC and Axin2 binds to β-catenin and phosphorylates it for degradation. Binding the appropriate Wnt proteins to Frz-Lrp5/6 co-receptor complex, it recruits and activates the cytoplasmic signalling protein Dvl which can inhibit the β-catenin destruction complex and maintain the expression of β-catenin. Stabilized cytosolic β-catenin translocates to the nucleus and interacts with TCF/LEF transcription factors to activate the downstream target genes involved in organ development.7,8
FIGURE 1.
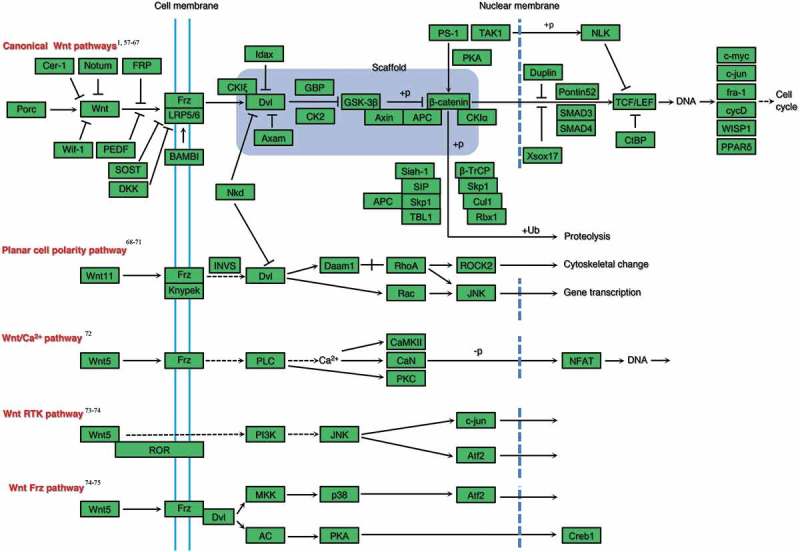
Canonical and non-canonical Wnt signalling pathways known or needed to be proven to participate in rat tooth germ development.1,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75.
Recently, advances in the understanding of the Wnt signalling pathway have been made. For example, our previous study suggested a novel inhibitory mechanism of non-canonical Wnt signalling pathway by the direct interaction between Lrp5/6 and Frz, through which the LRP5/6–Frz complex come into being with the function of maintains both canonical and non-canonical pathways in an inactive status at the basal level.9 Twa1 was identified as a new member of canonical Wnt signalling pathway, enhancing the Wnt pathway by accumulating nuclear β-catenin.10 The tumor specific protein C9orf140 compromised Wnt3a induced β-catenin accumulation to negative regulate the Wnt signalling pathway.11 All the novel advances founded in other tissues or species should be highlighted in tooth development.
ROLE OF WNT IN TOOTH DEVELOPMENT
The development of the tooth germ is the result of a temporal and spatial interaction between odontogenic epithelium and extraembryonic mesenchyme. The canonical Wnt signalling pathway involved in several stages of tooth formation.12 On day 11.5 of murine embryonic development, expression of members of Wnt signalling pathway, such as Lef1, Wnt10a, Wnt10b, can be detected in both upper and lower odontogenic regions.13 On day 12.5, nucleus β-catenin is expressed in both the epithelium and underlying mesenchyme. Inhibition of canonical Wnt signalling in this stage may arrest tooth development. For example, overexpression of Dkk112, a Wnt signalling inhibitor; conditional deletion of β-catenin driven by Prx-1-Cre14; or loss of Lef115, a downstream transcription factor of canonical Wnt pathway, lead to the arrest of tooth development at the bud stage. Furthermore, loss of function of Fgf4, a downstream molecule of Lef1/β-catenin, inhibits the proliferation of odontogenic epithelial cells.16 By contrast, enhanced expression of β-catenin by the K14 promoter in the dental epithelium induces ectopic tooth buds and leads to the formation of supernumerary.17 On day 13.5, the development of tooth germ enters the cap stage, which there is a dense signalling molecule region called the primary enamel knot. Wnt10, together with Shh, Bmp2, Fgf20, is restricted to a small cluster of placodal cells to form the early signalling center.18 There is evidence indicating that the enamel knot regulates the further formation of teeth. In the formation of this signalling center, Wnt/β-catenin may be the most upstream signal of Fgf4 and Fgf.2016,19 Different from the earlier stage, Wnt/β-catenin negatively regulate the odontogenic epithelial cell proliferation and tooth germ development by reducing the expression of Sema3A.20 On day 14.5, the development of tooth germ enters the bell stage, and a secondary enamel knot can be found in molars at this stage. The patterning of the secondary enamel knots determines the locations and heights of the tooth cusps. Ameloblasts and odontoblasts are generated during the bell stage. Wnt10a is expressed in both primary and secondary enamel knots; meanwhile, from E14.5, the expression of Wnt10a transmits from the epithelium to the mesenchyme and high expression is observed in the preodontoblasts that differentiate into DSPP-expressing odontoblasts.21 Scientists have also found that Wnt10a inhibits the proliferation of dental papilla cells and promotes their differentiation in the in vitro studies.21,22 Constitutive expression of β-catenin in ameloblasts of mice incisors results in the delayed mineralization and decreased amelogenesis-related proteins MMP20 and KLK.423 Transgenic mice have been constructed to identify the function of various molecules during tooth development in this stage. Overexpression of Wnt/β-catenin induces premature differentiation of odontoblasts and causes the teeth to produce large amounts of insufficiently mineralized dentin and low expression of DSPP in OC-Cre; Catnb(+/lox(ex3)) mutant mice.24,25 Conversely, decreased Wnt signalling activity in the early odontoblasts of OC-Cre; Wls(CO/CO) mutant mice cause a reduction of dentin deposition, resulting in a thinner dentin wall and wider pulp champer.26 PKP1 is the effector of Wnt pathway that controls the differentiation process of ameloblasts by regulating cell adhesion complex.27 WlsShh-Cre conditional knockout mice revealed that the Wnt ligand in dental epithelium is crucial for tooth differentiation in the late development stage.28 Mice overexpressed MMP20 have higher levels of β-catenin and fibroblasts invading the position of enamel, such that the original enamel becomes soft, thin and irregular.29
Animal studies on tooth development are no longer limited to rodents, scientists have done some investigations on large animals like miniature pigs.30–32 In situ hybridization, immunohistochemistry and microarray analysis of miniature pig tooth germs at cap stage, early bell stage and late bell stage revealed that Wnt signalling pathway play an essential role in the tooth development.30,32 Part of the expression pattern of Wnt signalling molecules in miniature pig tooth germs were similar to that of human and mice, such as Wnt3a, Wnt5a, β-catenin, Axin2, and Lef1 expressed in the cap and early bell stage.32 However, the expression of Wnt3a and Wnt5a in ameloblasts and Tcf4 in odontoblasts/pre-odontoblasts was never reported neither in human or mice.32 Due to the similarity between human and pigs in anatomy, physiology, and immune responses,33 much more researches should be done on pigs in the future.
ROLE OF WNT IN THE ROOT FORMATION
It has been shown that Wnt/β-catenin is not only involved in the tooth crown formation but also involved in the process of root formation accompanied with spatial and spatially specific expression. The expression and distribution of Wnt associated molecules was detected. Wnt3a can be detected in the HERS of mice mandibular first molar at postnatal 2 weeks but disappeared at 5 weeks.34 Bae et al. found that Wnt10a is specifically expressed in the root odontoblasts and β-catenin is expressed in odontoblasts, HERS cells, and periodontal ligament cells during the transition/root stage of mice tooth development.35 Paradoxically, the β-catenin degradation complex component Axin2 can be observed around the root sheath and dental papilla at PN 10 in mouse molars, and the expression remains at a high level in the root as this region continued to development and differentiate while the expression reduced in the odontoblasts.36 The expression of Axin2 indicated that Wnt signalling may be suppressed in the root development, which was contrary to those of Bae’s research35 mentioned above, possibly because other pathways were also involved in the root formation process and the mechanism of Wnt signalling in root development was unclear.
The absence of Wnt/β-catenin signalling has a negative effect on the differentiation of root dentin. Using the odontoblast-specific Ctnnb1 deletion, Zhang et al. found that ablation of β-catenin in odontoblasts led to aberrant HERS formation,37 as well as the formation of dentin and periodontal tissues is greatly hampered in the molar roots and the lingual portion of incisor in loss function of β-catenin.38 Tissue-specific inactivation of β-catenin in developing odontoblasts produces molars lacking roots and aberrantly thin incisors. Furthermore, root odontoblast differentiation is disrupted, followed by the loss of some HERS inner layer cells; the result suggests that there is a cell-autonomous requirement for Wnt/β-catenin signalling in the dental mesenchyme for root formation.39 Mechanism research has shown that the absence of Wnt signalling affected the secretion of dentin matrix and odontoblasts polarization.26,40 Vogel et al. found that the crown morphology of Notum-deficient mice was normal, but their surfaces were covered with severe dysplasia dentin and the roots were short.41 As a secretory enzyme, Notum regulates the Wnt signalling pathway by removing part of the palmitic Wnt protein. This result indicated that the inhibition of the Wnt/β-catenin signal leads to the obstruction of root dentin and cementum development. On the contrary, over activation of β-catenin during root development led to a shorter molar roots, thinner root dentin than the WT mice, as well as the downregulation of biglycan and DSPP and upregulation of DMP1 and DKK.125 These results indicated that the overactivated Wnt/β-catenin signalling inhibited the root dentin formation which is different to crown dentin. It can be seen that the regulation of Wnt/β-catenin on odontoblasts differentiation and matrix secretion in different stages of tooth development needs further study.
WNT AND THE ABNORMALITY OF TOOTH DEVELOPMENT
In mice, deletion of β-catenin in the odontoblasts leads to the incisor becoming much more smaller than the wild-type ones.39 Inactivation the expression of Gpr177 (Wnt ligand secretion mediator) in mouse odontogenic epithelium abrogates the dental epithelial tooth-forming capability and leads to the tooth development arrest at early cap stage.42 WIF1is a secretory Wnt inhibitor that is restrictly expressed in the enamel knot. Inhibiting the expression of WIF1 causes increased apoptosis and arrest of tooth development.43 Lef1/Tcfs regulates the development of tooth by combining with an upstream signal which is the enhancer of Shh, and deletion of the enhancer results in supernumerary teeth.44 Bcl9 and Bcl9l, co-transcription factors of the Wnt signalling pathway, highly expressed in the cytoplasm of ameloblasts, and deletion of these co-transcription factors leads to enamel malformation.45 Conditional knockout of β-catenin in the mouse incisor epithelium makes it short and blunt, with soft and irregular enamel.46 The in vitro cell experiment shows that the absence of β-catenin leads to defective differentiation, up-regulating E-cadherin and migration inhibition in ameloblasts.46 By contrast, the over-activation of Wnt signalling results in supernumerary teeth.47 The abnormal phenotypes of tooth development dysplasia connected with Wnt signalling pathway are summarized in Table 1.
TABLE 1.
Abnormal phenotypes of tooth development dysplasia connected with Wnt signalling pathway
| Gene | OMIM | Disease |
|---|---|---|
| Apc | 175100 | Gardner syndrome, Adenomatous polyposis coli |
| Axin2 | 608615 | Oligodontia-colorectal cancer syndrome |
| Dvl1 | 118210 | Charcot-Marie-Tooth disease type 2A |
| Kremen1 | 609898 | Ectodermal dysplasia including oligodontia |
| LRP6 | 616724 | Tooth agenesis |
| Wnt7a | 276820 | Al-Awadi-Raas-Rothschild syndrome |
| Wnt10b | 617073 | Tooth agenesis |
| Wnt10a | 257980 | Odontoonychodermal dysplasia |
| 150400 | Tooth agenesis |
EFFECT OF THE WNT SIGNALLING PATHWAY ON DENTIN REGENERATION
The essential role of the Wnt signalling pathway in tooth development has attracted scientists’ attention and has led to focus on its function in dentin regeneration. Hunter et al. found that the ideal healing of dental pulp after injury was closely related to the high expression of Wnt signalling molecules.48 Yoshioka et al. observed an accumulation of β-catenin in the pulp beneath the cavity of 9-weeks-old rat molars, suggesting that the preparation of cavity may activate the Wnt/β-catenin pathway to participate in the dentin regeneration process.49 The Wnt signalling activator LiCl and GSK3β inhibitor Tideglusib promoted the formation of tertiary dentin in vivo.50,51 These natural tooth repairing processes are potential new approaches to clinical tooth restoration. However, the presence of Wnt/β-catenin signalling does not always mean that it promotes odontoblast differentiation and dentin regeneration. For example, the overexpression of Wnt10a significantly increased the proliferation of DPSCs, but decreased the expression of odontoblast differentiation-related genes, such as DSPP, DMP1, ALP, and COL1A1, suggesting that overexpression of Wnt10a negatively regulates the differentiation of DPSCs into odontoblasts.52
CONCLUSIONS AND PERSPECTIVES
The development of tooth and roots involves several signalling pathways and growth factors. Our review mainly focuses on the Wnt signalling pathways during tooth development.
As is known, the Wnt signalling pathway can be classified into the canonical pathway and the non-canonical pathway. In the course of tooth development, scientists have focused more on the canonical Wnt signalling pathway. Recently, researches on the non-canonical Wnt signalling is increased. For example, both the ameloblasts and odontoblasts are polarized during tooth development, while non-canonical Wnt signalling pathway plays an important role in the cell polarized process.9,34 Thus, the function of the non-canonical Wnt signalling pathway may take part in the cell polarization during tooth development requires further study.
More importantly, there are often contradictory results when it comes to the role of Wnt signalling in tooth formation. For example, human mutations in APC and Axin2, the two components of the same β-catenin degradation complex, provoke opposite syndromes, hyperdontia, and hypodontia, respectively.47 Likewise, in non-mammalians, activation of Wnt signalling has been shown either yes53 or no54 to stimulate replacement tooth formation.
Studies of tooth development in large animals, like miniature pigs, revealed that Wnt signalling do play an important role in the development process, and the expression patterns were different from the mice to some extent. The similarities and differences between pigs, mice, and human make the miniature pig tooth germ an excellent model in the tooth morphogenesis and regeneration research.
In terms of the research methods, in situ hybridization and immunohistochemistry are traditional methods in scientific research. Tooth germ in vitro culture and renal capsule transplantation enable the role of Wnt signalling molecules in tooth development at the organ level to be investigated. RNA sequencing is a highly sensitive and accurate tool for detecting the whole transcriptome expression, enabling researchers to detect the expression changes in disease status, treatment response and any other required conditions. It is also applied in the researches of tooth germs development of both mice55 and rats5 in our previous studies. With the further study of Wnt signalling pathway, we hope to provide more theoretical evidence for tooth regeneration. Since the tooth morphogenesis is the result of sequential and reciprocal interactions between the dental epithelium and the underlying mesenchyme, the RNA-seq of separated dental epithelium and mesenchyme would be more powerful. This would be our next target in tooth development research.
The Wnt signalling pathway is not only involved in the tooth development but also in the regeneration of reparative dentin. For example, GSK3 antagonists promote the natural processes of reparative dentin formation to completely restore dentin in the mouse model.51 This novel approach has made Wnt signalling-related drugs ideally translatable in clinical therapy for dentistry. At present, there are no Wnt signalling pathway-related clinical trials for dental diseases, and we believe that it will appear in the near future.
In conclusion, both canonical and non-canonical Wnt signalling pathways are important in the regulation of tooth development. Drugs related to the components of the Wnt signalling pathway may be effective for tooth regeneration. Whether target Wnt signalling is ready for clinical treatment of tooth development dysplasia and regeneration of reparative dentin or not? With a deeper understanding of the role of Wnt signalling in tooth development, we hope that Wnt signalling targeting drugs will be successfully applied in dental therapy within this decade.
Funding Statement
This work was supported by the National Natural Science Foundation of China [81570965].
Highlights
The role of Wnt signalling pathway in crown morphogenesis
The role of Wnt signalling pathway in root formation
The role of Wnt signalling pathway in dentin regeneration
Author Contributions
X. L.: collection and assembly of data, data analysis; X. L. and S. L.: manuscript writing; J. Y.: samples collection of rat tooth germs; S. L. and S. Z.: conception and design, manuscript editing, and final approval of manuscript.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Kakugawa S, Langton PF, Zebisch M, Howell SA, Chang T-H, Liu Y, Feizi T, Bineva G, O'Reilly N, Snijders AP, et al. Notum deacylates Wnt proteins to suppress signalling activity. Nature. 2015;519:187–92. doi: 10.1038/nature14259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tamura M, Nemoto E.. Role of the Wnt signaling molecules in the tooth. Jpn Dent Sci Rev. 2016;52:75–83. doi: 10.1016/j.jdsr.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Z, Nor F, Oh M, Cucco C, Shi S, Nor JE. Wnt/beta-catenin signaling determines the vasculogenic fate of postnatal mesenchymal stem cells. Stem Cells. 2016;34:1576–87. doi: 10.1002/stem.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duan P, Bonewald LF. The role of the wnt/beta-catenin signaling pathway in formation and maintenance of bone and teeth. Int J Biochem Cell Biol. 2016;77:23–29. doi: 10.1016/j.biocel.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang J, Cai W, Lu X, Liu S, Zhao S. RNA-sequencing analyses demonstrate the involvement of canonical transient receptor potential channels in rat tooth germ development. Front Physiol. 2017;8:455. doi: 10.3389/fphys.2017.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Widelitz R. Wnt signaling through canonical and non-canonical pathways: recent progress. Growth Factors. 2005;23:111–16. doi: 10.1080/08977190500125746. [DOI] [PubMed] [Google Scholar]
- 7.Yang Z, Balic A, Michon F, Juuri E, Thesleff I. Mesenchymal Wnt/beta-catenin signaling controls epithelial stem cell homeostasis in teeth by inhibiting the antiapoptotic effect of Fgf10. Stem Cells. 2015;33:1670–81. doi: 10.1002/stem.1972. [DOI] [PubMed] [Google Scholar]
- 8.Yin X, Li J, Salmon B, Huang L, Lim WH, Liu B, Hunter DJ, Ransom RC, Singh G, Gillette M, et al. Wnt Signaling and Its Contribution to Craniofacial Tissue Homeostasis. J Dent Res. 2015;94:1487–94. doi: 10.1177/0022034515599772. [DOI] [PubMed] [Google Scholar]
- 9.Ren DN, Chen J, Li Z, Yan H, Yin Y, Wo D, Zhang J, Ao L, Chen B, Ito TK, et al. LRP5/6 directly bind to Frizzled and prevent Frizzled-regulated tumour metastasis. Nat Commun. 2015;6:6906. doi: 10.1038/ncomms7906. [DOI] [PubMed] [Google Scholar]
- 10.Lu Y, Xie S, Zhang W, Zhang C, Gao C, Sun Q, Cai Y, Xu Z, Xiao M, Xu Y, et al. Twa1/Gid8 is a beta-catenin nuclear retention factor in Wnt signaling and colorectal tumorigenesis. Cell Res. 2017;27:1422–40. doi: 10.1038/cr.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang J, Tang S, Xia J, Wen J, Chen S, Shu X, Huen MSY, Deng Y. C9orf140, a novel Axin1-interacting protein, mediates the negative feedback loop of Wnt/beta-catenin signaling. Oncogene. 2018;37:2992–3005. doi: 10.1038/s41388-018-0166-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu F, Chu EY, Watt B, Zhang Y, Gallant NM, Andl T, Yang SH, Lu -M-M, Piccolo S, Schmidt-Ullrich R, et al. Wnt/beta-catenin signaling directs multiple stages of tooth morphogenesis. Dev Biol. 2008;313:210–24. doi: 10.1016/j.ydbio.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dassule HR, McMahon AP. Analysis of epithelial-mesenchymal interactions in the initial morphogenesis of the mammalian tooth. Dev Biol. 1998;202:215–27. doi: 10.1006/dbio.1998.8992. [DOI] [PubMed] [Google Scholar]
- 14.Fujimori S, Novak H, Weissenbock M, Jussila M, Goncalves A, Zeller R, Galloway J, Thesleff I, Hartmann C. Wnt/beta-catenin signaling in the dental mesenchyme regulates incisor development by regulating Bmp4. Dev Biol. 2010;348:97–106. doi: 10.1016/j.ydbio.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sasaki T, Ito Y, Xu X, Han J, Bringas P Jr., Maeda T, Slavkin HC, Grosschedl R, Chai Y. LEF1 is a critical epithelial survival factor during tooth morphogenesis. Dev Biol. 2005;278:130–43. doi: 10.1016/j.ydbio.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 16.Kratochwil K, Galceran J, Tontsch S, Roth W, Grosschedl R. FGF4, a direct target of LEF1 and Wnt signaling, can rescue the arrest of tooth organogenesis in Lef1(-/-) mice. Genes Dev. 2002;16:3173–85. doi: 10.1101/gad.1035602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jarvinen E, Salazar-Ciudad I, Birchmeier W, Taketo MM, Jernvall J, Thesleff I. Continuous tooth generation in mouse is induced by activated epithelial Wnt/beta-catenin signaling. Proc Natl Acad Sci U S A. 2006;103:18627–32. doi: 10.1073/pnas.0607289103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jussila M, Thesleff I. Signaling networks regulating tooth organogenesis and regeneration, and the specification of dental mesenchymal and epithelial cell lineages. Cold Spring Harb Perspect Biol. 2012;4:a008425. doi: 10.1101/cshperspect.a008425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haara O, Harjunmaa E, Lindfors PH, Huh SH, Fliniaux I, Aberg T, Jernvall J, Ornitz DM, Mikkola ML, Thesleff I. Ectodysplasin regulates activator-inhibitor balance in murine tooth development through Fgf20 signaling. Development. 2012;139:3189–99. doi: 10.1242/dev.079558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujii S, Nagata K, Matsumoto S, Kohashi KI, Kikuchi A, Oda Y, Kiyoshima T, Wada N. Wnt/beta-catenin signaling, which is activated in odontomas, reduces Sema3A expression to regulate odontogenic epithelial cell proliferation and tooth germ development. Sci Rep. 2019;9:4257. doi: 10.1038/s41598-019-39686-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamashiro T, Zheng L, Shitaku Y, Saito M, Tsubakimoto T, Takada K, Takano-Yamamoto T, Thesleff I. Wnt10a regulates dentin sialophosphoprotein mRNA expression and possibly links odontoblast differentiation and tooth morphogenesis. Differentiation. 2007;75:452–62. doi: 10.1111/j.1432-0436.2006.00150.x. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Han D, Wang L, Feng H. Down-regulation of Wnt10a affects odontogenesis and proliferation in mesenchymal cells. Biochem Biophys Res Commun. 2013;434:717–21. doi: 10.1016/j.bbrc.2013.03.088. [DOI] [PubMed] [Google Scholar]
- 23.Fan L, Deng S, Sui X, Liu M, Cheng S, Wang Y, Gao Y, Chu C-H, Zhang Q. Constitutive activation of beta-catenin in ameloblasts leads to incisor enamel hypomineralization. J Mol Histol. 2018;49:499–507. doi: 10.1007/s10735-018-9788-x. [DOI] [PubMed] [Google Scholar]
- 24.Kim TH, Lee JY, Baek JA, Lee JC, Yang X, Taketo MM, Jiang R, Cho E-S. Constitutive stabilization of ss-catenin in the dental mesenchyme leads to excessive dentin and cementum formation. Biochem Biophys Res Commun. 2011;412:549–55. doi: 10.1016/j.bbrc.2011.07.116. [DOI] [PubMed] [Google Scholar]
- 25.Bae CH, Lee JY, Kim TH, Baek JA, Lee JC, Yang X, Taketo MM, Jiang R, Cho ES. Excessive Wnt/beta-catenin signaling disturbs tooth-root formation. J Periodontal Res. 2013;48:405–10. doi: 10.1111/jre.12018. [DOI] [PubMed] [Google Scholar]
- 26.Bae CH, Kim TH, Ko SO, Lee JC, Yang X, Cho ES. Wntless regulates dentin apposition and root elongation in the mandibular molar. J Dent Res. 2015;94:439–45. doi: 10.1177/0022034514567198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyazaki K, Yoshizaki K, Arai C, Yamada A, Saito K, Ishikawa M, Xue H, Funada K, Haruyama N, Yamada Y, et al. Plakophilin-1, a novel wnt signaling regulator, is critical for tooth development and ameloblast differentiation. PLoS One. 2016;11:e0152206. doi: 10.1371/journal.pone.0152206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiong Y, Fang Y, Qian Y, Liu Y, Yang X, Huang H, Huang H, Li Y, Zhang X, Zhang Z, et al. Wnt production in dental epithelium is crucial for tooth differentiation. J Dent Res. 2019;98:580–88. doi: 10.1177/0022034519835194. [DOI] [PubMed] [Google Scholar]
- 29.Shin M, Suzuki M, Guan X, Smith CE, Bartlett JD. Murine matrix metalloproteinase-20 overexpression stimulates cell invasion into the enamel layer via enhanced Wnt signaling. Sci Rep. 2016;6:29492. doi: 10.1038/srep29492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang F, Xiao J, Cong W, Li A, Wei F, Xu J, Zhang C, Fan Z, He J, Wang S. Stage-specific differential gene expression profiling and functional network analysis during morphogenesis of diphyodont dentition in miniature pigs, Sus Scrofa. BMC Genomics. 2014;15:103. doi: 10.1186/1471-2164-15-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang F, Li Y, Wu X, Yang M, Cong W, Fan Z, Wang J, Zhang C, Du J, Wang S. Transcriptome analysis of coding and long non-coding RNAs highlights the regulatory network of cascade initiation of permanent molars in miniature pigs. BMC Genomics. 2017;18:148. doi: 10.1186/s12864-016-3396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu X, Li Y, Wang F, Hu L, Li Y, Wang J, Zhang C, Wang S. Spatiotemporal expression of Wnt/beta-catenin signaling during morphogenesis and odontogenesis of deciduous molar in miniature pig. Int J Biol Sci. 2017;13:1082–91. doi: 10.7150/ijbs.20905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsunari H, Nagashima H, Watanabe M, Umeyama K, Nakano K, Nagaya M, Kobayashi T, Yamaguchi T, Sumazaki R, Herzenberg LA, et al. Blastocyst complementation generates exogenic pancreas in vivo in apancreatic cloned pigs. Proc Natl Acad Sci U S A. 2013;110:4557–62. doi: 10.1073/pnas.1222902110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He Q, Yan H, Wo D, Liu J, Liu P, Zhang J, Li L, Zhou B, Ge J, Li H, et al. Wnt3a suppresses Wnt/beta-catenin signaling and cancer cell proliferation following serum deprivation. Exp Cell Res. 2016;341:32–41. doi: 10.1016/j.yexcr.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 35.Bae CH, Kim TH, Chu JY, Cho ES. New population of odontoblasts responsible for tooth root formation. Gene Expr Patterns. 2013;13:197–202. doi: 10.1016/j.gep.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 36.Lohi M, Tucker AS, Sharpe PT. Expression of Axin2 indicates a role for canonical Wnt signaling in development of the crown and root during pre- and postnatal tooth development. Dev Dyn. 2009;NA-NA. doi: 10.1002/dvdy.22047. [DOI] [PubMed] [Google Scholar]
- 37.Zhang R, Teng Y, Zhu L, Lin J, Yang X, Yang G, Li T. Odontoblast beta-catenin signaling regulates fenestration of mouse Hertwig’s epithelial root sheath. Sci China Life Sci. 2015;58:876–81. doi: 10.1007/s11427-015-4882-8. [DOI] [PubMed] [Google Scholar]
- 38.Zhang R, Yang G, Wu X, Xie J, Yang X, Li T. Disruption of Wnt/beta-catenin signaling in odontoblasts and cementoblasts arrests tooth root development in postnatal mouse teeth. Int J Biol Sci. 2013;9:228–36. doi: 10.7150/ijbs.5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim TH, Bae CH, Lee JC, Ko SO, Yang X, Jiang R, Cho ES. beta-catenin is required in odontoblasts for tooth root formation. J Dent Res. 2013;92:215–21. doi: 10.1177/0022034512470137. [DOI] [PubMed] [Google Scholar]
- 40.Lim WH, Liu B, Cheng D, Hunter DJ, Zhong Z, Ramos DM, Williams BO, Sharpe PT, Bardet C, Mah S-J, et al. Wnt signaling regulates pulp volume and dentin thickness. J Bone Miner Res. 2014;29:892–901. doi: 10.1002/jbmr.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vogel P, Read RW, Hansen GM, Powell DR, Kantaputra PN, Zambrowicz B, Brommage R. Dentin dysplasia in notum knockout mice. Vet Pathol. 2016;53:853–62. doi: 10.1177/0300985815626778. [DOI] [PubMed] [Google Scholar]
- 42.Zhu X, Zhao P, Liu Y, Zhang X, Fu J, Yu HM I, Qiu M, Chen Y, Hsu W, Zhang Z. Intra-epithelial requirement of canonical Wnt signaling for tooth morphogenesis. J Biol Chem. 2013;288:12080–89. doi: 10.1074/jbc.M113.462473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee MJ, Kim EJ, Li L, Jung HS. Roles of Wnt inhibitory factor 1 during tooth morphogenesis. Cell Tissue Res. 2015;362:61–68. doi: 10.1007/s00441-015-2170-3. [DOI] [PubMed] [Google Scholar]
- 44.Seo H, Amano T, Seki R, Sagai T, Kim J, Cho SW, Shiroishi T. Upstream enhancer elements of shh regulate oral and dental patterning. J Dent Res. 2018;97:1055–63. doi: 10.1177/0022034518758642. [DOI] [PubMed] [Google Scholar]
- 45.Cantu C, Pagella P, Shajiei TD, Zimmerli D, Valenta T, Hausmann G, Basler K, Mitsiadis TA. A cytoplasmic role of Wnt/beta-catenin transcriptional cofactors Bcl9, Bcl9l, and Pygopus in tooth enamel formation. Sci Signal. 2017;10:eaah4598. doi: 10.1126/scisignal.aah4598. [DOI] [PubMed] [Google Scholar]
- 46.Guan X, Xu M, Millar SE, Bartlett JD. Beta-catenin is essential for ameloblast movement during enamel development. Eur J Oral Sci. 2016;124:221–27. doi: 10.1111/eos.12261. [DOI] [PubMed] [Google Scholar]
- 47.Lu X, Yu F, Liu J, Cai W, Zhao Y, Zhao S, Liu S. The epidemiology of supernumerary teeth and the associated molecular mechanism. Organogenesis. 2017;13:71–82. doi: 10.1080/15476278.2017.1332554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hunter DJ, Bardet C, Mouraret S, Liu B, Singh G, Sadoine J, Dhamdhere G, Smith A, Tran XV, Joy A, et al. Wnt acts as a prosurvival signal to enhance dentin regeneration. J Bone Miner Res. 2015;30:1150–59. doi: 10.1002/jbmr.2444. [DOI] [PubMed] [Google Scholar]
- 49.Yoshioka S, Takahashi Y, Abe M, Michikami I, Imazato S, Wakisaka S, Hayashi M, Ebisu S. Activation of the Wnt/beta-catenin pathway and tissue inhibitor of metalloprotease 1 during tertiary dentinogenesis. J Biochem. 2013;153:43–50. doi: 10.1093/jb/mvs117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ishimoto K, Hayano S, Yanagita T, Kurosaka H, Kawanabe N, Itoh S, Ono M, Kuboki T, Kamioka H, Yamashiro T, et al. Topical application of lithium chloride on the pulp induces dentin regeneration. PLoS One. 2015;10:e0121938. doi: 10.1371/journal.pone.0121938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neves VCM, Babb R, Chandrasekaran D, Sharpe PT. Promotion of natural tooth repair by small molecule GSK3 antagonists. Sci Rep. 2017;7. doi: 10.1038/srep39654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Z, Guo Q, Tian H, Lv P, Zhou C, Gao X. Effects of WNT10A on proliferation and differentiation of human dental pulp cells. J Endod. 2014;40:1593–99. doi: 10.1016/j.joen.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 53.Gaete M, Tucker AS, Schubert M. Organized emergence of multiple-generations of teeth in snakes is dysregulated by activation of Wnt/beta-catenin signalling. PLoS One. 2013;8:e74484. doi: 10.1371/journal.pone.0074484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huysseune A, Soenens M, Elderweirdt F. Wnt signaling during tooth replacement in zebrafish (Danio rerio): pitfalls and perspectives. Front Physiol. 2014;5:386. doi: 10.3389/fphys.2014.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu F, Cai W, Jiang B, Xu L, Liu S, Zhao S. A novel mutation of adenomatous polyposis coli (APC) gene results in the formation of supernumerary teeth. J Cell Mol Med. 2018;22:152–62. doi: 10.1111/jcmm.13303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003;116:2627–34. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- 57.van Den Heuvel M, Harryman-Samos C, Klingensmith J, Perrimon N, Nusse R. Mutations in the segment polarity genes wingless and porcupine impair secretion of the wingless protein. Embo J. 1993;12:5293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.MacDonald BT, He X. Frizzled and LRP5/6 receptors for Wnt/beta-catenin signaling. Cold Spring Harb Perspect Biol. 2012;4:a007880–a007880. doi: 10.1101/cshperspect.a007880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Poggi L, Casarosa S, Carl M. An eye on the Wnt inhibitory factor Wif1. Front Cell Dev Biol. 2018;6:167. doi: 10.3389/fcell.2018.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.He X, Cheng R, Park K, Benyajati S, Moiseyev G, Sun C, Olson LE, Yang Y, Eby BK, Lau K, et al. Pigment epithelium-derived factor, a noninhibitory serine protease inhibitor, is renoprotective by inhibiting the Wnt pathway. Kidney Int. 2017;91:642–57. doi: 10.1016/j.kint.2016.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Larraguibel J, Weiss AR, Pasula DJ, Dhaliwal RS, Kondra R, Van Raay TJ. Wnt ligand-dependent activation of the negative feedback regulator Nkd1. Mol Biol Cell. 2015;26:2375–84. doi: 10.1091/mbc.E14-12-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kadoya T, Kishida S, Fukui A, Hinoi T, Michiue T, Asashima M, Kikuchi A. Inhibition of Wnt signaling pathway by a novel axin-binding protein. J Biol Chem. 2000;275:37030–37. doi: 10.1074/jbc.M005984200. [DOI] [PubMed] [Google Scholar]
- 64.Ishitani T, Ninomiya-Tsuji J, Nagai S, Nishita M, Meneghini M, Barker N, Waterman M, Bowerman B, Clevers H, Shibuya H, et al. The TAK1-NLK-MAPK-related pathway antagonizes signalling between beta-catenin and transcription factor TCF. Nature. 1999;399:798–802. doi: 10.1038/21674. [DOI] [PubMed] [Google Scholar]
- 65.Kobayashi M, Kishida S, Fukui A, Michiue T, Miyamoto Y, Okamoto T, Yoneda Y, Asashima M, Kikuchi A. Nuclear localization of Duplin, a beta-catenin-binding protein, is essential for its inhibitory activity on the Wnt signaling pathway. J Biol Chem. 2002;277:5816–22. doi: 10.1074/jbc.M108433200. [DOI] [PubMed] [Google Scholar]
- 66.Zorn AM, Barish GD, Williams BO, Lavender P, Klymkowsky MW, Varmus HE. Regulation of Wnt signaling by Sox proteins: XSox17 alpha/beta and XSox3 physically interact with beta-catenin. Mol Cell. 1999;4:487–98. [DOI] [PubMed] [Google Scholar]
- 67.Kim TW, Kwak S, Shin J, Kang BH, Lee SE, Suh MY, Kim J-H, Hwang I-Y, Lee J-H, Choi J, et al. Ctbp2-mediated beta-catenin regulation is required for exit from pluripotency. Exp Mol Med. 2017;49:e385. doi: 10.1038/emm.2017.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Uysal-Onganer P, Kypta RM. Wnt11 in 2011 - the regulation and function of a non-canonical Wnt. Acta Physiol (Oxf). 2012;204:52–64. doi: 10.1111/j.1748-1716.2011.02297.x. [DOI] [PubMed] [Google Scholar]
- 69.Gao B. Wnt regulation of planar cell polarity (PCP). Curr Top Dev Biol. 2012;101:263–95. doi: 10.1016/B978-0-12-394592-1.00008-9. [DOI] [PubMed] [Google Scholar]
- 70.Jones C, Chen P. Planar cell polarity signaling in vertebrates. Bioessays. 2007;29:120–32. doi: 10.1002/bies.20526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wharton KA Jr., Zimmermann G, Rousset R, Scott MP. Vertebrate proteins related to drosophila naked cuticle bind dishevelled and antagonize Wnt signaling. Dev Biol. 2001;234:93–106. doi: 10.1006/dbio.2001.0238. [DOI] [PubMed] [Google Scholar]
- 72.De A. Wnt/Ca2+ signaling pathway: a brief overview. Acta Biochim Biophys Sin (Shanghai). 2011;43:745–56. doi: 10.1093/abbs/gmr079. [DOI] [PubMed] [Google Scholar]
- 73.Green J, Nusse R, van Amerongen R. The role of Ryk and Ror receptor tyrosine kinases in Wnt signal transduction. Cold Spring Harb Perspect Biol. 2014;6:a009175–a009175. doi: 10.1101/cshperspect.a009175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Voloshanenko O, Schwartz U, Kranz D, Rauscher B, Linnebacher M, Augustin I, Boutros M. beta-catenin-independent regulation of Wnt target genes by RoR2 and ATF2/ATF4 in colon cancer cells. Sci Rep. 2018;8:3178. doi: 10.1038/s41598-018-20641-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pan B, Huang XF, Deng C. Chronic administration of aripiprazole activates GSK3beta-dependent signalling pathways, and up-regulates GABAA receptor expression and CREB1 activity in rats. Sci Rep. 2016;6:30040. doi: 10.1038/srep30040. [DOI] [PMC free article] [PubMed] [Google Scholar]


