Abstract
Introduction
Alzheimer's disease (AD) is associated with synapse loss. Souvenaid, containing the specific nutrient combination Fortasyn Connect, was designed to improve synapse formation and function. The NL-ENIGMA study explored the effect of Souvenaid on synapse function in early AD by assessing cerebral glucose metabolism (CMRglc) with 18F-fluorodeoxyglucose ([18F]FDG) positron emission tomography (PET).
Methods
We conducted an exploratory double-blind randomized controlled single-center trial. Fifty patients with mild cognitive impairment or mild dementia with evidence of amyloid pathology (cerebrospinal fluid or PET) were stratified for MMSE (20–24 and 25–30) and randomly 1:1 allocated to 24-week daily administration of 125 mL Souvenaid (n = 25) or placebo (n = 25). Dynamic 60-minute [18F]FDG-PET scans (21 frames) with arterial sampling were acquired at baseline and 24 weeks. CMRglc was estimated by quantitative (Ki) and semiquantitative (standardized uptake value ratio, reference cerebellar gray matter) measurements in five predefined regions of interest and a composite region of interest. Change from baseline in CMRglc was compared between treatment groups by analysis of variance, adjusted for baseline CMRglc and MMSE stratum. Additional exploratory outcome parameters included voxel-based analyses by Statistical Parametric Mapping.
Results
No baseline differences between treatment groups were found (placebo/intervention: n = 25/25; age 66 ± 8/65 ± 7 years; female 44%/48%; MMSE 25 ± 3/25 ± 3). [18F]FDG-PET data were available for quantitative (placebo n = 19, intervention n = 18) and semiquantitative (placebo n = 20, intervention n = 22) analyses. At follow-up, no change within treatment groups and no statistically significant difference in change between treatment groups in CMRglc in any regions of interest were found by both quantitative and semiquantitative analyses. No treatment effect was found in the cerebellar gray matter using quantitative measures. The additional Statistical Parametric Mapping analyses did not yield consistent differences between treatment groups.
Discussion
In this exploratory trial, we found no robust effect of 24-week intervention with Souvenaid on synapse function measured by [18F]FDG-PET. Possible explanations include short duration of treatment.
Keywords: Alzheimer's disease, Nutritional intervention, Souvenaid, Randomized clinical trial, Synapse formation, Synapse activity, [18F]FDG-PET
Highlights
-
•
Alzheimer's disease (AD) is associated with synaptic loss in early stages.
-
•
Cerebral glucose metabolism reflects synapse function and density.
-
•
Souvenaid is designed to improve synapse function and formation in AD.
-
•
We found no effect of 24-week intervention with Souvenaid on synapse function in AD.
1. Introduction
The NL-ENIGMA study—a Dutch study exploring the Effect of a specific Nutritional Intervention on cerebral Glucose Metabolism in early Alzheimer's disease (AD)—has been designed to explore the effect of Souvenaid on synapse function in early AD. Detailed rationale and background information have been published [1]. Souvenaid, containing the specific nutrient combination Fortasyn Connect, has been designed to improve synapse formation and function in AD [2], [3], [4]. Souvenaid has been reported to have a small but positive effect on memory function [5], [6], an effect on brain network organization as assessed with electroencephalography [7] and an effect on brain phospholipid metabolism based on magnetic resonance spectroscopy [8], suggesting a positive effect on synapse function.
Aim of the present study was to further explore the effect of Souvenaid on synapse function. We focused on cerebral glucose metabolism as assessed with 18F-fluorodeoxyglucose ([18F]FDG) positron emission tomography (PET), as a surrogate marker for synapse function and density [9], [10]. We hypothesized that the intake of Souvenaid would increase glucose metabolism after 24-week intervention compared with placebo.
2. Methods
2.1. Patients
Patients diagnosed with mild cognitive impairment (MCI) or mild dementia due to AD were recruited from the Alzheimer Center Amsterdam outpatient memory clinic. Diagnoses were made in a multidisciplinary consensus team, consisting of a neurologist, neuropsychologist, and neuroradiologist, and according to the core clinical criteria of the National Institute on Aging–Alzheimer's Association [11], [12], [13]. Presence of AD pathology was evidenced by abnormal AD biomarkers cerebrospinal fluid tau/amyloid-β 1–42 ratio >0.52 or abnormal [11C] Pittsburgh compound B or [18F]florbetaben PET scan [1], [14]. Further inclusion criteria included 1) age between 50 and 85 years; 2) Mini–Mental State Examination (MMSE) ≥ 20; and 3) availability of a study partner. Most important exclusion criteria included medical conditions and use of concomitant medication or high doses of nutritional supplements possibly interfering with study parameters. All exclusion criteria and restrictions during participation are described previously [1]. Main exploratory parameters were assessed in per-protocol populations, which were defined before database was locked.
2.2. Procedures
Patients eligible for participation were provided with oral and written study information. Written informed consent was obtained from patients and caregivers. The study followed the Helsinki Declaration's principles, and the local Medical Ethics Review Committee reviewed and approved the study. The Dutch Trial Register number for this study is NTR4718. Screening and baseline visit were scheduled on the same day or with a maximum interval of four weeks. We collected study parameters at baseline visit and after 24.7 ± 1.3 weeks of intervention. Baseline and follow-up magnetic resonance imaging (MRI) and PET scans were scheduled on the same time of the day to limit influences of the circadian rhythm on outcome parameters (both between and within patients).
At baseline, patients were 1:1 randomized to receive the intervention product, containing the specific nutrient combination Fortasyn Connect (Nutricia N.V., Zoetermeer, The Netherlands; for the nutritional composition, see Table 1) or the iso-caloric placebo drink, lacking the specific nutrient combination, but otherwise identical to the intervention product. Randomization was stratified based on the MMSE score at screening (group 1: MMSE 20–24; group 2: MMSE 25–30). Details of randomization were unknown to patients, the investigators, site staff, and study staff from Danone Nutricia Research. Patients consumed products, provided in 125-mL bottles, once daily at breakfast for a period of 24.7 ± 1.3 weeks. We checked product compliance with participant and partner at every visit and telephone call.
Table 1.
Nutritional composition of Fortasyn Connect
| Component | Amount per daily dose∗ |
|---|---|
| Eicosapentaenoic acid | 300 mg |
| Docosahexaenoic acid | 1200 mg |
| Phospholipids | 106 mg |
| Choline | 400 mg |
| Uridine monophosphate | 625 mg |
| Vitamin E | 40 mg |
| Selenium | 60 μg |
| Vitamin B12 | 3 μg |
| Vitamin B6 | 1 mg |
| Folic acid | 400 μg |
| Vitamin C | 80 mg |
One bottle (125 mL) a day.
2.3. Main exploratory outcome parameters
Main exploratory outcome parameters included the effect of the 24-week intervention with intervention or placebo product on cerebral glucose metabolism, assessed with [18F]FDG-PET imaging using quantification of regional cerebral metabolism rate for glucose (CMRglc) by
-
1.
absolute quantitative values using arterial sampling and kinetic analysis and
-
2.
relative semiquantitative standardized uptake value ratios (SUVrs) with the cerebellum as the normalization region and within a predefined standard uptake time interval of 45–60 minutes after injection.
Main outcome parameters were explored using region of interest (ROI) analyses.
2.4. Additional exploratory outcome parameters
In addition, the main parameters as described previously were explored using additional anatomic ROIs, using voxel-based analyses, and using a previously validated AD discrimination tool (i.e. the Probability of Alzheimer's disease (PALZ) tool [15]). Methodologic details are provided in Supplementary Material.
Additional exploratory outcome parameters of the NL-ENIGMA study, as defined previously [1], will be analyzed and published separately.
2.5. PET assessment and analyses
Dynamic 60-minute [18F]FDG-PET scans (21 frames) were performed on a Philips TF PET-CT “Ingenuity” scanner. All scans, at baseline and follow-up, were made on the same scanner. Patients were in fasting state for at least eight hours preceding the scanning. PET scans were also preceded by the placement of an arterial cannula in one of the radial or brachial arteries. A head holder with band restricted movements of the head. PET scans were preceded by a low-dose CT scan for attenuation correction of PET data. Scans were performed with dimmed lights. Simultaneously with an injection of 187 ± 8 MBq [18F]FDG, dissolved in 5 mL of saline, a 60-minute dynamic emission scan was initiated. Standard corrections for dead time, decay, attenuation, randoms, and scatter were performed.
2.5.1. Coregistration to MRI
For coregistration of PET images, MRIs were acquired on a 3T whole-body MR system (Signa HDxt; GE Medical Systems Milwaukee, WI, USA) using an eight-channel head coil at baseline and follow-up visits. The scan protocol included a T1-weighted three-dimensional magnetization-prepared rapid acquisition gradient echo (slice thickness 1 mm, 180 slices, matrix size 256x256, voxel size 1 x 1 x 1 mm, echo time 3.2 ms, repetition time 8.2 ms, flip angle 12°). MRIs were aligned to corresponding PET images using a mutual-information algorithm.
2.5.2. Quantitative values
Dynamic scanning and blood sampling started together with tracer administration. Arterial blood was withdrawn continuously by a continuous well-sampler (5 mL/min for the first 5 minutes, 2.5 mL/min thereafter until 60 minutes after injection) and manually at set times (5, 10, 20, 40, and 60 minutes). Continuous withdrawal was interrupted briefly for the collection of manual blood samples (5 mL). The [18F]FDG concentrations of the manual samples were determined by high-performance liquid chromatography, which values were used to calibrate the continuous sampler. The plasma input function was derived by multiplying the measured whole-blood curve with the average plasma-to-blood ratios obtained from the discrete samples. [18F]FDG influx rate constants (Ki) images were calculated using the Patlak method with the plasma input function [16]. Because net [18F]FDG uptake, described by Ki, is directly proportional to glucose metabolic rate by multiplication with the plasma glucose concentration, the Ki results of the present study were valid for CMRglc as well.
2.5.3. Semiquantitative values
Semiquantitative SUVr were normalized to cerebellar gray matter and measured within a predefined standard uptake time interval of 45–60 minutes after injection.
2.5.4. ROI-based analyses
Quantitative and semiquantitative measures were first analyzed using predefined ROIs based on 1) an anatomic template and 2) a predefined explicit mask. Anatomic ROIs, as defined by the Hammers template [17], were delineated on coregistered MRI scans. Next, time-activity curves were generated by projecting defined ROIs onto the dynamic PET frames. Five anatomic ROIs were selected based on embedding ROIs that were previously identified in a meta-analysis focusing on detecting attenuation of cognitive decline (‘MetaROIs’), including 209 cross-sectional or correlational and 31 longitudinal [18F]FDG-PET studies in MCI and AD—i.e., left and right inferolateral remainder of the parietal lobes (embedding the angular gyri), bilateral posterior cingulate gyri, left and right middle and inferior temporal gyri, and the (volume weighted) composite score of these five anatomical ROIs. In addition, the comparison was repeated, analyzing group differences in terms of MetaROIs using the publicly available MetaROIs mask [18], including left and right angular gyri, bilateral posterior cingulate gyri, left and right middle/inferior temporal gyri, and the composite score of these five MetaROIs. Statistical analyses were performed for each MetaROI.
2.6. Statistical analyses
Considerations for the chosen sample size of 40 study completers were described previously [1]. Analyses were performed using SPSS, version 22 (IBM SPSS Statistics, Version 22.0, Released 2013; IBM Corp., Armonk, NY). Baseline characteristics were analyzed for the intention-to-treat population using χ2 tests, analysis of variance, or nonparametric tests when appropriate. Continuous variables were log-transformed when not normally distributed. Efficacy analyses were performed on the per-protocol populations as they were defined before unblinding in a data review meeting: 1) Analyses of absolute quantitative measures were performed on the PP-PETq (per-protocol population for the quantitative PET measure), that is, patients who completed follow-up without uncertainty of product compliance at the study staff and for whom reliable PET measures were available of both baseline and follow-up PET scans, including successful arterial blood sampling, and 2) analyses of relative semiquantitative measures were performed on the PP-PET (per-protocol population for semiquantitative PET measures), that is, patients who completed follow-up without uncertainty of product compliance at the study staff and for whom reliable PET measures were available of both baseline and follow-up PET scans. Additional analyses were performed on PP-PET+ and PP-PETq+ populations, in which in addition to the criteria described for PP-PET and PP-PETq, patients strictly followed criteria for use of nutritional supplements.
For analyses based on predefined anatomic ROIs, MetaROIs, and PALZ scores, derived values (quantitative and semiquantitative time-activity curves per [composite] ROI and PALZ scores) were analyzed separately using SPSS. Changes in main outcome measures were compared between the intervention and placebo groups using analysis of covariance, adjusted for the baseline value of the outcome measure of interest and adjusted for the MMSE score at screening. Significance level for analyses of outcome variables was set at P < .05 in a two-sided test. Owing to the explorative character of this study and expected correlation between outcomes, no statistical correction for multiple testing was made.
3. Results
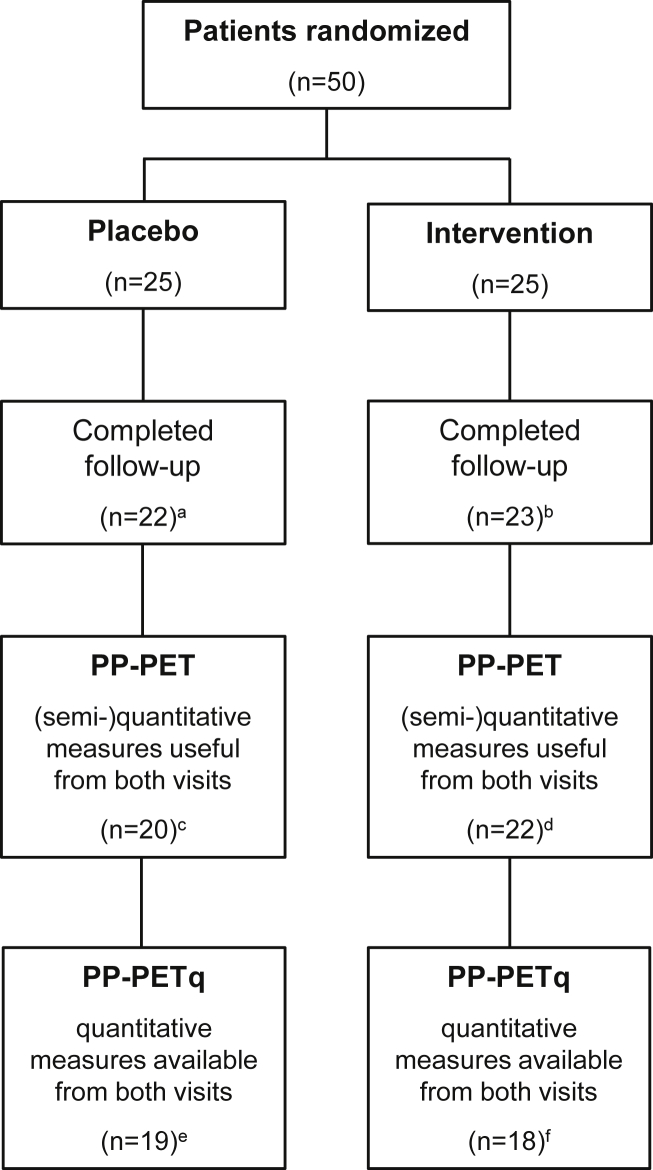
In total, 50 patients were randomized to intervention between March 2015 and August 2017. Mean age was 66 ± 7 years, and mean MMSE score was 25 ± 3. Patients were randomized at baseline to either the intervention product or the placebo product (n = 25, of whom n = 11 in MMSE stratum 20–24, and n = 14 in MMSE stratum 25–30 in both study groups; Fig. 1). Baseline characteristics of all randomized patients are summarized in Table 2; there were no differences between study groups. Product compliance was good in both treatment groups (in the PP-PET population, the percentage of days between baseline and 24-week visit that the study product was used based on participants' diary was 99% in the intervention group and 98% in the placebo group). One patient (2%) discontinued the study after six days because of second thoughts regarding the possibility to be randomized to a placebo product in combination with restrictions to use own high-dosed supplements. Seven patients (14%) were excluded from the PP-PET population because PET scan quality was not acceptable due to head motion (n = 4) or technical problems during scanning (n = 1), serious doubt by investigators about product compliance (n = 1), or discontinuation of the study after unsuccessful placement of the arterial cannula at baseline (n = 1). Another five patients (10%) were excluded from PP-PETq population because placement of the arterial cannula was not successful (n = 4) or due to malfunction of the arterial sampler (n = 1), during follow-up PET scan. Final per-protocol populations included 37 patients for quantitative analyses (PP-PETq population; randomized to either intervention product [n = 18] or placebo [n = 19]) and 42 patients for semiquantitative analyses (PP-PET population; randomized to either intervention product [n = 22] or placebo [n = 20]). For additional subgroup analyses, five patients were excluded from the PP-PET+ and PP-PETq+ population because they did not strictly follow criteria for use of nutritional supplements during the washing-out period (n = 4) or during the complete intervention period (n = 1). Two serious adverse events were reported—one elective re-replacement of hip prosthesis one week after follow-up visit (but before the last telephone visit at 26 weeks) in the intervention group and one transient ischemic attack in the placebo group. On a total of 36 adverse events, four adverse events were judged by the treating physician as possibly related to the study product.
Fig. 1.
Patient disposition. Abbreviations: PET, positron emission tomography; PP-PET, per-protocol population for all PET measures; PP-PETq, per-protocol population for the quantitative PET measure. a Discontinuation of participants due to patient withdrawal (n = 1), head motion (n = 1), or technical problems (n = 1) during baseline PET scan; b Discontinuation of patients because of unsuccessful placement of the arterial cannula (n = 1) or head motion (n = 1) during baseline PET scan; c Exclusion from PP-PET because of head motion during follow-up PET scan (n = 2); d Exclusion from PP-PET because of product incompliance (n = 1); e Exclusion from PP-PETq because of unsuccessful placement of the arterial cannula at follow-up PET scan (n = 1); f Exclusion from PP-PETq because of unsuccessful placement of the arterial cannula (n = 2) or technical issues with arterial sampling (n = 2) during follow-up PET scan. For additional subgroup analyses, five patients were excluded from the PP-PET+ and PP-PETq+ population because patients did not strictly follow criteria for use of nutritional supplements during the washing-out period (n = 2 in placebo group, n = 2 in intervention group) or during follow-up (n = 1 in placebo group), although strictly requested.
Table 2.
Baseline characteristics intention-to-treat population
| N | Placebo group, n = 25 | Intervention group, n = 25 | |
|---|---|---|---|
| Age (years) | 50 | 66 ± 8 | 65 ± 7 |
| Female | 50 | 11 (44) | 12 (48) |
| Education | 50 | 6 (4–7) | 5 (4–7) |
| Positive family history∗ | 50 | 7 (28) | 6 (24) |
| Apolipoprotein E ε4 carrier | 46 (25:21) | 21 (84) | 16 (76) |
| Body weight (kg) | 50 | 75 ± 13 | 73 ± 17 |
| BMI (kg/m2) | 45 (23:22) | 24 ± 3 | 25 ± 4 |
| Systolic blood pressure (mmHg) | 49 (24:25) | 147 ± 18 | 151 ± 23 |
| Diastolic blood pressure (mmHg) | 49 (24:25) | 86 ± 9 | 88 ± 11 |
| Pulse (/min) | 49 (24:25) | 61 ± 11 | 66 ± 7 |
| Fasting glucose before PET | 50 | 5.2 ± 0.52 | 5.4 ± 0.72 |
| MMSE† | 50 | 25 ± 3 | 25 ± 3 |
| RAVLT immediate recall (sum of 5 trials) | 50 | 28 (8–39) | 29 (14–41) |
| RAVLT delayed recall | 49 (24:25) | 2 (0–6) | 2 (0–10) |
| TMT version A (sec) | 50 | 48 (18–140) | 46 (27–225) |
| TMT version B (sec) | 42 (21:21) | 85 (47–299) | 136 (56–334) |
| MTA (mean of left and right)† | 50 | 1.5 (0–3) | 1.0 (0–3) |
| WMH† | 50 | 1 (0–2) | 1 (0–2) |
| CSF tau/amyloid-β 1–42† | 44 (22:22) | 1.35 ± 0.83 | 1.27 ± 0.46 |
| Abnormal amyloid PET scan | 20 (13:7) | 13 (100) | 7 (100) |
NOTE. Data are presented in mean ± standard deviation, median (minimum-maximum), or n (%). Education is calculated according to the Verhage scale (1–7, respectively, low-high education [19]). Using ANOVA, Kruskal-Wallis, or χ2 analyses when appropriate, no statistically significant differences between groups were found.
Abbreviations: AD, Alzheimer's disease; CSF, cerebrospinal fluid; MMSE, Mini–Mental State Examination; PET, positron emission tomography; MRI, magnetic resonance imaging; RAVLT, Rey Auditory Learning Verbal Learning Test; TMT, Trail Making Test time to complete; MTA, medial temporal lobe atrophy visual rating scale (0–4) in which higher scores reflect more severe atrophy [20]; WMH, white matter hyperintensities based on the Fazekas rating scale (0–3) in which higher scores reflex more white matter lesions [21]; CSF tau/amyloid-β 1–42, cerebrospinal fluid total tau/amyloid β 1–42, in which a value > 0.52 has found to be associated with AD [14].
Positive family history = first-degree family member with AD before age 66 years.
Screening variables: MMSE at screening visit, MRI within one year before inclusion, CSF, or amyloid PET at any time before baseline.
3.1. ROI-based analyses
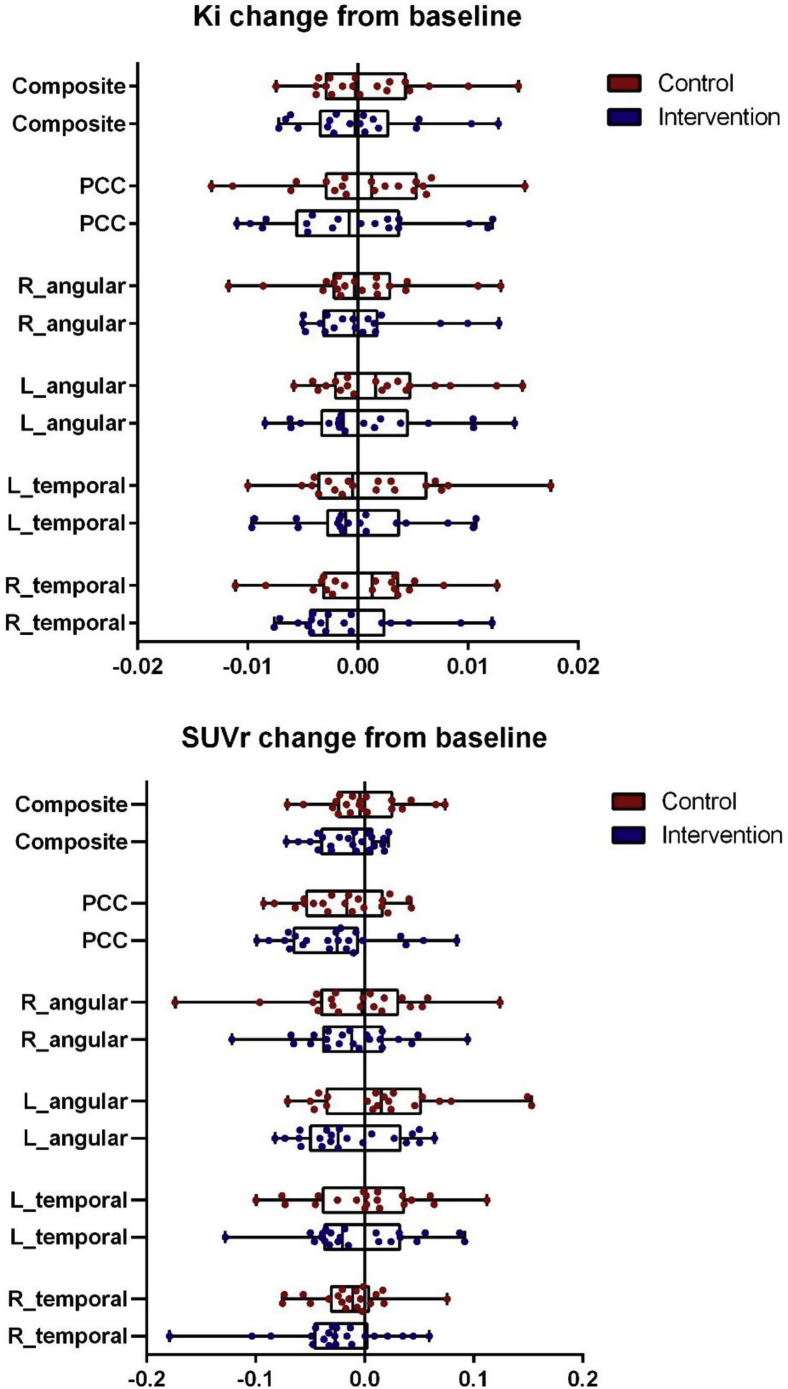
Quantitative and semiquantitative values were first derived per the predefined anatomic template-based ROI, and a composite score of these five ROIs was calculated. Quantitative baseline composite scores were 0.025 ± 0.006 in the intervention group and 0.026 ± 0.005 in the placebo group (P = .47); semiquantitative baseline composite scores were 1.02 ± 0.10 in the intervention group and 1.01 ± 0.09 in the placebo group (P = .71). Change in glucose metabolism over time was explored using calculated delta (follow-up minus baseline) values for each ROI and outcome measure separately (data shown in Tables 3, Table 4 and Fig. 2). Only the semiquantitative delta value of the inferolateral remainder of the left parietal lobe reached borderline significance (−0.01 ± 0.04 in the intervention group vs. 0.02 ± 0.06 in the placebo group, P = .05). Delta quantitative values of the compound ROI were −0.002 ± 0.003 in the intervention and −0.001 ± 0.004 in the placebo group (P = .57); semiquantitative delta values of the compound score were −0.02 ± 0.03 in the intervention group compared with −0.00 ± 0.04 in the placebo group (P = .14).
Table 3.
Quantitative (Ki) [18F]FDG-PET characteristics per template-based ROI in PP-PETq population
| Variable (mean ± SD) | Placebo group, n = 19 | Intervention group, n = 18 |
|---|---|---|
| Inferolateral remainder of the left parietal lobe (embedding the angular gyrus) | ||
| Baseline | 0.027 ± 0.005 | 0.026 ± 0.006 |
| Follow-up | 0.027 ± 0.004 | 0.025 ± 0.005 |
| Delta | 0.000 ± 0.004 | −0.001 ± 0.004 |
| Inferolateral remainder of the right parietal lobe (embedding the angular gyrus) | ||
| Baseline | 0.025 ± 0.006 | 0.024 ± 0.006 |
| Follow-up | 0.024 ± 0.005 | 0.022 ± 0.005 |
| Delta | −0.002 ± 0.005 | −0.002 ± 0.004 |
| Bilateral posterior cingulate gyrus | ||
| Baseline | 0.030 ± 0.006 | 0.029 ± 0.008 |
| Follow-up | 0.028 ± 0.006 | 0.026 ± 0.005 |
| Delta | −0.002 ± 0.006 | −0.003 ± 0.005 |
| Left middle/inferior temporal gyrus | ||
| Baseline | 0.026 ± 0.004 | 0.025 ± 0.005 |
| Follow-up | 0.025 ± 0.004 | 0.023 ± 0.003 |
| Delta | −0.001 ± 0.004 | −0.002 ± 0.004 |
| Right middle/inferior temporal gyrus | ||
| Baseline | 0.025 ± 0.005 | 0.023 ± 0.006 |
| Follow-up | 0.023 ± 0.004 | 0.020 ± 0.005 |
| Delta | −0.002 ± 0.004 | −0.003 ± 0.003 |
| Composite of ROIs | ||
| Baseline | 0.026 ± 0.005 | 0.025 ± 0.006 |
| Follow-up | 0.025 ± 0.004 | 0.023 ± 0.004 |
| Delta | −0.001 ± 0.004 | −0.002 ± 0.003 |
NOTE. Quantitative (Ki) values for ROIs (regions of interest) derived using the Hammers template [17] in the PP-PETq population. Using ANOVA, no statistically significant differences between groups were found (baseline, follow-up, and difference [follow-up minus baseline values] analyzed separately).
Abbreviations: ANOVA, analysis of variance; [18F] FDG, 18F-fluorodeoxyglucose; PET, positron emission tomography; ROI, region of interest.
Table 4.
Semiquantitative (SUVr) [18F]FDG-PET characteristics per template-based ROI in PP-PET population
| Variable (mean ± SD) | Placebo group, n = 20 | Intervention group, n = 22 |
|---|---|---|
| Inferolateral remainder of the left parietal lobe (embedding the angular gyrus) | ||
| Baseline | 1.04 ± 0.09 | 1.07 ± 0.11 |
| Follow-up | 1.06 ± 0.08 | 1.05 ± 0.11 |
| Delta | 0.02 ± 0.06 | −0.01 ± 0.04∗ |
| Inferolateral remainder of the right parietal lobe (embedding the angular gyrus) | ||
| Baseline | 0.96 ± 0.14 | 0.97 ± 0.12 |
| Follow-up | 0.95 ± 0.12 | 0.96 ± 0.13 |
| Delta | −0.01 ± 0.06 | −0.01 ± 0.05 |
| Bilateral posterior cingulate gyrus | ||
| Baseline | 1.12 ± 0.10 | 1.13 ± 0.15 |
| Follow-up | 1.10 ± 0.11 | 1.11 ± 0.14 |
| Delta | −0.02 ± 0.04 | −0.03 ± 0.05 |
| Left middle/inferior temporal gyrus | ||
| Baseline | 1.00 ± 0.07 | 1.01 ± 0.10 |
| Follow-up | 1.00 ± 0.07 | 1.00 ± 0.10 |
| Delta | 0.00 ± 0.05 | −0.01 ± 0.05 |
| Right middle/inferior temporal gyrus | ||
| Baseline | 0.94 ± 0.09 | 0.93 ± 0.12 |
| Follow-up | 0.92 ± 0.09 | 0.90 ± 0.14 |
| Delta | −0.01 ± 0.03 | −0.03 ± 0.05 |
| Composite of ROIs | ||
| Baseline | 1.01 ± 0.09 | 1.02 ± 0.10 |
| Follow-up | 1.01 ± 0.08 | 1.00 ± 0.10 |
| Delta | 0.00 ± 0.04 | −0.02 ± 0.03 |
NOTE. Semiquantitative (SUVr) values for ROIs (regions of interest) derived using the Hammers template [17] in the PP-PET population. Using ANOVA, no statistically significant differences between groups were found (baseline, follow-up, and difference [follow-up minus baseline values] analyzed separately), except for delta values of the inferior lateral remainder of the left parietal lobe (∗P = .05).
Abbreviations: ANOVA, analysis of variance; [18F] FDG, 18F-fluorodeoxyglucose; PET, positron emission tomography; ROI, region of interest; SUVr, standardized uptake value ratio.
Fig. 2.
Boxplots showing the difference between follow-up and baseline quantitative (Ki) and semiquantitative (SUVr) values. Abbreviations: SUVr, standardized uptake value ratio; PCC, bilateral posterior cingulate gyrus; R_angular, inferolateral remainder of the right parietal lobe (embedding the angular gyrus); L_angular, inferolateral remainder of the left parietal lobe (embedding the angular gyrus); L_temporal, left middle/inferior temporal gyrus; R_temporal, right middle/inferior temporal gyrus.
Change in glucose metabolism over time was further explored using delta values as dependent variables in analyses of covariance, adjusted for the baseline value of interest and adjusted for total MMSE score at screening visit. No differences were found between the intervention and placebo groups (in the composite ROI, P = .18 and P = .22 for quantitative and semiquantitative measures, respectively). In addition, change in glucose metabolism in the cerebellar gray matter was estimated by quantitative analysis to assess the effect of the intervention on the normalization region of the semiquantitative analyses. No treatment effect on the normalization area was observed (mean change intervention 0.001 ± 0.006; placebo 0.001 ± 0.005; P = .20).
When the explicit MetaROIs [18] were applied instead of the anatomic template-based ROIs, paired t-tests between baseline and follow-up showed a decrease of semiquantitative measures of the bilateral posterior cingulate (−0.032 ± 0.052, P = .014) in the placebo group and a decrease of the left angular gyrus (−0.026 ± 0.055, P = .036) and composite MetaROI (−0.022 ± 0.041, P = .020) in the intervention group. The quantitative measures did however not support these findings (−0.003 ± 0.007, P = .083; 0.001 ± 0.005, P = .258; 0.002 ± 0.005, P = .154). Quantitative delta values of the composite ROI were −0.002 ± 0.005 in the intervention and −0.001 ± 0.004 in the placebo group (P = .874); semiquantitative delta values of the compound score were −0.02 ± 0.04 in the intervention group and −0.01 ± 0.04 in the placebo group (P = .282). When the quantitative and semiquantitative delta values were assessed by analysis of covariance, adjusting for baseline value and MMSE score at screening visit, no differences were found between the intervention and placebo groups.
In addition, main parameters were explored in additional template-based ROIs, using voxel-based analyses and using a previously validated AD discrimination tool [15]. Results are provided in Supplementary Material.
4. Discussion
Previous studies described effects of the specific nutrient combination on memory function, functional connectivity, and brain network organization (based on electroencephalography), suggesting that this combination of nutrients influences synapse function in AD [5], [6], [7]. In the NL-ENIGMA study, exploring the effect of a nutritional intervention on synapse function and formation using ROI-based and voxel-based [18F]FDG-PET analyses, we were not able to find robust differences between the intervention and placebo groups after the 24-week intervention in MCI or mild dementia due to AD. Findings are possibly hampered due to the short follow-up duration.
The absence of significant signal in the present study could be related to methodological aspects. First, the placebo group did not show substantial decrease of cerebral metabolism over 24 weeks; ROI-based analyses resulted in a delta composite score of anatomic template-based ROIs of −0.001 ± 0.004 using quantitative measures and 0.00 ± 0.04 using semiquantitative measures, and similar results were found when using the specific MetaROI coordinates (−0.001 ± 0.004 using quantitative measures and −0.01 ± 0.04 using semiquantitative measures). When comparing baseline CMRglc with follow-up, the placebo group only significantly decreased in semiquantitative (but not quantitative) values in the bilateral posterior cingulate. Most likely, the follow-up duration of 24 weeks was too short to capture substantial changes in glucose metabolism in this placebo group with relative mild disease severity. Alternatively, it could be speculated that the expected decrease in metabolism after 24 weeks in the placebo group was limited as a result of compensatory hypermetabolism in the least impaired (predementia) patients. This is a phenomenon previously found in cognitive normal patients with amyloid burden [22], [23] and apolipoprotein E (APOE) ε4–positive genotype [24] and that was found to be present in MCI but absent in dementia due to AD [25]. However, when difference in the change of cerebral glucose metabolism over time (delta quantitative and semiquantitative measures) between MMSE strata (group 1: MMSE 20–24; group 2: MMSE 25–30) was further analyzed in anatomic ROIs using analysis of variance, no differences were found between MMSE strata (data not shown).
Second, the follow-up duration of 24 weeks may have been too short because 1) more time is needed to reliably capture disease progression by [18F]FDG-PET and 2) treatment should have sufficient duration to have its beneficial effect. In the last few years, multiple clinical studies with Souvenaid have been performed and especially those in the early phases of AD have yielded positive treatment effects on various clinical outcome measures. For instance, in patients with mild AD, Scheltens et al. [5] reported improved memory as measured by the delayed verbal recall task of the Wechsler Memory Scale–revised after a 12-week follow-up. Also in mild AD, Scheltens et al. [6] showed an improvement on the neuropsychological test battery memory domain and electroencephalography functional connectivity in the delta band after 24 weeks. In contrast, a recent large study by Soininen et al. [26] investigated the effect of Souvenaid in prodromal AD subjects, a population close to the current population with MCI or mild dementia due to AD. In this study, a positive effect on the clinical dementia rating sum of boxes and hippocampal and ventricular volume on MRI was found after 2 years of treatment, although the effects were not yet observed at the study's 1-year half-way point. The results of these studies combined with the effects found in our study indicate that a study duration of 2 years might be preferable to capture potential treatment effects.
Third, [18F]FDG-PET has been suggested as an appropriate measure to assess synapse function [9], [10], [25], but it is unclear whether this measure is suitable to capture changes over time in synapse formation and function, which is the target of interest of the current nutritional intervention. The possibility that results could be driven by the use of restricted high-dosed concomitant supplements was checked with repetition of analyses in PP-PET+ and PP-PETq+ populations, yielding similar results.
In this exploratory trial, two outcome measures of CMRglc were used: quantitative (Ki) and semiquantitative (SUVr) measures. The main rationale behind this methodological approach is that the SUVr uses a normalization region to produce an estimation of the CMRglc. This normalization region, in our case the cerebellar gray matter, might also be influenced by the intervention [1]. However, no effect of the intervention was found on the normalization region when analyzed using absolute quantitative measures (Ki). Therefore, the semiquantitative measure most likely produces reliable estimations of the CMRglc in our study.
Various additional analyses were done to exclude the possibility of negative results due to the PET-analysis methodology. Primary results were based on predefined ROIs from literature [18], therefore additionally other ROIs were analyzed but none of these yielded significant results (Supplementary Table Ia and Ib). When exploratory voxel-based analyses were performed, the placebo group was characterized by a lower uptake in the left inferior temporal gyrus at follow-up compared with baseline value (quantitative measures), suggesting local decrease in metabolism. Furthermore, a small increase in mean PALZ scores was seen (suggesting disease progression), albeit accompanied by large confidence intervals. None of these described methods produced significant or consistent differences between groups.
Strengths of our study include the double-blind randomized design, the availability of continuously drawn arterial blood during dynamic [18F]FDG-PET scanning, and the use of predefined ROIs as primary outcome. Another strength is that patients were included based on presence of abnormal AD biomarkers (i.e. cerebrospinal fluid tau/amyloid-β 1–42 > 0.52 [14] or abnormal amyloid PET), evidencing underlying AD pathology in included patients with mild dementia or MCI. Furthermore, the exploratory setting provides a methodological framework for further clinical trials in AD with [18F]FDG-PET.
In conclusion, quantitative and semiquantitative [18F]FDG-PET measures were not able to capture an effect of 24-week intervention with Souvenaid in an exploratory, randomized, controlled, double-blind trial including 50 patients with early AD. Possibly, follow-up duration is too short to capture robust treatment effect of a nutritional intervention on cerebral glucose metabolism.
Research in Context.
-
•
Systematic review: Alzheimer's disease is associated with synaptic loss in early stages. Glucose metabolism, measured with 18F-fluorodeoxyglucose positron emission tomography, is a direct index for synapse function and density. We searched PubMed for publications regarding the effect of nutritional interventions on glucose metabolism in Alzheimer's disease.
-
•
Interpretation: In an exploratory single-center clinical trial (1:1 randomization, double-blinded, n = 50), we were not able to capture increase in glucose metabolism after 24 weeks daily use of Souvenaid by quantitative or semiquantitative 18F-fluorodeoxyglucose positron emission tomography measures in early Alzheimer's disease.
-
•
Future directions: Present findings provide further information for future intervention studies, investigating the effect of a nutritional intervention on glucose metabolism. A longer follow-up duration is needed to capture decrease in glucose metabolism in the placebo group and to investigate whether Souvenaid has a stabilizing effect on synapse function and formation.
Acknowledgments
The authors would like to thank all the participants of the NL-ENIGMA study for their effort and commitment. Furthermore, the NL-ENIGMA study is a collaboration between the Amsterdam University Medical Centers (UMC) Alzheimer Center and Danone Nutricia Research. Amsterdam UMC is sponsor and responsible for all aspects of the clinical study. Danone Nutricia Research supported as follows:
-
•
Danone Nutricia Research was responsible for the production of the study products and the distribution of the study products to Amsterdam UMC.
-
•
Danone Nutricia Research contributed in safety monitoring.
-
•
Danone Nutricia Research contributed scientific expertise for the clinical study.
-
•
Study monitoring was the responsibility of Amsterdam UMC. Danone Nutricia Research performed co-monitoring.
-
•
Amsterdam UMC investigators have access to the final data set. Danone Nutricia Research provided additional statistical input.
Footnotes
The NL-ENIGMA study is an investigator-initiated study funded by the Netherlands Organization for Scientific Research (NWO) within the Food, Cognition and Behavior (FCB) initiative, project No. 057-13-003. N.M.E.S. and C.T.B. are funded by NWO (project nr 057-13-003). F.B. serves as a consultant to Biogen-Idec, Janssen Alzheimer Immunotherapy, Bayer-Schering, Merck-Serono, Roche, Novartis, Genzyme, and Sanofi-Aventis. He has received sponsoring from EU-H2020, NWO, SMSR, EU-FP7, TEVA, Novartis, and Toshiba; he is supported by the NIHRUCLH biomedical research center, and he is a member of editorial boards of Radiology, Brain, Neuroradiology, MSJ, and Neurology. C.E.T. serves on the advisory board of Fujirebio and Roche, received research consumables from Euroimmun, IBL, Fujirebio, Invitrogen, and Meso Scale Discovery, and performed contract research for IBL, Shire, Boehringer, Roche, and Probiodrug; and received grants from the European Commission, the Dutch Research Council (ZonMW), Association of Frontotemporal Dementia/Alzheimer's Drug Discovery Foundation, ISAO, and the Alzheimer's Drug Discovery Foundation. Dr. Teunissen received research consumables from Euroimmun, IBL, Fujirebio, Invitrogen, and Meso Scale Discovery and performed contract research for IBL, Shire, Boehringer, Roche, and Probiodrug. A.A. and L.M.v.B. are employees of Danone Nutricia Research. P.S. is fully employed by Amsterdam University Medical Centers and has received grant support (for the institution) from GE Healthcare, Nutricia Research, Piramal, and MERCK. In the past 2 years, he has received consultancy/speaker fees (paid to the institution) from Probiodrug, EIP Pharma, Piramal, and GE Healthcare. R.B., W.M.v.d.F., and B.N.M.v.B. report no conflicts of interest.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.trci.2019.08.002.
Supplementary Data
References
- 1.Scheltens N.M.E., Kuyper I.S., Boellaard R., Barkhof F., Teunissen C.E., Broersen L.M. Design of the NL-ENIGMA study: Exploring the effect of Souvenaid on cerebral glucose metabolism in early Alzheimer's disease. Alzheimers Dement (N Y) 2016;2:233–240. doi: 10.1016/j.trci.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wurtman R.J., Cansev M., Sakamoto T., Ulus I.H. Use of phosphatide precursors to promote synaptogenesis. Annu Rev Nutr. 2009;29:59–87. doi: 10.1146/annurev-nutr-080508-141059. [DOI] [PubMed] [Google Scholar]
- 3.van Wijk N., Broersen L.M., de Wilde M.C., Hageman R.J.J., Groenendijk M., Sijben J.W.C. Targeting synaptic dysfunction in Alzheimer's disease by administering a specific nutrient combination. J Alzheimers Dis. 2014;38:459–479. doi: 10.3233/JAD-130998. [DOI] [PubMed] [Google Scholar]
- 4.Mi W., van Wijk N., Cansev M., Sijben J.W.C., Kamphuis P.J.G.H. Nutritional approaches in the risk reduction and management of Alzheimer's disease. Nutrition. 2013;29:1080–1089. doi: 10.1016/j.nut.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 5.Scheltens P., Kamphuis P.J.G.H., Verhey F.R.J., Olde Rikkert M.G.M., Wurtman R.J., Wilkinson D. Efficacy of a medical food in mild Alzheimer's disease: A randomized, controlled trial. Alzheimers Dement. 2010;6:1–10.e1. doi: 10.1016/j.jalz.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Scheltens P., Twisk J.W.R., Blesa R., Scarpini E., Arnim von C.A.F., Bongers A. Efficacy of Souvenaid in mild Alzheimer's disease: results from a randomized, controlled trial. J Alzheimers Dis. 2012;31:225–236. doi: 10.3233/JAD-2012-121189. [DOI] [PubMed] [Google Scholar]
- 7.de Waal H., Stam C.J., Lansbergen M.M., Wieggers R.L., Kamphuis P.J.G.H., Scheltens P. The effect of Souvenaid on functional brain network organisation in patients with mild Alzheimer's disease: a randomised controlled study. PLoS one. 2014;9:e86558. doi: 10.1371/journal.pone.0086558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rijpma A., van der Graaf M., Lansbergen M.M., Meulenbroek O., Cetinyurek-Yavuz A., Sijben J.W. The medical food Souvenaid affects brain phospholipid metabolism in mild Alzheimer's disease: results from a randomized controlled trial. Alzheimers Res Ther. 2017;9:51. doi: 10.1186/s13195-017-0286-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reiman E.M., Chen K., Alexander G.E., Caselli R.J., Bandy D., Osborne D. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer's dementia. Proc Natl Acad Sci U S A. 2004;101:284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Wilde M.C., Kamphuis P.J.G.H., Sijben J.W.C., Scheltens P. Utility of imaging for nutritional intervention studies in Alzheimer's disease. Eur J Pharmacol. 2011;668:S59–S69. doi: 10.1016/j.ejphar.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 11.van der Flier W.M., Scheltens P. Amsterdam Dementia Cohort: Performing Research to Optimize Care. J Alzheimers Dis. 2018;62:1091–1111. doi: 10.3233/JAD-170850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albert M.S., DeKosky S.T., Dickson D., Dubois B., Feldman H.H., Fox N.C. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer‘s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R., Kawas C.H. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer‘s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duits F.H., Teunissen C.E., Bouwman F.H., Visser P.J., Mattsson N., Zetterberg H. The cerebrospinal fluid “Alzheimer profile”: easily said, but what does it mean? Alzheimers Dement. 2014;10:713–723.e2. doi: 10.1016/j.jalz.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 15.Herholz K., Salmon E., Perani D., Baron J.C., Holthoff V., Frölich L. Discrimination between Alzheimer dementia and controls by automated analysis of multicenter FDG PET. Neuroimage. 2002;17:302–316. doi: 10.1006/nimg.2002.1208. [DOI] [PubMed] [Google Scholar]
- 16.Patlak C.S., Blasberg R.G. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations. J Cereb Blood Flow Metab. 1985;5:584–590. doi: 10.1038/jcbfm.1985.87. [DOI] [PubMed] [Google Scholar]
- 17.Hammers A., Koepp M.J., Free S.L., Brett M., Richardson M.P., Labbé C. Implementation and application of a brain template for multiple volumes of interest. Hum Brain Mapp. 2002;15:165–174. doi: 10.1002/hbm.10016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landau S.M., Harvey D., Madison C.M., Koeppe R.A., Reiman E.M., Foster N.L. Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging. 2011;32:1207–1218. doi: 10.1016/j.neurobiolaging.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verhage F. Intelligence and religious persuasion. Ned Tijdschr Psychol. 1964;19:247–254. [PubMed] [Google Scholar]
- 20.Scheltens P., Launer L.J., Barkhof F., Weinstein H.C., van Gool W.A. Visual assessment of medial temporal lobe atrophy on magnetic resonance imaging: interobserver reliability. J Neurol. 1995;242:557–560. doi: 10.1007/BF00868807. [DOI] [PubMed] [Google Scholar]
- 21.Fazekas F., Chawluk J.B., Alavi A., Hurtig H.I., Zimmerman R.A. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol. 1987;149:351–356. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- 22.Oh H., Habeck C., Madison C., Jagust W. Covarying alterations in Aβ deposition, glucose metabolism, and gray matter volume in cognitively normal elderly. Hum Brain Mapp. 2014;35:297–308. doi: 10.1002/hbm.22173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson S.C., Christian B.T., Okonkwo O.C., Oh J.M., Harding S., Xu G. Amyloid burden and neural function in people at risk for Alzheimer's Disease. Neurobiol Aging. 2014;35:576–584. doi: 10.1016/j.neurobiolaging.2013.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yi D., Lee D.Y., Sohn B.K., Choe Y.M., Seo E.H., Byun M.S. Beta-amyloid associated differential effects of APOE ε4 on brain metabolism in cognitively normal elderly. Am J Geriatr Psychiatry. 2014;22:961–970. doi: 10.1016/j.jagp.2013.12.173. [DOI] [PubMed] [Google Scholar]
- 25.Cohen A.D., Klunk W.E. Early detection of Alzheimer's disease using PiB and FDG PET. Neurobiol Dis. 2014;72:117–122. doi: 10.1016/j.nbd.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soininen H., Solomon A., Visser P.J., Hendrix S.B., Blennow K., Kivipelto M. 24-month intervention with a specific multinutrient in people with prodromal Alzheimer's disease (LipiDiDiet): a randomised, double-blind, controlled trial. Lancet Neurol. 2017;16:965–975. doi: 10.1016/S1474-4422(17)30332-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.