Abstract
Objective
The objective of this study was to review the records of patients with excised abdominal wall endometriosis (AWE) to determine patient characteristics, diagnostic methods, presence of concurrent pelvic endometriosis and type of surgery.
Study design
Medical records from an 11-year period were searched to identify histologically confirmed AWE cases. Descriptive data were collected and analyzed. Two subgroups were differentiated: isolated AWE and pelvic endometriosis-associated AWE.
Results
Thirty-five women with AWE were included. The most common symptom was cyclic abdominal or parietal pain (68.6%); 17.1% of the women had no symptoms. Twenty-nine women (82.8%) had a history of gynecological or obstetrical surgery, most commonly cesarean section (CS). The mean interval between prior surgery and appearance of symptoms was 5.3 years. Six women (17.1%) had no prior surgery; all six presented with umbilical nodules, nulliparity and confirmed mild to severe pelvic endometriosis. Among all patients, 34.3% had concurrent pelvic endometriosis, 40% presented with isolated AWE and 25.7% had no pelvic exploration. Women with concurrent pelvic endometriosis had significantly lower parity, smaller nodule size and a higher likelihood of umbilical location than those with isolated AWE (p < 0.05). A history of CS was more commonly found in women with isolated AWE. The overall recurrence rate over the study period was 11.4%, with a mean follow-up period of 5.2 years.
Conclusions
AWE is an uncommon condition associated with long diagnostic and therapeutic delays. In patients with umbilical AWE and no surgical history, pelvic endometriosis is commonly present and should be highly suspected.
Keywords: Endometriosis, Abdominal wall, Cesarean section, Parietal repair
Introduction
Endometriosis is defined as ectopic endometrium-like tissue, generally located in the pelvis. Extrapelvic implants are rare, but they can be found in the bowel, lungs, kidneys, brain or anterior abdominal wall. Abdominal wall endometriosis (AWE) is defined as implantation of endometrial tissue outside the peritoneum, including lesions secondary to a surgical incision and those that arise spontaneously [1]. This terminology must be differentiated from scar endometrioma, which includes episiotomy nodules and excludes nodules in patients without a surgical history.
The reported incidence of AWE varies from 0.03 to 3.5% [2,3]. Most cases are associated with cesarean section (CS), with a relative risk of 27 for developing AWE in this population [2]. These nodules are often mistaken for granulomas, cysts, lipomas or hernias, resulting in underestimation of the incidence.
The most accepted hypothesis for the pathogenesis of AWE is that endometrial cells are directly implanted via an iatrogenic process. Other etiological theories have been proposed, such as lymphatic or hematogenous dissemination, metaplastic transformation and cell immunity modification [2,4,5].
The clinical presentation, time of occurrence and location are highly variable in AWE patients, making diagnosis difficult. In addition, there is no consensus regarding diagnostic methods. Most authors agree that surgical management with wide excision is the key to confirming a pathological diagnosis, avoiding recurrence and excluding malignancy [1,2,5].
We reviewed all cases of AWE managed at Geneva University Hospitals over an 11-year period and the current literature. The study aims were to assess demographic and clinical characteristics, diagnostic tools, surgical options, recurrence rates and association with pelvic endometriosis in patients with AWE.
Materials and methods
This retrospective, observational, descriptive study included a cohort of 35 women who underwent surgery at our institution from January 2007 to December 2017 and who had a confirmed diagnosis of AWE on histopathology. For each patient we reviewed history, characteristics, clinical presentation, diagnostic methods, nodule size and location, associated pelvic endometriosis, type of surgery and recurrence diagnosis.
We searched a prospectively maintained institutional database, using the search terms “cutaneous scar endometriosis,” “other endometriosis” and “endometriosis” as key words (International Classification of Diseases).
Approval from the local ethics committee was not required because of the type of collected data and their retrospective nature; data were stored in a secure anonymous database. All women who underwent resection of AWE provided written informed consent for surgical management of the disease.
Pelvic endometriosis lesions were determined on the basis of surgical intra-abdominal status according to the revised classification of the American Fertility Society (rAFS) [6]. We differentiated two subgroups: isolated AWE and pelvic-endometriosis-associated AWE.
We compared subgroup parameters with Student’s t test for normally distributed data and with Wilcoxon signed-rank test for non-normally distributed data. The incidence of post-CS AWE was estimated by calculating the CS delivery rate during the study period, on the assumption that all women would be referred to our center. Geneva University Hospital is both a tertiary/referral center and a primary-care institution because it is the only public hospital in the area. Patient history and follow-up during the study period were collected via medical record inspection.
Results
Thirty-five patients had confirmed endometriosis nodules of the abdominal wall that were surgically excised over an 11-year period from January 2007 to December 2017; the records of these patients were reviewed systematically. For abdominal wall lesions, the histopathological diagnosis was endometriosis. Associated pelvic lesions, when present, were characterized as deep infiltrative or superficial lesions.
Table 1 presents patients’ characteristics. The mean age at presentation was 36.05 years, with a standard deviation (SD) of 6.13 years.
Table 1.
Patient demographic data and study parameters.
| N | (%) | Mean | SD | |
|---|---|---|---|---|
| Age, years | 36.05 | 6.13 | ||
| Gravidity | 1.8 | 1.38 | ||
| Parity | 1.34 | 1.16 | ||
| Body mass index (kg/m2) | 25.55 | |||
| <18.5 | 1 | (2.8) | ||
| 18.5–24.9 | 14 | (40) | ||
| 25–29.9 | 12 | (34.3) | ||
| >30 | 7 | (20) | ||
| Unknown | 1 | (2.7) | ||
| Presenting symptoms | ||||
| Cyclic abdominal pain | 24 | (68.6) | ||
| Mass palpation | 9 | (25.7) | ||
| Cyclic bleeding | 4 | (11.4) | ||
| No symptom | 6 | (17.1) | ||
| Surgical History | ||||
| Cesarean section | 24 | (65.7) | ||
| Laparoscopy | 2 | (5.7) | ||
| Laparotomy | 3 | (8.5) | ||
| No prior surgery | 6 | (17.1) | ||
| Diagnostic tests | ||||
| Ultrasound (US) | 21 | (60) | ||
| US guided biopsy puncture | 5 | (14.3) | ||
| Computed tomography | 1 | (2.8) | ||
| Magnetic resonance imaging | 24 | (68.6) | ||
| None | 2 | (5.7) | ||
| ≥ 2 methods | 13 | (37.1) | ||
| Mass location | ||||
| Suprapubic | 25 | (71.4) | ||
| Umbilical | 10 | (28.6) | ||
| Layer involvement | ||||
| Suprafascial | 9 | (36) | ||
| Fascia involvement | 16 | (64) | ||
| Concurrent pelvic endometriosis | ||||
| Yes | 12 | (34.3) | ||
| No | 14 | (40) | ||
| Not explored | 9 | (25.7) | ||
| Recurrence | 4 | (11.4) | ||
| Resection results | ||||
| In toto | 33 | (94.3) | ||
| Positive margin | 2 | (5.7) | ||
| Primary mesh repair | 3 | (8.6) | ||
| Nodule size (cm) | 2.38 | 1.24 | ||
Six women (17.1%) had no history of prior surgery; all six presented with umbilical nodules, nulliparity and confirmed mild to severe pelvic endometriosis according to the rAFS. The remaining 29 women (82.8%) had a history of gynecological or obstetrical surgery, most commonly CS. In all CS cases, nodules were located in the incisional scar. The estimated incidence of AWE after CS was 0.23% (total number of CS during this period = 10,870). Two women had a history of operative laparoscopy, one for ovarian cystectomy and one for supracervical hysterectomy. The first patient presented with umbilical pain and bleeding 4 years after laparoscopy. She was found to have deep infiltrative pelvic endometriosis corresponding to rAFS stage III. The second patient presented with a left suprapubic nodule and minimal pelvic endometriosis (rAFS). Three other women had a history of laparotomy: two via midline incision for multiple myomectomy and one via mini-Pfannenstiel incision for tissue extraction after supracervical laparoscopic hysterectomy. Finally, one patient with an umbilical nodule and a surgical history had undergone appendectomy via McBurney laparotomy, followed by umbilical hernia repair and resection of the first nodule. Two years later she presented with a recurrent umbilical nodule; laparoscopy revealed rAFS stage IV pelvic endometriosis.
Most women (54.3%) were overweight (body mass index ≥ 25 kg/m2). The most common presenting symptom was cyclic abdominal or parietal pain (68.6%). Less frequently, women reported the presence of an abdominal mass (25.7%) or cyclic bleeding (11.4%). In some patients, multiple symptoms were present. Interestingly, all women presenting with cyclic bleeding had umbilical endometriosis. The mean interval between prior surgery and the appearance of AWE-related symptoms was 5.28 years (SD 3.7 years).
The mean nodule size (largest diameter) was 2.38 cm (SD 1.24 cm). Most nodules (71.4%) were found in the suprapubic area. Fascial and/or rectus abdominis muscle invasion was observed in 64% of women presenting with suprapubic nodules. Only three women required primary mesh repair, which represents 18.7% of the 16 women with nodules invading the fascia. These patients underwent parietal repair with polypropylene mesh (15 × 20 cm) placed in a retromuscular position. Two pathological specimens had positive margins but did not develop clinical recurrence. The overall recurrence rate was 11.4%, with a mean follow-up period of 5.2 years (SD 3.3 years). The mean time until recurrence was 3.4 years (SD 2.5 years).
Thirty-four percent of women with AWE had concurrent pelvic endometriosis; 40% presented with isolated AWE. Nine women had no pelvic exploration because there were no clinical or radiological signs suggestive of pelvic endometriosis. Analyses of the two subgroups are shown in Table 2. Women with concurrent pelvic endometriosis had significantly lower parity than those with isolated AWE. Nodule size was significantly larger in isolated AWE cases. The presence of umbilical nodules was significantly more common among women with pelvic endometriosis.
Table 2.
Comparison of study parameters according to presence of concurrent pelvic endometriosis.
| Isolated AWE (N = 14) | Concurrent pelvic endometriosis (N = 12) | P value | |
|---|---|---|---|
| Age (years) | 36.5 | 33.9 | 0.24 |
| Parity | 1.78 | 0.75 | 0.03 |
| Body mass index (kg/m2) | 26 | 24.6 | 0.16 |
| Cesarean section (%) | 71.4 | 41.6 | 0.12 |
| Nodule size (cm) | 3.1 | 1.6 | 0.003 |
| Umbilical nodule (%) | 14.2 | 66.6 | 0.006 |
| Suprapubic nodule (%) | 33.3 | 85.7 | 0.006 |
Discussion
The present study identified 35 cases of AWE treated at an academic and referral center. The interval between prior surgery and the appearance of AWE-related symptoms is among the longest reported in the literature, suggesting a possible delay in AWE recognition because of its rarity. The percentage of overweight women in our study was clearly higher than that in the general Swiss population (33%) [18]. This finding corroborates the observation of Khan et al. that women presenting with AWE have higher BMIs than controls [19].
Various rates of concurrent pelvic endometriosis have been reported in patients with AWE [[1], [2], [3], [4],7,8], ranging from 0% to 25%, which is close to our results (34.2%). Most authors have concluded that this rate is comparable to the rate of classical endometriosis in the general population and is thus not relevant [1,2,7]. However, prior studies have not analyzed these rates according to different patterns and with stratification of severity. Our comparison of subgroups according to the presence of concurrent pelvic endometriosis showed that parity was significantly higher among women with isolated AWE and that these patients presented with larger nodule size. In contrast, women with concurrent pelvic endometriosis were more likely to have an umbilical nodule and were significantly less fertile, which could be a consequence of endometrial disease. This finding contrasts with the case series of Filho et al., which investigated patients with umbilical nodules: five of their six patients had borne children. Although the women in that series reported dysmenorrhea, pelvic endometriosis was not explored with laparoscopy [20]. These results support the different patterns seen in our study population. If these patterns are consistent, we hypothesize that there may be two previously described pathogenic pathways in the development of AWE. Women presenting with isolated AWE likely experienced iatrogenic implantation of endometrial cells during surgery, whereas those with mild to severe pelvic endometriosis develop an abdominal lesion (mainly umbilical) as a result of lymphatic or hematogenous dissemination. Ridley and Edward conducted an experimental study in which they induced AWE in women by injecting endometrial tissue within the abdominal wall, supporting the first theory of pathogenesis [9]. Moreover, Wang et al. attributed the high prevalence of AWE after CS to parietal exposure to many endometrial cells and a high volume of blood, which creates a rich nutritional environment for cell implants [10].
Women with a history of laparoscopic supracervical hysterectomy may have an increased risk of developing AWE because the abdominal cavity is exposed to the endometrium, especially if uterine morcellation is performed without a containment device. We noted that the two patients with a history of midline laparotomy presented with an umbilical nodule, a finding that is similar to the observations of Vellido-Cotelo et al. [5]. Wicherek et al. [11] analyzed the obstetrical history of 81 women presenting with scar endometriosis after CS and found that CS performed before spontaneous onset of labor was associated with an increased risk of subsequent endometriosis. They hypothesized that women had high immune tolerance before the onset of labor, which permitted endometrial cell implantation (odds ratio = 2.18) [25]. Unfortunately, we were unable to collect all data concerning CS indications in this study. However, at least 11 patients (45%) had elective CS or premature birth during the period of enhanced immune tolerance. Oliveira et al. [12] found that elective CS was a risk factor for AWE (relative risk = 2), supporting Wicherek’s hypothesis.
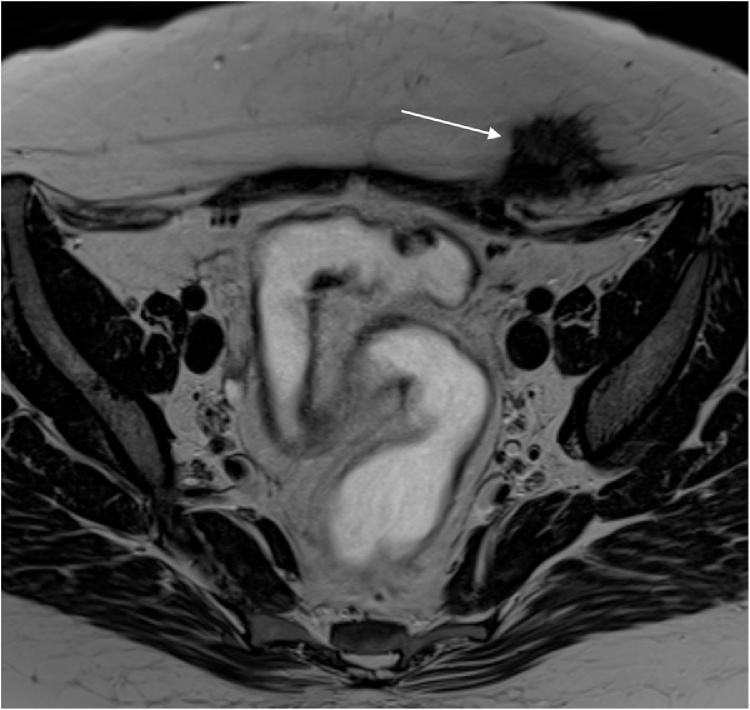
Lack of standardized diagnostic protocols for AWE can result in the need for multiple medical exams. Zhao and al. reported that 75% of fine-needle-aspirate samples had inconclusive cytology [7]. Studies have reported potential contamination and spread of ectopic endometrial implants linked to puncture; misdiagnosis of malignancy is another risk with fine-needle aspirates [23]. As recommended by Vellido-Cotelo et al., this procedure should remain a secondary diagnostic method in cases of clinical or imaging doubt [5]. Magnetic resonance imaging should be performed for nodules more than 2 cm in diameter and for those that are difficult to palpate (in patients with high BMI). MRI allows evaluation of relationships with neighboring structures and planning of multidisciplinary management for abdominal wall reconstruction (Fig. 1) [4,5,21]. The fact that the present study was conducted at a large and wealthy academic institution helps explain the high rate of magnetic resonance imaging in our cohort.
Fig. 1.
T2-weighted magnetic resonance imaging sequence showing spike-shaped abdominal wall endometriosis nodule in contact with right rectus abdominis muscle. The lesion contains hemorrhagic spots (white arrow).
Many authors do not recommend exploratory laparoscopy to look for possible concurrent pelvic endometriosis [13,14,20,29]. Because of the higher associated risks in patients without a surgical history, we recommend limiting this invasive procedure to women who present with incisional endometriosis and with symptoms compatible with associated pelvic endometriosis (dysmenorrhea, deep dyspareunia, pelvic pain).
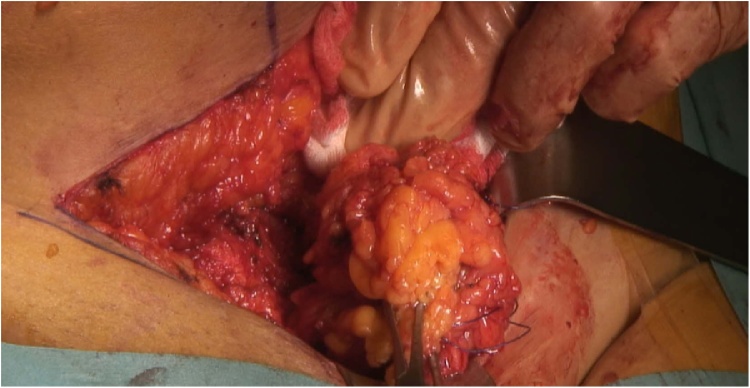
Some authors have suggested initiating medical therapy for endometriosis, such as oral contraceptives and gonadotropin-releasing hormone, to avoid recurrence after surgery [4,15]. Our study cannot support this recommendation because data regarding postoperative therapies were lacking. Although medical management is not an effective primary treatment for AWE, it is a feasible option for women close to menopause [1,2,22]. Women should be informed about the inability to rule out malignancy without resection of the lesion. Including patients in the decision-making process allows selection of the best treatment for individual patients. If primary surgical treatment is pursued, wide excision of nodules must be performed (Fig. 2) [1,2,5,17]. Because AWE is an uncommon condition, no prospective study has evaluated the size of safety margins [1]. Ding & Zhu and Rindos et al. proposed margins of at least 1 cm to avoid recurrence and/or malignant transformation [4,20]. The multivariate analysis of Zhao and al. showed that a lesion’s size and depth were risk factors for recurrence [7]. Because wide excision is the standard treatment, multidisciplinary management with cooperation between the general surgeon and gynecologist should be the rule, especially when a large area of fascia is involved and mesh repair is required. At our institution, we consider primary mesh repair for nodules that invade fascia or are larger than 3–4 cm. This cooperative management is also recommended by Rindos et al. [21]. Retromuscular mesh repair is usually preferred, depending on the clinical situation. In this young population, we generally do not offer intraperitoneal mesh placement [28].
Fig. 2.
Intraoperative view of large nodule resected with wide macroscopic margin.
Many preventive methods have been proposed to avoid AWE. Most of these involve the CS procedure. For example, the endometrium should be excluded during hysterorraphy, visceral and parietal peritoneum should be sutured, instruments used for hysterorraphy should be changed for closure and abundant abdominal lavage should be assured [1,2,7,16,24,26,27].
Strengths of this study include the number of cases and the volume of clinical data collected from a European referral center. Comparison of subgroups according to the presence of pelvic endometriosis offers a new perspective on the management of AWE and provides the basis for further investigations. Weaknesses include those inherent in any retrospective study, such as missing and/or imprecise information. The heterogeneity of diagnostic methods and management limited analysis.
In conclusion, AWE is an uncommon pathologic condition in which diagnostic and therapeutic delay must be avoided. Management of AWE differs according to individual surgical history. In patients with umbilical AWE without a surgical history, pelvic endometriosis should be strongly suspected and pelvic exploration should be discussed with the patient. Finally, we can expect AWE to be encountered more often as CS rates increase worldwide.
Declaration of Competing Interest
None.
Acknowledgment
We thank Rebecca Tollefson, DVM, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
References
- 1.Horton J.D., Dezee K.J., Ahnfeldt E.P., Wagner M. Abdominal wall endometriosis: a surgeon’s perspective and review of 445 cases. Am J Surg. 2008;196:207–212. doi: 10.1016/j.amjsurg.2007.07.035. [DOI] [PubMed] [Google Scholar]
- 2.Leite G.K.C., de Carvalho L.F.P., Korkes H., Guazzelli T.F., Kenj G. Scar endometrioma following obstetric surgical incisions: retrospective study on 33 cases and review of the literature. Sao Paulo Med J. 2009;127:270–277. doi: 10.1590/S1516-31802009000500005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uçar M.G., Şanlıkan F., Göçmen A. Surgical treatment of scar endometriosis following cesarean section, a series of 12 cases. Indian J Surg. 2015;77(Suppl 2):682–686. doi: 10.1007/s12262-013-0978-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ding Y., Zhu J. A retrospective review of abdominal wall endometriosis in Shanghai, China. Int J Gynaecol Obstet. 2013;121:41–44. doi: 10.1016/j.ijgo.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Vellido-Cotelo R., Muñoz-González J.L., Oliver-Pérez M.R. Endometriosis node in gynaecologic scars: a study of 17 patients and the diagnostic considerations in clinical experience in tertiary care center. BMC Womens Health. 2015;15:13. doi: 10.1186/s12905-015-0170-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adamson G.D. Endometriosis classification: an update. Curr Opin Obstet Gynecol. 2011;23:213–220. doi: 10.1097/GCO.0b013e328348a3ba. [DOI] [PubMed] [Google Scholar]
- 7.Zhao X., Lang J., Leng J., Liu Z., Sun D., Zhu L. Abdominal wall endometriomas. Int J Gynaecol Obstet. 2005;90:218–222. doi: 10.1016/j.ijgo.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Gunes M., Kayikcioglu F., Ozturkoglu E., Haberal A. Incisional endometriosis after cesarean section, episiotomy and other gynecologic procedures. J Obstet Gynaecol Res. 2005;31:471–475. doi: 10.1111/j.1447-0756.2005.00322.x. [DOI] [PubMed] [Google Scholar]
- 9.Ridley J.H., Edwards I.K. Experimental endometriosis in the human. Am J Obstet Gynecol. 1958;76:783–789. doi: 10.1016/0002-9378(58)90011-5. discussion 789-90. [DOI] [PubMed] [Google Scholar]
- 10.Wang P.H., Juang C.M., Chao H.T., Yu K.J., Yuan C.C., Ng H.T. Wound endometriosis: risk factor evaluation and treatment. J Chin Med Assoc. 2003;66:113–119. [PubMed] [Google Scholar]
- 11.Wicherek L., Klimek M., Skret-Magierlo J. The obstetrical history in patients with Pfannenstiel scar endometriomas--an analysis of 81 patients. Gynecol Obstet Invest. 2007;63:107–113. doi: 10.1159/000096083. [DOI] [PubMed] [Google Scholar]
- 12.de Oliveira M.A.P., de Leon A.C.P., Freire E.C., de Oliveira H.C. Risk factors for abdominal scar endometriosis after obstetric hysterotomies: a case-control study. Acta Obstet Gynecol Scand. 2007;86:73–80. doi: 10.1080/00016340601099346. [DOI] [PubMed] [Google Scholar]
- 13.Picod G., Boulanger L., Bounoua F., Leduc F., Duval G. Abdominal wall endometriosis after caesarean section: report of fifteen cases. Gynecol Obstet Fertil. 2006;34:8–13. doi: 10.1016/j.gyobfe.2005.11.002. (in French) [DOI] [PubMed] [Google Scholar]
- 14.Busard M.P.H., Mijatovic V., van Kuijk C., Hompes P.G.A., van Waesberghe J.H.T.M. Appearance of abdominal wall endometriosis on MR imaging. Eur Radiol. 2010;20:1267–1276. doi: 10.1007/s00330-009-1658-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J., Ding D., Liu X. Clinicopathological features of endometriosis in abdominal wall - clinical analysis of 151 cases. J Minim Invasive Gynecol. 2015;22:S174. doi: 10.1016/j.jmig.2015.08.644. [DOI] [PubMed] [Google Scholar]
- 16.Minaglia S., Mishell D.R., Ballard C.A. Incisional endometriomas after cesarean section: a case series. J Reprod Med. 2007;52:630–634. [PubMed] [Google Scholar]
- 17.Taburiaux L., Pluchino N., Petignat P., Wenger J.M. Endometriosis-associated abdominal wall cancer: a poor prognosis? Int J Gynecol Cancer. 2015;25:1633–1638. doi: 10.1097/IGC.0000000000000556. [DOI] [PubMed] [Google Scholar]
- 18.https://www.bfs.admin.ch/bfs/fr/home/statistiques/sante/determinants/exces-poids.html.
- 19.Khan Zaraq, Zanfagnin Valentina, El-Nashar Sherif A., Famuyide Abimbola O., Daftary Gaurang S., Hopkins Matthew R. Risk factors, clinical presentation, and outcomes for abdominal wall endometriosis. J Minim Invasive Gynecol. 2017;24(3):478–484. doi: 10.1016/j.jmig.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Filho Santos, Dos Paulo Vicente, Dos Santos Marcelo Protásio, Castro Samanta, Melo Valdinaldo Aragão D.E. Primary Umbilical Endometriosis. Rev Col Bras Cir. 2018;45(3):e1746. doi: 10.1590/0100-6991e-20181746. [DOI] [PubMed] [Google Scholar]
- 21.Rindos Noah B., Mansuria Suketu. Diagnosis and management of abdominal wall endometriosis: a systematic review and clinical recommendations. Obstet Gynecol Surv. 2017;72(2):116–122. doi: 10.1097/OGX.0000000000000399. [DOI] [PubMed] [Google Scholar]
- 22.Lopez-Soto Alvaro, Sanchez-Zapata Maria Isabel, Martinez-Cendan Juan Pedro, Reina Sebastian Ortiz, Bernal Mañas Carmen Maria, Solano Manuel Remezal. Cutaneous endometriosis: presentation of 33 cases and literature review. Eur J Obstet Gynecol Reprod Biol. 2018;221:58–63. doi: 10.1016/j.ejogrb.2017.11.024. [DOI] [PubMed] [Google Scholar]
- 23.Ecker A.M., Donnellan N.M., Shepherd J.P., Lee T.T.M. Abdominal wall endometriosis: 12 years of experience at a large academic institution. Am J Obstet Gynecol. 2014;211(363):e1–5. doi: 10.1016/j.ajog.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 24.Malutan A.M., Simon I., Ciortea R., Mocan-Hognogi R.F., Dudea M., Mihu D. Surgical scar endometriosis: a series of 14 patients and brief review of literature. Clujul Med. 2017;90:411–415. doi: 10.15386/cjmed-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lamblin G., Mathevet P., Buenerd A. Parietal endometriosis in abdominal scars. Report of 3 cases. J Gynecol Obstet Biol Reprod. 1999;28:271–274. (in French) [PubMed] [Google Scholar]
- 26.Zhu Z., MaM Al-Beiti, Tang L., Liu X., Lu X. Clinical characteristic analysis of 32 patients with abdominal incision endometriosis. J Obstet Gynaecol. 2008;28:742–745. doi: 10.1080/01443610802463744. [DOI] [PubMed] [Google Scholar]
- 27.Bektaş H., Bilsel Y., Sari Y.S. Abdominal wall endometrioma; a 10-year experience and brief review of the literature. J Surg Res. 2010;164:e77–81. doi: 10.1016/j.jss.2010.07.043. [DOI] [PubMed] [Google Scholar]
- 28.Brooks D.C., Cone J. 2018. Management of ventral hernias. Available at: https://www.uptodate.com/contents/management-of-ventral-hernias? search=managment%20of%20ventral%20hernias&source=search_result&selectedTitle=1∼150&usage_type=default&display_rank=1. [Google Scholar]
- 29.Boesgaard-Kjer Daniel, Boesgaard-Kjer Diana, Kjer Jens J.ørgen. Primary umbilical endometriosis (PUE) Eur J Obstet Gynecol Reprod Biol. 2017;209:44–45. doi: 10.1016/j.ejogrb.2016.05.030. [DOI] [PubMed] [Google Scholar]