Abstract
Organ transplantation is an effective therapeutic tool for treating many terminal diseases. However, one of the biggest challenges of transplantation is determining how to achieve the long-term survival of the allogeneic or xenogeneic transplant by, for example, preventing transplant rejection. In the current study, CD26 gene-knockout mice were used to investigate the potential role of CD26/dipeptidyl peptidase-4 (DPPIV) in allogeneic skin graft rejection by tail-skin transplantation. Compared with wild-type (CD26+/+) counterparts, CD26–/– mice showed reduced necrosis of grafts and delayed graft rejection after skin transplantation. Concentrations of serum IgG, including its subclasses IgG1 and IgG2a, were significantly reduced in CD26–/– mice during graft rejection. Moreover, after allogeneic skin transplantation, the secretion levels of the cytokines IFN-γ, IL-2, IL-6, IL-4, and IL-13 were significantly reduced, whereas the level of the cytokine IL-10 was increased in the serum of CD26–/– mice compared with that in the serum of CD26+/+ mice. Additionally, the concentration of IL-17 in serum and the percentage of cells secreting IL-17 in mouse peripheral blood lymphocytes (MPBLs) were both significantly lower, while the percentage of regulatory T cells (Tregs) was significantly higher in MPBLs of CD26–/– mice than in those of CD26+/+ mice. Furthermore, a lower percentage of CD8+ T cells in MPBLs and fewer infiltrated macrophages and T cells in graft tissues of CD26–/– mice were detected during graft rejection. These results indicate that CD26 is involved in allogeneic skin graft rejection and provides another hint that CD26 deficiency leads to less rejection due to lower activation and proliferation of host immune cells.
Keywords: Allogeneic graft rejection, CD26/DPPIV, Cytokine, IgG, T lymphocytes
Introduction
CD26, also known as dipeptidyl peptidase (DPPIV), is a multifunctional highly glycosylated integral type II transmembrane protein that is involved in a variety of biological processes.1 CD26 is widely expressed by epithelial and endothelial cells of different tissues, and its expression is strongly upregulated in activated T cells, B cells, and natural killer (NK) cells.2 As a serine protease, CD26 cleaves peptides selectively with proline or alanine at the penultimate position of the substrates to modulate their biological activities. A diverse range of bioactive peptides, such as some chemokines, peptide hormones, and neuropeptides, are indicated to be substrates of CD26/DPPIV.3 In addition to its peptidase activity, CD26 mediates cell adhesion through its interaction with fibronectin and collagen.4 Moreover, CD26 plays a crucial role in immune regulation.5 As a co-stimulator, CD26 is involved in T-cell activation and differentiation by its interaction with other molecules with essential cellular functions in T-cell responses, such as adenosine deaminase, CARMA1 and caveolin-1.5 Owing to its multidisciplinary characteristics, CD26 has been reported to be related to diverse different diseases, such as diabetes mellitus, HIV infection, cardiovascular disease, autoimmune diseases, and malignancies.6–8 Inhibition of DPPIV activity could alter the expression of immune response-related genes, and truncation of certain chemokines by CD26 may affect certain functions of T cells and immune responses. CD26 plays a potential role in different processes crucial for inflammation reaction and transplantation rejection, as well as wound healing. DPPIV inhibitors have recently emerged as antidiabetic drugs because of their reduction of blood glucose through their inhibition of the rapid degradation of incretin hormones glucagon-like peptide-1 and gastric inhibitory polypeptide.9 Furthermore, CD26 is also suggested as a therapeutic target for other diseases, such as chronic kidney disease, Middle East respiratory syndrome, coronavirus infection, and graft-versus-host disease (GVHD).10–12
Annually, >11 million people suffer from burn injuries worldwide,13 and skin transplantation is one of the most important approaches to treating large-area burns. However, skin transplantation also presents one of the challenges in clinical treatment. The long-term survival of allogeneic or xenogeneic skin grafts is difficult to achieve in clinical therapy because of immune rejection. After skin transplantation, dendritic cells of the donor skin migrate out of the graft and present donor antigens that can be recognized by the recipient T cells. Following allorecognition, the recipient T cells become activated, proliferate and secrete proinflammatory cytokines.14 Cytokines secreted by different T-cell subsets play a crucial role in the activation of effector T cells and macrophages.15 The inflammatory stage initiates the effector T cells and macrophages to arrive at the graft site to destroy the graft.16
Several observations indicate that CD26 contribute to the process of transplant rejection. First, CD26 acts as an important activation marker of T helper 1 (Th1) cells that are associated with the early stage of transplant rejection.17 Second, recent studies suggest that CD26 is a potential positive marker of interleukin (IL)-17-producing T cells (Th17) and a negative marker of regulatory T cells (Tregs).18, 19 The balance between Th17 and Tregs is critical for allograft rejection and immunological tolerance.15, 20 Moreover, recent studies reported that the strong expression of CD26 was associated with organ transplantation.21 Understanding the role of CD26, particularly its molecular mechanism in allogeneic graft rejection, is crucial in the development of a novel strategy for inhibiting graft rejection and improving the therapeutic effect in clinical organ transplantation.
In the present study, we compared allogeneic graft rejection between CD26+/+ and CD26–/– mice after tail-skin transplantation, measured the percentages of lymphocyte subsets in the spleen and blood of CD26+/+ and CD26–/– mice pretransplantation and posttransplantation, and analyzed the secretion levels of cytokines and antibodies in mice sera. Our results demonstrate that the deficiency of CD26 results in delayed and reduced graft immune rejection after murine allogeneic skin transplantation.
Materials and methods
Animals
The donor mice were 8-week-old male BALB/c mice. The recipient mice were 8–12-week-old homozygous CD26–/– mice on the C56BL/6 N genetic background22 and wild-type C57BL/6N mice. The mice were kept under specific pathogen-free conditions. Experiments were performed on males and females; there were no sex-related differences. The animals were treated according to the German Law on the Protection of Animals, and permission (G0071/14) was obtained from the State Animal Welfare Committees.
Murine tail-skin transplantation
To prepare the tail skin from donor mice, the donor mice were sacrificed, and the entire tail was swabbed with 70% ethanol. The tail skin of the donor was incised to an area of 1.0 × 1.0 cm2. The recipient mice were anesthetized with isoflurane inhalation, and a circumferential band was shaved on the back of the recipient mice. The shaved back was cleaned with 70% ethanol and allowed to dry. The back skin of the recipient mice was cut to a 1.0 × 1.0 cm2 graft bed. The bed skin was removed from the recipient, and the skin graft was placed into the graft bed. The four corners of the grafts were stitched, and the mice were wrapped with bandages. The mice were placed in a clean cage and heated by a red lamp until they moved freely.
Evaluation for necrosis of skin grafts
Seven days after transplantation, bandages were removed; skin grafts were monitored daily and recorded by photographs for up to 15 days. Skin rejection was scored based on the necrotic areas (wrinkled skin) of the skin graft. The necrotic areas were roughly estimated by visual inspection, and six (0–5) different score levels were defined according to the percentage of the necrotic area of the graft. Fully intact smooth grafts or grafts with <20% necrotic area scored a 5, those with 20–40% necrotic area scored a 4, those with 40–60% necrotic area scored a 3, and those with 60–80% necrotic area scored a 2, 80–100% (but not removed) scored a 1, and samples with the graft fully removed scored a 0.
Preparation of mouse spleen lymphocytes (MSLs) and mouse peripheral blood lymphocytes (MPBLs) and the analysis of lymphocyte subpopulations by flow cytometry
MSLs were isolated as published previously.23 For the separation of MPBLs, peripheral blood was collected with MiniCollect Tubes (Greiner Bio-One, Austria), and erythrocytes were lysed with lysing solution (BD FACSTM Lysing Solution). Following antibody staining, the subpopulation of lymphocytes was measured by flow cytometry. Mice monoclonal antibodies (mAbs) were used for multi-parameter flow cytometric analysis: allophycocyanin (APC)-conjugated anti-CD3, phycoerythrin (PE)-conjugated anti-NK1.1, fluorescein isothiocyanate (FITC)-conjugated anti-CD19, FITC-conjugated anti-CD4, PE-conjugated anti-CD8, and PE-conjugated anti-IL-17 were obtained from BioLegend (London United Kingdom); and PE-conjugated anti-CD25 and APC-conjugated anti-FoxP3 were provided by ImmunoTools (Friesoythe; Germany). Lymphocytes were incubated with antibodies in 1% (w/v) bovine serum albumin/phosphate-buffered saline (PBS) at 4 °C for 1 h in the dark. Cells were washed twice with PBS and analyzed by flow cytometry (BD Biosciences). WinMDI2.9 software was used to analyze the percentages of different lymphocyte subpopulations.
Measurement of immunoglobulin (Ig) production in sera
Blood samples were collected from mouse tails and clotted for 1 h at room temperature. After centrifugation, the sera were transferred into new tubes and stored at –20 °C for further analysis. To quantify serum Igs, we used an enzyme-linked immunosorbent assay (ELISA) as described previously by our group.23 Briefly, anti-mouse Ig polyclonal antibody (ImmunoTools, Friesoythe; Germany) was used as a coating Ab for IgG, IgG1, and IgG2a. Biotin-labelled anti-mouse IgG, IgG1, or IgG2a mAbs (BD Pharmingen) was used as a detecting Ab.
Measurement of cytokines secretion in serum
Interleukin concentrations in serum were determined by ELISA kits from eBioscience (interferon (IFN)-γ, IL-2, IL-4, and IL-6), R&D Systems (IL-10, IL-5, and IL-13), and Biolegend (IL-17). The procedure was performed according to the instructions provided by the corresponding manufacturer.
Immunohistofluorescence analysis
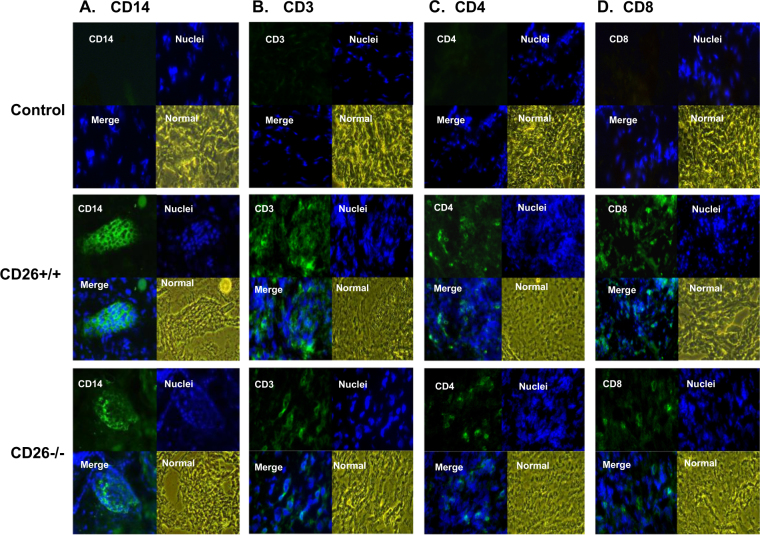
After tail-skin transplantation, the skin grafts of the recipient mice were collected on day 7 after transplantation and placed onto a prelabeled tissue base mold. The entire tissue block was covered with optimal cutting temperature compound, and the tissue block was stored at –20 °C within 1 month until it was ready for sectioning. The desired thickness of frozen sections was 5–10 μm. After frozen-section cutting, the tissue sections were fixed with pre-cooled acetone for 10 min and then washed with PBS. Tissue sections were first incubated with primary Abs overnight at 4 °C. After being washed with PBS, these sections were further incubated with FITC-labeled secondary Ab for 1 h. Following the washing steps, the sections were incubated with Hoechst 33,342 (Thermo Fisher Scientific) for nuclear staining. The Abs used in the present experiment were rat anti-mouse monoclonal Abs against CD14 (25 mg/mL) (R&D System), CD3 (25 mg/mL) (BioLegend), CD4 (25 mg/mL) (BioLegend), and CD8 (25 mg/mL) (ImmunoTools). The tissue from the donor mice served as a control.
Statistical analysis
All data were obtained from three or more independent experiments, and the values represent the means ± SD of at least seven mice in each group. Significant differences between CD26–/– mice and CD26+/+ mice were calculated by ANOVA-analysis. Differences between groups were considered significant at p < 0.05, p < 0.01, p < 0.005, and p < 0.001.
Results
Lower necrotic level and delayed rejection of skin graft in CD26–/– mice
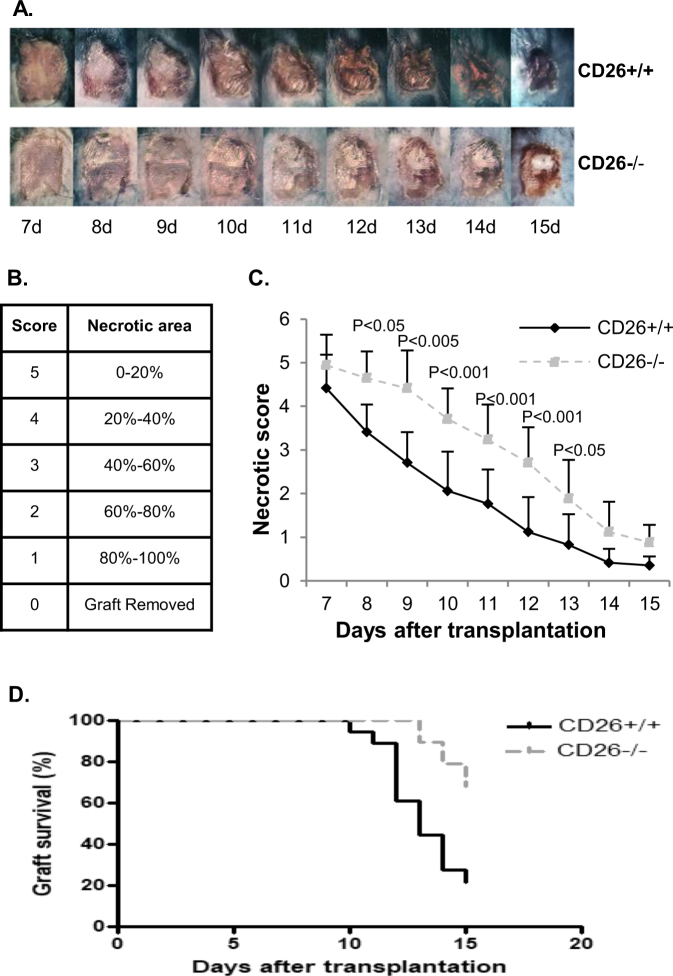
CD26+/+ mice and CD26–/– mice were transplanted with BALB/c tail skin (n ≥ 10 per group). Seven days after transplantation, bandages were removed; necrotic areas (black spots) of skin grafts were monitored daily and recorded by photographs up to 15 days posttransplantation (Fig. 1a). Six different score levels (0–5) were defined according to the necrotic area of the mouse skin graft posttransplantation (Fig. 1b). Statistical analysis indicated that the necrotic levels of skin grafts were lower in CD26–/– mice than in CD26+/+ mice from day 7 to day 15 (Fig. 1c), and the graft rejection of CD26–/– mice was significantly slower than that of CD26+/+ mice (p < 0.001) (Fig. 1d). This finding suggests involvement of CD26 in graft rejection.
Fig. 1. Allograft rejection of CD26+/+ and CD26–/– mice.
a Skin allograft appearance of CD26+/+ (upper panel) and CD26–/– mice (lower panel) from day 7 to 15 posttransplantation. c Statistical analysis of the necrotic levels of grafts from day 7 to day 15 posttransplantation. b Different score levels indicate different necrotic areas of the mice skin allograft. d The graft survival rate in CD26+/+ and CD26–/– mice within 15 days posttransplantation. The values in c and d represent the mean values ± SD of at least 17 mice at each time point. The data were analyzed with GraphPad 6; p-values were calculated with a Chi-square test
Markedly less IgG, particularly IgG1, in serum of CD26–/– mice after allogeneic skin transplantation
To understand the underlying molecular mechanisms of CD26 in allogeneic graft rejection, the production of IgG, as well as IgG1 and IgG2a, in mice serum at different time points was measured by ELISA after skin transplantation. The production of these antibodies in mice serum elevated rapidly until day 15 posttransplantation (Fig. 2). The production levels of IgG and IgG2a in CD26+/+ mice were highest on day 11, while maximum IgG1 production was reached on day 15 posttransplantation. However, on days 7 and 11 posttransplantation, the concentration levels of serum IgG in CD26–/– mice were significantly lower than those in CD26+/+ mice. The concentrations of IgG in CD26+/+ mice were 1.7- and 2-fold higher than those in CD26–/– mice (6640 vs. 3868 μg/mL, p < 0.05 and 12,027 vs. 6912 μg/mL, p < 0.05) on days 7 and 11, respectively (Fig. 2a). Further analysis demonstrated that the concentrations of both IgG isotypes IgG1 and IgG2a were also remarkably lower in CD26–/– mice; in particular, the levels of IgG1 on days 7 and 11 in the serum of CD26–/– mice were only approximately 44% of those in the serum of CD26+/+ mice (1054 μg/mL vs. 2355 μg/mL and 1247 μg/mL vs. 2868 μg/mL, respectively) (Fig. 2b, c). These results suggest a delayed and insufficient immune response of CD26-deficient mice to the allogeneic transplantation.
Fig. 2. Levels of IgG, IgG1, and IgG2a in the serum of mice after skin transplantation.
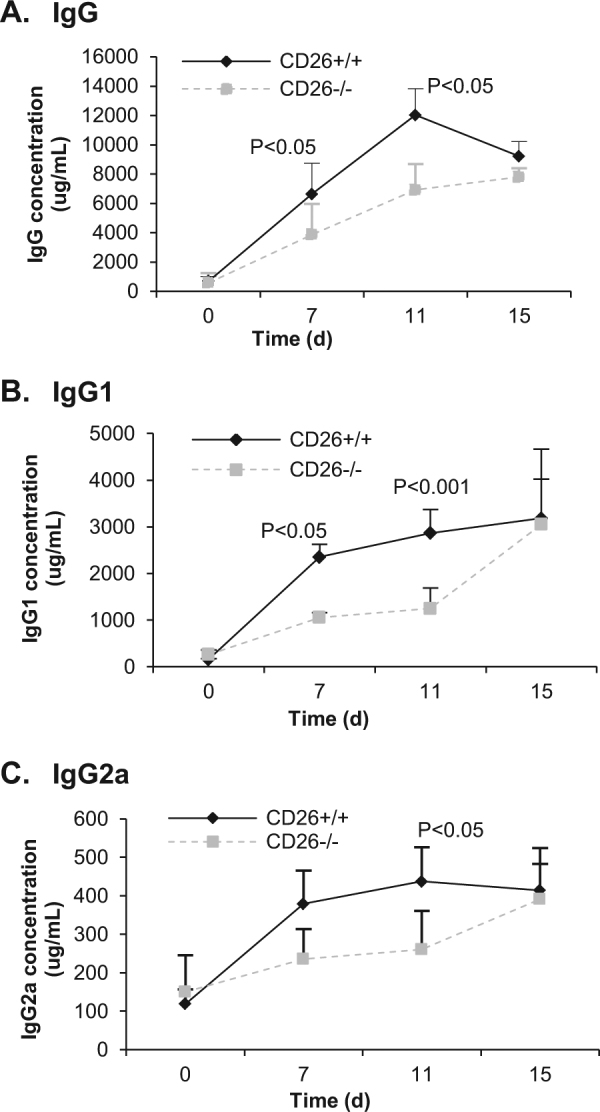
Blood was taken at the indicated time points after skin transplantation (day 0 represents the day before transplantation). Serum concentrations of a IgG, b IgG1, and c IgG2a were measured by ELISA. Values represent the means ± SD of at least eight mice at each time point.
Reduced percentage of CD8+ cells in the MPBLs of CD26–/– mice after allogeneic skin transplantation
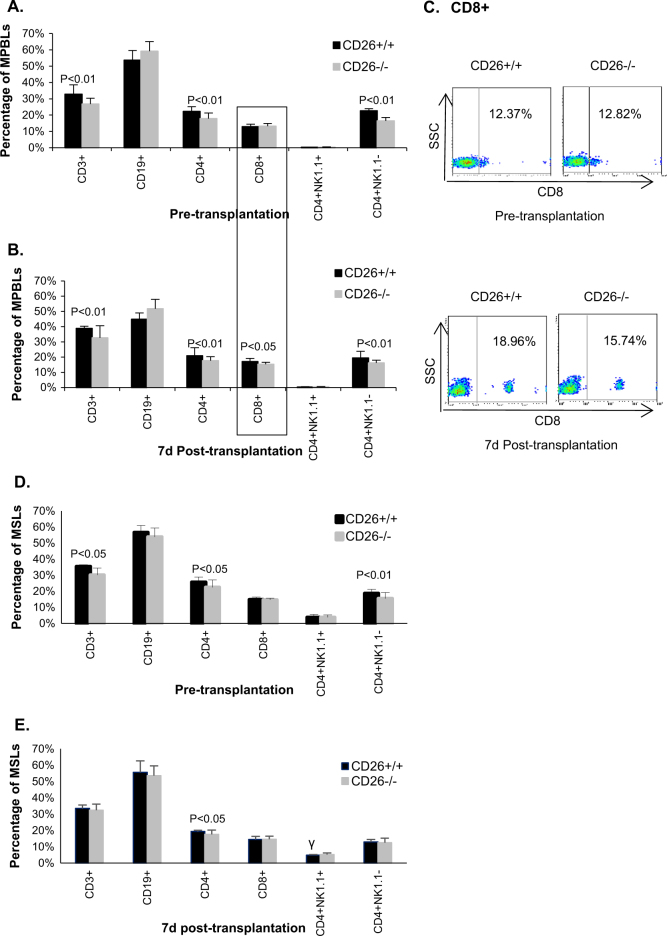
To clarify why CD26–/– mice produced lower IgGs after skin transplantation, the proliferation and differentiation of lymphocytes in both types of mice were investigated. As the highest distinct secretions of IgGs were observed on day 7 posttransplantation, the percentages of different lymphocyte subpopulations in MPBLs and MSLs of CD26+/+ mice and CD26–/– mice on days 0 (i.e., before transplantation) and 7 after skin transplantation were measured. Before transplantation, the percentages of CD3+, CD4+, and CD4+NK1.1– cells in both MPBLs and MSLs of CD26–/– mice were lower than those in CD26+/+ mice, while the percentages of CD8+ and CD19+ did not differ significantly between the two mice types (Fig. 3a, d). However, relative to pretransplantation, the percentage of CD3+ cells of MPBLs was increased, while that of CD19+ cells was decreased in both mice types on day 7 posttransplantation (Fig. 3b), indicating a proliferation of CD3 T lymphocytes after skin transplantation. Nevertheless, no significant changes were detected in the percentages of CD4+ and CD4+NK1.1– cells between pretransplantation and posttransplantation. Interestingly, the percentage of CD8+ cells showed no significant differences in MPBLs between CD26+/+ and CD26–/– mice before skin transplantation; however, at day 7 posttransplantation, CD8+ cells in CD26–/– MPBLs was 11% lower than that in CD26+/+ MPBLs (15.27 vs. 17.10%, p < 0.05), indicating a reduced activation and proliferation of CD8+ cells in CD26–/– mice in response to allogeneic transplantation.
Fig. 3. Percentages of MPBL and MSL subpopulations in mice.
Lymphocytes were prepared and analyzed by flow cytometry. Percentages of MPBL subpopulations before (a) and on day 7 posttransplantation (b). FACS dot plot of CD8+ cells in MBPLs of mice (c). Percentages of MSL subpopulations before (d) and on day 7 posttransplantation (e). The values represent the means ± SD of 8 mice. SSC side scatter
In MSLs, the percentage difference in CD3+, CD4+, and CD4+NK1.1– between the two types of mice decreased posttransplantation compared with pretransplantation levels. In CD26–/– mice, the percentage of CD4+ cells was lower than that in CD26+/+ mice before and after transplantation, while no significant percentage difference in CD3+ and CD4+NK1.1– cells between CD26–/– and CD26+/+ mice could be found posttransplantation.
Lower levels of IL-2, IFN-γ, IL-6, IL-4, and IL-13 but higher level of IL-10 in serum of CD26–/– mice after allogeneic skin transplantation
As shown, CD26–/– mice presented a lower percentage of CD4+ cells in both MPBLs and MSLs before and after skin transplantation and a lower percentage of CD8+ cells in MPBLs after skin transplantation. Further investigation was necessary to clarify the differentiation and effects of lymphocyte subpopulations of CD26–/– mice after skin transplantation. For this purpose, cytokine secretions, which serve as specific signals for lymphocyte differentiation and effects, were determined after skin transplantation.
The cytokine levels in serum at different time points were analyzed by ELISA. Figure 4 shows that all measured cytokines were secreted after skin transplantation. The concentrations of these cytokines peaked on day 7 posttransplantation, except for IL-13, which peaked on day 4 posttransplantation. Notably, the levels of Th1 cytokines, IL-2 and IFN-γ (Fig. 4a, b) in the serum of CD26–/– mice were much lower than that in the serum of CD26+/+ mice. On day 7 posttransplantation, the level of IFN-γ in the serum of CD26–/– mice was only one-third of that in the serum of CD26+/+ mice (3.54 pg/mL vs. 10.45 pg/mL) and the level of IL-2 in the serum of CD26–/– mice was approximately 60% of that in the serum of CD26+/+ mice. This finding suggests reduced differentiation and functions of Th1 cells in CD26–/– mice after allogeneic skin transplantation.
Fig. 4. Determination of cytokine secretion in the serum of CD26+/+ and CD26–/– mice by ELISA.
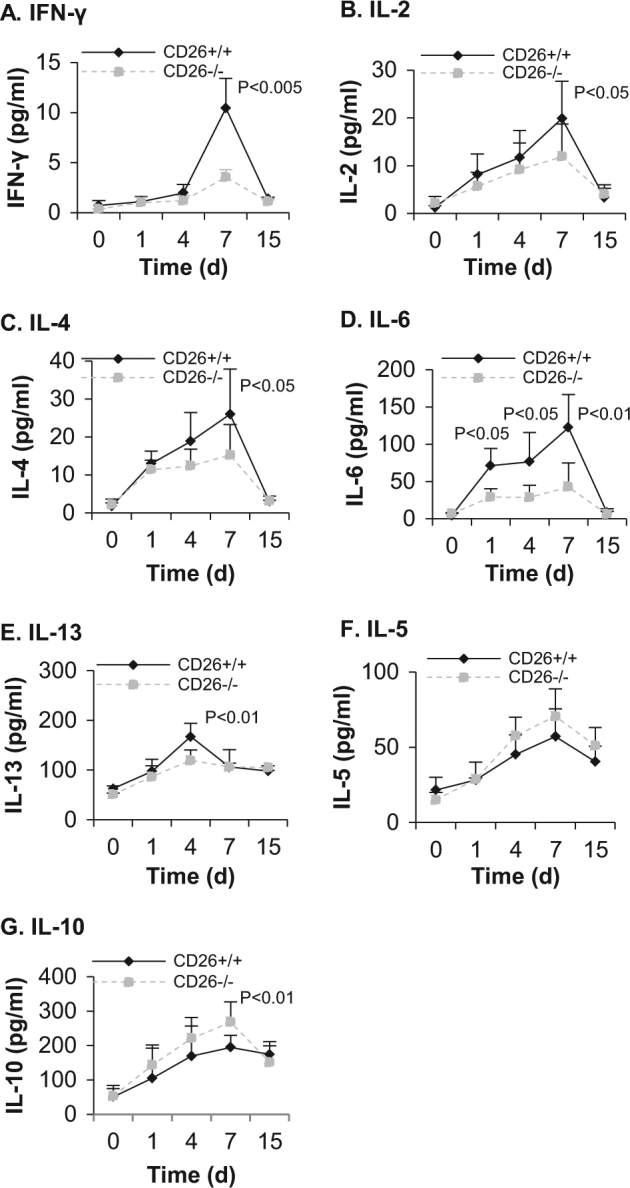
Blood was taken at the indicated times before and after allogeneic skin transplantation (day 0 represents the day before transplantation). The levels of IFN-γ (a), IL-2 (b), IL-4 (c), IL-6 (d), IL-13 (e), IL-5 (f) and IL-10 (g) were measured by ELISA. The values represent the means ± SD of at least seven mice at each time point
Interestingly, levels of different Th2 cytokines in the serum of CD26–/– mice were different compared with those in the serum of CD26+/+ mice. The level of IL-4 (Fig. 4c) in the serum of CD26–/– mice was significantly lower than that in the serum of CD26+/+ mice (15.26 pg/mL vs. 26.01 pg/mL at day 7 posttransplantation, p < 0.05), and the level of IL-13 (Fig. 4e) in CD26–/– mice was markedly lower than that in CD26+/+ mice (119.24 pg/mL vs. 167.38 pg/mL at day 4 posttransplantation, p < 0.01). In contrast, the concentration of IL-5 in serum between both types of mice did not show any significant differences (Fig. 4f), while the concentration of IL-10 in CD26–/– mice at day 7 posttransplantation was 38% higher than that in CD26+/+ mice (Fig. 4g). Notably, the IL-6 level in the serum of CD26–/– mice on day 1 after skin transplantation was significantly lower than that in the serum of CD26+/+ mice; on day 7, the concentration of IL-6 in CD26–/– mice was only one-third of that in CD26+/+ mice. This finding suggests a difference in the differentiation and immune response of Th2 cells in CD26–/– mice to allogeneic skin transplantation.
Decreased Th17 lymphocytes and increased Treg cells in CD26–/– mice after allogeneic skin transplantation
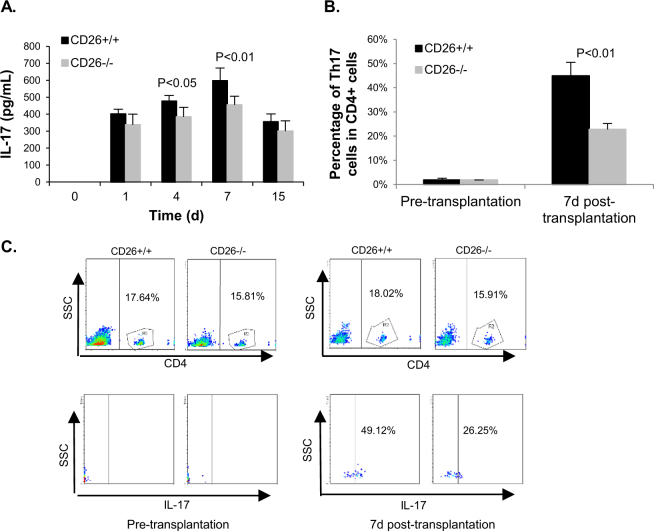
Given that IL-6 is essential to the differentiation of CD4+ cells into the Th17 subpopulation and the important role this subpopulation plays during inflammation, the amount of IL-17—which is a typical cytokine of the Th17 subset—was determined by ELISA. The release of IL-17 in the serum of both types of mice increased and peaked on day 7 after transplantation (Fig. 5a). The level of IL-17 was significantly lower in the serum of CD26–/– than that in CD26+/+ mice. On day 4 after transplantation, the concentration of IL-17 in CD26–/– mice was only 80% of that in CD26+/+ mice (385 pg/mL vs. 477 pg/mL), and on day 7, the level of IL-17 in CD26–/– was reduced to 76% of that in CD26+/+ mice (455 pg/mL vs. 597 pg/mL). The percentages of cells secreting IL-17 (Th17 cells) in CD4+ cells of MPBLs were comparable between the two mice types before transplantation, while the percentage of Th17 cells in CD4+ cells of CD26–/– mice was nearly half of that of CD26+/+ mice (22.77 vs. 44.88%, p < 0.01) on day 7 after transplantation (Fig. 5b).
Fig. 5. Analysis of the IL-17 secretion and percentage of Th17 cells in CD4+ cells of MPBLs in CD26+/+ and CD26–/– mice.
Blood was taken at the indicated times before and after allogeneic skin transplantation (day 0 represents the day before transplantation). The secretion of IL-17 in serum was analyzed by ELISA, and the percentages of Th17 cells in CD4+ cells were analyzed by flow cytometry. a The secretion of IL-17 in the serum of CD26+/+ mice and CD26–/– mice. b The percentage of Th17 cells in CD4+ cells of CD26+/+ and CD26–/– mice on the day before transplantation and on day 7 posttransplantation. The values represent the means ± SD of at least 7 mice at each time point. c Upper panels: Dot plot of percentage of CD4+ cells of MPBLs in CD26+/+ and CD26–/– mice. Lower panels: Dot plot of percentage of Th17 cells in CD4+ cells
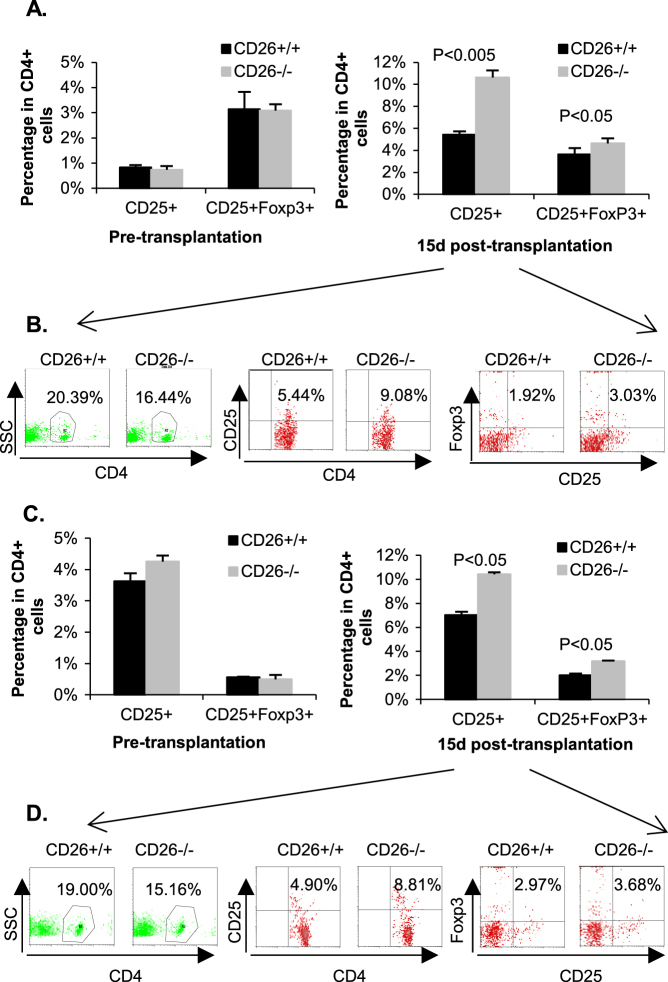
Given that the balance between Th17 and Tregs is critical for allograft rejection and immunological tolerance, the expression of biomarkers of Tregs (CD4+CD25+ and CD4+CD25+Foxp3+) was analyzed by flow cytometry. We found that the percentages of CD4+CD25+Foxp3+ in the peripheral blood and spleen did not exhibit any difference between CD26+/+ and CD26–/– mice before transplantation (left panel of Fig. 6a, c). However, on day 15 posttransplantation, the percentage of CD25+ cells in CD4+ cells of CD26–/– MPBLs was nearly two-fold that of CD26+/+ mice (10.62 vs. 5.42%), and the percentage of CD25+ in CD4+ cells of CD26–/– MSLs was 48% higher than that of CD26+/+ MSLs (10.39 vs. 7.00%) (right panel of Fig. 6a, c). Consistent with these data on day 15 posttransplantation, the percentage of CD25+Foxp3+ cells in CD4+ cells of CD26–/– MPBLs was 30% higher than that of CD26+/+ MPBLs (4.62 vs. 3.64%), while the percentage of CD25+Foxp3+ cells in the CD4+ subpopulation of CD26–/– MSLs was 50% higher than that of CD26+/+ MSLs (3.18 vs. 2.02%) (right panel of Fig. 6a, c).
Fig. 6. Percentage of Tregs in CD26+/+ and CD26–/– mice.
The lymphocytes of MPBLs and MSLs were separated before and on day 15 after transplantation. The percentages of Tregs (CD4+CD25+ and CD4+CD25+Foxp3+) in MPBLs and MSLs were analyzed by flow cytometry. a Percentages of CD25+ and CD25+Foxp3+ cells in CD4+ cells of MPBLs. The values represent the means ± SD of at least seven mice at each time point. b FACS analysis of the percentage of CD4+ cells in MPBLs and percentages of CD25+ and CD25+Foxp3+ cells in CD4+ cells of MPBLs in CD26+/+ and CD26–/– mice on day 15 after skin transplantation. c Percentages of CD25+ and CD25+Foxp3+ cells in CD4+ cells of MSLs. The values represent the means ± SD of at least seven mice at each time point. d FACS analysis of the percentage of CD4+ cells in MSLs and percentages of CD25+ and CD25+Foxp3+ cells in CD4+ cells of MSLs in CD26+/+ and CD26–/– mice on day 15 after skin transplantation. SSC side scatter
These data suggest that the deficiency of CD26 resulted in the reduced differentiation of CD4+ cells into Th17 cells while increasing the differentiation of CD4+ cells into Tregs, which could contribute to the immune tolerance and retarded graft rejection in CD26–/– mice observed after allogeneic transplantation.
Reduction of infiltration of macrophages and T lymphocytes in the graft tissue in CD26–/– mice
The infiltration of macrophages (CD14+) and T cells (CD3+, CD4+, and CD8+) in the graft tissue of CD26+/+ and CD26–/– mice was determined by immunohistofluorescence analysis. In graft tissues of both CD26+/+ and CD26–/– mice, clusters of CD14+ macrophages were detected on day 7 after transplantation (Fig. 7a); however, the frequency of these clusters in grafts of CD26–/– mice was obviously lower, the area of the clusters in the grafts of CD26–/– mice was relatively smaller, and the number of macrophages in the grafts of CD26–/– mice was significantly less than that in the grafts of CD26+/+ mice, suggesting reduced infiltration of macrophages in the grafts of CD26–/– mice (Fig. 7a). Infiltration of T lymphocytes (CD3+) was also found in the grafts of both types of mice on day 7 posttransplantation (Fig. 7b). As shown, the number of infiltrated CD3+ cells in CD26–/– mice was clearly less than that in CD26+/+ mice on day 7 after skin transplantation (Fig. 7b). Further analysis showed that the T-cell subsets of CD4+ cells and CD8+ cells were both infiltrated in the graft tissues of the two types of mice after skin transplantation. The numbers of infiltrated cells of both types, particularly CD8+ cells, were less distinct in CD26–/– mice than in CD26+/+ mice (Fig. 7c, d). However, no obvious infiltration of B lymphocytes (CD19+) was detected for either type of mouse after skin transplantation (data not shown).
Fig. 7. Determination of infiltrated macrophages and T lymphocytes in grafts of CD26+/+ and CD26–/– mice after skin transplantation.
The tail skin before transplantation was collected as a control; graft tissues of CD26+/+ and CD26–/– mice were obtained on day 7 after transplantation. The frozen sections of skin graft were stained with monoclonal antibody against mouse CD14, CD3, CD4, or CD8, and the nucleus was then counterstained with Hoechst 33,342. a–d Immunofluorescence analysis results of infiltrated CD14+ cells, CD3+ cells, CD4+ cells, or CD8+ cells in the grafts of CD26+/+ and CD26–/– mice, respectively. Photographs are shown at 400× magnification
Discussion
Finding an effective way to inhibit immune rejections is one of the most important strategies for supporting clinical transplantations. Recently, studies concerning organ transplantation demonstrated that the application of DPPIV inhibitor or the anti-CD26 mAb increased the engraftment of donor cells and decreased acute GVHD, respectively, and indicated CD26 as a novel target for therapeutic intervention in GVHD disease.11 To clarify the role of CD26 in allogeneic graft rejection, CD26 knockout mice were used in an allogeneic skin transplantation study. We found that CD26–/– mice presented a lower necrotic degree of grafts and delayed allograft rejection (Fig. 1).
CD26 is an activation marker of T and B lymphocytes and NK cells. As a costimulatory molecule, CD26 mediates T-cell signal transduction processes through its interaction with adenosine deaminase, CD45, caveolin-1, or CARMA1.5 Blockade of CD26-mediated T-cell co-stimulation induced anergy in CD4+ T cells.24 In the present work, a lower percentage of CD3+, as well as CD4+ cells, were found in MPBLs and MSLs of CD26–/– mice both pre- and post-skin transplantation (Fig. 3). In addition, infiltration of CD3+ and CD4+ cells was detected to a lower degree in the graft tissues of CD26–/– mice than in CD26+/+ mice after skin transplantation (Fig. 7b, c). This finding indicates that CD26 deficiency results in impaired development, maturation, and function of CD4+ cells, which corresponds to our previous findings.23 Interestingly, the percentage of CD8+ cells in MPBLs of CD26–/– mice was the same as in CD26+/+ mice before transplantation; however, the percentage was significantly lower in CD26–/– than in CD26+/+ mice after skin transplantation (Fig. 3). The infiltration number of CD8+ cells in the skin grafts was distinctly lower in CD26–/– than in CD26+/+ mice after skin transplantation. These findings suggest reduced proliferation, activation, and function of CD8+ cells in CD26–/– mice in response to allogeneic antigens. CD8+ T cells are a prominent component of the allogeneic T-cell repertoire induced after allogeneic transplantation in mice; their cytotoxic activity is directed toward donor major histocompatibility complex (MHC) class I peptides.25 The reduced percentage of CD8+ cells in CD26–/– mice (Fig. 3) after allogeneic transplantation may partly contribute to the reduced necrotic degree of grafts and delayed allograft rejection. Moreover, the percentages of CD3+, CD4+, and CD4+NK1.1– cells of MSLs were lower in CD26–/– mice than in CD26+/+ mice before transplantation, but the difference was reduced after transplantation. It was reported that the inhibition of CD26 increased donor cell homing and improved allogeneic engraftment.26 The decrease in the percentage difference of CD3+ and CD4+ cells of MSLs between the two types of mice after transplantation may be due to the increased homing of those cells to the spleen in CD26–/– mice.
Allograft rejection is primarily driven by host T cells. While all components of the innate and adaptive immune systems participate in graft rejection, T lymphocytes and, in particular, CD4+ cells are of paramount importance in this process.27 Once activated, CD4+ T cells primarily direct the progression of the response by secreting cytokines that activate, expand, and/or recruit other effector cells, such as macrophages, CD8+ T cells, and B cells.27, 28 Through further analysis of cytokine levels in both types of mice, markedly reduced secretion of IL-2, IFN-γ, IL-6, IL-17, IL-4, and IL-13 was found in the serum of CD26–/– mice after allogeneic transplantation (Fig. 4). This reduced secretion may have been caused by a lower number of CD4+ cells in CD26–/– mice before transplantation; however, impaired differentiation and function of CD4+ cells in response to the allogeneic antigen in CD26–/– mice should be considered. Low serum levels of IL-2, IFN-γ, and IL-6 indicate partly defective differentiation and function for Th1 cells, while low levels of IL-4 and IL-13 indicate insufficient differentiation and function of Th2 cells in CD26–/– mice.
As a key cytokine, IFN-γ exhibits diverse and potentially contradictory effects on organ allograft rejection.29 IL-2 is another Th1-associated cytokine that also has complex effects on allograft rejection.30 Both IFN-γ and IL-2 are pleiotropic cytokines and play an important role in the proliferation of T and B cells during the inflammatory reaction. The cytokines first act as molecules initiating T-cell growth and survive during the immune response and then reinforce the Th1 response with positive feedback.29, 30 In acute rejection, Th1 cells predominantly infiltrate into grafts, in which IL-2 and IFN-γ can induce the activation of NK cells and macrophages, which are strong weapons for destroying allografts.29, 30 Furthermore, IFN-γ induces the expression of class II MHC molecules and the secretion of IgG2a and IgG3 from activated B cells. In an acute rejection model, IFN-γ–/– mice showed delayed skin graft rejection.31 Several studies have reported that tolerance to allograft rejection is mediated at least in part by IL-4, a typical cytokine of Th2 cells, by promoting IL-10 and IgG1 production.32 Other studies have provided conflicting results, indicating that the administration of Th2 inhibitor prolongs cardiac allograft survival.33 IL-13, which shows the effects similar to those of IL-4, is another cytokine associated with the Th2 response; IL-13 shares a receptor chain (IL-4R α-chain) with IL-4 but differs in the target cells involved, which results in a series of different biological events.32 An increasing number of studies have demonstrated that the cytokines of both Th1 and Th2 cells, such as IL-2, IFN-γ, and IL-4, are capable of supporting B-cell clonal expansion and antibody synthesis.28, 34 CD26 deficiency results in impaired differentiation and function of Th1 and Th2 cells in CD26–/– mice. Low levels of Th1 cytokines IFN-γ and IL-2 could result in a decrease in the proliferation of CD8+ cells (Fig. 3) and activation of macrophages, thereby reducing the infiltration of CD8+ cells and macrophages into grafts (Fig. 7) during allograft rejection. On the other hand, low levels of IFN-γ, IL-2, and IL-4 in CD26–/– mice after allogeneic transplantation could impair the activation and differentiation of B cells, reducing antibody production (Fig. 2).
Immune rejection is a complex process that involves a cellular as well as a humoral immune response and is characterized by the production of antibodies by B lymphocytes. IgG, the main component of serum Igs and a common pathogenic antibody in patients with transplant rejection, plays an indispensable role in damaging grafts during transplant rejection.35 In our present work, low production of IgG and its subsets, IgG1 and IgG2a, was detected in the serum of CD26–/– mice (Fig. 2). This result corresponds to our previous findings demonstrating that antibody production was clearly lower in CD26–/– mice than in CD26+/+ mice either after ovalbumin immunization or after pokeweed mitogen stimulation.23, 36 This finding indicates that the differentiation of B cells was impaired by CD26 deficiency. In T-cell-dependent B-cell activation, an interaction between B cells and Th cells and certain cytokines of Th1 and Th2 are required to support B-cell clonal expansion and antibody synthesis. In CD26–/– mice, a low percentage of Th cells (CD4+) (Fig. 3) and low production of IL-2 and IL-4 (Fig. 4) could lead to impaired activation and differentiation of B cells, thus reducing IgG production. IgG is a major component that mediates allorecognition between exogenous antigens and recipient CD8+ cells during graft rejection.37 Low IgG production in CD26–/– mice (Fig. 2) may therefore result in the reduction of graft attack by effector cells.
Interestingly, the secretion level of IL-10 was higher in CD26–/– mice (Fig. 4G). Supporting our results, CD26/DPPIV blockade has been shown to improve lung allograft transplantation and increase the expression of IL-10.38 IL-10 is known to be an anti-inflammatory cytokine; IL-10 has been reported to downregulate the expression of Th1 and Th17 cytokines in the inflammation process, particularly during Treg cell signaling.39 Various cell types produce IL-10, including Th2 and macrophages, as well as regulatory T cells.40 In CD26–/– mice, the high levels of IL-10 secretion (Fig. 4g) might have been the result of a high percentage of Tregs (Fig. 6). Notably, in response to allogeneic transplantation, high levels of IL-6 were detected in the serum of CD26+/+ mice from the first day and peaked on day 7 after transplantation; however, only a small amount of IL-6 was detected in the serum of CD26–/– mice until day 7 after transplantation (Fig. 4d). Recent studies have demonstrated that IL-6 plays a very important role in regulating the balance between IL-17-producing Th17 cells and Tregs.41 Thus the low level of IL-17 and high percentage of Tregs observed in CD26–/– mice in this study (Figs. 5 and 6) could have been partly due to reduced IL-6 function and excess IL-10 activity.
In addition, it has been reported that human Th17 cells are characterized by high expression of CD26,18 which is a negative selection marker for human Tregs,19 suggesting that CD26 is involved in the differentiation and function of Th17 cells but is not related to Tregs. It is therefore not surprising that an impaired balance of differentiation of Th17 and Tregs was observed in CD26–/– mice. Although allograft rejection is traditionally associated with Th1 differentiation, many recent studies showed that Th17 cells and IL-17 were closely associated with allograft rejection.42 Th17 cells are a more recent addition to the T-cell paradigm, while IL-17, a key Th17 cytokine, is a pro-inflammatory factor.43 Accumulating evidence suggests that Th17 cells play a role in the development of chronic allograft injury in the transplantation of various organs. The hallmark of Th17 cell-mediated allograft rejection is IL-17's ability to recruit neutrophils, which are one of the first inflammatory effector cells capable of infiltrating the allograft after transplantation, thereby causing allograft damage.42 Conversely, Tregs play a crucial role in the immune tolerance and negative control of various immune responses through the suppression or downregulation of other effector T cells during immune rejection.44 It was reported that the inhibition of CD26/DPPIV promotes the secretion of TGF-β1 which is essential for the differentiation of Tregs.45 For the development and function of CD4+CD25+ Treg cells, forkhead transcription factor (Foxp3) is required.46 IL-10 is reported to be an important factor in maintaining FoxP3 expression.47 The high level of IL-10 observed in CD26–/– mice (Fig. 4g) may have benefitted the expression of FoxP3. The reduced Th17 activity and increased Tregs' percentage in MSLs and MPBLs of CD26–/– mice after allogeneic transplantation are assumed to be the most important reason for the reduced necrosis of the graft and delayed allograft rejection in CD26–/– mice.
Certain chemokines contribute to the infiltration of macrophages into graft tissues, such as monocyte chemotactic proteins (MCP-1, -2, -3), macrophage colony-stimulating factor (M-CSF or CSF-1), and chemokine ligand 5 (CCL5 or RANTES (regulated and normal T cell expressed and secreted)).48 As substrates of DPPIV/CD26, MCPs and RANTES can be truncated by DPPIV, followed by the alteration of their chemotactic.3 It was found that MCP with an amino-terminal Lys can be cleaved by CD26/DPPIV and may result in the inactivation of its chemotaxis; however, MCP with an NH2-terminal pGlu remained unaffected.49 The effect of CD26 on the different isoforms of MCP involved in macrophage chemotaxis during skin transplantation should be further investigated. The truncation of CCL5/RANTES by DPPIV was reported to decrease its binding to chemokine receptor 1 (CCR1) and CCR3 but increased its binding to CCR5, contributing to macrophage recruitment in renal grafts.48 The CD26 deficiency of CD26–/– mice likely reduced the activity of CCL5/RANTES binding to CCR5 and resulted in the reduction of macrophage infiltration into grafts.
CD26 is a multifunctional protein, exhibiting either enzymatic activity or interacting with different molecules. Recent studies have reported that CD26/DPPIV is involved in cutaneous wound healing. Higher rates of wound closure, revascularization, and cell proliferation were observed in CD26–/– mice, and DPPIV inhibitor showed a potential benefit in wound healing.50 In the case of skin transplantation, CD26 is involved not only in immune rejection but also in the wound healing process; thus CD26 plays a Janus-like role in engraftment and rejection. In the present work, the necrotic level in grafts and the levels of IgG and related cytokines in the serum of CD26–/– mice were markedly lower than those of CD26+/+ mice. However, from day 13 posttransplantation, the grafts were removed and the wounds healed quickly in CD26–/– mice. The reduction of allograft rejection in CD26–/– mice, as observed in our present work, may be partly counteracted by the enhancement of wound healing, where CD26 is involved in both processes.
In conclusion, our results indicate that CD26 is involved in allogeneic graft rejection. CD26 deficiency resulted in partly impaired differentiation of Th1, Th2, and Th17 subpopulations but increased the percentage of Tregs. In turn, reduced functions of Th1, Th2, and Th17 affected the differentiation of B cells and decreased the activities of CD8+ cells and macrophages, leading to lower production of IgGs and delayed allograft rejection in CD26–/– mice.
Outlook
Skin transplantation is a complex process. In the current work, we demonstrated that CD26 deficiency could result in delayed allograft rejection in allogeneic skin transplantation. The underlying mechanisms should be further investigated and elucidated, such as the activation and differentiation of B cells and macrophages after allogeneic transplantation in CD26–/– mice, the influence of CD26-deficient on activities of certain chemokines as well as on the infiltration of macrophages, and the mechanism that drives Tregs in CD26–/– mice. In addition, because CD26 plays important roles in both skin transplantation and wound healing, the extent to which wound healing affects graft survival/rejection in CD26–/– mice after allogeneic skin transplantation should also be clarified.
Acknowledgements
The authors would like to thank Dr. Didier Marguet (Centre d’Immunologie INSERM-CNRS de Marseille-Luminy, Marseille, France) for generously providing homozygous CD26–/– mice and Dr. Reinhard Gessner (Institut für Klinische Chemie, Medizinische Hochschule Hannover, Germany) for the valuable discussions regarding the present study. This work was supported by a grant from the Deutsche Forschungsgemeinschaft Bonn (Sonderforschungsbereich 366 and 449) and the China Scholarship Council.
Competing interests
The authors declare no competing interests.
References
- 1.Ansorge S, et al. Novel aspects of cellular action of dipeptidyl peptidase IV/CD26. Biol. Chem. 2011;392:153–168. doi: 10.1515/bc.2011.008. [DOI] [PubMed] [Google Scholar]
- 2.Fan H, et al. Dipeptidyl peptidase IV/CD26 in T cell activation, cytokine secretion and immunoglobulin production. Adv. Exp. Med. Biol. 2003;524:165–174. doi: 10.1007/0-306-47920-6_20. [DOI] [PubMed] [Google Scholar]
- 3.De Meester I, Korom S, Van Damme J, Scharpe S. CD26, let it cut or cut it down. Immunol. Today. 1999;20:367–375. doi: 10.1016/S0167-5699(99)01486-3. [DOI] [PubMed] [Google Scholar]
- 4.Loster K, Zeilinger K, Schuppan D, Reutter W. The cysteine-rich region of dipeptidyl peptidase IV (CD26) is the collagen-binding site. Biochem. Biophys. Res. Commun. 1995;217:341–348. doi: 10.1006/bbrc.1995.2782. [DOI] [PubMed] [Google Scholar]
- 5.Ohnuma K, Dang NH, Morimoto C. Revisiting an old acquaintance: CD26 and its molecular mechanisms in T cell function. Trends Immunol. 2008;29:295–301. doi: 10.1016/j.it.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Ohtsuki T, Tsuda H, Morimoto C. Good or evil: CD26 and HIV infection. J. Dermatol. Sci. 2000;22:152–160. doi: 10.1016/S0923-1811(99)00081-X. [DOI] [PubMed] [Google Scholar]
- 7.Panchapakesan U, Mather A, Pollock C. Role of GLP-1 and DPP-4 in diabetic nephropathy and cardiovascular disease. Clin. Sci. (Lond.) 2013;124:17–26. doi: 10.1042/CS20120167. [DOI] [PubMed] [Google Scholar]
- 8.Aytac U, Dang NH. CD26/dipeptidyl peptidase IV: a regulator of immune function and a potential molecular target for therapy. Curr. Drug Targets Immune Endocr. Metabol. Disord. 2004;4:11–18. doi: 10.2174/1568008043340035. [DOI] [PubMed] [Google Scholar]
- 9.Rohrborn D, Wronkowitz N, Eckel J. DPP4 in diabetes. Front. Immunol. 2015;6:386. doi: 10.3389/fimmu.2015.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Penno G, Garofolo M, Del Prato S. Dipeptidyl peptidase-4 inhibition in chronic kidney disease and potential for protection against diabetes-related renal injury. Nutr. Metab. Cardiovasc. Dis. 2016;26:361–373. doi: 10.1016/j.numecd.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Hatano R, et al. Prevention of acute graft-versus-host disease by humanized anti-CD26 monoclonal antibody. Br. J. Haematol. 2013;162:263–277. doi: 10.1111/bjh.12378. [DOI] [PubMed] [Google Scholar]
- 12.Baseler L, de Wit E, Feldmann H. A comparative review of animal models of Middle East respiratory syndrome coronavirus infection. Vet. Pathol. 2016;53:521–531. doi: 10.1177/0300985815620845. [DOI] [PubMed] [Google Scholar]
- 13.Peck MD. Epidemiology of burns throughout the world. Part I: Distribution and risk factors. Burns. 2011;37:1087–1100. doi: 10.1016/j.burns.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Benichou G, et al. Immune recognition and rejection of allogeneic skin grafts. Immunotherapy. 2011;3:757–770. doi: 10.2217/imt.11.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walsh PT, Strom TB, Turka LA. Routes to transplant tolerance versus rejection; the role of cytokines. Immunity. 2004;20:121–131. doi: 10.1016/S1074-7613(04)00024-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koh TJ, DiPietro LA. Inflammation and wound healing: the role of the macrophage. Expert Rev. Mol. Med. 2011;13:e23. doi: 10.1017/S1462399411001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willheim M, et al. Cell surface characterization of T lymphocytes and allergen-specific T cell clones: correlation of CD26 expression with T(H1) subsets. J. Allergy Clin. Immunol. 1997;100:348–355. doi: 10.1016/S0091-6749(97)70248-3. [DOI] [PubMed] [Google Scholar]
- 18.Bengsch B, et al. Human Th17 cells express high levels of enzymatically active dipeptidylpeptidase IV (CD26) J. Immunol. 2012;188:5438–5447. doi: 10.4049/jimmunol.1103801. [DOI] [PubMed] [Google Scholar]
- 19.Salgado FJ, et al. CD26: a negative selection marker for human Treg cells. Cytom. A. 2012;81:843–855. doi: 10.1002/cyto.a.22117. [DOI] [PubMed] [Google Scholar]
- 20.Afzali B, Lombardi G, Lechler RI, Lord GM. The role of T helper 17 (Th17) and regulatory T cells (Treg) in human organ transplantation and autoimmune disease. Clin. Exp. Immunol. 2007;148:32–46. doi: 10.1111/j.1365-2249.2007.03356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Leary H, Ou X, Broxmeyer HE. The role of dipeptidyl peptidase 4 in hematopoiesis and transplantation. Curr. Opin. Hematol. 2013;20:314–319. doi: 10.1097/MOH.0b013e32836125ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marguet D, et al. Enhanced insulin secretion and improved glucose tolerance in mice lacking CD26. Proc. Natl. Acad. Sci. USA. 2000;97:6874–6879. doi: 10.1073/pnas.120069197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan S, Marguet D, Dobers J, Reutter W, Fan H. Deficiency of CD26 results in a change of cytokine and immunoglobulin secretion after stimulation by pokeweed mitogen. Eur. J. Immunol. 2003;33:1519–1527. doi: 10.1002/eji.200323469. [DOI] [PubMed] [Google Scholar]
- 24.Ohnuma K, et al. Blockade of CD26-mediated T cell costimulation with soluble caveolin-1-Ig fusion protein induces anergy in CD4 + T cells. Biochem Biophys. Res. Commun. 2009;386:327–332. doi: 10.1016/j.bbrc.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 25.Gutierrez-Hoya A, et al. Role of CD8 regulatory T cells versus Tc1 and Tc17 cells in the development of human graft-versus-host disease. J. Immunol. Res. 2017;2017:1236219. doi: 10.1155/2017/1236219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peranteau WH, et al. CD26 inhibition enhances allogeneic donor-cell homing and engraftment after in utero hematopoietic-cell transplantation. Blood. 2006;108:4268–4274. doi: 10.1182/blood-2006-04-018986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanchez-Fueyo A, Markmann JF. Immune exhaustion and transplantation. Am. J. Transplant. 2016;16:1953–1957. doi: 10.1111/ajt.13702. [DOI] [PubMed] [Google Scholar]
- 28.Smith KM, et al. Th1 and Th2 CD4 + T cells provide help for B cell clonal expansion and antibody synthesis in a similar manner in vivo. J. Immunol. 2000;165:3136–3144. doi: 10.4049/jimmunol.165.6.3136. [DOI] [PubMed] [Google Scholar]
- 29.Hidalgo LG, Halloran PF. Role of IFN-gamma in allograft rejection. Crit. Rev. Immunol. 2002;22:317–349. doi: 10.1615/CritRevImmunol.v22.i4.50. [DOI] [PubMed] [Google Scholar]
- 30.Avni B, Grisariu S, Shapira MY. Interleukin-2: a double-edge sword in allogeneic stem cell transplantation. Immunotherapy. 2016;8:241–243. doi: 10.2217/imt.15.117. [DOI] [PubMed] [Google Scholar]
- 31.Ring GH, et al. Interferon-gamma is necessary for initiating the acute rejection of major histocompatibility complex class II-disparate skin allografts. Transplantation. 1999;67:1362–1365. doi: 10.1097/00007890-199905270-00012. [DOI] [PubMed] [Google Scholar]
- 32.Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annu. Rev. Immunol. 1999;17:701–738. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 33.Hsu LW, et al. Prolongation of heart allograft survival of rats treated by a Th2 inhibitor. Transpl. Immunol. 2003;11:385–388. doi: 10.1016/S0966-3274(02)00152-1. [DOI] [PubMed] [Google Scholar]
- 34.Crotty S. A brief history of T cell help to B cells. Nat. Rev. Immunol. 2015;15:185–189. doi: 10.1038/nri3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoffman W, Lakkis FG, Chalasani G. B cells, antibodies, and more. Clin. J. Am. Soc. Nephrol. 2016;11:137–154. doi: 10.2215/CJN.09430915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yan S, Gessner R, Dietel C, Schmiedek U, Fan H. Enhanced ovalbumin-induced airway inflammation in CD26-/- mice. Eur. J. Immunol. 2012;42:533–540. doi: 10.1002/eji.201041038. [DOI] [PubMed] [Google Scholar]
- 37.Stastny P, et al. Role of immunoglobulin (Ig)-G and IgM antibodies against donor human leukocyte antigens in organ transplant recipients. Hum. Immunol. 2009;70:600–604. doi: 10.1016/j.humimm.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 38.Yamada Y, et al. CD26 costimulatory blockade improves lung allograft rejection and is associated with enhanced interleukin-10 expression. J. Heart Lung Transplant. 2016;35:508–517. doi: 10.1016/j.healun.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 39.Wang S, et al. Interleukin-10 deficiency impairs regulatory T cell-derived neuropilin-1 functions and promotes Th1 and Th17 immunity. Sci. Rep. 2016;6:24249. doi: 10.1038/srep24249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 41.Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur. J. Immunol. 2010;40:1830–1835. doi: 10.1002/eji.201040391. [DOI] [PubMed] [Google Scholar]
- 42.Abadja F, Sarraj B, Ansari MJ. Significance of T helper 17 immunity in transplantation. Curr. Opin. Organ Transplant. 2012;17:8–14. doi: 10.1097/MOT.0b013e32834ef4e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat. Med. 2007;13:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 44.Walsh PT, Taylor DK, Turka LA. Tregs and transplantation tolerance. J. Clin. Invest. 2004;114:1398–1403. doi: 10.1172/JCI200423238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steinbrecher A, et al. Targeting dipeptidyl peptidase IV (CD26) suppresses autoimmune encephalomyelitis and up-regulates TGF-beta 1 secretion in vivo. J. Immunol. 2001;166:2041–2048. doi: 10.4049/jimmunol.166.3.2041. [DOI] [PubMed] [Google Scholar]
- 46.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4 + CD25 + regulatory T cells. Nat. Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 47.Murai M, et al. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat. Immunol. 2009;10:1178–1184. doi: 10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang X, Tian W, Sung YK, Qian J, Nicolls MR. Macrophages in solid organ transplantation. Vasc. Cell. 2014;6:5. doi: 10.1186/2045-824X-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Coillie E, et al. Functional comparison of two human monocyte chemotactic protein-2 isoforms, role of the amino-terminal pyroglutamic acid and processing by CD26/dipeptidyl peptidase IV. Biochemistry. 1998;37:12672–12680. doi: 10.1021/bi980497d. [DOI] [PubMed] [Google Scholar]
- 50.Baticic Pucar L, Pernjak Pugel E, Detel D, Varljen J. Involvement of DPP IV/CD26 in cutaneous wound healing process in mice. Wound Repair Regen. 2017;25:25–40. doi: 10.1111/wrr.12498. [DOI] [PubMed] [Google Scholar]