Abstract
Cardiovascular diseases (CVDs) are the leading causes of premature death and disability in people around the world. Therefore, the prevention and treatment of CVDs has become an important subject. In this study, we verified the thrombolytic activities of a nattokinase‐like protease named NK‐01 in vivo. Label‐free liquid chromatography–tandem mass spectrometry (LC‐MS/MS) technique was used in our study. NK‐01 could inhibit the activity of coagulation factors though the up‐regulation of proteinase C inhibitors and protein S. NK‐01 also could inhibit the angiotensinogen conversion to AngII and promote the degradation of kininogen to reduce the blood pressure. In addition, NK‐01 could increase the content of paraoxonase 1, which could prevent atherosclerosis. In our study, we found that NK‐01 cloud effect some key proteins which participant in CVDs associated metabolic processes such as coagulation system, blood pressure, and atherosclerosis. Taken together, the underlying molecular mechanisms for the biological beneficial of NK‐01 were investigated. Our proteomic study will provide further theoretical basis for application of NK in prevention or adjuvant treatment in biomedicine areas.
Keywords: cardiovascular disease, disease prevention, label free, nattokinase
1. INTRODUCTION
WHO reported that 17.7 million people died of cardiovascular diseases (CVDs) every year and that counts for 31% of all death in the world (McAloon et al., 2016). Although the formation of CVDs is a complex pathological process, it frequently occurs suddenly and leads to disability or death (World Health Organization, 2013). For example, atherosclerosis is the underlying pathology of CVDs. The process of atherosclerosis is the deposition of fat and cholesterol in the lumen of blood vessels, which results in the reduced elasticity of blood vessels and the formation of thrombus. Thrombus can easily cause coronary heart disease and stroke (Mendis et al., 2011).
Some effective ways had been taken to reduce the incidence and mortality of CVDs. The primary preventions of CVDs are healthy diet, regular physical activities, and prohibition of tobacco products. For secondary prevention, pharmacologic interventions are necessary. The data from clinical trials have proved that medicines, like aspirin, statins, and blood pressure medicines, played important roles in managing CVDs (Baigent et al., 2009, 2010; Law, Morris, & Wald, 2009). However, CVD is a chronic disease that requires long‐term medical treatment. The patients usually have poor adherence to drugs, and the benefits of medications cannot be fully realized (Haynes, McKibbon, & Kanani, 1996; O'Flaherty, Buchan, & Capewell, 2013). In addition, most drugs have some side effects (Beltowski, Wojcicka, & Jamroz‐Wisniewska, 2009; Hankey & Eikelboom, 2006; Klegerman, 2017; Toh et al., 2012). For example, thrombolytic agents could lead to the unwanted internal bleeding and their half‐lives are short in vivo Nordt & Bode, 2003). Statins could lower the plasma low‐density lipoprotein (LDL) cholesterol, and it could also cause sympathy and severe rhabdomyolysis (Beltowski et al., 2009). As an antiplatelet drug, aspirin plays an important role in the prevention of CVDs. But the curative effects of aspirin vary from person to person and some people showed poor antiplatelet effect, which was called “aspirin resistance” (Georgiadis et al., 2013).
Nattokinase is a kind of serine protease with strong thrombolytic activity (Sumi, Hamada, Tsushima, Mihara, & Muraki, 1987). It was extracted from fermented beans such as natto, douche, and tempeh and can be absorbed through the intestine (Fujita, Hong, et al., 1995). Nattokinase was widely studied as a dietary supplement and nutritional food, which has potential to prevent and treat CVDs (Dabbagh et al., 2014; Weng, Yao, Sparks, & Wang, 2017). Hongjie Chen et al. reported that nattokinase was a promising alternative in prevention and treatment of cardiovascular diseases by possessing a variety of favorable cardiovascular effects, such as fibrinolytic activity, antihypertensive, antiatherosclerotic, and lipid‐lowering, antiplatelet, and neuroprotective effects (Chen et al., 2018). The consumption of natto has been linked to a reduction in CVD mortality. Compared with other thrombolytic drugs, nattokinase has higher thrombolytic activity, which was about 4× higher compared with urokinase (Fujita, Hong, et al., 1995). Nattokinase is more sensitive to cross‐linked fibrin than fibrinogen, which could effectively prevent internal bleeding (Fujita, Ito, Hong, & Nishimuro, 1995). Ji Young Kim et al. found that nattokinase resulted in a reduction in systolic blood pressure (SBP) and diastolic blood pressure (DBP) of hypertensive patients (Kim et al., 2008). Ren nina et al. had proved that nattokinase also played an important role in preventing atherosclerosis of patients (N. N. Ren, Chen, Li, Mcgowan, & Lin, 2017).
Recently, we found a nattokinase‐like protease named NK‐01, which shared 99% sequence identity with the nattokinase (Genebank number: AHZ12722.1) and composed of multiple fragments. Through casein plate method, we showed that NK‐01 has better fibrinolytic activity than urokinase (Y. Ren, Pan, Lyu, & Liu, 2018). In this study, we confirmed the in vivo thrombolytic activity of NK‐01. Based on label‐free technology, we studied the effects of NK‐01 on the proteomic profiling of plasma proteins in rats and further analyzed the possible mechanisms of its biological functions in the prevention of cardiovascular diseases.
2. MATERIALS AND METHODS
2.1. Chemicals
Nattokinase NK‐01 was prepared in our laboratory. Urokinase was obtained from Nanda Co., Ltd, Pelltobarbitalum Natricum was purchased from Solarbio. Trypsin was purchased from Promega, and iodoacetamide was purchased from Sigma. MeOH, formic acid, CAN, and TCEP were purchased from ThermoFisher Scientific.
2.2. Animals
Male SD rats (~300 g, 8 week) were purchased from DaRenFuChen Animal Co., Ltd. Rats were customized in cages in air‐conditioned animal room under a photoperiod schedule of 12‐hr light/12‐hr dark cycles at 25 ± 2°C. The rats were fed with normal food and tap water for 1 week before the experiments. Rats were fasted overnight before experiments with free access to tap water. All experiments with animals were carried out in accordance with the guidelines of Care and Use of Laboratory Animals published by China National Institute of Health.
2.3. Thrombolytic activity of NK‐01 in vivo
Fifteen rats were divided into negative control group, NK‐01 group, and positive control group. Rats were anesthetized by 3% Pelltobarbitalum Natricum (1 ml/kg body weight). Rats in negative control group and NK‐01 group were injected with 0.5 ml 0.9% NaCl and 0.5 ml NK‐01 (5,000 FU) into duodenal. Rats in positive control group were treated with urokinase (50,000 U) by tail vein injection. Four hours after injection, the blood of all rats was collected with tubes containing 3.2% sodium citrate solution (9:1, v/v). Blood was centrifuged at 3,000 g for 15 min at room temperature. Subsequently, the citrated plasma was used for analysis of thrombotic indexes (PT, APTT, TT, and FIB).
2.4. Preparation of proteomic samples
Twelve rats were divided into two groups: negative control group and NK‐01 group. Rats were anesthetized as mentioned above and were injected with 0.5 ml 0.9% NaCl and 0.5 ml NK‐01 (5,000 FU) into duodenal. Four hours after injection, the blood of all rats was collected with tubes containing 3.2% sodium citrate solution (9:1, v/v) and was centrifuged at 3,000 g for 15 min at room temperature.
A moderate amount of plasma, PBS, and protease inhibitors were added into 0.22‐μm membrane filter centrifuge tube with loaded packing (Thermo 191085305) and incubated for 1 hr at room temperature. Then, the tubes were centrifuged at 500 g for 1 min and the filtrates were collected. The packing was cleaned with PBS twice to collect the filtrates. All of the filtrates were concentrated by 3K ultrafiltration. The concentrated plasma samples were replaced with 8 M urea for three repeated times to appropriate volume. Finally, the protein concentrations of plasma samples were determined by bicinchoninic acid (BCA) method.
2.5. Protein digestion and peptides quantification
The plasma samples were digested with trypsin solution according to the standard procedure. The peptides were re‐suspended with 2% ACN, 0.1% TFA, and Sep‐Pak desalination. Then, each sample was vacuum dried. The peptides were finally quantified by Thermo Fisher Scientific (Thermo 23275).
2.6. Mass spectrometry analysis
Experiments were performed on a Q Exactive mass spectrometer coupled with Easy‐nLC 1,200. Peptide of each sample (0.5 μg/μl) was injected for nano LC‐MS/MS analysis. The peptide (2 μg) was loaded onto a C18‐reversed phase column (75 μm × 25 cm, Thermo) in buffer A (2% acetonitrile and 0.1% Formic acid) and separated with a linear gradient of buffer B (80% acetonitrile and 0.1% Formic acid) at a flow rate of 300 nl/min. In order to fully separate the peptides in plasma, the gradient elution program was set as follows: The concentration of (B) in the mobile phase was 0% at the initial, and then a linear increase from 3% to 6% (B) from 0 to 2 min, followed by a linear increase from 6% to 23% (B) from 2 to 105 min, and a linear increase from 23% to 29% (B) from 105 to 130 min, further a linear increase from 29% to 100% (B) from 130 to 149 min, finally 100% constant 100% (B) from 149 to 155 min. Electrospray ionization was carried out in the positive ionization mode. The electrospray voltage was 1.8 kV. Q Exactive mass spectrometer was operated in the data‐dependent mode to switch automatically between MS and MS/MS acquisition. Resolution for MS survey scan was set to 70 K for full‐scan MS spectra (m/z 350–1300) and 17.5 K for MS/MS scan. In all cases, one microscan was recorded using dynamic exclusion of 18 s. For MS/MS, normalized collision energy was set at 30 s.
2.7. Sequence database searching
MS/MS spectra were searched using PEAKS against Rattus norvegicus (29,978 entries) and the decoy database as the following parameters. The highest score for a given peptide mass (best match to that predicted in the database) was used to identify parent proteins. The parameters for protein searching were set as follows: tryptic digestion with up to two missed cleavages, carbamidomethylation of cysteines as fixed modification, and oxidation of methionines and protein N‐terminal acetylation as variable modifications. Peptide spectral matches were validated based on q‐values at a 1% false discovery rate.
2.8. Statistical analysis
All results were expressed as mean ± standard deviation (), and statistical analysis was performed using Student's t test for comparison of two groups. In order to avoid problems such as the omission of identification results, the peptide error of the database search needs to be controlled within ± 10 ppm. To define the differentially expressed proteins, either a fold change (FC)>1.2 or a FC < 1/1.2 was set as the threshold. p‐value (<.05) was set as significant level.
3. RESULTS AND DISCUSSIONS
3.1. Thrombolytic activity of NK‐01 in vivo
The levels of prothrombin time (PT), activated partial thromboplastin time (APTT), thrombin time (TT), and fibrinogen (FIB) are important indicators to judge the pathological changes of coagulation system in the body. The levels of PT, APTT, and TT are decreased in the persons with thrombotic diseases. However, the level of FIB is usually increased. And it was reported that the level of PT could be decreased after treated with nattokinase (Kapoor, Harde, Jain, Panda, & Panda, 2015) And it was also reported that nattokinase not only possesses plasminogen activator activity, but also directly digests fibrin through limited proteolysis. Chien‐Hsun Hsia et al. conducted a clinical trial and showed that oral administration of nattokinase could be considered as a CVD nutraceutical by decreasing plasma levels of fibrinogen, factor VII, and factor VIII (Hsia et al., 2009). Yuko Kurosawa1 determined the quantitative effects of a single dose of nattokinase administration on coagulation/fibrinolysis parameters in healthy male subjects. They found that a single dose of NK administration could enhance fibrinolysis and anticoagulation via several different pathways simultaneously. (Chen et al., 2018).
It was shown in Figure 1 that the levels of PT, APTT, and TT were significantly increased in the male rats treated with NK‐01 compared with the negative control group (p < .05). However, the level of FIB in the rats treated with NK‐01 was significantly decreased compared with the negative control group (p < .05). The result indicated that NK‐01 had potential in vivo antithrombotic activities.
Figure 1.
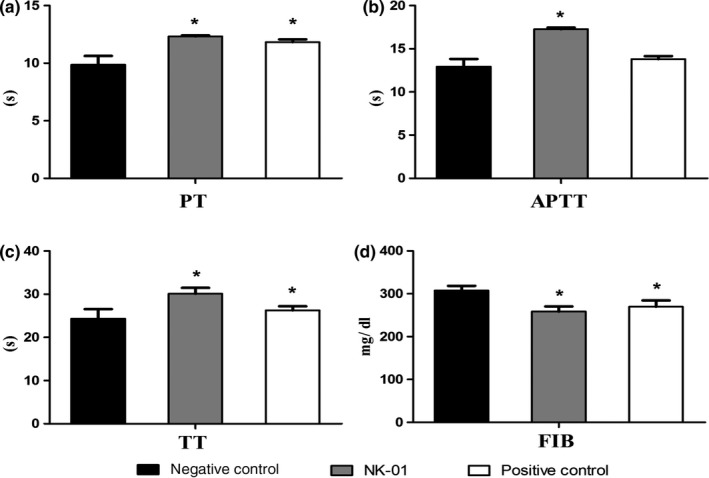
Changes of PT, APTT, TT, and FIB levels in rat. (a: PT, b: APTT, c: TT, d: FIB)
3.2. Identification of proteins from quantitative proteomic analysis
A total of 254,117 spectra were obtained from the LC‐MS/MS proteomic analysis. After data filtering, a total of 64,302 spectra were matched to 4,086 peptides, which were mapped to 665 proteins and 516 protein groups. Among all the identified proteins, approximately 57% of the proteins included 1–3 peptides and 43% of the proteins included at least 4 peptides (Figure 2a). In addition, the proteins were identified with high sequence coverage, 65% of proteins were identified with more than 10% of the sequence coverage, and 54% of proteins were identified with more than 20% of the sequence coverage (Figure 2b). Also, 42% of proteins were identified with the molecular weight less than 40 kDa and 58% of the proteins were identified with the molecular weight larger than 40 kDa (Figure 2c).
Figure 2.
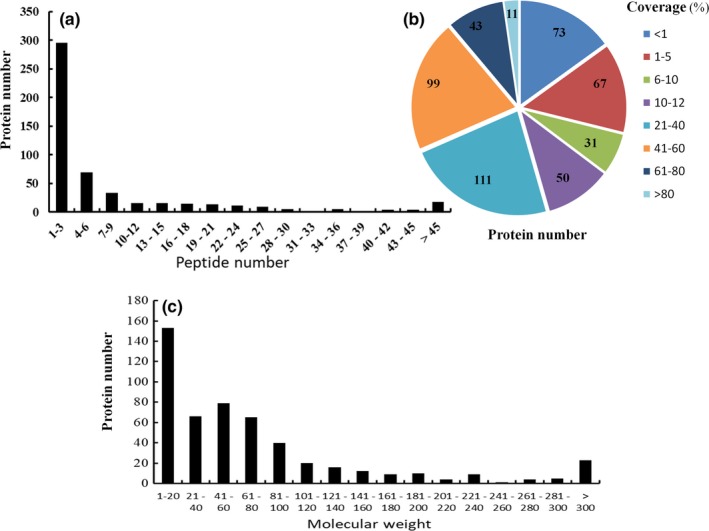
Distribution of peptide number, protein coverage, and protein molecular weight. (a) Distribution and number of peptides matched to proteins. (b) Coverage of proteins by identified peptides. (c) Protein molecular weight distribution
3.3. Analysis of differentially expressed proteins
Three biological replicate experiments were performed on the serum from two groups of rats. The experimental results showed that a total of 665 proteins were detected and 453 proteins could be quantified. Among them, there were 73 differentially expressed proteins including 49 up‐regulated (FC > 1.2, *p < .05) proteins and 24 down‐regulated proteins (FC < 0.83, *p < .05). The basic information for the up‐regulated proteins and down‐regulated proteins was shown in Table 1.
Table 1.
List of relevant proteins identified as significantly differentially expressed in rats
| Accession | FC (experiment/control) | Description |
|---|---|---|
| M0RE02 | 479.58 | Uncharacterized protein |
| F1M0B7 | 16.01 | Uncharacterized protein |
| M0RBL2 | 13.94 | Uncharacterized protein |
| A0A0G2K458 | 10.58 | Uncharacterized protein |
| D3ZEP5 | 9.92 | Uncharacterized protein |
| O09171 | 9.58 | Betaine—homocysteine S‐methyltransferase |
| M0R7M5 | 5.95 | Uncharacterized protein |
| M0RC23 | 5.40 | Uncharacterized protein |
| A0A0G2JY98 | 4.26 | Uncharacterized protein |
| F1M7I8 | 4.05 | Similar to Ig variable region light chain |
| M0RDL2 | 3.23 | Uncharacterized protein |
| P01836 | 3.07 | Ig kappa chain C region A allele |
| M0R816 | 2.99 | Uncharacterized protein |
| A0A0G2KAH4 | 2.76 | Dedicator of cytokinesis 6 |
| D4ACR1 | 2.69 | Uncharacterized protein |
| P23785 | 2.66 | Granulins |
| D3ZWC1 | 2.57 | Uncharacterized protein |
| P02770 | 2.56 | Serum albumin |
| D3Z9Z7 | 2.54 | Collagen beta(1‐O)galactosyltransferase 2 |
| F1LXY6 | 2.50 | Uncharacterized protein |
| P02761 | 2.34 | Major urinary protein |
| A0A0G2JSH5 | 2.10 | Serum albumin |
| M0R979 | 2.08 | Uncharacterized protein |
| A0A0G2K0N6 | 2.04 | Uncharacterized protein |
| M0RBP7 | 2.03 | Uncharacterized protein |
| Q5M890 | 1.92 | Apolipoprotein N |
| F1LZH0 | 1.86 | Uncharacterized protein |
| D4A183 | 1.84 | Similar to Vanin‐3 (Predicted) |
| Q9EPI1 | 1.81 | Xylosyltransferase 1 (Fragment) |
| F1MAK3 | 1.81 | Rho GTPase‐activating protein 32 |
| Q6AYS3 | 1.69 | Carboxypeptidase |
| D3ZP12 | 1.68 | Zinc finger CCCH type containing 7 A (Predicted) |
| P04916 | 1.68 | Retinol‐binding protein 4 OS = R |
| A0A0G2JX36 | 1.64 | Uncharacterized protein |
| P55159 | 1.64 | Serum paraoxonase/arylesterase 1 |
| P53813 | 1.64 | Vitamin K‐dependent protein S |
| M0RBX3 | 1.63 | Uncharacterized protein |
| E9PSL7 | 1.63 | Citron rho‐interacting serine/threonine kinase |
| M0R4C5 | 1.62 | Uncharacterized protein |
| F1LVL2 | 1.59 | Inducible T‐cell co‐stimulator ligand |
| Q6P734 | 1.55 | Plasma protease C1 inhibitor |
| M0R628 | 1.49 | Uncharacterized protein |
| A0A0G2K5X3 | 1.48 | Uncharacterized protein |
| A0A0G2K980 | 1.43 | Uncharacterized protein |
| D3ZCX6 | 1.32 | RNA exonuclease 1 homolog |
| A0A0G2K151 | 1.29 | Apolipoprotein E |
| A0A0G2K531 | 1.28 | Glutathione peroxidase |
| P01015 | 1.26 | Angiotensinogen |
| A0A0G2K4I9 | 1.25 | Coagulation factor IX |
| A0A0G2JUY3 | 0.32 | Uncharacterized protein |
| B1WC21 | 0.68 | Fibulin−1 |
| P01883 | 0.21 | Ig delta chain C region (Fragment) |
| A0A0G2K9Z5 | 0.25 | Uncharacterized protein |
| E9PTD7 | 0.18 | Kin of IRRE‐like 3 (Drosophila) |
| A0A0H2UHM3 | 0.62 | Haptoglobin |
| P02651 | 0.67 | Apolipoprotein A‐IV |
| P08932 | 0.69 | T‐kininogen 2 |
| P01805 | 0.65 | Ig heavy chain V region IR2 |
| A0A0G2K2D9 | 0.38 | Uncharacterized protein |
| A0A0G2K926 | 0.48 | Alpha−1‐inhibitor III |
| A0A0G2JZL1 | 0.76 | Uncharacterized protein |
| O88278 | 0.73 | Cadherin EGF LAG seven‐pass G‐type receptor 3 |
| P01855 | 0.49 | Ig epsilon chain C region |
| P20766 | 0.63 | Ig lambda−1 chain C region |
| A0A0G2JZN1 | 0.60 | Uncharacterized protein |
| A0A140TAE6 | 0.82 | Enoyl‐[acyl‐carrier‐protein] reductase mitochondrial |
| F7FIX1 | 0.38 | Uncharacterized protein |
| F1M9L4 | 0.60 | Cation‐transporting ATPase |
| D3ZFC6 | 0.79 | Inter‐alpha‐trypsin inhibitor heavy chain family member 4 |
| G3V7H3 | 0.58 | Complement factor D |
| Q68FP1 | 0.72 | Gelsolin |
| A0A1W2Q642 | 0.47 | Uncharacterized protein |
| D3ZF92 | 0.67 | Tumor necrosis factor receptor superfamily member 21 |
3.4. Influence of NK‐01 on key serum proteins involved in the thrombolysis and blood coagulation process
As shown in Figure 3a, a total of 17 proteins that are associated with thrombolysis and coagulation processes, such as prothrombin, plasminogen, and coagulation factors, were detected. The proteinase C1 inhibitors and protein S were up‐regulated in normal rats after treatment with NK‐01, and others had no significant changes in normal rats after treated with high dose of NK‐01.
Figure 3.
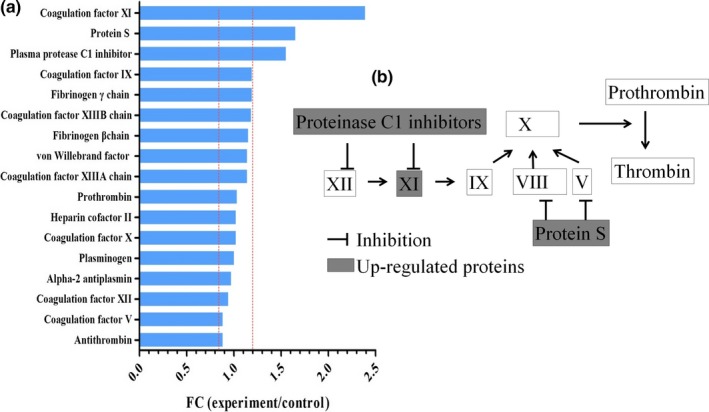
Identified proteins associated with fibrinolysis and coagulation system. (a) Showed the seventeen proteins associated with fibrinolysis and coagulation system. The dot lines mean the thresholds of the significantly differentially expressed proteins. (b) Showed the metabolic pathway which the proteinase C1 and protein S participate in and the function of them
The roles of proteinase C1 inhibitors and protein S were illustrated in Figure 3b (Castoldi & Hackeng, 2008; Schurmann et al., 2014). Our data indicated that NK‐01 could affect the process of blood coagulation by influencing the activities of coagulation factors.
In addition, most of the 17 proteins that were detected had no significant fold change, suggesting that high dose of NK‐01 treatment cannot cause disorder in the thrombolytic process. Therefore, NK‐01 had good biosafety in terms of thrombolysis.
3.5. Influence of NK‐01 on key proteins involved in blood pressure regulation
Among the proteins detected, there were five proteins that were related to the regulation of blood pressure. The angiotensinogen was significantly up‐regulated, and the T‐kininogen 2 was significantly down‐regulated as shown in Figure 4a. Angiotensinogen and T‐kininogen 2 all played important roles in regulating blood pressure (Figure 4b).
Figure 4.
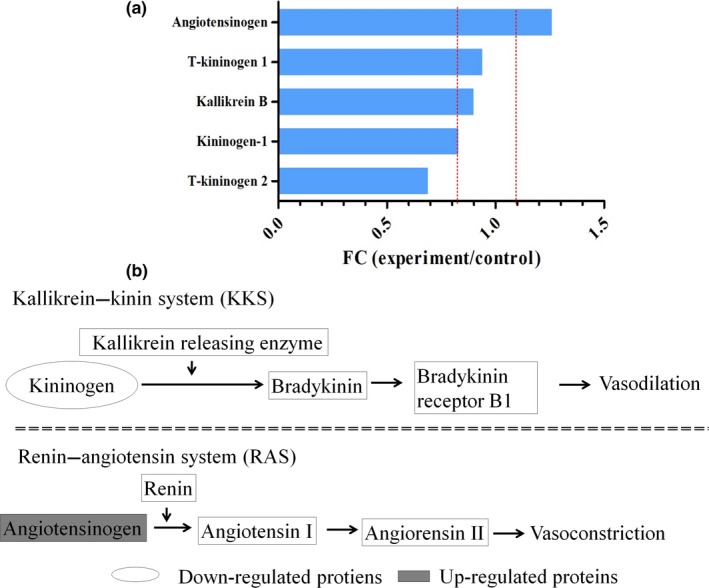
Identified proteins associated with regulation of blood pressure. (a) Showed the five proteins associated with regulation of blood pressure. The dot lines mean the thresholds of the significantly differentially expressed proteins. (b) Showed metabolic pathway which the Angiotensinogen and T‐kininogen 2 participant and the function of them
Renin plays an important role in renin–angiotensin system (RAS) and could convert angiotensinogen to angiotensin I. Then, angiotensin I was converted to angiotensin II, which could raise the blood pressure directly (Lavoie & Sigmund, 2003). In addition, the kallikrein–kinin system (KKS) could lower blood pressure and negatively regulate RAS. In the KKS, kininogen is degraded into kinin, which is helpful for controlling the blood pressure (Regoli & Gobeil, 2017). It was reported that nattokinase could lower the blood pressure and had impact on the renin activity (Kim et al., 2008). Nattokinase could inhibit angiotensin I converting enzyme by a mixed type (the decrease in Vmax and the increase in Km value) to suppress blood pressure. (Murakami, Yamanaka, Ohnishi, Fukayama, & Yoshino, 2012). In our study, angiotensinogen was up‐regulated and the kininogen was down‐regulated in rats treated with NK‐01. This implied that nattokinase could inhibit the conversation of angiotensinogen and promote the degradation of kininogen. In this way, nattokinase could regulate the blood pressure.
3.6. Influence of NK‐01 on key proteins involved in blood lipid regulation
It was shown in Figure 5 that a total of 13 proteins that were associated with regulation of blood lipids were detected. Paraoxonase/arylesterase 1, apolipoprotein N, and apolipoprotein E were all up‐regulated, while apolipoprotein A‐IV was down‐regulated (Figure 5) Paraoxonase/arylesterase 1, a high‐density lipoprotein (HDL)‐associated enzyme, could inhibit the oxidation of LDL (Mackness, Quarck, Verreth, Mackness, & Holvoet, 2006). After treatment with NK‐01, the paraoxonase/arylesterase 1 was significantly up‐regulated in rats. Therefore, NK‐01 had the potential to prevent atherosclerosis. According to our results, the Apo E was up‐regulated and the Apo A‐IV was down‐regulated in normal rats after treatment with NK‐01. The possible reason might be because that the rats were fasted overnight and need more LDL to maintain normal metabolic.
Figure 5.
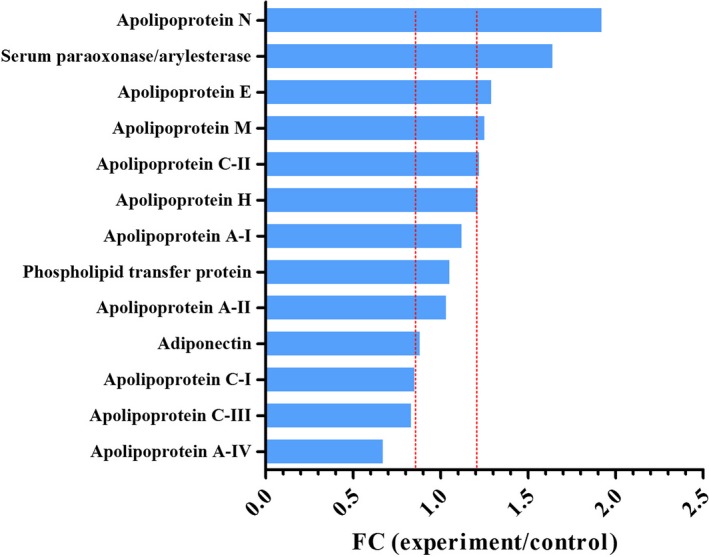
Identified proteins associated with regulation of blood lipids. This figure showed the thirteen proteins associated with regulation of lipids. The dot lines mean the thresholds of the significantly differentially expressed proteins
CONFLICT OF INTEREST
The authors declare that they do not have any conflict of interest. All the authors approve the submission of this manuscript and will take responsibility for any conflict of interest.
ETHICAL APPROVAL
This study was approved by the Institutional Review Board of Ocean University of China, and the study's protocols and procedures were ethically reviewed and approved by China National Institute of Health.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (81302684, 2016), the Natural Science Foundation of Shandong Province (ZR2013CM044, 2013), and Key Research and Development Program of Shandong Province (2018GSF121038,2018).
Pan X, Liang P, Teng L, et al. Study on molecular mechanisms of nattokinase in pharmacological action based on label‐free liquid chromatography–tandem mass spectrometry. Food Sci Nutr. 2019;7:3185–3193. 10.1002/fsn3.1157
Contributor Information
Weizhi Liu, Email: liuweizhi@mail.ouc.edu.cn.
Yan Yang, Email: 172812969@qq.com.
REFERENCES
- Baigent, C. , Blackwell, L. , Collins, R. , Emberson, J. , Godwin, J. , Peto, R. , … Zanchetti, A. (2009). Aspirin in the primary and secondary prevention of vascular disease: Collaborative meta‐analysis of individual participant data from randomised trials. Lancet, 373(9678), 1849–1860. 10.1016/s0140-6736(09)60503-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baigent, C. , Blackwell, L. , Emberson, J. , Holland, L. E. , Reith, C. , Bhala, N. , … Collins, R. (2010). Efficacy and safety of more intensive lowering of LDL cholesterol: A meta‐analysis of data from 170,000 participants in 26 randomised trials. Lancet, 376(9753), 1670–1681. 10.1016/S0140-6736(10)61350-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltowski, J. , Wojcicka, G. , & Jamroz‐Wisniewska, A. (2009). Adverse effects of statins ‐ mechanisms and consequences. Current Drug Safety, 4(3), 209–228. [DOI] [PubMed] [Google Scholar]
- Castoldi, E. , & Hackeng, T. M. (2008). Regulation of coagulation by protein S. Current Opinion in Hematology, 15(5), 529 10.1097/MOH.0b013e328309ec97 [DOI] [PubMed] [Google Scholar]
- Chen, H. , McGowan, E. M. , Ren, N. , Lal, S. , Nassif, N. , Shad‐Kaneez, F. , … Lin, Y. (2018). Nattokinase: A Promising Alternative in Prevention and Treatment of Cardiovascular Diseases. Biomark Insights, 13, 1177271918785130 10.1177/1177271918785130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabbagh, F. , Negahdaripour, M. , Berenjian, A. , Behfar, A. , Mohammadi, F. , Zamani, M. , … Ghasemi, Y. (2014). Nattokinase: Production and application. Applied Microbiology and Biotechnology, 98(22), 9199–9206. 10.1007/s00253-014-6135-3 [DOI] [PubMed] [Google Scholar]
- Fujita, M. , Hong, K. , Ito, Y. , Misawa, S. , Takeuchi, N. , Kariya, K. , & Nishimuro, S. (1995). Transport of nattokinase across the rat intestinal tract. Biological &/and Pharmaceutical Bulletin, 18(9), 1194–1196. 10.1248/bpb.18.1194 [DOI] [PubMed] [Google Scholar]
- Fujita, M. , Ito, Y. , Hong, K. , & Nishimuro, S. (1995). Characterization of nattokinase‐degraded products from human fibrinogen or cross‐linked fibrin. Fibrinolysis, 9(3), 157–164. 10.1016/S0268-9499(95)80005-0 [DOI] [Google Scholar]
- Georgiadis, A. L. , Cordina, S. M. , Vazquez, G. , Tariq, N. , Suri, M. F. , Lakshminarayan, K. , … Qureshi, A. I. (2013). Aspirin treatment failure and the risk of recurrent stroke and death among patients with ischemic stroke. Journal of Stroke and Cerebrovascular Diseases: the Official Journal of National Stroke Association, 22(2), 100–106. 10.1016/j.jstrokecerebrovasdis.2011.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankey, G. J. , & Eikelboom, J. W. (2006). Aspirin resistance. Lancet, 367(9510), 606–617. 10.1016/s0140-6736(06)68040-9 [DOI] [PubMed] [Google Scholar]
- Haynes, R. B. , McKibbon, K. A. , & Kanani, R. (1996). Systematic review of randomised trials of interventions to assist patients to follow prescriptions for medications. Lancet, 348(9024), 383–386. [DOI] [PubMed] [Google Scholar]
- Hsia, C. H. , Shen, M. C. , Lin, J. S. , Wen, Y. K. , Hwang, K. L. , Cham, T. M. , & Yang, N. C. (2009). Nattokinase decreases plasma levels of fibrinogen, factor VII, and factor VIII in human subjects. Nutrition Research, 29(3), 190–196. 10.1016/j.nutres.2009.01.009 [DOI] [PubMed] [Google Scholar]
- Kapoor, R. , Harde, H. , Jain, S. , Panda, A. K. , & Panda, B. P. (2015). Downstream Processing, Formulation Development and Antithrombotic Evaluation of Microbial Nattokinase. Journal of Biomedical Nanotechnology, 11(7), 1213–1224. 10.1166/jbn.2015.2071 [DOI] [PubMed] [Google Scholar]
- Kim, J. Y. , Gum, S. N. , Paik, J. K. , Lim, H. H. , Kim, K.‐C. , Ogasawara, K. , … Lee, J. H. (2008). Effects of nattokinase on blood pressure: A randomized, controlled trial. Hypertension Research, 31(8), 1583–1588. 10.1291/hypres.31.1583 [DOI] [PubMed] [Google Scholar]
- Klegerman, M. E. (2017). Translational initiatives in thrombolytic therapy. Frontiers of Medicine, 11(1), 1–19. 10.1007/s11684-017-0497-8 [DOI] [PubMed] [Google Scholar]
- Lavoie, J. L. , & Sigmund, C. D. (2003). Minireview: Overview of the renin‐angiotensin system–an endocrine and paracrine system. Endocrinology, 144(6), 2179–2183. 10.1210/en.2003-0150 [DOI] [PubMed] [Google Scholar]
- Law, M. R. , Morris, J. K. , & Wald, N. J. (2009). Use of blood pressure lowering drugs in the prevention of cardiovascular disease: Meta‐analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ, 338, b1665 10.1136/bmj.b1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackness, B. , Quarck, R. , Verreth, W. , Mackness, M. , & Holvoet, P. (2006). Human paraoxonase‐1 overexpression inhibits atherosclerosis in a mouse model of metabolic syndrome. Arteriosclerosis, Thrombosis, and Vascular Biology, 26(7), 1545–1550. 10.1161/01.ATV.0000222924.62641.aa [DOI] [PubMed] [Google Scholar]
- McAloon, C. J. , Boylan, L. M. , Hamborg, T. , Stallard, N. , Osman, F. , Lim, P. B. , & Hayat, S. A. (2016). The changing face of cardiovascular disease 2000–2012: An analysis of the world health organisation global health estimates data. International Journal of Cardiology, 224, 256–264. 10.1016/j.ijcard.2016.09.026 [DOI] [PubMed] [Google Scholar]
- Mendis, S. , Puska, P. , Norrving, B. , Mendis, S. , Puska, P. , & Norrving, B. (2011). Global atlas on cardiovascular disease prevention and control. Geneva: World Health Organization. [Google Scholar]
- Murakami, K. , Yamanaka, N. , Ohnishi, K. , Fukayama, M. , & Yoshino, M. (2012). Inhibition of angiotensin I converting enzyme by subtilisin NAT (nattokinase) in natto, a Japanese traditional fermented food. Food & Function, 3(6), 674–678. 10.1039/c2fo10245e [DOI] [PubMed] [Google Scholar]
- Nordt, T. K. , & Bode, C. (2003). Thrombolysis: Newer thrombolytic agents and their role in clinical medicine. Heart, 89(11), 1358–1362. 10.1136/heart.89.11.1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Flaherty, M. , Buchan, I. , & Capewell, S. (2013). Contributions of treatment and lifestyle to declining CVD mortality: Why have CVD mortality rates declined so much since the 1960s? Heart, 99(3), 159–162. 10.1136/heartjnl-2012-302300 [DOI] [PubMed] [Google Scholar]
- Regoli, D. , & Gobeil, F. (2017). Kallikrein‐kinin system as the dominant mechanism to counteract hyperactive renin‐angiotensin system. Canadian Journal of Physiology and Pharmacology, 95(10), 1117–1124. 10.1139/cjpp-2016-0619 [DOI] [PubMed] [Google Scholar]
- Ren, N. N. , Chen, H. J. , Li, Y. , Mcgowan, G. W. , & Lin, Y. G. (2017). A clinical study on the effect of nattokinase on carotid artery atherosclerosis and hyperlipidaemia. Zhonghua Yi Xue Za Zhi, 97(26), 2038–2042. [DOI] [PubMed] [Google Scholar]
- Ren, Y. , Pan, X. , Lyu, Q. , & Liu, W. (2018). Biochemical characterization of a fibrinolytic enzyme composed of multiple fragments. Acta Biochimica Et Biophysica Sinica (Shanghai), 50(2), 227–229. 10.1093/abbs/gmx125 [DOI] [PubMed] [Google Scholar]
- Schürmann, D. , Herzog, E. , Raquet, E. , Nolte, M. , May, F. , Müller‐Cohrs, J. , … Pragst, I. (2014). C1‐esterase inhibitor treatment: Preclinical safety aspects on the potential prothrombotic risk. Thrombosis and Haemostasis, 112(5), 960–971. 10.1160/th13-06-0469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumi, H. , Hamada, H. , Tsushima, H. , Mihara, H. , & Muraki, H. (1987). A novel fibrinolytic enzyme (nattokinase) in the vegetable cheese Natto; a typical and popular soybean food in the Japanese diet. Experientia, 43(10), 1110–1111. 10.1007/BF01956052 [DOI] [PubMed] [Google Scholar]
- Toh, S. , Reichman, M. E. , Houstoun, M. , Ross Southworth, M. , Ding, X. , Hernandez, A. F. , … Hennessy, S. (2012). Comparative risk for angioedema associated with the use of drugs that target the renin‐angiotensin‐aldosterone system. Archives of Internal Medicine, 172(20), 1582–1589. 10.1001/2013.jamainternmed.34 [DOI] [PubMed] [Google Scholar]
- Weng, Y. , Yao, J. , Sparks, S. , & Wang, K. Y. (2017). Nattokinase: An oral antithrombotic agent for the prevention of cardiovascular disease. International Journal of Molecular Sciences, 18(3), 523 10.3390/ijms18030523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2013). Prevention of cardiovascular disease: Pocket guidelines for assessment and management of cardiovascular risk: (WHO/ISH cardiovascular risk prediction charts for the African Region). Tetrahedron Letters, 54(22), 2817–2820. [Google Scholar]


