Abstract
Extra virgin olive oil (EVOO), appraised for its healthy properties, represents an important element for the economy of several countries of the Mediterranean area, including Italy. Our study aimed to evaluate some biochemical characteristics (polyphenols and volatile compounds) as well as the antioxidant activity of three EVOOs obtained from the varieties Ravece, Ogliarola, and Ruvea antica, grown in the same field of an Irpinian village, Montella, in the Campania region, Southern Italy. Extra virgin olive oil Ruvea antica contained the greatest amount of total polyphenols and showed the highest antioxidant activity. Principal component analysis of the aromatic profiles indicated that the three EVOOs could be easily discriminated according to the cultivar. 1‐Hexanol, 2‐hexen‐1‐ol, 3‐pentanone, representing the most abundant volatiles of the EVOO Ruvea antica, and 2‐hexenal, which resulted the main component in EVOOs Ogliarola and Ravece, could be considered as markers to discriminate these three EVOOs, according to the ReliefF feature selection algorithm.
Keywords: antioxidant activity, extra virgin olive oil, polyphenols, statistical analysis, volatile compounds
1. INTRODUCTION
Extra virgin olive oil (EVOO) is the most precious product obtained by the milling of the fruits of the olive tree (Olea europaea L. subsp. europaea var. europaea), one of the most worldwide cultivated plants (Antolini, 1997). At present, about 1,500 cultivars and/or varieties are known, as well as 3,000 synonyms of registered cultivars, ecotypes, and local varieties, which overall contribute to the enormous capital of olive germplasm. Italy, Spain, France, Tunisia, and Greece have the highest number of cultivars and varieties (Doveri & Baldoni, 2007). Extra virgin olive oil can be considered as a “lipid fruit juice” obtained from the fresh fruits of the olive by physical and mechanical processes. The strong link between the cultivar and the territory of cultivation makes it a product with particular characteristics, especially from the point of view of organoleptic, nutritional, and healthy properties. The EVOO plays a key role in the eating habits of the Mediterranean peoples, and since ancient time, it is very popular in several dietary regimes, and still today it represents a pillar of the Mediterranean diet. The variety/cultivar, climatic conditions of the cultivation site, agronomic practices, degree of ripening, storage conditions, and techniques of fruit processing are all factors that may affect the quality and the sensorial characteristics of EVOO, as well as its biological properties (Lazzez, Perri, Caravita, Khlif, & Cossentini, 2008). The variety certainly represents one of the most important factors in determining the quality of the oil, which can vary greatly also for genetic reasons (Tura, Failla, Bassi, Pedo, & and, 2008). However, olives of the same variety, cultivated under different environmental conditions or in diverse geographical areas, can produce EVOOs with different organoleptic characteristics and healthy properties (Angerosa, Basti, Vito, & Lanza, 1999). Concurrently, fruits from different cultivars grown under the same environmental conditions could produce oils with different biochemical characteristics (Gorzynik‐Debicka et al., 2018). In the composition of EVOOs, volatile organic compounds (VOCs) and polyphenols are of great importance. Volatile organic compounds are strongly related to oil aroma perceived during the assay of the product (Salas, Harwood, & Martinez‐Force, 2013). They are produced at the beginning of the malaxation, during cell structure rupture, due to enzymatic reactions in the presence of oxygen. C6 aldehydes, C6 alcohols, and their corresponding esters, together with smaller amounts of C5 carbonyl compounds, are the main constituents of VOCs (60%–80%). Specifically, hexan‐1‐ol, hexanal, E‐2‐hexenal, and 3‐methylbutan‐1‐ol generally dominate the VOCs pattern of the most common EVOOs from Mediterranean regions (Salas et al., 2013). volatile organic compounds profile can depend on cultivar and on degree of maturation (Angerosa et al., 1999).
The Mediterranean diet is the golden standard for healthy nutrition. It is characterized mainly by a high intake of fruit, vegetables, and cereals, which are rich in phytochemicals (Fratianni et al., 2016). Among these compounds, polyphenols stimulated particular attention, due to their versatility of action, being able to protect against oxidative stress and to inhibit the proliferation of cancer cells (Del Rio, Costa, Lean, & Crozier, 2010). The beneficial effects of the Mediterranean diet are also attributed to the EVOO (Visioli & Bernardini, 2011), which, even if more expensive than olive oil, is richer in polyphenols, vitamins, phytosterols, etc., concurring to reduce the risk of cardiovascular events (Estruch et al., 2013), so that US Food and Drug Administration compared it to a real drug. Extra virgin olive oil is rich in polyphenols ranging between 50 and 1,000 mg gallic acid equivalents (GAE)/kg of product (Gorzynik‐Debicka et al., 2018). Oleuropein, quercetin, and hydroxytyrosol, some of the main polyphenols present in EVOO, have antioxidant activity and ascertained effects in protecting against the coronary artery disease (Manna et al., 2002) or cancer (Owen et al., 2000).
The aim of our work was to determine the biochemical composition of three EVOOs obtained from traditional varieties of olives cultivated in the same field of Montella, a little village of the Irpinia region, Southern Italy, harvested in the same period and processed by cold pressure. Three varieties, Ogliarola, Ravece, and Ruvea antica, in particular, attracted our attention. These are typical varieties of the Mediterranean area, diffused in Campania. Tree of Ogliarola has a medium foliage, with elliptical‐lanceolate leaves. It produces a low number of flowers. Its fruits are black and elliptical, with a weight of 2–4 g. The endocarp has a weight of 0.3–0.45 g. Ravece tree has a high foliage density. Leaves are elliptical‐lanceolate. Fruits are elongated, purple, and have a weight of 4–6 g; the endocarp is heavy (weight >0.45 g). Ruvea antica tree has medium foliage. Leaves are elliptical‐lanceolate and longer more than 7 cm. Its fruits are purple, elliptical, and show a weight of 2–4 g. The endocarp has a weight of 0.3–0.45 g (Di Vaio & Nocerino, 2012). The biochemical characterization of resulting EVOOs involved the total antioxidant activity and the polyphenol content. The polyphenolic profile and VOCs were also evaluated. Statistical analysis allowed us to correlate some of the biochemical characteristics of the EVOOs; in particular, the antioxidant activity was correlated with total polyphenols and the singular components, identified in the oil by UPLC. Principal component analysis (PCA) of the aromatic profiles (obtained by Gas Chromatography/Mass Spectrometry) was carried out to discriminate oil samples according to cultivar. Moreover, a feature selection algorithm was used to identify and select putative volatile markers responsible for EVOO varieties discrimination.
2. MATERIALS AND METHODS
2.1. Materials
Caffeic, ferulic, p‐coumaric, gallic, and chlorogenic acids, catechin, quercetin, 3‐hydroxytyrosol, spiraeoside, oleuropein, daidzein, luteolin, naringenin, formononetin, 2,2‐diphenyl‐1‐picrylhydrazyl (DPPH), HPLC‐grade ethanol, 4‐methyl‐2‐pentanol, and acetonitrile were purchased from Sigma‐Aldrich. Apigenin and hyperoside were purchased from Extrasynthese. Ethanol was obtained from Romil. Ultrapure water from a Milli‐Q system (Millipore) with a resistivity at 25°C of 18 MΩ ∗ cm was used throughout the analyses. Helium (Rivoira) at a purity of 99.999% was the GC carrier gas. The SPME glass vials and the fibers were from Supelco; the capillary GC‐MS column HP‐Innowax (30 m × 0.25 mm × 0.5 μm) was purchased from Agilent J&W (Agilent Technologies Inc.).
2.2. Plant material
The EVOOs used in this study were produced in the same year by cold pressing of three different varieties (Ruvea antica, Ogliarola, and Ravece) grown in the same field located in the Montella village, in the Irpinia Province, Campania region, Southern Italy. Prof. Vincenzo De Feo identified the varieties. Voucher specimens of the three varieties were stored in the herbarium of the Department of Pharmacy, University of Salerno.
2.3. Polyphenol analysis and free radical scavenging capacity
To isolate the phenolic fraction of the three EVOOs, 1.5 g of sample was mixed with 1.5 ml of hexane and charged onto cartridges SPE C18. Polyphenols were eluted through 3 ml of methanol 100% and recovered; this step was repeated other two times. The three residues were collected, grouped, dried, and re‐suspended with 1 ml of methanol. The samples were filtered (mesh = 0.20 μm). The method of Singleton and Rossi (Singleton & Rossi, 1965) was used to evaluate the content of total polyphenols present in the three EVOO samples. Quantification was determined by using gallic acid as standard and reading the absorbance at 760 nm through a Cary UV/Vis spectrophotometer (Varian). Results were expressed as μg gallic acid equivalent (GAE)/g of EVOO ± standard deviation (SD).
The free radical scavenging activity was determined using the stable radical 2,2‐diphenyl‐1‐picrylhydrazyl (DPPH assay) (Brand‐Williams, Cuvelier, & Berset, 1995). The analysis was performed in microplates by adding 15 μl of extract to 300 μl of a methanol DPPH solution (0.153 M). Next, the absorbance at λ = 517 nm was spectrophotometrically measured (Cary 50 MPR, Varian). The absorbance of DPPH without antioxidant (control sample) was used for baseline measurements. The scavenging activity was expressed as effectiveness (%) of the sample to inhibit DPPH radical activity during a 60‐min incubation.
Polyphenol profile was determined through UPLC (ultra high‐performance liquid chromatography) by using an ACQUITY Ultra Performance system linked to a PDA 2996 photodiode array detector (Waters), setting the UV detection wavelength at 280 nm, following the method of Fratianni and coworkers (Fratianni et al., 2016). Quantification of known components was performed by comparing the peak areas on the chromatograms of samples with those obtained from standard solutions.
2.4. Analysis of VOCs profiles
The optimization of SPME parameters was achieved by examining samples of a commercial EVOO bought at a local supermarket. SPME GC‐MS volatile analysis was accomplished according to Romero and coworkers (Romero, Garcıa‐Gonzalez, Aparicio‐Ruiz, & Morales, 2015), but using the DVB/CAR/PDMS (50/30 μm) fiber. For the sample preparation, 2 g of each sample was put into a 20‐mL headspace vial with screw cap (Supelco) and 4‐methyl‐2‐pentanol to a final concentration of 1.5 mg/g was added as an internal standard to guarantee the analytical reproducibility. Subsequently, vials, closed with a Teflon (PTFE) septum and an aluminum cap (Chromacol) and stirred, were put in the instrument dry block heater and held at 40°C for 10 min. After the equilibration time, the extraction and injection processes were automatically carried out using an autosampler MPS 2 (Gerstel).
Volatiles were analyzed by gas chromatography‐quadrupole mass spectrometry (GC‐qMS), introducing the SPME fiber into the injector port of the gas chromatographer, model GC 7890A, Agilent hyphenated with a mass spectrometer 5975C. Once desorbed, metabolites were directly transferred to the capillary column HP‐Innowax for the analysis. The oven temperature program was initially set at 40°C for 3 min, increased to 200°C at 30°C/min, and then ramped to 240°C at 30°C/min, holding for 1 min. Volatiles were investigated according to the instrumental parameters as reported in the literature (Cozzolino, Martignetti, et al., 2016; Cozzolino, Pace, et al., 2016). Each sample was analyzed in duplicate in a randomized sequence where blanks were also run. Volatile metabolites recorded in the headspace of the extra virgin olive oils under study were identified by three diverse methods, as previously reported (Cozzolino, Martignetti, et al., 2016; Cozzolino, Pace, et al., 2016). The areas of the identified volatiles were determined from the total ion current (TIC), and the semiquantitative data of each metabolite (Relative Peak Area, RPA%) were considered in relation to the area of the peak of 4‐methyl‐2‐pentanol, used as internal standard.
2.5. Statistical analysis
Data were expressed as the mean ± standard deviation (SD) of triplicate measurements, and antioxidant activity was correlated with polyphenols. As concerns VOCs, analysis of variance (ANOVA) was used to compare results and significance was accepted at p < .05. Principal component analysis (PCA) was then used to relate the obtained values and as an explorative tool for the preliminary visualization of the separation of the different EVOO samples, according to their VOCs profiles. Last, the ReliefF (Kononenko, Simec, & Robnik‐Sikonja, 1997) feature selection algorithm was used to identify potential markers, among VOCs, responsible for EVOO discrimination.
3. RESULTS AND DISCUSSION
3.1. Total polyphenol content and antioxidant activity
The analysis of total polyphenols (TPF, Table 1) indicates that the amount of TPF present in the three EVOOs ranged between 156.96 and 324.27 μg GAE/g of EVOO. These values were coherent with the data of Di Giovacchino and coworkers (Di Giovacchino, Sestili, & Di Vincenzo, 2002). Ruvea antica and Ogliarola exhibited a content of TPF very close to that exhibited by some monovarietal Spanish EVOOS, such as “Cordoba” and “Sevilla” (Oliveras‐Ferraros et al., 2011). The antioxidant activity, evaluated through the DPPH test (whose results were expressed as percentage inhibition of DPPH, Table 1), indicated that the EVOO of Ruvea antica showed the best antioxidant activity (33.8%). Its efficacy was superior to the Ogliarola EVOO (20.7%) and mainly to the Ravece EVOO, which exhibited an effectiveness in inhibiting the DPPH radical even by half than Ruvea antica. The three EVOOS showed a perfect degree of correlation between the total polyphenol content and the antioxidant activity (corr = 99.99, Figure 1). A high correlation between polyphenols and antioxidant activity has been also demonstrated by Samaniego Sanchez and coworkers (Samaniego Sánchez et al., 2007).
Table 1.
Total polyphenols (expressed as μg GAE/g of EVOO ± SD) and antioxidant activity (evaluated through the DPPH and expressed as percentage ± SD) of the three polyphenolic extracts from Ogliarola, Ravece, and Ruvea antica EVOOs
| Variety | Total polyphenols | Antioxidant activity | ||
|---|---|---|---|---|
| μg GAE/g of EVOO | SD | % | SD | |
| Ogliarola | 198.5 | ±14.7 | 20.7 | ±2.227 |
| Ravece | 156.97 | ±12.91 | 16.2 | ±1.601 |
| Ruvea antica | 324.275 | ±6.91 | 33.8 | ±0.368 |
Figure 1.
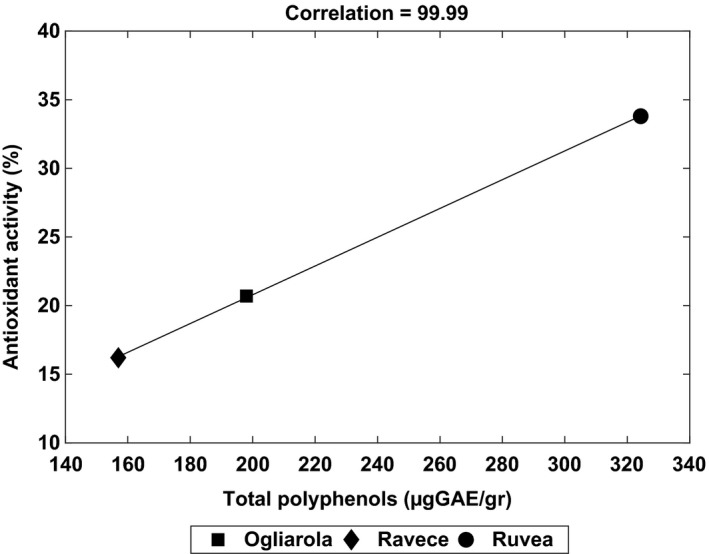
Correlation between total polyphenols found in the three EVOOs (expressed as μg GAE/g of product) and the antioxidant activity (calculated through the DPPH test and expressed as percentage)
3.2. Polyphenol profile
The amount (expressed as μg GAE/g of EVOO) of polyphenols identified through UPLC analysis is shown in Table 2. In our study, we tried to eliminate as many variables as possible: For this reason, we took into consideration three olive cultivars grown in the same plot of land, collected, and processed in the same period using the same technology. The resulting EVOOs exhibited a quite varied polyphenols profile, both for the presence and the amount of constituents. Quercetin resulted very abundant in all samples. It ranged between 28.34 μg GAE/g of oil in Ravece and 47.65 μg GAE/g in Ogliarola, with an intermediate value (34.43 μg GAE/g) in Ruvea antica. Oleuropein, representing an abundant polyphenol in Ogliarola and Ruvea antica, was found at concentrations much lower in EVOO Ravece (9.30 μg GAE/g), the 5.93% of the total polyphenols. This molecule is an ester of hydroxytyrosol; it gives rise from the mevalonic acid pathway (Omar, 2010). Since an inverse relationship between fruit ripening and content of oleuropein occurs during the development of olive fruit (Kalua, Allen, Bedgood, Bishop, & Prenzler, 2005), the fruits of Ravece, though collected in the same period, were probably more mature respect to Ogliarola and mainly to Ruvea antica. This could be confirmed also by the presence/absence of the flavonoid apigenin, which was detected only in the polyphenolic extract of Ruvea antica: Taking into account that apigenin gives rise from naringenin, a variable degree of immaturity might be considered. Probably, the EVOO Ruvea antica was the most immature, conversely to EVOO Ravece, the most mature, with EVOO Ogliarola exhibiting an intermediate rate of maturity. Furthermore, taking into consideration the amount of oleuropein and naringenin, we might hypothesize that the EVOO Ogliarola could be closer, from the point of view of maturity, to Ravece rather than Ruvea antica. Such hypothesis is supported also by the total polyphenols content and the antioxidant activity of the EVOO Ogliarola, which values were more similar to those of the EVOO Ravece than to EVOO Ruvea antica. Spiraeoside (quercetin‐4′‐glucoside), a derivative of quercetin, was detected in similar amounts (18.78 and 18.67 μg GAE/g, respectively) in EVOOs Ogliarola and Ruvea antica. Concurrently, we found luteolin in Ravece and Ruvea antica (9.77 and 5.09 μg GAE/g, respectively), but not in Ogliarola. The polyphenols identified in the three EVOOs are well known highly bioavailable molecules. The presence of high amounts of oleuropein, whose absorption in the body is about 55%–60% (Omar, 2010), is very significant, given the numerous and key effects of such metabolite including antioxidant, anti‐inflammatory, anticancer, antiatherogenic activities, and cardioprotective, antihyschaemic and hypolipidemic properties (Visioli & Galli, 2002). Concomitantly, the high content of quercetin contributes to improve the biological value of the three EVOOs. The amount of quercetin and its derivative spiraeoside in the EVOOs Ogliarola and Ruvea antica represented the 51.74% and 35.04%, respectively, of the polyphenols. A so high amount of these compounds is certainly essential: Like other flavonoids, they can affect the cellular function, by mediating gene expression and signal transduction rather than through a direct antioxidant effect (Nemeth et al., 2003). Dietary quercetin and other flavonoids are absorbed by a little percentage (5%–10%) in the small intestine; the residue of these molecules moves to the colon, where they are metabolized by the gut microbiota, influencing its composition. These molecules exert potential prebiotic effect, protecting from intestinal dysbiosis and all alterations interesting microbiota, and finally, they can concur to significantly influence host biochemistry and host susceptibility to diseases (Nazzaro, Fratianni, d’Acierno, & Coppola, 2013; Tamura et al., 2017).
Table 2.
Polyphenolic profile of the three EVOOs resulting from the UPLC analysis (expressed as μg GAE/g of EVOO)
| Polyphenols | Ogliarola | Ravece | Ruvea antica |
|---|---|---|---|
| Gallic acid | 0.00 | 0.00 | 0.00 |
| 3 Hydroxytyrosol | 3.68 | 0.68 | 3.57 |
| Catechin | 2.13 | 0.00 | 1.40 |
| p‐Coumaric acid | 0.00 | 0.45 | 0.36 |
| Quercetin‐4′‐glucoside (spiraeoside) | 18.78 | 0.00 | 18.67 |
| Oleuropein | 31.23 | 9.30 | 41.58 |
| Daidzein | 8.18 | 0.00 | 7.66 |
| Luteolin | 0.00 | 9.77 | 5.09 |
| Quercetin | 47.65 | 28.34 | 34.43 |
| Apigenin | 0.00 | 0.00 | 10.34 |
| Naringenin | 7.90 | 10.31 | 21.05 |
| Formononetin | 8.82 | 7.55 | 7.37 |
Considering the almost complete linearity between the total polyphenol content and the antioxidant activity (corr = 99.9, Figure 1), we used a statistical approach to evaluate the putative influence of some singular polyphenols on the antioxidant activity exhibited by the three extracts. This approach was previously used to study the influence of polyphenols on some biochemical characteristics and biological properties of different ecotypes of Phaseolus vulgaris L. (Ombra et al., 2016). The analysis was performed taking into consideration the most abundant molecules present in the three extracts, which resulted, by the UPLC analysis, quercetin, oleuropein, spiraeoside, formononetin, naringenin, and luteolin. The results are shown in Figure 2. Naringenin, present in all three polyphenolic extracts, at amounts ranging between 7.90 and 21.05 μg GAE/g EVOO, did not seem to affect the antioxidant activity in marked way until 10.31 μg GAE/g. Its effect seemed stronger upper such threshold, so that, at twice amounts, a doubling of the antioxidant activity was observed (Figure 2a). Oleuropein appeared to exhibit a linear behavior, with an antioxidant activity growing concurrently to its amounts (corr = 88.75, Figure 2b). This molecule seemed to be the main responsible for the antioxidant activity exhibited by the three polyphenol extracts, although it did not represent the most abundant molecule. Therefore, the noticeable antioxidant activity of oleuropein is reported, mainly as a scavenger of chain‐propagating lipid peroxyl radicals within the membranes (Saija et al., 1998). Quercetin, the most abundant molecule detected in all three extracts (present at amount ranging from 28.34 to 47.65 μg/g of EVOO, Table 2), exhibited a different behavior. It apparently was able to influence positively the antioxidant activity up to a concentration of 34.43 μg/g. On the other hand, at higher values, quercetin seemed to exert an inhibitory action on the antioxidant activity, which decreased from 34% to 20.7% (Figure 2c). A similar behavior could be also attributed to luteolin (Figure 2d), which amounts in the three extracts ranged between zero and 9.77 μg GAE/g. Like quercetin, it apparently exerted an antioxidant activity (34%) until a specific threshold (that could be ascribable to 5.09 μg GAE/g); after which, increasing its content until 9.77 μg GAE/g, the antioxidant activity decreased from 34% to 16%. Formononetin (corr = −37.98) showed a variable trend so that increasing its amount until a certain percentage (7.6%) the antioxidant activity decreased, increasing again as the molecule's content increased (Figure 2e). Our results corroborated the hypothesis that a certain bioactive compound can modify its properties in the presence of other compounds. In the case of spiraeoside, for instance, it is possible that its influence on the antioxidant activity can be negligible (Figure 2f), although its presence in the extracts of EVOOs Ruvea antica and Ogliarola was practically the same.
Figure 2.
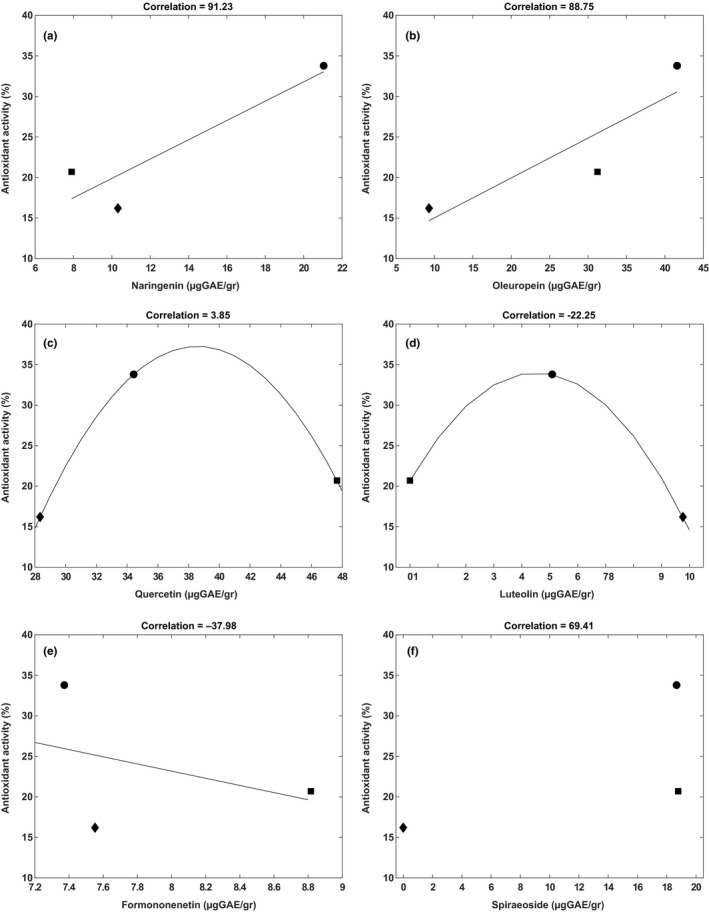
Correlation between the amount of some polyphenols with the antioxidant activity (calculated through the DPPH test and expressed as percentage) for Ogliarola (■), Ravece (♦), and Ruvea antica (●)
3.3. Volatile compounds analysis
The analysis of EVOO volatile compounds was performed through the SPME sampling followed by GC‐MS (Torri, Sinelli, & Limbo, 2010). SPME, as an alternative technique for fractionation of volatiles from interfering non‐volatile matrix compounds, is a pre‐concentration technology, which integrates sample extraction, concentration, and sample introduction into a single solvent‐free step, preventing the production of artifacts compared with conventional solvent extraction procedures (Pawliszyn, 2012). A total of 49 VOCs were identified, which belonged to hydrocarbons (3), aldehydes (11), alcohols (12), ketones (5), esters (5), carboxylic acids (6), terpenes (6), and others (1). Identification of volatile metabolites was carried out by (a) associating the GC retention time and mass spectra with those of pure commercial standards, when available, (b) matching MS spectra for each putative compound with those of NIST and Wiley libraries (https://chemdata.nist.gov/dokuwiki/doku.php?xml:id=chemdata:ridatabase; https://www.sisweb.com/software/wiley-registry.htm), and (c) comparing the Kovats indexes calculated by using a C8‐C40 n‐alkane series to literature data for similar chromatographic conditions. SPME GC‐MS semiquantitative data, calculated as the percent ratio of the respective peak area relative to the peak area of 4‐methyl‐2‐pentanol, used as internal standard, were subject to a one‐way ANOVA, in order to investigate the effect of cultivar on the identified VOCs. Table 3 reports the ANOVA of the 49 detected volatiles, the abbreviation code, the experimental and literature reported Kovats index, and the identification methods. Data reported in this table showed that the three cultivars share 30 common metabolites. Anyway, some VOCs can be considered cultivar‐specific flavor compounds, as they are present only in one cultivar. At this concern, heptane (H1), 2‐methyl butanal (Ald1), heptanal (Ald5), 1‐octanol (Al10), and 2‐methyl ethyl butanoate (E2) are found only in EVOO Ogliarola, while butanoic acid (A2) and α‐muurolene (T5) were exclusively present in EVOO Ravece. 1‐Butanol‐3‐methyl (Al2) was detected only in the VOCs profile of Ruvea antica. Aldehydes were the most abundant VOCs in Ogliarola and Ravece EVOOs, representing the 84.7% and 64.0% of total volatile compounds, respectively. Among aldehydes, (E) 2‐hexenal produced by the LOX pathway was notably predominant, representing a percentage of 79.8% of total volatiles in Ogliarola and 59.6% in Ravece. These results agree with previous studies reporting (E) 2‐hexenal among the principal volatiles normally found in VOCs of EVOOs produced in the Mediterranean basin (Salas et al., 2013). This compound provides the typical “green note” of olive oil and has been reported to be negatively correlated with the maturity and degree of oxidation of virgin olive oils (Pouliarekou et al., 2011). Alcohols were the most abundant volatiles present in the EVOO of Ruvea antica, representing the 58.2% of the total VOCs. The principal alcohols were (E) ‐hexen‐1‐ol (37.4%) and 1‐hexanol (15%), both deriving from the LOX pathway and showing a characteristic odor described as green, grassy, leafy. These compounds, on the other hand, were present only at lower concentration in Ogliarola (1.2% and 1.8%, respectively) and in Ravece EVOOs (4.2% and 14.1%, respectively).
Table 3.
Volatile organic compounds (VOCs) in the three EVOOs
| VOCs | Code | RIt/RIsp | ID | Ogliarola | Ravece | Ruvea antica | p | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Hydrocarbons | ||||||||||
| Heptane | H1 | 700/700 | RI/MS/S | 1.23 | a | nd | b | nd | b | *** |
| Octane | H2 | 800/800 | RI/MS/S | 3.28 | a | 2.11 | ab | 1.19 | b | ** |
| Toluene | H3 | 1021/1022 | RI/MS/S | 0.75 | a | 0.52 | ab | 0.33 | b | * |
| Aldehydes | ||||||||||
| Butanal 2‐methyl | Ald1 | 878/880 | RI/MS/S | 0.54 | a | nd | b | nd | b | *** |
| Hexanal | Ald2 | 1057/1058 | RI/MS/S | 10.38 | a | 9.72 | a | 3.97 | b | * |
| 2‐Pentenal (E) | Ald3 | 1111/1111 | RI/MS/S | 0.83 | a | 0.69 | a | nd | b | *** |
| 3‐Hexenal (Z) | Ald4 | 1124/1126 | RI/MS | 1.53 | a | 1.98 | a | nd | b | *** |
| Heptanal | Ald5 | 1180/1182 | RI/MS/S | 0.36 | a | nd | b | nd | b | *** |
| 2‐Hexenal (E) | Ald6 | 1213/1207 | RI/MS/S | 292.63 | a | 208.48 | a | 31.24 | b | *** |
| Octanal | Ald7 | 1292/1287 | RI/MS/S | 0.53 | 0.41 | 3.26 | ns | |||
| 2‐Heptenal (Z) | Ald8 | 1324/1320 | RI/MS/S | 0.61 | a | 0.28 | ab | nd | b | *** |
| 2,4‐Hexadienal | Ald9 | 1394/1397 | RI/MS | 0.71 | a | 0.54 | a | nd | b | *** |
| 2,4‐Heptadienal | Ald10 | 1465/1451 | RI/MS | 0.71 | a | 0.72 | a | 0.35 | b | ** |
| Nonanal | Ald11 | 1391/1385 | RI/MS/S | 1.64 | 1.16 | 11.62 | ns | |||
| Alcohols | ||||||||||
| 1‐Penten‐3‐ol | Al1 | 1156/1157 | RI/MS/S | 2.75 | 3.46 | 2.18 | ns | |||
| 1‐Butanol‐3‐methyl | Al2 | 1211/1212 | RI/MS/S | nd | b | nd | b | 0.72 | a | *** |
| 1‐Pentanol | Al3 | 1256/1255 | RI/MS/S | nd | b | 0.42 | b | 1.44 | a | *** |
| 2‐Penten‐1‐ol (Z) | Al4 | 1319/1312 | RI/MS/S | 0.30 | 0.61 | 0.42 | ns | |||
| 2‐Penten‐1‐ol (E) | Al5 | 1326/1327 | RI/MS | 2.80 | ab | 4.56 | a | 1.79 | b | ** |
| 1‐Hexanol | Al6 | 1358/1360 | RI/MS/S | 4.52 | b | 14.85 | b | 30.63 | a | *** |
| 3‐Hexen‐1‐ol (E) | Al7 | 1368/1351 | RI/MS/S | nd | b | 0.43 | a | 0.40 | a | ** |
| 3‐Hexen‐1‐ol (Z) | Al8 | 1385/1390 | RI/MS/S | 4.21 | b | 10.43 | a | 4.45 | b | ** |
| 2‐Hexen‐1‐ol (E) | Al9 | 1405/1400 | RI/MS/S | 6.63 | b | 49.26 | a | 76.08 | a | *** |
| 1‐Octanol | Al10 | 1562/1562 | RI/MS/S | 0.28 | a | nd | b | nd | b | *** |
| Benzyl alcohol | Al11 | 1866/1865 | RI/MS/S | 0.16 | 0.11 | 0.10 | ns | |||
| Phenylethyl alcohol | Al12 | 1901/1859 | RI/MS/S | 0.69 | a | 0.34 | b | 0.21 | b | *** |
| Ketones | ||||||||||
| 3‐Pentanone | K1 | 984/984 | RI/MS/S | 2.27 | b | 6.36 | b | 20.51 | a | *** |
| 2‐Pentanone‐4‐methyl | K2 | 1003/1006 | RI/MS/S | 9.99 | ab | 16.26 | a | 6.60 | b | ** |
| 1‐Penten‐3‐one | K3 | 1012/1014 | RI/MS/S | 8.32 | a | 2.87 | b | nd | b | *** |
| 2‐Octanone | K4 | 1286/1285 | RI/MS/S | 0.07 | 0.05 | 0.07 | ns | |||
| 6‐Methyl‐5‐hepten‐2‐one | K5 | 1338/1336 | RI/MS/S | 0.34 | 0.32 | 0.33 | ns | |||
| Esters | ||||||||||
| Ethyl acetate | E1 | 853/854 | RI/MS/S | 0.14 | b | 0.11 | b | 0.33 | a | *** |
| 2‐Methyl ethyl butanoate | E2 | 1034/1033 | RI/MS/S | 0.16 | a | nd | b | nd | b | *** |
| Hexyl acetate | E3 | 1277/1307 | RI/MS/S | 0.24 | a | 0.16 | a | nd | b | *** |
| 3‐Hexen‐1‐ol acetate (Z) | E4 | 1321/1390 | RI/MS | 0.57 | b | 2.47 | a | 0.93 | b | ** |
| Methyl salicylate | E5 | 1767/1760 | RI/MS/S | 0.34 | 0.34 | 0.23 | ns | |||
| Carboxylic acids | ||||||||||
| Propanoic acid | A1 | 1543/1523 | RI/MS/S | 0.53 | 0.41 | 0.34 | ns | |||
| Butanoic acid | A2 | 1633/1619 | RI/MS/S | nd | b | 0.13 | a | nd | b | *** |
| Hexanoic acid | A3 | 1851/1850 | RI/MS/S | 0.76 | 0.72 | 0.61 | ns | |||
| Heptanoic acid | A4 | 1918/1913 | RI/MS/S | 0.44 | ab | 0.77 | a | nd | b | ** |
| Octanoic acid | A5 | 2048/2046 | RI/MS/S | 1.57 | 2.72 | 1.14 | ns | |||
| Nonanoic acid | A6 | 2174/2171 | RI/MS/S | 0.90 | ab | 1.42 | a | 0.12 | b | * |
| Terpenes | ||||||||||
| dl‐Limonene | T1 | 1183/1185 | RI/MS/S | 0.29 | a | nd | b | 0.18 | a | ** |
| cis β‐Ocimene | T2 | 1252/1245 | RI/MS/S | 0.46 | b | 0.93 | a | 0.44 | b | * |
| α‐Copaene | T3 | 1481/1488 | RI/MS | 0.21 | b | 1.72 | a | 0.39 | b | *** |
| α‐Bergamotene | T4 | 1579/1580 | RI/MS | nd | b | 0.19 | a | 0.12 | ab | ** |
| α‐Muurolene | T5 | 1721/1722 | RI/MS | nd | b | 0.22 | a | nd | b | *** |
| α‐Farnesene | T6 | 1721/1751 | RI/MS/S | 0.57 | a | nd | b | 0.18 | b | *** |
| Others | ||||||||||
| Aniline | O1 | 1753/1754 | RI/MS/S | 0.49 | 0.73 | 0.64 | ns | |||
Data are the mean values of three samples. For each metabolite, the mean values followed by a different letters are significantly different (p < .05) according to one‐way ANOVA test (ns = not significant; *, **, *** = significant at values < .05, .01, .001). Concentrations were calculated as RPA (%). ID = Identification method as indicated by the following: RI: Kovats retention index on the HP‐Innowax column; MS: NIST and Wiley libraries spectra; S: co‐injection with authentic standard compounds on the HP‐Innowax column. RIt/RIsp: Relative theoretical retention index versus Relative retention index experimentally calculated against n‐alkanes (C8‐C20) on the HP‐Innowax column.
The variety could strongly affect the abundance of volatile compounds, which in turn have revealed to be extremely valuable as varietal markers (Kalua et al., 2005). For this reason, the volatile profiles of the three EVOOs were subjected to multivariate statistical analysis with the aim to build models able to explain the variations of the metabolic content dependently from genotype, and to identify putative volatile markers useful for cultivar discrimination. Exploratory data analysis performed by PCA was applied to evaluate the effectiveness of VOCs profiles on the cultivar distinction. The cross‐validation test showed that two principal components were required to explain the total variability of analyzed parameters. The eigenvalues of the covariance matrix demonstrated that the two principal components (PCs) accounted for more than the 93% of the total explained variance. Principal component analysis model generated the 2D‐plots presented in Figure 3, where the presence of three distinctive clusters corresponding to the different cultivars is highlighted. This result confirms that, considering the volatile profile, the variety dominated over other factors (geography, maturity stage, and quality) in distinction of EVOOs of different ecotypes, even when, as in our case, produced in a very small area and with the same agriculture practices and postharvest handling (Lin et al., 2013; Zhang et al., 2010). Moreover, the ReliefF algorithm, a filter‐based method for feature selection, showed that the four most important potential marker volatiles responsible for the discrimination of the three olive oil cultivars are 1‐hexenol (Al6), 2‐hexen‐1‐ol (Al9), 3‐pentanone (K1), and 2‐hexenal (Ald6). These findings are consistent with PCA data (Figure 3), according to the fact that Al6, Al9, and K1 are the most abundant volatiles in EVOO Ruvea antica, while Ald6 is the main compound both in the EVOOs Ogliarola and Ravece.
Figure 3.
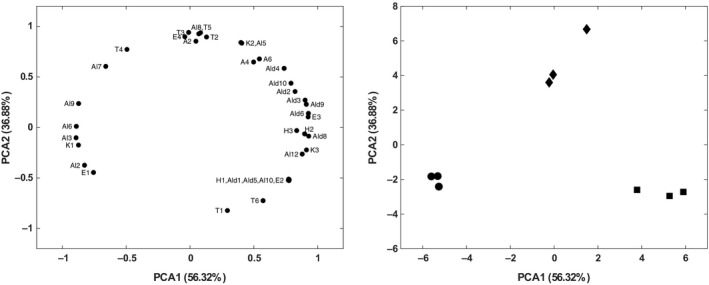
2D‐PCA plots of the VOCs profiles of the three EVOOs. The loadings (left) and the three (scaled) samples of Ogliarola (■), Ravece (♦), and Ruvea antica (●) in the transformed space (right)
4. CONCLUSION
Data obtained in this research clearly confirm the influence of genetic and environmental factors in determining the organoleptic properties of olive oil and permitted a distinction of the three EVOOs studied on the basis of their volatile constituents.
CONFLICT OF INTEREST
Authors declare that they do not have any conflict of interests.
ETHICAL APPROVAL
Human and animal testing was unnecessary for this study.
ACKNOWLEDGMENTS
This work was funded within the Project “SALVE,” PSR 2007‐2013, mis. 214, action f2, by the Campania Regional Council, Italy.
Fratianni F, Cozzolino R, Martignetti A, et al. Biochemical composition and antioxidant activity of three extra virgin olive oils from the Irpinia Province, Southern Italy. Food Sci Nutr. 2019;7:3233–3243. 10.1002/fsn3.1180
REFERENCES
- Angerosa, F. , Basti, C. , Vito, R. , & Lanza, B. (1999). Effect of fruit stone removal on the production of virgin olive oil volatile compounds. Food Chemistry, 67, 295–299. 10.1016/S0308-8146(99)00138-7 [DOI] [PubMed] [Google Scholar]
- Antolini, P. (1997). II grande manuale dell’ulivo e dell’olio: storia, coltivazione, lavorazione, produzione, itinerari e ricette gastronomiche. Giorgio Mondadori Editore, Milano, (pp. 1–175). ISBN: 883741515X BID: UM10028959. [Google Scholar]
- Brand‐Williams, W. , Cuvelier, M. E. , & Berset, C. (1995). Use of a free radical method to evaluate antioxidant activity. LWT ‐ Food Science and Technology, 28, 25–30. 10.1016/S0023-6438(95)80008-5 [DOI] [Google Scholar]
- Cozzolino, R. , Martignetti, A. , Pellicano, M. P. , Stocchero, M. , Cefola, M. , Pace, B. , & De Giulio, B. (2016). Characterisation of volatile profile and sensory analysis of fresh‐cut radicchio di Chioggia stored in air or modified atmosphere. Food Chemistry, 192, 603–611. 10.1016/j.foodchem.2015.07.045 [DOI] [PubMed] [Google Scholar]
- Cozzolino, R. , Pace, B. , Cefola, M. , Martignetti, A. , Stocchero, M. , Fratianni, F. , … De Giulio, B. (2016). Assessment of volatile profile as potential marker of chilling injury of basil leaves during postharvest storage. Food Chemistry, 213, 361–368. 10.1016/j.foodchem.2016.06.109 [DOI] [PubMed] [Google Scholar]
- Del Rio, D. , Costa, L. , Lean, M. , & Crozier, A. (2010). Polyphenols and health: What compounds are involved? Nutrition, Metabolism and Cardiovascular Diseases, 20, 1–6. 10.1016/j.numecd.2009.05.015 [DOI] [PubMed] [Google Scholar]
- Di Giovacchino, L. , Sestili, S. , & Di Vincenzo, D. (2002). Influence of olive processing on virgin olive oil quality. European Journal of Lipid Science and Technology, 104, 587–601. [Google Scholar]
- Di Vaio, C. , & Nocerino, S. (2012). Caratteristica morfologica e bio‐agronomica delle cultivar campane di olivo In Imago (Ed.), Il germoplasma dell’olivo in Campania, a cura di Claudio Di Vaio (pp 17–68). Dragoni (CE), Italy: http://agricoltura.regione.campania.it/pubblicazioni/pdf/germoplasma_olivo.pdf [Google Scholar]
- Doveri, S. , & Baldoni, L. (2007).Olive, In Kole C., (Ed.), Fruits and nuts. Genome mapping and molecular breeding in plants (Vol. 4, pp. 253–264). Berlin, Heidelberg, Germany: Springer. [Google Scholar]
- Estruch, R. , Ros, E. , Salas‐Salvadó, J. , Covas, M.‐I. , Corella, D. , Arós, F. , … Martínez‐González, M. A. (2013). Primary prevention of cardiovascular disease with a mediterranean diet. New England Journal of Medicine, 368(14), 1279–1290. 10.1056/NEJMoa1200303 [DOI] [PubMed] [Google Scholar]
- Fratianni, F. , Ombra, M. N. , Cozzolino, A. , Riccardi, R. , Spigno, P. , Tremonte, P. , … Nazzaro, F. (2016). Phenolic constituents, antioxidant, antimicrobial and anti‐proliferative activities of different endemic Italian varieties of garlic (Allium sativum L.). Journal of Functional Foods, 21, 240–248. 10.1016/j.jff.2015.12.019 [DOI] [Google Scholar]
- Gorzynik‐Debicka, M. , Przychodzen, P. , Cappello, F. , Kuban‐Jankowska, A. , Marino Gammazza, A. , Knap, N. , … Gorska‐Ponikowska, M. (2018). Potential health benefits of olive oil and plant polyphenols. International Journal of Molecular Sciences, 19, 686 10.3390/ijms19030686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalua, C. M. , Allen, M. S. , Bedgood, D. R. , Bishop, A. G. , & Prenzler, P. D. (2005). Discrimination of olive oils and fruits into cultivars and maturity stages based on phenolic and volatile compounds. Journal of Agriculture and Food Chemistry, 53, 8054–8062. 10.1021/jf051233i [DOI] [PubMed] [Google Scholar]
- Kononenko, I. , Simec, E. , & Robnik‐Sikonja, M. (1997). Overcoming the myopia of inductive learning algorithms with RELIEFF. Applied Intelligence, 7, 39–55. 10.1023/A:1008280620621 [DOI] [Google Scholar]
- Lazzez, A. , Perri, E. , Caravita, M. A. , Khlif, M. , & Cossentini, M. (2008). Influence of olive maturity stage and geographical origin on some minor components in virgin olive oil of the chemlali variety. Journal of Agriculture and Food Chemistry, 56, 982–988. 10.1021/jf0722147 [DOI] [PubMed] [Google Scholar]
- Lin, J. , Zhang, P. , Pan, Z. , Xu, H. , Luo, Y. , & Wang, X. (2013). Discrimination of oolong tea (Camellia sinensis) varieties based on feature extraction and selection from aromatic profiles analysed by HS‐SPME/GC–MS. Food Chemistry, 141, 259–265. 10.1016/j.foodchem.2013.02.128 [DOI] [PubMed] [Google Scholar]
- Manna, C. , D’Angelo, S. , Migliardi, V. , Loffredi, E. , Mazzoni, O. , Morrica, P. , … Zappia, V. (2002). Protective effect of the phenolic fraction from virgin olive oils against oxidative stress in human cells. Journal of Agriculture and Food Chemistry, 50, 6521–6526. 10.1021/jf020565+ [DOI] [PubMed] [Google Scholar]
- Nazzaro, F. , Fratianni, F. , d’Acierno, A. , & Coppola, R. (2013). Gut microbiota and polyphenols: A strict connection enhancing human health In: Rai R. V. (Ed.), Advances in food biotechnology (pp. 335–349). New York, NY: Wiley. [Google Scholar]
- Nemeth, K. , Plumb, G. W. , Berrin, J. G. , Juge, N. , Jacob, R. , Naim, H. Y. , … Kroon, P. A. (2003). Deglycosylation by small intestinal epithelial cell β‐glucosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. European Journal of Nutrition, 42, 29–42. 10.1007/s00394-003-0397-3 [DOI] [PubMed] [Google Scholar]
- Oliveras‐Ferraros, C. , Fernandez‐Arroyo, S. , Vazquez‐Martin, A. , Lozano‐Sanchez, J. , Cufı, S. , Joven, J. , … Menendez, J. A. (2011). Crude phenolic extracts from extra virgin olive oil circumvent de novo breast cancer resistance to HER1/HER2‐targeting drugs by inducing GADD45‐sensed cellular stress, G2/M arrest and hyperacetylation of histone H3. International Journal of Oncology, 38, 1533–1547. 10.3892/ijo.2011.993 [DOI] [PubMed] [Google Scholar]
- Omar, S. H. (2010). Oleuropein in olive and its pharmacological effects. Scientia Pharmaceutica, 78(2), 133–154. 10.3797/scipharm.0912-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ombra, M. , d’Acierno, A. , Nazzaro, F. , Riccardi, R. , Spigno, P. , Zaccardelli, M. , … Fratianni, F. (2016). Phenolic composition and antioxidant and antiproliferative activities of the extracts of twelve common bean (Phaseolus vulgaris L.) endemic ecotypes of Southern Italy before and after cooking. Oxidative Medicine and Cellular Longevity, 2016, 1398298 10.1155/2016/1398298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen, R. , Giacosa, A. , Hull, W. , Haubner, R. , Spiegelhalder, B. , & Bartsch, H. (2000). The antioxidant/anticancer potential of phenolic compounds isolated from olive oil. European Journal of Cancer, 36, 1235–1247. 10.1016/S0959-8049(00)00103-9 [DOI] [PubMed] [Google Scholar]
- Pawliszyn, J. (2012). Solid‐phase microextraction in perspective In Pawliszyn J. (Ed.), Handbook of solid phase microextraction (pp. 1–12). Amsterdam, the Netherlands: Elsevier. [Google Scholar]
- Pouliarekou, E. , Badeka, A. , Tasioula‐Margari, M. , Kontakos, S. , Longobardi, F. , & Kontominas, M. G. (2011). Characterization and classification of western greek olive oils according to cultivar and geographical origin based on volatile compounds. Journal of Chromatography A, 1218, 7534–7542. 10.1016/j.chroma.2011.07.081 [DOI] [PubMed] [Google Scholar]
- Romero, I. , Garcıa‐Gonzalez, D. L. , Aparicio‐Ruiz, R. , & Morales, M. (2015). Validation of SPME–GCMS method for the analysis of virgin olive oil volatiles responsible for sensory defects. Talanta, 134, 394–401. 10.1016/j.talanta.2014.11.032 [DOI] [PubMed] [Google Scholar]
- Saija, A. , Trombetta, D. , Tomaino, A. , Cascio, R. L. , Princi, P. , Uccella, N. , … Castelli, F. (1998). In vitro evaluation of the antioxidant activity and biomembrane interaction of the plant phenols oleuropein and hydroxytyrosol. International Journal of Pharmaceutics, 166, 123–133. 10.1016/S0378-5173(98)00018-0 [DOI] [Google Scholar]
- Salas, J. J. , Harwood, J. L. , & Martinez‐Force, E. (2013). Lipid metabolism in olive: Biosynthesis of triacylglycerols and aroma components In Aparicio R., & Harwood J. (Eds.) Handbook of Olive Oil (2nd ed., pp. 97–127). Berlin, Heidelberg, Germany: Springer; 10.1007/978-1-4614-7777-8 [DOI] [Google Scholar]
- Samaniego Sánchez, C. , Troncoso González, A. M. , García‐Parrilla, M. C. , Quesada Granados, J. J. , López García de la Serrana, H. , & López Martínez, M. C. (2007). Different radical scavenging tests in virgin olive oil and their relation to the total phenol content. Analytica Chimica Acta, 593, 103–107. 10.1016/j.aca.2007.04.037 [DOI] [PubMed] [Google Scholar]
- Singleton, V. L. , & Rossi, J. A. (1965). Colorimetry of total phenolics with phosphomolybdic‐phosphotungstic acid reagents. American Journal of Enology and Viticulture, 16, 144–158. [Google Scholar]
- Tamura, M. , Hoshi, C. , Kobori, M. , Takahashi, S. , Tomita, J. , Nishimura, M. , & Nishihira, J. (2017). Quercetin metabolism by fecal microbiota from healthy elderly human subjects. PLoS ONE, 12, e0188271 10.1371/journal.pone.0188271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torri, L. , Sinelli, N. , & Limbo, S. (2010). Shelf life evaluation of fresh‐cut pineapple by using an electronic nose. Postharvest Biology and Technology, 56, 239–245. 10.1016/j.postharvbio.2010.01.012 [DOI] [Google Scholar]
- Tura, D. , Failla, O. , Bassi, D. , Pedo, S. , & Serraiocco, A. (2008). Cultivar influence on virgin olive (Olea europea L.) oil flavor based on aromatic compounds and sensorial profile. Scientia Horticulturae, 118, 139–148. 10.1016/j.scienta.2008.05.030 [DOI] [Google Scholar]
- Visioli, F. , & Bernardini, E. (2011). Extra virgin olive oil’s polyphenols: Biological activities. Current Pharmaceutical Design, 17, 786–804. 10.2174/138161211795428885 [DOI] [PubMed] [Google Scholar]
- Visioli, F. , & Galli, C. (2002). Biological properties of olive oil phytochemicals. Critical Reviews in Food Science and Nutrition, 42, 209–221. 10.1080/10408690290825529 [DOI] [PubMed] [Google Scholar]
- Zhang, J. , Li, L. I. , Gao, N. , Wang, D. , Gao, Q. , & Jiang, S. (2010). Feature extraction and selection from volatile compounds for analytical classification of Chinese red wines from different varieties. Analytica Chimica Acta, 662, 137–142. 10.1016/j.aca.2009.12.043 [DOI] [PubMed] [Google Scholar]


