Abstract
Purpose
To report the first case of Acanthamoeba keratitis treated with oral miltefosine in the United States.
Observations
A 17-year-old female with a history of orthokeratology contact lens wear presented after five months of left eye pain, redness, and photophobia. She was previously treated with antivirals and topical corticosteroids for presumed herpetic disease. She was found to have a large central ring infiltrate and corneal cultures were positive for Acanthamoeba. The infection progressed despite hourly PHMB 0.02% and chlorhexidine 0.02%, and oral vorizonazole. The patient was started on oral miltefosine 50 mg 3 times per day. Following one week of treatment, repeat cultures were positive for Acanthamoeba and therefore, the concentration of chlorhexidine was increased from 0.02% to 0.06% and PHMB was changed to propamidine isetionate (Brolene 0.1%). There was definite clinical improvement after five weeks of treatment with oral miltefosine, topical chlorhexidine 0.06% and propamidine isetionate 0.1%.
Conclusions and importance
Acanthamoeba keratitis is a challenging entity to treat and often associated with a poor prognosis. Oral miltefosine may offer additional therapeutic benefit in cases of refractory Acanthamoeba keratitis.
Keywords: Miltefosine, Acanthamoeba keratitis, Amoebic keratitis
1. Introduction
Acanthamoeba keratitis is a rare but potentially devastating parasitic infection of the cornea. The primary risk factor is a history of contact lens wear, especially with poor contact lens hygiene and exposure to hot tubs or swimming pools.1 Early signs of infection include epitheliopathy followed by deeper stromal involvement and a ring infiltrate.2 Clinical diagnosis can be challenging due to the nonspecific nature of the early signs of infection.
Acanthamoeba are particularly fastidious organisms because they can exist in an active trophozoite form and a dormant cyst form. The cysts are resistant to chlorination and biocides, and can survive temperatures as low as 0–2 °C.3, 4, 5 Topical biguanides and diamidines are frequently used in combination to treat Acanthamoeba keratitis. The biguanides polyhexamethyele biguanide (PHMB) 0.02% to 0.06% and chlorhexidine 0.02% to 0.06%, and the diamidines propamidine 0.1% and hexamidine 0.1% are the most commonly used as they are cysticidal.6 Despite appropriate treatment, infection can persist or progress necessitating enucleation in severe cases.7
Miltefosine is an alkylphosphocholine that has demonstrated efficacy in other protozoal infections such as visceral leishmaniasis, Trichomonas vaginalis, Trypanosoma cruzi, and Entamoeba histolytica.8, 9, 10, 11 In December 2016, oral miltefosine received the US Food and Drug Administration's Orphan Drug Designation for the treatment of Acanthamoeba keratitis.
Here, we present the first case of Acanthamoeba keratitis successfully treated with oral miltefosine in conjunction with topical anti-amoebic agents.
2. Case report
A 17-year-old female with a history of orthokeratology contact lens wear presented with pain, redness, and photophobia of the left eye for five months. She denied any preceding trauma or exposure to fresh water. She had been previously treated for several months with antivirals and topical corticosteroids for presumed herpetic disease prior to her presentation to our clinic.
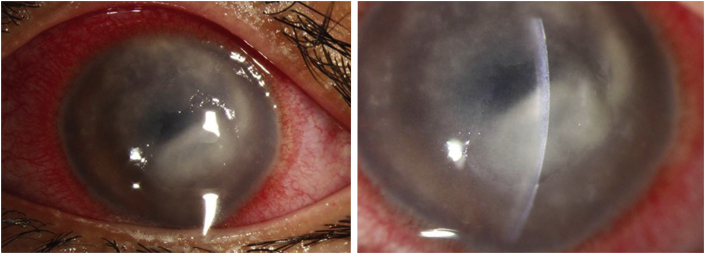
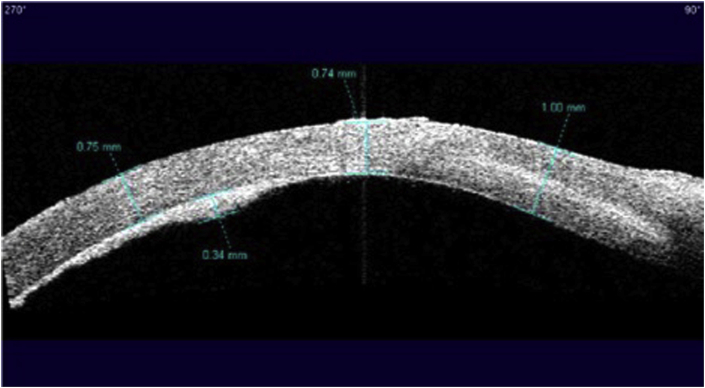
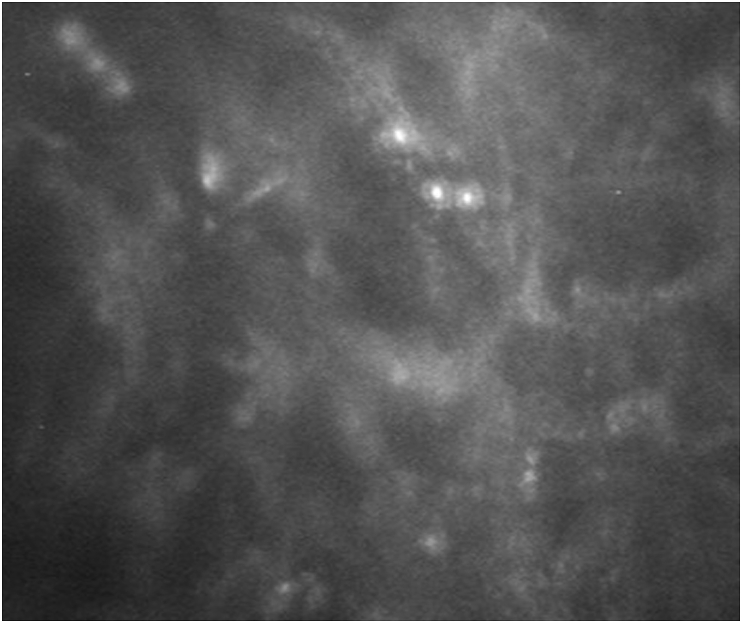
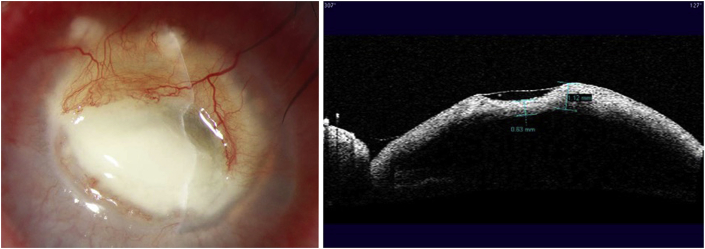
On examination, her uncorrected distance visual acuity (UDVA) in the affected left eye was hand motions. On slit lamp exam, she was found to have moderate ptosis, severe conjunctival injection, a 7.0 mm × 7.5 mm ring infiltrate with diffuse opacity involving 75% of the cornea with moderate stromal edema (Fig. 1). There were also infiltrates along the superior limbus. The AC was formed but there was a limited view of the intraocular structures. Anterior segment OCT demonstrated a retro-corneal plaque (Fig. 2) and B-scan of the posterior pole was unremarkable. Her contralateral right eye was normal. Confocal microscopy demonstrated a few frames suspicious for Acanthamoeba cysts (Fig. 3). Corneal scrapings were positive for Acanthamoeba and very rare number of coagulase negative staphylococcus. Herpes simplex virus-1, herpes simplex virus-2, and varicella zoster virus polymerase chain reaction (PCR) testing were negative, and fungal and acid-fast bacilli cultures showed no growth. She was started on hourly topical PHMB 0.02% alternating with chlorhexidine 0.02%, moxifloxacin 0.5% four times a day, cyclopentolate 1% three times a day and was kept on valacyclovir 500 mg twice a day.
Fig. 1.
Slit lamp photos at presentation to our institution. She had severe conjunctival injection, a 7.0 mm × 7.5 mm ring infiltrate with diffuse opacity involving 75% of the cornea with moderate stromal edema and infiltrates along the superior limbus.
Fig. 2.
Anterior segment OCT at presentation demonstrating a retro-corneal plaque.
Fig. 3.
Confocal microscopy at presentation was suspicious for Acanthamoeba cysts.
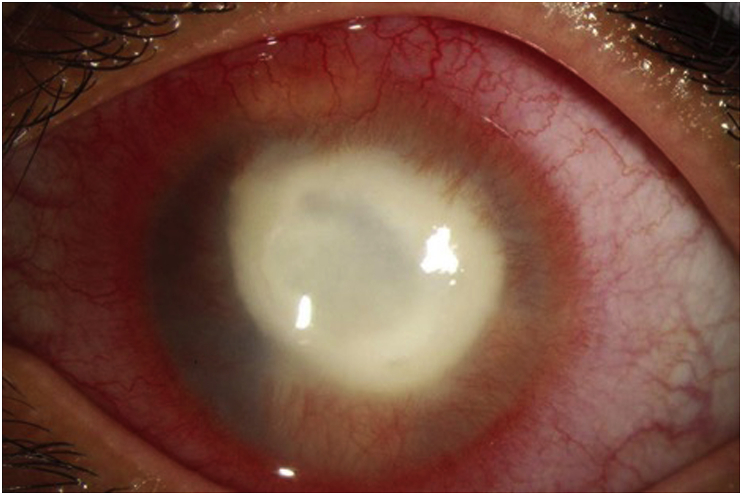
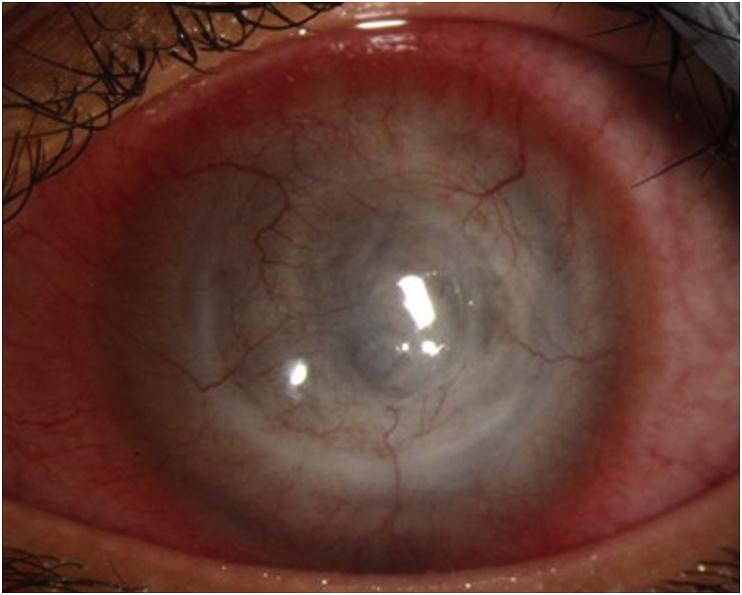
Despite two weeks of topical treatment, the ring infiltrate was becoming more dense (Fig. 4). An orbital MRI demonstrated abnormal thickening and enhancement of the anterior margin of the left globe, involving the cornea, ciliary body, and iris. Mild left proptosis was noted but no intraorbital extension was appreciated. Voriconazole 200 mg twice a day was added to the regimen and a request for oral miltefosine was submitted to her insurance for approval (which later was denied). Valacyclovir was discontinued at this time. Six weeks later (8 weeks after presentation), Profunda Inc. provided the medication free of charge, at which time voriconazole was discontinued and oral miltefosine 50 mg 3 times per day was initiated.
Fig. 4.
Slit lamp photo two weeks after presentation demonstrating clinical worsening with a denser ring infiltrate.
One week later (9 weeks after presentation), an enlarging epithelial defect with associated stromal necrosis and 50% thinning along the superior margin of the ulcer was noted (Fig. 5). Chlorhexidine and PHMB were held for 2 days to re-culture the cornea. The necrotic stroma was excised, and the patient was instructed to resume chlorhexidine 0.02% and PHMB 0.02% 4 times per day and was started on doxycycline 50 mg twice a day and vitamin C 1000 mg once a day. Oral miltefosine 50 mg 3 times a day was continued throughout this time. The culture result was positive for Acanthamoeba and therefore the concentration of chlorhexidine was increased from 0.02% to 0.06% and the dosing increased to every hour. PHMB was changed to propamidine isetionate (Brolene 0.1%) every 2 hours while awake.
Fig. 5.
Slit lamp photo and anterior segment OCT nine weeks after presentation demonstrating 50% thinning along the superior margin of the ulcer.
Following five weeks of oral miltefosine with concomitant topical chlorhexidine and propamidine, clinical improvement was noted with decreased pain, conjunctival injection, and cornea opacity. Chlorhexidine and propamidine dosing was decreased to every 2 hours. Oral miltefosine was decreased to 50 mg twice a day secondary to gastrointestinal side effects.
Four months following the initial presentation, further improvement was observed (Fig. 6) and propamidine isetionate 0.1% and chlorhexidine 0.06% were decreased to four times per day and doxycycline and vitamin C were discontinued. At this point, the patient was only taking miltefosine once a day due to nausea. She was instructed to complete a two-month course of miltefosine while staying on propamidine isetionate 0.1% and chlorhexidine 0.06% four times per day.
Fig. 6.
Slit lamp photo after eight weeks of oral miltefosine, six weeks of chlorhexidine 0.06%, three weeks of propamidine isetionate 0.1% (4 months after presentation) demonstrating improvement with a decreased density of the infiltrate and no areas of focal thinning.
Topical propamidine 0.1% and chlorhexidine 0.06% were used for one year and she has since been doing well off of medication. One year following the initial presentation, her visual acuity remained hand motions. There was significant scarring of the cornea with severe opacification and diffuse vascularization. There was no epithelial defect and no evidence of recurrence.
3. Discussion
Acanthamoeba keratitis generally has a poor prognosis. Treatment for Acanthamoeba keratitis is frequently delayed because of the nonspecific early signs resulting in misdiagnosis. Furthermore, the dormant cyst form of the organism is difficult to eradicate, leading to persistent and recurrent disease. In our patient, despite two months of aggressive topical therapy and oral voriconazole, repeat cultures were positive for Acanthamoeba. Increasing the concentration and frequency of topical medications is an option but this increases the risk of ocular surface toxicity. Therapeutic penetrating keratoplasty (TPK) has the potential to eradicate the infection by encompassing the infected cornea. However, clear margins free of Acanthamoeba would not have been possible in our patient given the involvement of the limbus. Kitzmann et al. analyzed 31 eyes with Acanthamoeba keratitis and found that those who underwent TPK had a significantly higher rate of repeat keratoplasty, recurrent Acanthamoeba keratitis, glaucoma, persistent epithelial defects, and endophthalmitis compared to those who underwent optical penetrating keratoplasty.12
Experiments with rodents have yielded favorable outcomes using topical miltefosine for Acanthamoeaba keratitis. Polat et al. inoculated Acanthamoeba into 60 hamster eyes and found that topical miltefosine 65.12 μg/mL had a higher cure rate than propamidine 0.1% plus polyhexanide 0.02%.13 In a follow up study using rat eyes, Polat et al. compared treatment with topical miltefosine 65.12 μg/mL, chlorhexidine 0.02%, polyhexanide 0.02% (PHMB), propamidine, combination of miltefosine and chlorhexidine, combination of miltefosine and PHMB, and combination of miltefosine and propamidine and found that the best results were in the miltefosine plus PHMB group. In addition, they found that miltefosine had no negative effect on cell viability.14 Recently, Tavassoli et al. reported the first use of oral miltefosine in a patient with refractory Acanthamoeba keratitis in the United Kingdom.7 They used a dose of 50 mg 3 times a day and started the medication two weeks before planned enucleation to minimize extra-ocular spread. Although this therapy was brief and still resulted in the patient losing the globe, no adverse events were reported and the patient even experienced symptomatic and clinical improvement a week after initiating treatment.
Oral miltefosine is a potentially powerful adjunct therapy for refractory cases of Acanthamoeba keratitis. It is designated a FDA orphan drug and its use for Acanthamoeba is considered off label in the United States. The recommended dose is one 50 mg capsule twice a day for patients 30–44 kg and one 50 mg capsule three times a day for patients greater than 45 kg. The recommended duration of treatment is 28 days for leishmaniasis but regimens extending treatment to six weeks has been documented for mucosal leishmaniasis.15 The manufacturer generally recommends treating with oral miltefosine for 2 months for Acanthamoeba keratitis. It is recommended that topical treatment be continued with oral miltefosine. We asked our patient to complete a two-month course in the hope that this would eradicate all of the cysts. It is recommended that the medication be administered with food to mitigate gastrointestinal adverse reactions. Known risks of the medication include embryo-fetal toxicity, infertility, renal toxicity, hepatic toxicity, thrombocytopenia, and gastrointestinal side effects. A pregnancy test should be performed in female patients of childbearing age prior to initiating treatment and the patient advised on the use of appropriate contraception. Complete blood count, renal and hepatic function should also be monitored.
The advantages of oral miltefosine include its oral administration, which avoids further ocular surface toxicity, and the addition of a new treatment option for Acanthamoeba keratitis. There is very limited data on the use of oral miltefosine for Acanthamoeba keratitis in humans. There has only been one previous case report of the use of systemic miltefosine for the treatment of Acanthamoeba keratitis and scleritis prior to enucleation to reduce the risk of extraocular spread.7
In our patient's case, multiple variables were changing over the time period that miltefosine was implemented, so we cannot definitively conclude that our patient's improvement was a result of the oral miltefosine. However, since the diamidines are not thought to be particularly cysticidal, miltefosine may have had a significant contribution.16 Nevertheless, we demonstrate the safety and potential efficacy of oral miltefosine in a refractory case of severe Acanthamoeba keratitis with limbal involvement. Systemic miltefosine provides an addition to the armamentarium of treatment options for severe Acanthamoeba keratitis.
4. Conclusion
We report the first case in the United States of oral miltefosine used in the successful treatment of refractory Acanthamoeba keratitis. Oral miltefosine appears to be safe and potentially effective in the treatment of Acanthamoeba keratitis.
Patient consent
Consent to publish the case report was not obtained. This report does not contain any personal information that could lead to the identification of the patient.
Funding
No funding or grant support.
Conflicts of interest
The following authors have no financial disclosures: KH, CL, CT.
Authorship: All authors attest that they meet the current ICMJE criteria for Authorship.
Acknowledgements
National Eye Institute P30-026877 and Research to Prevent Blindness.
References
- 1.Brown A.C., Ross J., Jones D.B. Risk factors for acanthamoeba keratitis-A multistate case-control study, 2008-2011. Eye Contact Lens. 2018;44(Suppl 1):S173–S178. doi: 10.1097/ICL.0000000000000365. [DOI] [PubMed] [Google Scholar]
- 2.Tu E.Y., Joslin C.E., Sugar J., Shoff M.E., Booton G.C. Prognostic factors affecting visual outcome in Acanthamoeba keratitis. Ophthalmology. 2008;115:1998–2003. doi: 10.1016/j.ophtha.2008.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Jonckheere J., van de Voorde H. Differences in destruction of cysts of pathogenic and nonpathogenic Naegleria and Acanthamoeba by chlorine. Appl Environ Microbiol. 1976;31:294–297. doi: 10.1128/aem.31.2.294-297.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khunkitti W., Lloyd D., Furr J.R., Russell A.D. Acanthamoeba castellanii: growth, encystment, excystment and biocide susceptibility. J Infect. 1998;36:43–48. doi: 10.1016/s0163-4453(98)93054-7. [DOI] [PubMed] [Google Scholar]
- 5.Brown T.J., Cursons R.T. Pathogenic free-living amebae (PFLA) from frozen swimming areas in Oslo, Norway. Scand J Infect Dis. 1977;9:237–240. doi: 10.3109/inf.1977.9.issue-3.16. [DOI] [PubMed] [Google Scholar]
- 6.Alkharashi M., Lindsley K., Law H.A., Sikder S. Medical interventions for acanthamoeba keratitis. Cochrane Database Syst Rev. 2015:CD010792. doi: 10.1002/14651858.CD010792.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tavassoli S., Buckle M., Tole D., Chiodini P., Darcy K. The use of miltefosine in the management of refractory Acanthamoeba keratitis. Contact Lens Anterior Eye. 2018;(41):400–402. doi: 10.1016/j.clae.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Rocha D.A., de Andrade Rosa I., de Souza W., Benchimol M. Evaluation of the effect of miltefosine on Trichomonas vaginalis. Parasitol Res. 2014;113:1041–1047. doi: 10.1007/s00436-013-3738-z. [DOI] [PubMed] [Google Scholar]
- 9.Machado P.R., Penna G. Miltefosine and cutaneous leishmaniasis. Curr Opin Infect Dis. 2012;25:141–144. doi: 10.1097/QCO.0b013e3283509cac. [DOI] [PubMed] [Google Scholar]
- 10.Santa-Rita R.M., Santos Barbosa H., Meirelles M.N., de Castro S.L. Effect of the alkyl-lysophospholipids on the proliferation and differentiation of Trypanosoma cruzi. Acta Trop. 2000;75:219–228. doi: 10.1016/s0001-706x(00)00052-8. [DOI] [PubMed] [Google Scholar]
- 11.Seifert K., Duchêne M., Wernsdorfer W.H. Effects of miltefosine and other alkylphosphocholines on human intestinal parasite Entamoeba histolytica. Antimicrob Agents Chemother. 2001;45:1505–1510. doi: 10.1128/AAC.45.5.1505-1510.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kitzmann A.S., Goins K.M., Sutphin J.E., Wagoner M.D. Keratoplasty for treatment of Acanthamoeba keratitis. Ophthalmology. 2009;116:864–869. doi: 10.1016/j.ophtha.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 13.Polat Z.A., Obwaller A., Vural A., Walochnik J. Efficacy of miltefosine for topical treatment of Acanthamoeba keratitis in Syrian hamsters. Parasitol Res. 2012;110:515–520. doi: 10.1007/s00436-011-2515-0. [DOI] [PubMed] [Google Scholar]
- 14.Polat Z.A., Walochnik J., Obwaller A., Vural A., Dursun A., Arici M.K. Miltefosine and polyhexamethylene biguanide: a new drug combination for the treatment of Acanthamoeba keratitis. Clin Exp Ophthalmol. 2014;42:151–158. doi: 10.1111/ceo.12120. [DOI] [PubMed] [Google Scholar]
- 15.Soto J., Rea J., Valderrama M. Efficacy of extended (six weeks) treatment with miltefosine for mucosal leishmaniasis in Bolivia. Am J Trop Med Hyg. 2009;81:387–389. [PubMed] [Google Scholar]
- 16.Pérez-Santonja J.J., Kilvington S., Hughes R., Tufail A., Matheson M., Dart J.K. Persistently culture positive acanthamoeba keratitis: in vivo resistance and in vitro sensitivity. Ophthalmology. 2003;110:1593–1600. doi: 10.1016/S0161-6420(03)00481-0. [DOI] [PubMed] [Google Scholar]