Abstract
CD19-targeted chimeric antigen receptor-T (CAR-T) cells with CD28 or 4-1BB (28z CAR-T and BBz CAR-T) have shown great promise to treat relapsed or refractory (r/r) B cell non-Hodgkin’s lymphoma (B-NHL). However, comparison of their clinical outcomes has never been reported. This study investigated their efficacy and adverse events in B-NHL therapy. Six patients with r/r B-NHL were initially enrolled and infused with 28z or BBz CAR-T cells at a dose of 0.75–5 × 105/kg. These CAR-T cells showed similar antitumor efficacies, with a complete response (CR) rate of 67% within 3 months. BBz CAR-T was well tolerated. However, severe cytokine release syndrome and immune effector cell-associated neurotoxicity syndrome occurred in the 28z CAR-T cohort, resulting in the termination of further evaluation of 28z CAR-T. Three more patients were enrolled to investigate BBz CAR-T cells in-depth at an escalated dose (1 × 106/kg). All cases achieved CR within 3 months, and only grade 1/2 adverse events occurred. This study suggests that 4-1BB is more beneficial for the clinical performance of CAR-T cells than CD28 in CD19-targeted B-NHL therapy, at least under our manufacturing process.
Keywords: CD19-targeted chimeric antigen receptor-T cells, co-stimulatory domain, B cell non-Hodgkin’s lymphoma
Graphical Abstract
Introduction
Non-Hodgkin’s lymphoma (NHL) is one of the most common hematologic malignancies worldwide.1 Despite progress in chemotherapy and molecular targeted therapy for certain types of B cell NHL (B-NHL), a large number of patients relapse or are resistant to these regimens.2 These intractable patients have a poor prognosis. Chimeric antigen receptor-T (CAR-T) cell therapy is considered a most promising approach in the treatment of hematological malignancies such as B cell acute lymphoblastic leukemia and diffuse large B cell lymphoma (DLBCL) that are relapsed or refractory (r/r) to conventional treatments.3, 4, 5 Currently, two CD19-targeted CAR-T products, axicabtagene ciloleucel (marketed as Yescarta) and tisagenlecleucel (marketed as Kymriah), have been approved to treat certain types of r/r B-NHL.6, 7, 8 Many other clinical studies of CD19-targeted CAR-T cells are ongoing for B-NHL therapy.9, 10, 11 In addition, CAR-T cells targeting other cell surface antigens of B-NHL or exhibiting special functions have also been investigated.12, 13
CAR-T cells are engineered by the transduction of T cells with CAR molecules comprising an antigen recognition single-chain variable fragment (scFvs), a transmembrane domain, and an intracellular signaling domain, such as CD3ξ.14 To confer T cells with a great capacity for expansion, activity, and persistence, the signaling domains of co-stimulatory receptors, including CD28 (T cell-specific surface glycoprotein CD28) and 4-1BB (also known as tumor necrosis factor (TNF) receptor superfamily member 9), have been incorporated together with the cytoplasmic domain of the CD3ξ chain, generating the second-generation CAR-T.15, 16, 17 Although remarkable antitumor activities of second-generation CAR-T cells have been observed and the effects of CAR-T cells with distinct co-stimulatory domains have been investigated in parallel in some pre-clinical studies,18, 19, 20, 21, 22, 23 there is a lack of clinical studies comparing the performances of CD28 and 4-1BB functionalized CAR-T cells in parallel. Previous mechanistic studies identified that CD28-based CAR-T cells usually elicit a robust proliferative response and yield effector memory T cells, whereas 4-1BB co-stimulation induces a progressive response and can endow CAR-T cells with enhanced persistence and central memory differentiation.19 Nevertheless, choice of the co-stimulatory domain is still controversial and may be associated with the structures of CAR molecules and the histopathology of target diseases. In this study, we aimed to compare the therapeutic efficacy and safety of 4-1BB and CD28 co-stimulated CAR-T cells in patients with CD19-positive NHL. This study will clearly show the different contributions of 4-1BB and CD28 to the clinical outcomes of CD19-targeted CAR-T cells in B-NHL therapy, providing evidence for selection of the co-stimulatory domain in the design of CAR molecules against such aggressive lymphoma.
Results
Study Design and Characteristics of Patients to Be Infused with CAR-T Cells at the Dose of 5 × 105/kg
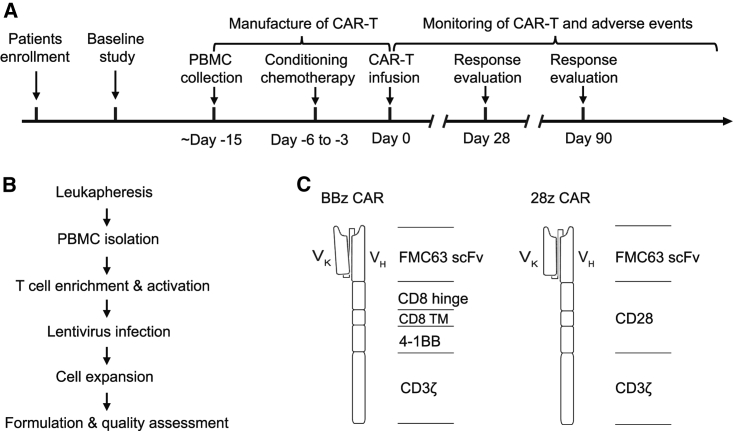
The clinical trial was a single-center phase I/IIa study to assess the safety and efficacy of CD19 CAR-T products with 4-1BB or CD28 co-stimulatory molecules (named BBz CAR-T and 28z CAR-T) in patients with r/r B-NHL, and was conducted according to the protocol shown in Figure 1A. Patients were pre-treated with fludarabine (25 mg/m2/day) and cyclophosphamide (250 mg/m2/day) for 3 consecutive days. The CAR-T cell product was infused 48 h after the pre-conditioning regimen. The patients were followed by monitoring peripheral blood CAR-T cell numbers and adverse events. At 1 and 3 months after infusion, patients underwent positron emission tomography-computed tomography (PET/CT) scanning for tumor burden evaluation.
Figure 1.
Schematic Design of the Clinical Study, CAR-T Manufacture, and CAR Molecules
(A) Clinical study design. (B) CAR-T manufacturing process. (C) Composition of BBz CAR and 28z CAR.
Patients were recruited and treated at Peking University Cancer Hospital, Beijing, China. Between May 2018 and June 2018, six patients with marginal zone B cell lymphoma (MZL), DLBCL, or follicular lymphoma (FL) were enrolled into this phase I/IIa clinical trial (Table 1). All patients previously received multiple rounds of chemotherapy, radiotherapy, or targeted therapy (Table 1; Table S1). The patients were divided into two groups irrespective of sex, age, weight, tumor subtype, tumor burden, and stage, and were planned to be infused with either BBz CAR-T or 28z CAR-T at the dose of 5 × 105/kg after receiving conditioning chemotherapy (Table 1). The infusion doses of 28z CAR-T cells were slightly decreased in two patients based on the severity of adverse events in cases after CAR-T treatment.
Table 1.
Patient Characteristics, Treatment, and Clinical Responses
| Patient No. | Sex | Age (years) | Weight (kg) | Diagnosis/Stage | Disease Status | Prior Regimens | Conditioning Regimen before T Cell Infusion | CAR-T Regimen | CAR-T Dose | Baseline Tumor Burden (SPD, mm2) | 1-Month Response (SPD, mm2) | 3-Month Response (SPD, mm2)a |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BBz-1 | male | 49 | 70 | MZL/IV | primary refractory | R-CHOP × 6, CHOP × 2, Ibrutinib | Flu/Cy | BBz CAR-T | 5 × 105/kg | 814 | CR (0) | CR (0) |
| BBz-2 | male | 34 | 78 | DLBCL/I | primary refractory | R-CHOP × 5, RT | Flu/Cy | BBz CAR-T | 5 × 105/kg | 1,044 | PD (1,505) | PD (3,266) |
| BBz-3 | male | 59 | 92 | FL/IV | primary refractory | R-CHOP × 4, (R-DDP+VP-16+DXM) × 2, GEMOX × 3 | Flu/Cy | BBz CAR-T | 5 × 105/kg | 7,472 | CR (2,131) | CR (1,616) |
| 28z-1 | male | 34 | 58 | FL/IV | primary refractory | R-CHOP × 3, CHOP × 5 | Flu/Cy | 28z CAR-T | 5 × 105/kg | 5,123 | CR (1,487) | CR (1,126) |
| 28z-2 | female | 47 | 56 | FL/II | primary refractory | R-CHOP × 6, RICE × 3 | Flu/Cy | 28z CAR-T | 4 × 105/kg | 446 | CR (316) | CR (226) |
| 28z-3 | male | 21 | 49 | DLBCL/IV | primary refractory | R-CHOP × 8, DICE × 1, RT, GEMOX × 3 | Flu/Cy | 28z CAR-T | 7.5 × 104/kg | 7,439 | – | – |
| BBz-4 | male | 65 | 75 | FL | primary refractory | CHOP × 8, CHOP × 3, DICE × 4 | Flu/Cy | BBz CAR-T | 1 × 106/kg | 3,679 | CR (1,952) | CR |
| BBz-5 | female | 50 | 46 | DLBCL/IV | primary refractory | R-CHOP × 7, R-DICE × 2 | Flu/Cy | BBz CAR-T | 1 × 106/kg | 1,829 | CR (497) | CR |
| BBz-6 | female | 36 | 56 | FL/IV | primary refractory | R-CHOP × 6, BGB-3111 × 93 weeks | Flu/Cy | BBz CAR-T | 1 × 106/kg | 1,910 | CR (808) | CR |
CR, complete response; Cy, cyclophosphamide; DICE, dexamethasone, ifosfamide, cisplatin, etoposide; DLBCL, diffuse large B cell lymphoma; DXM, dexamethasone; FL, follicular lymphoma; Flu, fludarabine; GEMOX, gemcitabine, oxaliplatin; MZL, marginal zone B cell lymphoma; PD, progressive disease; R-CHOP, rituximab, cyclophosphamide, adriamycin, vincristine, prednisone; R-DDP, rituximab, cisplatin; RICE, rituximab, ifosfamide, cisplatin, etoposide; RT, radiation therapy.
SPD was not available at 3 months for patients BBz-4, BBz-5, and BBz-6 because they underwent PET/CT scan only at 3 months.
Generation and Characteristics of BBz CAR-T and 28z CAR-T Cells
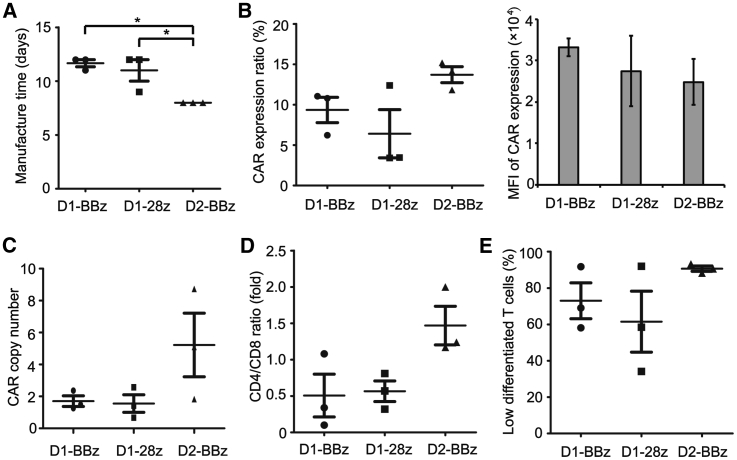
Clinically applicable CAR-T cells were successfully prepared by isolation of T cells from the peripheral blood mononuclear cells (PBMCs) of each patient and transduction of lentiviral vectors expressing CARs that included an FMC63-derived CD19-specific scFv, a 4-1BB or CD28 co-stimulatory molecule, and a CD3ζ intracellular signaling domain (Figures 1B and 1C). The nucleotide sequences of BBz CAR and 28z CAR are shown in Figure S1. The average manufacturing time of both CAR-T types was approximately 11 days (patient BBz-1, 12; patient BBz-2, 12; patient BBz-3, 11; patient 28z-1, 9; patient 28z-2, 12; patient 28z-3, 12; Figure 2A). Before infusion, CAR-T cells were characterized by their CAR expression level, cellular composition, and differentiation status. The expression ratios of BBz CAR and 28z CAR were 9.4% and 6.4%, respectively (patient BBz-1, 10.79%; patient BBz-2, 6.22%; patient BBz-3, 11.05%; patient 28z-1, 12.38%; patient 28z-2, 3.46%; patient 28z-3, 3.41%; Figure 2B). The mean fluorescence intensity of BBz CAR and 28z CAR in T cells was similar (Figure 2B). The average copy numbers of BBz CAR and 28z CAR were 1.70 and 1.55 (patient BBz-1, 1.49; patient BBz-2, 2.36; patient BBz-3, 1.26; patient 28z-1, 0.67; patient 28z-2, 1.42; patient 28z-3, 2.57; Figure 2C). The CD4/CD8 ratio was approximately 1/2 for both CAR-T cells (patient BBz-1, 0.34; patient BBz-2, 0.1; patient BBz-3, 1.08; patient 28z-1, 0.81; patient 28z-2, 0.57; patient 28z-3, 0.32; Figure 2D). About 73% of the BBz CAR-T and 62% of the 28z CAR-T cells were found to be subsets of naïve and stem cell memory T cells (CD45RA+CD62L+), central memory T cells (CD45RA−CD62L+), and effector memory T cells (CD45RA−CD62L−) based on flow cytometry analysis of CD45RA and CD62L expression (patient BBz-1, 91.76%; patient BBz-2, 58.15%; patient BBz-3, 69.07%; patient 28z-1, 91.97%; patient 28z-2, 34.20%; patient 28z-3, 58.43%; Figure 2E; Figure S2). These results demonstrated that both BBz CAR-T and 28z CAR-T cells were successfully generated using a similar manufacturing process and exhibited similar characteristics.
Figure 2.
Characteristics of the Manufactured BBz CAR-T and 28z CAR-T Cells
(A) The time taken to manufacture BBz CAR-T and 28z CAR-T cells for each patient. (B) The expression ratio and mean fluorescence intensity (MFI) of CARs in BBz CAR-T and 28z CAR-T cells. CAR-T cells were stained with FITC-anti-CAR scFv and analyzed using flow cytometry. (C) The copy number of BBz CAR and 28z CAR in each CAR-T cell. (D) The CD4/CD8 ratio of BBz CAR-T and 28z CAR-T cells. (E) The differentiation status of BBz CAR-T and 28z CAR-T cells. CAR-T cells were stained with APC-anti-CD45RA and APC-Cy7-anti-CD62L antibodies, and analyzed using flow cytometry. CD45RA+CD62L+, CD45RA−CD62L+, and CD45RA−CD62L− were regarded as lowly differentiated cells. *p < 0.05; the error bars represent the SD (n = 3). D1, at the dose of ∼5 × 105/kg; D2, at the dose of 1 × 106/kg.
Clinical Responses of Patients Treated with BBz CAR-T or 28z CAR-T Cells
Clinical response was evaluated at 1 and 3 months after infusion using PET/CT scanning. As shown in Table 1 and Figure S3, two of the three patients receiving BBz CAR-T (BBz-1 and BBz-3) or 28z CAR-T (28z-1 and 28z-2) cell therapy achieved a complete response (CR). One patient (BBz-2) treated with BBz CAR-T cells showed progressive disease. One patient (28z-3) in the 28z CAR-T cell group died on day 10. Peripheral blood CAR-T cell number in each patient was monitored from day 4 to day 120. All subjects exhibited CAR-T cell expansion in peripheral blood (Figure S4). The variations in BBz CAR-T and 28z CAR-T cell quantities were similar in vivo. CAR-T cells proliferated and reached a peak level on days 9–11 in all patients (Figure S4). In addition, the peak values of both types of CAR-T cells were in the same order of magnitude (Table 2). However, slower decrease of BBz CAR-T than 28z CAR-T was observed from 14 days to 4 months after infusion (Table 2). To further support the activity and persistence of CAR-T cells, we examined the ratio of CD19+ B cells in the peripheral blood of each patient (Table S2). Most patients exhibited an extremely low level of B cells within 4 months, indicating the presence of CAR-T cells and their capacity to kill CD19+ cells. Only patient 28z-1 showed a dramatic increase of B cells at months 3 and 4. Meanwhile, the B cell level of patient 28z-2 was also higher than that of the BBz cohort. These results revealed that BBz CAR-T and 28z CAR-T cells exhibited similar antitumor efficacy at the dose of 0.75–5 × 105/kg, but longer persistence was observed for BBz CAR-T cells after engraftment.
Table 2.
Peak Level and Residual Number of CAR-T Cells in Each Patient after Infusion
| Patient No. | Peak Level of CAR-T Cells, 105/L (%) | Time to Reach Peak Level | No. of CAR-T Cells in Peripheral Blood after Infusion (105/L) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Day 10 | Day 14 | Day 21 | Day 28 | Month 2 | Month 3 | Month 4 | |||
| BBz-1 | 6,302.01 (44) | day 10 | 6,302.01 | 1,697.26 | 687.6 | 719.9 | 65.72 | 114.4 | 152.28 |
| BBz-2 | 2,864.16 (40) | day 10 | 2,864.16 | 118.9 | 111 | 118.37 | 210 | 49.68 | – |
| BBz-3 | 1,373.04 (19) | day 14 | 5.85 | 1,373.04 | 200.88 | 259.2 | 25.95 | 47.74 | 71.38 |
| 28z-1 | 7,694.36 (52) | day 10 | 6,520.66 | 602.28 | 118.4 | 51.06 | 333.35 | 120.52 | 47.5 |
| 28z-2 | 1,674.45 (37) | day 10 | 1,674.45 | 76.5 | 88.75 | 14.7 | 43.99 | 34.08 | 22.4 |
| 28z-3 | 208.50 (14) | day 10 | – | – | – | – | – | – | – |
| BBz-4 | 11,170.18 (69) | day 7 | 5,664.63 | 1,666.62 | 1,106.64 | 242.64 | 67.1 | 70.72 | 46.8 |
| BBz-5 | 11,554.95 (73) | day 10 | 11,554.95 | 1,011.15 | 180.95 | 203.00 | 10.88 | 56.76 | 31.82 |
| BBz-6 | 6,924.61 (43) | day 10 | 6,924.61 | 166.14 | 143.36 | 97.17 | 47.73 | 132.3 | 26.4 |
Adverse Events of BBz CAR-T and 28z CAR-T Cells
The adverse events associated with BBz CAR-T and 28z CAR-T cells were monitored from day 4 to day 120 (Table 3). BBz CAR-T cells were infused to three patients at the dose of 5 × 105/kg according to the preliminary design, and only one patient experienced grade 1 cytokine release syndrome (CRS) (Table 3). However, after patient 28z-1 received the adoptive transfer of 28z CAR-T cells at the dose of 5 × 105/kg, grade 2 CRS occurred. Therefore, a lower dose (4 × 105/kg) was used to treat patient 28z-2, who also experienced severe CRS (grade 3) afterward (Table 3). Patient 28z-2 was given tocilizumab 10 days after CAR-T infusion, and CRS was alleviated afterward. Considering the adverse events, patient 28z-3 was treated with 28z CAR-T cells at a dose of 7.5 × 104/kg (Table 1). During the clinical investigation, patient 28z-3 was injected with 10 mg of dexamethasone and 400 mg of tocilizumab to limit CRS; however, the patient still died of grade 5 CRS on day 10 after emergent rescue (Table 3).
Table 3.
Adverse Events after BBz CAR-T or 28z CAR-T Cell Infusion
| Adverse Events, n (%) | Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
Grade 5 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose 1 |
Dose 2 |
Dose 1 |
Dose 2 |
Dose 1 |
Dose 2 |
Dose 1 |
Dose 2 |
Dose 1 |
Dose 2 |
||||||
| BBz | 28z | BBz | BBz | 28z | BBz | BBz | 28z | BBz | BBz | 28z | BBz | BBz | 28z | BBz | |
| Blood System Disorders | |||||||||||||||
| Neutropenia | – | 1 (33) | 1 (33) | 2 (67) | – | 2 (67) | 1 (33) | – | – | – | 1 (33) | – | – | – | – |
| Anemia | 1 (33) | 2 (67) | 1 (33) | – | 1 (33) | 1 (33) | – | – | – | – | – | – | – | – | – |
| Thrombocytopenia | – | 2 (67) | 1 (33) | – | – | – | – | – | – | – | – | – | – | – | |
| CRS | 1 (33) | – | 2 (67) | – | 2 (67) | – | – | – | – | – | – | – | – | 1(33) | – |
| Alanine aminotransferase increased | 1 (33) | – | 1 (33) | – | – | – | – | – | – | – | – | – | – | – | – |
| Aspartate aminotransferase increased | – | – | 1 (33) | – | – | – | – | – | – | – | – | – | – | – | – |
| Bilirubin increased | 2 (67) | 1 (33) | 1 (33) | – | – | – | – | – | – | – | – | – | – | – | – |
| Elevated CRP | 1 (33) | 1 (33) | 2 (67) | – | 2 (67) | – | – | – | – | – | – | – | – | – | – |
| Diarrhea | 1 (33) | 1 (33) | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Fever (>38.5°C) | 1 (67) | 1 (33) | 2 (67) | – | 1 (33) | – | – | – | – | – | – | – | – | – | – |
| Systolic pressure decreased | – | 1 (33) | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Tachycardia | 1 (33) | 1 (33) | 1 (33) | – | – | – | – | – | – | – | – | – | – | – | – |
| ICANS | – | 1 (33) | 1 (33) | – | – | – | – | 1 (33) | – | – | – | – | – | – | – |
| Headache | – | 1 (33) | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Seizure | – | – | – | – | – | – | – | 1 (33) | – | – | – | – | – | – | – |
| Other Symptoms | |||||||||||||||
| Frequent micturition | 1 (33) | – | – | – | – | – | – | – | – | – | – | – | – | ||
| Cough and expectoration | – | 2 (67) | – | – | – | – | – | – | – | – | – | – | – | ||
| Limb edema | – | 1 (33) | – | – | – | – | – | – | – | – | – | – | – | ||
| Chest stuffiness | – | 1 (33) | – | – | 1 (33) | – | – | – | – | – | – | – | – | ||
| Nausea and vomiting | – | – | – | – | 1 (33) | – | – | – | – | – | – | – | – | ||
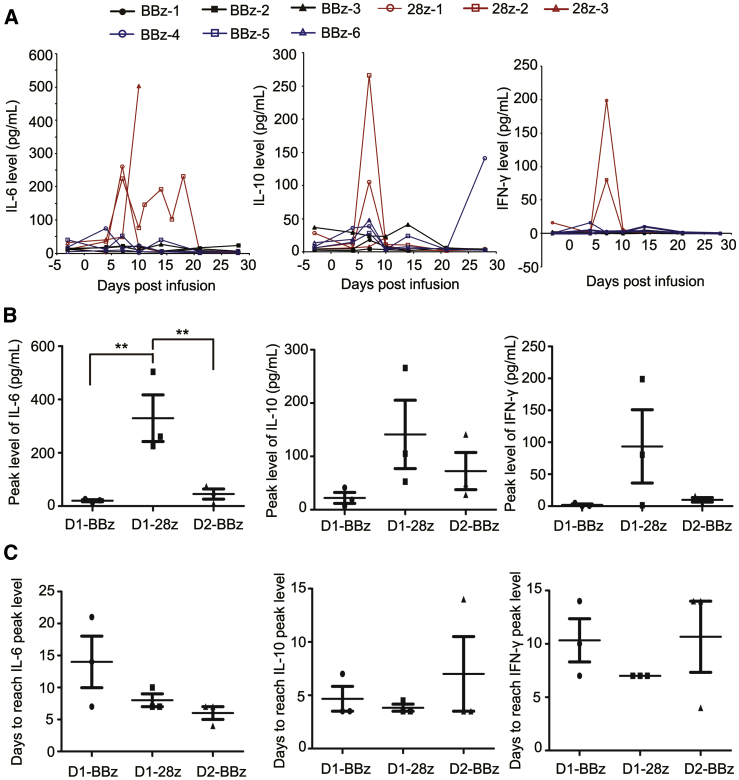
Peripheral blood inflammatory cytokines and growth factors were analyzed using both the cytometric bead array (Figures S5A–S5F) and Luminex assay (Figures S6 and S7). Infusion of either BBz CAR-T or 28z CAR-T cells induced an elevation in multiple interleukins (ILs), such as IL-6 and IL-10, and inflammatory cytokines, such as granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon-inducible protein-10 (IP-10), and monocyte chemotactic protein 1 (MCP-1). In addition, the expression of interferon-γ (IFN-γ) also increased in two patients (28z-1 and 28z-2) in the 28z CAR-T cohort (Figure 3A). Notably, the peak levels of IL-6, IL-10, G-CSF, and GM-CSF were much higher in 28z CAR-T cell-treated patients than in BBz CAR-T cell-treated patients (median IL-6 level: 329.9 pg/mL for 28z CAR-T, 20.3 pg/mL for BBz CAR-T; median IL-10 level: 141.2 pg/mL for 28z CAR-T, 22.2 pg/mL for BBz CAR-T; Figure 3B; Figure S7). Two (28z-2 and 28z-3) of the three patients in the 28z CAR-T cell group developed immune effector cell-associated neurotoxicity syndrome (ICANS), whereas BBz CAR-T did not induce any neurotoxicity in three patients (Table 3). We examined the levels of CAR-T cells and cytokines in the cerebrospinal fluid in patient 28z-2 on day 11. The ratio of CAR-T cells was 26% in cerebrospinal fluid. In addition, the concentration of IL-6 in cerebrospinal fluid reached 313.1 pg/mL and was more than 2-fold greater than that in peripheral blood (Table S3). All patients in the BBz CAR-T group showed a normal level or mild elevation of C-reactive protein (Figure S8A). In contrast, 28z CAR-T cell engraftment led to a marked increase in C-reactive protein, especially in patient 28z-2 (peak level, 107.5 mg/L). Most routine blood indices were similar in patients from both cohorts, except that platelets were decreased in two patients (28z-1 and 28z-2) after 28z CAR-T cell infusion (Figure S8B; Table S4). Severe toxicities (grade ≥3) detected by routine blood tests and blood biochemistry were observed only in patients treated with 28z CAR-T cells (Table 3). These results strongly suggested that BBz CAR-T cells showed a favorable safety profile compared with that of 28z CAR-T cells.
Figure 3.
Comparison of Cytokine Levels in Patients after Infusion of BBz CAR-T or 28z CAR-T Cells
(A) Expression of IL-6, IL-10, and IFN-γ in the peripheral blood of patients after infusion of BBz CAR-T or 28z CAR-T cells for different time intervals. (B) Peak level of IL-6, IL-10, and IFN-γ in each group of patients. (C) Time intervals to reach the peak level of IL-6, IL-10, and IFN-γ in each group of patients. **p < 0.01; the error bars represent SD (n = 3). D1, at the dose of ∼5 × 105/kg; D2, at the dose of 1 × 106/kg.
Clinical Outcomes of BBz CAR-T Cells at the Dose of 1 × 106/kg
Based on the good safety profile of BBz CAR-T cells at the dose of 5 × 105/kg, we further evaluated the efficacy and adverse events of this CAR-T cell type at an escalated dose. Three more patients with r/r DLBCL or FL were enrolled. The characteristics of these patients are shown in Table 1. Patients were infused with BBz CAR-T cells at a dose of 1 × 106/kg after receiving conditioning chemotherapy (Table 1). The manufacturing time of these BBz CAR-T cells was all 8 days (Figure 2A). The average BBz CAR expression ratio in T cells was 13.72% (patient BBz-4, 11.83%; patient BBz-5, 15.19%; patient BBz-6, 14.13%; Figure 2B), and the average copy number of BBz CAR was 5.22 in each cell (patient BBz-4, 1.83; patient BBz-5, 5.10; patient BBz-6, 8.73; Figure 2C). The CD4/CD8 ratio was 2.00, 1.24, and 1.17 for patient BBz-4, patient BBz-5, and patient BBz-6, respectively (Figure 2D). Most of the BBz CAR-T cells were subsets of naive, stem cell memory, central memory, and effector memory T cells (patient BBz-4, 88.15%; patient BBz-5, 93.30%; patient BBz-6, 90.55%; Figure 2E). At 1 and 3 months after CAR-T infusion, patients underwent PET/CT scanning, and tumor burden for each patient was analyzed. All three patients achieved CR (Table 1; Figure S9) and exhibited a low or normal level of B cells in peripheral blood (Table S2). The number of BBz CAR-T cells in peripheral blood was higher than that in the patients treated at the dose of 5 × 105/kg, and the average peak number was about 2.8 times that at the low dose (Table 2; Figure S10). Regarding the adverse events, only grade 1 and 2 toxicities were observed (Table 3). The level and kinetics of cytokines including IL-6, IL-10, and IFN-γ in peripheral blood were similar to those induced by BBz CAR-T at the dose of 5 × 105/kg (Figure 3; Figure S11). These results demonstrate that BBz CAR-T cells exhibited high antitumor activity and favorable safety profile at the dose of 1 × 106/kg in r/r B-NHL patients.
Discussion
This study compared the therapeutic efficacy and adverse events of CAR-T cells with CD28 or 4-1BB co-stimulatory domains in a single-center phase I/IIa clinical trial of r/r B-NHL therapy, irrespective of the baseline characteristics of patients and the CAR-T manufacturing process. Response evaluation revealed that the two types of CAR-T cells had similar antitumor activities. However, 4-1BB co-stimulation conferred a more favorable safety profile on the CAR-T cells in comparison with CD28 co-stimulation.
To date, the majority of clinical studies have focused on the second generation of CAR-T cells engineered with CD28 or 4-1BB co-stimulatory molecules.6, 24, 25, 26, 27, 28 The manufacturing process is regarded as critical for the potency of CAR-T cell therapy;29, 30 therefore, we produced clinical-grade 28z CAR-T and BBz CAR-T cells using an identical manufacturing process including T cell isolation, lentivirus transduction, T cell expansion, and final product preparation. The two types of CAR-T cells showed similar characteristics before infusion. Thus, we believe that in the present study, the variations in the clinical outcomes of these CAR-T cells were independent of the manufacturing process. To exclude possible interference factors, we established two cohorts irrespective of patient gender, age, weight, disease status, and previous treatment lines. All patients received the same conditioning chemotherapy and identical dose of CAR-T cells. These design principles ensured the parallel comparison of the two types of CAR-T cells.
Previous studies have reported that the CD28 and 4-1BB signaling molecules endow T cells with distinct functionalities.18, 19, 20, 21, 22, 23 CD28 co-stimulation has been validated to induce a brisk T cell activation, promoting T cells to differentiate into cells with an effector memory phenotype. In contrast, 4-1BB can lead to a relatively progressive and long-lasting response. CAR-T cells with 4-1BB show enhanced persistence and central memory differentiation.19 A recent study showed that CD28 CAR is capable of eliciting faster and stronger signaling compared with 4-1BB, resulting in T cell differentiation into an effector phenotype and reduced antitumor activity of CAR-T cells.18 Consistently, our study demonstrated that the in vivo persistence of BBz CAR-T cells was higher than that of 28z CAR-T cells (Figure S4; Table 2). Although the antitumor activity of these CAR-T cells was similar within 3 months after infusion (Table 1), we speculated that BBz CAR-T cells are likely to show superior antitumor efficacy over a longer period due to the contribution of 4-1BB to T cell survival and central memory differentiation. Patient BBz-2 showed progressive disease after infusion of BBz CAR-T cells (Table 1). We observed a relatively higher decrease of CAR-T cell number in this patient, which may account for the disease progression. The therapeutic efficacy and persistence of BBz or 28z CAR-T cells was not associated with the baseline tumor burden (mean sum of perpendicular diameter [SPD]: 3,110 [814, 7,442] versus 4,336 [446, 7,439]; p = 0.70; Table 1). In addition, the therapeutic efficacy may be also correlated with the differentiation of CAR-T cells in vivo, calling for further in-depth investigation.
The greater advantage of BBz CAR-T cells compared with 28z CAR-T cells is their favorable safety profile. Within 3 months after administration, only mild toxicities were observed in patients infused with BBz CAR-T cells. Grade 2 or higher CRS and ICANS occurred only in the 28z CAR-T cohort (Table 3). In particular, one patient (28z-3) died as a result of severe adverse events following 28z CAR-T cell infusion. We did not observe differences in the differentiation status and proliferation rate of CAR-T cells between patient 28z-3 and the other patients. The death of patient 28z-3 was also independent of the tumor burden, which was similar to that of patient BBz-3. Although patient 28z-3 was the youngest, it was regarded that younger patients could gain more benefits from CAR-T therapy because of a more active immune system. Thus, the death of patient 28z-3 was irrespective of age, tumor burden, and infusion dose. This case was included to summarize the adverse events and represented a grade 5 adverse event.
CRS and ICANS are two common CAR-T-related toxicities that should receive greater attention during CAR-T therapy.31 We observed that 28z CAR-T cells induced higher expression of certain cytokines compared with BBz CAR-T cells (Figure 3A; Figure S7). The cytokine release was also not correlated with the baseline tumor burden. In addition, ICANS occurred only in the 28z CAR-T cohort at the low dose level, and one patient developed grade 1 ICANS after infusion of BBz CAR-T at the escalated dose level (Table 3). Interestingly, we observed that the ratio of CAR-T cells in the cerebrospinal fluid of patient 28z-2 (treated with 28z CAR-T cells) was 26% on day 11, indicating that a large number of CAR-T cells are present in the brain area. The level of IL-6 in cerebrospinal fluid was more than 2-fold greater than that in the peripheral blood of this patient on day 11 (Table S3). Based on the different functional mechanisms of CD28 and 4-1BB, we deduced that the severe side effects of 28z CAR-T cells may result from the rapid and out-of-control immune response induced by CD28 stimulation. The slow and steady behavior of 4-1BB stimulation is beneficial for the safety of CAR-T cell therapy.
In conclusion, our clinical investigations suggested that the 4-1BB co-stimulatory domain was conducive to CD19-targeted CAR-T therapy against B-NHL, at least under our current manufacturing process. Although the hinge and transmembrane regions were different between BBz CAR and 28z CAR, our results showed that the co-stimulatory domain was more critical for the function of CAR-T cells. Nevertheless, it would be valuable to further investigate whether 4-1BB is more competent than CD28 for CAR-T cells against other targets such as CD123 and B cell maturation antigen in hematological malignancies and in solid tumors with their complicated microenvironment.
Materials and Methods
Patient Eligibility Criteria
Patients with CD19-positive B-NHL who showed progressive disease after treatment with rituximab- or doxorubicin-containing regimens were enrolled. Other inclusion criteria were as follows: (1) patients with evaluable disease lesions; (2) age ≥18 years; (3) expected lifespan of over 3 months; (4) an Eastern Cooperative Oncology Group (ECOG) score of 0–2 points; and (5) women of childbearing age with a negative blood pregnancy test. Patients with graft-versus-host disease or a history of CNS disease were excluded. Patients should not have received any chemotherapy or radiotherapy within 3 days, or systemic steroids within 5 days before apheresis.
Clinical Study Design
The clinical trial (ClinicalTrials.gov identifier: NCT03528421) was a single-center phase I/IIa study to assess the safety and efficacy of CD19 CAR-T products in patients with r/r B-NHL. This study had planned to enroll 15 cases into each of the BBz CAR-T and 28z CAR-T groups. Three dose levels (5 × 105/kg, 1 × 106/kg, and 3 × 106/kg) were set up for each cohort, and three cases were used for evaluation of each dose. Six more cases would be recruited for extensive investigation in each cohort. Once dose-limiting toxicity (DLT) occurred in more than two cases, the recruitment should be terminated at this dose. This study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board at Peking University Cancer Hospital. All participants were informed of the possible risks and side effects of the therapy and provided signed informed consent.
A schematic design of the clinical trial is shown in Figure 1A. Apheresis was performed in all eligible patients for isolation of PBMCs and subsequent production of CAR-T cells. The period of CAR-T manufacture and quality control was 8–15 days. Patients were pre-treated with fludarabine (25 mg/m2/day) and cyclophosphamide (250 mg/m2/day) for 3 consecutive days. On day 0, patients were infused with BBz CAR-T or 28z CAR-T cells. The patients were followed by monitoring peripheral blood CAR-T cell numbers and adverse events that were associated with routine blood analysis, blood biochemistry, C-reactive protein, CRS, and ICANS. Adverse events were evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. The severity of CRS and ICANS was assessed according to the reported gradings.31 At 1 and 3 months after infusion, patients underwent PET/CT scanning for response evaluation based on the revised criteria for response assessment,32 and tumor burden was calculated according to previous reports.33
Vector Construction and Lentivirus Production
The CD19-targeted 28z CAR contained an FMC63-derived CD19-specific scFv, a CD28 co-stimulatory molecule, and the intracellular signaling domain of CD3ζ. The BBz CAR was composed of an FMC63-derived CD19-specific scFv, the hinge and transmembrane domains of CD8α, the intracellular domain of 4-1BB, and the intracellular domain of CD3ζ. The PCR products of both CAR molecules were ligated to the third-generation EF1α promoter-based lentiviral transfer plasmid pLenti6.3/V5 (Thermo Fisher, Waltham, MA, USA). Lentivirus stock was prepared by transient transfection of transfer plasmid, packaging plasmids (pLP1 and pLP2; Thermo Fisher), and envelope plasmid (pLP/VSVG; Thermo Fisher) to 293T cells using polyethyleneimine (Polysciences, Warrington, PA, USA), collection of the culture medium 48 and 72 h after transfection, ultrafiltration of the culture medium, and subsequent purification of the lentiviral particles using Core 700 chromatography (GE Healthcare, Chicago, IL, USA).
Preparation of CAR-T Cells
CAR-T cells were produced using the good manufacturing practice (GMP) facilities at Immunochina Pharmaceuticals (Beijing, China), and the manufacturing process is shown in Figure 1B. In brief, the PBMCs from each patient were prepared using Ficoll (GE Healthcare, Chicago, IL, USA) density centrifugation leukapheresis. The PBMCs were washed in saline twice, and T cells were isolated and activated using CD3/CD28 magnetic beads (Thermo Fisher) at a T cell/bead ratio of 1:1. T cells were cultured in X-VIVO 15 medium (Lonza Group, Basel, Switzerland) supplemented with 500 U/mL IL-2 at a density of 1.5 × 106/mL. Cells were transduced with GMP-grade lentiviral vector carrying the CAR components. Cell viability and transduction efficiency were monitored for 5–7 days after lentivirus transduction. When the CAR-T cells had sufficiently expanded for patient infusion, the cells were cryopreserved and underwent quality-control evaluations including viability, potency, CAR expression ratio, replication-competent lentivirus, sterility, mycoplasma contamination, and endotoxin levels. All CAR-T cell products met the defined specifications.
Flow Cytometry Analysis of CAR-T Cells
Flow cytometry was used to analyze CAR expression, the CD4/CD8 ratio, and the differentiation status of the manufactured CAR-T cells. In brief, CAR-T cells (1 × 106) were suspended in 100 μL of Dulbecco’s PBS (DPBS; Thermo Fisher) and incubated with fluorescent molecule-labeled antibodies for 30 min. After washing in DPBS twice, the cells were analyzed using a flow cytometer (NOVOCYTE 2060R; ACEA Biosciences, San Diego, CA, USA). The cells were stained with allophycocyanin (APC)-anti-CD3 (BD Biosciences, San Jose, CA, USA) and fluorescein isothiocyanate (FITC)-anti-CAR that was developed by Immunochina Pharmaceuticals to specifically recognize the scFv of CAR. Phycoerythrin (PE)-anti-CD4 and FITC-anti-CD8 (BD Biosciences) were used to label CAR-T cells to determine the CD4/CD8 ratio. APC-anti-CD45RA and FITC-anti-CD62L (BioLegend, San Diego, CA, USA) were chosen to evaluate the differentiation status of CAR-T cells.
Cytometric Bead Array Assay of Cytokines
The cytometric bead array human Th1/Th2/Th17 kit (BD Biosciences) was used to measure IL-2, IL-4, IL-6, IL-10, TNF, IFN-γ, and IL-17A protein levels in each sample. Cytokine standards were prepared by serial dilution of lyophilized human Th1/Th2/Th17 cytokines according to the manufacturer’s instructions. Capture beads were added to the serum samples from each patient, cytokine standards, and negative control, and were incubated in the dark for 30 min. Flow cytometry was used to detect the cytokine levels.
Luminex Assay
The human cytokine and chemokine magnetic bead panel kit (Millipore, Merck, Darmstadt, Germany) was used to analyze the serum levels of 29 cytokines and chemokines in each patient. Serum samples were incubated with the magnetic beads, followed by labeling with fluorescent molecule-conjugated secondary antibodies according to the manufacturer’s instructions. The levels of candidate cytokines and chemokines were evaluated using the Luminex 200 system (Millipore, Merck).
Statistical Analysis
The two-sided Wilcoxon-Mann-Whitney test was used to compare the two experimental groups. Statistical analysis of more than three groups was based on one-way ANOVA and Tukey’s multiple comparison test. Analyses were performed using SPSS and GraphPad Prism software. A threshold of p < 0.05 was considered statistically significant for all analyses.
Author Contributions
Z.Y., T.H., X.-a.L., and J.Z. conceived and designed the study; Z.Y., X.W., W.Z., N.L., M.T., Y.X., L.P., C.Z., W.L., and L.D. performed clinical examinations; Z.Y., F.Q., Y.D., T.H., X.-a.L., and Y.S. analyzed and interpreted the data; F.Q., X.-a.L., and T.H. designed the CAR and prepared the CAR-T cell product; Y.D., X.-a.L., Z.Y., and J.Z. wrote the manuscript; all authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China ( 81600164), Beijing Municipal Natural Science Foundation ( 7172046), and Beijing Municipal Administration of Hospitals Incubating Program (PX2017001).
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.omto.2019.08.002.
Contributor Information
Yuqin Song, Email: songyuqin622@163.com.
Jun Zhu, Email: zhu-jun2017@outlook.com.
Supplemental Information
References
- 1.Shankland K.R., Armitage J.O., Hancock B.W. Non-Hodgkin lymphoma. Lancet. 2012;380:848–857. doi: 10.1016/S0140-6736(12)60605-9. [DOI] [PubMed] [Google Scholar]
- 2.Novelli S., Sierra J., Briones J. New therapies in non-Hodgkin lymphoma. Expert Rev. Anticancer Ther. 2015;15:349–359. doi: 10.1586/14737140.2015.1002773. [DOI] [PubMed] [Google Scholar]
- 3.June C.H., O’Connor R.S., Kawalekar O.U., Ghassemi S., Milone M.C. CAR T cell immunotherapy for human cancer. Science. 2018;359:1361–1365. doi: 10.1126/science.aar6711. [DOI] [PubMed] [Google Scholar]
- 4.Kochenderfer J.N., Rosenberg S.A. Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors. Nat. Rev. Clin. Oncol. 2013;10:267–276. doi: 10.1038/nrclinonc.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frey N.V., Porter D.L. CAR T-cells merge into the fast lane of cancer care. Am. J. Hematol. 2016;91:146–150. doi: 10.1002/ajh.24238. [DOI] [PubMed] [Google Scholar]
- 6.Neelapu S.S., Locke F.L., Bartlett N.L., Lekakis L.J., Miklos D.B., Jacobson C.A., Braunschweig I., Oluwole O.O., Siddiqi T., Lin Y. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017;377:2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chow V.A., Shadman M., Gopal A.K. Translating anti-CD19 CAR T-cell therapy into clinical practice for relapsed/refractory diffuse large B-cell lymphoma. Blood. 2018;132:777–781. doi: 10.1182/blood-2018-04-839217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salmikangas P., Kinsella N., Chamberlain P. Chimeric Antigen Receptor T-Cells (CAR T-Cells) for Cancer Immunotherapy—Moving Target for Industry? Pharm. Res. 2018;35:152–159. doi: 10.1007/s11095-018-2436-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Makita S., Yoshimura K., Tobinai K. Clinical development of anti-CD19 chimeric antigen receptor T-cell therapy for B-cell non-Hodgkin lymphoma. Cancer Sci. 2017;108:1109–1118. doi: 10.1111/cas.13239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Enblad G., Karlsson H., Gammelgård G., Wenthe J., Lövgren T., Amini R.M., Wikstrom K.I., Essand M., Savoldo B., Hallböök H. A Phase I/IIa Trial Using CD19-Targeted Third-Generation CAR T Cells for Lymphoma and Leukemia. Clin. Cancer Res. 2018;24:6185–6194. doi: 10.1158/1078-0432.CCR-18-0426. [DOI] [PubMed] [Google Scholar]
- 11.Bao F., Wan W., He T., Qi F., Liu G., Hu K., Lu X.A., Yang P., Dong F., Wang J., Jing H. Autologous CD19-directed chimeric antigen receptor-T cell is an effective and safe treatment to refractory or relapsed diffuse large B-cell lymphoma. Cancer Gene Ther. 2019;26:248–255. doi: 10.1038/s41417-018-0073-7. [DOI] [PubMed] [Google Scholar]
- 12.Bluhm J., Kieback E., Marino S.F., Oden F., Westermann J., Chmielewski M., Abken H., Uckert W., Höpken U.E., Rehm A. CAR T Cells with Enhanced Sensitivity to B Cell Maturation Antigen for the Targeting of B Cell Non-Hodgkin’s Lymphoma and Multiple Myeloma. Mol. Ther. 2018;26:1906–1920. doi: 10.1016/j.ymthe.2018.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang C.M., Wu Z.Q., Wang Y., Guo Y.L., Dai H.R., Wang X.H., Li X., Zhang Y.J., Zhang W.Y., Chen M.X. Autologous T Cells Expressing CD30 Chimeric Antigen Receptors for Relapsed or Refractory Hodgkin Lymphoma: An Open-Label Phase I Trial. Clin. Cancer Res. 2017;23:1156–1166. doi: 10.1158/1078-0432.CCR-16-1365. [DOI] [PubMed] [Google Scholar]
- 14.June C.H., Sadelain M. Chimeric Antigen Receptor Therapy. N. Engl. J. Med. 2018;379:64–73. doi: 10.1056/NEJMra1706169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Stegen S.J., Hamieh M., Sadelain M. The pharmacology of second-generation chimeric antigen receptors. Nat. Rev. Drug Discov. 2015;14:499–509. doi: 10.1038/nrd4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J., Jensen M., Lin Y., Sui X., Chen E., Lindgren C.G., Till B., Raubitschek A., Forman S.J., Qian X. Optimizing adoptive polyclonal T cell immunotherapy of lymphomas, using a chimeric T cell receptor possessing CD28 and CD137 costimulatory domains. Hum. Gene Ther. 2007;18:712–725. doi: 10.1089/hum.2007.028. [DOI] [PubMed] [Google Scholar]
- 17.Hombach A.A., Rappl G., Abken H. Arming cytokine-induced killer cells with chimeric antigen receptors: CD28 outperforms combined CD28-OX40 “super-stimulation”. Mol. Ther. 2013;21:2268–2277. doi: 10.1038/mt.2013.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salter A.I., Ivey R.G., Kennedy J.J., Voillet V., Rajan A., Alderman E.J., Voytovich U.J., Lin C., Sommermeyer D., Liu L. Phosphoproteomic analysis of chimeric antigen receptor signaling reveals kinetic and quantitative differences that affect cell function. Sci. Signal. 2018;11 doi: 10.1126/scisignal.aat6753. eaat6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawalekar O.U., O’Connor R.S., Fraietta J.A., Guo L., McGettigan S.E., Posey A.D., Jr., Patel P.R., Guedan S., Scholler J., Keith B. Distinct Signaling of Coreceptors Regulates Specific Metabolism Pathways and Impacts Memory Development in CAR T Cells. Immunity. 2016;44:380–390. doi: 10.1016/j.immuni.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 20.Vinay D.S., Kwon B.S. Role of 4-1BB in immune responses. Semin. Immunol. 1998;10:481–489. doi: 10.1006/smim.1998.0157. [DOI] [PubMed] [Google Scholar]
- 21.Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat. Rev. Immunol. 2003;3:609–620. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- 22.Priceman S.J., Gerdts E.A., Tilakawardane D., Kennewick K.T., Murad J.P., Park A.K., Jeang B., Yamaguchi Y., Yang X., Urak R. Co-stimulatory signaling determines tumor antigen sensitivity and persistence of CAR T cells targeting PSCA+ metastatic prostate cancer. OncoImmunology. 2017;7:e1380764. doi: 10.1080/2162402X.2017.1380764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhong Q., Zhu Y.M., Zheng L.L., Shen H.J., Ou R.M., Liu Z., She Y.L., Chen R., Li C., Huang J. Chimeric Antigen Receptor-T Cells with 4-1BB Co-Stimulatory Domain Present a Superior Treatment Outcome than Those with CD28 Domain Based on Bioinformatics. Acta Haematol. 2018;140:131–140. doi: 10.1159/000492146. [DOI] [PubMed] [Google Scholar]
- 24.Park J.H., Rivière I., Gonen M., Wang X., Sénéchal B., Curran K.J., Sauter C., Wang Y., Santomasso B., Mead E. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N. Engl. J. Med. 2018;378:449–459. doi: 10.1056/NEJMoa1709919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramos C.A., Ballard B., Zhang H., Dakhova O., Gee A.P., Mei Z., Bilgi M., Wu M.F., Liu H., Grilley B. Clinical and immunological responses after CD30-specific chimeric antigen receptor-redirected lymphocytes. J. Clin. Invest. 2017;127:3462–3471. doi: 10.1172/JCI94306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu Y., Wu Z., Luo Y., Shi J., Yu J., Pu C., Liang Z., Wei G., Cui Q., Sun J. Potent Anti-leukemia Activities of Chimeric Antigen Receptor-Modified T Cells against CD19 in Chinese Patients with Relapsed/Refractory Acute Lymphocytic Leukemia. Clin. Cancer Res. 2017;23:3297–3306. doi: 10.1158/1078-0432.CCR-16-1799. [DOI] [PubMed] [Google Scholar]
- 27.Guo Y., Feng K., Liu Y., Wu Z., Dai H., Yang Q., Wang Y., Jia H., Han W. Phase I Study of Chimeric Antigen Receptor-Modified T Cells in Patients with EGFR-Positive Advanced Biliary Tract Cancers. Clin. Cancer Res. 2018;24:1277–1286. doi: 10.1158/1078-0432.CCR-17-0432. [DOI] [PubMed] [Google Scholar]
- 28.Katz S.C., Burga R.A., McCormack E., Wang L.J., Mooring W., Point G.R., Khare P.D., Thorn M., Ma Q., Stainken B.F. Phase I Hepatic Immunotherapy for Metastases Study of Intra-Arterial Chimeric Antigen Receptor-Modified T-cell Therapy for CEA+ Liver Metastases. Clin. Cancer Res. 2015;21:3149–3159. doi: 10.1158/1078-0432.CCR-14-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Köhl U., Arsenieva S., Holzinger A., Abken H. CAR T Cells in Trials: Recent Achievements and Challenges that Remain in the Production of Modified T Cells for Clinical Applications. Hum. Gene Ther. 2018;29:559–568. doi: 10.1089/hum.2017.254. [DOI] [PubMed] [Google Scholar]
- 30.Gardner R.A., Finney O., Annesley C., Brakke H., Summers C., Leger K., Bleakley M., Brown C., Mgebroff S., Kelly-Spratt K.S. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. 2017;129:3322–3331. doi: 10.1182/blood-2017-02-769208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee D.W., Santomasso B.D., Locke F.L., Ghobadi A., Turtle C.J., Brudno J.N., Maus M.V., Park J.H., Mead E., Pavletic S. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol. Blood Marrow Transplant. 2019;25:625–638. doi: 10.1016/j.bbmt.2018.12.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheson B.D., Fisher R.I., Barrington S.F., Cavalli F., Schwartz L.H., Zucca E., Lister T.A., Alliance, Australasian Leukaemia and Lymphoma Group. Eastern Cooperative Oncology Group. European Mantle Cell Lymphoma Consortium. Italian Lymphoma Foundation. European Organisation for Research. Treatment of Cancer/Dutch Hemato-Oncology Group. Grupo Español de Médula Ósea. German High-Grade Lymphoma Study Group. German Hodgkin’s Study Group. Japanese Lymphorra Study Group. Lymphoma Study Association. NCIC Clinical Trials Group. Nordic Lymphoma Study Group. Southwest Oncology Group. United Kingdom National Cancer Research Institute Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J. Clin. Oncol. 2014;32:3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hay K.A., Hanafi L.A., Li D., Gust J., Liles W.C., Wurfel M.M., López J.A., Chen J., Chung D., Harju-Baker S. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood. 2017;130:2295–2306. doi: 10.1182/blood-2017-06-793141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.