Highlights
-
•
Ultrasound improves the bioactives content from green propolis in a lower time.
-
•
Optimization allowed good extraction of artepillin C and p-coumaric acid.
-
•
Green propolis had similar effects to synthetic antioxidant in emulsion systems.
-
•
Green propolis did not change the S. cerevisiae membrane.
Keywords: Green propolis, Ultrasound-assisted extraction, Antioxidant, In vivo assays
Abstract
Green propolis presents a potential source of bioactive compounds, responsible for its antioxidant capacity. The effects of ethanol concentration, solid-solvent ratio, and extraction time were evaluated in regard to the total phenolic content (TPC) and antioxidant capacity of the extracts by the use of central composite rotatable designs. Optimum extraction conditions lead to significant reduction of extraction time compared to conventional extraction methods. Under optimum conditions, extracts were composed of 1614.80 mg GAE. g−1 and 807 mg artepillin C. g−1. Extracts were effective in retarding the oxidation in oil-in-water emulsions subjected to accelerated tests. Green propolis extracts (up to 200 mg.kg−1) did not increase Saccharomyces cerevisiae cell damage after 4 h of exposure, indicating its antioxidant effect and potential innocuity. Results demonstrated the antioxidant properties of the propolis extract was similar or better than the ones from synthetic antioxidants and warrant further investigation to determine its potential industrial application.
1. Introduction
Propolis is considered a natural source of antioxidants and it is presented as a resinous material collected from different plant parts and synthetized by bees (Andrade, Denadai, de Oliveira, Nunes, & Narain, 2017) with the aid of salivary enzymes. Propolis extracts present both antimicrobial and antioxidant activities, which makes them a potential ingredient for subsequent use by the pharmaceutical, cosmetic, and food industries (Kubiliene et al., 2015).
The native Brazilian plant Baccharis dracunculifolia is the main source of green propolis in Brazil (Teixeira, Negri, Meira, Message, & Salatino, 2005). This specific propolis has a particular phenolic composition which includes p-coumaric acid and artepillin C, contributing to its effective antioxidant capacity (Simões et al., 2004). The concentration and composition of the propolis extract depend on the choice and effectiveness of extraction method used (Bittencourt et al., 2015). Processing parameters such as sample-to-solvent ratio, temperature, and extraction time have a significant effect on the diversity and concentration of compounds present in the final extract (Bulduk, Gezer, & Cengiz, 2015).
Recent research has shown that the use of ultrasound-assisted extraction enables the extraction of a wide range of compounds at shorter reaction times with reduced use of organic solvents and use of lower temperatures, the latter leading to reduced energy consumption and being particularly important to avoid thermal degradation of some active compounds (Briones-Labarca et al., 2015, Chemat et al., 2017).
Ultrasound assisted extraction enhancement is usually attributed to several mechanisms resulting from the acoustic cavitation phenomena (formation, growth, and implosion of bubbles). In general, enhanced reactivity is attributed to increased mass transfer due to particle size reduction, the latter being associated with the collapse of the cavitation bubbles (Corrales et al., 2008, Morelli and Prado, 2012, Chemat et al., 2017).
Because extraction conditions have a significant impact on extraction yields and product functionality, response surface methodology (RSM), a practical and important tool to identify the interaction of several processing parameters was used to reduce the number of experiments, allowing to calculate and evaluate the experimental error (Rodrigues & Iemma, 2014). With the aid of response surface methodology, it is possible to verify the optimal point of extraction, where the responses, such as antioxidant capacity, are superior.
The antioxidant capacity of an extract may be evaluated through several in vitro methods (Espín et al., 2000, Fukumoto and Mazza, 2000). However, there is a need to represent its function in cellular systems, for example in eukaryotic microorganisms, such as Saccharomyces cerevisiae (Soares, Andreazza, & Salvador, 2005).
The objective of this work was to develop a process for antioxidant recovering from green propolis using ultrasound-assisted extraction, to evaluate the antioxidant capacity of extract obtained under optimum conditions in oil-in-water emulsion under accelerated conditions, and to evaluate the toxicity of the extract in yeast cells. This research may improve the process of obtaining bioactive compounds from green propolis through the use of ultrasound, indicating for better alternatives to the use of synthetic antioxidants.
2. Materials and methods
2.1. Chemicals and reagents
Folin–Ciocalteau reagent, p-coumaric acid, AAPH (2,2′-azobis (2-amidinopropane)), butylhydroxyanisole (BHA), butylhydroxytoluene (BHT), terc-butylhydroquinone (TBHQ), cumene hydroperoxide, propidium iodide (PI), phosphate buffered solution, ABTS (2.2′-azinobis-(3-ethylbenzothiazoline-6-acid)), fluorescein and Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) were obtained from Sigma–Aldrich Chemical Co. (St. Louis, MO, USA). Artepillin C (3,5-diprenyl-4-hydroxycinnamic acid) was obtained from Wako (Osaka, Japan). The refined soybean oil was purchased at a local market. Other reagents were p.a grade.
2.2. Green propolis
Raw green propolis sample were kindly provided by Natucentro Indústria e Apiários Centro Oeste Ltda. (19° 59′ 46′’S, 45° 48′ 38′’ W) (Bambuí, Minas Gerais, Brazil). Propolis samples were collected over a period of one year (2016), obtained from five beehives, they were pooled, comminuted with aid of liquid nitrogen until homogenized, then they were packed in dark low-density polyethylene bags (identified by the month of collection) and stored at −18 °C until used. In this study sample collected during spring season was used.
2.3. Experimental design and statistical analyses
Extractions were performed in a 28 W/L ultrasonic bath USC-14000 (Sppencer, Indaiatuba, Brazil) and experimental parameters were determined following two sequential central composite rotatable designs (CCRD), including 4 central points each one. The factorial designs were developed sequentially in order to get as close as possible to an optimal region with the least amount of sample and extraction time. The independent variables in first design were extraction time (5–45 min) (x1) and solids-to-solvent ratio (1:10 – 1:30 (w/v)) (x2) with the ethanol concentration in hydroalcoholic extracting solution maintained at 65%. The second experimental design was planned according to the results obtained in the first one and the variables evaluated were ethanol/distillated water concentration in extracting solution (0–99% ethanol, v/v) (x1) and solids-to-solvent ratio (1:20–1:50 (w/v)) (x2), keeping the time constant in 20 min. In order to minimize the extraction errors, sample-to-solvent ratio for both designs were adjusted to a total volume of 30 mL, therefore, it was possible to guarantee that all samples have the same contact surface for ultrasonic waves to break cell walls and release phenolic compounds from green propolis. The temperature of extraction process was maintained at 25 °C. The dependent variables obtained for these designs were total phenolic content (TPC) and antioxidant capacity.
After each extraction run, samples were centrifuged at 4695 g for 15 min in a refrigerated centrifuge model CF16RN (Hitachi KOKI, Tokyo, Japan) kept at 25 °C, and subsequently filtered using a Whatman n. 2 filter paper. The extracts was evaporated in rotary evaporator model R-215 (Buchi Labortechnik AG, Flawil, Switzerland) at 45 °C and then they were stored under refrigeration at 7 °C in amber flasks until analysis.
For statistical analysis, the responses were firstly submitted to one-way ANOVA, evaluated in relation to the coefficient of determination (R2) and F-value at 95% of probability, using the software Statistica 12.0 (Statsoft, USA). The effects analysis was performed, and terms considered not significant at the 5% probability level were eliminated from the model and incorporated into the residue. The regions of interest of the response surfaces generated were studied, in order to determine the optimal process condition.
2.4. Total phenolic content (TPC) and in vitro antioxidant capacity
2.4.1. Determination of total phenolic content (TPC)
TPC of the green propolis extracts was determined according to the Folin-Ciocalteau spectrophotometric method, described by Singleton, Orthofer, and Lamuela-Raventos (1999). An aliquot of 0.5 mL of the hydroalcoholic extract and 2.5 mL of the Folin-Ciocalteau: water solution (1:10 v/v) were transferred to a test tube. The mixture was vortexed and after five minutes at room temperature 2.0 mL of the sodium carbonate (p.a grade) 4% (m/v) solution were added. Then, the mixture was agitated again and kept at rest for 120 min in the dark and room temperature. Absorbance was read at 740 nm using a spectrophotometer UV mini-1240 (Shimadzu, Kyoto, Japan). The results were calculated using a standard curve of gallic acid (p.a grade) with known concentrations (5–60 μg mL−1), and expressed as mg of gallic acid equivalent (GAE).g−1 of green propolis. Measurements were performed in triplicate.
2.4.2. ABTS radical scavenging capacity
The ABTS [2.2′-azinobis-(3-ethylbenzothiazoline-6-acid)] assay was performed according to the method described by Re et al. (1999). The ABTS radical was diluted with ethanol of analytical grade until reaching an absorptivity value of 0.70 ± 0.02 at 734 nm. The dilutions were prepared with the extracts, and an aliquot of 30 mL from each extract dilution was transferred to test tubes with 3.0 mL of the ABTS radical. Absorbance was determined at 734 nm using a spectrophotometer UV mini-1240 (Shimadzu, Kyoto, Japan) after 6 min of reaction. All tests were run in triplicate and the average value was calculated. Trolox was used as a standard and the curve was generated at known concentrations (250–1000 µg mL−1). The results were expressed as μmol TEAC.g−1 of sample.
2.4.3. Antioxidant capacity by ORAC method
The antioxidant capacity was determined according to Chisté et al. (2011), through thermodecomposition of AAPH (2,2′-azobis (2-amidinopropane) radical using Spectramax M3 microplates (Molecular Devices, Sunnyvale, United States). The reagents were diluted in 75 mM potassium phosphate buffer, pH 7.4. For the analysis, 30 μL extract/standard/blank plus 60 μL fluorescein 4.066 mM and 110 μL of AAPH 76 mm were added in a microplate. The fluorescence signal was monitored every minute during 2 h at 485 ± 20 nm of excitation and 528 ± 20 nm of emission until the total decay of fluorescence. Trolox was used as standard control at concentrations of 12.5–400 μM and the results were calculated according to the difference between the area under the fluorescence decay curve in the presence of the sample (AUC antioxidant) and in its absence (AUC blank). The results were expressed in μmol TEAC.g−1 sample and measurements were performed in triplicate.
2.5. Identification and quantification of phenolic compounds
Compounds identification was performed according to Szliszka et al. (2013) on a high-performance liquid chromatography system (Prominence, Shimadzu, Japan) coupled with a quadruple time of flight (MicroTOF-QII, Bruker Daltonics, USA) with an electrospray ionization (ESI) source. Separation was achieved on a Luna C18 (2) column (250 × 3 mm, 5 μm, Phenomenex). 10 μL aliquot were filtered through a 0.22 μm filter (Millipore). The mobile phase consisted of 0.1% aqueous acetic acid (v/v) (A) and methanol (B). The gradient program was applied as follows: 0–90 min at 30–70% B; 90–100 min at 90% B; 100–107 min at 90–30% B; and 107–120 min at 30% B. The outlet of HPLC was split (1:10) and introduced into the ESI source at 4–62 min and into waste at 0–4 min and 62–70 min. The flow rate was 0.5 mL/min and the column was operated at 40 °C. The electrospray source was operated in negative mode at 3000 V. Other parameters used: nebulizer gas (nitrogen): 35 psi; drying gas (nitrogen): 6 L/min at 220 °C. For quantification of selected compounds the analysis was performed according to Alencar et al. (2007), injecting 10 μL of the extract in a chromatograph LC 10 (Shimadzu, Kyoto, Japan) coupled to a photodiode array (PDA) SPD-M10AVp (Shimadzu, Kyoto, Japan) and detector UV–Vis SPD-20AV (Shimadzu, Kyoto, Japan). The analytical column used was a Varian C-18 RP (250 mm × 4.6 mm, 5 μm) with temperature maintained at 30 °C. The mobile phase used was water:acetic acid (1:19 v/v) as solvent A and methanol as solvent B with elution in gradient mode. The gradient started with 30% until 40% of B in 15 min up to 50% of B in 30 min, 60% of B in 45 min, 75% of B in 65 min, 75% of B in 85 min, 90% of B in 95 min and 30% of B in 105 min. The chromatograms were processed using the Class-VP software. Authentic standards of p-coumaric acid and 3,5-diprenyl-4-hydroxycinnamic acid (artepillin C) were used for quantification in ultraviolet at wavelengths between 300 and 310 nm.
2.6. Lipid emulsion oxidation essay
The ability of the propolis extract to retard lipid oxidation in oil-in-water lipid emulsions was evaluated and compared with synthetic antioxidants. The method for emulsions preparation was adopted according to Huang, Hopia, Schwarz, Frankel, and German (1996). Oil-in-water emulsions were prepared by adding 200 g of refined, bleached, deodorized soybean oil, 180 g of distilled water, and 20 g of Polysorbate 20 (Tween 20) in a beaker. The mixture was mixed in a Turrax sample homogenizer T25 basic (Ika, Staufen, Germany) for 1 min at 18000 rpm.
Propolis extracts (100 mg.kg−1, based on the phenolic content) were added to the emulsions and compared with synthetic antioxidants (Butylhydroxyanisole (BHA), Butylhydroxytoluene (BHT) and Terc-butylhydroquinone (TBHQ), also added at 100 mg.kg−1. To each antioxidant, it was realized triplicate aliquots of emulsions distributed in 10 mL glass test tubes and stored in an oven 315 SE (FANEM, São Paulo, Brazil) at constant temperature of 40 °C. The samples were collected to analysis after each storage period (0, 3, 6, and 9 days). The evaluation of the oxidation degree of the emulsion was carried out by hydroperoxide content, according to the procedures of Branco and Castro (2011) with some modifications. Oil sample fractions (300 µL) were mixed with 1.50 mL of isooctane/2-propanol solution (3:1, v/v), which resulted in a final volume of 1.80 mL. The mixture was vortexed and. 25 µL of the mixture were added to a solution of methanol/1-butanol (2:1, v/v). An indicator thiocyanate/ferrous solution was prepared by mixing 500 µL of 3.94 M thiocyanate solution with 500 µL of 0.072 M Fe2+ solution. The thiocyanate/ferrous solution (30 µL) was added to the methanol/1-butanol mixture, vortexed and incubated at room temperature for 20 min. Samples absorbance values were registered at 510 nm using a spectrophotometer UV mini-1240 (Shimadzu, Kyoto, Japan). Hydroperoxide content was determined by a standard curve with known concentrations of cumene hydroperoxide. The results were expressed as mg cumene hydroperoxide equivalent.kg−1 oil. Analysis of variance (ANOVA) was performed using the SAS 9.3 application and, when applicable, the data were compared by Tukey test at significantly different at p ≤ 0.5.
2.7. Interaction of the extracts with yeast cells
In vivo antioxidant capacity methodologies are an important tool to provide information about the action and effect of antioxidants (Stinco et al., 2015), producing results that may be complementary to in vitro methodologies. With the use of yeasts, it is possible to verify cell viability in the presence of bioactive compounds and if this compound produces some toxicity to cells. To procedure the in vivo study, 300 mL of green propolis extract were prepared according to the optimal conditions and then it was dried (-55 °C and 200–300 mmHg) in Lyophilizer L108 (Liotop, São Carlos, Brazil). The Saccharomyces cerevisiae strain BY4742 (Wild Type) was used, according to the procedure proposed by Cruz et al. (2019). For the isolation and the cultivability essays, the strain was kept on yeast extract-peptone-dextrose (YPD). While for the culture preparation the YPD was used without agar. After that, the culture was stored at −18 °C and sub-cultured once per month. All the dilutions were done in phosphate buffered solution (Sigma–Aldrich Chemical Co. (St. Louis, MO, USA). Subcultures of yeast were performed by placing one isolated colony in 250 mL conical flasks containing 100 mL of YPD medium. Flasks were placed in a rotary shaker Excella E24 (New Brunswick Scientific TM, Edison, USA) at 250 rpm, 25 °C for 48 h. Cultures were performed by transferring 1 mL of subcultures into a flask containing 100 mL of fresh YPD medium and cells were grown at 25 °C during 24 h in a rotary shaker at 250 rpm. At harvest, cell density was 108 cell/mL. Cell suspension (20 mL) was centrifuged in shaker model 5810R for 5 min at 2800 g and 25 °C (Eppendorf, Hamburg, Germany). Cell pellet was washed twice and after that the optical density (OD) was adjusted in 0.5 at 600 nm using a GeneQuant 1300 spectrophotometer (GE Healthcare®, Little Chalfont, United Kingdom) in phosphate buffered solution (PBS).
2.7.1. Plasma membrane integrity.
Yeast cells with OD 0.5 (after the pre-culture) were exposed to four different green propolis dried extract concentrations (0, 50, 100, and 200 mg.kg−1) for 4 h. After the exposure period, cells were centrifuged for 10 min at 5000 g and 25 °C, washed twice with PBS and then marked with propidium iodide (PI) to present at 2 µg/mL final concentration. Antioxidant effects of green propolis extracts were evaluated after the oxidative treatment with tert-butyl hydroperoxide (t-BOOH). Yeast cells were exposed to 18 mM of t-BOOH for 24 h. Then the cells were centrifuged for 10 min at 5.000 × g and 25 °C and washed twice with PBS and then marked with propidium iodide (PI). Flow cytometry was performed in FACSAria™ II flow cytometer (BD Biosciences, San Jose, USA), with excitation/emission set at 493/636 nm to indicate the number of cells with cytoplasmic membrane lesion. The control experiment was performed the cell solution (OD 0.5) marked with PI, without the addition of the propolis extract. The results were expressed in percentage of cells with intact membrane.
3. Results and discussion
3.1. Extraction process
The results of the parameters of extraction according to the first factorial design are shown in Table 1. TPC in green propolis extracts ranged from 316.4 to 533.9 mg GAE.g−1, demonstrating that the extraction yields of conditions evaluated were in accordance with the Brazilian legislation, which requires a minimum of 5% (w/w) of phenolic compounds in commercial propolis extracts (Brasil, 2001).
Table 1.
Real values of the independent variables and observed responses to the first factorial design.
| Independent variables (real values) |
Responses |
|||
|---|---|---|---|---|
| Runa | Extraction time (min) | Propolis-to-solvent ratio (w/v)* | YTPC (mg GAE.g−1)b | YTAA (µmol TEAC.g−1)c |
| 1 | 11 | 1:13 | 334.2 ± 13.2 | 2452.0 ± 7.0 |
| 2 | 39 | 1:13 | 351.9 ± 15.6 | 3123.1 ± 12.2 |
| 3 | 11 | 1:27 | 405.3 ± 17.7 | 3077.6 ± 12.5 |
| 4 | 39 | 1:27 | 481.8 ± 17.5 | 3326.4 ± 13.7 |
| 5 | 5 | 1:20 | 344.8 ± 13.1 | 2210.2 ± 7.3 |
| 6 | 45 | 1:20 | 316.4 ± 9.3 | 3524.0 ± 18.8 |
| 7 | 25 | 1:10 | 413.3 ± 9.6 | 3064.5 ± 15.6 |
| 8 | 25 | 1:30 | 533.9 ± 13.1 | 3253.8 ± 17.9 |
| 9 | 25 | 1:20 | 450.9 ± 15.5 | 3245.3 ± 14.8 |
| 10 | 25 | 1:20 | 464.7 ± 17.6 | 3214.7 ± 15.0 |
| 11 | 25 | 1:20 | 494.5 ± 13.2 | 3255.1 ± 19.6 |
| 12 | 25 | 1:20 | 411.4 ± 12.5 | 3272.9 ± 16.1 |
The results were expressed as mean ± standard deviation.
Tests performed in random order.
Total Phenolic Content.
Total Antioxidant Capacity
All assays were performed with a total volume of 30 mL (Degree of hydration of the extractive solution: 65% ethanol).
The regression model TPC (Eq. (1)) was significant at 95% confidence level (Fregression 44.92 > Ftabulated 4.39) and able to explain 87% of variation between the observed and predicted values. According to the predictive model extraction yields (TPC) are favored by higher dilution of raw propolis in extracting solution and by the use of extraction time around the central point (25 min). The results of antioxidant capacity by ORAC method ranged to 2210.2 from 3524.0 μmol TEAC.g−1 sample. Eq. (2) represent coded models for ORAC, with 86% of variation was explained by the adjusted model, and the Fregression 50.6 > Ftabulated 4, indicating that the model was valid and predictive.
| (1) |
| (2) |
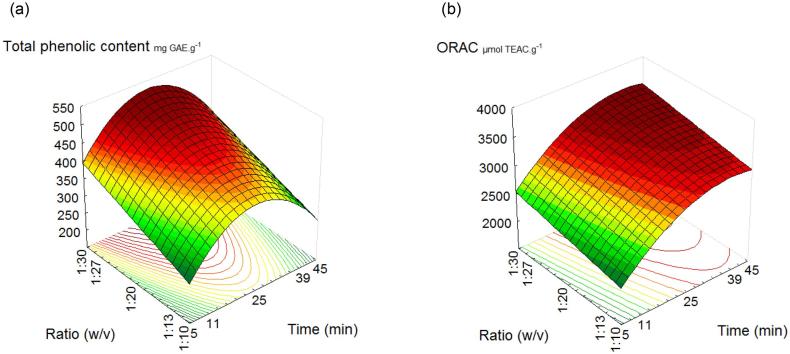
Based on the predictive models and contour curves, that representing the adjusted models (Fig. 1), it was possible to identify the region that presented both higher amount of TPC (a) and antioxidant capacity by ORAC (b) to be applied in extraction process. Observing Fig. 1, it is possible to verify that the best results for these dependent variables were observed above 20 min and at 1:30 sample-to-solvent ratio (w/v). Oldoni et al. (2015) found values of Brazilian propolis’s TPC ranging from 2.4 to 5.7 mg GAE.g−1 sample, using a time variation from 5 to 45 min. Their results were lower than our (316.4–533.9 mg GAE.g−1), using a similar extraction time. Considering the presence of wax in crude propolis samples (30–40%), conventional extraction process requires a long time under low temperatures (up to 12 h) to separate the wax fraction from raw sample prior to extract phenolics compounds, as performed by some authors (Oldoni et al., 2015, Hatano et al., 2012). Oliver et al. (2014) observed how ultrasound acted on wax through microscopy and verified that the procedure enabled a redistribution of the sample wax, facilitating substrate accessibility. Due to its ability to break down and redistribute the particles, it is possible to infer that ultrasound enabled propolis waxy material mixes with the extract, avoiding the overnight step.
Fig. 1.
Response surface plots of Total Phenolic Content (a) and antioxidant capacity by ORAC method (b) of green propolis extract affected by extraction time and propolis:solvent ratio.
Considering the time extraction, Tiwari et al., 2009, Maran et al., 2013 affirm that most of the phenolic compounds are broken down in the first 20 min of extraction and after that the ultrasound process can induce the degradation of these compounds in the sample. However, as it was observed in our work, the TPC content in extracts showed a small reduction only after 40 min.
From the experimental results of the first design, a second factorial design was applied setting time at 20 min and ranging propolis-to-solvent from 1:10 to 1:30 (w/v), as can be observed in Table 2. Extraction time was kept at 20 min because expressive results of antioxidant capacity and total phenolic content was achieved, keeping the extraction time as low as possible, as we can observe on surfaces responses (Fig. 1). According to obtained results, TPC was strongly influenced by ethanol concentration in extracting solution. Data showed the positive effect of increasing ethanol concentration on both TPC and antioxidant capacity, even though using less sample amount. The analysis of variance (ANOVA) of the second design for total phenolic content (TPC), antioxidant capacity by ABTS and ORAC are presented in Table 3. Adjusted models for all dependent variables presented calculated F value higher than tabulated values and great coefficient of determination (R2), which means they are valid and suitable for predicting purposes.
Table 2.
Real values of the independent variables and observed responses to the second factorial design.
| Runa | Independent variables (real values) |
Responses |
|||
|---|---|---|---|---|---|
| Ethanol (%) | Propolis-to-solvent ratio(w/v)* | YTPC (mg GAE.g−1)b | YORAC (µmol TEAC.g−1)c | YABTS (µmol TEAC.g−1)d | |
| 1 | 14.4 | 1:24.3 | 132.9 ± 0.7 | 5003.7 ± 163.5 | 2665.5 ± 13.7 |
| 2 | 84.6 | 1:24.3 | 646.5 ± 2.0 | 4925.6 ± 109.4 | 2901.9 ± 24.0 |
| 3 | 14.4 | 1:45.6 | 125.3 ± 1.0 | 3439.1 ± 2.2 | 1155.3 ± 8.1 |
| 4 | 84.6 | 1:45.6 | 651.0 ± 4.9 | 8107.4 ± 112.0 | 4689.0 ± 32.2 |
| 5 | 0 | 1:35 | 57.9 ± 1.3 | 21.3 ± 102.9 | 408.6 ± 2.4 |
| 6 | 99 | 1:35 | 1614.8 ± 28.2 | 13244.5 ± 62.8 | 13412.1 ± 49.4 |
| 7 | 49.5 | 1:20 | 278.6 ± 0.9 | 3551.8 ± 27.8 | 1219.3 ± 40.3 |
| 8 | 49.5 | 1:50 | 212.3 ± 0.5 | 2569.1 ± 177.4 | 1439.5 ± 3.4 |
| 9 | 49.5 | 1:35 | 719.4 ± 3.8 | 7605.7 ± 152.9 | 2417.4 ± 2.4 |
| 10 | 49.5 | 1:35 | 719.0 ± 1.9 | 7650.6 ± 110.9 | 2417.4 ± 7.3 |
| 11 | 49.5 | 1:35 | 719.0 ± 3.8 | 8464.1 ± 113.4 | 2574.0 ± 43.8 |
| 12 | 49.5 | 1:35 | 718.5 ± 3.8 | 6702.4 ± 76.8 | 2260.7 ± 55.60 |
The results were expressed as mean ± standard deviation.
Tests performed in random order.
Total Phenolic Content.
Total antioxidant capacity by ORAC.
Total antioxidant capacity by ABTS.
All assays were performed with a total volume of 30 mL and the extraction time was maintained in 20 min.
Table 3.
Regression models, coefficient of determination (R2) and analysis of variance (ANOVA) (Fcal and Ftab) of the estimated regression models for TPC, ABTS and ORAC for the second factorial design.
| Reparameterized Regression Model* | R2 | Fcal | Ftab | Sum of squares | Degree of freedom | Mean square | |
|---|---|---|---|---|---|---|---|
| TPC | TPC = 719.42 + 405.52x1 + 20.86x12 − 12.10x2 − 276.35x22 + 3.02x1x1 | 89% | 49.84 | 4.39 | |||
| Regression | 1.800.374 | 5 | 1.800.374 | ||||
| Residual | 216.636 | 6 | 36.106 | ||||
| Total | 2.051.725 | 11 | |||||
| ABTS | ABTS = 1,728.68 + 2771.36x1 + 2108.40x12 | 70% | 21.62 | 4.26 | |||
| Regression | 90.615.769 | 2 | 90.615.769 | ||||
| Residual | 37.698.340 | 9 | 4.188.704 | ||||
| Total | 128.314.109 | 11 | |||||
| ORAC | ORAC = 7323.20 + 2913.03x1 − 2080.33x22 + 1186.60x1x2 | 70% | 28.00 | 4.00 | |||
| Regression | 101.894.516 | 3 | 101.894.516 | ||||
| Residual | 29.069.510 | 8 | 3.633.689 | ||||
| Total | 130.964.026 | 11 | |||||
Reparameterized models include regression coefficients statistically significant at p < 0.05.
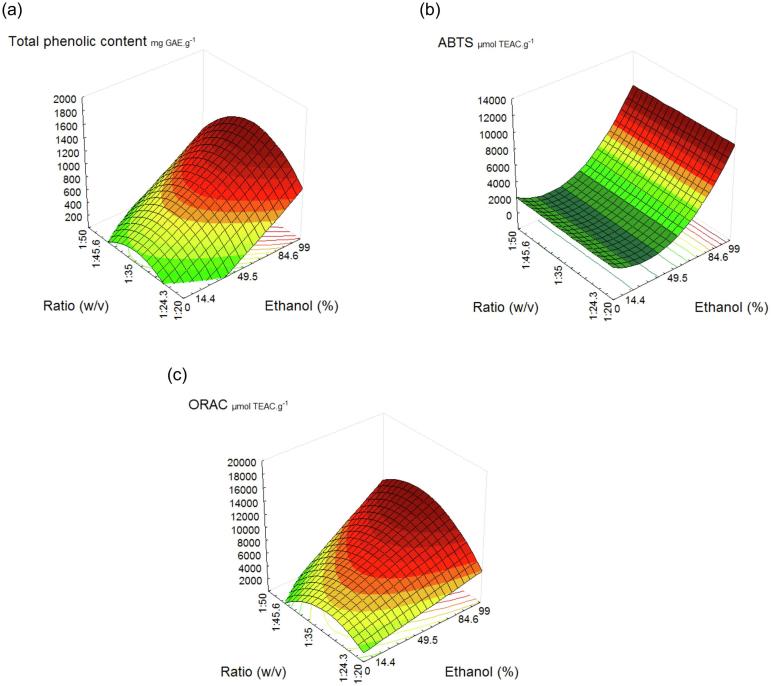
The effect of the independent variables on the TPC, ABTS, and ORAC are shown in Fig. 2. The increase in TPC and antioxidant capacity (ABTS and ORAC) was observed with an increase in the ethanol concentration in extracting solution. The total phenolic content of our work, when compared to the results of Bittencourt et al. (2015), was more than 8 times higher. It is important to note that these authors studied Brazilian propolis extracted by traditional methods, using agitation and heating. The minimum value found for the antioxidant capacity measured by ABTS method was 408.6 μmol TEAC.g−1, and the maximum value was 13412.1 μmol TEAC.g−1. Palomino, García, Gil, Rojano, and Durango (2009), analyzing the antioxidant capacity of propolis extracts from different locations prepared by maceration with 96% ethanol, obtained values ranging from 455.5 to 1091 μmol TEAC.g−1.
Fig. 2.
Response surface plots of Total Phenolic Content (a) and antioxidant capacity by ABTS (b) and ORAC (c) methods of green propolis extract affected by ethanol concentration and propolis:solvent ratio.
Optimal conditions for recovering green propolis active compounds effectively using the ultrasound-assisted extraction were obtained by using 99% of ethanol solution and 1:35 propolis:solvent ratio (w/v), over 20 min. Under these conditions it was possible to obtain extracts with high antioxidant capacity without the use of the overnight step for wax separation, indicating the application of more than one experimental design were crucial to improve yield of antioxidant recovery from green propolis.
3.1.1. Identification and quantification of phenolic compounds in green propolis extract
The identified compounds in the green propolis extracted under optimal conditions were: caffeic acid, isomers of caffeoylquinic acid, p-coumaric acid, isomers of dicaffeoylquinic acid, hesperidin, tricaffeoylquinic acid, 3-prenylcinnamic acid (drupanine), artepillin C, and baccharin. Selected compounds, p-coumaric acid and artepillin C, were subject to quantification analysis and their standards presented retention times of 14.3 and 76.1 min, respectively. The major phenolic compound in green propolis extract obtained under optimized condition was the 4-hydroxy-3,5-diprenyl (artepillin C), at concentration of 807.6 ± 57.7 mg artepillin C.g−1 of sample. Concentration of p-coumaric was 45.6 ± 0.2 mg.g–1 of sample. The formation of these two substances occurs from the same compounds: the cinnamic acid and its derivatives (Salatino et al., 2005, Teixeira et al., 2005) and it can be a justification for the concomitant increase of both compounds in selected extract samples. Castro et al. (2007) observed that artepillin C and p-coumaric represented the majority in their analyzed sample too. The difference among studies about the concentrations of these types of compounds may be due to the geographic location and harvesting season (Inoue et al., 2007, Kumazawa et al., 2004).
3.2. Application green propolis extract in oil emulsion and in vivo assay
3.2.1. Oxidation of oil emulsion
Antioxidants may act in a variety of ways on a model emulsion system, as reducing agents, which convert the hydroperoxides into stable compounds. However, the formation of hydroperoxides and secondary products during oxidation process can cause oxidative damage in the emulsion system (Carocho and Ferreira, 2013, Espinosa et al., 2015). Antioxidant effects of green propolis extract, BHA, BHT, and TBHQ are shown in Table 4.
Table 4.
Hydroperoxide content of soybean oil emulsions added of synthetic antioxidants (TBHQ, BHA, and BHT) and green propolis extract.
| Treatments | Hydroperoxides (mg/kg of oil) |
|||
|---|---|---|---|---|
| 0 day | 3 days | 6 days | 9 days | |
| Control | 7.5 ± 0.8Ab | 9.1 ± 0.72Ab | 25.7 ± 0.10ABa | 29.5 ± 0.38ABCa |
| TBHQ | 10.7 ± 0.63Aa | 11.0 ± 0.08Aa | 12.5 ± 0.01Ca | 18.7 ± 0.14Ca |
| BHA | 7.5 ± 0.11Ac | 16.4 ± 0.14Abc | 28.1 ± 0.08Aa | 33.5 ± 0.08Aa |
| BHT | 6.5 ± 0.01Ab | 11.4 ± 0.01Aa | 12.9 ± 0.07Cab | 23.5 ± 0.01ABCa |
| Green Propolis | 7.4 ± 0.10Aa | 19.4 ± 1.15Aa | 12.4 ± 0.05Ca | 16.7 ± 0.14Ca |
Different capital letters in the columns and different lowercase letters in the lines differ significantly from each other by Tukey test (p < 0.05).
There was no significantly difference among treatments from day 0 to day 3. It worth mention that at day 6, oxidation degree among TBHQ, BHT, and Green Propolis did not differ statistically from each other. However, these three mentioned treatments differed significantly from the Control and BHA treatments. On the ninth day of storage under accelerated conditions the hydroperoxide content of emulsions containing green propolis extract did not differ and presented similar results when compared to emulsions containing TBHQ, which was considered the most effective synthetic antioxidant in the study. Nevertheless, both TBHQ and Green Propolis differed significantly from the BHA treatment, which in turn had a higher hydroperoxide content than the control treatment. Ye, Wang, Duncan, Eigel, and O’Keefe (2015) found similar results in their study, verifying that antioxidant BHA exceeded the peroxide values in soybean oil when compared to control sample after a few days of induced oxidation. It can be said that some factors may have influenced results of peroxide values, such as: oxygen concentration, interfacial rheological properties and particle size (Waraho, McClements, & Decker, 2011).
3.2.2. In vivo assay with yeast cells
Although useful as preliminary tools, antioxidant capacity in vitro tests do not offer a good representation of physiological processes. Antioxidants perform a physiologically important activity and information obtained in vivo not only complements, but also are necessary to evaluate the effects and safety of these compounds.
The use of yeast Saccharomyces cerevisiae as a model microorganism to test the interaction of antioxidant activity or even the toxicity of several substances is highlighted not only by the similarity of this microorganism with higher eukaryotic organisms, but also by the ease and safety in handling, high reproducibility and low cost of analysis. Therefore, S. cerevisiae was used in this study to evaluate the interaction of the extracts with the cells by means of cell membrane analysis (Hassan, 2011). Flow cytometry is used frequently to collect quantitative information about specific cell in specific regions such as damage to DNA or membrane. This information can provide important data on cell stability when exposed to different reagents (Georgakoudi et al., 2004).
In this study flow cytometry was used to evaluate the membrane integrity of Saccharomyces cerevisiae exposed for 4 h to green propolis dried extracts at different concentrations (0, 50, 100, and 200 mg.kg−1). The percentage of cells with the intact membrane observed for the control was 98.9% ± 1.1. For the green propolis dried extracts exposed cell, values were 99.5% ± 0.5; 98.6% ± 1.0; 98.5% ± 1.2 for the concentrations of 50, 100, and 200 mg.kg−1, respectively. Green propolis dried extracts did not change the cell membrane in an expressive way, being this a good indicator of the innocuity of these extracts, since the integrity of the membrane is directly linked to cell viability and its normal functions (Grégori et al., 2001).
To assess the antioxidants effect, the cell solutions were exposed for 4 h to the same concentrations of green propolis in the presence of t-BOOH (18 mM). The percentage of cells with intact membrane were 28.2 ± 0.3; 99.6% ± 1.5; 97.6% ± 1.3, and 98.4% ± 1.1 for the concentrations of 0, 50, 100, and 200 mg.kg−1, respectively. These results indicate that green propolis compounds were also to protect plasma membrane lipids from oxidation induced by t-BOOH.
4. Conclusion
This study indicated the optimization of ultrasound-assisted extraction of antioxidant compounds from raw green propolis allowed a significant reduction in the extraction time, avoiding the overnight step normally applied for wax separation. Thus, the optimization of extraction process allowed an improvement on bioactive compounds recovery. Under optimized condition, the extracts were rich in artepillin C, which is considered the chemical marker for green propolis. Its extract showed a similar effect to TBHQ in oil-in-water emulsion during oven test, with the advantage of not being toxic to the S. cerevisiae cells. Besides this, dried extracts presented antioxidant effect in in vivo model, being a potential product to be applied in food systems.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Funding: This work was supported by São Paulo Research Foundation – FAPESP (grant 2014/18227-4, and grant 2015/50437-1) and it was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brazil – CAPES (Finance Code 001). Authors thanks the Natucentro Indústria e Apiários Centro Oeste Ltda for samples and Dr. Thalita R. Augusto-Obara (University of Sao Paulo) for her help in the lab analysis.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2019.100054.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Alencar S.M., Oldoni T.L.C., Castro M.L., Cabral I.S.E., Costa-Neto C.M., Cury J.A.…Ikegaki M. Chemical composition and biological activity of a new type of Brazilian propolis: Red propolis. Journal of Ethnopharmacology. 2007;113:278–283. doi: 10.1016/j.jep.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Andrade J.K.S., Denadai M., de Oliveira C.S., Nunes M.L., Narain N. Evaluation of bioactive compounds potential and antioxidant activity of brown, green and red propolis from Brazilian northeast region. Food Research International. 2017;101:129–138. doi: 10.1016/j.foodres.2017.08.066. [DOI] [PubMed] [Google Scholar]
- Bittencourt M.L., Ribeiro P.R., Franco R.L., Hilhorst H.W., De Castro R.D., Fernandez L.G. Metabolite profiling, antioxidant and antibacterial activities of Brazilian propolis: Use of correlation and multivariate analyses to identify potential bioactive compounds. Food Research International. 2015;76(3):449–457. doi: 10.1016/j.foodres.2015.07.008. [DOI] [PubMed] [Google Scholar]
- Branco G.F., Castro I.A. Optimization of oil oxidation by response surface methodology and the application of this model to evaluate antioxidants. Journal of the American Oil Chemists' Society. 2011;88(11):1747–1758. [Google Scholar]
- Brasil, Ministério Da Agricultura. (2001). Instrução Normativa n° 3 – ANEXO VI – Regulamento técnico para fixação de identidade e qualidade de própolis, de 19 de janeiro de 2001. Diário Oficial da República Federativa do Brasil.
- Briones-Labarca V., Plaza-Morales M., Giovagnoli-Vicuña C., Jamett F. High hydrostatic pressure and ultrasound extractions of antioxidant compounds, sulforaphane and fatty acids from Chilean papaya (Vasconcellea pubescens) seeds: Effects of extraction conditions and methods. LWT-Food Science and Technology. 2015;60(1):525–534. [Google Scholar]
- Bulduk I., Gezer B., Cengiz M. Optimization of ultrasound-assisted extraction of morphine from capsules of Papaver somniferum by response surface methodology. International Journal of Analytical Chemistry. 2015;2015 doi: 10.1155/2015/796349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carocho M., Ferreira I.C. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food and Chemical Toxicology. 2013;51:15–25. doi: 10.1016/j.fct.2012.09.021. [DOI] [PubMed] [Google Scholar]
- Castro M.L., Cury J.A., Rosalen P.L., Alencar S.M., Ikegaki M., Duarte S., Koo H. Própolis do sudeste e nordeste do Brasil: influência da sazonalidade na atividade antibacteriana e composição fenólica. Química Nova. 2007;30(7):1512–1516. [Google Scholar]
- Chemat F., Rombaut N., Sicaire A.G., Meullemiestre A., Fabiano-Tixier A.S., Abert-Vian M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrasonics Sonochemistry. 2017;34:540–560. doi: 10.1016/j.ultsonch.2016.06.035. [DOI] [PubMed] [Google Scholar]
- Chisté R.C., Mercadante A.Z., Gomes A., Fernandes E., Da Costa Lima J.L.F., Bragagnolo N. In vitro scavenging capacity of annatto seed extracts against reactive oxygen and nitrogen species. Food Chemistry. 2011;127(2):419–426. doi: 10.1016/j.foodchem.2010.12.139. [DOI] [PubMed] [Google Scholar]
- Corrales M., Toepfl S., Butz P., Knorr D., Tauscher B. Extraction of anthocyanins from grape by-products assisted by ultrasonics, high hydrostatic pressure or pulsed electric fields: A comparison. Innovative Food Science and Emerging Technologies. 2008;9(1):85–91. [Google Scholar]
- Cruz R.G., Beney L., Gervais P., Lira S.P., Vieira T.M.F.S., Dupont S. Comparison of the antioxidant property of acerola extracts with synthetic antioxidants using an in vivo method with yeasts. Food Chemistry. 2019;277:698–705. doi: 10.1016/j.foodchem.2018.10.099. [DOI] [PubMed] [Google Scholar]
- Espín J.C., Soler-Rivas C., Wichers H.J. Characterization of the total free scavenger capacity of vegetable oils and oil fractions using 2,2 diphenyl-1-picrylhydrazyl radical. Journal of Agricultural and Food Chemistry. 2000;48(3):648–656. doi: 10.1021/jf9908188. [DOI] [PubMed] [Google Scholar]
- Espinosa R.R., Inchingolo R., Alencar S.M., Rodriguez-Estrada M.T., Castro I.A. Antioxidant activity of phenolic compounds added to a functional emulsion containing omega-3 fatty acids and plant sterol esters. Food Chemistry. 2015;182:95–104. doi: 10.1016/j.foodchem.2015.02.130. [DOI] [PubMed] [Google Scholar]
- Fukumoto L.R., Mazza G. Assessing antioxidant and prooxidant activities of phenolic compounds. Journal of Agricultural and Food Chemistry. 2000;48(8):3597–3604. doi: 10.1021/jf000220w. [DOI] [PubMed] [Google Scholar]
- Georgakoudi I., Solban N., Novak J., Rice W.L., Wei X., Hasan T., Lin C.P. In vivo flow cytometry: A new method for enumerating circulating cancer cells. Cancer Research. 2004;64(15):5044–5047. doi: 10.1158/0008-5472.CAN-04-1058. [DOI] [PubMed] [Google Scholar]
- Grégori G., Citterio S., Chiani A., Labra M., Sgorbati S., Brown S., Denis M. Resolution of viable and membrane-compromised bacteria in freshwater and marine waters based on analytical flow cytometry and nucleic acid double staining. Applied and Environmental Microbiology. 2001;67(10):4662–4670. doi: 10.1128/AEM.67.10.4662-4670.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatano A., Nonaka T., Yoshino M., Mok-Ryeon A.H.N., Tazawa S., Araki Y., Kumazawa S. Antioxidant activity and phenolic constituents of red propolis from Shandong, China. Food Science and Technology Research. 2012;18(4):577–584. [Google Scholar]
- Hassan H.M.M. Antioxidant and immunostimulating activities of yeast (Saccharomyces cerevisiae) autolysates. World Applied Sciences Journal. 2011;15(8):1110–1119. [Google Scholar]
- Huang S.W., Hopia A., Schwarz K., Frankel E.N., German J.B. Antioxidant activity of a-tocopherol and trolox in different lipid substrates: Bulk oils vs oil-in-water emulsions. Journal of Agricultural and Food Chemistry. 1996;44(2):444–452. [Google Scholar]
- Inoue H.T., Sousa E.A., Orsi R.O., Funari S.C., Barreto L.M.R.C., Dib A. Produção de própolis por diferentes métodos de coleta. Archivos Latinoamericanos de Producción Animal. 2007;15(2):65–69. [Google Scholar]
- Kubiliene L., Laugaliene V., Pavilonis A., Maruska A., Majiene D., Barcauskaite K., Savickas A. Alternative preparation of propolis extracts: Comparison of their composition and biological activities. BMC Complementary and Alternative Medicine. 2015;15(1):156. doi: 10.1186/s12906-015-0677-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumazawa S., Hamasaka T., Nakayama T. Antioxidant activity of propolis of various geographic origins. Food Chemistry. 2004;84(3):329–339. [Google Scholar]
- Maran J.P., Manikandan S., Nivetha C.V., Dinesh R. Ultrasound assisted extraction of bioactive compounds from Nephelium lappaceum L. fruit peel using central composite face centered response surface design. Arabian Journal of Chemistry. 2013;10(1):S1145–S1157. [Google Scholar]
- Morelli L.L., Prado M.A. Extraction optimization for antioxidant phenolic compounds in red grape jam using ultrasound with a response surface methodology. Ultrasonics Sonochemistry. 2012;19(6):1144–1149. doi: 10.1016/j.ultsonch.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Oldoni T.L.C., Oliveira S.C., Andolfatto S., Karling M., Calegari M.A., Sado R.Y.…Lima V.A. Chemical characterization and optimization of the extraction process of bioactive compounds from propolis produced by selected bees apis mellifera. Journal of the Brazilian Chemical Society. 2015;26(10):2054–2062. [Google Scholar]
- Oliver C.M., Mawson R., Melton L.D., Dumsday G., Welch J., Sanguansri P., Augustin M.A. Sequential low and medium frequency ultrasound assists biodegradation of wheat chaff by white rot fungal enzymes. Carbohydrate Polymers. 2014;111:183–190. doi: 10.1016/j.carbpol.2014.04.028. [DOI] [PubMed] [Google Scholar]
- Palomino L.R.G., García C.M.P., Gil J.H.G., Rojano B.A., Durango R.D.L. Determination of phenolic content and evaluation of antioxidant activity of propolis from antioquia (COLOMBIA) Revista de la facultad de química farmacéutica. 2009;16(3):388–395. [Google Scholar]
- Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Rodrigues M.I., Iemma A.F. CRC Press; 2014. Experimental design and process optimization. [Google Scholar]
- Salatino A., Teixeira E.W., Negri G., Message D. Origin and chemical variation of Brazilian propolis. Evidence-Based Complementary and Alternative Medicine. 2005;2(1):33–38. doi: 10.1093/ecam/neh060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simões L.M.C., Gregório L.E., Da Silva Filho A.A., De Souza M.L., Azzolini A.E.C.S., Bastos J.K., Lucisano-Valim Y.M. Effect of Brazilian green propolis on the production of reactive oxygen species by stimulated neutrophils. Journal of Ethnopharmacology. 2004;94(1):59–65. doi: 10.1016/j.jep.2004.04.026. [DOI] [PubMed] [Google Scholar]
- Singleton V.L., Orthofer R., Lamuela-Raventos R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods in Enzymology. 1999;299:152–178. [Google Scholar]
- Soares D.G., Andreazza A.C., Salvador M. Avaliação de compostos com atividade antioxidante em células da levedura Saccharomyces cerevisiae. Revista Brasileira de Ciências Farmacêuticas. 2005;41(1):95–100. [Google Scholar]
- Stinco Carla M., Baroni M.V., Naranjo R.D.D.P., Wunderlin D.A., Heredia F.J., Meléndez-Martínez A.J., Vicario I.M. Hydrophilic antioxidant compounds in orange juice from different fruit cultivars: Composition and antioxidant activity evaluated by chemical and cellular based (Saccharomyces cerevisiae) assays. Journal of Food Composition and Analysis. 2015;37:1–10. [Google Scholar]
- Szliszka E., Kucharska A.Z., Sokol-Ietowska A., Mertas A., Czuba Z.P., Król W. Chemical composition and anti-inflammatory effect of ethanolic extract of Brazilian green propolis on activated J774A.1 macrophages. Evidence-Based Complementary and Alternative Medicine. 2013;2013:1–13. doi: 10.1155/2013/976415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira E.W., Negri G., Meira R.M., Message D., Salatino A. Plant origin of green propolis: Bee behavior, plant anatomy and chemistry. Evidence-based complementary and alternative medicine. 2005;2(1):85–92. doi: 10.1093/ecam/neh055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari B.K., Donnell C.P., Cullen P.J. Effect of sonication on retention of anthocyanins in blackberry juice. Journal of Food Engineering. 2009;93(2):166–171. [Google Scholar]
- Waraho T., McClements D.J., Decker E.A. Mechanisms of lipid oxidation in food dispersions. Trends in Food Science & Technology. 2011;22(1):3–13. [Google Scholar]
- Ye L., Wang H., Duncan S., Eigel W., O’Keefe S. Antioxidant activities of Vine Tea (Ampelopsis grossedentata) extract and its major component dihydromyricetin in soybean oil and cooked ground beef. Food Chemistry. 2015;172:416–422. doi: 10.1016/j.foodchem.2014.09.090. https://www.sciencedirect.com/science/article/pii/S0308814614014666 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.