Abstract
The recent FDA approval of the neurosteroid, brexanolone (allopregnanolone), as a treatment for women with postpartum depression, and successful trials of a related neuroactive steroid, SGE-217, for men and women with major depressive disorder offer the hope of a new era in treating mood and anxiety disorders based on the potential of neurosteroids as modulators of brain function. This review considers potential mechanisms contributing to antidepressant and anxiolytic effects of allopregnanolone and other GABAergic neurosteroids focusing on their actions as positive allosteric modulators of GABAA receptors. We also consider their roles as endogenous “stress” modulators and possible additional mechanisms contributing to their therapeutic effects. We argue that further understanding of the molecular, cellular, network and psychiatric effects of neurosteroids offers the hope of further advances in the treatment of mood and anxiety disorders.
Keywords: Allopregnanolone, Brexanolone, SGE-217, Steroid enantiomers, Tonic inhibition
1. Introduction
Psychiatric illnesses are major causes of disability and death. In the United States (US), mental illnesses account for at least one-third of all disabilities across the human life-span (Friedrich, 2017; Mokdad et al., 2004; Murray, 2013). Patients with severe psychiatric disorders also die considerably earlier than expected with causes of death including metabolic and cardiovascular illnesses, violence and suicide; in the US, the suicide rate has risen over the past 20 years and now claims more than 40,000 lives per year. Major depression and anxiety disorders are among the most common psychiatric illnesses and are leading contributors to disability and suicide (Friedrich, 2017). Current treatments, including medications, neuromodulation methods and evidence-based forms of psychotherapy, can be effective but, under the best of circumstances, up to one-third of patients fail to respond to treatment. Even among those who respond, relapses are common (Conway et al., 2017). Additionally, since the advent of the selective serotonin reuptake inhibitors (SSRIs) more than 30 years ago, pharmacological treatments have changed very little. Thus, there is substantial need to better understand the biology of depression and anxiety and to develop treatments with novel mechanisms of action. The recent FDA approvals of esketamine for treatment resistant major depression and brexanolone for postpartum depression offer new hope. Brexanolone is a cyclodextrin-based formulation of the neurosteroid, allopregnanolone (AlloP), suitable for intravenous infusion (Kanes et al., 2017; Meltzer-Brody et al., 2018). AlloP is a potent and effective positive allosteric modulator (PAM) of GABA-A receptors (GABAARs) and we will discuss its effects on these receptors as well as other possible mechanisms contributing to its antidepressant and anxiolytic effects (Majewska et al., 1986; Belelli and Lambert, 2005; Esser et al., 2006; Zorumski et al., 2013).
2. Neurosteroids, neuroactive steroids & GABAARs
The term “neurosteroid” was coined by Baulieu in the early 1980's to describe endogenous steroids that are synthesized in the central nervous system from cholesterol or sterol precursors (Baulieu, 1997). AlloP is the prototype for these neurosteroids, but is only one of multiple neurosteroids that have been identified. Subsequent work showed that both endogenous and exogenous steroids can modulate nervous system function and, to account for this broader array of molecules, Paul and Purdy (1992) proposed the term “neuroactive steroid” (NAS). In this review, we will use the terms neurosteroid and NAS interchangeably but will point out specific instances where we are describing the effects of endogenously produced neurosteroids.
Although most endogenous neurosteroids have minimal or no actions at classical nuclear hormone receptors (Rupprecht et al., 1993; Rupprecht and Holsboer, 1999), it is clear that these steroids can alter the function of multiple receptors, ion channels and intracellular targets. Thus it is unlikely that any of the endogenous steroids are completely selective for one specific target. However, synthetic NAS can be made to be highly selective for a given target. The recent successful clinical trials with brexanolone and an orally-active synthetic NAS, SGE-217 (zuranolone), both of which have potent GABAAR activity, for postpartum depression and major depression highlight the likely importance of GABAARs as a target for understanding the psychotropic effects of these agents. Hence we will focus heavily on their GABAergic actions in this review (Gunduz-Bruce et al., 2019; Kanes et al., 2017; Martinez Botella et al., 2017; Meltzer-Brody et al., 2018).
To describe the effects of neurosteroids on GABAARs, it is important to understand the complexities of these receptors, especially since not all superficially similar GABAAR modulators have antidepressant action. GABAARs are pentameric chloride channels that are activated (gated) by the neurotransmitter, GABA. To date, 19 GABAAR subunits have been identified (α1-6, β1-3, γ1-3, δ, ε, ρ1-3, π, Ɵ) and most native GABAARs express three subunits (usually with 2α, 2β and a third subunit, although receptors with only one or two subunit types have been described) (Chuang and Reddy, 2018; Johnston, 2005; Korpi and Sinkkonen, 2006; Olsen and Sieghart, 2009). Importantly, different types of GABAARs mediate different forms of inhibition in the brain. Phasic (synaptic) inhibition usually involves GABAARs that express γ2 subunits, while more persistent, tonic inhibition is mediated by extrasynaptic receptors that often, although not always, contain a δ-subunit (Belelli et al., 2009; Brickley and Mody, 2012; Farrant and Nusser, 2005; Glykys et al., 2008; Prenosil et al., 2006). Receptors mediating tonic inhibition typically differ from those mediating phasic inhibition in their higher sensitivity to GABA, slower ion channel kinetics and weaker desensitization. NAS appear to affect GABAARs promiscuously, with little selectivity for various subtypes, although, as will be discussed later, some evidence suggests preferential effects on δ-subunit expressing extrasynaptic receptors (Belelli and Lambert, 2005; Stell et al., 2003; Wohlfarth et al., 2002).
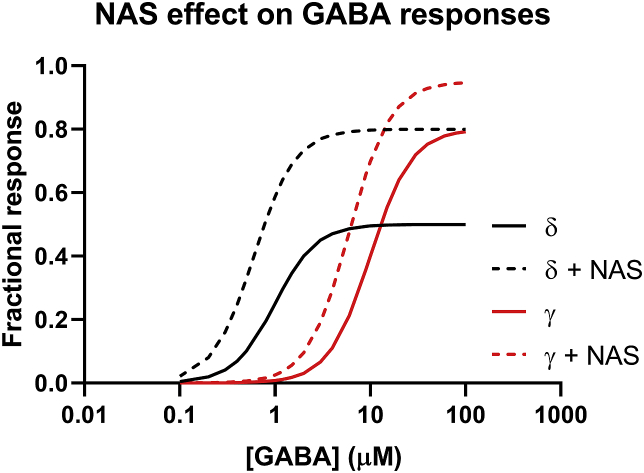
Broadly speaking, NAS have one of three effects on GABAARs. Certain NAS are GABAAR PAMs, enhancing the actions of GABA at the broad range of these receptors expressed in brain. AlloP (3α-hydroxy-5α-pregnan-20-one) is the prototype for these GABAergic PAMs, but PAM activity is shared by other 5α-reduced (planar) neurosteroids (e.g. 3α-hydroxy-5α-tetrahydro-deoxycorticosterone,THDOC) and by certain 5β-reduced (bent) NAS (e.g. SGE-217, a NAS in late stage human trials for major depression, and the endogenous neurosteroid, pregnanolone) (Majewska et al., 1986; Belelli and Lambert, 2005; Martinez Botella et al., 2017). Structurally, GABA PAMs typically have a hydrogen bond donor in the 3α-position of the steroid A-ring (a hydroxyl group in AlloP) and a hydrogen bond acceptor in the 17β-position of the steroid D-ring (a methylketone for AlloP). The PAMs have similar actions in that they enhance the effects of sub-saturating concentrations of GABA at sub-micromolar NAS concentrations (Puia et al., 1990). At somewhat higher concentrations, PAMs can directly open GABAARs in the absence of GABA (referred to as direct channel gating) via a site independent of the GABA binding site (Alvarez et al. (2019). A primary effect of these modulators appears to make GABA and other agonists more efficacious; in addition to shifting the EC50 for orthosteric agonists to lower concentrations, the maximum effect of partial agonists is enhanced (Wohlfarth et al., 2002) (Fig. 1).
Fig. 1.
Typical pharmacological effects of NAS PAMs on GABAA receptor function at the whole-cell level. NAS at a modest, fixed concentration usually shift the GABA EC50 value to the left and increase the maximum GABA response, i.e., increase agonist efficacy. Because GABA is a lower efficacy agonist on δ-containing receptors than on γ2-containing receptors, NAS may have a somewhat larger effect on δ containing receptors.
A second class of NAS act as negative allosteric modulators (NAMs) at GABAARs, and are activation-dependent, non-competitive inhibitors. Examples of NAMs include steroids that are sulfated at the 3-position of the steroid A-ring (as opposed to having a hydroxyl group in the 3α-position in NAS PAMs). Examples include pregnenolone sulfate (PREGS) and dehydroepiandrosterone sulfate (DHEAS) (Eisenman et al., 2003; Seljeset et al., 2018; Shen et al., 2000). Steroids that have a hydroxyl group in the 3-beta position are also GABAAR NAMS at sufficiently high concentrations (Wang et al., 2002). Interestingly, these NAMs can inhibit the effects of NAS PAMs and have sometimes been considered to be antagonists of NAS PAMs, although they are not selective for NAS PAMs (Wang et al., 2002). Most sulfated NAS also allosterically modulate NMDA-type glutamate receptors. Some have PAM activity, and others have NAM activity at these receptors. The NMDA receptor actions are of clinical neuropsychiatric interest, and several groups, including ours, are interested in the medicinal chemistry of sulfated NAS around NMDA receptors. The physiological (as opposed to pharmacological) significance of the sulfated neurosteroids remains unclear given discrepancies in their reported brain levels (Gibbs et al., 2006).
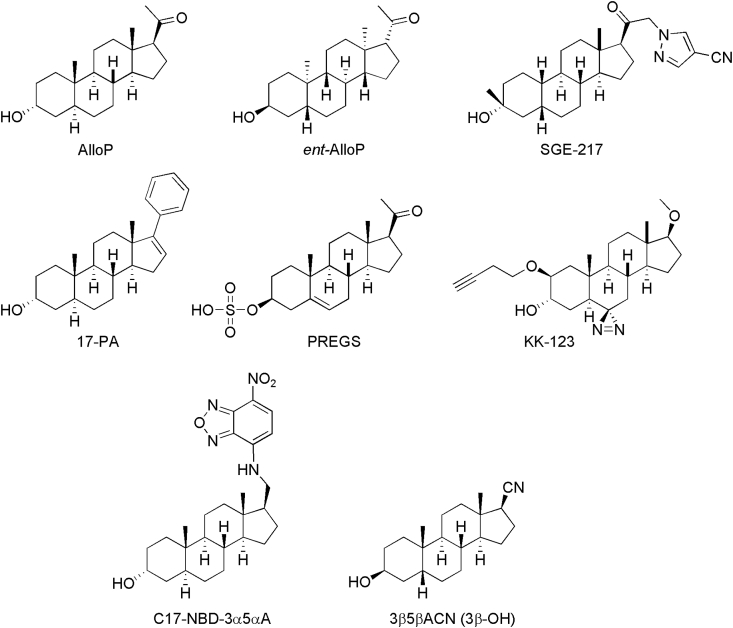
Other NAS have little or no intrinsic activity at GABAARs, yet some of these steroids can inhibit the action of NAS PAMs. Somewhat surprisingly, the ability of certain neutral allosteric ligands (NALs) to inhibit NAS PAMs shows selectivity for 5α-over 5β-reduced NAS PAMs. The reasons for this apparent selectivity remain uncertain. An example of a NAL is (3α,5α)-17-phenylandrost-16-en-3-ol (17-PA) (Mennerick et al., 2004). Fig. 2 shows structures of several of the steroids discussed in this section and elsewhere in this paper.
Fig. 2.
The figure shows structures of AlloP and several other NAS discussed in the text.
3. Effects of neurosteroids on GABAARs
The remainder of this review will focus on effects of natural and synthetic NAS PAMs, including actions on GABAARs and possible additional mechanisms that may contribute to antidepressant effects. In this section, we highlight five main points about NAS PAMs that emphasize important aspects of their actions and contribute to considerations for future drug development.
3.1. Point #1. Neurosteroids are potent, effective and broad spectrum GABAAR PAMs
AlloP and its structural analogues enhance the efficacy of GABA at GABAARs and have threshold effects at nanomolar concentrations (Majewska et al., 1986; Puia et al., 1990; Belelli and Lambert, 2005). Thus, these steroids are among the most potent GABAAR PAMs identified to date, rivaling benzodiazepines. In addition to high potency, the PAMs are highly effective modulators enhancing responses to low concentrations of GABA by 10-fold or greater, similar to barbiturates (Fig. 1). At the single channel level, NAS exert three major actions to enhance receptor function. These actions include increasing the duration of long channel open times, decreasing activation-dependent closed times within clusters of channel openings and increasing the frequency of long channel openings (Akk et al., 2004). Some NAS exhibit all of these effects while others have only one or two effects; decreases in intra-cluster closed times appear to be particularly important for the prolongation of decay of GABA-mediated synaptic currents (Chakrabarti et al., 2016).
NAS PAMs are also broad-spectrum enhancers of GABAARs, potentiating responses at both synaptic (phasic) and extrasynaptic (tonic) receptors (Herd et al., 2007). Some evidence suggests that the PAMs may have preferential effects on GABAARs that express δ-subunits and thus have major effects on tonic inhibition (Stell et al., 2003). It is clear that NAS PAMs augment tonic inhibition but whether this reflects selective effects on δ-expressing receptors is uncertain. An alternative hypothesis is that δ-expressing receptors are low efficacy channels with GABA serving as a partial agonist; the large enhancement of these tonic currents may reflect the increase in efficacy of GABA as a partial agonist at δ-containing receptors, a mechanism that may be shared by other PAMs (Feng and Forman, 2018; Shu et al., 2012). Recent studies have convincingly demonstrated that GABAARs with δ-subunits are activated by synaptically released GABA and contribute to phasic inhibition (Sun et al., 2018); thus, effects on δ-receptors may not be selective for tonic inhibition. Despite these caveats, it is clear that NAS augment both tonic and phasic inhibition in contrast to benzodiazepines, which primarily augment phasic inhibition. Attempts are underway to identify NAS that may have more selectivity for δ-expressing or other subtypes of GABAARs (Shu et al., 2012). Also, given that other GABAAR PAMs (e.g. benzodiazepines) do not appear to exhibit antidepressant benefit, a bias toward δ-containing receptors (and augmenting tonic inhibition) is an attractive explanation for the unique psychoactive profile of NAS. It is also important to consider other subtypes of GABAARs and how they might contribute to antidepressant and anxiolytic actions. Recent studies suggest that actions at GABAARs expressing α2 subunits involved in both tonic and phasic inhibition contribute to anxiolytic-like effects of NAS but not to antidepressant effects (Durkin et al., 2018). This finding echoes work showing that the anxiolytic effect of benzodiazepines primarily involves α2 -containing GABAARs (Low et al., 2000) and raises the possibility that agents with selective effects on mood or anxiety can be developed.
3.2. Point #2. High potency does not mean high affinity for GABAARs
NAS are highly lipophilic molecules with log P's (octanol/water partition coefficients) typically ranging from about 2 to 5 (Chisari et al., 2009, 2010). This means that NAS accumulate preferentially in hydrophobic regions of cells including plasma membranes. Thus, for a steroid such as AlloP (with a logP above 4), accumulation in hydrophobic regions can lead to concentrations that are 10,000-fold higher than in aqueous environments. Studies examining NAS with logPs that differ over the range of 2 to >4 demonstrated that potency for potentiating GABAARs correlates well with logP, with the highest potency (lowest EC50) being observed with the most lipophilic of the steroids (in the case of this study, AlloP > THDOC > alphaxalone > alphadolone (Chisari et al., 2009, 2010).
Given the high lipophilicity of NAS, it is important to consider whether high aqueous potency reflects high receptor affinity. Unfortunately, there are no acceptable binding assays for these steroids to test this possibility. As an alternative, Shu et al. (2004) took advantage of the fact that cyclodextrins can be used as “molecular sponges” to extract NAS from membranes and cells. Using a NAS PAM with a fluorescent NBD group at the C-11 steroid position, these studies showed that the prolonged decay of currents directly gated by the steroid was unlikely to result from high affinity drug-receptor interactions but rather reflected the high intramembranous concentration of the NAS. Similarly, the slow decay of intracellular fluorescence produced by NAS accumulation was also greatly speeded by cyclodextrin and the decay of GABAAR currents and cellular fluorescence were closely correlated. Similar results were obtained examining potentiation of GABA-gated currents by NAS (Chisari et al., 2010; Shu et al., 2007). These and related studies also provided evidence that NAS access their site(s) of action on GABAARs via the plasma membrane (Akk et al., 2005, 2007) and that the ability of NAS to accumulate intracellularly can lead to situations where intracellular pools are either a sink or a source for steroid acting at intramembranous sites on receptors (Jiang et al., 2016; Li et al., 2007).
3.3. Point #3. GABAAR pharmacophore considerations are important
While NAS hydrophobicity is important for optimizing the potency and likely efficacy of NAS on GABAARs, it is also important to consider how NAS interact with their sites of action on receptors. The most direct support for this issue comes from studies using NAS enantiomers. Dating to studies in the late 1990's, it has been known that the effects of NAS PAMs on GABAARs are enantioselective, with selectivity being greater for 5α-reduced NAS compared to 5β-reduced steroids (Covey et al., 2000, 2001; Wittmer et al., 1996) (Fig. 2). Studies of enantiomeric steroids are important because enantiomers (unlike stereoisomers) have identical physico-chemical properties, including logP, and differ only in their rotation of polarized light (Alakoskela et al., 2007). Studies of NAS enantiomers provided the first strong evidence that NAS interact with chiral (likely protein) sites rather than exerting effects by changes in membrane properties, even though 5α-reduced steroids increase the liquid disordered state of membranes resulting in higher lateral membrane mobility (Balleza et al., 2015; Sacchi et al., 2015). Using fluorescent enantiomeric steroids, Chisari et al. (2009) found that only NAS with the natural configuration of natural neurosteroids were able to potentiate GABA currents while both enantiomers accumulated identically in cell membranes and intracellular compartments.
Detailed structure-activity studies have determined the basis for the enantioselectivity of NAS, with the size and position of the functional group on the D-ring being a critical determinant (Katona et al., 2008). This structural information made possible the preparation of enantiomeric steroid PAMs with high activity at GABAARs (Krishnan et al., 2012; Qian et al., 2014). It is unlikely that these active enantiomeric steroids are metabolized in the same way as endogeneous NAS and this could lead to enantiomeric NAS with longer in vivo half-lives and less interference with endogenous steroid biotransformations. A longer duration of anticonvulsant activity has been found with the active enantiomers of androsterone and etiocholanolone (Zolkowska et al., 2014).
Enantiomers may be attractive tool compounds to explore the importance of GABAARs in the psychoactive effects of NAS. Some of the biological effects of NAS-like compounds do not show strong enantioselectivity, including some anti-inflammatory and neuroprotective effects of NAS PAMs (Langmade et al., 2006), and the NAM effects on GABAARs of sulfated NAS (Nilsson et al., 1998). If GABA inactive enantiomers retain effects on circuits and behaviors indicative of antidepressant actions, the role of GABAARs in clinical benefit would be seriously questioned.
3.4. Point #4. NAS interact with multiple sites in hydrophobic regions of GABAARs
While studies using enantiomers provided support for the idea that NAS act directly on GABAARs, more definitive evidence for direct interactions came from studies of Hosie et al., 2006, Hosie et al., 2008 using site-directed receptor mutagenesis. These investigators found that NAS interact with multiple amino acid residues in α1 subunits located in transmembrane (TM) spanning regions 1 and 4. In TM1 key loci included glutamine-241 (Q241) and tryptophan-245 (W245), while key sites in TM4 included asparagine-407 (N407) and tyrosine-410 (Y410). These sites contribute to the ability of NAS to enhance the function of GABAARs. This work also raised the possibility that direct gating of GABA channels by NAS may involve a separate site involving threonine-236 (T236) in α1 subunits and tyrosine-284 (Y284) in β2 subunits. Subsequent studies using NAS photoaffinity labels in β3 homopentamers and in α1β3 pentamers identified phenylalanine-301 (F301) in TM3 as a target for NAS interactions (Chen et al., 2012b, 2019).
Advances in structural biology provided further insights and clarifications into how NAS PAMs interact with GABAARs. Based on elegant cryo-electron microscopic and x-ray crystallographic structures of chimeric GABAARs, it appears that NAS promote potentiation and gating via a single interfacial site that involves TM1, TM3 and TM4. In this model, the steroid 3α-hydroxyl group appears to interact with Q241 in TM1 along with W245, consistent with hydrophobic stacking of steroid rings. The hydrogen bond acceptor on the steroid D-ring interacts with TM3 at a site analogous to F301 (Laverty et al., 2017). Other structural evidence indicates that the NAS binding site is coupled to helices that form the channel pore and modulate the receptor conformation underlying receptor desensitization (Miller et al., 2017).
Recent studies using novel NAS photoaffinity labels and click chemistry that allow bio-orthogonal protein labeling provide evidence of even further complexity in how NAS interact with GABAARs (e.g. KK-123, Fig. 2). Using α1β3 pentamers, these studies indicate that NAS interact with at least 7 sites in 3 clusters, representing both intersubunit and intrasubunit interactions (Chen et al., 2019). This study provides support for intrasubunit interactions between TM4 and TM1 of α1 subunits including homologous sites for N407 and Y410 outlined above, as well as intersubunit interactions between Q241 in TM1 of α1 and F301 in the TM3 region of β3. In addition, intrasubunit sites within the TM3 and TM4 regions of β3 were also identified; these latter novel residues are located toward the extracellular portion of β3. Based on mutagenesis experiments, it appears that all of these sites contribute to the effects of NAS on GABAARs with the α1 intrasubunit and β3-α1 intrasubunit sites being most critical for NAS activity; furthermore, it does not appear that there are separate sites for receptor potentiation and direct gating. At present, the roles of the intrasubunit sites on β3 are the least understood. See Alvarez et al. (2019) for an overview of sites contributing to NAS effects.
3.5. Point #5. NAS accumulate intracellularly and interact with Golgi and specific intracellular proteins
Studies using fluorescent NAS and photoaffinity labels highlight the fact that these molecules also readily accumulate in intracellular compartments. In particular, studies using a clickable photoaffinity label (e.g. KK-123, Fig. 2) indicate preferential labeling of Golgi, although specific protein targets have not yet been identified. Interestingly, intracellular accumulation and Golgi labeling are not enantioselective, and prior studies have indicated that certain actions of NAS including effects on inflammatory responses and perhaps neuronal survival, unlike GABAAR effects, are also not enantioselective (Jiang et al., 2016; Langmade et al., 2006). Furthermore, a similar pattern of cellular accumulation in Golgi is evident regardless of whether the cells are alive or dead at the time of labeling. Thus, the role of active transport mechanism in the profile of accumulation remains unclear.
Photoaffinity labeling studies have begun to identify several potential intracellular NAS targets. These include labeling of voltage-dependent anion channel-1 (VDAC-1), a key mitochondrial protein involved in regulating the mitochondrial permeability transition pore (mPTP), at glutamate-73 (Darbondi-Tonkabon et al., 2003); this particular residue also interacts with cholesterol (Budelier et al., 2017). NAS PALs also label β-tubulin, a microtubule protein, at cysteine-354. This latter site is the locus that binds colchicine (Chen et al., 2012a). The function of these intracellular sites for NAS remains uncertain.
4. How do NAS act as antidepressants and anxiolytics?
It is safe to say that we presently don't understand exactly how AlloP and other NAS PAMs act as antidepressants. Our discussion to this point has emphasized prominent GABAAR effects that are likely to contribute to psychotropic actions. However, other GABA enhancing drugs (e.g. benzodiazepines) do not appear to be effective antidepressants, so NAS seem to be unique among GABAAR PAMs. This suggests that NAS may have additional (or alternative) targets that are important for antidepressant effects. However, as highlighted above, NAS, in contrast to benzodiazepines, augment GABA via multiple GABAARs. Some evidence also suggests that changes in endogenous NAS levels could participate in the etiology of some depressive illnesses. In this section we will discuss aspects of endogenous neurosteroid actions within brain as a framework for understanding recent clinical findings.
4.1. Lessons from rodent models
Neurosteroids including AlloP are synthesized endogenously in brain. Several cell types including astrocytes and neurons exhibit neurosteroid synthesis in response to various stimuli (Rupprecht and Holsboer, 1999). Importantly, in the presence of stressors, neurons acutely increase production of 5α-reduced neurosteroids, and this neurosteroidogensis is observed primarily within excitatory neurons (Agis-Balboa et al., 2006; Saalman et al., 2007; Tokuda et al., 2010, 2011) (Fig. 3A). Both behavioral and metabolic/cellular stressors greatly enhance neurosteroid production. This effect was shown initially and dramatically by Purdy et al. (1991) using a forced swim behavioral task. AlloP levels in brain increased within minutes of the stress and remained elevated for over 2 h, exceeding levels that were observed in the periphery. Other behavioral stresses including foot shock have yielded similar results (Barbaccia et al., 1997). Studies over the past decade have shown that cellular and metabolic stressors also acutely enhance local production of AlloP in CA1 hippocampal pyramidal neurons. These stressors include exposure to high ethanol concentrations (Izumi et al., 2007; Tokuda et al., 2011), acetaldehyde (Tokuda et al., 2013), ammonia (Izumi et al., 2013) and corticosterone (Izumi et al., 2015). Similarly, there is evidence that high pressure stress results in acute AlloP production in retinal ganglion cells where it plays a neuroprotective role (Ishikawa et al., 2014). Interestingly, the various activating stressors result in stimulation of N-methyl-D-aspartate glutamate receptors (NMDARs), and in cases where this has been studied, the increases in neurosteroids can be blocked by NMDAR antagonists administered during the period of stressor exposure (Guarneri et al., 1998; Ishikawa et al., 2014; Kimoto et al., 2001; Tokuda et al., 2011; Zorumski et al., 2013). Also low concentrations of NMDA itself (1–10 μM) can promote neurosteroid synthesis in CA1 pyramidal neurons (Tokuda et al., 2011). Taken together, these studies strongly suggest that AlloP-type neurosteroids can be viewed as acute, “on-demand” stress modulators (Gunn et al., 2011). How cellular stress activates AlloP synthesis is not entirely clear, although there is evidence that under toxic conditions activation of stress responses in endoplasmic reticulum (ER) promotes movement of cholesterol from ER to mitochondria where cholesterol undergoes side chain cleavage to pregnenolone, the first and critical step leading to neurosteroid (AlloP) synthesis (Barbero-Camps et al., 2014).
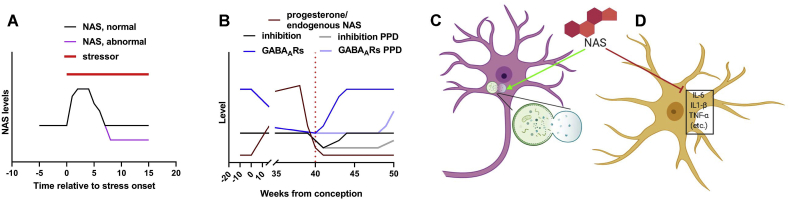
Fig. 3.
Possible endogenous and exogenous NAS roles in depression-like syndromes. A, B. Endogenous NAS levels change as a result of stress and postpartum hormonal changes. In stress (panel A), acute stressors increase NAS levels, but more chronic stress may lead to a state of NAS deficiency, contributing to symptoms. B. In pregnancy, NAS levels rise, and GABA receptor expression decreases, yielding stable inhibition. Following the sudden loss of NAS at parturition (dotted red line), recovery of GABA receptor expression lags, creating a hyperexcitable state that may persist and participate in symptomology of postpartum depression. C, D. Two intracellular pathways through which NAS may have underappreciated beneficial effects: by activating autophagy (panel C) and by inhibiting neuroinflammation (panel D). See text for details and citations. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
From the perspective of major depression and other psychiatric illnesses it is important to consider what happens with more prolonged stress. This issue has been studied in most detail in models of chronic behavioral stress (Locci and Pinna, 2017; Pinna, 2019). Although levels of AlloP increase acutely with behavioral stress, over several weeks of ongoing social isolation, rodents exhibit a variety of stress-like behaviors including changes in response to contextual fear tasks and the ability to extinguish fear responses (Evans et al., 2012; Pinna et al., 2003; Pibiri et al., 2008). These changes correlate with decreases in the levels of AlloP and its precursor 5α-dihydroprogesterone (DHP) with no changes observed in levels of pregnenolone or progesterone (Dong et al., 2001). Changes in neurosteroid levels are accompanied by decreases in brain transcription and protein expression of 5α-reductase (5AR), a key enzyme in neurosteroid synthesis. Another enzyme in steroid synthesis, 3α-hydroxysteroid reductase, shows no change (Dong et al., 2001; Agis-Balboa et al., 2007). Treatment with agents that inhibit 5AR mimic the effects of chronic stress on AlloP levels and behavior, and treatments that enhance AlloP levels in chronically stressed animals can reverse behavioral changes (Pibiri et al., 2008). Based on these results, it appears that, unlike acute stress, chronic stress results in a state of relative AlloP deficiency and/or synthetic capability that contributes to changes in depressive- and anxiety-like behaviors (Fig. 3A). Thus, NAS may be more appropriately conceptualized as modulators of brain stress states than as strict antidepressants.
4.2. Are changes in neurosteroid levels in rodent stress models relevant to human major depression?
Evidence gathered over the past 20 years examining changes in neurosteroids in major depression are suggestive, but not definitive. Multiple studies have documented decreases in the levels of AlloP in cerebrospinal fluid (CSF) and blood in patients with major depression (Girdler and Klatzkin, 2007; Romeo et al., 1998; Schule et al., 2011; Strohle et al., 1999; Uzunova et al., 1998). In some of these studies, the levels of the neurosteroids increase following successful treatment of depression and these increases correlate with clinical improvement (Uzunova et al., 1998). There is also tentative evidence for changes in the expression of key enzymes in neurosteroid synthetic pathways. Consistent with a decrease in levels of AlloP in depression, Agis-Balboa et al. (2014) observed about 50% decrease in the expression of 5AR-type I in pyramidal neurons of prefrontal cortex in a small sample of postmortem brains from patients with major depression. Interestingly, patients with post-traumatic stress disorder (PTSD), a disorder that is highly comorbid with major depression, also have deficits in AlloP levels. Based on the ratio of AlloP to its precursor, 5α-dihydroprogesterone, it appears that men and women differ in which neurosteroid synthetic enzymes are altered. Men exhibit defects consistent with changes in 5AR and women demonstrate abnormalities in 3α-hydroxysteroid dehydrogenase (Pineles et al., 2018; Rasmusson et al., 2019).
4.3. Is postpartum depression a unique case?
Postpartum depression is an important subtype of depression that has long been thought to involve changes in neurosteroids (Maguire, 2019; Maguire and Mody, 2008; Mody, 2019; Rincon-Cortes et al., 2019). While milder, transient changes in mood are seen in many women following pregnancy, PPD is a serious and persistent disorder affecting up to 15% of women in the perinatal period, with timing of onset extending from the third trimester of pregnancy to 6 months or so following pregnancy (Pawluski et al., 2017; Stewart and Vigod, 2016). During pregnancy, levels of steroid hormones, including neurosteroids such as AlloP increase dramatically and the levels of these steroids rapidly decline following birth of the baby (Fig. 3B). Rodent models have provided strong support for the idea that the increased levels of AlloP during pregnancy likely contribute to changes in the expression of GABAAR subunits, particularly δ-subunits that may contribute most to tonic inhibition (Mody, 2019). When the levels of AlloP plummet in the postpartum period there appears to be a sudden imbalance in the ratio of excitation to inhibition (E/I imbalance) (Fig. 3B, black line), and these changes contribute to a hyperexcitable state (Isaacson and Scanziani, 2011) that can be manifest as stress-like and depressive-like behaviors. These behaviors can be corrected by agents that enhance tonic inhibition including NAS (Maguire and Mody, 2008; Melon et al., 2018). Interestingly, one model for postpartum psychiatric illness involves knockout/knockdown of expression of δ-subunits. These female mice show normal behavior in the absence of pregnancy, but exhibit marked behavioral changes in the postpartum period, including stress-like behaviors and poor infant care (with infanticide) (Maguire, 2019; Maguire and Mody, 2008). Agents that increase tonic inhibition, including NAS, can reverse the behavioral changes (Maguire, 2019).
The rapid and apparently sustained improvement of women with PPD following intravenous infusions of brexanolone are consistent with the hypothesis that directly replenishing AlloP is highly effective in treating this disorder (Kanes et al., 2017; Meltzer-Brody et al., 2018). However, there is also accumulating evidence that another NAS, SGE-217, is effective in treating both women and men with major depression, particularly women outside the postpartum period, suggesting that NAS may be effective across a broader range of psychiatric illnesses (Gunduz-Bruce et al., 2019). It is also notable that in the clinical trials done to date NAS exhibit beneficial effects on both depression and anxiety symptoms.
4.4. How do changes in neurosteroid levels influence the effects of exogenous NAS?
Studies in rodent chronic stress models and in humans suggest that major depression may represent a set of disorders that involve states of relative neurosteroid deficiency (Fig. 3A). Such conditions could contribute to changes in input-output relationships and network activity that have been described in hippocampus and other neural circuits in depression-like conditions (Airan et al., 2007; Small et al., 2011; Duman et al., 2019; Popoli et al., 2012). If this hypothesis is correct, then it becomes important to consider the prevailing “neurosteroid state” in understanding how exogenously administered NAS affect neurons and circuits (Nin et al., 2008). Under such a model, defects in 5AR and neurosteroid synthesis would result in defects in the ability to mount acute neurosteroid responses to stressors. States of neurosteroid deficiency could in turn influence the effects that an exogenous steroid exerts on local activity and network function. In the case of AlloP deficiency, an exogenously applied steroid would encounter neurons with low levels of intracellular endogenous steroids; in such a case, the intracellular pool of steroids would be low and the intracellular compartment could serve as a “sink” for exogenous NAS (Jiang et al., 2016; Li et al., 2007). Under conditions of active neurosteroid synthesis, exogenous NAS would encounter neurons in which the intracellular pool of steroids enhances access to membranous receptors, under the assumption that the intracellular pool has capacity limits.
In prior studies, we have encountered conditions in hippocampal neurons that provide tentative support for the intracellular pool hypothesis (Jiang et al., 2016; Li et al., 2007). For example, young postnatal neurons grown in culture have limited capacity to synthesize neurosteroids. In these neurons, exogenously applied NAS readily access intracellular compartments, resulting in a dampening of NAS effects on inhibitory responses evoked by activation of GABAARs at the neuronal soma (Jiang et al., 2016). When CA1 hippocampal neurons are more mature (e.g. postnatal day 30 in hippocampal slices), we found that neurosteroids may contribute to forms of inhibition of spike firing activated by stimulation proximal to pyramidal neuron cell bodies (Izumi et al., 2007; Tokuda et al., 2010). Surprisingly, we found that inhibitors of neurosteroid synthesis (5AR inhibitors) or function (the NAS NAL, 17-PA) dampen this form of proximal inhibition (Izumi et al., 2007; Tokuda et al., 2010), while treatments that increase neurosteroid staining enhance this form of spike inhibition (Tokuda et al., 2010). Using an antibody against 5α-reduced neurosteroids, we observed that pyramidal neurons of juvenile rodents show variable staining under baseline conditions and that the level of staining is increased by neuronal stressors (Tokuda et al., 2011; Izumi et al., 2015; Zorumski and Izumi, 2012).
5. Beyond GABA: other possible contributors to the effects of NAS
Increasing evidence indicates that changes in GABA activity in the brain are likely to be important in the biology of major depression (Duman et al., 2019; Luscher et al., 2011; Mohler, 2012; Luscher and Mohler, 2019). Thus, the ability of NAS PAMs to broadly enhance both tonic and phasic inhibition mediated by GABAARs forms a leading hypothesis for mechanisms underlying psychotropic actions of these agents. Robust PAM activity provides a way to enhance tonic and phasic inhibition in key neural circuits that contribute to depression-like behavior (Airan et al., 2007; Duman et al., 2019). NAS differ from benzodiazepines, which require certain receptor subunit compositions for effective modulation (Rudolph and Mohler, 2006), but it is less clear how NAS differ from other allosteric GABA modulators that have broad actions, including barbiturates and certain anesthetics. Hence, there is interest in understanding whether alternative or additional NAS targets may contribute to antidepressant and other psychotropic actions.
In addition to direct effects on GABAARs, NAS can promote the expression and trafficking of GABAAR subunits likely via phosphorylation of specific subunits by protein kinase C and perhaps other kinases (Abramian et al., 2014). Mechanisms underlying the ability of NAS to promote kinase activation and receptor trafficking are not entirely clear, but changes in GABAAR subunit expression can result in prolonged increases in tonic inhibition in dentate gyrus. Interestingly, not all NAS share this ability to enhance tonic inhibition via metabotropic changes in subunit expression and trafficking; enhanced subunit expression is observed with AlloP and THDOC but not with the synthetic NAS, ganaxolone (Modgil et al., 2017). Changes in GABAAR expression provide a mechanism by which NAS can have lasting effects on brain function that persist beyond the period of drug exposure. An intriguing target that could contribute to NAS-mediated changes in subunit phosphorylation and expression are membrane progesterone receptors, a class of G-protein coupled receptors that are expressed in brain and activated by AlloP (Thomas and Pang, 2012).
AlloP and other NAS PAMs also alter the function of ion channels other than GABAARs. In particular, certain of these agents inhibit low voltage activated (LVA) calcium channels (T-type channels). AlloP is a partial inhibitor of T-channels and acts on T-channels at concentrations that overlap with those needed for effects on GABAARs, with an EC50 of 0.9 μM for T-channels expressed in rodent dorsal root ganglion neurons. Effects on T-channels, like effects on GABAARs, are enantioselective with the unnatural enantiomer being an order of magnitude less potent than AlloP (Pathirathna et al., 2005). Importantly, T-channels contribute significantly to burst firing in many regions of the brain and increases in burst firing in lateral habenula appear to contribute to depressive-like behaviors in rodent models; inhibiting these channels has antidepressant-like effects (Yang et al., 2018). AlloP and another NAS that has prominent effects on T-channels but no effects on GABAARs (e.g. (3β,5β,17β)-3-hydroxy-androstane-17-carbonitrile (3β5βACN), also called 3β-OH or B260 in other publications, Fig. 2) (Todorovic et al., 2004) inhibit burst firing in pyramidal neurons in the subiculum at concentrations that are likely relevant to clinical use (Joksimovic et al., 2019). Thus, effects on T-channels and burst firing could be important for some clinical effects of NAS.
Earlier we described intracellular accumulation of exogenously applied NAS including prominent interactions with Golgi. It is thus possible that intracellular targets could contribute to therapeutic effects (Fig. 3C and D). Importantly, AlloP-type NAS do not have prominent actions on classical nuclear steroid hormone receptors although it is possible that metabolism to other steroids (e.g., 5α-dihydroprogesterone) could result in progesterone-like actions (Rupprecht and Holsboer, 1999, Rupprecht et al., 1993). The most certain intracellular NAS targets identified to date, based on photoaffinity labeling, are VDAC-1 (Darbandi-Tonkabon et al., 2003) and β-tubulin (Chen et al., 2012a,b); specific protein targets in Golgi remain unknown. VDAC is an important mitochondrial protein that helps to regulate the mitochondrial permeability transition pore (mPTP) that is important in regulating movement of molecules across the external mitochondrial membrane and also participating in intrinsic cell death pathways. There is evidence that AlloP and perhaps other NAS can inhibit mPTP as a possible mechanism contributing to neuroprotection (Sayeed et al., 2009), although AlloP does not alter voltage-dependent gating of VDAC-1 (Cheng et al., 2019). A NAS photoaffinity label interacts with a specific amino acid in VDAC, glutamate-73 (Darbandi-Tonkabon et al., 2003), but it is presently unknown whether this interaction contributes to changes in mPTP or other effects on cellular function. Glu-73 along with threonine-83 and three other sites are also labeled by cholesterol photoaffinity labels, suggesting a more general regulatory mechanism for steroids on VDAC (Budelier et al., 2017; Cheng et al., 2019); other studies indicate that there is overlap in sites for NAS and cholesterol in other proteins (Budelier et al., 2019).
Certain psychiatric illnesses including depression are thought to involve altered mitochondrial function and energy metabolism (Manji et al., 2012). NAS can promote cellular energy production via effects on glycolytic and respiration-mediated energy metabolism, with AlloP having its most prominent effects on respiration (Grimm et al., 2014). Perhaps related to cellular energy metabolism, AlloP has been reported to promote autophagy as a mechanism to dampen cellular stress (Liao et al., 2009; Kim et al., 2012). Autophagy involves cellular machinery that degrades and recycles cellular components to maintain energy in response to stressful conditions (Galluzzi et al., 2016) (Fig. 3C).
NAS also have anti-inflammatory effects that could contribute to their therapeutic actions (Langmade et al., 2006; Noorbakhsh et al., 2014; VanLandingham et al., 2006) (Fig. 3D). Recent evidence indicates that effects on inflammation may occur via toll-like receptor-4 (Balan et al., 2019; Murugan et al., 2019), a receptor that is important in regulating the function of monocytes and microglia. Additionally, NAS photolabel the microtubule protein, β-tubulin, at cysteine-354, the same amino acid at which colchicine acts (Chen et al., 2012a,b). Interestingly, colchicine has anti-inflammatory actions but it is unknown whether NAS interactions with β-tubulin contribute to anti-inflammatory effects. Other studies indicate that certain NAS can alter the function of microtubules and that these effects contribute to antidepressant-like effects in rodent models (Bianchi and Baulieu, 2012).
AlloP and related NAS also increase the expression of certain nuclear receptors including pregnane xenobiotic receptors (PXR) and the liver xenobiotic receptors (LXR) (Frye et al., 2012; Irwin et al., 2014), and AlloP and other steroids are direct LXR agonists (Fan et al., 2013; Lamba et al., 2004; Langmade et al., 2006). Effects on these receptors could provide mechanisms by which NAS modulate synthesis of other intracellular modulators, including steroids, and regulate endoplasmic reticulum cellular stress mechanisms (Rong et al., 2013). These effects may contribute to anti-inflammatory and neuroprotective actions (Langmade et al., 2006).
Finally, there is evidence that several types of clinically-used antidepressants promote neurogenesis in the dentate gyrus of adult rodents (Santarelli et al., 2003). These new neurons intercalate into the circuitry of the dentate gyrus and help to dampen production of corticosterone and other stress modulators (Snyder et al., 2011; Surget et al., 2011); the new dentate neurons also help to correct stress-related behaviors and changes in hippocampal signaling in chronic stress models (Airan et al., 2007). AlloP also enhances dentate neurogenesis Irwin et al. (2012), 2014; Wang et al. (2010), an effect that could contribute to antidepressant actions as well as neuro-restorative effects (Charalampopoulos et al., 2008). Mechanisms contributing to NAS-induced neurogenesis are not certain, although effects on GABAARs could help the survival and function of these neurons, particularly during phases of development where GABA functions as an excitatory modulator of neurons. In this light it is interesting that certain antidepressants, including fluoxetine, can increase AlloP production (Pibiri et al., 2008).
6. Summary & future considerations
Psychiatry is entering an exciting era as evidenced by the advent of the first novel pharmacological treatments for major depression to arrive on the scene in more than 30 years. These novel treatments, including NAS, offer hope for patients with severe, complex and refractory psychiatric syndromes. In this review we have focused on unique aspects of NAS highlighting both their role as endogenous stress modulators and their prospects for new directions in therapeutics. NAS clearly target major signaling systems in brain as exemplified by their prominent actions on GABAARs. Yet, effects on other ion channels such as T-type calcium channels and intracellular actions must continue to be studied to determine their possible roles in psychopharmacology. It is possible that novel synthetic NAS can be developed that exhibit more selective actions than AlloP, and these agents could lead to alternative forms of treatment. It is also possible that NAS with activities at multiple receptors, for example combined GABA PAM and NMDA NAM activity, can be developed that might have even greater therapeutic efficacy taking advantage of both the NAS and ketamine advances (Mennerick et al., 2001; Roth et al., 2004).
Important considerations for NAS going forward include side effects, abuse potential and interactions with other medications and drugs of abuse. In clinical studies to date, brexanolone and SGE-217 have been well tolerated with sedation and dizziness being the most prominent side effects (Kanes et al., 2017; Meltzer-Brody et al., 2018; Gunduz-Bruce et al., 2019). These drugs have also been administered to patients taking traditional antidepressants but potential interactions with other medications remain uncertain. The abuse potential of NAS also remains uncertain, but needs to be examined given the GABAergic nature of the PAMs.
We have focused this review on possible mechanisms contributing to antidepressant effects of NAS PAMs. At this point, these agents are being considered for use in other disorders, including insomnia, anxiety disorders and essential tremor. Recent studies of psychiatric illnesses highlight the complex and overlapping genetics among these syndromes (Brainstorm Consortium, 2018; Smoller et al., 2019), and the possibility that the different syndromes share a common underlying psychopathology factor (Caspi and Moffitt, 2018) and share neurocircuitry dysfunction across diagnoses (Etkin, 2019). This symptomatic complexity and genetic and circuitry overlap suggest that powerful new treatments like NAS may not prove to be illness specific, but rather may be effective across a range of psychiatric disorders and possibly extend to primary neurological disorders such as epilepsy and neurodegenerative disorders.
Declaration of competing interest
CZ and SP are members of the Scientific Advisory Board of Sage Therapeutics and CZ, SP and DC have stock in Sage Therapeutics. Sage Therapeutics was not involved in writing or review of this manuscript.
Acknowledgments
Work in the authors' labs is supported by MH101874 (CZ and SM), MH114866 (CZ), MH110550 (DC), MH101874, AA026753 (SM), Sage Therapeutics (SM), the Taylor Family Institute for Innovative Psychiatric Research (CZ, SP, DC, SM) and the Bantly Foundation (CZ).
References
- Abramian A.M., Comenencia-Ortiz E., Modgil A., Vien T.N., Nakamura Y., Moore Y.E., Maguire J.L., Terunuma M., Davies P.A., Moss S.J. Neurosteroids promote phosphorylation and membrane insertion of extrasynaptic GABAA receptors. Proc. Natl. Acad. Sci. (USA) 2014;111:7132–7137. doi: 10.1073/pnas.1403285111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agis-Balboa R.C., Guidotti A., Pinna G. 5α-reductase type I expression is downregulated in the prefrontal cortex/Brodmann's area 9 (BA9) of depressed patients. Psychopharmacology. 2014;231:3569–3580. doi: 10.1007/s00213-014-3567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agis-Balboa R.C., Pinna G., Pibiri F., Kadriu B., Costa E., Guidotti A. Down-regulation of neurosteroid biosynthesis in corticolimbic circuits mediates social isolation-induced behavior in mice. Proc. Natl. Acad. Sci. (USA) 2007;104:18736–18741. doi: 10.1073/pnas.0709419104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agis-Balboa R.C., Pinna G., Zhubi A., Maloku E., Veldic M., Costa E., Guidotti A. Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proc. Natl. Acad. Sci. (USA) 2006;103:14602–14607. doi: 10.1073/pnas.0606544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airan R.D., Meltzer L.A., Roy M., Gong Y., Chen H., Deisseroth K. High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science. 2007;317:819–823. doi: 10.1126/science.1144400. [DOI] [PubMed] [Google Scholar]
- Akk G., Bracamontes J.R., Covey D.F., Evers A., Dao T., Steinbach J.H. Neuroactive steroids have multiple actions to potentiate GABAA receptors. J. Physiol. (London) 2004;558:59–74. doi: 10.1113/jphysiol.2004.066571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G., Covey D.F., Evers A.S., Steinbach J.H., Zorumski C.F., Mennerick S. Mechanisms of neurosteroid interactions with GABAA receptors. Pharmacol. Ther. 2007;116:35–57. doi: 10.1016/j.pharmthera.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akk G., Shu H.-J., Wang C., Steinbach J.H., Zorumski C.F., Covey D.F., Mennerick S. Neurosteroid access to the GABA-A receptor. J. Neurosci. 2005;25:11606–11613. doi: 10.1523/JNEUROSCI.4173-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alakoskela J.M., Covey D.F., Kinnunen P.K. Lack of enantiomeric specificity in the effects of anesthetic steroids on lipid bilayers. Biochim. Biophys. Acta. 2007;1768:131–145. doi: 10.1016/j.bbamem.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Alvarez L.D., Pecci A., Estrin D.A. In search of GABAA receptor's neurosteroid binding sites. J. Med. Chem. 2019;62:5250–5260. doi: 10.1021/acs.jmedchem.8b01400. [DOI] [PubMed] [Google Scholar]
- Balan I., Beattie M.C., O'Buckley T.K., Aurelian L., Morrow A.L. Endogenous neurosteroid (3α,5α)3-hydroxypregnan-20-one inhibits Toll-like-4 receptor activation and pro-inflammatory signaling in macrophages and brain. Sci. Rep. 2019;9:1220. doi: 10.1038/s41598-018-37409-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleza D., Sacchi M., Vena G., Galloni D., Puia G., Facci P., Alessandrini A. Effects of neurosteroids on a model membrane including cholesterol: a micropipette aspiration study. Biochim. Biophys. Acta. 2015;1848:1268–1276. doi: 10.1016/j.bbamem.2015.01.017. [DOI] [PubMed] [Google Scholar]
- Barbaccia M.L., Roscetti G., Trabucchi M., Purdy R.H., Mostallino M.C., Concas A., Biggio G. The effects of inhibitors of GABAergic transmission and stress on brain and plasma allopregnanolone concentrations. Br. J. Pharmacol. 1997;120:1582–1588. doi: 10.1038/sj.bjp.0701046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbero-Camps E., Fernandez A., Baulies A., Martinez L., Fernandez-Checa J.C., Colell A. Endoplasmic reticulum stress mediates amyloid β neurotoxicity via mitochondrial cholesterol trafficking. Am. J. Pathol. 2014;184:2066–2081. doi: 10.1016/j.ajpath.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulieu E.E. Neurosteroids: of the nervous system, by the nervous system, for the nervous system. Recent Prog. Horm. Res. 1997;52:1–32. [PubMed] [Google Scholar]
- Belelli D., Harrison N.L., Maguire J., Macdonald R.L., Walker M.C., Cope D.W. Extrasynaptic GABAA receptors: form, pharmacology, and function. J. Neurosci. 2009;29:12757–12763. doi: 10.1523/JNEUROSCI.3340-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D., Lambert J.J. Neurosteroids: endogenous regulators of the GABAA receptor. Nat. Rev. Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Bianchi M., Baulieu E.E. 3β-methoxy-pregnenolone (MAP4343) as an innovative therapeutic approach for depressive disorders. Proc. Natl. Acad. Sci. (USA) 2012;109:1713–1718. doi: 10.1073/pnas.1121485109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainstorm Consortium Analysis of shared heritability in common disorders of the brain. Science. 2018;360 doi: 10.1126/science.aap8757. pii: eaap.8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley S.G., Mody I. Extrasynaptic GABAA receptors: their function in the CNS and implications for disease. Neuron. 2012;73:23–34. doi: 10.1016/j.neuron.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budelier M.M., Cheng W.W.L., Bergdoll L., Chen Z.W., Janetka J.W., Abramson J., Krishnan K., Mydock-McGrane L., Covey D.F., Whitelegge J.P., Evers A.S. Photoaffinity labeling wit cholesterol analogues precisely maps a cholesterol-binding site in voltage-dependent anion channel-1. J. Biol. Chem. 2017;292:9294–9304. doi: 10.1074/jbc.M116.773069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budelier M.M., Cheng W.W.L., Chen Z.W., Bracamontes J.F., Sugasawa Y., Krishnan K., Mydock-McGrane L., Covey D.F., Evers A.S. Common binding sites for cholesterol and neurosteroids on a pentameric ligand-gated ion channel. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2019;186:128–136. doi: 10.1016/j.bbalip.2018.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A., Moffit T.E. All for one and one for all: mental disorders in one dimension. Am. J. Psychiatry. 2018;175:831–844. doi: 10.1176/appi.ajp.2018.17121383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S., Qian M., Krishnan K., Covey D.F., Mennerick S., Akk G. Comparison of steroid modulation of spontaneous inhibitory postsynaptic currents in cultured hippocampal neurons and steady-state single-channel currents from heterologously expressed α1β2γ2L GABA(A) receptors. Mol. Pharmacol. 2016;89:399–406. doi: 10.1124/mol.115.102202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charalampopoulos I., Remboutsika E., Margioris A.N., Gravanis A. Neurosteroids as modulators of neurogenesis and neuronal survival. Trends Endocrinol. Metab. 2008;19:300–307. doi: 10.1016/j.tem.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Chen Z.W., Bracamontes J.R., Budelier M.M., Germann A.L., Shin D.J., Kathiresan K., Qian M.X., Manion B., Cheng W.W.L., Reichert D.E., Akk G., Covey D.F., Evers A.S. Multiple functional neurosteroid binding sites on GABA-A receptors. PLoS Biol. 2019;17 doi: 10.1371/journal.pbio.3000157. 10:1371/jounal.pbio.3000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z.W., Chen L.H., Akentieva N., Lichti C.F., Darbandi R., Hastings R., Covey D.F., Reichert D.E., Townsend R.R., Evers A.S. A neurosteroid analogue photolabeling reagent labels the cochicine-binding site on tubulin: a mass spectrometric analysis. Electrophoresis. 2012;33:666–674. doi: 10.1002/elps.201100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z.W., Manion B., Townsend R.R., Reichert D.E., Covey D.F., Steinbach J.H., Sieghart W., Fuchs K., Evers A.S. Neurosteroid analog photolabeling of a site in the third transmembrane domain of the β3 subunit of the GABA(A) receptor. Mol. Pharmacol. 2012;82:408–419. doi: 10.1124/mol.112.078410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng W.W.L., Budelier M.M., Sugasawa Y., Bergdoll L., Queralt-Martin M., Rosencrans W., Rostovtseva T.K., Chen Z.-W., Abramson J., Krishnan K., Covey D.F., Whitelegge J.P., Evers A.S. Multiple neurosteroid and cholesterol binding sites in voltage-dependent anion channel-1 determined by photo-affinity labeling. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2019;(19) doi: 10.1016/j.bbalip.2019.06.004. pii:S1388-1981. 30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisari M., Eisenman L.N., Covey D.F., Mennerick S., Zorumski C.F. The sticky issue of neurosteroids and GABAA receptors. Trends Neurosci. 2010;33:299–306. doi: 10.1016/j.tins.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisari M., Eisenman L.N., Krishnan K., Bandyopadhyaya A.K., Wang C., Taylor A., Benz A., Covey D.F., Zorumski C.F., Mennerick S. The influence of neuroactive steroid lipophilicity on GABAA receptor modulation: evidence for a low affinity interaction. J. Neurophysiol. 2009;102:1254–1264. doi: 10.1152/jn.00346.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang S.-H., Reddy D.S. Genetic and molecular regulation of extrasynaptic GABA-A receptors in the brain: therapeutic insights for epilepsy. JPET. 2018;364:180–197. doi: 10.1124/jpet.117.244673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway C.R., George M.S., Sackeim H.A. Toward and evidence-based, operational definition of treatment-resistant depression: when enough is enough. JAMA Psychiatry. 2017;74:9–10. doi: 10.1001/jamapsychiatry.2016.2586. [DOI] [PubMed] [Google Scholar]
- Covey D.F., Evers A.S., Mennerick S., Zorumski C.F., Purdy R.H. Recent developments in structure-activity relationships for steroid modulators of GABA(A) receptors. Brain Res. Rev. 2001;37:91–97. doi: 10.1016/s0165-0173(01)00126-6. [DOI] [PubMed] [Google Scholar]
- Covey D.F., Nathan D., Kalkbrenner M., Nilsson K.R., Hu Y., Zorumski C.F., Evers A.S. Enantioselectivity of pregnanolone-induced gamma-aminobutyric acid (A) receptor modulation and anesthesia. JPET. 2000;293:1009–1016. [PubMed] [Google Scholar]
- Darbandi-Tonkabon R., Hastings W.R., Zeng C.M., Akk G., Manion B.D., Bracamontes J.R., Steinbach J.H., Mennerick S.J., Covey D.F., Evers A.S. Photoaffinity labeling with a neuroactive steroid analogue. 6-azi-pregnanolone labels voltage-dependent anion channel-1 in rat brain. J. Biol. Chem. 2003;278:13196–13206. doi: 10.1074/jbc.M213168200. [DOI] [PubMed] [Google Scholar]
- Dong E., Matsumoto K., Uzunova V., Sugaya I., Takahata H., Nomura H., Watanabe H., Costa E., Guidotti A. Brain 5α-dihydroprogesterone and allopregnanolone synthesis in a mouse model of protracted social isolation. Proc. Natl. Acad. Sci. (USA) 2001;98:2849–2854. doi: 10.1073/pnas.051628598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman R.S., Sanacora G., Krystal J.H. Altered connectivity in depression: GABA and glutamate neurotransmitter deficits and reversal by novel treatments. Neuron. 2019;102:75–90. doi: 10.1016/j.neuron.2019.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durkin E.J., Muessig L., Herit T., Lumb M.J., Patel R., Thomas P., Bright D., Jurd R., Moss S.J., Dickenson A.H., Cacucci F., Smart T.G. BioRxiv. 2018. [DOI]
- Eisenman L.N., He Y., Fields C., Zorumski C.F., Mennerick S. Activation dependent properties of pregnenolone sulfate inhibition of GABAA receptor-mediated current. J. Physiol. (London) 2003;550:679–691. doi: 10.1113/jphysiol.2003.043810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser C., Romeo E., Baghai T.C., Di Michele F., Schule C., Pasini A., Zwanzger P., Padgerg F., Rupprecht R. Neuroactive steroids as modulators of depression and anxiety. Neuroscience. 2006;138:1041–1048. doi: 10.1016/j.neuroscience.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Etkin A. A reckoning and research agenda for neuroimaging in psychiatry. Am. J. Psychiatry. 2019;176:507–511. doi: 10.1176/appi.ajp.2019.19050521. [DOI] [PubMed] [Google Scholar]
- Evans J., Sun Y., McGregor A., Connor B. Allopregnanolone regulates neurogenesis and depressive/anxiety-like behavior in a social isolation rodent model of chronic stress. Neuropharmacology. 2012;63:1315–1326. doi: 10.1016/j.neuropharm.2012.08.012. [DOI] [PubMed] [Google Scholar]
- Fan J., Shimizu Y., Chan J., Wilkinson A., Ito A., Tontonoz P., Eullaghan E., Galea L.A.M., Pfeifer T., Wellington C.L. Hormonal modulators of glial ABCA1 and apoE levels. J. Lipid Res. 2013;54:3139–3150. doi: 10.1194/jlr.M042473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M., Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat. Rev. Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Feng H.J., Forman S.A. Comparison of αβδ and αβγ GABA-A receptors: allosteric modulation and identification of subunit arrangement by site-selective general anesthetics. Pharmacol. Res. 2018;133:289–300. doi: 10.1016/j.phrs.2017.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich M.J. Depression is the leading cause of disability around the world. J. Am. Med. Assoc. 2017;317:1517. doi: 10.1001/jama.2017.3826. [DOI] [PubMed] [Google Scholar]
- Frye C.A., Paris J.J., Walf A.A., Rusconi J.C. Effects and mechanisms of 3α5α-THP on emotion, motivation and reward functions involving pregnane xenobiotic receptor. Front. Neurosci. 2012;5:1–18. doi: 10.3389/fnins.2011.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L., Bravo-San Pedro J.M., Blomgren K., Kroemer G. Autophagy in acute brain injury. Nat. Rev. Neurosci. 2016;17:467–484. doi: 10.1038/nrn.2016.51. [DOI] [PubMed] [Google Scholar]
- Gibbs T.T., Russek S.J., Farb D.H. Sulfated steroids as endogenous modulators. Pharmacol. Biochem. Behav. 2006;84:555–567. doi: 10.1016/j.pbb.2006.07.031. [DOI] [PubMed] [Google Scholar]
- Girdler S.S., Klatzkin R. Neurosteroids in the context of stress: implications for depressive disorders. Pharmacol. Ther. 2007;116:125–139. doi: 10.1016/j.pharmthera.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glykys J., Mann E.O., Mody I. Which GABAA receptor subunits are necessary for tonic inhibition in the hippocampus? J. Neurosci. 2008;28:1421–1426. doi: 10.1523/JNEUROSCI.4751-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm A., Schmitt K., Lang U.E., Mensah-Nyagan A.G., Eckert A. Improvement of neuronal bioenergetics by neurosteroids: implications for age-related neurodegenerative disorders. Biochim. Biophys. Acta. 2014;1842:2427–2438. doi: 10.1016/j.bbadis.2014.09.013. [DOI] [PubMed] [Google Scholar]
- Guarneri P., Russo D., Cascio C., De Leo G., Piccoli F., Guarneri R. Induction of neurosteroid synthesis by NMDA receptors in isolated rat retina: a potential early event in excitotoxicity. Eur. J. Neurosci. 1998;10:1752–1763. doi: 10.1046/j.1460-9568.1998.00191.x. [DOI] [PubMed] [Google Scholar]
- Gunduz-Bruce H., Silber C., Kaul I., Rothschild A.J., Riesenberg R., Sankoh A.J., Li H., Lasser R., Zorumski C.F., Rubinow D.R., Paul S.M., Jonas J., Doherty J.J., Kanes S.J. Trial of SGE-217 in patients with major depressive disorder. N. Engl. J. Med. 2019 doi: 10.1056/NEJMoa1815981. (in press) [DOI] [PubMed] [Google Scholar]
- Gunn B.G., Brown A.R., Lambert J.J., Belelli D. Neurosteroids and GABAA receptor interactions: a focus on stress. Front. Neurosci. 2011;5:1–20. doi: 10.3389/fnins.2011.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herd M.B., Belelli D., Lambert J.J. Neurosteroid modulation of synaptic and extrasynaptic GABAA receptors. Pharmacol. Ther. 2007;116:20–34. doi: 10.1016/j.pharmthera.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Hosie A.M., Clarke L., da Silva H., Smart T.G. Conserved site for neurosteroid modulation of GABAA receptors. Neuropharmacology. 2008;56:149–154. doi: 10.1016/j.neuropharm.2008.07.050. [DOI] [PubMed] [Google Scholar]
- Hosie A.M., Wilkins M.E., da Silva H.M.A., Smart T.G. Endogenous neurosteroids regulate GABAA receptors via two discrete transmembrane sites. Nature. 2006;444:486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- Irwin R.W., Solinsky C.M., Brinton R.D. Frontiers in therapeutic development of allopregnanolone for Alzheimer's disease and other neurological disorders. Front. Cell. Neurosci. 2014;8:203. doi: 10.3389/fncel.2014.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin R.W., Wang J.M., Chen S., Brinton R.D. Neuroregenerative mechanisms of allopregnanolone in Alzheimer's disease. Front. Endocrinol. 2012;2:1–14. doi: 10.3389/fendo.2011.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson J.S., Scanziani M. How inhibition shapes cortical activity. Neuron. 2011;72:231–243. doi: 10.1016/j.neuron.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M., Yoshitomi T., Zorumski C.F., Izumi Y. Neurosteroids are endogenous neuroprotectants in an ex vivo glaucoma model. Investig. Ophthalmol. Vis. Sci. 2014;55:8531–8541. doi: 10.1167/iovs.14-15624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi Y., Murayama K., Tokuda K., Krishnan K., Covey D.F., Zorumski C.F. GABAergic neurosteroids mediate the effects of ethanol on long-term potentiation in rat hippocampal slices. Eur. J. Neurosci. 2007;26:1881–1888. doi: 10.1111/j.1460-9568.2007.05809.x. [DOI] [PubMed] [Google Scholar]
- Izumi Y., O'Dell K.A., Zorumski C.F. Corticosterone enhances the potency of ethanol against hippocampal long-term potentiation via local neurosteroid synthesis. Front. Cell. Neurosci. 2015;9:254. doi: 10.3389/fncel.2015.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi Y., Svrakic N., O'Dell K., Zorumski C.F. Ammonia inhibits long-term potentiation via neurosteroid synthesis in hippocampal pyramidal neurons. Neuroscience. 2013;233:166–173. doi: 10.1016/j.neuroscience.2012.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Shu H.-J., Krishnan K., Qian M., Taylor A.A., Covey D.F., Zorumski C.F., Mennerick S. A clickable neurosteroid photolabel reveals selective Golgi compartmentalization with preferential impact on proximal inhibition. Neuropharmacology. 2016:193–206. doi: 10.1016/j.neuropharm.2016.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston G.A.R. GABAA receptor channel pharmacology. Curr. Pharmaceut. Des. 2005;11:1867–1885. doi: 10.2174/1381612054021024. [DOI] [PubMed] [Google Scholar]
- Joksimovic S.M., Izumi Y., Joksimovic S.L., Tesic V., Krishnan K., Snake B., Jevtovic-Todorovic V., Covey D.F., Zorumski C.F., Todorovic S.M. A novel neurosteroid hypnotic blocks T-type calcium channel-dependent rebound burst firing in the juvenile rat subiculum and suppresses LTP at the CA1-subiculum synapse. Br. J. Anaesth. 2019;122:643–651. doi: 10.1016/j.bja.2019.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanes S., Colquhoun H., Gunduz-Bruce H., Raines S., Arnold R., Schacterle A., Doherty J., Epperson N.C., Deligiannidis K.M., Riesenberg R., Hoffmann E., Rubinow D., Jonas J., Paul S., Meltzer-Brody S. Brexanolone (SAGE-547 injection) in post-partum depression: a randomised controlled trial. Lancet. 2017;390:480–489. doi: 10.1016/S0140-6736(17)31264-3. [DOI] [PubMed] [Google Scholar]
- Katona B.W., Krishnan K., Cai Z.Y., Manion B.D., Benz A., Taylor A., Evers A.S., Zorumski C.F., Mennerick S., Covey D.F. Neurosteroid analogues. 12. Potent enhancement of GABA-mediated chloride currents at GABA-A receptors by ent-androgens. Eur. J. Med. Chem. 2008;43:107–113. doi: 10.1016/j.ejmech.2007.02.017. [DOI] [PubMed] [Google Scholar]
- Kim H.N., Lee S.-J., Koh J.-Y. The neurosteroids, allopregnanolone and progesterone, induce autophagy in cultured astrocytes. Neurochem. Int. 2012;60:125–133. doi: 10.1016/j.neuint.2011.11.015. [DOI] [PubMed] [Google Scholar]
- Kimoto T., Tsurugizawa T., Ohta Y., Makino J., Tamura H.-O., Hojo Y., Takata N., Kawato S. Neurosteroid synthesis by cytochrome P450-containing systems localized in the rat brain hippocampal neurons: N-methyl-D-aspartate and calcium-dependent synthesis. Endocrinology. 2001;142:3578–3589. doi: 10.1210/endo.142.8.8327. [DOI] [PubMed] [Google Scholar]
- Korpi E.R., Sinkkonen S.T. GABA(A) receptor subtypes as targets for neuropsychiatric drug development. Pharmacol. Ther. 2006;109:12–32. doi: 10.1016/j.pharmthera.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Krishnan K., Manion B.D., Taylor A., Bracamontes J., Steinbach J.H., Reichert D.E., Evers A.S., Zorumski C.F., Mennerick S., Covey D.F. Neurosteroid analogues. 17. Inverted binding orientations of androsterone enantiomers at the steroid potentiation site on γ-aminobutyric acid type A receptors. J. Med. Chem. 2012;55:1334–1345. doi: 10.1021/jm2014925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamba V., Yasuda K., Lamba J.K., Assem M., Davila J., Strom S., Schuetz E.G. PXR (NR1I2): splice variants in human tissues, including brain, and identification of neurosteroids and nicotine as PXR activators. Toxicol. Appl. Pharmacol. 2004;199:251–265. doi: 10.1016/j.taap.2003.12.027. [DOI] [PubMed] [Google Scholar]
- Langmade S.J., Gale S.E., Frolov A., Mohri I., Suzuki K., Mellon S.H., Walkley S.U., Covey D.H., Schaffer J.E., Ory D.S. Pregnane X receptor (PXR) activation: a mechanism for neuroprotection in a mouse model of Niemann-Pick C disease. Proc. Natl. Acad. Sci. (USA) 2006;103:13807–13812. doi: 10.1073/pnas.0606218103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laverty D., Thomas P., Field M., Andersen O.J., Gold M.G., Biggin P.C., Gielen M., Smart T.G. Crystal structures of a GABAA-receptor chimera reveal new endogenous neurosteroid-binding sites. Nat. Struct. Mol. Biol. 2017;24:977–985. doi: 10.1038/nsmb.3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao G., Cheung S., Galeano J., Ji A.X., Qin Q., Xiaoning B. Allopregnanolone treatment delays cholesterol accumulation and reduces autophagic/lysosomal dysfunction and inflammation in NPC1-/- mouse brain. Brain Res. 2009;1270:140–151. doi: 10.1016/j.brainres.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Shu H.-J., Wang C., Mennerick S., Zorumski C.F., Covey D.F., Steinbach J.H., Akk G. Neurosteroid migration to intracellular compartments reduces steroid concentration in the membrane and diminishes GABA-A receptor potentiation. J. Physiol. (London) 2007;584:789–800. doi: 10.1113/jphysiol.2007.142794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low K., Crestani F., Keist R., Benke D., Brunig I., Benson J.A., Fritschy J.M., Rulicke T., Bluethmann H., Mohler H., Rudolph U. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- Locci A., Pinna G. Neurosteroid biosynthesis down-regulation and changes in GABAA receptor subunit composition: a biomarker axis in stress-induced cognitive and emotional impairment. Br. J. Pharmacol. 2017;174:3226–3241. doi: 10.1111/bph.13843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher B., Mohler H. Brexanolone, a neurosteroid antidepressant, vindicates the GABAergic deficit hypothesis of depression and may foster resilience. F1000Research. 2019;8:751. doi: 10.12688/f1000research.18758.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher B., Shen Q., Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Mol. Psychiatry. 2011;16:383–406. doi: 10.1038/mp.2010.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J. Neuroactive steroids and GABAergic involvement in the neuroendocrine dysfunction associated with major depression and postpartum depression. Front. Cell. Neurosci. 2019;13:83. doi: 10.3389/fncel.2019.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire J., Mody I. GABA(A)R plasticity during pregnancy: relevance to postpartum depression. Neuron. 2008;59:207–213. doi: 10.1016/j.neuron.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska M.D., Harrison N.L., Schwartz R.D., Barker J.L., Paul S.M. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Manji H., Kato T., Di Prospero N.A., Ness S., Beal M.F., Krams M., Chen G. Impaired mitochondrial function in psychiatric disorders. Nat. Rev. Neurosci. 2012;13:293–307. doi: 10.1038/nrn3229. [DOI] [PubMed] [Google Scholar]
- Martinez Botella G., Salituro F.G., Harrison B.L., Beresis R.T., Bai Z., Blanco M.-J., Belfort G.M., Dai J., Loya C.M., Ackley M.A., Althaus A.L., Grossman S.J., Hoffmannm E., Doherty J.J., Robichaud A.J. Neuroactive steroids. 2. 3α-hydroxy-3β-methyl-21-(4-cyano-1H-pyrazol-1’-yl)-19-nor-5β-pregnan-20-one (SAGE-217): a clinical next generation neuroactive steroid positive allosteric modulator of the (γ-aminobutyric acid)A receptor. J. Med. Chem. 2017;60:7810–7819. doi: 10.1021/acs.jmedchem.7b00846. [DOI] [PubMed] [Google Scholar]
- Melon L., Hammond R., Lewis M., Maguire J. A novel, synthetic, neuroactive steroid is effective at decreasing depression-like behaviors and improving maternal care in preclinical models of postpartum depression. Front. Endocrinol. 2018;9:703. doi: 10.3389/fendo.2018.00703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer-Brody S., Colquhoun H., Riesenberg R., Epperson C.N., Deligiannidis K.M., Rubinow D.R., Li H., Sankoh A.J., Clemson C., Schacterle A., Jonas J., Kanes S. Brexanolone injection in post-partum depression: two multicenter, double-blind, randomized, placebo-controlled, phase 3 trials. Lancet. 2018;392:1058–1070. doi: 10.1016/S0140-6736(18)31551-4. [DOI] [PubMed] [Google Scholar]
- Mennerick S., He Y., Jiang X., Manion B.D., Wang M., Shute A., Benz A., Evers A.S., Covey D.F., Zorumski C.F. Selective antagonism of 5α-reduced neurosteroid effects at GABAA receptors. Mol. Pharmacol. 2004;65:1191–1197. doi: 10.1124/mol.65.5.1191. [DOI] [PubMed] [Google Scholar]
- Mennerick S., Zeng C.-M., Benz A., Shen W., Izumi Y., Evers A.S., Covey D.F., Zorumski C.F. Effects on GABAA receptors of a neuroactive steroid that negatively modulates glutamate neurotransmission and augments GABA neurotransmission. Mol. Pharmacol. 2001;60:732–741. [PubMed] [Google Scholar]
- Miller P.S., Scott S., Masiulis S., De Colibus L., Pardon E., Steyaert J., Aricescu A.R. Structural basis for GABAA receptor potentiation by neurosteroids. Nat. Struct. Mol. Biol. 2017;24:986–992. doi: 10.1038/nsmb.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modgil A., Parakala M.L., Ackley M.A., Doherty J.J., Moss S.J., Davies P.A. Endogenous and synthetic neuroactive steroids evoke sustained increases in the efficacy of GABAergic inhibition via a protein kinase C-dependent mechanism. Neuropharmacology. 2017;113:314–322. doi: 10.1016/j.neuropharm.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody I. GABAAR modulator for postpartum depression. Cell. 2019;176:1. doi: 10.1016/j.cell.2018.12.016. [DOI] [PubMed] [Google Scholar]
- Möhler H. The GABA system in anxiety and depression and its therapeutic potential. Neuropharmacology. 2012;62:42–53. doi: 10.1016/j.neuropharm.2011.08.040. [DOI] [PubMed] [Google Scholar]
- Mokdad A.H., Marks J.S., Stroup D.F., Gerberding J.E. Actual causes of death in the United States, 2000. J. Am. Med. Assoc. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Murray C.J., US Burden of Disease Collaborators The state of US health, 1990-2010: burden of disease, injuries and risk factors. J. Am. Med. Assoc. 2013;310:591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugan S., Jakka P., Namani S., Mujumdar V., Radhakrishnan G. The neurosteroid pregnenolone promotes degradation of key proteins in the innate immune signaling to suppress inflammation. J. Biol. Chem. 2019;294:4596–4607. doi: 10.1074/jbc.RA118.005543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson K.R., Zorumski C.F., Covey D.F. Neurosteroid analogues. 6. The synthesis and GABAA receptor pharmacology of enantiomers of dehydroepiandrosterone sulfate, pregnenolone sulfate, and (3 alpha, 5 beta)-3-hydroxypregnan-20-one sulfate. J. Med. Chem. 1998;41:2604–2613. doi: 10.1021/jm980148h. [DOI] [PubMed] [Google Scholar]
- Nin M.S., Salles F.B., Azeredo L.A., Frazon A.P.G., Gomez R., Barros H.M.T. Antidepressant effect and changes of GABA-A receptor γ2 subunit mRNA after hippocampal administration of allopregnanolone in rats. J. Psychopharmacol. 2008;22:477–485. doi: 10.1177/0269881107081525. [DOI] [PubMed] [Google Scholar]
- Noorbakhsh F., Baker G.B., Power C. Allopregnanolone and neuroinflammation: a focus on multiple sclerosis. Front. Cell. Neurosci. 2014;8:134. doi: 10.3389/fncel.2014.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R.W., Sieghart W. GABAA receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–148. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathirathna S., Brimelow B.C., Jagodic M.M., Krishnan K., Jiang X., Zorumski C.F., Mennerick S., Covey D.F., Todorovic S.M., Jevtovic-Todorovic V. New evidence that both T-type calcium channels and GABAA channels are responsible for the potent analgesic effects of 5α-reduced neuroactive steroids. Pain. 2005;114:429–443. doi: 10.1016/j.pain.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Paul S.M., Purdy R.H. Neuroactive steroids. FASEB J. 1992;6:2311–2322. [PubMed] [Google Scholar]
- Pawluski J.L., Lonstein J.S., Fleming A.S. The neurobiology of postpartum anxiety and depression. Trends Neurosci. 2017;40:106–120. doi: 10.1016/j.tins.2016.11.009. [DOI] [PubMed] [Google Scholar]
- Pibiri F., Nelson M., Guidotti A., Costa E., Pinna G. Decreased corticolimbic allopregnanolone expression during social isolation enhances contextual fear: a model relevant for posttraumatic stress disorder. Proc. Natl. Acad. Sci. (USA) 2008;105:5567–5572. doi: 10.1073/pnas.0801853105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineles S.L., Nillni Y.I., Pinna G., Irvine J., Webb A., Arditte Hall K.A., Hauger R., Miller M.W., Resick P.A., Orr S.P., Rasmusson A.M. PTSD in women is associated with a block in conversion of progesterone to the GABAergic neurosteroids allopregnanolone and pregnanolone measured in plasma. Psychoneuroendocrinology. 2018;93:133–141. doi: 10.1016/j.psyneuen.2018.04.024. [DOI] [PubMed] [Google Scholar]
- Pinna G. Animal models of PTSD: the socially isolated mouse and the biomarker role of allopregnanolone. Front. Behav. Neurosci. 2019;13:114. doi: 10.3389/fnbeh.2019.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna G., Dong E., Matsumoto K., Costa E., Guidotti A. In socially isolated mice, the reversal of brain allopregnanolone down-regulation mediates the anti-aggressive action of fluoxetine. Proc. Natl. Acad. Sci. (USA) 2003;100:2035–2040. doi: 10.1073/pnas.0337642100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoli M., Yan Z., McEwen B.S., Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat. Rev. Neurosci. 2012;13:22–37. doi: 10.1038/nrn3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prenosil G.A., Schneider-Gasser E.M., Rudolph U., Kreist R., Fritschy J.M., Vogt K.E. Specific subtypes of GABAA receptors mediate phasic and tonic forms of inhibition in hippocampal pyramidal neurons. J. Neurophysiol. 2006;96:846–857. doi: 10.1152/jn.01199.2005. [DOI] [PubMed] [Google Scholar]
- Puia G., Santi M.R., Vicini S., Pritchett D.B., Purdy R.H., Paul S.M., Seeburg P.H., Costa E. Neurosteroids act on recombinant human GABAA receptors. Neuron. 1990;4:759–765. doi: 10.1016/0896-6273(90)90202-q. [DOI] [PubMed] [Google Scholar]
- Purdy R.H., Morrow A.L., Moore P.H., Paul S.M. Stress-induced elevations of γ-aminobutyric acid type A receptor-active steroids in the rat brain. Proc. Natl. Acad. Sci. (USA) 1991;88:4553–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian M., Krishnan K., Kudova E., Li P., Manion B.D., Taylor A., Elias G., Akk G., Evers A.S., Zorumski C.F., Mennerick S., Covey D.F. Neurosteroid analogues. 18. Structure-activity studies of ent-steroid potentiators of γ-aminobutyric acid type A receptors and comparison of their activities with those of alphaxalone and allopregnanolone. J. Med. Chem. 2014;57:171–190. doi: 10.1021/jm401577c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson A.M., King M.W., Valovski I., Gregor K., Scioli-Salter E., Pineles S.L., Hamouda M., Nillni Y.I., Anderson G.M., Pinna G. Relationships between cerebrospinal fluid GABAergic neurosteroid levels and symptom severity in men with PTSD. Psychoneuroendocrinology. 2019;102:95–104. doi: 10.1016/j.psyneuen.2018.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincón-Cortés M., Herman J.P., Lupien S., Maguire J., Shansky R.M. Stress: influence of sex, reproductive status and gender. Neurobiol. Stress. 2019;10:100155. doi: 10.1016/j.ynstr.2019.100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo E., Strohle A., Spalletta G., di Michele F., Hermann B., Holsboer F., Pasini A., Rupprecht R. Effects of antidepressant treatment on neuroactive steroids in major depression. Am. J. Psychiatry. 1998;155:910–913. doi: 10.1176/ajp.155.7.910. [DOI] [PubMed] [Google Scholar]
- Rong X., Albert C.J., Hong C., Duerr M.A., Chamberlain B.T., Tarling E.J., Ito A., Gao J., Wang B., Edwards P.A., Jung M.E., Ford D.A., Tontonoz P. LXRs regulate ER stress and inflammation through dynamic modulation of membrane phospholipid composition. Cell Metabol. 2013;18:685–697. doi: 10.1016/j.cmet.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth B.L., Sheffler D.J., Kroeze W.K. Magic shotguns versus magic bullets: selectively non-selective drugs for mood disorders and schizophrenia. Nat. Rev. Drug Discov. 2004;3:353–359. doi: 10.1038/nrd1346. [DOI] [PubMed] [Google Scholar]
- Rudolph U., Mohler H. GABA-based therapeutic approaches: GABAA receptor subtype functions. Curr. Opin. Pharmacol. 2006;6:18–23. doi: 10.1016/j.coph.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Rupprecht R., Holsboer F. Neuroactive steroids: mechanisms of action and neuropsychopharmacological perspectives. Trends Neurosci. 1999;22:410–416. doi: 10.1016/s0166-2236(99)01399-5. [DOI] [PubMed] [Google Scholar]
- Rupprecht R., Reul J.M.H.M., Trapp T., van Steensel R., Wetzel C., Kamm K., Zieglgansberger W., Holsboer F. Progesterone receptor-mediated effects of neuroactive steroids. Neuron. 1993;11:523–530. doi: 10.1016/0896-6273(93)90156-l. [DOI] [PubMed] [Google Scholar]
- Saalman Y.B., Kirkcaldie M.T.K., Waldron S., Calford M.B. Cellular distribution of the GABAA receptor-modulating 3α-hydroxy, 5α-reduced pregnane steroids in the adult rat brain. J. Neuroendocrinol. 2007;19:272–284. doi: 10.1111/j.1365-2826.2006.01527.x. [DOI] [PubMed] [Google Scholar]
- Sacchi M., Balleza D., Vena G., Puia G., Facci P., Alessandrini A. Effects of neurosteroids on a model lipid bilayer including cholesterol: an atomic force microscopy study. Biochim. Biophys. Acta. 2015;1848:1258–1267. doi: 10.1016/j.bbamem.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Santarelli L., Saxe M., Gross C., Surget A., Battaglia F., Dulawa S., Weisstraub N., Lee J., Duman R., Arancio O., Belzung C., Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Sayeed I., Parvez S., Wall B., Siemen D., Stein D.G. Direct inhibition of the mitochondrial permeability transition pore: a possible mechanism for better neuroprotective effects of allopregnanolone over progesterone. Brain Res. 2009;1263:165–173. doi: 10.1016/j.brainres.2009.01.045. [DOI] [PubMed] [Google Scholar]
- Schule C., Eser D., Baghai T.C., Nothdurfter C., Kessler J.S., Rupprecht R. Neuroactive steroids in affective disorders: target for novel antidepressant or anxiolytic drugs? Neuroscience. 2011;191:55–77. doi: 10.1016/j.neuroscience.2011.03.025. [DOI] [PubMed] [Google Scholar]
- Seljeset S., Bright D.P., Thomas P., Smart T.G. Probing GABA-A receptors with inhibitory neurosteroids. Neuropharmacology. 2018;136:23–36. doi: 10.1016/j.neuropharm.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W., Mennerick S., Covey D.F., Zorumski C.F. Pregnenolone sulfate modulates inhibitory synaptic transmission by enhancing GABAA receptor desensitization. J. Neurosci. 2000;20:3571–3579. doi: 10.1523/JNEUROSCI.20-10-03571.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu H.-J., Bracamontes J., Taylor A., Wu K., McCollum M., Akk G., Manion B., Evers A.S., Krishnan K., Covey D.F., Zorumski C.F., Steinbach J.H., Mennerick S. Characteristics of α4/δ-containing GABAA receptors expressed in Xenopus oocytes. Br. J. Pharmacol. 2012;165:2228–2243. doi: 10.1111/j.1476-5381.2011.01690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu H.-J., Eisenman L.N., Jinadasa D., Covey D.F., Zorumski C.F., Mennerick S. Slow actions of neuroactive steroids at GABA-A receptors. J. Neurosci. 2004;24:6667–6675. doi: 10.1523/JNEUROSCI.1399-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu H.-J., Zeng C.M., Wang C., Covey D.F., Zorumski C.F., Mennerick S. Cyclodextrins sequester neuroactive steroids and differentiate mechanisms that rate limit steroid actions. Br. J. Pharmacol. 2007;150:164–175. doi: 10.1038/sj.bjp.0706973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small S.A., Schobel S.A., Buxton R.B., Witter M.P., Barnes C.A. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat. Rev. Neurosci. 2011;12:585–601. doi: 10.1038/nrn3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoller J.W., Andreassen O.A., Edenberg H.J., Raraone S.V., Glatt S.J., Kendler K.S. Psychiatric genetics and the structure of psychopathology. Mol. Psychiatry. 2019;24:409–420. doi: 10.1038/s41380-017-0010-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder J.S., Soumier A., Brewer M., Pickel J., Cameron H.A. Adult hippocampal neurogenesis buffers stress responses and depressive behaviors. Nature. 2011;476:458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell B.M., Brickley S.G., Tang C.Y., Farrant M., Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by δ subunit-containing GABAA receptors. Proc. Natl. Acad. Sci. (USA) 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart D.E., Vigod S. Postpartum depression. N. Engl. J. Med. 2016;375:2177–2186. doi: 10.1056/NEJMcp1607649. [DOI] [PubMed] [Google Scholar]
- Strohle A., Romeo E., Hermann B., Pasini A., Spalletta G., di Michele F., Holsboer F., Rupprecht R. Concentrations of 3 alpha-reduced neuroactive steroids and their precursors in plasma of patients with major depression and after clinical recovery. Biol. Psychiatry. 1999;45:274–277. doi: 10.1016/s0006-3223(98)00328-x. [DOI] [PubMed] [Google Scholar]
- Sun M.Y., Shu H.-J., Benz A., Bracamontes J., Akk G., Zorumski C.F., Steinbach J.H., Mennerick S.J. Chemogenetic isolation reveals synaptic contribution of δ GABA-A receptors in mouse dentate granule neurons. J. Neurosci. 2018;38:8128–8145. doi: 10.1523/JNEUROSCI.0799-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surget A., Tanti A., Leonardo E.D., Laugeray A., Rainer Q., Touma C., Palme R., Griebel G., Ibarguen-Vargas Y., Hen R., Belzung C. Antidepressants recruit new neurons to improve stress response regulation. Mol. Psychiatry. 2011;16:1177–1188. doi: 10.1038/mp.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P., Pang Y. Membrane progesterone receptors: evidence for neuroprotective, neurosteroid signaling and neuroendocrine functions in neuronal cells. Neuroendocrinology. 2012;96:162–171. doi: 10.1159/000339822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorovic S.M., Pathirathna S., Brimelow B.C., Jagodic M.M., Ko S.-H., Jiang X., Nilsson K.R., Zorumski C.F., Covey D.F., Jevtovic-Todorovic V. 5β-reduced neuroactive steroids are novel voltage-dependent blockers of T-type Ca2+ channels in rat sensory neurons in vitro and potent peripheral analgesics in vivo. Mol. Pharmacol. 2004;66:1223–1235. doi: 10.1124/mol.104.002402. [DOI] [PubMed] [Google Scholar]
- Tokuda K., Izumi Y., Zorumski C.F. Ethanol enhances neurosteroidogenesis in hippocampal pyramidal neurons by paradoxical NMDA receptor activation. J. Neurosci. 2011;31:9905–9909. doi: 10.1523/JNEUROSCI.1660-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda K., Izumi Y., Zorumski C.F. Locally-generated acetaldehyde contributes to the effects of ethanol on neurosteroids and LTP in the hippocampus. Neurol. Clin. Neurosci. 2013;1:138–147. doi: 10.1111/ncn3.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda K., O'Dell K.A., Izumi Y., Zorumski C.F. Midazolam inhibits hippocampal long-term potentiation and learning through dual central and peripheral benzodiazepine receptor activation and neurosteroidogenesis. J. Neurosci. 2010;30:16788–16795. doi: 10.1523/JNEUROSCI.4101-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzunova V., Sheline Y., Davis J.M., Rasmusson A., Uzunov D.P., Costa E., Guidotti A. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc. Natl. Acad. Sci. (USA) 1998;95:3239–3244. doi: 10.1073/pnas.95.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanLandingham J.W., Cutler S.M., Virmani S., Hoffman S.W., Covey D.F., Krishnan K., Hammes S.R., Jamnongjit M., Stein D.G. The enantiomer of progesterone acts as a molecular neuroprotectant after traumatic brain injury. Neuropharmacology. 2006;51:1078–1085. doi: 10.1016/j.neuropharm.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Wang J.M., Singh C., Liu L., Irwin R.W., Chen S., Chung E.J., Thompson R.E., Brinton R.D. Allopregnanolone reverses neurogenic and cognitive deficits in mouse model of Alzheimer's disease. Proc. Natl. Acad. Sci. (USA) 2010;107:6498–6503. doi: 10.1073/pnas.1001422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., He Y., Eisenman L.N., Fields C., Zeng C.-M., Mathews J., Benz A., Fu T., Zorumski E., Steinbach J.H., Covey D.F., Zorumski C.F., Mennerick S. 3β-Hydroxypregnane steroids are pregnenolone sulfate-like GABAA-receptor antagonists. J. Neurosci. 2002;22:3366–3375. doi: 10.1523/JNEUROSCI.22-09-03366.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmer L.L., Hu Y., Kalkbrenner M., Evers A.S., Zorumski C.F., Covey D.F. Enantioselectivity of steroid-induced γ-aminobutyric acidA receptor modulation and anesthesia. Mol. Pharmacol. 1996;50:1581–1586. [PubMed] [Google Scholar]
- Wohlfarth K.M., Bianchi M.T., Macdonald R.L. Enhanced neurosteroid potentiation of ternary GABA (A) receptors containing the delta subunit. J. Neurosci. 2002;22:1541–1549. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Cui Y., Sang K., Dong Y., Ni Z., Ma S., Hu H. Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature. 2018;554:317–322. doi: 10.1038/nature25509. [DOI] [PubMed] [Google Scholar]
- Zolkowska D., Dhir A., Krishnan K., Covey D.F., Rogawski M.A. Anticonvulsant potencies of the enantiomers of the neurosteroids androsterone and etiocholanolone exceed those of the natural forms. Psychopharmacology. 2014;231:3325–3332. doi: 10.1007/s00213-014-3546-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorumski C.F., Izumi Y. NMDA receptors and metaplasticity: mechanisms and possible roles in neuropsychiatric disorders. Neurosci. Biobehav. Rev. 2012;36:989–1000. doi: 10.1016/j.neubiorev.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorumski C.F., Paul S.M., Izumi Y., Covey D.F., Mennerick S. Neurosteroids, stress and depression: potential therapeutic opportunities. Neurosci. Biobehav. Rev. 2013;37:109–122. doi: 10.1016/j.neubiorev.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]