Abstract
Mobile phone-based health interventions (mHealth) are viewed as an attractive approach to foster behaviour change, and found to be effective in promoting physical activity and healthy diets. The present study aims to investigate whether mHealth with advice for dietary and lifestyle modifications would reduce 10-year cardio vascular disease (CVD) risk among overweight or obese adults aged 35–64 years in Sri Lanka. A two-group parallel-arm randomized controlled trial (RCT) was conducted in Colombo district, recruiting 1200 individuals aged 35–64 years with a body mass index (BMI) of ≥25 kgm−2. Participants were randomly assigned either to mHealth package (intervention arm, n = 600) or usual care (control arm, n = 600). The intervention package contains a series of dietary and lifestyle improvement messages, a mobile application to register participants, and a web application to deliver these messages. Participants in the intervention arm receive 2 voice and 2 text messages per week to their mobile phones for a period of 12 months. The primary outcome (10-year CVD risk) will be assessed according to sex, age, smoking status, blood pressure, serum cholesterol and glycaemic status. Data are collected at enrollment and after 12 months of intervention on: dietary practices, physical activity, smoking, anthropometry, body composition, blood pressure, fasting plasma glucose, HbA1c and lipid profile. Analysis of effect will be performed by intention-to-treat principle, comparing the outcomes between intervention and control arms. The study resulted in a comprehensive mHealth nutrition and lifestyle package (mHENAL) and successfully completed recruitment and baseline assessment of participants. The message delivery is in progress.
Keywords: mHealth, Mobile phone-based, Nutrition intervention, Lifestyle, Cardiovascular disease, Obesity
1. Introduction
Cardiovascular disease (CVD) is the leading cause of death worldwide, and the number of deaths due to CVD is increasing every year [1]. Globally, there were 17.3 million estimated deaths due to cardiovascular diseases in 2013, with age-standardized mortality rate of 293 per 100,000 population [2]. Hospital statistics in Sri Lanka indicated a rapid rise in morbidity and mortality due to CVD during the past 2 decades [3], and this would cause a significant impact on quality of life of individuals and country's economic growth. There is no national level estimate of prevalence of CVD, however, a prevalence of 6.9% in adults aged 30 years and above was reported in a sub-urban area [4]. The 10-year CVD risk is a measure of an individual's risk based on a combination of risk factors, and the proportion at high risk has been reported in the Sri Lanka non-communicable disease risk factor survey (STEPS 2015) and in a cohort study [5,6].
Abnormal lipids, smoking, hypertension, diabetes, abdominal obesity, psychosocial factors, low consumption of fruits and vegetables, alcohol, and physical inactivity account for most of the CVD risk worldwide [7,8]. Sri Lanka diabetes and cardiovascular study has reported high prevalence of diabetes (10.3%), pre-diabetes (11.5%), hypertension (23.7%), dyslipidaemia (77.4%) and metabolic syndrome (24.3%) in adults [[9], [10], [11], [12]]. According to STEPS 2015, 25% of men and 34% of women aged 18–69 years were either overweight or obese [5], and evidence shows that there a rapid increase in obesity in Sri Lanka [13]. Adoption of a healthy diet is preferable or complementary to long-term medication in the general population in order to prevent or delay the onset of CVD and to reduce the burden on health services [7]. With the evolution of mobile phone technology and its wider usage, mobile health (mHealth) is viewed as an attractive and promising approach to foster behaviour change [14,15]. mHealth is defined as medical and public health practice supported by mobile devices, such as mobile phones, patient monitoring devices, personal digital assistants and other wireless devices [16]. Effects of mHealth on illness have been demonstrated through randomized controlled trials (RCT) with various outcomes such as diabetes [14], hypertension [15], weight gain [17], physical activity [18] and diet [14,15,17,18]. For example, a RCT conducted in Latin America found that a mHealth intervention with monthly motivational counseling calls and weekly personalized text messages for 12 months was associated with a small reduction in bodyweight and an improvement in dietary habits [15]. In a systematic review of trials from developing countries, it was revealed that e− and mHealth interventions were effective in promoting physical activity and healthy diet [19]. The review recommended the need of more rigorous study designs in future interventional studies.
A gap of knowledge exists with respect to evidence on effective interventions that would address dietary and lifestyle causes, and thereby reduce CVD risk specially in targeted individuals in the community. Our primary research question is to examine whether a nutrition and lifestyle improvement intervention based on mobile phones would be effective in reducing CVD risk. In this study, we aim to investigate whether mHealth that included voice and text messages containing advice for dietary and lifestyle modifications for 12-month period would reduce 10-year cardiovascular disease risk among overweight or obese adults aged 35–64 years in a predominantly urban Sri Lankan population. The study also aims to evaluate the effects of the mHealth intervention on body mass index, dietary practices and physical activity.
2. Materials and methods
2.1. Overview
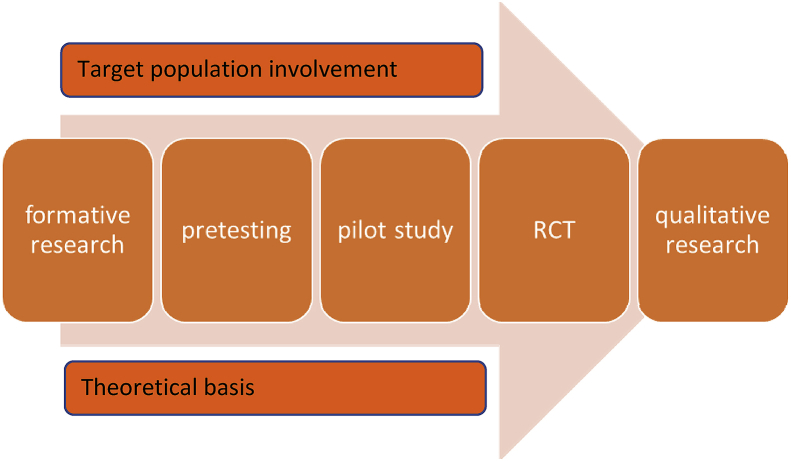
The study design followed an established process for development and testing of mHealth interventions as illustrated in Fig. 1 [20]. The steps in this process are: A formative research, compiling and pretesting the content, development of mHealth application and a pilot study, a RCT, and further qualitative research.
Fig. 1.
Steps in the process for mHealth intervention development and testing
Adapted with permission from Whittaker et al. 2012 [20].
Formative research was carried out at the inception to obtain views from a wide range of stakeholders through focus groups discussions (FGD) and in-depth interviews (IDI). The formative study explored contextual information required to plan, develop and implement the intervention. Twelve FGD comprising 6–8 persons aged 35–64 years in each were conducted in different socio-economic strata and occupational settings. Both male and female were included as participants in each FGD. Of the 12 FGDs, 7 were conducted among persons from the middle socio-economic class, while 3 from the lower and 2 from the upper. The participants included both employed and unemployed persons, and covered a spectrum of occupations such as casual workers, factory workers, teachers, health care providers, managerial staff, entrepreneurs and professionals. Twenty IDI were conducted under three categories of stakeholders namely, medical professionals, paramedical professionals, and information and communication technology experts. Nutrition experts were present under the medical and paramedical professional categories.
2.2. Compiling and pre-testing the content
The intervention package contained a series of messages on nutrition and lifestyle practices aiming a change participants’ behaviour. These contents were prepared conforming to the best scientific evidence in principles and practice of nutrition and healthy lifestyles, including food based dietary guidelines [21], my plate model [22,23] and global recommendations on physical activity for health [24]. The content and expression of messages were underpinned by relevant theories for behaviour change applicable for m-health interventions [25]. The messages cover healthy dietary practices (e.g., Serve one-half your plate with vegetables, one quarter with rice or other starchy food, and the remaining quarter with proteins such as fish, meat, egg or dhal; Avoid eating processed meats such as sausages, ham, bacon, and meat balls; Minimize the amount of salt you add to your food, and especially do not add salt to rice; Limit to 1 tea spoon of sugar per cup of tea or drink tea without sugar; Drink at least eight glasses of water a day) and heathy lifestyles (e.g., Engage in physical exercise at least 30 minutes a day; Sleep at least 7 hours in the night; Stop smoking, and; Cut down amount of alcohol intake). The messages were concise, simple and easy to comprehend by the target audience. Each message was available in local languages (Sinhalese and Tamil), and pretested among a sample of 30 individuals including linguistic and communication experts. Following appropriate corrections, the messages were sequenced in a meaningful order.
2.3. Developing mHealth application and pilot study
The same messages were developed both in voice and text form. The voice messages were recorded in male and female voices alternately in MP3 format, and the text messages in Unicode text. Some massages were made interactive by including questions for the participant to respond via SMS with yes/no options or values such as body weight and number of days engaged in exercise.
In order to operationalize message delivery, two interlinked applications, a mobile and a web application, were developed by a software development company (Data Management Systems Ltd.) in consultation with the investigators. The mobile application was designed to register participants for the m-health at enrollment to the trial in the community. The web application was designed to run an online system to administer the m-health package. The web application has facilities to assign users (Project Assistants) who would subsequently register participants using the mobile application, and schedule text and voice message delivery for participants through a mobile network. The web application also has the facility to generate reports such as participant details, message delivery status, participant's receipt of these messages, and user replies. Both mobile and web applications were password protected, and accessible by the investigators only through user accounts. The package was tailor made to suit any type of mobile phone of the end-user, either feature phone or smart phone.
A leading mobile company (Mobitel Private Ltd.) was engaged as the mobile network provider to host and deliver the mHealth intervention according to an automated schedule. Connectivity services and gateway facilities were set up by the network provider to facilitate sending of domestic Short Message Services (SMS) and Out Bound Dialing (OBD) to the study participants of the intervention arm. A survey platform was established and hosted in a dedicated server facility connected to the mobile network.
A pilot study was carried-out using few mock participants, as well as a sample (n = 20) of eligible participants for 6 weeks. Following the pilot testing, updates were done by rectifying the shortcomings.
2.4. The randomized controlled trial (RCT)
2.4.1. Study design and setting
The RCT is based on the hypothesis that the “mHealth intervention” compared to “usual care” would reduce cardiovascular disease risk by one-third among overweight or obese individuals in the age group 35–64 years. The RCT is a two-group, parallel-arm, superiority trial with an allocation ratio of 1:1 for intervention and control groups, respectively. The RCT is conducted in Colombo district which has a population of 2,324,349, almost 11% of the country's total population. It is the most urban district in Sri Lanka, with 78% of its residents living in urban areas [26]. The community health services in the district are provided through 15 Medical Officer of Health (MOH) areas under the provincial health services and 05 MOH areas under the Colombo Municipal Council. The latter was excluded due to its complexity, heterogeneity and different organizational structure for community health services. Potential participants for RCT were identified from the communities in the aforementioned 15 MOH areas.
2.4.2. Participants
The eligible participants fulfilled the following inclusion criteria: 35–64 years of age; body mass index (BMI) of 25 kgm−2 or above; and no intention to move out from area in the succeeding two years. Individuals having a history of any cardiovascular event (myocardial infarction, unstable angina or stable angina, past coronary artery procedure, stroke or transient ischaemic attack), renal artery or kidney disease, peripheral vascular disease, congestive heart failure, and cancer or treatment for cancer in the last 2 years were excluded. Currently pregnant or breastfeeding mothers and anyone who has a recent weight loss at least 5% in the preceding 6 months, musculoskeletal conditions that precluded walking for 30 min, and using medication or other active lifestyle interventions known to affect body weight were also excluded. Absence of a mobile phone for personal use was not a reason for exclusion since those individuals would be provided a mobile phone for the purpose of the study.
2.4.3. Intervention
The eligible participants were assigned either to mHealth nutrition and lifestyle package (the intervention) or routine care (the control) after obtaining informed consent. The field investigators using the mobile application registered participants for the intervention. Upon registration, a welcome message is sent to participant's mobile phone. Since then, automated messages are delivered at a frequency of 2 voice and 2 text messages per week for 12 months. The option for direct calling from participant to investigator or vice-versa is available through a dedicated hotline for any assistance or clarification. The flow diagram of the communication pathways is illustrated in Appendix A.
If a participant does not wish to follow the intervention with or without a valid reason, he or she is allowed to discontinue it. The message delivery will be discontinued temporarily if a participant travels abroad, but resumed if she or he returns within a month. Intervention will be discontinued, if the data monitoring committee (DMC) recommends termination of trial.
Participants in both arms were not prevented attending any ongoing health promotional activities including any nutrition and healthy lifestyle interventions. However, they were advised not to follow any mHealth or eHealth interventions related to nutrition and healthy lifestyle or share the messages with others. The participants, irrespective of the arm, were instructed to continue their ongoing medical therapies if any, or to seek medical advice if any medical condition was detected during the baseline assessment.
2.4.4. Outcomes
The primary outcome is 10-year CVD risk, which is based on sex, age, smoking status, blood pressure, serum cholesterol (lipid profile), and glycaemic status. The 10-year CVD risk will be calculated and classified according to National Cholesterol Education Program Adult Treatment Panel-III (NECP-ATP-III) [27], and World Health Organization/International Society of Hypertension (WHO/ISH) [28] risk prediction tools. These global CVD risk scores are known to predict the likelihood of CVD events with reasonable predictive validity in the population [6,29,30]. The secondary outcomes are body weight, body mass index, waist circumference, abdominal obesity, waist-hip ratio, body composition (i.e., body fat percentage and fat free mass), dietary intake and physical activity. Median estimated 10-year CVD risk will be compared between the intervention and control arms on enrollment at the baseline and on completion of the 12-month intervention.
2.4.5. Sample size
Sample size was calculated using the primary outcome measure, the 10-year CVD risk using the formula described by Machin and Fayers [31] (Appendix B). The prevalence of 10-year CVD risk of 30% or above was approximately 14% in adults 35–64 years of age according to a previous study in a sub-urban area [6]. The present trial is primarily expected to recognize a reduction of 10-year CVD risk by one-third from its current value, from 14% to 8.5% as clinically significant following the intervention. A total sample size of 1200 is required to detect a difference at least of this size in 10-year CVD risk prevalence in the intervention group compared to the control, with a two-sided α-error of 0.05, a power of 80%, and an anticipated loss-to-follow up of 14%.
This sample size would also allow the study to detect a difference in the following secondary outcomes between the control and intervention arms respectively: Obesity (30% vs 20%); ‘unhealthy’ diet (50% vs 40%) and physical inactivity (33% vs 25%).
2.4.6. Recruitment
Participants were selected in two stages from the 15 MOH areas: In the first stage, 5 to 6 sub-divisions within each MOH area were selected. In the second stage, about 30–35 participants from each sub-division were identified and screened for eligibility. Only one eligible participant was chosen from a given family. As illustrated in the trial profile in Fig. 2, of the 2518 participants screened for eligibility, 1318 were excluded due to the reasons given in the text box.
Fig. 2.
mHealth nutrition and lifestyle intervention (mHENAL) trial profile.
2.4.7. Assignment of intervention/randomization
Each participant was randomized either to intervention or control arm, using stratified block randomization method. Stratification was based on sex (female or male), age category (35–49 years or 50–64 years) and BMI category (overweight or obese). Allocation sequence was generated in blocks of 2 for 600 blocks in total. This process would ensure almost equal number of participants between the 2 arms within each stratum. Block randomization is a commonly used technique in clinical trial design to reduce bias and achieve balance in the allocation of participants to treatment arms, especially when the sample size is small [32]. A web-based application, Research Randomizer (www.randomizer.org), was used to generate a random set of sequencing blocks [33].
2.4.8. Implementation of RCT
Those assigned to intervention arm were registered for the m-health nutrition and lifestyle intervention package by a field investigator using the mobile application. During registration, basic socio-demographic data (date of birth and sex), anthropometric data (weight and height), smoking and alcohol habits, mobile phone number of the participant, and the preferred language and time of the day to receive messages were recorded. These details were submitted via internet to the web application to synchronize the automated message delivery. The existing mobile phone connection of the participants was used to deliver the intervention. Those participants who did not possess a personal mobile phone were provided with a basic mobile phone (feature phone) and a new mobile connection for the purpose of the study.
Individuals in the control group are expected to continue their usual care/practices without any active intervention or interruption by the study. The usual care may include: visits to healthy lifestyle centre, nutrition clinics or any other health professionals, and following available information, education and communication material. There is no active promotion of the IEC material or clinic follow-up for the participants in the control group.
There is no blinding of participants about the assignment of intervention since the intervention occurs with their knowledge. Data collectors, except those who will be performing post-intervention qualitative inquiry, are unaware of the trial arm of the participants.
2.5. Data collection, management and analysis
2.5.1. Data collection methods
Data collection is done at the enrollment and after 12 months of intervention. Data collectors were graduates in Sociology or Health Promotion, and appropriately trained on administering questionnaire, anthropometry and ethical standards. Multiple data collection methods were used to gather necessary information including a face-to-face questionnaire-based interview, anthropometry, body composition assessment, examination for blood pressure and investigation of blood samples. Data collection at baseline was performed in two steps: firstly, a face-to-face questionnaire-based interview, and subsequently, anthropometric measurements, examination for blood pressure and collection of blood sample for biochemical investigations.
The timeline of RCT as per Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) template is given in Appendix C. Enrollment of participants and baseline assessments took place simultaneously, during the first 6 months. The intervention duration of 12 months was based on the potential time period for CVD risk reduction [15,18,34,35].
2.5.2. Questionnaire
A structured questionnaire was designed to obtain data on socio-demographics, smoking habits, alcohol consumption, chronic illness, dietary practices, dietary intake and physical activity. The survey questionnaire was pretested and modified accordingly. Certain sections of the questionnaire were adapted from the standard surveys or previously validated tools as described below.
Questions related to tobacco use, alcohol consumption, chronic illness and dietary practices were derived from the WHO STEPS instrument used in Sri Lanka non-communicable disease risk factor survey [5]. Dietary intake was assessed using food frequency questionnaire (FFQ) which was validated for Sri Lanka by Jayawardena et al. [36,37], with permission from the author. The FFQ consisted of 8 food groups containing the main foods comprising Sri Lankan adult diet, a total of 85 items and 12 color photographs to identify serving size. The FFQ has been validated against 7-day weighed-intake dietary records with significant positive correlations (p < 0.05) between the two methods for energy, carbohydrate, protein, fat and dietary fiber intake [37].
Physical activity was assessed by a short version of Physical Activity Questionnaire, which had been validated for urban settings in Sri Lanka by Arambepola et al. [38], with permission from the author. The tool comprises 16 questions and collects information on physical activity participation in three circumstances: activity at work, travel to and from places, and recreational activities as well as sedentary behaviour.
Participants in batches of 20–30 were requested to attend a pre-arranged location (a clinic centre, primary health care facility, community centre, office of a government organization or a community-based organization) in 12-hour overnight fasting state for subsequent assessments.
During the follow up of participants in the intervention arm, user dynamics and responses are extracted via monthly reports generated through the web application. On completion, a qualitative feedback will be obtained from participants about their experiences, strengths and areas for further improvement.
2.5.3. Anthropometry, body composition and blood pressure
Weight, height, waist circumference and hip circumference were measured by trained data collectors following standard procedure for anthropometric measurements [39]. Digital weighing scale (Seca 813, Hamburg, Germany), stadiometer (Seca 213, Hamburg, Germany), and ergonomic circumference measuring tape (Seca 201, Hamburg, Germany) were used. Body composition was assessed by 4-point, bio impedance analyzer (Omron HBF 375, Kyoto, Japan). The values given by the body composition analyzer for fat mass and fat free mass for the whole body and its regions were recorded. The measurements were made between 8.00 and 10.00 a.m., under uniform conditions regarding food and fluids of the subjects. Blood pressure was checked by a medically qualified investigator using digital blood pressure apparatus (Omron HEM 7320, Kyoto, Japan), as per the recommended procedure by the manufacturer. Measurements were validated against standard mercury sphygmomanometer, in every 10th participant to ensure reliability.
2.5.4. Biochemical investigations
Blood samples (6 ml of blood per person) were drawn in 12-hour fasting state using sterile venipuncture procedure by a phlebotomist. Blood samples were tested in 2 accredited laboratories using the recommended methods: HbA1c using high-performance liquid chromatographic assay (Bio-Rad D10, USA); fasting plasma glucose using glucose oxidase method (Randox Imola, UK); total cholesterol using cholesterol oxidase method (Randox Imola, UK); HDL cholesterol by direct clearance method (Randox Imola, UK) and triglycerides by GPO-PAP method (Randox Imola, UK). LDL cholesterol was calculated using Friedewald equation in samples with triglyceride less than 400 mg/dL [40]. All biochemical investigations were performed under direct supervision of a Chemical Pathologist.
2.5.5. Measures to enhance compliance and retention
The strategies adopted to enhance adherence to protocol included the options for participants to choose their preferred language and time slot, and monthly messages sent in question form requesting the participant to reply. Quarterly (once in 3 months) reminders by direct calling would also enhance the compliance. Individual compliance is monitored by automated status reports generated via web application every month.
Each participant was provided with his or her baseline report consisting of anthropometry, body composition, blood pressure, blood glucose, HbA1c and lipid profile, and informed the status by the principal investigator or a medically qualified research assistant. A referral letter was given for further medical care as required. All participants will be called individually to attend the endline assessment. Deviations from the protocol will be assessed in the final survey. Those who discontinue the trial will be interviewed over the phone for reasons for discontinuation.
2.6. Data management
2.6.1. Data entry
Data will be eneterd in SPSS software (Version 19.0). Food frequency data will be entered in a MS Excel spreadsheet designed to quantify the daily intake of food items, which will eventually be transferred to NutriSurvey (2007) software to generate nutrient profile. NutriSurvey is a free software for non-commercial use, which had been customized previously for Sri Lankan food by Jayawardena et al. [36,41].
Double entry will be performed for 20% of the data sheets in order to assess the reliability of data entry, which will be verified by simple cross-tabulations and appropriate statistical tests. Cronbach's alpha will be used to assess the internal consistency of the food frequency and physical activity questionnaire. Validity checks for key variables will be carried out by generating frequency distributions, cross-tabulations, correlations and Q-Q plots.
2.6.2. Statistical methods
Baseline characteristics (sex, age, BMI, smoking status, systolic and diastolic blood pressure, fasting plasma glucose, HbA1c, total cholesterol, HDL, LDL, VLDL and triglycerides) will be categorized according to standard cut-off values.
The primary outcome measure, the 10-year CVD risk, is calculated using WHO/ISH risk prediction charts, Framingham risk equation and NCEP-ATP-III tool [6,27,28]. The secondary outcomes include intake of ‘healthy’ diet, physical activity, waist-hip ratio, body fat percentage and fat fee mass etc.
Statistical significance for the difference of baseline characteristics and primary and secondary outcomes between the 2 arms will be examined by independent samples t-test (numeric data) and chi-square test (categorical data). Furthermore, baseline and endline comparison of the outcomes within the same arm will be tested for significance using paired t-test and Mc-Nemar test.
The analysis will be extended to estimate the relative risk ratio of 12-month to baseline 10-year CVD risk to compare the intervention arm with the control arm. Regression analyses will be performed using the endline 10-year CVD risk as the dependent variable, and the treatment arm (intervention or control) as the main predictor variable. Both binary logistic regression (having 10-year CVD risk of ≥30% in contrast to <30%) and linear regression (10-year CVD risk in a continuum) analyses will be performed. The potential covariates include education level, employment status, urbanicity of living area, body mass index, waist-hip ratio, dietary intake and physical activity. We would use constrained longitudinal data analysis (cLDA) model for analysis of treatment effects on 10-year CVD risk. In the cLDA model the baseline value as well as the post-randomization values are modeled as dependent variables [42].
Possible sub-group analyses by age, sex and BMI, mobile phone usage, and illness categories will be carried out to examine whether effects are modified by such parameters.
Analysis of effect will be based on intention-to-treat principle which would include all randomly assigned participants to address the issues due to non-compliance and missing outcome data. A modified approach described by Gupta [43] would be followed to include all randomized subjects only if complete outcome data are available at the follow-up assessment at 12 months, despite protocol deviations.
Statistical analyses will be guided by analytical procedure described in the Randomized Clinical Trials: Design, Practice and Reporting [31].
2.6.3. Data monitoring
A four-member DMC comprising a medical specialist, biostatistician, monitoring and evaluation expert, and representative of an ethics review committee was established. The DMC is independent of the sponsors and investigators and has no competing interests. The investigators are obliged to report any adverse outcome of the trial to the DMC immediately, and a regular summary of data in a prescribed format, every 3 months during implementation phase of the trial. The report structure includes the enrollment status (number of participants screened, eligible, consented, and randomized), subject status (number continued, completed or discontinued trial), stopping rules, safety concerns, protocol deviations and maintenance of quality of data.
An interim analysis report will be sent every 3 months to the DMC. The DMC is vested with the authority to recommend termination of trial based on its evaluation of these reports. The trial can be terminated due to safety concerns, outstanding benefit, or futility. In the case of major adverse events occurring among one or more participants the study would be terminated immediately pending further investigation. The anticipated major adverse events include any accident while receiving a voice message or reading a text message, a cardiac event or accident while engaging in physical exercise; a sickness attributed to dietary modification that required medical attention. The statistical criteria for outstanding benefit will consider a low p value (p < 0.001) as the threshold, a minimum sample size of 600 and a margin of 15% reduction in 10-year CVD risk, in an interim analysis. The study has not defined any statistical criteria for futility, but as a general rule, it is considered if the participation in the mHealth intervention drops below 33% in the intervention arm.
There is no separate auditing process of the trial. The study progress is reviewed by the following organizations via regular progress reports as part of their project monitoring process: National Research Council; Research and Higher Degrees Committee and Ethics Review Committee of the Faculty of Medicine, University of Colombo; and Sri Lanka Clinical Trials registry.
2.7. Ethics and dissemination
2.7.1. Ethics
The study is conducted conforming to standards of involving human subjects in research. Ethics clearance was obtained from the Ethics Review Committee of the Faculty of Medicine, University of Colombo (Ref No: EC-16-061). The trial has been registered in the Sri Lanka Clinical Trials Registry hosted by the Sri Lanka Medical Association (SLCTR/2016/018). Administrative clearance was obtained from the Ministry of Health, Nutrition and Indigenous Medicine, and stakeholder briefings were held with the MOH staff prior to the enrollment. So far there were no deviations from the protocol, however a mechanism is in place to report any deviations.
Informed written consent was obtained from the potential trial participants at enrollment before randomization. The participants were informed regarding the purpose and potential benefits and risks of the study, both verbally and through an information sheet. The contact details of the PI have been provided to inform any unintended effects of trial interventions or any behaviour change as a consequence of it.
Biological specimens will not be used in ancillary studies. The identification details such as name, address and contact number is detached from the main questionnaire and kept separately, and a specific identification number is used to link up the two. Strict confidentiality of data is assured, and no information will be revealed in a manner that participants’ identity could be found. None of the contractual agreements with funders or other parties has requested access to data. Paper based data records will be destroyed after 5 years. All identification details of electronic data will be deleted permanently after 5 years.
There are no anticipated risks to participants by engaging in the intervention. The participants have freedom to withdraw even without prior notice or without any reason. Participants have been advised to continue their regular medication or health care irrespective of the intervention arm. Any individuals detected with health issues which have not been paid medical attention are referred for appropriate medical/health care through a referral letter. Medical treatment or advise is not provided by the investigators.
2.7.2. Dissemination
All participants were informed the results of the investigations performed at the enrollment, and will be given the corresponding figures after one year. The proposed research will result in a mHealth nutrition and lifestyle improvement package, and policy relevant evidence regarding its feasibility and effectiveness. The outcome of research will be disseminated at a seminar for national level stakeholders with the aim of providing policy relevant evidence to scale up the above package in the health system. Publications in indexed journals and presentations at international conferences will be made to inform the wider scientific community. Web-based and mass media-based communications will also be made for the benefit of the public.
Essential details of the protocol are available in Sri Lanka clinical trials registry (https://slctr.lk/trials/slctr-2016-018). The full protocol will not be made available to the public since it has personal data of participants. Additional details such as questionnaire and code books will be shared on request for non-commercial purposes.
3. Discussion
According to literature, the evidence on the effects of mHealth on cardiometabolic conditions and other non-communicable diseases within the heath care systems is limited in low- and middle income countries due to lack of methodological rigor despite several mHealth projects [19,44,45]. Our study will be an innovative approach since mHealth has not been previously tested for CVD risk reduction in Sri Lanka. If the mHealth nutrition and lifestyle improvement package is proven to be effective, then there is a great potential for integration it into the existing health system and scaling up for other parts of the country. Eventually, this would contribute to a slowdown in the rapidly increasing disease burden and mortality due to cardio-vascular disease.
We selected Colombo district as the study setting, since it is the most urban district with the highest burden of non-communicable disease in the country [5,46]. The inclusion criteria of middle-aged individuals who are overweight or obese, capture the most vulnerable group for CVD. Although the proposed study is a population-based trial, the intervention is delivered at individual level. Thus, we designed and conducted an individually randomized trial as opposed to a cluster randomized trial which is common in community-based interventions. One advantage of individually randomized trial over the cluster randomized trial is the smaller sample size for the same statistical power since there is no clustering effect. Further, there is a higher possibility to equalize distribution of background characteristics and certain confounding variables between the 2 arms through stratification by age groups, sex and BMI categories before random allocation of intervention. Since these 3 factors are key predictors of CVD risk we assume that the primary and secondary outcomes are also equally distributed between the 2 arms at the baseline.
The methods of this study followed an established process for development and testing of mHealth interventions, that has been described previously by the Clinical Trial Research Unit, University of Auckland, New Zealand [20]. The trial was preceded by a formative study including stakeholders from different capacities and levels. Engagement of target population and key stakeholders in formative research is a strength of the proposed trial to develop a tailor-made intervention that will enhance acceptability, in addition to the scientific rigor of the RCT design. The formative study helped us to identify and understand the community attitudes, interests and behaviour, as well as needs of the individuals. Furthermore, formative study led the investigators to find the best technological solution for the mHealth application and its integration to a mobile network. Formative study is an integral part of large-scale community trials [20].
The proposed design, i.e., the two-arm, parallel-group RCT is consistent with many other mHealth trials. Larger sample size of the present RCT (n = 1200) in contrast to many previous RCT on mHealth is a strength. In consistent with many previous trials, the outcomes will be evaluated after 12 months of intervention [15,17].
The messages were carefully developed through a series of discussions with nutrition experts, clinical specialists and behavioral scientists. The intervention package was underpinned by appropriate health behavioral change theories or models. The investigators considered some of the influential theories/models such as health belief model (HBF), social cognitive theory (SCT), information-motivation-behavioral skills model (IMB), and tailored health communications (THC) [25]. Use of both voice and text messages has added benefit than a single mode, since the participants can read the text messages in a sequence later on. Almost all messages are applicable to general middle-aged population irrespective of their disease status other than those excluded on enrollment. Customization the programme to a certain level, i.e., preferred language, time of the day, and smoking and drinking status would lead to a more personalized intervention.
The risk of bias in this trial has been minimized by taking adequate precautions, as per criteria described in the Cochrane Risk of Bias Assessment Tool [47]. The precautions to minimize biases include random sequence generation (selection bias), blinding of the outcome assessment (detection bias), and use of standardized tools and procedure (measurement bias).
There was no restriction for any participant to attend healthy lifestyle centers (HLC) or similar facilities that are being implemented currently by the Ministry of Health. Based on the low utilization of HLC at present [48], we anticipated a small number of trial participants who would be attending such centers during the trial period. Even that number will be equally distributed due to randomization, so that confounding effect would be minimal. Information about their engagement in such activities will be obtained at the endline survey, in order to make adjustment in the analysis of effectiveness of the m-Health intervention.
Intention-to-treat analysis would avoid problems due to non-compliance and missing outcome data, and provide a more conservative estimate of the treatment effect. We would attempt an updated approach as described by Gupta (2011) to include all randomized subjects only if outcome data are available, while making an attempt to assess these participants at the endline despite their protocol non-adherence [43].
The constrained longitudinal data analysis (cLDA) model proposed for analysis of treatment effects on 10-year CVD risk provides fairly robust results in the presence of missing data. Another benefit is that it assumes no differences of 10-year CVD risk between two arms, therefore can remedy the missing data issue at baseline to some point [49]. In addition, a mixed-effects model comprising time effect (12 month follow-up compared with baseline), group effect (intervention vs control), and group-by-time interaction will be tried out to examine such effects.
The present mHealth trial is simple, low-cost and compatible with any type of mobile phones either feature phones or smart phones, therefore, is more likely to be integrated into existing health-care systems in a low- and middle-income country including Sri Lanka. The trial, if found effective, would offer a feasible and scalable solution to reduce cardiovascular disease risk among middle-aged adults who are overweight or obese, through improving their nutrition and healthy lifestyle behaviour.
Trial status
All participants have being recruited, and the intervention is in progress.
Clinical trial registration
The trial was registered in the Sri Lanka Clinical Trials Registry (SLCTR/2016/018). Available at https://slctr.lk/trials/slctr-2016-018.
Ethics approval and consent to participate
Ethics clearance has been obtained from the Ethics Review Committee of the Faculty of Medicine, University of Colombo (Ref No: EC-16-061). Informed written consent was obtained by the data collectors from the potential trial participants at enrollment before randomization to assign the intervention.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Conflicts of interest
The authors declare that there are no conflicts of interest to report or on any of the components of this trial.
Funding
The study is primarily funded by the National Research Council, Sri Lanka (NRC -15-145). Additional financial support is provided by the University of Colombo, Sri Lanka (Uni. Res. Grants 2017) and Medical Research Institute of the Ministry of Health, Nutrition and Indigenous Medicine, Sri Lanka (MRI 11-2018). All 3 funding agencies reviewed the protocol anonymously prior to approval of financial support and monitor the progress of the study through annual progress reports. None of these agencies will have any intervening or ultimate authority over any of the following: study design; collection, management, analysis, and interpretation of data; writing of the report; or the decision to submit the report for publication.
Roles of protocol contributors
US conceptualized the research, conducted literature review, developed the methodology including the study instruments, developed the intervention package, and wrote the manuscript.
PK provided inputs in the background, guidelines for measurements, provided inputs for the intervention package and critically reviewed the manuscript.
DNF reviewed the protocol, and provided expertise in the development of study tools and intervention package.
NSK reviewed the protocol, and conducted literature review, and provided expertise for nutrition related inputs in the study tools and intervention package and revised the manuscript.
KP provided inputs in literature survey, translated and pretested study tools, coordinated field work, collected data and assisted in developing the intervention package.
RJ provided the food frequency questionnaire validated by him previously, contributed to the development of the m-health package, assisted in data management and revised the manuscript.
GK supervised collection and laboratory testing of the blood samples, drafted the sections on methods and reviewed and revised the manuscript.
MJD reviewed the protocol, improved the methods, and provided expertise in the development of study tools, and edited the manuscript.
Acknowledgements
The authors wish to acknowledge the Ministry of Health, Nutrition and Indigenous Medicine for granting permission and facilitating this study. The project was primarily funded by the National Research Council, Sri Lanka (NRC 15-145) with additional support from the University of Colombo, Sri Lanka (Uni. Res. Grants 2017), and Medical Research Institute, Sri Lanka (MRI 11-2018). We specially thank Prof. Manuj C. Weerasinghe for guidance in qualitative research, Mr. Dhanajaya Premarathna of Data Management Systems Ltd. for software development and Mr. Nalin Senarathne and team at Mobitel (Pvt) Ltd. for connectivity services. Support by following persons is appreciated with gratitude: Dr. Claudio Umesh, Dr. Samitha Prasanna, Mr. Nimal Rathnasiri, Ms. Iresha Sanjeewani, Ms. Priya Jayawardena, Ms. Kanchana Kumudumali, and Ms. Krishni Fernando.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.conctc.2019.100453.
Contributor Information
Upul Senarath, Email: upul@commed.cmb.ac.lk.
Prasad Katulanda, Email: prasad.katulanda@yahoo.com.
Dulitha N. Fernando, Email: dulithafernando@hotmail.com.
Nishan S. Kalupahana, Email: skalupahana@pdn.ac.lk.
Kunarathinam Partheepan, Email: coolparthee@gmail.com.
Ranil Jayawardena, Email: ranil@physiol.cmb.ac.lk.
Gaya Katulanda, Email: gwijeweera@yahoo.com.
Michael J. Dibley, Email: michael.dibley@sydney.edu.au.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Lozano R., Naghavi M., Foreman K. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012 doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naghavi M., Wang H., Lozano R. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015 doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ministry of Health Nutrition and Indigenous Medicine . 2015. Annual Health Bulletin. Colombo, 2015. [Google Scholar]
- 4.Jayawardene J.B., Samarutilake G.D.N., Zackie M.H.M., De Silva V., Karunanayake A., Weerasooriya M.A. Prevalence of coronary artery disease in a semi urban population in Southern Sri Lanka. Ceylon Med. J. 2017;62:34–39. doi: 10.4038/cmj.v62i1.8432. [DOI] [PubMed] [Google Scholar]
- 5.Ministry of Health Nutrition and Indigenous Medicine . 2015. Non-communicable Disease Risk Factor Survey. Sri Lanka, Colombo. [Google Scholar]
- 6.Ranawaka U.K., Wijekoon C.N., Pathmeswaran A., Kasturiratne A., Gunasekera D., Chackrewarthy S., Kato N., Wickramasinghe A.R. Risk estimates of cardiovascular diseases in a Sri Lankan community. Ceylon Med. J. 2016;11:11–17. doi: 10.4038/cmj.v61i1.8253. [DOI] [PubMed] [Google Scholar]
- 7.Yusuf S., Hawken S., Ôunpuu S., Dans T., Avezum A., Lanas F., McQueen M., Budaj A., Pais P., Varigos J., Lisheng L. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. The Lancet. 2004;364(9438):937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 8.Gamlath L., Nandasena S., Hennadige Padmal De Silva S., Linhart C., Ngo A., Morrell S., Nathan S., Sharpe A., Taylor R. Differentials in cardiovascular risk factors and diabetes by socioeconomic status and sex in kalutara, Sri Lanka, Asia-Pacific. J. Public Health. 2017;29 doi: 10.1177/1010539517709028. [DOI] [PubMed] [Google Scholar]
- 9.Katulanda P., Constantine G.R., Mahesh J.G., Sheriff R., Seneviratne R.D.A., Wijeratne S., Wijesuriya M., McCarthy M.I., Adler A.I., Matthews D.R. Prevalence and projections of diabetes and pre-diabetes in adults in Sri Lanka - Sri Lanka Diabetes, Cardiovascular Study (SLDCS) Diabet. Med. 2008 doi: 10.1111/j.1464-5491.2008.02523.x. [DOI] [PubMed] [Google Scholar]
- 10.Katulanda P., Ranasinghe P., Jayawardena R., Constantine G.R., Rezvi Sheriff M.H., Matthews D.R. The prevalence, predictors and associations of hypertension in Sri Lanka: a cross-sectional population based national survey. Clin. Exp. Hypertens. 2014 doi: 10.3109/10641963.2013.863321. [DOI] [PubMed] [Google Scholar]
- 11.Katulanda P., Dissanayake H.A., De Silva S.D.N., Katulanda G.W., Liyanage I.K., Constantine G.R., Sheriff R., Matthews D.R. Prevalence, patterns, and associations of dyslipidemia among Sri Lankan adults—Sri Lanka Diabetes and Cardiovascular Study in 2005–2006. J. Clin. Lipidol. 2018;12 doi: 10.1016/j.jacl.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Katulanda P., Ranasinghe P., Jayawardana R., Sheriff R., Matthews D.R. Metabolic syndrome among Sri Lankan adults: prevalence, patterns and correlates. Diabetol. Metab. Syndrome. 2012 doi: 10.1186/1758-5996-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jayawardena R., Byrne N.M., Soares M.J., Katulanda P., Hills A.P. The obesity epidemic in Sri Lanka revisited. Asia Pac. J. Public Health. 2015;27 doi: 10.1177/1010539512464650. [DOI] [PubMed] [Google Scholar]
- 14.Ramachandran A., Kumar R., Nanditha A., Snehalatha C., Krishnamoorthy S., Joshi P., Tesfaye F. 2018. mDiabetes Initiative Using Text Messages to Improve Lifestyle and Health-Seeking Behaviour in India; pp. 1–8. [Google Scholar]
- 15.Rubinstein A., Miranda J.J., Beratarrechea A., Diez-Canseco F., Kanter R., Gutierrez L., Bernabé-Ortiz A., Irazola V., Fernandez A., Letona P., Martínez H., Ramirez-Zea M., Alasino A.A., Cuesta L.L., Moscoso B.N.B., Surichaqui J.E., Estrada L.P., Ramírez C.M., de la Cruz G.R., Drago J.C.S., Loayza J.A.Z., Carrara C., Giardini G., Guevara J., Juárez A.M., Salguero J., Lewitan D., Urtasún M. Effectiveness of an mHealth intervention to improve the cardiometabolic profile of people with prehypertension in low-resource urban settings in Latin America: a randomised controlled trial. Lancet Diabetes Endocrinol. 2016 doi: 10.1016/S2213-8587(15)00381-2. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization . 2011. mHealth: New Horizons for Health through Mobile Technologies: Global Health Observatory for eHealth Series. [Google Scholar]
- 17.Partridge S.R., McGeechan K., Hebden L., Balestracci K., Wong A.T., Denney-Wilson E., Harris M.F., Phongsavan P., Bauman A., Allman-Farinelli M. Effectiveness of a mHealth lifestyle program with telephone support (TXT2BFiT) to prevent unhealthy weight gain in young adults: randomized controlled trial. JMIR mHealth and uHealth. 2015;3(2):e66. doi: 10.2196/mhealth.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin S.S., Feldman D.I., Blumenthal R.S., Jones S.R., Post W.S., McKibben R.A., Michos E.D., Ndumele C.E., Ratchford E.V., Coresh J., Blaha M.J. mActive: a randomized clinical trial of an automated mHealth intervention for physical activity promotion. J. Am. Heart Assoc. 2015;9(11) doi: 10.1161/JAHA.115.002239. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Müller A.M., Alley S., Schoeppe S., Vandelanotte C. The effectiveness of e-& mHealth interventions to promote physical activity and healthy diets in developing countries: a systematic review. Int. J. Behav. Nutr. Phys. Act. 2016;13 doi: 10.1186/s12966-016-0434-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whittaker R., Merry S., Dorey E., Maddison R. A development and evaluation process for mhealth interventions: examples from New Zealand. J. Health Commun. 2012 doi: 10.1080/10810730.2011.649103. [DOI] [PubMed] [Google Scholar]
- 21.Ministry of Health . second ed. Nutrition Division, Ministry of Health; Colombo: 2011. Food Based Dietary Guidelines for Sri Lankans.http://203.94.76.60/departmnt/NutritionDivision/NutritionGuidelines/FBDG-English.pdf [Google Scholar]
- 22.Jayawardena R. Colombo Medical Faculty Publishers; Colombo: 2017. My Rice Plate - an Evidence Based Approach to Lose Excess Body Weight. [Google Scholar]
- 23.Jayawardena R., Fernando P., Lokunarangoda N., Pathirana A.K. Effects of the “plate model” as part of dietary intervention on modification of selected cardiometabolic risk factors in post-myocardial infarction patients: study protocol for a randomized controlled trial. Trials. 2017;18:1–11. doi: 10.1186/s13063-017-2057-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization . Geneva World Heal. Organ.; 2011. Global Recommendations on Physical Activity for Health, 18-64 Years Old. [Google Scholar]
- 25.Cho Y.M., Lee S., Islam S.M.S., Kim S.Y. Theories applied to m-health interventions for behavior change in low- and middle-income countries: a systematic review. Telemed. e-Health. 2018;24 doi: 10.1089/tmj.2017.0249. tmj.2017.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Department of Census and Statistics . 2012. Census of Population and Housing, Colombo. [Google Scholar]
- 27.NCEP . NIH Publ; 2001. Third Report of the National Cholesterol Education Program (NCEP) [Google Scholar]
- 28.Mendis S., Lindholm L.H., Anderson S.G., Alwan A., Koju R., Onwubere B.J.C., Kayani A.M., Abeysinghe N., Duneas A., Tabagari S., Fan W., Sarraf-Zadegan N., Nordet P., Whitworth J., Heagerty A. Total cardiovascular risk approach to improve efficiency of cardiovascular prevention in resource constrain settings. J. Clin. Epidemiol. 2011 doi: 10.1016/j.jclinepi.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Otgontuya D., Oum S., Buckley B.S., Bonita R. Assessment of total cardiovascular risk using WHO/ISH risk prediction charts in three low and middle income countries in Asia. BMC Public Health. 2013;13 doi: 10.1186/1471-2458-13-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jukema J.W., Mccormack T., Catapano A.L. 2010. Barriers to Cardiovascular Disease Risk Scoring and Primary Prevention in Europe; pp. 727–739. [DOI] [PubMed] [Google Scholar]
- 31.Machin D., Fayers P.M. 2011. Randomized Clinical Trials: Design, Practice and Reporting. [Google Scholar]
- 32.Suresh K. An overview of randomization techniques: an unbiased assessment of outcome in clinical research. J. Hum. Reprod. Sci. 2011 doi: 10.4103/0974-1208.82352. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Urbaniak Geoffrey C., Plous S. 1997. Research Randomizer.https://www.randomizer.org [Google Scholar]
- 34.Rees K., Dyakova M., Wilson N., Ward K., Thorogood M., Brunner E. Dietary advice for reducing cardiovascular risk. Cochrane Database Syst. Rev. 2013 doi: 10.1002/14651858.CD002128.pub4. [DOI] [PubMed] [Google Scholar]
- 35.Maruthur N.M., Wang N.Y., Appel L.J. Lifestyle interventions reduce coronary heart disease risk results from the premier trial. Circulation. 2009 doi: 10.1161/CIRCULATIONAHA.108.809491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jayawardena R., Swaminathan S., Byrne N.M., Soares M.J., Katulanda P., Hills A.P. Development of a food frequency questionnaire for Sri Lankan adults. Nutr. J. 2012;11:2–7. doi: 10.1186/1475-2891-11-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jayawardena R., Byrne N.M., Soares M.J., Katulanda P., Hills A.P. Springerplus; 2016. Validity of a Food Frequency Questionnaire to Assess Nutritional Intake Among Sri Lankan Adults. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arambepola C., Ekanayake R., Fernando D. Gender differentials of abdominal obesity among the adults in the district of Colombo, Sri Lanka. Prev. Med. 2007 doi: 10.1016/j.ypmed.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention . 2007. Anthropometry Procedures Manual. Atlanta. [Google Scholar]
- 40.Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 41.Erhardt Juergen. 2007. NutriSurvey for Windows. [Google Scholar]
- 42.Liang K.Y., Zeger S. Longitudinal data analysis of continuous and discrete responses for pre–post designs. Sankhya: The Indian Journal of Statistics (Series B) 2000;62:134–148. [Google Scholar]
- 43.Gupta S. Intention-to-treat concept: a review. Perspect. Clin. Res. 2011 doi: 10.4103/2229-3485.83221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beratarrechea A., Lee A.G., Willner J.M., Jahangir E., Ciapponi A., Rubinstein A. The impact of mobile health interventions on chronic disease outcomes in developing countries: a systematic review. Telemedicine and e-Health. 2014;20(1):75–82. doi: 10.1089/tmj.2012.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beratarrechea A., Diez-Canseco F., Irazola V., Miranda J., Ramirez-Zea M., Rubinstein A A. Use of m-health technology for preventive interventions to tackle cardiometabolic conditions and other non-communicable diseases in Latin America-challenges and opportunities. Prog. Cardiovasc. Dis. 2016;58(6):661–673. doi: 10.1016/j.pcad.2016.03.003. 1. [DOI] [PubMed] [Google Scholar]
- 46.Department of Census and Statistics . 2014. National Survey on Self-Reported Health in Sri Lanka.http://www.statistics.gov.lk/social/National Survey on Self-reported Health-2014.pdf Colombo. [Google Scholar]
- 47.Higgins J.P.T., Green S., editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. http://handbook.cochrane.org [updated March 2011] Available from: [Google Scholar]
- 48.Mallawaarachchi D.V., Wickremasinghe S.C., Somatunga L.C., Siriwardena V.T., Gunawardena N.S. Healthy Lifestyle Centres: a service for screening noncommunicable diseases through primary health-care institutions in Sri Lanka. WHO South-East Asia journal of public health. 2016;5(2):89. doi: 10.4103/2224-3151.206258. 1. [DOI] [PubMed] [Google Scholar]
- 49.Liu G.F., Lu K., Mogg R., Mallick M., Mehrotra D.V. Should baseline be a covariate or dependent variable in analyses of change from baseline in clinical trials? Stat. Med. 2009;28(20):2509–2530. doi: 10.1002/sim.3639. 10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.