ABSTRACT
Background: Esophageal cancer is a highly aggressive neoplasm. Targeted therapy has been proven to be a promising way for cancer therapy. Here, we report a novel anti-CD147 antibody for esophageal cancer therapy, which is a chimeric antibody with modified glycoform in Fc region.
Methods: ADCC assay was used to explore the antitumor efficacy of Metuzumab against esophageal cancer in vitro. Wound healing assay and Boyden Chamber invasion assay were performed to explore whether Metuzumab could inhibit migration and invasion of esophageal cancer in vitro. Insulin-like growth factors 1 (IGF-1) and PI3k/Akt was assayed for elaborating antagonistic mechanism of Metuzumab in migration and invasion of esophageal cancer cells. Subcutaneous xenograft nude mouse model was used to investigate the antitumor efficacy of Metuzumab against esophageal cancer in vivo. The esophageal cancer tissue microarrays (TMA) was examined for identification of association of CD147 with lymph node metastasis, and the footpad xenograft nude mouse model was used to explore whether Metuzumab could inhibit lymph node metastasis of esophageal cancer in vivo.
Results: The results showed that Metuzumab exhibited higher ADCC compared to the wild type antibody cHAb18. Metuzumab inhibited migration and invasion of esophageal cancer through blockade of CD147 in vitro. The results of Western blot showed Metuzumab might inhibit migration and invasion of esophageal cancer cells through suppressing activation of PI3k/Akt and expression of IGF-1. Experiments in vivo showed that Metuzumab exhibited significant antitumor efficacy and inhibited lymph node metastasis of esophageal cancer in xenograft models. The immunochemical staining of TMA showed CD147 was high-expressed on various kinds of esophageal cancer tissues and associated with the grade of lymph node-metastasis.
Conclusions: The in vitro and in vivo study demonstrated dual effects of Metuzumab in effectively mediating ADCC by activating effector cells, and inhibiting metastasis of esophageal cancer through blockade the function of CD147, providing justification for moving Metuzumab forward to clinical development in esophageal cancer.
KEYWORDS: Esophageal cancer, Metuzumab, ADCC
Introduction
Esophageal cancer is a highly aggressive neoplasm, which is the eighth leading cause of cancer-related mortality worldwide.1 According to the data released by GLOBOCAN 2018, more than 500 thousands of people died of esophageal cancer each year.1 Surgery, in conjunction with radiation and chemotherapy, is the major treatment for esophageal cancer.2 However, the 5-year overall survival rate remains poor because of high rate of tumor recurrence and lymph node metastasis.3
In recent years, targeted therapies brought hope for patients with esophageal cancer.4 Immunotherapy using mAbs is considered as an effective and safe method for the treatment of esophageal cancer.5 Ramucirumab (anti-VEGF receptor 2 antibody) has been reported to improve survival as second-line therapy in phase III trials.6 Claudiximab, against a tight cell junction protein CLDN18.2, have encouraging effects in esophageal cancer therapy. It exerted its dual mode of action by interfering function of CLDN18.2 and activating complement-dependent cellular cytotoxicity (CDCC) and antibody-dependent cellular cytotoxicity (ADCC).7 However, the mAbs have poor curative effect on tumor metastasis. Thus, mAbs that inhibit tumor proliferation and metastasis at the same time could be exciting to pursue in esophageal cancer.
CD147, also known as EMMPRIN (extracellular matrix metalloproteinase inducer) or Basigin, is a type I transmembrane glycoprotein, belonging to the immunoglobulin superfamily.8 It is well established that CD147 is broadly and highly expressed in various types of cancer tissues, such as esophageal cancer, hepatocellular carcinoma, breast cancer, etc.9–12 CD147 expressed on cancer cells induces the production of various matrix metalloproteinases (MMPs) in cancer cells and fibroblasts, leading to degradation of extracellular matrix, which facilitates cancer cell invasion and metastasis.13 Other functions of CD147 include inducing angiogenesis, regulating metabolism, and conferring resistance to chemotherapeutic drugs.14 The overexpression of CD147 in cancer tissues is correlated with poor prognosis of patients with several types of solid tumors, including esophageal cancer.15,16 These suggest that the effects of a CD147 targeting agent may result in inhibition of tumor proliferation, invasion, and metastasis.
To date, clinical studies examining CD147 therapeutic antibodies have been limited to hepatocellular cancer (HCC). We previously developed a therapeutical anti-HCC radioimmunological agent, Licartin (generic name, Iodine [131I] Metuximab injection), which was generated by labeling131I with the murine anti-CD147 mAb fragment HAb18 F(ab’)2. Licartin has been proven efficient and safe in clinical practice and approved for the clinical therapy of HCC in China (approval number S20060064).17 However, radiation and the human anti-mouse antibody (HAMA) response limit the administration of Licartin.
Metuzumab is a novel IgG1 chimeric monoclonal antibody targeting the CD147 antigen. The constant fragment (Fc) domain of Metuzumab is modified with a variant glycoform that enhances its antibody-dependent cell-mediated cytotoxicity (ADCC) potency and efficacy by increasing its affinity to the activating Fcγ receptor of effector cells. In this manuscript, we describe the in vitro and in vivo properties of this novel anti-CD147 antibody.
Results
Avidity assay for Metuzumab
To assess the binding activity of Metuzumab for CD147, we first performed a surface plasmon resonance (SPR) assay. The result demonstrated that two CD147 antibodies, Metuzumab and cHAb18, bind the CD147 antigen with nearly identical avidity (KD values of 3.62 × 10−10M and 4.75 × 10−10M, respectively) (Figure 1(b)). The results indicated that Metuzumab retained high avidity for CD147 antigen, and the binding activity was not altered with a variant glycosylation pattern.
Figure 1.
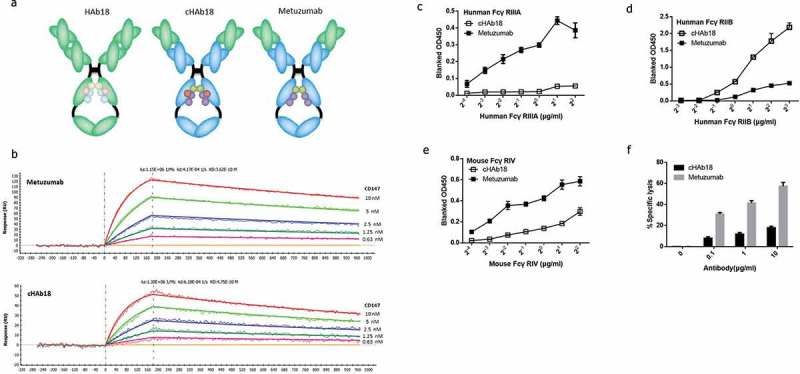
Development of Metuzumab.
(a) Diagram of the development of Metuzumab. (b) Binding affinity for CD147 of Metuzumab and the wild-type antibody cHAb18. Surface plasmon resonance traces of 10μg/mL Metuzumab (top) and 10μg/mL cHAb18 (bottom) binding to CD147. The binding curves for CD147 were determined at0.63, 1.25, 2.5, 5 and 10 nM. (c) Metuzumab exhibited increased binding affinity for human FcγRIIIA (an activating FcγR) compared with cHAb18. (d) Metuzumab exhibited decreased binding affinity for human FcγRIIB (an inhibitory FcγR) compared withcHAb18. (e) Metuzumab exhibited increased binding affinity for mouse FcγRIV (an activating FcγR) compared with cHAb18. (The binding of FcγRs was determined by ELISA.) (f) Metuzumab enhanced ADCC compared with the wild-type antibody cHAb18 with effector cells derived from nude mice. The error bars represent the SEMs.
Metuzumab exhibited higher binding affinity for activating FcγRs but lower binding affinity for inhibitory FcγR
We then used ELISA to assess whether the binding activity of Metuzumab to the activating or inhibitory FcγRs were changed according to its modified glycosylation. The result showed that the binding affinity for human FcγRIIIA (an activating FcγR) (Figure 1(c)) of Metuzumab was markedly increased and that its binding affinity for human FcγRIIB (an inhibitory FcγR)(Figure 1(d)) was decreased compared with cHAb18.We also assessed whether the binding activity of Metuzumab to mouse activating FcR could be enhanced, because only when Metuzumab was able to bind to the activating FcγR on the surface of mouse immune cells, it could exert ADCC in mouse xenografts models. Our results showed that Metuzumab displayed increased affinity for mouse FcγRIV compared with cHAb18 (Figure 1(e)). Thus, Metuzumab presents substantially increased binding affinity for both human and mouse activating FcγRs and decreased affinity for human inhibitory FcγR.
Moreover, we used nude mouse splenocytes as effector cells in an ADCC assay for the Eca-109 cell line. The result showed that Metuzumab enhances ADCC compared with the wild type antibody cHAb18 using effector cells derived from nude mice (Figure 1(f)), which was similar to the result obtained using human effector cells, suggesting that a nude mice xenograft model may be an applicable model for testing the therapeutic benefit of Metuzumab.
Metuzumab augmented ADCC in esophageal cancer cell lines
Variations in composition of the carbohydrate, saccharide, or sugar molecule, have been shown to affect the affinity of IgG for FcγRs that link IgG-mediated immune response with cellular effector functions. To assess whether Metuzumab facilitates immune effector cell function, we performed in vitro ADCC assay using PBMC cells as effectors and two human esophageal cancer cell lines as targets. The real-time cell analysis showed that cell viability was sustainedly inhibited in Metuzumab, cHAb18 or Cetuximab group; however, the cell viability was increased sharply during continuous culture with Hu-IgG or without antibody treatment. Metuzumab significantly augmented ADCC in esophageal cancer cell lines compared to the wild type antibody cHAb18. (Figure 2(a–d)).
Figure 2.

Metuzumab augmented ADCC against esophageal cancer cells.
(a)(b) Cell viability of Eca-109 and TE-1 cells in existence of PBMC and mAb. (c)(d) Metuzumab enhanced ADCC compared to the wild type antibody cHAb18 in both two esophageal cancer cell lines. Error bars represent SEMs. (e) ADCC mediated by Metuzumab was suppressed by granzyme B and perforin inhibitors. (f) Metuzumab increased IFN-γ production by effector cells.
Metuzumab-mediated ADCC against esophageal cancer was mediated by perforin/granzyme B
We further explored the mechanism through which effector cells mediate cytotoxicity against Metuzumab-bearing esophageal cancer cells. Direct and antibody-dependent NK cell killing can occur by perforating the cell membrane with perforin followed activating caspases with granzyme B. The incubation of PBMCs (containing NK cells) with the specific perforin inhibitor CMA (Concanamycin A), specific granzyme B inhibitor DCI (3,4-dichloroisocoumarin), or both CMA and DCI before the co-culture of PBMCs with the target esophageal cancer cells Eca-109 (E/T ratio 50:1) resulted in a significant reduction in the magnitude of ADCC induced by Metuzumab (DCI: 54.8% decrease, P = .0083,; CMA: 53.7% decrease, P = .0079;DCI +CMA: 72.3% decrease, P = .0013) compared with DMSO (Figure 2(e)). The suppression of ADCC mediated by cHAb18 was also observed with granzyme B and perforin inhibitors in a similar manner but lower. The suppression of ADCC supported the finding that perforin and granzyme B play important roles in ADCC induced by Metuzumab.
Metuzumab increased IFN-γ secretion
IFN-γ released from NK cells is another important cytokine involved in innate immune-mediated tumor cell clearance. The production of IFN-γ can be stimulated by the engagement of FcγR on the surface of NK cells and serves as a reliable marker of their activation. We stimulated PBMCs (containing NK cells) from healthy donors with the esophageal cancer cells Eca-109 and antibody or antibody alone for 24 h. The supernatants were harvested and analyzed to detect the presence of soluble IFN-γ. The results showed that the existence of tumor cells activated NK cells to release IFN-γ and that Metuzumab enhanced this function. Our results showed that Metuzumab induced an increased production of IFN-γ in the absence of tumor cells compared with cHAb18 (1.27-fold higher; P < .0001) or medium control (1.58-fold higher; P < .0001). Metuzumab induced a remarkable increased production of IFN-γ in the presence of tumor cells compared with cHAb18 (3.11-fold higher; P < .0001) or medium control (3.01-fold higher; P < .0001) (Figure 2(f)).
Metuzumab inhibited growth of subcutaneous xenograft esophageal cancer
To investigate whether Metuzumab has antitumor efficacy in vivo, we used Eca-109 subcutaneous xenograft esophageal cancer models in nude mice. Animals were randomized to receive human IgG control (2mg/kg), cHAb18 (2mg/kg), and Metuzumab (HcHAb18) (0.4mg/kg, 2mg/kg or 10mg/kg).
Our resuts showed that the tumor weight was significantly inhibited with Metuzumab treatment in a dose-dependent manner. Even one-fifth dose of Metuzumab had significant tumor-inhibiting effects compared with cHAb18 group (P < .001) (Figure 3).
Figure 3.

Metuzumab inhibited growth of subcutaneous xenograft esophageal cancer.
(a)(b) Inhibited effects of Metuzumab (HcHAb18) (0.4mg/kg, 2mg/kg or 10mg/kg) in xenograft esophageal cancer model compared with human IgG control (2mg/kg) and cHAb18 (2mg/kg).
Metuzumab inhibited migration and invasion of esophageal cancer cell lines in vitro
To evaluate whether Metuzumab can inhibit migration of esophageal cancer cells, we measured the migration potential of Eca-109 cells co-cultured with Metuzumab (0.1μg/mL, 1μg/mL, or 10μg/mL) or cHAb18 (10μg/mL) by wound healing assay. Metuzumab treatment (0.1μg/mL, 1μg/mL, 10μg/mL) significantly decreased the migration distance (Figure 4(a)) by 16.03%, 38.16%, 66.41% (Figure 4(c)) respectively, compared with control group. Similar results were found in cHAb18 treatment group.
Figure 4.

Metuzumab inhibited migration and invasion of esophageal cancer cell lines in vitro.
(a)(c) Metuzumab inhibited migration of esophageal cancer cell Eca-109. (b)(d) Metuzumab inhibited invasion of esophageal cancer cell Eca-109. Error bars represent SEMs. (e) Quantitative real-time PCR was specifically performed for IGF-1 after Metuzumab treatment. *P < .05, **P < .01, ****P < .0001 versus normal group; (f) Representative Western blot analysis of CD147, IGF-1, AKT and p-AKT in Eca-109.
We used in vitro invasion assay to explore whether Metuzumab can inhibit invasion of Eca-109 cells. The results showed that Metuzumab treatment groups (0.1μg/mL, 1μg/mL and 10μg/mL) significantly decreased cells invasion (Figure 4(b,d)) by 29.37%, 38.61%, 65.34%, respectively, compared with control group. Similar results were found in cHAb18 treatment group.
Our previous work showed that CD147 can regulate expression of IGF-1 to influence the migration of microglia cells.18 This is actually enlightening to identify the relationship between IGF-1 and the metastasis of esophageal cancer. Therefore, we examined the expression of IGF-1 (Figure 4(e,f)) and PI3k/Akt pathway in Eca-109 cells treated with Metuzumab, the results of quantitative real-time PCR and Western blot showed that expression of IGF-1 was decreased with Metuzumab treatment. Vice versa, the expression of IGF-1 was markedly increased by over-expression of CD147. AKT phosphorylation of Eca-109 was inhibited with Metuzumab treatment and could be recovered by over-expression of CD147. This result indicated that the impeditive effects of Metuzumab on IGF-1 activation might be associated with the inhibition of the phosphorylated AKT signaling pathways.
Metuzumab inhibited lymph node metastasis of xenograft esophageal cancer
To investigate whether Metuzumab inhibits lymph node metastasis in vivo, we used Eca-109 footpad xenograft esophageal cancer models in nude mice (Figure 5). Animals were randomized to receive human IgG control, cHAb18 (2mg/kg), or Metuzumab (2mg/kg) treatment. The results showed that numbers of mice with metastasis to armpit lymph nodes significantly were decreased in Metuzumab treatment groups (P < .001), compared with control group. These results indicate that Metuzumab inhibited distant lymphatic metastasis of esophageal cancer in vivo.
Figure 5.
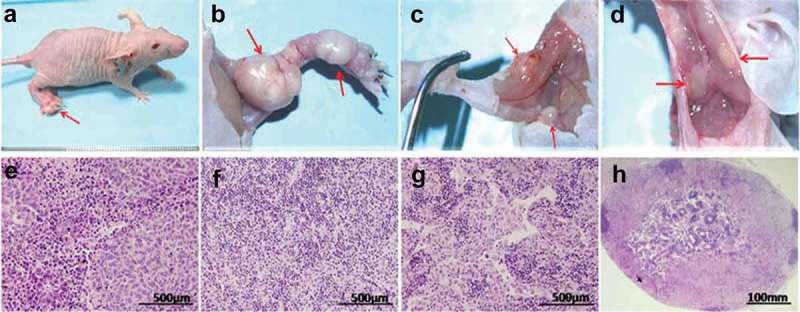
Metuzumab inhibited lymph node metastasis of xenograft esophageal cancer.
(a) Xenograft on footpad. (b) Carcinoma on footpad and popliteal fossa. (c) Enlarged lymph nodes on groin. (d) Enlarged lymph nodes on armpit; HE staining of (e) carcinoma on footpad, (f) normal lymph nodes, (g)(h) carcinoma in lymph nodes.
HAb18G/CD147 is highly expressed on esophageal cancer tissues, and associated with lymphatic metastasis
To evaluate whether HAb18G/CD147 is highly expressed on esophageal cancer tissues, we used two-step Envision immunohistochemical technique on tissue microarrays (TMAs) (Figure 6) which included 40 samples of human esophageal carcinoma tissues and eight samples of normal esophageal tissues.
Figure 6.

HAb18G/CD147 is highly expressed on esophageal cancer.
(a) Benign (b) Squamous cell carcinoma (c) Adeno-carcinoma (d) Small cell esophagus cancer
Result showed that HAb18G/CD147 is high-expressed on various kinds of esophageal cancer tissues (Table 2). We further analyzed the association between HAb18G/CD147 and lymphatic metastasis in esophageal cancer and found that the strongly positive cases was significantly associated with the grade of lymph node-metastasis (Table3). Thus, HAb18G/CD147 is associated with lymphatic metastasis in esophageal cancer.
Discussion
Antibody drug is a promising biological therapy for varieties of cancers in recent years. The mechanisms of tumor cell killing by antibodies can be summarized as follows: direct action of the antibody (through receptor blockade or agonist activity) delivery of a drug or cytotoxic agent;19 immune-mediated cell killing mechanisms, including complement-dependent cytotoxicity (CDC), antibody-dependent cellular cytotoxicity (ADCC) and regulation of T cell function.20 The Fc function of antibodies is particularly important for mediating tumor cell killing through CDC and ADCC.21 All of these approaches have been successfully applied in the clinic.
ADCC is one of the immune system’s killing mechanisms.22 In ADCC, antibody firstly binds to its target on tumor cells, and following this, the Fc portion is recognized by FcγR on effector cells, such as Natural killing (NK) cells, which comprise 10–20% of peripheral blood mononuclear cells (PBMC).23 Interaction of the Fc with the FcγR activates effector cells, resulting in the release of molecules contained in cytotoxic granules, such as perforin, granzymes, and granulysin, which leads to lysis of tumor cells. The ability of therapeutic antibodies to induce ADCC depends on their binding affinity to both the target and to the activating FcγR. The Fc portion of a therapeutic antibody may therefore have an important role in its mechanism of action through its influence on ADCC.
ADCC characterizes mAb as a “bridge” to link immune cells such as NK cells, monocytes and macrophages to target cells like cancer cells and kill them. It can occur if Fc portion of mAb binds to activating receptor FcγRs on effector cells. By contrast, inhibitory FcγRs will supress this immune response.24 Methods for improving affinity of mAb Fc portion to activating FcγRs are generally ways to enhance ADCC effects.
One approach to optimizing FcγR affinity is amino acid modification of mAb, using alanine scanning and site-directed mutagenesis, computational structure-based design, and selection-based methods.25 Another method for enhancing FcγR affinity is glycosylation modification of mAb. It has been proved that low level of fucosylation of mAb can increase ADCC by enhancing its binding to activated Fc receptor but reducing its binding to inhibitory Fc receptor. Approaches to block fucosylation modification of mAb mainly focus on glycoengineering of CHO lines, including generation of cells with inducible expression of the enzyme β (1,4)-N-acetylglucosaminyltransferase III (GnTIII), and genetic knockout of the enzyme α-1,6-fucosyltransferase (FUT8).26
In our study, we expressed our antibody Metuzumab in a patented engineered CHO host cell (Patent No.US8084222 B2, Eureka Therapeutics, Inc.) which leading to glycoform modification in Fc region of Metuzumab and enhanced its ADCC effects. The host cell was manufactured by treatment of chemical mutagen and selection of LCA-toxin. The chemical mutagen may possibly produce host cells with variety of glycosylation patterns such as ones with high or low level of fucosylation. LCA-toxin specifically targeted and eliminated cells with high fucosylation and enriched mutant cells with low levels. Therefore, when Metuzumab was expressed in such cell, it will have a low level of fucosylation and enhanced ADCC effects.
Our results showed Metazumab retained high binding activity to its antigen CD147 after glycosylation modification but enhanced efficiency against both two esophageal cancer cell lines in presence of human effector cells, comparing to its parental chimeric mAb cHAb18. The mechanism could be that Metuzumab had higher affinity for activating Fc receptor (FcγRIIIA in human and FcγRIV in mice). The measurement of in vivo activity of ADCC was not easily tested. Usually, immunodeficient mice such as nude mice or NOD/SCID mice or NOD/SCID/gammaCnull (NOG) mice have been frequently used.27 In some cases, mice with severe immunodeficiency were transplanted with human tumors, and subsequently given human PBMC and therapeutic antibody. Our study indicated that nude mice xenograft model could be a handy in vivo model for testing the therapeutic benefit of Metuzumab without given human PBMC, for mouse effector cell may work as well.
CD147 has been proved to be associated with tumor progression. The results of our study showed that Metuzumab inhibited migration and invasion of esophageal cancer cells in vitro. Insulin-like growth factor 1 (IGF1) is a growth and differentiation factor and has been confirmed to induce EMT and lymphangiogenesis of cancers.28,29 Therofore, metastasis of esophageal cancer will be attenuated if inhibiting IGF-1 activation, Our previous data showed that IGF-1 was down-regulated in microglial cell when knocking down CD147. To further clarify the antagonistic mechanism of Metuzumab, we examined the IGF-1 in esophageal cancer cells treated with Metuzumab. The results showed that Metuzumab decreased expression of IGF-1 via PI3K-AKT pathway. This suggests that Metuzumab might inhibit metastasis of esophageal cancer through suppressing IGF-1 expression of esophageal cancer.
The footpad and subcutaneous xenograft nude mouse models were used to explore the anti-growth and anti-metastasis efficacy of Metuzumab against esophageal cancer in vivo. The results revealed that Metuzumab exhibited dual mode of therapy effects in nude mouse xenograft models.
Methods
Development of Metuzumab
Metuzumab was established by recombinant antibody engineering technology. The variable region of the heavy and light chains of HAb18 IgG (targeting CD147, derived from mouse) was inserted into an expression vector that contained the constant region of a human antibody. We named the chimeric antibody cHAb18.The Metuzumab was obtained from cHAb18 by modifying its glycosylation form in an engineered CHO cell, which was characterized by producing proteins with a variant glycosylation pattern, particularly a low level of fucosylation. We used the cHAb18 antibody expressed by normal CHO host cells as a control in the study. The cHAb18 antibody contained an identical amino acid sequence as that of Metuzumab with the exception of the wild-type glycoform (Figure 1(a)).
Binding activity determination
Surface plasmon resonance (SPR) measurements for calculating binding activity were performed using ProteOn XPR36 (Bio-Rad). The antibody avidity for CD147 was determined by immobilizing Metuzumab or cHAb18 antibody on a GLC Chip (Bio-Rad) and injecting CD147 over the antibody-bound surface. All of the experiments were performed at 25℃ using PBST as the running buffer [Phosphate Buffered Saline pH 7.4 and 0.05% (v/v) Tween 20] unless noted otherwise. A ProteOn XPR36 biosensor was equipped with a GLC chip (Bio-Rad) and coupling reagents (10 mM sodium acetate, pH 4.5, Sulfo-NHS (N-hydroxysulfosuccinimide), 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride [EDC], and ethanolamine). Antibodies at 10μg/mL were immobilized on the GLC chip surface at 25μl/min. CD147 in two-fold serial dilutions (five concentrations starting at 10nM) was injected over the antibody-bound surface for 3 min at 50μl/min and then subjected to a 12-min dissociation phase. After each cycle, the surface was regenerated by injecting glycine buffer (10 mM, pH 1.5). Kinetic analyses were performed by the global fitting of the binding data with a 1:1 Langmuir binding model using the ProteOn Manager™ software (Bio-Rad).
Assay for mAb binding to Fc receptors
The binding of Metuzumab and cHAb18 to human FcRs was determined using a sandwich ELISA assay. Metuzumab and cHAb18 were coated in 96-well plates. Recombinant human Fc gamma RIIIA (CD16a), recombinant mouse Fc gamma RIV (CD16-2) and recombinant human Fc gamma RIIB (CD32b/c) were purchased from R&D systems. Rabbit-anti-His [HRP] was used as the secondary antibody.
Cell lines and animal
Human esophageal cancer cell lines: Eca-109 and TE-1 were obtained from Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences. Cells were cultured in RPMI 1640 medium (Gibco, Grand Island, NE, USA), supplemented with 10% fetal calf serum (FBS), 1% penicillin/streptomycin and 2% L-glutamine at 37 °C in a humidified atmosphere of 5% CO2.
BALB/C nude mice (Beijing HFK Bioscience, Beijing Co., Ltd, China) were maintained in a pathogen-free facility, kept in micro-isolator cages, and used when 4–6 weeks old. FMMU’s Institutional Animal Care and Use Committee approved all experiments.
Antibody-dependent cell mediated cytotoxicity
Human blood samples were collected from healthy volunteer donors with informed consent using a protocol approved by the Institutional Review Board of Fourth Military Medical University. Human peripheral blood mononuclear cells (PBMC) were isolated from whole blood by centrifugation through a Ficoll-hypaque gradient. Effector cells (PBMC cells) were cocultured for 24 h with Eca-109 or TE-1 at an effector to target cell ratio (E/T) of 50:1. Metuzumab, cHAb18, Hu-IgG or Cetuximab was added in culture solution, respectively. Cell viability was assessed using the xCELLigence real-time cell analysis (RTCA) system (ACEA Biosciences, San Diego, CA, USA). ELISAs were done according to the respective manufacturer protocols: human IFN-γ (R & D Systems, Minneapolis, MN); human Granzyme B (eBioscience, San Diego, CA); human Perforin-1 (Abcam, Cambridge, MA).
Wound healing assay
Eca-109 and TE-1 cells were seeded and cultured until confluent in 24 well plates. The monolayer was scratched with a pipette tip to create a cross in each well. Cells were washed twice with medium to remove the detached cells. Cells were cultured for additional 24 h with fresh medium containing different concentration of Metuzumab or cHAb18. Photos for the wound were taken with same configuration of the microscope. The gap distance was quantitatively evaluated by Image-Pro Plus 6.0.
Invasion assay
Cell invasion assay was performed using 24-well Transwell units with an 8-mm pore size polycarbonate filter (Millipore, MA, USA) according to the manufacturer’s instructions. Tumor cells (2 × 105) pre-incubated with antibodies were suspended in 0.1 mL RPMI 1640 containing 0.1%BSA and added into the upper compartment of the Transwell unit and incubated for 36 h at 37 °C in a humidified atmosphere containing 5% CO2. The number of cells that had invaded through the filter into the lower compartment was determined using a colorimetric crystal violet assay.
Quantitative real-time reverse transcription PCR
Reverse transcription, followed by a quantitative PCR analysis (qRT–PCR), was performed to check expression of IGF1. The sequences of specific primers for IGF-1 were listed as follows: IGF-1 (forward: 5ʹ-CTTCACACCTCTTCTACCTGGCG-3ʹ; reverse: 5ʹ-AAGTAAAAGCCCCTCGGTCCACA-3ʹ). The qRT–PCR was run in CFX96 Real-time system (Bio-Rad, Hercules, CA, USA) according to standard protocol. Briefly, thermal cycling conditions were an initial activation step for 10 min at 95°C followed by 40 cycles at 95°C for 15 s then 58°C for 1 min and 72°C for 15 sec. No template control (NTC) consisting of H2O was included in each run. A melt curve analysis was performed to ensure specific amplification. At least three independent experiments were performed. The results were normalized as the fold change for each mRNA of target gene toβ-Actin, which was calculated from standard curves using the 2-ΔΔCT method.
Western blot analysis
Cells lysates were prepared in lysis buffer (RIPA with 1mM phenylmethanesulfonyl fluoride and1X proteinase inhibitor per mL), and protein concentration was determined by BCA protein assay kit (Sangon Biotech, Shanghai, China). Equal amounts (20μg) of protein were electrophoresed on a 10% sodium dodecyl sulfate (SDS) polyacrylamide gel and transferred onto a 0.22 mm PVDF membrane from Millipore (Bedford, MA, USA). The primary antibodies were used to probe the membranes at 4°C overnight. The membranes were washed and incubated with secondary antibodies for 60 min. Secondary antibodies were chosen according to the primary antibodies origin. After three washes with TBST, protein band signals were detected with an enhanced chemiluminescence system (Millipore). The density of the band was standardized to that of tubulin.
Subcutaneous xenograft nude mouse model
Log-phase cell cultures of Eca-109 cells were harvested with 1 mM EDTA in phosphate-buffered saline (PBS), washed three times with serum-free RPMI-1640, diluted by PBS at a cell density of 1 × 107/mL. A 0.4 mL insulin syringe (Becton Dickinson) was used to inject the cell inoculum into the subcutaneous on the right side of the back of BALB/C nude mice.
Tumor-bearing animals that were subjected to control (human IgG, 2mg/kg), cHAb18 (2mg/kg), or Metuzumab (0.4, 2, or 10mg/kg) were sacrificed 5 weeks after initiation of administration. The tumor tissues were collected from the different cohorts, and the weights were determined to assess the tumor growth.
Footpad xenograft nude mouse model
Eca-109 cells were harvested as the method above and diluted by PBS at a cell density of 4 × 107/mL. Five-week-old male BALB/C nude mice were injected in the left hind leg footpad with 2 × 106 Eca-109 cells in 50 µl of PBS. Mice were treated with control (human IgG, 2mg/kg) or Metuzumab (2mg/kg) 6 ~ 7 days after injection when tumors were 2 ~ 3 mm in diameter. Tumor-bearing animals were sacrificed 4 weeks later. The tumor tissue on footpads and lymph nodes ipsilateral were collected from the different cohorts. H&E staining was used to detect tumor metastasis in lymph nodes.
Tissue microarrays and immunohistochemical
Tissue microarrays (TMAs) which included 40 samples of human esophageal carcinoma tissues and eight samples of normal esophageal tissues were purchased from Shaanxi Alenabio Co., Ltd (Xi’an, China). Two-step Envision immunohistochemical technique (Dako, Carpinteria, CA, USA) was used to detect the expression of CD147 in esophageal carcinoma tissues. The sections were incubated with the primary antibody HAb18 mAb. Immunopositivity was independently evaluated by two pathologists who were blinded to clinical data.
Statistical analysis
One-way ANOVA, Student’st test and chi-square test were performed with GraphPad Prism 5 software for windows (GraphPad Software, Inc.). A probability of less than 0.05 was considered statistically significant.
Funding Statement
This study was supported by National Natural Science Foundation of China (No. 81872129).
Abbreviations
- IGF-1
Insulin-like growth factors 1
- TMA
tissue microarrays
- CDCC
complement-dependent cellular cytotoxicity
- ADCC
antibody-dependent cellular cytotoxicity
- EMMPRIN
extracellular matrix metalloproteinase inducer
- MMPs
matrix metalloproteinases
- HCC
hepatocellular cancer
- HAMA
human anti-mouse antibody
- Fc
constant fragment
- SPR
Surface plasmon resonance
- FBS
fetal calf serum
- PBMC
peripheral blood mononuclear cells
- qRT–PCR
quantitative real-time PCR
- NTC
no template control
- CMA
Concanamycin A
- DCI
3,4-dichloroisocoumarin
- CDC
complement-dependent cytotoxicity
- NK cells
Natural killing cells
- GnTIII
β (1,4)-N-acetylglucosaminyltransferase III
- FUT8
α-1,6-fucosyltransferase
Acknowledgments
We thank all the staff in the laboratory.
Authors’ contributions
YL, HY, XMY, and YW conceived and designed the study. MW, SZ, QS, YMZ, RZS performed the experiments. MW and SZ wrote the paper. YL, HY, XMY, and YW reviewed and edited the manuscript. All authors read and approved the manuscript.
DeclarationsEthics approval and consent to participate
The research design was approved by the Ethics Committee. All procedures are conducted in conformity to ethical standards on human.
Consent for publication
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Disclosure of potential conflict of interest
The authors declare that they have no competing interests.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A.. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018. November;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Nakajo K, Yoda Y, Hori K, Takashima K, Sinmura K, Oono Y, Ikematsu H, Yano T. Technical feasibility of endoscopic submucosal dissection for local failure after chemoradiotherapy or radiotherapy for esophageal squamous cell carcinoma. Gastrointest Endosc. 2018;88(4):637–646. doi: 10.1016/j.gie.2018.06.033. [DOI] [PubMed] [Google Scholar]
- 3.Ohashi S, Miyamoto S, Kikuchi O, Goto T, Amanuma Y, Muto M. Recent advances from basic and clinical studies of esophageal squamous cell carcinoma. Gastroenterology. 2015;149(7):1700–1715. doi: 10.1053/j.gastro.2015.08.054. [DOI] [PubMed] [Google Scholar]
- 4.Belkhiri A, El-Rifai W. Advances in targeted therapies and new promising targets in esophageal cancer. Oncotarget. 2015;6(3):1348–1358. doi: 10.18632/oncotarget.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Ran Y, Yu L, Hu H, Zhou Z, Sun L, Lou J, Yang Z. Monoclonal antibody to human esophageal cancer endothelium inhibits angiogenesis and tumor growth. Anticancer Res. 2006;26(4B):2963–2970. [PubMed] [Google Scholar]
- 6.Wilke H, Muro K, Van CE, Oh SC, Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, et al.; RAINBOW Study Group . Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014. October;15(11):1224–1235. doi: 10.1016/S1470-2045(14)70420-6. [DOI] [PubMed] [Google Scholar]
- 7.Lyons TG, Ku GY. Systemic therapy for esophagogastric cancer: targeted therapies. Chin Clin Oncol. 2017. October;6(5):48. doi: 10.21037/cco. [DOI] [PubMed] [Google Scholar]
- 8.Kanekura T, Miyauchi T, Tashiro M, Muramatsu T. Basigin, a new member of the immunoglobulin superfamily: genes in different mammalian species, glycosylation changes in the molecule from adult organs and possible variation in the N-terminal sequences. Cell Struct Funct. 1991;16:23–30. [DOI] [PubMed] [Google Scholar]
- 9.Feng F, Wang B, Sun X, Zhu Y, Tang H, Nan G, Wang L, Wu B, Huhe M, Liu S, et al. Metuzumab enhanced chemosensitivity and apoptosis in non-small cell lung carcinoma. Cancer Biol Ther. 2017;18(1):51–62. doi: 10.1080/15384047.2016.1276126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li H, Jiang C, Wu D, Shi S, Liao M, Wang J, Li Y, Xu Z. The prognostic and clinicopathologic characteristics of CD147 and esophagus cancer: A meta-analysis. PLoS One. 2017;12(7):e0180271. doi: 10.1371/journal.pone.0180271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, Huang Q, Long X, Zhang J, Huang X, Aa J, Yang H, Chen Z, Xing J. CD147 reprograms fatty acid metabolism in hepatocellular carcinoma cells through Akt/mTOR/SREBP1c and P38/PPARalpha pathways. J Hepatol. 2015;63(6):1378–1389. doi: 10.1016/j.jhep.2015.07.039. [DOI] [PubMed] [Google Scholar]
- 12.Knutti N, Huber O, Friedrich K. CD147 (EMMPRIN) controls malignant properties of breast cancer cells by interdependent signaling of Wnt and JAK/STAT pathways. Mol Cell Biochem. 2019;451(1–2):197–209. [DOI] [PubMed] [Google Scholar]
- 13.Nasry WHS, Wang H, Jones K, Dirksen WP, Rosol TJ, Rodriguez-Lecompte JC, Martin CK. CD147 and cyclooxygenase expression in feline oral squamous cell carcinoma. Vet Sci. 2018;5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang H, Chen B. CD147 in ovarian and other cancers. Int J Gynecol Cancer. 2013;23(1):2–8. doi: 10.1097/IGC.0b013e3182749139. [DOI] [PubMed] [Google Scholar]
- 15.Xiao W, Zhao S, Shen F, Liang J, Chen J. Overexpression of CD147 is associated with poor prognosis, tumor cell migration and ERK signaling pathway activation in hepatocellular carcinoma. Exp Ther Med. 2017;14(3):2637–2642. doi: 10.3892/etm.2017.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu S, Li Y, Mi L, Zhang Y, Zhang L, Gong L, Han X, Yao L, Lan M, Chen Z, et al. Clinical impact of HAb18G/CD147 expression in esophageal squamous cell carcinoma. Dig Dis Sci. 2011;56(12):3569–3576. doi: 10.1007/s10620-011-1812-x. [DOI] [PubMed] [Google Scholar]
- 17.Xu J, Shen ZY, Chen XG, Zhang Q, Bian HJ, Zhu P, Xu HY, Song F, Yang XM, Mi L, et al. A randomized controlled trial of licartin forpreventing hepatoma recurrence after liver transplantation. Hepatology. 2007;45(2):269–276. doi: 10.1002/hep.21465. [DOI] [PubMed] [Google Scholar]
- 18.Houghton AN, Scheinberg DA. Monoclonal antibody therapies-a ‘constant’ threat to cancer. Nat Med. 2000;6(4):373–374. doi: 10.1038/74621. [DOI] [PubMed] [Google Scholar]
- 19.Yin J, Xu WQ, Ye MX, Zhang Y, Wang HY, Zhang J, Li Y, Wang YS. Up-regulated basigin-2 in microglia induced by hypoxia promotes retinal angiogenesis. J Cell Mol Med. 2017;21(12):3467–3480. doi: 10.1111/jcmm.13256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6(4):443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 21.Weiner GJ. Rituximab: mechanism of action. Semin Hematol. 2010;47:115–123. doi: 10.1053/j.seminhematol.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barkas T, Al-Khateeb SF. Serum factors influencing antibody-directed cell-mediated cytotoxicity (ADCC) and their effects on the detection of immune complexes by inhibition of ADCC. Immunology. 1979;36(2):299–306. [PMC free article] [PubMed] [Google Scholar]
- 23.Timonen T. Natural killer cells: endothelial interactions, migration, and target cell recognition. J Leukoc Biol. 1997;62(6):693–701. doi: 10.1002/jlb.62.6.693. [DOI] [PubMed] [Google Scholar]
- 24.Jiang XR, Song A, Bergelson S, Arroll T, Parekh B, May K, Chung S, Strouse R, Mire-Sluis A, Schenerman M. Advances in the assessment and control of the effector functions of therapeutic antibodies. Nat Rev Drug Discov. 2011;10(2):101–111. doi: 10.1038/nrd3365. [DOI] [PubMed] [Google Scholar]
- 25.Desjarlais JR, Lazar GA, Zhukovsky EA, Chu SY. Optimizing engagement of the immune system by anti-tumor antibodies: an engineer’s perspective. Drug Discov Today. 2007;12(21-22:898–910. doi: 10.1016/j.drudis.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 26.Yamane-Ohnuki N, Kinoshita S, Inoue-Urakubo M, Kusunoki M, Iida S, Nakano R, Wakitani M, Niwa R, Sakurada M, Uchida K, et al. Establishment of FUT8 knockout Chinese hamster ovary cells: an ideal host cell line for producing completely defucosylated antibodies with enhanced antibody-dependent cellular cytotoxicity. Biotechnol Bioeng. 2004;87(5):614–622. doi: 10.1002/bit.20151. [DOI] [PubMed] [Google Scholar]
- 27.Nimmerjahn F, Ravetch J. Fcγ receptors as regulators of immune responses. Nature Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 28.Zhou Y, Zhang Z, Wang N, Chen J, Zhang X, Guo M, John Zhong L, Wang Q. Suppressor of cytokine signalling-2 limits IGF1R-mediated regulation of epithelial-mesenchymal transition in lung adenocarcinoma. Cell Death Dis. 2018. April 1;9(4):429. doi: 10.1038/s41419-018-1111-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Björndahl M, Cao R, Nissen LJ, Clasper S, Johnson LA, Xue Y, Zhou Z, Jackson D, Hansen AJ, Cao Y. Insulin-like growth factors 1 and 2 induce lymphangiogenesis in vivo. Proc Natl Acad Sci USA. 2005;102:15593–15598. doi: 10.1073/pnas.0507865102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


